Abstract
Reg (Regenerating gene) gene was originally isolated from rat regenerating islets and its encoding protein was revealed as an autocrine/paracrine growth factor for β cells. Rat Reg gene is activated in inflammatory conditions for β cell regeneration. In human, although five functional REG family genes (REG Iα, REG Iβ, REG III, HIP/PAP, and REG IV) were isolated, their expressions in β cells under inflammatory conditions remained unclear. In this study, we found that combined addition of IL-6 and dexamethasone (Dx) induced REG Iα and REG Iβ expression in human 1.1B4 β cells. Promoter assay revealed that a signal transducer and activator of transcription- (STAT-) binding site in each promoter of REG Iα (TGCCGGGAA) and REG Iβ (TGCCAGGAA) was essential for the IL-6+Dx-induced promoter activation. A Janus kinase 2 (JAK2) inhibitor significantly inhibited the IL-6+Dx-induced REG Iα and REG Iβ transcription. Electrophoretic mobility shift assay and chromatin immunoprecipitation revealed that IL-6+Dx stimulation increased STAT3 binding to the REG Iα promoter. Furthermore, small interfering RNA-mediated targeting of STAT3 blocked the IL-6+Dx-induced expression of REG Iα and REG Iβ. These results indicate that the expression of REG Iα and REG Iβ should be upregulated in human β cells under inflammatory conditions through the JAK/STAT pathway.
1. Introduction
Decreased functional β cell mass is one of hallmarks in both type 1 and type 2 diabetes. Regeneration of pancreatic β cells has been shown to occur at a basal rate in normal adult tissues and to increase under certain conditions such as pregnancy and obesity [1–3]. However, the mechanism of human pancreatic β cell regeneration still remains to be elucidated.
Reg (Regenerating gene) gene product, Reg protein, could be responsible for the regenerative process [4–6]. The Reg gene was originally isolated from regenerating pancreatic islets from depancreatized rats, and it encodes a 16 kDa autocrine/paracrine growth factor for β cells [7, 8]. The Reg and Reg-related genes were isolated and revealed to constitute a multigene family, the Reg family, which consists of four subtypes (types I, II, III, and IV) based on the primary structures of the encoded proteins of the genes [4, 5, 9, 10]. The type I Reg gene, Reg I, was the first isolated Reg gene from the regenerating islets [7]. In rat and mouse, Reg I protein is not expressed in pancreatic β cells in physiological conditions but is induced during islet regeneration [4, 7, 11, 12]. Therefore, the Reg I gene induction, which occurs in response to inflammatory mediators such as interleukin- (IL-) 6 and glucocorticoid analogue dexamethasone (Dx) in rat RINm5F β cells [13], is considered to be one of the crucial events in β cell regeneration. In human, five functional REG family genes (REG Iα, REG Iβ, REG III, HIP/PAP, and REG IV) were isolated [4, 7, 14–19]. However, which human REG gene is expressed in β cells during regeneration is still obscure mainly because of their restrict supply of human islets. Induction of proliferation/regeneration in human β cells must be beneficial for both type 1 and type 2 diabetes treatment/prevention [20]. The human REG genes would play an important role in β cell proliferation/regeneration in human as the Reg I gene does in rodents. Since there are many differences in cell proliferation between rodent and human β cells [21], it is crucial to investigate the expression of human REG genes in human β cells. Recently, β cell line, 1.1B4, was established and becomes available as human pancreatic β cells [22]. In the present study, we investigated the induction of human REG family genes in inflammatory conditions in 1.1B4 human β cells.
2. Materials and Methods
2.1. Cell Culture and Reagents
Human 1.1B4 cells, an insulin-releasing human pancreatic β cell line (European Collection of Cell Culture, Salisbury, UK) [22, 23], and rat RINm5F cells were grown in RPMI 1640 medium (NACALAI TESQUE, Kyoto, Japan) containing 10% (v/v) fetal calf serum, 100 units/mL penicillin G, and 100 μg/mL streptomycin (Wako Pure Chemical Industries, Osaka, Japan) as described [23]. The cells were maintained in a 5% CO2-95% air, water-saturated atmosphere at 37°C. Recombinant human IL-6, tumor necrosis factor- (TNF-) α, and interferon- (IFN-) γ were purchased from Roche Applied Science (Indianapolis, IN). Recombinant human IL-1β, IL-22, IFN-β, IL-8, and H2O2 were obtained from Wako Pure Chemical Industries. Recombinant human platelet-derived growth factor (PDGF) was obtained from PeproTech (Rocky Hill, NJ). Sodium palmitate (Sigma, St. Louis, MO) was prepared as albumin-bound form by stirring at 45°C with defatted bovine serum albumin (Cosmo Bio Co., Tokyo, Japan). Dexamethasone (Dx) was purchased from MP Biomedicals (Santa Ana, CA). S-Nitroso-N-acetylpenicillamine (SNAP), a NO donor [24], and AG490, a Janus kinase 2 (JAK2) inhibitor [25], were obtained from Calbiochem (Billerica, MA) and were dissolved in dimethyl sulfoxide as stock solutions. Streptozotocin (STZ) (Sigma) was dissolved in citrate buffer (pH 4.5) and further diluted with cell culture media just before use. 3-Aminobenzamide (3AB) was purchased from Tokyo Kasei Co. (Tokyo, Japan).
2.2. Promoter Assays
The reporter constructs were prepared by inserting the 5′-flanking regions of human REG family genes (REG Iα, GenBank Accession No. J05412; −1190~+26, −569~+26, −462~+26, −168~+26, −146~+26, −130~+26, REG Iβ, GenBank Accession No. D17291; −978~+31, −155~+31, −139~+31, REG III, GenBank Accession No. AB161039; −1550~+64, HIP/PAP, GenBank Accession No. X79987; −1520~+17, REG IV, GenBank Accession No. AL592186; −1053~+22) upstream of a firefly luciferase reporter gene in pGL3-Basic vector (Promega, Madison, WI). For construction of the promoter region mutants used in this study, the indicated sequences in the “−146” REG Iα/luciferase constructs and “−155” REG Iβ/luciferase constructs were substituted by primers using PCR. The cells were grown in 24-well plates and were transfected with reporter plasmids by lipofection. Briefly, 0.8 μg of each reporter plasmid and 0.08 μg of pCMV-SPORT-β-galactosidase (Life Technologies, Carlsbad, CA), as an internal control, were mixed with Lipofectamine 2000 (Life Technologies) per well in a 24-well plate. After 24 h incubation, the cells were treated with indicated amount of stimulants, 100 nM Dx, 20 ng/mL IL-6, 300 U/mL IL-1β, 370 U/mL TNF-α, 100 U/mL IFN-γ, 60 U/mL IL-1β + 185 U/mL TNF-α + 14 U/mL IFN-γ, 10 ng/mL IL-22, 1500 U/mL IFN-β, 100 nM IL-8, and 1 U/mL PDGF, amino acids at a concentration five times greater than that in basal RPMI, 100 μM palmitate, 10 μM H2O2, 250 μM SNAP, 2 mM STZ, and 2 mM 3AB, and combinations thereof. After 24 h treatment, the cells were harvested and processed for luciferase assay as described [13, 26]. In cases of STZ and STZ + Dx, the media containing STZ or STZ + Dx were changed with fresh media after 2 h treatment, and cells were further incubated for 22 h before harvest.
2.3. Quantitative Real-Time RT-PCR
Total RNA was isolated using the RNeasy Plus Mini Kit (Qiagen, Hilden, Germany) from 1.1B4 cells, and cDNA was synthesized from total RNA as a template using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) for the template of real-time PCR as described [23, 27, 28]. The cDNA was subjected to PCR with the following primers: β-actin (NM_001101) sense, 5′-GCGAGAAGATGACCCAGA-3′ and antisense, 5′- CAGAGGCGTACAGGGATA-3′; REG Iα (NM_002909) sense, 5′-AGGAGAGTGGCACTGATGACTT-3′ and antisense, 5′-TAGGAGACCAGGGACCCACTG-3′; REG Iβ (NM_006507) sense 5′-GCTGATCTCCTCCCTGATGTTC-3′ and antisense 5′-GGCAGCTGATTCGGGGATTA-3′; REG III (AB161037) sense 5′-GAATATTCTCCCCAAACTG-3′ and antisense 5′-GAGAAAAGCCTGAAATGAAG-3′; HIP/PAP (NM_138937) sense 5′-AGAGAATATTCGCTTAATTCC-3′ and antisense 5′-AATGAAGAGACTGAAATGACA-3′; REG IV (AY007243) sense 5′-ATCCTGGTCTGGCAAGTC-3′ and antisense 5′-CGTTGCTGCTCCAAGTTA-3′. All the PCR primers were synthesized by Nihon Gene Research Laboratories (Sendai, Japan). Real-time PCR was performed using KAPA SYBR FAST qPCR Master Mix (Kapa Biosystems, Boston, MA) and the Thermal Cycler Dice Real-Time System (Takara, Otsu, Japan) as described [23, 27, 28]. PCR was performed with an initial step of 3 min at 95°C followed by 40 cycles of 3 s at 95°C and 20 s at 60°C for β-actin, REG III, and HIP/PAP and 40 cycles of 3 s at 95°C and 20 s at 64°C for REG Iα, REG Iβ, and REG IV. The level of target mRNA was normalized to the mRNA level of β-actin as an internal standard.
2.4. Electrophoretic Mobility Shift Assay (EMSA)
Nuclear extracts were prepared as described previously and stored at −80°C until use [13, 29]. EMSAs were performed essentially as described previously [13]. DNA probes for EMSAs were synthesized as oligonucleotides. The sequences of the individual oligonucleotides in the sense orientation were as follows: probe 1, 5′-AGTGTGTGCCGGGAAAAGGCTCATA-3′ (nt. −148~−124 of human REG Iα promoter; nt. 1048–1072 of J05412), probe M1, 5′-AGTGTGCAGTAGGAAAAGGCTCATA-3′.
DNA-protein binding reactions were performed by incubation of the nuclear extracts in a solution containing 10 mM HEPES (pH 7.8), 50 mM KCl, 5 mM MgCl2, 1 mM EDTA, 10% glycerol, 5 mM DTT, 1 mg/mL BSA, 0.7 mM PMSF, 50 ng/μL of poly(dI-dC) (Roche) for 10 min at room temperature, followed by an additional 30 min incubation with 32P-end-labeled probe at room temperature. For supershift assays, 1 μg of anti-signal transducer and activator of transcription (STAT)3 antibody (Santa Cruz Biotechnology, SC-482X, Santa Cruz, CA) [30] was added to the samples and incubated for 15 min before incubation with the labeled probe. DNA-protein complexes were separated on 4% nondenaturing acrylamide gels and detected by autoradiography.
2.5. Chromatin Immunoprecipitation (ChIP) Assay
ChIP assay was performed using a ChIP-IT Express kit (Active Motif, Carlsbad, CA) following the manufacturer's instructions as described previously [31]. Briefly, 1.1B4 cells were treated with or without IL-6 + Dx and cross-linked with 1% formaldehyde for 10 min at room temperature. After washing and treatment with glycine Stop-Fix solution, the cells were lysed and nuclei were subjected to ultrasonic disruption for preparing DNA fragments ranging in size from 200 to 1500 bp. Immunoprecipitation was carried out overnight at 4°C using an incubation mixture containing sheared chromatin, protein G magnetic beads, and 3 μg of anti-STAT3 antibody (Santa Cruz Biotechnology, SC-482X). The immune complexes were precipitated, eluted, reverse cross-linked, and treated with proteinase K. The resulting DNA samples were subjected to PCR amplification (1 cycle of 94°C for 3 min, 35 cycles of 94°C for 30 s, 62°C for 30 s, 72°C for 1 min) of the REG Iα promoter using specific forward (5′-ACCTTGGACTTAGACAGCTTG-3′) and reverse (5′-ACCACGTCATTTAAGCAAAAGG-3′) primers designed to amplify the promoter region (−212 to −46 nucleotides in relation to the transcription start site). The PCR products were resolved by electrophoresis in a 2.5% agarose gel containing ethidium bromide for visualization.
2.6. RNA Interference (RNAi)
RNAi was performed using Silencer Select predesigned small interfering RNAs (siRNAs) (Life Technologies) [23] directed against human STAT3 gene. The sense sequence of siRNA (5′-GCACCUUCCUGCUAAGAUUtt-3′) was synthesized by Nihon Gene Research Laboratories. As a control, siRNA-scramble (Ambion, Life Technologies) [23] was also used. Transfection of siRNA to 1.1B4 cells was carried out using Lipofectamine RNAiMAX (Life Technologies). Cells were transfected with 5 pmol of siRNA in a 24-well culture dish (4 × 105 cells/mL) as described [23].
2.7. Data Analysis
Results are expressed as mean ± SE. Statistical significance was determined by Student's t-test using GraphPad Prism software (GraphPad Software, La Jolla, CA).
3. Results
3.1. Activation of Human REG Family Gene Promoters in β Cells
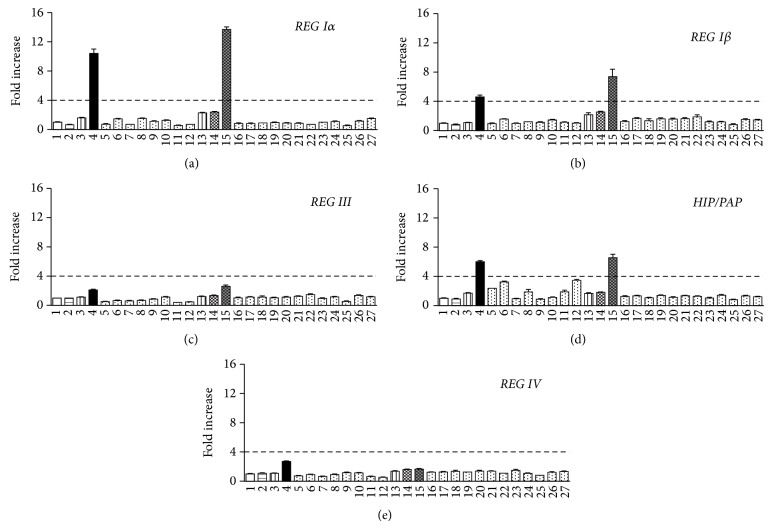
We first tested various stimuli on human REG family gene transcription in human 1.1B4 β cells. As Reg gene expression was observed in the phase of transient β cell proliferation: for example, in pancreatic islets of diabetic BB/Wor//Tky rats during the remission phase of diabetes [32], in islets of nonobese diabetic mice during active diabetogenesis [33] and pancreatic ductal cells, which are thought to be progenitor cells of β cells, during differentiation and proliferation in a mouse model of autoimmune diabetes [34, 35], and inflammation and cell death in and/or around islets was involved in these cases, we used inflammatory cytokines such as IL-1β, TNF-α, and IFN-γ [36], as well as pancreatic β cell toxic agents, such as palmitate [37, 38], H2O2 [39, 40], SNAP [41, 42], and STZ [43, 44]. Other factors, which were reported to upregulate Reg gene expression in β cells, such as IL-6 [13, 31], glucocorticoid [13, 31], IL-22 [6, 45], IFN-β [35], PDGF [46], and amino acids [46], were tested. We also used IL-8, which was reported to stimulate REG Iα promoter activity in gastric endocrine carcinoma cells [47]. As shown in Figure 1(a), IL-6 and IL-22 modestly increased the REG Iα promoter activity (columns 3 and 13). When IL-6 and the glucocorticoid analogue Dx were added together, the promoter activity was remarkably increased (columns 3 and 4). In contrast, the combination of IL-22 and Dx did not further increase the promoter activity (columns 13 and 14). The combination of IL-22 and IL-6 + Dx did not result in the synergistic response (columns 14 and 15). Treatments with other stimulants were ineffective or resulted in only small changes in promoter activity of REG Iα gene compared to IL-6 + Dx. These results showed that IL-6 + Dx was the most effective inducer for human REG Iα gene transcription in human β cells. IL-6 + Dx also enhanced the promoter activities of REG Iβ and HIP/PAP significantly; however, the fold increases were much smaller compared to that of REG Iα (Figures 1(a), 1(b), and 1(d)). Although the transcription of REG III and REG IV was induced to some extent by IL-6 + Dx, the increments were quite small (Figures 1(c) and 1(e)). The other treatments did not induce REG Iα, REG Iβ, REG III, HIP/PAP, nor REG IV transcription as much as IL-6 + Dx did.
Figure 1.
Effects of cytokines, β-cell toxic agents, and growth factors on the transcription of (a) REG Iα, (b) REG Iβ, (c) REG III, (d) HIP/PAP, and (e) REG IV in human 1.1B4 β cells. Cells were transfected with the human REG family gene reporter plasmids and treated as follows: 1, No addition; 2, Dx (100 nM); 3, IL-6 (20 ng/mL); 4, IL-6 + Dx; 5, IL-1β (300 U/mL); 6, IL-1β + Dx; 7, TNF-α (370 U/mL); 8, TNF-α + Dx; 9, IFN-γ (100 U/mL); 10, IFN-γ + Dx; 11, IL-1β (60 U/mL) + TNF-α (185 U/mL) + IFN-γ (14 U/mL); 12, IL-1β + TNF-α + IFN-γ + Dx; 13, IL-22 (10 ng/mL); 14, IL-22 + Dx; 15, IL-22 + IL-6 + Dx; 16, IFN-β (1500 U/mL); 17, IFN-β + Dx; 18, IL-8 (100 nM); 19, IL-8 + Dx; 20, PDGF (1 U/mL); 21, PDGF + Dx; 22, amino acids (5 times greater than that in RPMI 1640 medium); 23, palmitate (100 μM); 24, H2O2 (10 μM); 25, SNAP (250 μM); 26, STZ (2 mM); 27, STZ + Dx. The promoter activity was normalized for variations in transfection efficiency using β-galactosidase activity as an internal standard. Fold increase is calculated by dividing the promoter activity of stimulated cells by that of unstimulated cells (column 1). The broken line corresponds to 4-fold increase. Data are expressed as means ± SE for each group ((a) N = 3-4, (b–e) N = 3). Transcriptional activities of no. 4 and 15 (IL-6 + Dx and IL-22 + IL-6 + Dx) in panels (a), (b), and (d) were significantly (P < 0.005) and prominently (over 4 fold) increased.
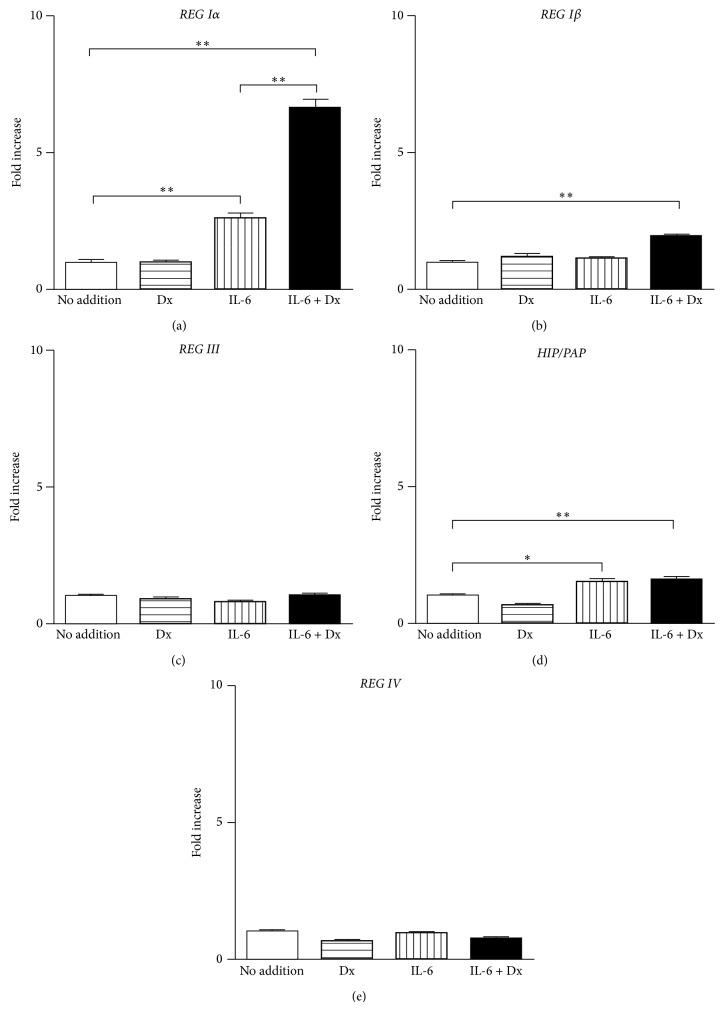
To determine whether the IL-6 + Dx-induced expression of REG family gene was restricted only in 1.1B4 cells, we investigated the effects of IL-6 + Dx on the promoter activities of human REG genes in rat RINm5F β cells. Similarly in 1.1B4 cells, the transcription of REG Iα was synergistically increased by the combined addition of IL-6 and Dx (Figure 2(a)). The REG Iβ transcription was also significantly induced by IL-6 + Dx in RINm5F cells (Figure 2(b)); however, the increase was not so large as that in 1.1B4 cells. The transcriptional activity of HIP/PAP was also increased by IL-6 and IL-6 + Dx about 1.5-fold (Figure 2(d)). On the other hand, the transcriptional activities of REG III and REG IV were never induced by Dx, IL-6, nor IL-6 + Dx (Figures 2(c) and 2(e)). These results indicated that activation of human type I REG gene transcription by IL-6 + Dx is general feature in pancreatic β cells.
Figure 2.
Effects of Dx and IL-6 on the promoter activity of (a) REG Iα, (b) REG Iβ, (c) REG III, (d) HIP/PAP, and (e) REG IV in rat RINm5F cells. RINm5F cells were transfected with the human REG family gene reporter plasmids and treated without (no addition) or with Dx (100 nM), or IL-6 (20 ng/mL), or IL-6 + Dx. The promoter activity was normalized for variations in transfection efficiency using β-galactosidase activity as an internal standard. Fold increase is calculated by dividing the promoter activity of stimulated cells by that of unstimulated cells (No addition). Data are expressed as means ± SE for each group (N = 3). * P < 0.01; ** P < 0.005.
3.2. Induction of mRNAs for REG Iα and REG Iβ by IL-6 + Dx in β Cells
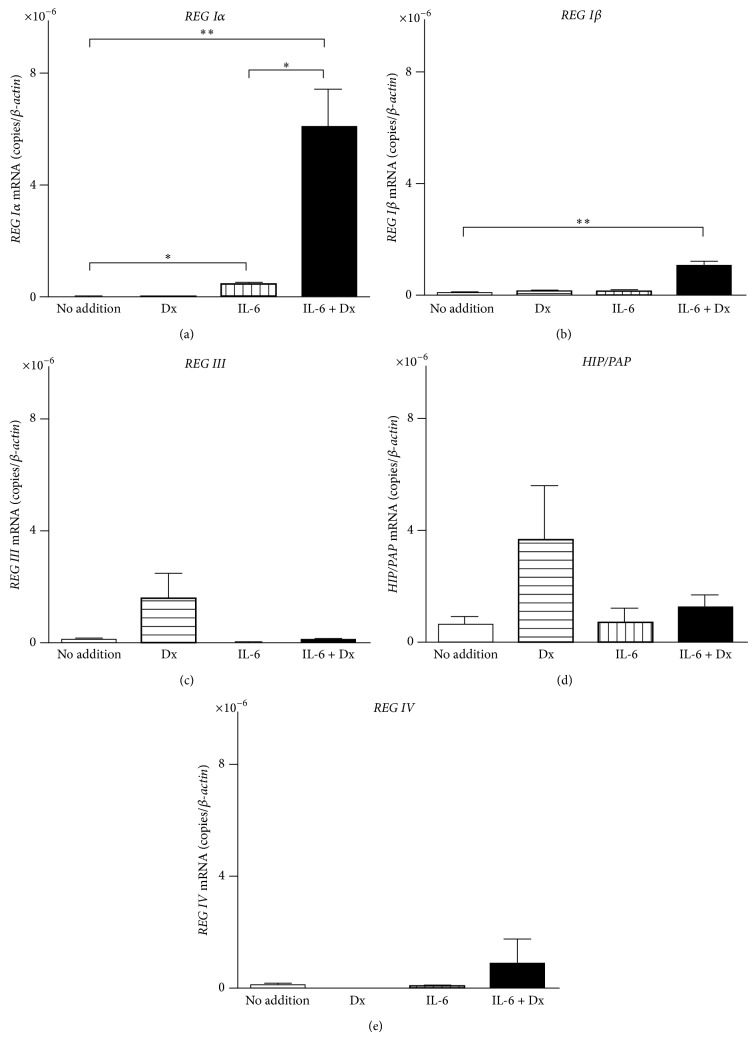
We next analyzed the mRNA levels of intrinsic human REG family genes in 1.1B4 cells treated with IL-6 and Dx by real-time RT-PCR. The mRNA levels of REG Iα and REG Iβ were significantly increased by the addition of IL-6 + Dx (Figures 3(a) and 3(b)). On the other hand, the mRNA levels of REG III, HIP/PAP, and REG IV were not significantly increased by the addition of neither IL-6, Dx, nor IL-6 + Dx (Figures 3(c), 3(d) and 3(e)). These results strongly suggest that type I REG family genes (REG Iα and REG Iβ) were induced in human β cells under inflammatory conditions by increased levels of circulating IL-6 and glucocorticoids.
Figure 3.
The mRNA levels of (a) REG Iα, (b) REG Iβ, (c) REG III, (d) HIP/PAP, and (e) REG IV in human 1.1B4 cells treated without (no addition) or with Dx (100 nM), or IL-6 (20 ng/mL), or IL-6 + Dx. Data are represented as the ratio of the number of target mRNA copies to the number of β-actin mRNA copies and expressed as means ± SE for each group (N = 4). * P < 0.05; ** P < 0.005.
3.3. IL-6 + Dx Response Element in REG Gene Promoter
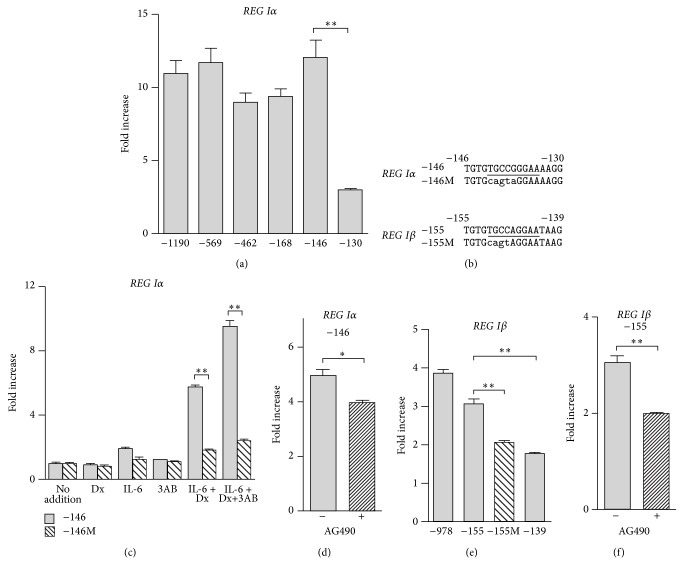
To identify the region essential for REG Iα induction by IL-6 + Dx, the reporter plasmids containing progressively deleted promoter fragments of REG Iα (−1190~+26, −569~+26, −462~+26, −168~+26, −146~+26, −130~+26) upstream of luciferase gene were transfected into 1.1B4 cells. Deletion of the REG Iα promoter from position of −1190 to −146 retained a significant increase in response to IL-6 + Dx in luciferase activity (Figure 4(a)). In contrast, further deletion to position −130 caused the loss of inducibility. These data suggest that the region between −146 and −131 is essential for the IL-6 + Dx-induced REG Iα transcription. The sequence analysis revealed the presence of elements homologous to the STAT-binding site between position −142 and −134 (TGCCGGGAA) (Figure 4(b)). Site-directed mutagenesis was conducted within the luciferase construct “−146,” and the influence of the mutation (“−146 M” in Figure 4(b)) on the amplitude of luciferase induction by IL-6 + Dx was monitored. The replacement of the STAT-binding sequence to the sequence CAGTAGGAA, which disrupted the binding of STAT (“−146 M”), significantly blocked the IL-6 + Dx-induced transcriptional activation of REG Iα in 1.1B4 cells (Figure 4(c)).
Figure 4.
IL-6 + Dx-induced transcription of REG Iα and REG Iβ in human 1.1B4 β cells is dependent on the JAK/STAT pathway. (a) IL-6 + Dx-induced REG Iα promoter activation in 1.1B4 cells. (b) Sequences of the human REG Iα and REG Iβ promoters. Putative STAT-binding site is underlined. Nucleotide substitutions in the STAT-binding site are indicated by lower-case letters. (c) Effects of site-directed mutation on IL-6 + Dx-induced REG Iα promoter activation. (d) Effects of AG490, a JAK inhibitor, on IL-6 + Dx-induced REG Iα promoter activity. 1.1B4 cells were transfected with indicated construct. (e) IL-6 + Dx-induced REG Iβ promoter activation in deleted and mutated promoter. (f) Effects of JAK inhibitor on IL-6 + Dx-induced REG Iβ promoter activity. Fold increase is calculated by dividing the promoter activity of IL-6 + Dx treated cells by that of untreated cells. Data are expressed as means ± SE for each group ((a) N = 4, (c) N = 3-4, (d) N = 3, (e) N = 3-4, (f) N = 4). * P < 0.05; ** P < 0.005.
In rat RINm5F cells, the cis-element of rat Reg I gene for induction in response to IL-6 + Dx was the −81~−70 region, which corresponds to the −76~−65 region of human REG Iα gene [13]. In RINm5F cells, poly(ADP-ribose) polymerase (PARP) was found to bind to the cis-element of Reg I gene and was involved in the active transcriptional DNA/protein complex formed by the stimulation of IL-6 + Dx. The DNA/protein complex formation was inhibited by the autopoly(ADP-ribosyl)ation of PARP in the complex. Thus, PARP inhibitors, such as 3AB, enhanced the DNA/protein complex formation for Reg I gene transcription [13]. To clarify whether PARP was involved in the IL-6 + Dx-induced human REG Iα transcription, we treated 1.1B4 cells with IL-6 + Dx + 3AB and measured the promoter activities of the constructs “−146” and “−146 M.” As shown in Figure 4(c), 3AB further enhanced the IL-6 + Dx-induced promoter activity of construct “−146.” On the other hand, the promoter activity of mutant construct “−146 M” was not induced as much as that of “−146” by IL-6 + Dx + 3AB, suggesting that STAT rather than PARP played a major role in the induction of human REG Iα transcription by IL-6 + Dx.
STAT proteins are activated by JAKs, and IL-6 induces activation of JAKs [48, 49]. Pretreatment of 1.1B4 cells with a JAK2 inhibitor, AG490 (50 μM), for 4 h significantly inhibited the inducibility of REG Iα promoter by IL-6 + Dx (Figure 4(d)). These results suggest that the JAK/STAT signaling pathway is involved in the IL-6 + Dx-induced REG Iα gene transcription in β cells.
As the nucleotide sequence of promoter region of REG Iα and REG Iβ is very similar, we searched possible STAT-binding sequence in the REG Iβ promoter and found that there is a STAT-binding site between position −151 and −143, which corresponds to the STAT-binding site of REG Iα (the −142~−134 region) as shown in Figure 4(b). The deletion and mutational analyses revealed that the STAT-binding site was essential to the IL-6 + Dx-induced REG Iβ transcription (Figure 4(e)). Moreover, AG490 almost completely blocked the induction of REG Iβ promoter by IL-6 + Dx (Figure 4(f)). Thus, it is suggested that IL-6 + Dx-induced REG Iβ transcription also depends on the JAK/STAT signaling pathway.
3.4. STAT3 Binding to the REG Iα Promoter
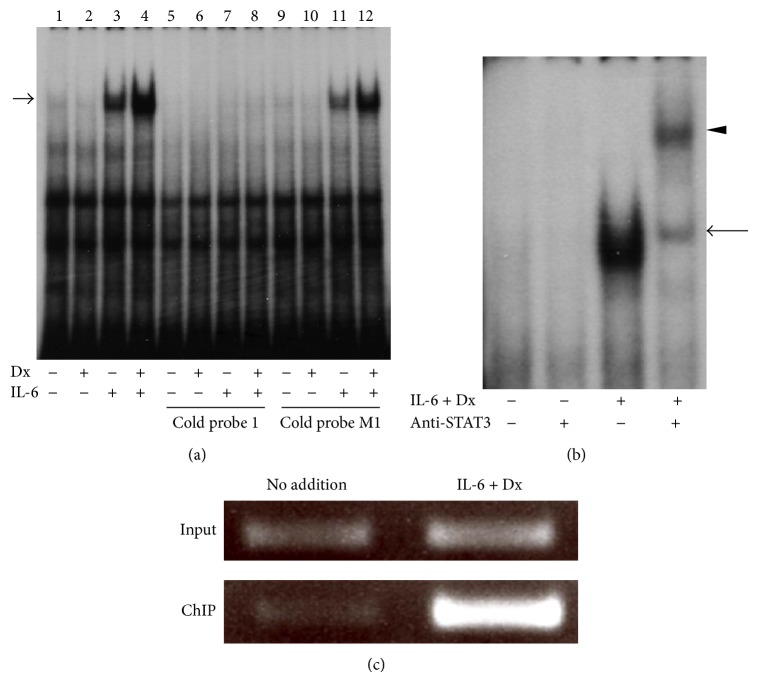
To further investigate whether STAT protein interacts with the REG Iα promoter in vitro and in vivo, we performed EMSA and ChIP assays, respectively. Treatment of Dx did not change the nuclear protein binding to the probe 1 containing the STAT-binding sequence (TGCCGGGAA) (Figure 5(a), lane 2). In contrast, treatment of IL-6 resulted in an increase in nuclear protein binding to the probe (lane 3), and the intensity of shift band was further increased by the combined addition of IL-6 and Dx (lane 4). The nuclear protein binding induced by IL-6 + Dx was competed with an excess of unlabeled probe (lane 8). On the other hand, excess amount of unlabeled mutated probe (probe M1), which disrupted the STAT-binding site, could not competitively block the binding to the labeled probe (lane 12). These results showed the binding specificity of the IL-6 + Dx-induced complex. An antibody for STAT3 produced a supershift band (Figure 5(b)), suggesting that STAT3 was involved in this nuclear protein-DNA complex formed by the IL-6 + Dx stimulation. Furthermore, immunoprecipitations of cross-linked DNA-protein complexes derived from IL-6 + Dx-treated 1.1B4 cells with a STAT3 antibody contained the STAT-binding element of the human REG Iα gene (Figure 5(c)). These results showed that STAT3 binding to the REG Iα promoter was induced by IL-6 + Dx in β cells.
Figure 5.
IL-6 + Dx-increased STAT3 binding to the REG Iα promoter. (a) Detection of DNA-protein complexes using EMSA. Nuclear extracts (2.5 μg protein) prepared from 1.1B4 cells, treated without or with Dx (100 nM), or IL-6 (20 ng/mL), or IL-6 + Dx, were incubated with a 32P-labeled probe 1. Nuclear extracts from untreated cells were applied onto lanes 1, 5, and 9; those from Dx-treated cells were applied onto lanes 2, 6, and 10; those from IL-6-treated cells were applied onto lanes 3, 7, and 11; and those from IL-6 + Dx-treated cells were applied onto lanes 4, 8, and 12. Lanes 1–4 contain no competitor; lanes 5–8 contain 100 × unlabeled probe 1; lanes 9–12 contain 100 × unlabeled probe M1. An arrow marks IL-6 + Dx-inducible complex migration. (b) Supershift assay of STAT3 containing complex. EMSAs were performed in the presence or absence of STAT3 antibodies as indicated. An arrow marks IL-6 + Dx-inducible complex migration. An arrowhead indicates supershift complex by anti-STAT3 antibody. (c) ChIP assay showing IL-6 + Dx increases in STAT3 binding to the REG Iα promoter. 1.1B4 cells were treated without or with IL-6 (20 ng/mL) + Dx (100 nM). ChIPs were performed with anti-STAT3 antibody, followed by PCR with primers specific for the REG Iα promoter. A representative result from three experiments is shown.
3.5. STAT3 Is Necessary for the IL-6 + Dx-Induced Type I REG Gene Expression
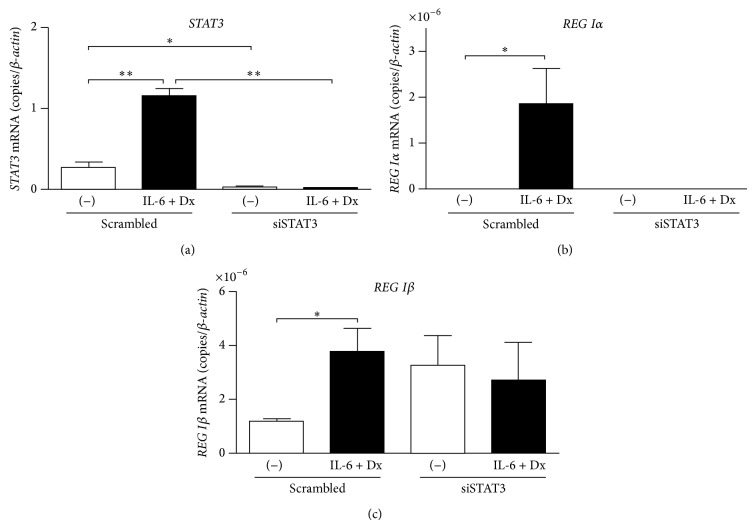
Involvement of STAT3 in the IL-6 + Dx-induced expressions of REG Iα and REG Iβ was further demonstrated by siRNA. 1.1B4 cells were transfected with scrambled siRNA control or STAT3-specific siRNA and were stimulated with IL-6 + Dx. We confirmed that STAT3-siRNA suppressed both basal and IL-6 + Dx-induced STAT3 mRNA (Figure 6(a)). In the cells treated with the STAT3-siRNA, the IL-6 + Dx-induced upregulation of REG Iα and REG Iβ was abolished (Figures 6(b) and 6(c)). These results clearly showed the essential requirement of STAT3 in the IL-6 + Dx-induced type I REG gene expression in β cells.
Figure 6.
Effects of STAT3 knockdown on IL-6 + Dx-induced mRNA expression of REG Iα and REG Iβ in human β cells. 1.1B4 cells were transfected with scrambled siRNA control or STAT3-specific siRNA and then stimulated without or with IL-6 (20 ng/mL) + Dx (100 nM). The mRNA levels of (a) STAT3, (b) REG Iα, and (c) REG Iβ were measured by real-time RT-PCR using β-actin as an endogenous control. Data are represented as the ratio of the number of target mRNA copies to the number of β-actin mRNA copies and expressed as means ± SE for each group ((a) N = 4, (b) N = 3-4, (c) N = 3). * P < 0.05; ** P < 0.0005.
4. Discussion
Reg family genes are involved in cell proliferation and regeneration in several tissues. In rat, type I Reg family gene (Reg I) is induced during pancreatic β cell regeneration [4, 7]. The completion of the human genome sequencing project revealed the complete set of REG genes (REG Iα, REG Iβ, REG III, HIP/PAP, and REG IV) [4, 5, 50, 51]. However, in human, it is not clear which REG gene is involved in the β cell regeneration. In this study, we found that among five functional human REG genes, REG Iα and REG Iβ gene expressions were significantly enhanced by the combined addition of IL-6 and Dx in human β cells. The rat Reg I gene expression is activated by IL-6 + Dx [13], and we and others have shown that human REG Iα protein promotes the proliferation of RINm5F cells and mouse islets [52, 53]. Therefore, in human, as well as in rat [13, 31], the type I REG proteins could be induced under inflammatory conditions, in which the levels of circulating IL-6 and glucocorticoids are increasing and function as a growth factor for β cell proliferation.
We demonstrated that IL-6 + Dx induced the type I REG gene (REG Iα and REG Iβ) expression through the JAK/STAT3 pathway. It has been reported that in gastric cancer cells, IL-6 enhanced REG Iα transcription through the STAT3 activation [54, 55]. In addition, the enhancement of REG Iα promoter activity by IL-6 was reported also in colon cancer cells [56] and in salivary ductal cells [57]. Thus, involvement of STAT3 in REG Iα gene expression is not restricted in pancreatic β cells but rather common to diverse cell types. However, in β cells, Dx is required in addition of IL-6 for the induction, since IL-6 alone only slightly induced REG Iα expression. We recently reported that HGF gene transcription was also induced by IL-6 + Dx through STAT3 activation in pancreatic β cells [31]. It is possible that glucocorticoids are necessary for STAT3 activation in addition to IL-6 in pancreatic β cells. The role of glucocorticoids in the REG Iα induction is currently under investigation.
The IL-6 + Dx-induced increases, in the promoter activity and the mRNA level of REG Iα, were much larger than those of REG Iβ. The reason of the difference of inducibility between REG Iα and REG Iβ is not clear. The expression patterns of two genes are different in pancreas [58]. In addition, we recently reported that, in 1.1B4 cells treated by intermittent hypoxia, REG Iα gene expression but not REG Iβ gene expression was increased [23]. Thus, although the JAK/STAT pathway seems to be essential to the induction of both type I REG genes, these two genes should be regulated independently by other transcription factors under physiological and pathological conditions. Previous [23] and current studies suggest that REG Iα gene rather than REG Iβ gene should be much more important for β cells in the circumstances in which β cells are damaged.
IL-22 was reported to induce REG Iα transcription via STAT3 activation in human colon cancer cells [59] and to induce Reg I and Reg II in NOD mouse islets [6, 45, 60]. In addition, Shioya et al. reported IL-22 receptor expression in human pancreatic β cells [61]. However, in the present study, either IL-22 alone or IL-22 + Dx only modestly increased the REG Iα and REG Iβ promoter activities in 1.1B4 cells. Therefore, STAT3 may not be fully activated by IL-22 nor IL-22 + Dx in human pancreatic β cells.
In rat RINm5F cells, the cis-element of rat Reg I gene for induction in response to IL-6 + Dx was the −81~−70 region [13]. This region in rat Reg I promoter corresponds to the −76~−65 region of the human REG Iα gene, which is downstream of the STAT-binding site (−142~−134). In reporter assays, the promoter activity of the construct “−130” was not enhanced by the stimulation of IL-6 + Dx. Moreover, the combined addition of 3AB with IL-6 + Dx did not enhance the promoter activity of mutant construct “−146 M” as much as that of “−146.” These results suggest that although PARP was involved in the IL-6 + Dx-induced activation of REG Iα promoter, STAT binding to the −142~−134 region played a major role for the induction of REG Iα transcription in human β cells.
In this study, we found that among five human REG family genes, the expressions of REG Iα and REG Iβ were significantly enhanced by IL-6 + Dx. These results suggest that, under inflammatory conditions, human type I REG proteins could be increased and function as growth factors for β cells to facilitate proliferation. Furthermore, we showed that the JAK/STAT pathway was involved in the IL-6 + Dx-induced human type I REG gene transcription in β cells. Therefore, polymorphisms and/or mutations in REG Iα and REG Iβ promoters as well as other polymorphisms/mutations of factors involved in the JAK/STAT pathway may be involved in diabetes incidence/pathology.
Acknowledgments
This work was supported in part by Grant-in-Aid for Young Scientists (B) (25860220) from Japan Society for the Promotion of Science (JSPS) and Grant-in-Aid for Practical Application Research from Japan Technology Agency (JST).
Conflict of Interests
The authors report no conflict of interests regarding the publication of this paper.
References
- 1.Meier J. J. Beta cell mass in diabetes: a realistic therapeutic target? Diabetologia. 2008;51(5):703–713. doi: 10.1007/s00125-008-0936-9. [DOI] [PubMed] [Google Scholar]
- 2.Jurczyk A., Bortell R., Alonso L. C. Human β-cell regeneration: progress, hurdles, and controversy. Current Opinion in Endocrinology, Diabetes and Obesity. 2014;21(2):102–108. doi: 10.1097/med.0000000000000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weir G. C., Bonner-Weir S. Islet β cell mass in diabetes and how it relates to function, birth, and death. Annals of the New York Academy of Sciences. 2013;1281(1):92–105. doi: 10.1111/nyas.12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okamoto H., Takasawa S. Recent advances in the Okamoto model: The CD38-cyclic ADP-ribose signal system and the regenerating gene protein (Reg)-Reg receptor system in β-cells. Diabetes. 2002;51(supplement 3):S462–S473. doi: 10.2337/diabetes.51.2007.s462. [DOI] [PubMed] [Google Scholar]
- 5.Takasawa S., Okamoto H. Pancreatic β-cell death, regeneration and insulin secretion: roles of poly(ADP-ribose) polymerase and cyclic ADP-ribose. International Journal of Experimental Diabetes Research. 2002;3(2):79–96. doi: 10.1080/15604280214485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh B., Nikoopour E., Huszarik K., Elliott J. F., Jevnikar A. M. Immunomodulation and regeneration of islet beta cells by cytokines in autoimmune type 1 diabetes. Journal of Interferon and Cytokine Research. 2011;31(10):711–719. doi: 10.1089/jir.2011.0025. [DOI] [PubMed] [Google Scholar]
- 7.Terazono K., Yamamoto H., Takasawa S., et al. A novel gene activated in regenerating islets. The Journal of Biological Chemistry. 1988;263(5):2111–2114. [PubMed] [Google Scholar]
- 8.Watanabe T., Yonemura Y., Yonekura H., et al. Pancreatic beta-cell replication and amelioration of surgical diabetes by Reg protein. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(9):3589–3592. doi: 10.1073/pnas.91.9.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu J.-L., Cui W., Li B., Lu Y. Possible roles of Reg family proteins in pancreatic islet cell growth. Endocrine, Metabolic & Immune Disorders: Drug Targets. 2008;8(1):1–10. doi: 10.2174/187153008783928361. [DOI] [PubMed] [Google Scholar]
- 10.Parikh A., Stephan A.-F., Tzanakakis E. S. Regenerating proteins and their expression, regulation and signaling. BioMolecular Concepts. 2012;3(1):57–70. doi: 10.1515/bmc.2011.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Unno M., Nata K., Noguchi N., et al. Production and characterization of Reg knockout mice: reduced proliferation of pancreatic β-cells in Reg knockout mice. Diabetes. 2002;51(supplement 3):S478–S483. doi: 10.2337/diabetes.51.2007.s478. [DOI] [PubMed] [Google Scholar]
- 12.Unno M., Yonekura H., Nakagawara K.-I., et al. Structure, chromosomal localization, and expression of mouse reg genes, reg I and reg II: a novel type of reg gene, reg II, exists in the mouse genome. The Journal of Biological Chemistry. 1993;268(21):15974–15982. [PubMed] [Google Scholar]
- 13.Akiyama T., Takasawa S., Nata K., et al. Activation of Reg gene, a gene for insulin-producing β-cell regeneration: poly(ADP-ribose) polymerase binds Reg promoter and regulates the transcription by autopoly(ADP-ribosyl)ation. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(1):48–53. doi: 10.1073/pnas.240458597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watanabe T., Yonekura H., Terazono K., Yamamoto H., Okamoto H. Complete nucleotide sequence of human reg gene and its expression in normal and tumoral tissues: the reg protein, pancreatic stone protein, and pancreatic thread protein are one and the same product of the gene. The Journal of Biological Chemistry. 1990;265(13):7432–7439. [PubMed] [Google Scholar]
- 15.Moriizumi S., Watanabe T., Unno M., et al. Isolation, structural determination and expression of a novel reg gene, human reg Iβ . Biochimica et Biophysica Acta. 1994;1217(2):199–202. doi: 10.1016/0167-4781(94)90035-3. [DOI] [PubMed] [Google Scholar]
- 16.Lasserre C., Simon M.-T., Ishikawa H., et al. Structural organization and chromosomal localization of a human gene (HIP/PAP) encoding a C-type lectin overexpressed in primary liver cancer. European Journal of Biochemistry. 1994;224(1):29–38. doi: 10.1111/j.1432-1033.1994.tb19991.x. [DOI] [PubMed] [Google Scholar]
- 17.Dusetti N. J., Frigerio J.-M., Fox M. F., Swallow D. M., Dagorn J.-C., Iovanna J. L. Molecular cloning, genomic organization, and chromosomal localization of the human pancreatitis-associated protein (PAP) gene. Genomics. 1994;19(1):108–114. doi: 10.1006/geno.1994.1019. [DOI] [PubMed] [Google Scholar]
- 18.Nata K., Liu Y., Xu L., et al. Molecular cloning, expression and chromosomal localization of a novel human REG family gene, REG III . Gene. 2004;340(1):161–170. doi: 10.1016/j.gene.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 19.Hartupee J. C., Zhang H., Bonaldo M. F., Soares M. B., Dieckgraefe B. K. Isolation and characterization of a cDNA encoding a novel member of the human regenerating protein family: Reg IV. Biochimica et Biophysica Acta. 2001;1518(3):287–293. doi: 10.1016/s0167-4781(00)00284-0. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi N., Yuasa T., Okitsu T. Regenerative medicine for diabetes mellitus. Cell Transplantation. 2009;18(5-6):491–496. doi: 10.1177/096368970901805-602. [DOI] [PubMed] [Google Scholar]
- 21.Bernal-Mizrachi E., Kulkarni R. N., Scott D. K., Mauvais-Jarvis F., Stewart A. F., Garcia-Ocaña A. Human β-cell proliferation and intracellular signaling part 2: still driving in the dark without a road map. Diabetes. 2014;63(3):819–831. doi: 10.2337/db13-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCluskey J. T., Hamid M., Guo-Parke H., McClenaghan N. H., Gomis R., Flatt P. R. Development and functional characterization of insulin-releasing human pancreatic beta cell lines produced by electrofusion. The Journal of Biological Chemistry. 2011;286(25):21982–21992. doi: 10.1074/jbc.m111.226795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ota H., Itaya-Hironaka A., Yamauchi A., et al. Pancreatic β cell proliferation by intermittent hypoxia via up-regulation of Reg family genes and HGF gene. Life Sciences. 2013;93(18-19):664–672. doi: 10.1016/j.lfs.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 24.Kumari S., Sammut I. A., Giles G. I. The design of nitric oxide donor drugs: s-nitrosothiol tDodSNO is a superior photoactivated donor in comparison to GSNO and SNAP. European Journal of Pharmacology. 2014;737:168–176. doi: 10.1016/j.ejphar.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 25.Nielsen M., Kaltoft K., Nordahl M., et al. Constitutive activation of a slowly migrating isoform of Stat3 in mycosis fungoides: tyrphostin AG490 inhibits Stat3 activation and growth of mycosis fungoides tumor cell lines. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(13):6764–6769. doi: 10.1073/pnas.94.13.6764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamauchi A., Takahashi I., Takasawa S., et al. Thiazolidinediones inhibit REG Iα gene transcription in gastrointestinal cancer cells. Biochemical and Biophysical Research Communications. 2009;379(3):743–748. doi: 10.1016/j.bbrc.2008.12.113. [DOI] [PubMed] [Google Scholar]
- 27.Yoshimoto K., Fujimoto T., Itaya-Hironaka A., et al. Involvement of autoimmunity to REG, a regeneration factor, in patients with primary Sjögren's syndrome. Clinical and Experimental Immunology. 2013;174(1):1–9. doi: 10.1111/cei.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ota H., Tamaki S., Itaya-Hironaka A., et al. Attenuation of glucose-induced insulin secretion by intermittent hypoxia via down-regulation of CD38. Life Sciences. 2012;90(5-6):206–211. doi: 10.1016/j.lfs.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 29.Takasawa S., Ikeda T., Akiyama T., et al. Cyclin D1 activation through ATF-2 in Reg-induced pancreatic β-cell regeneration. FEBS Letters. 2006;580(2):585–591. doi: 10.1016/j.febslet.2005.12.070. [DOI] [PubMed] [Google Scholar]
- 30.Niu G., Wright K. L., Ma Y., et al. Role of Stat3 in regulating p53 expression and function. Molecular and Cellular Biology. 2005;25(17):7432–7440. doi: 10.1128/mcb.25.17.7432-7440.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakagawa K., Takasawa S., Nata K., et al. Prevention of Reg I-induced β-cell apoptosis by IL-6/dexamethasone through activation of HGF gene regulation. Biochimica et Biophysica Acta. 2013;1833(12):2988–2995. doi: 10.1016/j.bbamcr.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 32.Ishii C., Kawazu S., Tomono S., et al. Appearance of a regenerating (reg) gene protein in pancreatic islets of remission BB/Wor//Tky rats. Endocrine Journal. 1993;40(2):269–273. doi: 10.1507/endocrj.40.269. [DOI] [PubMed] [Google Scholar]
- 33.Baeza N. J., Moriscot C. I., Renaud W. P., Okamoto H., Figarella C. G., Vialettes B. H. Pancreatic regenerating gene overexpression in the nonobese diabetic mouse during active diabetogenesis. Diabetes. 1996;45(1):67–70. doi: 10.2337/diab.45.1.67. [DOI] [PubMed] [Google Scholar]
- 34.Anastasi E., Ponte E., Gradini R., et al. Expression of Reg and cytokeratin 20 during ductal cell differentiation and proliferation in a mouse model of autoimmune diabetes. European Journal of Endocrinology. 1999;141(6):644–652. doi: 10.1530/eje.0.1410644. [DOI] [PubMed] [Google Scholar]
- 35.Planas R., Alba A., Carrillo J., et al. Reg (regenerating) gene overexpression in islets from non-obese diabetic mice with accelerated diabetes: role of IFNβ . Diabetologia. 2006;49(10):2379–2387. doi: 10.1007/s00125-006-0365-6. [DOI] [PubMed] [Google Scholar]
- 36.Souza K. L. A., Gurgul-Convey E., Elsner M., Lenzen S. Interaction between pro-inflammatory and anti-inflammatory cytokines in insulin-producing cells. Journal of Endocrinology. 2008;197(1):139–150. doi: 10.1677/JOE-07-0638. [DOI] [PubMed] [Google Scholar]
- 37.Cnop M., Hannaert J. C., Hoorens A., Eizirik D. L., Pipeleers D. G. Inverse relationship between cytotoxicity of free fatty acids in pancreatic islet cells and cellular triglyceride accumulation. Diabetes. 2001;50(8):1771–1777. doi: 10.2337/diabetes.50.8.1771. [DOI] [PubMed] [Google Scholar]
- 38.Sommerweiss D., Gorski T., Richter S., Garten A., Kiess W. Oleate rescues INS-1E β-cells from palmitate-induced apoptosis by preventing activation of the unfolded protein response. Biochemical and Biophysical Research Communications. 2013;441(4):770–776. doi: 10.1016/j.bbrc.2013.10.130. [DOI] [PubMed] [Google Scholar]
- 39.Lenzen S. Oxidative stress: the vulnerable β-cell. Biochemical Society Transactions. 2008;36(3):343–347. doi: 10.1042/bst0360343. [DOI] [PubMed] [Google Scholar]
- 40.Gul A. S. D., Fadillioglu E., Karabulut I., Yesilyurt A., Delibasi T. The effects of oral carvacrol treatment against H2O2 induced injury on isolated pancreas islet cells of rats. Islets. 2013;5(4):149–155. doi: 10.4161/isl.25519. [DOI] [PubMed] [Google Scholar]
- 41.Nakata M., Uto N., Maruyama I., Yada T. Nitric oxide induces apoptosis via Ca2+-dependent processes in the pancreatic β-cell line MIN6. Cell Structure and Function. 1999;24(6):451–455. doi: 10.1247/csf.24.451. [DOI] [PubMed] [Google Scholar]
- 42.Kwon K.-B., Kim E.-K., Lim J.-G., et al. Protective effect of Coptidis Rhizoma on S-nitroso-N-acetylpenicillamine (SNAP)-induced apoptosis and necrosis in pancreatic RINm5F cells. Life Sciences. 2005;76(8):917–929. doi: 10.1016/j.lfs.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 43.Yamamoto H., Uchigata Y., Okamoto H. Streptozotocin and alloxan induce DNA strand breaks and poly(ADP-ribose) synthetase in pancreatic islets. Nature. 1981;294(5838):284–286. doi: 10.1038/294284a0. [DOI] [PubMed] [Google Scholar]
- 44.Uchigata Y., Yamamoto H., Kawamura A., Okamoto H. Protection by superoxide dismutase, catalase, and poly(ADP-ribose) synthetase inhibitors against alloxan- and streptozotocin-induced islet DNA strand breaks and against the inhibition of proinsulin synthesis. The Journal of Biological Chemistry. 1982;257(11):6084–6088. [PubMed] [Google Scholar]
- 45.Hill T., Krougly O., Nikoopour E., et al. The involvement of interleukin-22 in the expression of pancreatic beta cell regenerative Reg genes. Cell Regeneration. 2013;2(1, article 2):11. doi: 10.1186/2045-9769-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Francis P. J., Southgate J. L., Wilkin T. J., Bone A. J. Expression of an islet regenerating (reg) gene in isolated rat islets: effects of nutrient and non-nutrient growth factors. Diabetologia. 1992;35(3):238–242. doi: 10.1007/bf00400923. [DOI] [PubMed] [Google Scholar]
- 47.Yoshino N., Ishihara S., Rumi M. A. K., et al. Interleukin-8 regulates expression of Reg protein in Helicobacter pylori-infected gastric mucosa. The American Journal of Gastroenterology. 2005;100(10):2157–2166. doi: 10.1111/j.1572-0241.2005.41915.x. [DOI] [PubMed] [Google Scholar]
- 48.Kishimoto T. IL-6: From its discovery to clinical applications. International Immunology. 2010;22(5):347–352. doi: 10.1093/intimm/dxq030. [DOI] [PubMed] [Google Scholar]
- 49.Cheon H., Yang J., Stark G. R. The functions of signal transducers and activators of transcriptions 1 and 3 as cytokine-inducible proteins. Journal of Interferon & Cytokine Research. 2011;31(1):33–40. doi: 10.1089/jir.2010.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Y.-W., Ding L.-S., Lai M.-D. Reg gene family and human diseases. World Journal of Gastroenterology. 2003;9(12):2635–2641. doi: 10.3748/wjg.v9.i12.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jin C. X., Hayakawa T., Ko S. B. H., Ishiguro H., Kitagawa M. Pancreatic stone protein/regenerating protein family in pancreatic and gastrointestinal diseases. Internal Medicine. 2011;50(15):1507–1516. doi: 10.2169/internalmedicine.50.5362. [DOI] [PubMed] [Google Scholar]
- 52.Shervani N. J., Takasawa S., Uchigata Y., et al. Autoantibodies to REG, a beta-cell regeneration factor, in diabetic patients. European Journal of Clinical Investigation. 2004;34(11):752–758. doi: 10.1111/j.1365-2362.2004.01419.x. [DOI] [PubMed] [Google Scholar]
- 53.Gross D. J., Weiss L., Reibstein I., et al. Amelioration of diabetes in nonobese diabetic mice with advanced disease by linomide-induced immunoregulation combined with Reg protein treatment. Endocrinology. 1998;139(5):2369–2374. doi: 10.1210/endo.139.5.5997. [DOI] [PubMed] [Google Scholar]
- 54.Sekikawa A., Fukui H., Fujii S., et al. REG Iα protein may function as a trophic and/or anti-apoptotic factor in the development of gastric cancer. Gastroenterology. 2005;128(3):642–653. doi: 10.1053/j.gastro.2004.12.045. [DOI] [PubMed] [Google Scholar]
- 55.Sekikawa A., Fukui H., Fujii S., et al. REG Iα protein mediates an anti-apoptotic effect of STAT3 signaling in gastric cancer cells. Carcinogenesis. 2008;29(1):76–83. doi: 10.1093/carcin/bgm250. [DOI] [PubMed] [Google Scholar]
- 56.Sekikawa A., Fukui H., Fujii S., et al. Possible role of REG Iα protein in ulcerative colitis and colitic cancer. Gut. 2005;54(10):1437–1444. doi: 10.1136/gut.2004.053587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fujimura T., Fujimoto T., Itaya-Hironaka A., et al. Interleukin-6/Stat pathway is responsible for the induction of REG Iα, a new auto-antigen in Sjögren’s syndrome patients, in salivary duct epithelial cells. Annals of the Rheumatic Diseases. 2014;73(supplement 2):865–866. doi: 10.1136/annrheumdis-2014-eular.2813. [DOI] [Google Scholar]
- 58.Sanchez D., Figarella C., Marchand-Pinatel S., Bruneau N., Guy-Crotte O. Preferential expression of reg Iβ gene in human adult pancreas. Biochemical and Biophysical Research Communications. 2001;284(3):729–737. doi: 10.1006/bbrc.2001.5033. [DOI] [PubMed] [Google Scholar]
- 59.Sekikawa A., Fukui H., Suzuki K., et al. Involvement of the IL-22/REG Iα axis in ulcerative colitis. Laboratory Investigation. 2010;90(3):496–505. doi: 10.1038/labinvest.2009.147. [DOI] [PubMed] [Google Scholar]
- 60.Sigh B., Hill T., Krougly O., et al. Role of IL-22 in tissue regeneration in autoimmunity. The Journal of Immunology. 2013;190(meeting abstract supplement, 195.11) [Google Scholar]
- 61.Shioya M., Andoh A., Kakinoki S., Nishida A., Fujiyama Y. Interleukin 22 receptor 1 expression in pancreas islets. Pancreas. 2008;36(2):197–199. doi: 10.1097/MPA.0b013e3181594258. [DOI] [PubMed] [Google Scholar]