Abstract
With the increasing use of antifungals such as amphotericin B, itraconazole, voriconazole, caspofungin, and terbinafine (TRB) in patients at high risk for invasive aspergillosis, resistance of Aspergillus fumigatus to these agents will ultimately emerge. Due to the limited availability of molecular genetics for A. fumigatus, few studies have addressed its mechanisms of resistance to antifungals. We transformed A. fumigatus protoplasts with a pyrG-based A. fumigatus genomic DNA library (constructed in the multicopy nonintegrating vector pRG3-AMA1-NotI, which also has the pyr-4 gene for selection). We obtained one pyrG+ transformant that grew in medium containing a fungicidal concentration (0.625 μg/ml) of TRB. To determine whether TRB resistance in that transformant was plasmid dependent, we evicted the plasmid and found concomitant loss of uracil prototrophy and TRB resistance. DNA sequence analysis identified the gene responsible for TRB resistance as the A. fumigatus squalene epoxidase gene (ERG1), which encodes the target enzyme of TRB. Authentic A. fumigatus ERG1, amplified from the genome and cloned into pRG3-AMA1-NotI, also conferred TRB-specific resistance. This molecular approach has the potential to enhance our knowledge of the mechanisms of A. fumigatus resistance to modern antifungals.
Invasive aspergillosis (IA), which is typically caused by Aspergillus fumigatus, is now a common cause of death in patients with hematologic malignancies and bone marrow transplant recipients (6, 12). In addition to the profound and persistent net state of immunosuppression in patients who develop IA, intrinsic or secondary antifungal resistance may also account for failure of antifungal treatment (6, 12, 18). However, due to the limited availability of molecular tools for A. fumigatus, few studies have examined genes and pathways that may be associated with the resistance of this fungus to antifungals (13).
Currently, four classes of antifungals licensed in the United States have activity against Aspergillus species: polyenes (e.g., amphotericin B [AMB] and its lipid formulations), triazoles (e.g., itraconazole [ITC], voriconazole [VRC]), echinocandins (e.g., caspofungin [CAS]), and the allylamines (e.g., terbinafine [TRB]) (12). The activity of these antifungals when used alone remains suboptimal, with >50% of all patients experiencing failure of first-line therapy for IA (14). Because of this and the fact that A. fumigatus resistance to some of these antifungal agents both in vitro and in vivo has already been reported (13, 18), the strategy of combining antifungal agents having different mechanisms of action has become appealing in recent years (14). Conceptually, combination therapy for IA is supported by several studies that have demonstrated synergistic interactions between various antifungals against A. fumigatus both in vitro and in animal models: CAS plus nikkomycin Z (2, 38; K. E. Manavathu, G. J. Alangaden, J. L. Cutright, and P. H. Chandrasekar, Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother., abstr. M-853, 2002), CAS plus VRC (9, 27, 28), and ITC plus TRB (19, 31, 39). Because of the extensive use of antifungals either alone or in various combinations in clinical practice, resistance of A. fumigatus is anticipated to ultimately emerge, thus creating the need for studying the underlying mechanisms of resistance to individual antifungals or their combinations.
Fortunately, following the establishment of efficient transformation systems in the genetically tractable nonpathogenic mold Aspergillus nidulans, DNA-mediated transformation systems in A. fumigatus are now well established (1, 5). Transformation-based approaches have already been used for other fungi to study their mechanisms of resistance to antifungals (16, 17, 23, 26, 34). These studies have shown that the presence of extra copies of genes encoding target enzymes or efflux transporters is a common mechanism of resistance to antifungal agents (13, 16, 17, 23, 26, 34). We therefore looked for genes that, when increased in number, resulted in resistance of A. fumigatus to antifungals by transforming a pyrG− plasmid-based genomic DNA library into a pyrG− A. fumigatus strain. We used TRB, a fungicidal allylamine (32), as the selection drug to screen for pyrG+ TRB-resistant transformants. We found that extra copies of the gene encoding the known target of TRB (squalene epoxidase) resulted in a high level resistance of A. fumigatus to TRB. We believe that this screening strategy has promise for further investigation of dose-dependent gene resistance of A. fumigatus to various antifungals.
MATERIALS AND METHODS
Strains, media, and antifungals.
The Aspergillus isolates and plasmids we used are given in Table 1. We used standard methods for preparing Luria-Bertani medium and plasmid extraction as described previously (33). The uracil auxotroph A. fumigatus strain AF293.1 (23) was used as the transformation recipient strain. This strain is a derivative of A. fumigatus strain AF293, which has been used in the A. fumigatus sequencing project (Fungal Research Trust [www.aspergillus.man.ac.uk]). To prepare protoplasts, we incubated fresh conidia of the pyrG− strain AF293.1 in malt extract glucose (MAG) liquid medium (2% malt extract, 0.2% peptone, 1% dextrose, trace elements, 1 mg of pyroxidine/liter, and 8.8 mg of riboflavin/liter) (23) supplemented with uracil (5 mM) and uridine (10 mM) at room temperature for 24 h followed by incubation at 37°C until germ tube emergence was evident on 10 to 20% of the germinating conidia (assessed by phase-contrast microscopy). After pelleting the germlings via benchtop centrifugation at maximum speed for 5 min, we digested the germlings in 40 ml of a lytic mix (50 mM citric acid, 0.4 M ammonium sulfate [adjusted to pH 5.8 with KOH], 10 mM magnesium sulfate, 5 mg of bovine serum albumin fraction V/ml, 1 mM dithiothreitol, 100 μl of beta-glucuronidase [G-0762; Sigma Chemical Co., St. Louis, Mo.], and 200 μl of glucanex [Novo Ltd., Bagsvaerd, Denmark]) at 32°C for 2 to 3 h. We then centrifuged the digested protoplasts and washed them twice in 40 ml of washing buffer (50 mM citric acid, 0.4 mM ammonium sulfate, and 1% sucrose; pH 5.8 with KOH). Finally, we suspended the protoplasts in 1 ml of 0.6 M KCl, 100 mM CaCl2, and 10 mM Tris-HCl, pH 7.5, and incubated them at 4°C for 3 h until transformation. To screen for pyrG+ TRB-resistant transformants, we used TRB-containing (0.625 μg/ml) minimal medium (70 mM NaNO3, 7 mM KCl, 2 mM MgSO4, 12 mM KPO4 [pH 6.8], trace elements, and 1% dextrose) minus-uracil plates supplemented with 0.2 M sucrose. One molar sucrose was used in the top agar as an osmotic stabilizer for the protoplasts.
TABLE 1.
Strains and plasmids used in the present study
| Strain or plasmid | Relevant characteristics | Source |
|---|---|---|
| Strains | ||
| AF293.1 | pyrG− A. fumigatus | Osherov et al. (23) |
| TRBR | pyrG+ TRB-resistant A. fumigatus transformant | This study |
| rTRBR | AF293.1 retransformed with the plasmid pRG3-AMA1-TRBR isolated from TRBR | This study |
| TRBR-E | AF293.1 transformed with the empty plasmid pRG3-AMA1-NotI | This study |
| TRBR-L | Strain derived from TRBR after plasmid eviction | This study |
| TRBR-G | AF293.1 transformed with the plasmid pRG3-AMA1-TRBRG | This study |
| Plasmids | ||
| pRG3-AMA1-TRBR | Plasmid recovered from the TRB-resistant candidate TRBR | This study |
| pRG3-AMA1-TRBRG | Recombinant plasmid with the complete A. fumigatus squalene epoxidase gene | This study |
Stock solutions of TRB (Novartis, Titusville, N.J.), VRC (Pfizer Inc., New York, N.Y.), ITC (Janssen Pharmaceutica, Titusville, N.J.), and AMB (Pharma-Tek, Huntington, N.Y.) were prepared in 100% dimethyl sulfoxide and stored at −80°C until use. To prepare TRB-containing plates, we cooled the agar to 48°C before adding the appropriate drug concentration.
Construction of A. fumigatus genomic library.
A genomic library for AF293 was made by using a pRG3-AMA1-NotI vector (G. S. May, personal communication). This vector (24) is based on pUC19 and contains the pyr-4 and AMA-1 genes, which allow autonomous nonintegrating replication of the plasmid (24, 25). Furthermore, this is a shuttle vector that allows transformation in Escherichia coli. The library has an average insert size of 6 kb and consists of approximately 20,000 recombinant clones; therefore, it is expected to thoroughly represent all genes.
A. fumigatus transformation and screening for TRB-resistant colonies.
We performed a previously described method (23) of polyethylene glycol-mediated transformation of pyrG− strain AF293.1 protoplasts (inoculum, 6 × 106) with 0.5 μg of the A. fumigatus genomic DNA library. Transformed protoplasts were then plated at 6 × 106/plate on TRB-containing (0.625 μg/ml) minimal medium minus uracil (MM-U) plates. In pilot experiments, we had found that TRB at a concentration of 0.625 μg/ml in MM-U plates killed both protoplasts and conidia of AF293.1 (data not shown). To estimate the transformation efficiency, an inoculum of 6 × 106 transformed AF293.1 protoplasts/plate was plated in parallel on MM-U plates. For additional control purposes, AF293.1 protoplasts that were transformed with the empty plasmid pRG3-AMA1-NotI (0.5 μg) were plated on MM-U plates containing TRB (0.625 μg/ml). All of the plates were incubated at 37°C for 3 to 4 days. TRB-resistant pyrG+ transformants were then identified and retested via spreading on MM-U with TRB (0.625 μg/ml) plates and incubation at 37°C for 3 days. Only one such transformant (named TRBR) from a total of 5 × 104 transformants was identified and retested. We then randomly selected four pyrG+ TRB-resistant individual colonies of that transformant (named TRBR1, TRBR2, TRBR3, and TRBR4) and retested their susceptibilities to TRB as well as to AMB, ITC, and VRC.
TRB susceptibility testing.
We confirmed TRB resistance analysis using two independent methods. First, we used a disk diffusion assay in which growth of the strains TRBR1 and TRBR2 was examined by plating approximately 106 fresh conidia on MM-U plates. Fifteen micrograms (15 μl from a 1-mg/ml TRB stock solution) of TRB was placed on a [1/4]-in.-diameter paper disk (Schleicher & Schuell, Keene, N.H.). The radius of the zone of growth inhibition caused by TRB was measured after 48 h of growth at 37°C. We used conidia of AF293.1 transformed with the empty plasmid pRG3-AMA1-NotI as a control. Second, we measured the MICs of TRB for TRBR1, TRBR2, TRBR3, and TRBR4 by performing a broth microdilution method according to the National Committee for Clinical Laboratory Standards (NCCLS) M38-A guidelines (21). TRBR1, TRBR2, TRBR3, and TRBR4 were pregrown on MM-U plates containing TRB (at a concentration equal to one-fourth of the TRB screening concentration). The lack of uracil and presence of TRB were used to maintain the selection of the pyrG+ plasmid containing the gene conferring TRB resistance in these transformants. For the same purpose, we used the selective liquid MM-U medium instead of the RPMI 1640 medium recommended in the NCCLS M38-A method. We prepared 96-well microtiter plates that contained TRB at concentrations ranging from 0.06 to 256 μg/ml. The TRB MIC was determined visually after 48 h of growth at 37°C as the lowest TRB concentration that resulted in complete inhibition of visible growth. Candida parapsilosis ATCC 22019 was used as a quality control strain (21). Antifungal cross-resistance among TRBR1, TRBR2, TRBR3, and TRBR4 was determined by further measuring the MICs of AMB, ITC, and VRC using the NCCLS M38-A method. Again, conidia of AF293.1 transformed with the empty plasmid pRG3-AMA1-NotI were used as controls. All of the antifungal MICs were determined in duplicate in two independent experiments.
Testing of whether TRB-resistant colonies were plasmid dependent.
Because supplementation of uracil allows for a gradual loss of the plasmid in the uracil prototrophic TRB-resistant transformant candidate TRBR (TRBR1, TRBR2, TRBR3, and TRBR4), we serially subcultured these four independent colonies of TRBR on plates containing the nutrient-rich MAG medium supplemented with uracil and uridine. After each of these serial passages (n = 3 to 5), we determined whether each TRBR strain had lost the plasmid by inoculating it on MM-U plates. Colonies that were able to grow on uracil-containing MM plates but not MM-U plates were considered to have lost the plasmid. Colonies that had lost the plasmid (named TRBR-L) were then retested for their susceptibility to TRB as well as the other antifungals (AMB, ITC, and VRC) under the same conditions as proposed in the NCCLS M38-A method, but using MM plus uracil (MM+U) liquid medium instead of RPMI 1640.
Recovery of the plasmid conferring TRB resistance.
We transformed the genomic DNA (5 ng) from the TRB-resistant transformant TRBR into electrocompetent E. coli DH10B cells (Invitrogen Corp., Carlsbad, Calif.). Plasmids recovered from the individual ampicillin-resistant transformant colonies were divided into separate groups according to the restriction patterns by using BamHI and SmaI. We selected one representative plasmid (pRG3-AMA1-TRBR-1C and pRG3-AMA1-TRBR-4B) from each of these groups to transform back into AF293.1 protoplasts; the resulting transformants were named rTRBR transformants. We then measured the susceptibility of the resulting transformants to TRB, AMB, ITC, and VRC using the NCCLS microdilution method on liquid MM-U medium. The susceptibility of AF293.1 transformed with the empty plasmid pRG3-AMA1-NotI (0.5 μg) was again used as a control.
Sequence analysis of the plasmids conferring TRB resistance.
The plasmids conferring specific TRB resistance were then sequenced using a 3730x1 DNA analyzer (Applied Biosystems, Tokyo, Japan). Using the A. fumigatus Basic Local Alignment Search Tool (BLAST) database (The Institute for Genomic Research [http://www.tigr.org]), we searched the sequence of the A. fumigatus genomic fragment contained in the plasmid conferring TRB resistance. To look for gene homologues in other fungi, we searched the National Center for Biotechnology Information BLAST nucleotide database (http://www.ncbi.nlm.nih.gov).
Subcloning of the DNA fragment containing the complete A. fumigatus gene conferring TRB resistance.
Based on the results of the BLAST analyses, we were able to identify the gene conferring TRB resistance and the corresponding amino acid sequence. To amplify the genomic region of the gene (named the TRB resistance gene, or TRBRG), we designed PCR primers that contained the cleavage sites of the restriction enzymes KpnI and NotI. The forward primer (A, 5′-ACGGGTACCTCCCGTTATAGGTTTGCAGC-3′) starts 1,135 bp upstream of the potential open reading frame (ORF), and the reverse primer (B, 5′-ATAAGAATGCGGCCGCATCCTCGACGTTGTCTTGA-3′) ends 1,457 bp downstream of the potential ORF. Genomic DNA (50 ng) of AF293 was used as a template for the PCR. The PCR cycling parameters were as follows: 95°C for 5 min followed by 30 cycles at 95°C for 30 s, 60°C for 1 min, 72°C for 1 min and 30 s and, finally, 72°C for 15 min. The PCR product that represented the full-length A. fumigatus TRBRG was then digested with both NotI and KpnI and subcloned into the same pRG3-AMA1-NotI, which was digested with NotI and KpnI. The resulting plasmid was termed pRG3-AMA1-TRBRG.
Testing if extra copies of the plasmid pRG3-AMA1-TRBRG containing the complete A. fumigatus TRBRG result in TRB resistance.
We transformed the recombinant plasmid pRG3-AMA1-TRBRG into AF293.1 protoplasts. We tested the susceptibility of the pyrG+ retransformant (termed TRBR-G) to TRB, AMB, ITC, and VRC using the NCCLS microdilution method in the liquid MM-U medium. AF293.1 protoplasts transformed with the empty pRG3-AMA1-NotI (0.5 μg) were again used as controls.
RESULTS
Isolation of a pyrG+ transformant having a high level of TRB resistance.
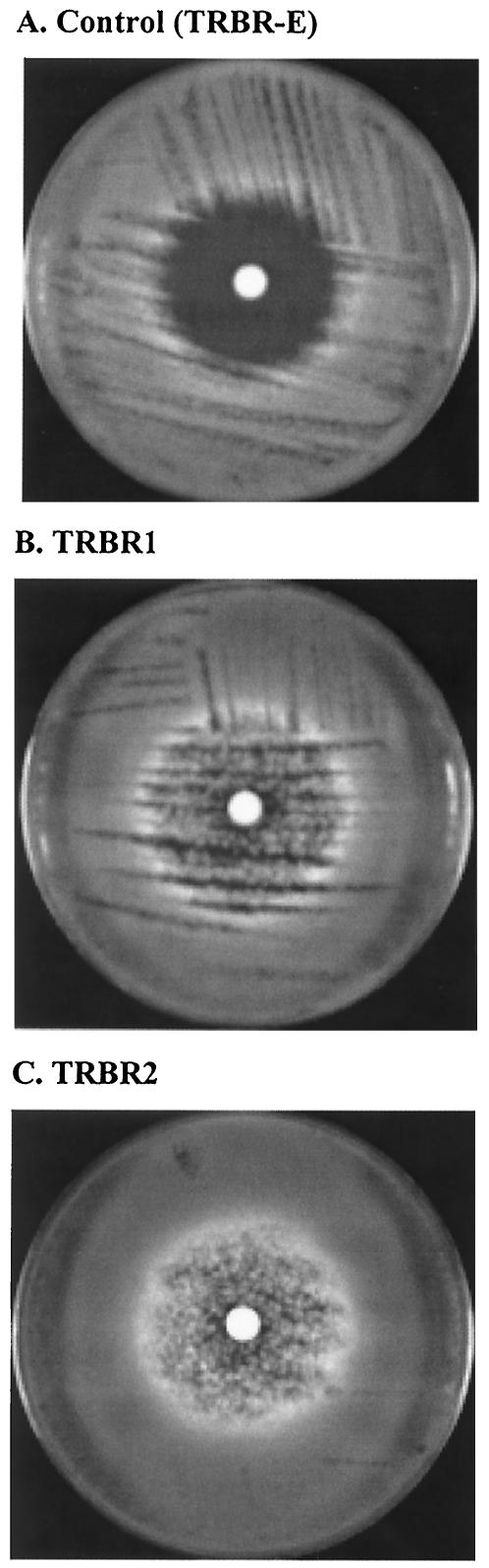
After transforming the genomic DNA library into pyrG− AF293.1 protoplasts, we identified one TRB-resistant pyrG+ candidate (TRBR) out of 5 × 104 such pyrG+ transformants. We then measured the susceptibility of TRBR to TRB as well as to AMB, ITC, and VRC, using both disk diffusion susceptibility testing and the NCCLS M38-A method. Disk diffusion testing of two independent TRBR colonies (TRBR1 and TRBR2) showed resistance to TRB (Fig. 1B and C), compared to the control strain TRBR-E, which was derived from pyrG− AF293.1 protoplasts transformed with the empty plasmid, that was susceptible to TRB (Fig. 1A). Similarly, using the NCCLS M38-A method, we found that the TRBR transformant exhibited a high level of TRB resistance (with a MIC greater than nine twofold dilutions above that of the control strain, TRBR-E), while the MICs of AMB, ITC, and VRC for TRBR were comparable to that for the control TRBR-E. This resistance was specific to TRB in TRBR, as the MICs of AMB, ITC, and VRC did not change when compared with those for the control strain (Table 2).
FIG. 1.
Disk diffusion susceptibility testing of TRB against A. fumigatus conidia, in which 106 conidia (100-μl aliquot of a 107-conidia/ml suspension) were spread on each of the MM-U plates. Each disk contained 15 μg of TRB, and the plates were incubated at 37°C for 72 h. (A) Conidia derived from pyrG− AF293.1 protoplasts transformed with the empty plasmid pRG3-AMA1-NotI (TRBR-E), which are susceptible to TRB. (B) Independent colony of the TRBR1 TRB-resistant pyrG+ transformant. (C) Independent colony of the TRBR2 TRB-resistant pyrG+ transformant.
TABLE 2.
MICs of antifungal agents in the A. fumigatus strains tested in this studya
| Straind | MIC (μg/ml)
|
|||
|---|---|---|---|---|
| TRB | AMB | ITC | VRC | |
| TRBR | >256 | 1 | 1 | 0.5 |
| rTRBR | >256 | 1 | 1 | 0.5 |
| TRBR-G | >256 | 1 | 1 | 0.5 |
| TRBR-E | 0.5 | 1 | 1 | 0.5 |
| TRBR-Lb | 1 | 1 | 1 | 0.5 |
| AF293.1b | 1 | 1 | 1 | 0.5 |
| QC strainc | 1 | 1 | 0.5 | 0.25 |
NCCLS M38-A microdilution method; liquid MM-U medium was used instead of liquid RPMI 1640 medium to maintain selection.
Liquid MM+U medium was used instead of the recommended liquid RPMI 1640 medium.
C. parapsilosis ATCC 22019. QC, quality control.
TRBR, conidia of the pyrG+ TRB-resistant transformant; rTRBR, conidia derived from retransformation of AF293.1 protoplasts with plasmid obtained from the initial TRBR pyrG+ transformant; TRBR-G, conidia derived from transformation of AF293.1 protoplasts with the complete A. fumigatus squalene epoxidase gene; TRBR-E, conidia derived from transformation of AF293.1 protoplasts with the empty plasmid; TRBR-L, conidia of the strain derived from TRBR after plasmid eviction.
TRB resistance is plasmid dependent.
Following serial passages of the four independent colonies of the initial TRB-resistant pyrG+ transformant TRBR on the nutrient-rich MAG solid medium supplemented with uracil and uridine, two of the colonies lost the plasmid (TRBR-L). Loss of plasmid in TRBR-L was indicated by the lack of growth of TRBR-L in the absence of uracil. The MIC of TRB for two TRBR-L colonies was the same as that for the control TRBR-E (Table 2). Hence, loss of plasmid was associated with concomitant loss of both uracil prototrophy and TRB resistance, indicating that TRB resistance of the TRBR pyrG+ transformant is plasmid mediated.
Retransformation of the pyrG− AF293.1 protoplasts with the plasmid recovered from the TRBR transformant resulted in TRB resistance.
We recovered two types of plasmids from the TRB-resistant transformant TRBR, based on different restriction patterns: pRG3-AMA1-1C and pRG3-AMA1-4B. We then retransformed both of these plasmids back into the protoplasts of the pyrG− strain AF293.1. As shown in Table 2, each of the corresponding rTRBR transformants had a similarly high level of TRB-specific resistance that was comparable with that of TRBR. Again, the susceptibilities of rTRBR to AMB, ITC, and VRC were identical to those of the control, TRBR-E.
Identification of the squalene epoxidase gene via sequencing.
Sequence analysis indicated that both plasmids (pRG3-AMA1-TRBR-1C and pRG3-AMA1-TRBR-4B) contained a different DNA fragment. The sequences from both DNA fragments matched to sequences of the squalene epoxidase gene, which codes for the target enzyme of TRB in Candida albicans (7) and Saccharomyces cerevisiae (8).
Subcloning of the DNA fragment containing the complete A. fumigatus squalene epoxidase gene.
Using PCR, we amplified the complete A. fumigatus squalene epoxidase gene (TRBRG) from the AF293 genomic DNA. The size of the PCR product was 3,807 bp. We then cloned this PCR product into the plasmid pRG3-AMA1-NotI to create the resulting plasmid, pRG3-AMA1-TRBRG. Sequence analysis using the National Center for Biotechnology Information BLAST nucleotide database showed that this 3,807-bp DNA fragment contained a potential ORF from bp 1136 to 2350 (GenBank accession number AY532916). Alignment between the amino acid sequence of this potential ORF and the C. albicans squalene epoxidase gene ERG1 (GenBank accession number D88252) (7) and S. cerevisiae squalene epoxidase gene ERG1 (M64994) (8) was performed using the CLUSTAL_W1.82 software (European Bioinformatics Institute [www.ebi.ac.uk]). The amino acid sequence was 41 and 40% identical to the ERG1 genes of C. albicans and S. cerevisiae, respectively (Fig. 2).
FIG. 2.
Amino acid sequence alignment of the A. fumigatus squalene epoxidase gene ORF, which is contained within TRBRG (bp 1136 to 2350; GenBank accession number AY532916), C. albicans squalene epoxidase gene ERG1 (GenBank accession number D88252), and S. cerevisiae squalene epoxidase gene ERG1 (GenBank accession number M64994) was performed by using CLUSTAL_W1.82 at the European Bioinformatics Institute website (www.ebi.ac.uk). The asterisks indicate the residues in the column that are identical in all sequences in the alignment. A colon indicates conservative amino acid substitutions, and a period indicates semiconservative amino acid substitutions. CA-SE, C. albicans squalene epoxidase; SC-SE, S. cerevisiae squalene epoxidase; AF-SE, A. fumigatus squalene epoxidase.
Resistance of A. fumigatus to TRB is conferred by extra copies of the A. fumigatus squalene epoxidase gene.
The recombinant plasmid pRG3-AMA1-TRBRG (squalene epoxidase gene) was transformed back into the pyrG− AF293.1 protoplasts. The resulting transformants again had a high level of TRB-specific resistance (TRB MIC, ≥256 μg/ml) according to the NCCLS M38-A method. This resistance was similar to that of the initial TRB-resistant transformant (TRBR), whereas the AF293.1 strain that was transformed with the empty plasmid pRG3-AMA1-NotI was susceptible to TRB (MIC = 1 μg/ml) (Table 2).
DISCUSSION
Our investigators previously developed a pyrG-based transformation system in A. fumigatus (23, 24). Using this approach, we found that heterologous overexpression of extra copies of the P450 14α-demethylase gene in A. nidulans confers ITC resistance in A. fumigatus (23). In the present study, we isolated a TRB-resistant A. fumigatus strain (TRBR) by transforming a high copy number of an A. fumigatus genomic DNA library constructed in the vector pRG3-AMA1-NotI into the A. fumigatus recipient strain AF293.1 (23-25). Susceptibility testing using two independent methodologies indicated that this TRB-resistant transformant had a high level of TRB resistance when compared with control. To determine whether this resistance was plasmid dependent, we evicted the plasmid from the TRB-resistant transformant by serially subculturing it on nutrient-rich medium. We found that loss of the plasmid resulted in both uracil auxotrophy and restoration of TRB susceptibility. This finding demonstrates that the TRB resistance of TRBR was entirely associated with the plasmid.
Our molecular approach is based on transformation of a DNA library that contains different gene fragments. The number of copies of gene fragments that are transformed in different transformants is variable. Hence, we believe that the facts that TRB-resistant colonies appeared hazy in a disk diffusion assay and that the appearance of resistance was variable from colony to colony (as indicated by the slightly different patterns of growth in Fig. 1B and C) were possibly due to the variable number of copies or the different DNA fragments containing the ERG1 gene in each of the transformants. The hyphae within the zone looked normal (data not shown), and in liquid assays, the TRB-resistant colonies exhibited normal growth at TRB concentrations that inhibited the control colonies.
We also found that TRBR did not exhibit cross-resistance to AMB and triazoles, which have different modes of action. TRB, as well as other allylamine antifungals, inhibits squalene epoxidase, a key enzyme that catalyzes the epoxidation of squalene to (3S)-2,3-oxidosqualene in the ergosterol biosynthetic pathway (32). This specific inhibition results in intracellular accumulation of toxic squalene levels and decreased ergosterol production. High intracellular squalene concentrations have been postulated to interfere with normal fungal membrane function in C. albicans. TRB, a medically important oral allylamine (29, 32), has been extensively used for the treatment of onychomycosis and dermatophytosis (4, 37). TRB has also been suggested to have a role in the treatment of systemic mold infections, such as IA (35, 36) and zygomycosis (3), either alone or in combination with triazoles.
The prevalence and mechanisms of intrinsic or secondary TRB resistance in different fungi have not been well studied (13, 18). Some studies have shown that acquisition of efflux-mediated multidrug resistance results in resistance to TRB. More specifically, overexpression of the multidrug resistance transporter CaMDR1 in C. albicans has been associated with increased efflux of TRB and TRB resistance in addition to azole resistance (13). Another study demonstrated that membrane alterations leading to asymmetry of the membrane lipid phase in C. albicans could also contribute to development of TRB and azole resistance (11). In S. cerevisiae, functional expression of the C. albicans ATP-binding cassette transporters Cdr1p and Cdr2p has also been associated with both TRB and azole resistance (16, 17, 34). In addition, Klobucnikova et al. (10) found that a single point mutation in the S. cerevisiae gene ERG1, the target gene for TRB, resulted in TRB and ITC resistance instead of specific TRB resistance.
On the other hand, Leber et al. (15), by using mutagenesis and gene replacement experiments, recently demonstrated that several other single point mutations in the ERG1 gene resulted in TRB resistance without cross-resistance to azoles. Several other studies have also demonstrated specific resistance of fungi to allylamines (20, 22; C. S. Osborne and B. Favre, Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother. abstr. M-471, 2002). In one of those studies, Mukherjee et al. (20) reported that Trichophyton rubrum strains isolated from patients with onychomycosis that did not respond to TRB therapy were intrinsically resistant to TRB. Because these strains showed specific resistance to squalene epoxidase inhibitors, the authors speculated that mutations in the squalene epoxidase gene, the target enzyme of TRB, could have been responsible for their resistance to TRB. Osborne and Favre (Osborne and Favre, 42nd ICAAC) further found that amino acid substitutions due to squalene epoxidase gene mutations resulted in specific TRB resistance in T. rubrum clinical isolates. They also reported that T. rubrum has little capacity for developing TRB resistance even after prolonged exposure on agar plates containing TRB (22). Nonetheless, the mechanisms of TRB resistance in molds such as A. fumigatus have not been as well studied to date. In one of the few studies, Rocha et al. (30) suggested that a single gene mutation could be responsible for TRB resistance in A. nidulans. As an extension of the previous studies, we describe here for the first time that the presence of extra copies of the A. fumigatus squalene epoxidase gene confers TRB resistance to A. fumigatus. However, the clinical relevance of resistance to TRB in A. fumigatus remains unexplored.
In conclusion, we used a DNA transformation system in A. fumigatus and, as a proof of principle, showed that the presence of extra copies of the TRB target gene resulted in TRB-specific resistance in A. fumigatus. Thus, we believe that this genetic screening approach shows promise as a tool for conducting studies pertaining to the mechanisms of resistance to antifungals or the increasingly used antifungal combinations in IA.
REFERENCES
- 1.Brakhage, A. A., and K. Langfelder. 2002. Menacing mold: the molecular biology of Aspergillus fumigatus. Annu. Rev. Microbiol. 56:433-455. [DOI] [PubMed] [Google Scholar]
- 2.Chiou, C., N. Mavrogiorgos, E. Tillem, R. Hector, and T. J. Walsh. 2001. Synergy, pharmacodynamics, and time-sequenced ultrastructural changes of the interaction between nikkomycin Z and the echinocandin FK463 against Aspergillus fumigatus. Antimicrob. Agents Chemother. 45:3310-3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dannaoui, E., J. W. Mouton, J. F. Meis, and P. E. Verweij, and Eurofung Network. 2002. Efficacy of antifungal therapy in a nonneutropenic murine model of zygomycosis. Antimicrob. Agents Chemother. 46:1953-1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Degreef, H. J., and P. R. DeDoncker. 1994. Current therapy of dermatophytosis. J. Am. Acad. Dermatol. 31:S25-S30. [DOI] [PubMed] [Google Scholar]
- 5.d'Enfert, C., G. Weidner, P. C. Mol., and A. A. Brakhage. 1999. Transformation systems of Aspergillus fumigatus: new tools to investigate fungal virulence, p. 149-166. In A. A. Brakhage, J. Bernhard, and A. Schmidt (ed.), Aspergillus fumigatus: biology, clinical aspects and molecular approaches to pathogenicity. Karger, New York, N.Y. [DOI] [PubMed]
- 6.Denning, D. W. 1998. Invasive aspergillosis. Clin. Infect. Dis. 26:781-803. [DOI] [PubMed] [Google Scholar]
- 7.Favre, B., and N. S. Ryder. 1997. Cloning and expression of squalene epoxidase from the pathogenic yeast Candida albicans. Gene 189:119-126. [DOI] [PubMed] [Google Scholar]
- 8.Jandrositz, A., F. Turnowsky, and G. Hoegenauer. 1991. The gene encoding squalene epoxidase from Saccharomyces cerevisiae: cloning and characterization. Gene 107:155-160. [DOI] [PubMed] [Google Scholar]
- 9.Kirkpatrick, R. W., S. Perea, B. J. Coco, and T. F. Patterson. 2002. Efficacy of caspofungin alone and in combination with voriconazole in a guinea pig model of invasive aspergillosis. Antimicrob. Agents Chemother. 46:2564-2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klobucnikova, V., P. Kohut, R. Leber, S. Fuchsbichler, N. Schweighofer, F. Turnowsky, and I. Hapala. 2003. Terbinafine resistance in a pleiotropic yeast mutant is caused by a single point mutation in the ERG1 gene. Biochem. Biophys. Res. Commun. 309:666-671. [DOI] [PubMed] [Google Scholar]
- 11.Kohli, A., Smriti, K. Mukhopadhyay, A. Rattan, and R. Prasad. 2002. In vitro low-level resistance to azoles in Candida albicans is associated with changes in membrane lipid fluidity and asymmetry. Antimicrob. Agents Chemother. 46:1046-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kontoyiannis, D. P., and G. P. Bodey. 2002. Invasive aspergillosis in 2002: an update. Eur. J. Clin. Microbiol. Infect. Dis. 21:161-172. [DOI] [PubMed] [Google Scholar]
- 13.Kontoyiannis, D. P., and R. E. Lewis. 2002. Antifungal drug resistance of pathogenic fungi. Lancet 359:1135-1144. [DOI] [PubMed] [Google Scholar]
- 14.Kontoyiannis, D. P., and R. E. Lewis. 2003. Combination chemotherapy for invasive fungal infections: what laboratory and clinical studies tell us so far. Drug Resist. Update 6:257-269. [DOI] [PubMed] [Google Scholar]
- 15.Leber, R., S. Fuchsbichler, V. Klobucnikova, N. Schweighofer, E. Pitters, K. Wohlfarter, M. Lederer, K. Landl, C. Ruckenstuhl, I. Hapala, and F. Turnowsky. 2003. Molecular mechanism of terbinafine resistance in Saccharomyces cerevisiae. Antimicrob. Agents Chemother. 47:3890-3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manuela, S. M., B. Willinger, R. Egner, G. Ecker, and K. Kuchler. 2003. Reversal of antifungal resistance mediated by ABC efflux pumps from Candida albicans functionally expressed in yeast. Int. J. Antimicrob. Agents 22:291-300. [DOI] [PubMed] [Google Scholar]
- 17.Manuela, S. M., B. Willinger, G. Krapf, S. Enzinger, E. Presterl, and K. Kuchler. 2003. The Candida albicans Cdr2p ATP-binding cassette (ABC) transporter confers resistance to caspofungin. Mol. Microbiol. 48:225-235. [DOI] [PubMed] [Google Scholar]
- 18.Moore, C. B., N. Sayers, J. Mosquera, J. Slaven, and D. W. Denning. 2000. Antifungal drug resistance in Aspergillus. J. Infect. 41:203-220. [DOI] [PubMed] [Google Scholar]
- 19.Mosquera, J., C. B. Moore, P. A. Warn, and D. W. Denning. 2002. In vitro interaction of terbinafine with itraconazole, fluconazole, amphotericin B and 5-flucytosine against Aspergillus spp. J. Antimicrob. Chemother. 50:189-194. [DOI] [PubMed] [Google Scholar]
- 20.Mukherjee, P. K., S. D. Leidich, N. Isham, I. Leitner, N. S. Ryder, and M. A. Ghannoum. 2003. Clinical Trichophyton rubrum strain exhibiting primary resistance to terbinafine. Antimicrob. Agents Chemother. 47:82-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi. Proposed standard. NCCLS document M38-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 22.Osborne, C. S., B. Hofbauer, B. Favre, and N. S. Ryder. 2003. In vitro analysis of the ability of Trichophyton rubrum to become resistant to terbinafine. Antimicrob. Agents Chemother. 47:3634-3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Osherov, N., D. P. Kontoyiannis, A. Romans, and G. S. May. 2001. Resistance to itraconazole in Aspergillus nidulans and Aspergillus fumigatus is conferred by extra copies of the A. nidulans P-450 14α-demethylase gene, pdmA. J. Antimicrob. Chemother. 48:75-81. [DOI] [PubMed] [Google Scholar]
- 24.Osherov, N., J. Mathew, and G. S. May. 2000. Polarity-defective mutants of Aspergillus nidulans. Fungal Genet. Biol. 31:181-188. [DOI] [PubMed] [Google Scholar]
- 25.Osherov, N., and G. S. May. 2000. Conidial germination in Aspergillus nidulans requires RAS signaling and protein synthesis. Genetics 155:647-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Osherov, N., G. S. May, N. D. Albert, and D. P. Kontoyiannis. 2002. Overexpression of Sbe2p, a Golgi protein, results in resistance to caspofungin in Saccharomyces cerevisiae. Antimicrob. Agents Chemother. 46:2462-2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perea, S., G. Gonzalez, W. A. Fothergill, R. W. Kirkpatrick, G. M. Rinaldi, and T. F. Patterson. 2002. In vitro interaction of caspofungin acetate with voriconazole against clinical isolates of Aspergillus spp. Antimicrob. Agents Chemother. 46:3039-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petraitis, V., R. Petraitiene, A. A. Sarafandi, A. M. Kelaher, C. A. Lyman, H. E. Casler, T. Sein, A. H. Groll, J. Bacher, N. A. Avila, and T. J. Walsh. 2003. Combination therapy in treatment of experimental pulmonary aspergillosis: synergistic interaction between an antifungal triazole and an echinocandin. J. Infect. Dis. 187:1834-1843. [DOI] [PubMed] [Google Scholar]
- 29.Petranyi, G., J. G. Meingassner, and H. Mieth. 1987. Antifungal activity of the allylamine derivative terbinafine in vitro. Antimicrob. Agents Chemother. 31:1365-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rocha, E. M. F., C. B. Almeida, and N. M. Marinez-Rossi. 2002. Identification of genes involved in terbinafine resistance in Aspergillus nidulans. Lett. Appl. Microbiol. 35:228-232. [DOI] [PubMed] [Google Scholar]
- 31.Ryder, N. S., and I. Leitner. 2001. Synergistic interaction of terbinafine with triazoles or amphotericin B against Aspergillus species. Med. Mycol. 39:91-95. [DOI] [PubMed] [Google Scholar]
- 32.Ryder, N. S., and B. Favre. 1997. Antifungal activity and mechanism of action of terbinafine. Rev. Contemp. Pharmacother. 8:275-287. [Google Scholar]
- 33.Sambrook, J., and W. D. Russell. 1989. Molecular cloning. Cold Spring Harbor Press, Cold Spring Harbor, N.Y.
- 34.Sanglard, D., F. Ischer, M. Monod, and J. Bille. 1997. Cloning of Candida albicans genes conferring resistance to azole antifungal agents: characterization of CDR2, a new multidrug ABC transporter gene. Microbiology 143:405-416. [DOI] [PubMed] [Google Scholar]
- 35.Schiraldi, G. F., M. D. Colombo, S. Harari, S. Lo Cicero, G. Ziglio, M. Ferrarese, D. Rossato, and E. Soresi. 1996. Terbinafine in the treatment of non-immunocompromised compassionate cases of bronchopulmonary aspergillosis. Mycoses 39:5-12. [DOI] [PubMed] [Google Scholar]
- 36.Schiraldi, G. F., S. Lo Cicero, M. D. Colombo, D. Rossato, M. Ferrarese, and E. Soresi. 1996. Refractory pulmonary aspergillosis: compassionate trial with terbinafine. Br. J. Dermatol. 134(Suppl. 46):25-29. [DOI] [PubMed] [Google Scholar]
- 37.Sigurgeirsson, B., J. H. Olafsson, J. B. Steinsson, C. Paul, S. Billstein, and E. G. Evans. 2002. Long-term effectiveness of treatment with terbinafine vs itraconazole in onychomycosis: a 5-year blinded prospective follow-up study. Arch. Dermatol. 138:353-357. [DOI] [PubMed] [Google Scholar]
- 38.Stevens, A. D. 2000. Drug interaction studies of a glucan synthase inhibitor (LY 303366) and a chitin synthase inhibitor (Nikkomycin Z) for inhibition and killing of fungal pathogens. Antimicrob. Agents Chemother. 44:2547-2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Te Dorsthorst, D. T., P. E. Verweij, J. F. Meis, N. C. Punt, and J. W. Mouton. 2002. Comparison of fractional inhibitory concentration index with response surface modeling for characterization of in vitro interaction of antifungals against itraconazole-susceptible and -resistant Aspergillus fumigatus isolates. Antimicrob. Agents Chemother. 46:702-707. [DOI] [PMC free article] [PubMed] [Google Scholar]