Summary
Background
Patients with chronic lymphocytic leukaemia (CLL) with TP53 aberrations respond poorly to first-line chemoimmunotherapy resulting in early relapse and short survival. We investigated the safety and activity of ibrutinib in previously untreated and relapsed or refractory CLL with TP53 aberrations.
Methods
In this investigator-initiated, single-arm phase 2 study we enrolled eligible adult patients with active CLL with TP53 aberrations at the National Institutes of Health Clinical Center (Bethesda, MD, USA). Patients received 28-day cycles of ibrutinib 420 mg orally once daily until disease progression or the occurrence of limiting toxicities. The primary endpoint was overall response to treatment at 24 weeks in all evaluable patients. This study is registered with ClinicalTrials.gov number NCT01500733, and is fully enrolled.
Findings
Between Dec 22, 2011 and Jan 2, 2014 we enrolled 51 patients; 47 had CLL with deletion 17p13.1 and four carried a TP53 mutation in the absence of deletion 17p13.1. All patients had active disease requiring therapy. 35 enrolled patients had previously untreated CLL and 16 had relapsed or refractory disease. Median follow-up was 24 months (IQR 12·9-27·0). 33 previously untreated patients and 15 patients with relapsed or refractory CLL were evaluable for response at 24 weeks. 32 (97%; 95% CI 86-100) of 33 previously untreated patients achieved an objective response, including partial response in 18 patients (55%) and partial response with lymphocytosis in 14 (42%). One patient had progressive disease at 0·4 months. 12 (80%; 95% CI 52-96) of the 15 patients with relapsed or refractory CLL had an objective response: six (40%) achieved a partial response and six (40%) a partial response with lymphocytosis; the remaining three (20%) patients had stable disease. Grade 3 or worse treatment-related adverse events were neutropenia in 12 (24%) patients (grade 4 in one [2%] patient), anaemia in seven (14%) patients, and thrombocytopenia in five (10%) patients (grade 4 in one [2%] patient). Grade 3 pneumonia occurred in three (6%) patients, and grade 3 rash in one (2%) patient.
Interpretation
The activity and safety profile of single-agent ibrutinib in CLL with TP53 aberrations is encouraging and supports its consideration as a novel treatment option for patients with this high-risk disease in both first-line and second-line settings
Funding
Intramural Research Program of the National Heart, Lung, and Blood Institute and the National Cancer Institute, Danish Cancer Society, Novo Nordisk Foundation, National Institutes of Health Medical Research Scholars Program, and Pharmacyclics Inc.
Introduction
Chemoimmunotherapy is the standard of care for patients who need treatment for chronic lymphocytic leukaemia (CLL).1 Patients with TP53 aberrations respond less well to treatment than do those without this high-risk genetic lesion resulting in early relapse and inferior survival.1,2 Deletion of chromosome band 17p13.1 results in loss of one allele of the tumour suppressor gene TP53, and the other allele is often incapacitated through point mutations.3 The P53 pathway induces cell death in response to DNA damage, and its inactivation is associated with resistance to chemotherapy. In previously untreated patients the prevalence of deletion 17p13.1 and TP53 mutations, which often occur together, ranges from 7% to 11·5%.1,4,5 However, selection of chemotherapyresistant clones during treatment increases the prevalence of TP53 aberrations at relapse.3
The identification of appropriate treatment options for patients with TP53 aberrations is a research priority.3,6 Allogeneic stem-cell transplantation has long been viewed as the only option for long-term disease control in these patients. Consensus guidelines recommend allogeneic stem-cell transplantation during first remission for patients with CLL and TP53 aberrations.7 However, allogeneic stem-cell transplantation is not broadly applicable due to the advanced age of most patients with CLL, and transplant-related mortality remains a concern. Alternative treatment options are scarce. The anti-CD52 antibody alemtuzumab is active in CLL with TP53 aberrations but most responses are not durable and the agent is associated with a high incidence of grade 3 or worse infections.8,9 Promising new options include kinase inhibitors such as ibrutinib or idelalisib, which have shown activity in relapsed or refractory patients with high-risk cytogenetic abnormalities, including deletion 17p13.1.10-12 With the advent of these novel therapies the role of allogeneic stem-cell transplantation for patients with CLL with TP53 aberrations is being re-assessed.13
Ibrutinib (PCI-32765) is an orally bioavailable, covalent inhibitor of Bruton's tyrosine kinase (BTK).14 Once-daily administration causes sustained inactivation of the kinase resulting in inhibition of B-cell receptor signalling and tumour-microenvironment interactions.15 In a 2013 report of a phase 1b-2 study,11 71% of patients in the ibrutinib 420 mg dosing group with relapsed or refractory CLL achieved a response according to International Workshop on Chronic Lymphocytic Leukemia (IWCLL) 2008 criteria. An additional 20% of patients had partial responses with lymphocytosis. More recently, a randomised comparison of ibrutinib versus the anti-CD20 antibody ofatumumab showed superior activity of ibrutinib over ofatumumab, including improved survival.10 Based on these studies and the encouraging activity of ibrutinib in patients with deletion 17p13.1, ibrutinib received full Food and Drug Administration approval in the USA on July 28, 2014 for patients with CLL who have received at least one previous therapy, and for those with CLL with deletion 17p13.1. Approval by the European Commission for patients who have received at least one previous therapy, or in first-line CLL patients in the presence of deletion 17p13.1 or TP53 mutation in those unsuitable for chemotherapy was granted on Oct 17, 2014.
In view of the encouraging activity of ibrutinib in CLL and the few treatment options for patients with TP53 aberrations, we aimed to assess the safety and activity of ibrutinib in this high-risk patient population.
Methods
Study Design and Participants
For this phase 2, open-label, single-center, investigator-initiated study, between Dec 22, 2011, and Jan 2, 2014, we enrolled previously untreated patients with CLL and patients with relapsed or refractory disease at the NIH Clinical Center in Bethesda, MD, USA. Eligibility criteria included: diagnosis of CLL, including small lymphocytic lymphoma according to WHO criteria, with deletion 17p13.1 identified by interphase fluorescence in-situ hybridisation (FISH) in at least 10% of nuclei scored or presence of TP53 mutation in the absence of deletion 17p13.1; active disease requiring therapy according to the IWCLL criteria;16 age 18 years or older; ECOG performance status of 0, 1, or 2; neutrophil count of at least 500 cells per μL. Exclusion criteria included: being younger than 18 years; transformed disease; autoimmune haemolytic anaemia or thrombocytopenia necessitating steroid therapy; impaired hepatic function (total bilirubin level ≥1·5×the upper limit of normal [ULN] unless due to Gilbert's disease, or aspartate aminotransferase or alanine aminotransferase ≥2·5×ULN unless caused by infiltration of the liver), impaired renal function (creatinine concentration ≥2·0 mg/dL or glomerular filtration rate ≤50 mL/min), active Hepatitis B infection, HIV infection, concomitant corticosteroid treatment at doses equivalent to prednisone more than 20 mg per day, and anticoagulation with warfarin. For previously treated patients, a 4-week washout period was required.
Ethics approval for the study was granted by the National Heart, Lung, and Blood Institute institutional review board. All patients provided written informed consent.
Procedures
Pretreatment assessments included a complete medical history; physical examination; ECOG performance evaluation; complete blood count; liver and renal function tests; β2-microglobulin; serum free light chains; viral serologies for HIV and hepatitis B; lymphocyte phenotyping; bone marrow aspiration and biopsy, peripheral blood flow cytometry, and CT scans of the neck, chest, abdomen, and pelvis. Flow cytometry was used to show that all patients had circulating tumour cells consistent with CLL. Pretreatment lymph node biopsies were obtained in all patients with superficial lymphadenopathy. Interphase FISH was done on peripheral blood or bone marrow in the National Cancer Institute Clinical Cytogenetics Laboratory with the Vysis probe (Abbott Molecular, Des Plaines, IL, USA); results from accredited external testing facilities were accepted. Sequencing of the immunoglobulin heavy chain variable gene (IGHV) was done by LBP in the laboratory of CG (Copenhagen, Denmark).
Patients received ibrutinib 420 mg administered orally once daily on a continuous schedule until disease progression or toxicity necessitated drug discontinuation. Each cycle was 28 days (give or take 4 days). Ibrutinib treatment was paused in cases of grade 4 neutropenia lasting for longer than 7 days or any grade 4 thrombocytopenia. Patients who had a first occurrence of grade 3 diarrhoea, constitutional symptoms, or infection were restarted at the same dose when the toxicity resolved to grade 1 severity or less or to baseline. For any other grade 3 adverse event, the dose was reduced by 140 mg. For new-onset or symptomatic atrial fibrillation (grade 2), ibrutinib treatment was paused and a cardiac assessment was done. After resolution of a first occurrence of atrial fibrillation of up to grade 2 severity, the study drug could be restarted at the same dose. Safety monitoring was done every other week for the first month, then monthly until month 6, and every 3 months thereafter. Laboratory tests as part of safety monitoring included complete blood counts, and acute care, hepatic, and mineral panels.
Outcomes
The primary endpoint was overall response to treatment after six cycles of therapy at 24 weeks. Secondary endpoints were safety, overall survival, progression-free survival, best response, and nodal response. Data cutoff for study analysis was Aug 1, 2014. Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 4.0. Haematological adverse events were graded according to IWCLL criteria.16
Responses were assessed based on IWCLL 2008 criteria, incorporating the recent updates on partial response with lymphocytosis (appendix p 2).16,17 Clinical response was assessed at each visit. At 2, 6, and 12 months, radiological assessments were done. Responses reported here are based on these radiological assessments. Bone marrow evaluation was undertaken in 42 patients at 8 weeks, and in 47 patients at 24 weeks. The bone marrow at 8 weeks was omitted in the first patients and one patient refused bone marrow biopsy at 24 weeks. Bone marrow samples were assessed by immunostaining. FISH for deletion 17p13.1 was repeated on blood samples at 24 weeks. The frequency of deletion 17p13.1 in all CLL cells was calculated as 1 divided by the proportion of CD19+ cells in all lymphocytes multiplied by the proportion of cells with deletion 17p13.1. Spleen volume was calculated from CT scans with the Vitrea Core Workstation Server, version 6.6 (Vital Images, Minnetonka, MN, USA).
Statistical Analysis
We used a Simon's minimax two-stage design to test the null hypothesis that the proportion of patients achieving an objective response in the study was 15% or lower versus the alternative hypothesis that it was 40% or higher, with a type I error of 0·05 and 90% power. We established that a sample size of 27 patients evaluable for the primary endpoint was needed. Initially, both untreated and relapsed or refractory patients were enrolled. On Aug 24, 2012, the study was amended by the investigators to allow enrolment of an entire cohort of 27 previously untreated patients, in addition to a cohort of 16 patients with relapsed or refractory disease. We enrolled eight additional previously untreated patients to account for the possibility of non-treatment-related discontinuation before 6 months. The safety and survival analysis was based on the intention-to-treat population. Objective responses were assessed in patients who were evaluable at 24 weeks.
We used descriptive statistics to summarise the findings in each of the defined cohorts, including the primary endpoint. We used the Kaplan-Meier method to estimate overall survival (defined as time from study enrolment to death) and progression-free survival (defined as time from study enrolment to progression or death). We estimated the cumulative incidence of progression by considering deaths without relapse as competing risk events. We compared survival probabilities by the log-rank test and cumulative incidence by the Gray's test. R version 3.1.1 was used for statistical analyses.
This study is registered with ClinicalTrials.gov, number NCT01500733.
Role of the funding source
his study was funded by the Intramural Research Program of the National Heart, Lung, and Blood Institute and the National Cancer Institute, National Institutes of Health. The study was designed by the investigators, a draft of the protocol was submitted to Pharmacyclics (who provided the study drug) for comments, and all data were collected by the investigators and stored at the National Institutes of Health. The investigators analysed the data and wrote the report. A draft was submitted to Pharmacyclics for comments. The corresponding author had full access to all the data in the study and had final responsibility for the content of the report and the decision to submit for publication.
Results
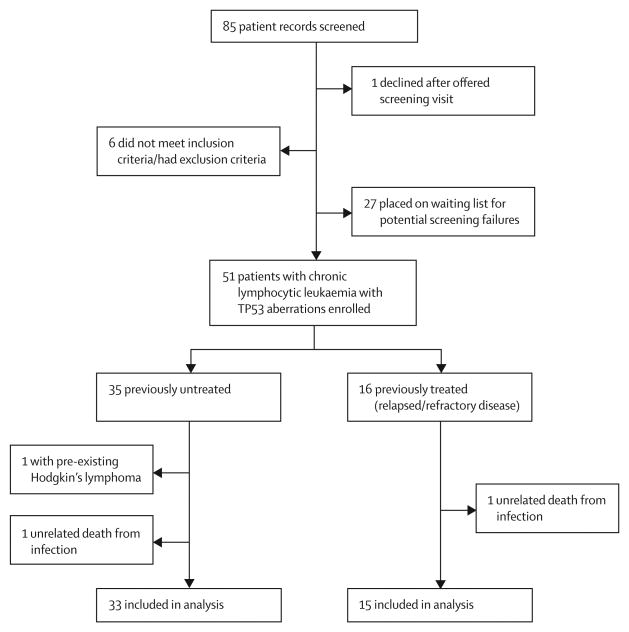
Between Dec 22, 2011 and Jan 2, 2014, we screened 85 patient records and enrolled 51 eligible patients with CLL, of whom 35 had previously untreated CLL and 16 had relapsed or refractory disease (figure 1, table 1). Most patients had advanced Rai stage and IGHV-unmutated disease (table 1). 47 patients had deletion 17p13.1 identified by interphase fluorescence in-situ hybridisation in at least 10% of nuclei scored and four patients (two in each cohort) had a TP53 mutation in the absence of deletion 17p13.1. 35 patients were previously untreated, 16 had relapsed or refractory disease. At the time of analysis, median follow-up for all patients was 24 months (IQR 12·9-27·0). Median follow-up for the previously untreated cohort was 15 months (IQR 12·5-25·7) and for the relapsed or refractory cohort was 26 months (25·4–28·3). 42 (82%) of 51 enrolled patients are still on treatment without disease progression. Nine (18%) of 51 patients discontinued treatment. Reasons for treatment discontinuation were disease progression in five (10%) patients, and three (6%) deaths. One patient, who was found to have Hodgkin's lymphoma that predated enrolment into the study, was taken off study and is included in the safety analysis only (figure 1, appendix p 1). Progressive disease was caused by Richter's transformation in three patients. An additional two patients had prolymphocytic transformation. Median time to progression for these five patients was 7·5 months (IQR 7·2–15·0).
Figure 1. Trial profile.
Table 1. Baseline characteristics.
| Previously untreated CLL (n=35) | Relapsed/refractory CLL* (n=16) | |
|---|---|---|
| Age (years) | 62 (33-82) | 62 (56-79) |
|
| ||
| Sex | ||
| Female | 12 (34%) | 8 (50%) |
| Male | 23 (66%) | 8 (50%) |
|
| ||
| Rai stage III/IV | 22 (63%) | 12 (75%) |
|
| ||
| Bulky adenopathy (≥5 cm)† | 8 (23%) | 8 (50%) |
|
| ||
| Splenomegaly (≥315 mL)† | 30 (86%) | 14 (88%) |
|
| ||
| IGHV unmutated‡ | 22 (63%) | 12 (75%) |
|
| ||
| % CLL cells with deletion 17p13.1 | 61 (13-97%) | 42 (12-94%) |
|
| ||
| β2 microglobulin ≥3 mg/dL | 25 (71%) | 13 (81%) |
Data are median (range) or n (%). CLL= chronic lymphocytic leukaemia.
Median of four previous treatments (range 1–7). Previous treatment regimens included nucleoside analogue in 13 patients (81%), alkylator in eight (50%), bendamustine in nine (56%), rituximab in 14 (88%), ofatumumab in two (13%), and allogeneic stem-cell transplantation in one (6%) patients.
Assessed by CT scan.
Unmutated IGHV indicates <2% change in IGHV sequence compared with germline. At initiation of ibrutinib treatment, Rai stage ≥ III disease was present in 10 (59%) of the 17 patients with IGHV-mutated CLL and in 24 (71%) of the 34 patients with IGHV-unmutated CLL.
At 24 weeks 48 patients were evaluable for response (33 previously untreated patients and 15 relapsed/refractory patients). 44 (92%; 95% CI 84–100) of these 48 patients achieved an objective response; 24 (50%) had a partial response, and 20 (42%) patients achieved an objective response(95% CI 84-99.5), 24 (50%)a partial response with lymphocytosis (table 2). 32 (97%; 95% CI 86–100) of 33 previously untreated patients achieved an objective response including partial response in 18 (55%) and partial response with lymphocytosis in 14 (42%). One previously untreated patient had progressive disease at 0·4 months. 12 (80%; 95% CI 52-96) of the 15 patients with relapsed or refractory CLL had an objective response: six (40%) patients achieved a partial response, six (40%) a partial response with lymphocytosis, and three patients had stable disease. The proportion of patients achieving an overall response in subgroups based on IGHV mutation status, age, Rai disease stage, β2-microglobulin, or the presence of bulky lymphadenopathy or splenomegaly was similar (appendix p 3). However, the distribution of partial response and partial response with lymphocyotsis showed some variation (appendix p 3), consistent with different rates of resolution of the treatment-induced lymphocytosis between patients.11,18
Table 2. Response to treatment.
| All evaluable patients (n=48) |
Previously untreated patients (n=33) |
Relapsed or refractory patients (n=15) |
|
|---|---|---|---|
| Response at 24 weeks | |||
|
| |||
| Complete response | .. | .. | .. |
| Partial response | 24 (50%) | 18 (55%) | 6 (40%) |
| Partial response with lymphocytosis | 20 (42%) | 14 (42%) | 6 (40%) |
| Stable disease | 3 (6%) | .. | 3 (20%) |
| Progressive disease | 1 (2%) | 1 (3%) | .. |
|
| |||
| Best response | |||
|
| |||
| Complete response | 5 (10%) | 4 (12%) | 1 (7%) |
| Partial response | 32 (67%) | 23 (70%) | 9 (60%) |
| Partial response with lymphocytosis | 8 (17%) | 5 (15%) | 3 (20%) |
| Stable disease | 2 (4%) | .. | 2 (13%) |
| Progressive disease | 1 (2%) | 1 (3%) | .. |
Data are n (%)
The rate and depth of response increased with time. Partial response was recorded in six (13%) of 48 patients at 8 weeks and in 24 (50%) at 24 weeks. Conversely, a partial response with lymphocytosis was recorded in 24 (50%) patients at 8 weeks and 20 (42%) patients at 24 weeks. The best response achieved was objective response in 45 (94%; 95% CI 87–100) of all 48 evaluable patients, in 32 (97%; 86–100) of the 33 evaluable patients with previously untreated disease, and in 13 (87%; 69·5–100) of the 15 evaluable patients with relapsed or refractory disease. Five (10%) of all evaluable patients had a complete response as best response, of whom four had IGHV-unmutated CLL, and all except for one were previously untreated. Median time to complete response was 48 weeks (IQR 48–48).
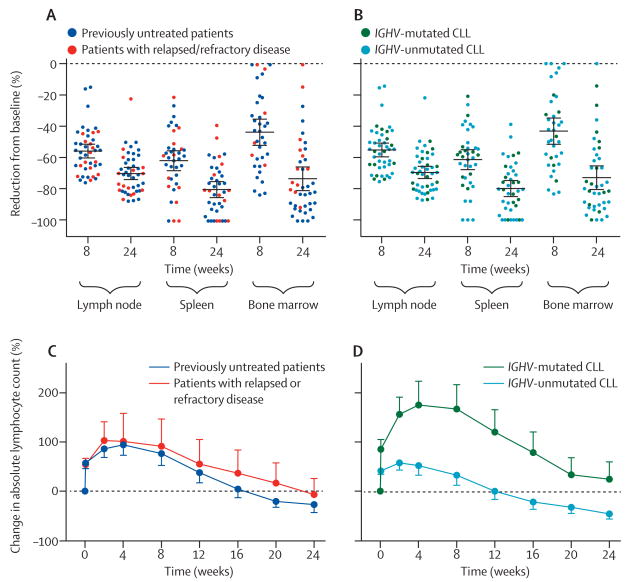
Rapid disease control was achieved in all tissue compartments, reaching at least a 50% mean reduction in tumour burden in both spleen and lymph nodes at 8 weeks, with further improvements on continued therapy (figure 2A, 2B).Responses in the bone marrow seemed to be somewhat slower than in the spleen and lymph nodes. At 8 weeks, at least a 50% decrease in tumour burden in the bone marrow, lymph node, and spleen was recorded in 16 (44%) of 36, 31 (70%) of 44, and 30 (79%) of 38 patients with complete data, respectively. At the completion of 24 weeks of treatment, the proportion of patients with at least a 50% reduction in tumour burden in bone marrow, lymph node, and spleen was 34 (83%) of 41, 42 (93%) of 45, and 38 (95%) of 40 patients with complete data, respectively. Reasons for incomplete data include omission of bone marrow biopsies or insufficient material (n=2 at 8 weeks, n=7 at 24 weeks), missing scans (n=2 at 8 weeks, n=1 at 24 weeks), and absence of pathological lymphadenopathy (n=2) or splenomegaly (n=7) at baseline. In three patients, no residual CLL infiltrate was detected in the bone marrow by immunohistochemistry. The quality of tumour response in the tissue sites did not differ by previous treatment history or IGHV mutation status (figure 2C, 2D). The characteristic treatment-induced lymphocytosis was reported in most patients, and the degree and kinetics did not differ in previously untreated patients compared with those with relapsed or refractory disease (figure 2C). By contrast, patients with IGHV-mutated CLL had a greater relative increase and slower resolution of lymphocytosis than did those with IGHV-unmutated CLL (figure 2D).
Figure 2. Disease control in diff erent anatomic compartments.
(A, B) Reduction of tumour burden in each compartment for each patient during treatment. (A) Previously untreated patients vs patients with relapsed or refractory disease. (B) Patients with IGHV-mutated CLL vs patients with IGHV-unmutated CLL. The mean is indicated by the central black horizontal lines; error bars are 95% CI. The mean decrease in tumour burden at 24 weeks in lymph node was 69·34% (SEM 1·98), in spleen 79·75% (SEM 2·66), and in bone marrow 73·49% (SEM 3·83). Disease reduction was similar in subgroups defined by previous treatment history or by IGHV mutation status. (C, D) Mean (SEM) percentage change in lymphocyte count during treatment compared with baseline. (C) Previously untreated patients vs patients with relapsed or refractory disease. (D) Patients with IGHV-mutated CLL vs patients with IGHV-unmutated CLL. IGHV=immunoglobulin heavy chain variable gene. CLL=chronic lymphocytic leukaemia.
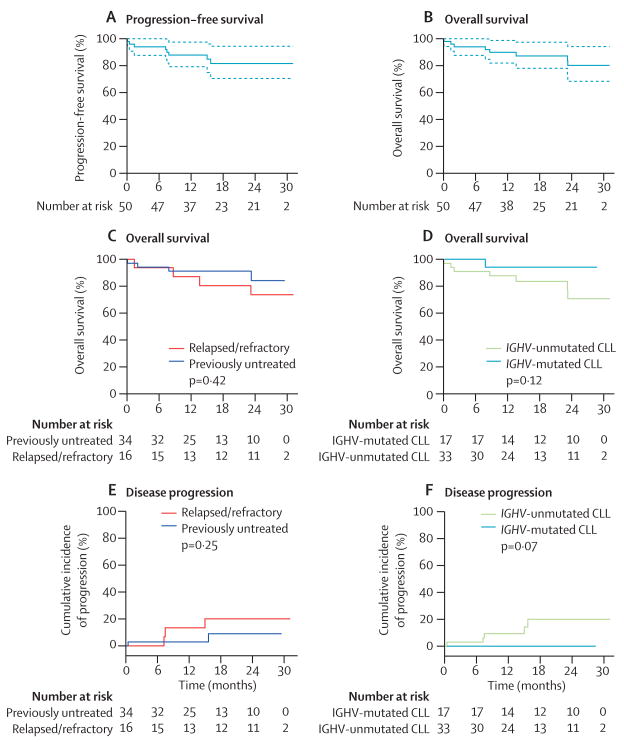
Estimated progression-free survival for all patients on an intention-to-treat basis at 24 months was 82% (95% CI 71–94), and overall survival at 24 months was 80% (68–94) (figure 3A, 3B). At final follow-up for this report (Aug 1, 2014), eight (16%) patients had died: five (10%) from progressive disease, two (4%) from infection, and one (2%) patient with sudden unexplained death that was judged to be possibly treatment related (appendix p 1). The estimated overall survival at 24 months was 84% (95% CI 72–100) for previously untreated patients and 74% (57–100) for patients with relapsed or refractory disease (figure 3C); it was 94% (95% CI 84–100) in IGHV-mutated and 71% (53–94) in IGHV-unmutated CLL (figure 3D). The cumulative incidence of progression at 24 months was 20% (95% CI 5–44) in patients with relapsed or refractory disease, 9% (1–27) in previously untreated patients (figure 3E), and 20% (7–39) for IGHV-unmutated CLL (figure 3F). None of the patients with IGHV-mutated CLL have progressed so far.
Figure 3. Estimates of progression-free and overall survival, and of cumulative incidence of disease progression.
(A) Progression-free survival and (B) overall survival for all patients. (C) Overall survival in subgroups by treatment history and (D) by IGHV mutation status. The p value was calculated by log-rank test. (E) Estimated cumulative incidence of disease progression in subgroups by treatment history and (F) by IGHV mutation status. The p value was calculated by Gray's test. IGHV=immunoglobulin heavy chain variable gene. CLL=chronic lymphocytic leukaemia.
To assess whether or not deletion 17p13.1 confers resistance to ibrutinib, we established the proportion of CLL cells carrying deletion 17p13.1 at baseline and after 24 weeks on treatment. In 43 evaluable patients, the proportion of CLL cells with deletion 17p13.1 decreased in 20 (47%) patients (median decrease 48% [IQR 18·7–82·8%]), increased in 20 (47%) patients (median increase 12% [4·2–43·9%]), and remained unchanged in three (6%) patients (appendix p 4). In the 20 patients with a relative increase in the frequency of deletion 17p13.1, none progressed, 18 (90%) had a clinical response at 24 weeks, and two (10%) patients had stable disease. All of these patients are still continuing in the study on treatment without progression at data cutoff (Aug 1, 2014). Of the four patients who developed progressive disease beyond 24 weeks, two were included in this analysis, in both of whom the proportion of CLL cells with deletion 17p13.1 was decreased compared with baseline at 24 weeks.
Treatment with ibrutinib was well tolerated. Most nonhaematological adverse events were of grade 1 or 2 severity (table 3). The most frequent possibly treatmentrelated adverse events were arthralgia, diarrhoea, rash, nail ridging, bruising, and muscle spasms or cramps. The most common non-haematological grade 3 adverse event was pneumonia in three (6%) patients. No grade 3 or worse bleeding events occurred. One patient (2%) required a dose reduction for grade 3 rash. No other adverse events required dose reductions or withdrawal of patients from the study. Grade 3 or 4 haematological adverse events were neutropenia in 12 (24%) patients, anaemia in seven (14%) patients, and thrombocytopenia in five (10%) patients (table 3). Cytopenias mainly occurred during the first few months on therapy, improved with continuous treatment, and were not associated with infections or bleeding.
Table 3. Treatment-related adverse events in all 51 patients.
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
|---|---|---|---|---|
| Arthralgia | 24 (47%) | 6 (12%) | .. | .. |
| Diarrhoea | 25 (49%) | 1 (2%) | .. | .. |
| Rash | 23 (45%) | .. | 1 (2%) | |
| Nail Ridging | 22 (43%) | .. | .. | .. |
| Bruising | 17 (33%) | .. | .. | .. |
| Muscle spasm or cramps | 15 (29%) | 1 (2%) | .. | .. |
| Neutropenia* | 1 (2%) | 2 (4%) | 11 (22%) | 1 (2%) |
| Fatigue | 13 (25%) | .. | .. | .. |
| Bilirubin Increase | 10 (20%) | .. | .. | .. |
| Lung infection (pneumonia) | .. | 6 (12%) | 3 (6%) | .. |
| Anaemia | 2 (4%) | .. | 7 (14%) | .. |
| Alkaline phosphatase increase | 6 (12%) | 2 (4%) | .. | .. |
| Alanine aminotransferase increase | 7 (14%) | 1 (2%) | .. | .. |
| Aspartate aminotransferase increase | 7 (14%) | 1 (2%) | .. | .. |
| Dyspepsia | 8 (16%) | .. | .. | .. |
| Mucositis | 8 (16%) | .. | .. | .. |
| Thrombocytopenia | 3 (6%) | .. | 4 (8%) | 1 (2%) |
| Hair Texture Changes | 7 (14%) | .. | .. | .. |
| Oedema in limbs | 3 (6%) | 1 (2%) | .. | .. |
| Epistaxis | 3 (6%) | 1 (2%) | .. | .. |
| Skin ulceration | 4 (8%) | .. | .. | .. |
| Dry skin | 3 (6%) | .. | .. | .. |
| Nausea | 3 (6%) | .. | .. | .. |
| Myalgia | 2 (4%) | 1 (2%) | .. | .. |
| Subconjunctival haemorrhage | 2 (4%) | 1 (2%) | .. | .. |
| Fever | 2 (4%) | .. | .. | .. |
| Cognitive disturbance | 2 (4%) | .. | .. | .. |
| Creatine kinase increase | 2 (4%) | .. | .. | .. |
| Headache | 2 (4%) | .. | .. | .. |
| Tendonitis | 2 (4%) | .. | .. | .. |
| Alopecia | 1 (2%) | .. | .. | .. |
| Anorexia | 1 (2%) | .. | .. | .. |
| Atrial fibrillation | .. | 1 (2%) | .. | .. |
| Chills | 1 (2%) | .. | .. | .. |
| Constipation | 1 (2%) | .. | .. | .. |
| Dry eyes | 1 (2%) | .. | .. | .. |
| Hoarseness | 1 (2%) | .. | .. | .. |
| Palpitations | 1 (2%) | .. | .. | .. |
| Pruritus | 1 (2%) | .. | .. | .. |
| Pulmonary infiltrates | 1 (2%) | .. | .. | .. |
| Purpura | 1 (2%) | .. | .. | .. |
| Sinusitis | .. | 1 (2%) | .. | .. |
| Urinary tract infection | .. | 1 (2%) | .. |
Four patients received granulocyte colony-stimulating factor support with complete resolution of neutropenia. No neutropenic fever events occurred.
Discussion
TP53 aberrations identify a high-risk group of patients with CLL for whom no standard treatment exists. Responses to front-line chemoimmunotherapy are short lived.1,2 Ibrutinib has shown activity in relapsed and refractory patients including in those with deletion 17p13.1.10,11 We report mature results from a phase 2 trial of single-agent ibrutinib for patients with CLL with TP53 aberrations including—to the best of our knowledge—the largest experience in previously untreated patients receiving this targeted agent as first-line therapy (panel). Ibrutinib induced responses in 32 (97%) of the 33 evaluable, previously untreated patients; the estimated rate of progression was 9% (95% CI 1–27) at 24 months. This finding contrasts with responses in around 68% of patients and median progression-free survival of less than 12 months when first-line fludarabine, cyclophosphamide, and rituximab are used to treat these high-risk patients.1 Our study includes 35 patients with TP53 aberrationsreceiving ibrutinib as first-line therapy―a number that is similar to the combined 30 previously untreated patients with deletion 17p13.1 who were treated with fludarabine, cyclophosphamide, and rituximab in the two largest studies done so far.1,21 Another non-chemotherapy based regimen active in CLL with TP53 aberrations is alemtuzumab with methylprednisolone, which in first line achieved a response in 88% of patients and a median progression-free survival of 18·3 months (95% CI 15·6–not available).9 Longer follow-up with ibrutinib is needed to better assess durability of response and the possible need for subsequent treatment.
Safety and tolerability of treatment with ibrutinib was favourable and similar to findings reported in other studies.10,11,22 Only one patient needed a dose reduction and no patients discontinued treatment because of adverse events.
In the phase 1b–2 study of single-agent ibrutinib in relapsed or refractory CLL, the estimated progression-free survival at 26 months for patients with deletion 17p13.1 was 57%, but more than 90% for patients without deletion 17p13.1 or 11q22.3.11 We therefore sought to investigate whether deletion 17p13.1 confers resistance to ibrutinib that would lead to relapse as reported with chemoimmunotherapy. However, sequential FISH analysis provided no evidence for the selection of resistant subclones with deletion 17p13.1 during ibrutinib therapy, which supports a probably P53-independent mechanism of action.
The type of response that occurred with ibrutinib differs from that achieved with chemoimmunotherapy. Deep remissions, at least during the first year on therapy, were rare, even in previously untreated patients. However, as we and others have previously reported, residual disease that persists during ibrutinib therapy consists mostly of quiescent cells with a low proliferative rate.18,23,24 Consistently, the presence of residual disease does not seem to predict treatment failure, by contrast with the experience with chemoimmunotherapy.24
Disease progression on treatment was reported in only five (10%) patients and occurred at a median of 7·5 months (IQR 7·2–15·0). Notably, all five patients had transformation, which manifested as Richter's syndrome in three patients and as prolymphocytic transformation in two. In retrospect, transformation might have already been present at study entry in two patients (appendix p 1). In addition to TP53 aberrations, all the patients who progressed had additional high-risk disease features at baseline: all had IGHV-unmutated CLL, two had MYC amplification (a rare ultra-high-risk event in CLL), and three had been heavily pretreated. Recently, Richter's transformation was reported in 23% of patients with deletion 17p13.1 after a median of 12 months from first-line therapy, mainly with fludarabine, cyclophosphamide, and rituximab.2 Thus, the rate of transformation on ibrutinib seems to be lower than reported with other frequently used first-line therapies for high-risk patients. However, additional follow-up will be needed to better assess the long-term transformation rate in patients treated with ibrutinib.
Tumour–microenvironment interactions and B-cell receptor signalling are pivotal pathogenic pathways in CLL and are targets for new therapeutic approaches.25–27 The substantial reduction in disease burden and the notable reduction in intracellular signalling, cellular activation, and proliferation throughout the different anatomical compartments is consistent with the role of BTK as a central node in many signalling pathways.14,27 Recently, acquired mutations in BTK and PLCg2 that confer resistance to ibrutinib have been reported.28,29 However, the low frequency of these mutations suggests that tumour cells cannot easily bypass the block in intracellular signalling imposed by ibrutinib.
In conclusion, single-agent ibrutinib induced durable responses in CLL with TP53 aberrations. Treatment failure in previously untreated patients was rare. Subclones with deletion 17p13.1 were equally sensitive to ibrutinib as were those not carrying the deletion, which is consistent with a P53-independent mechanism of action of the drug. The quality and duration of response might be improved further with combination therapy.30 These data support the use of ibrutinib as first-line therapy of CLL with TP53 aberrations. Longer follow-up will be needed to assess the effect of this targeted agent on long-term survival, which will clarify whether or not ibrutinib could eventually replace allogeneic stem-cell transplantation for these high-risk patients.
Supplementary Material
Panel: Research in context.
Systematic review
We designed this study in 2011 to investigate novel treatment options for patients with TP53 aberrations. Although chemoimmunotherapy with fludarabine, cyclophosphamide, and rituximab was firmly established as an effective regimen for most patients with chronic lymphocytic leukaemia (CLL), patients with TP53 aberrations had poor outcomes.1 We searched PubMed for articles published in English between Jan 1, 2006, and June, 2011 for treatment options in CLL with TP53 aberrations or deletion 17p13.1, using the search terms “chronic lymphocytic leukemia”, “TP53”, “deletion 17p”, “alemtuzumab”, “flavopiridol”, and “lenalidomide”. In particular, alemtuzumab,8,9 lenalidomide,19 and flavopiridol,20 were shown to have activity in at least a subset of these high-risk patients (summarised by Badoux and colleagues6). However, responses to these agents were often short lived. Allogeneic stem-cell transplantation was known to provide long-term disease control and consensus guidelines recommend this transplantation approach in first remission for patients with CLL and deletion 17p13.1.7 Early data about ibrutinib presented at scientific meetings suggested it had good activity in CLL, irrespective of whether or not classical high-risk disease features were present.
Interpretation
Several studies have now established ibrutinib as an effective and well-tolerated treatment option for relapsed or refractory patients with CLL with TP53 aberrations.10,11 Ibrutinib is now approved in both the USA and Europe for patients with relapsed disease and as first-line therapy for CLL with deletion 17p13.1 (USA and EU) or TP53 aberrations (EU). Our study provides mature data for the largest cohort so far of such high-risk patients receiving ibrutinib as first-line therapy. Although progressive disease manifested as transformation in 10% of patients on this study, the rate of transformation on ibrutinib seems to be lower than previously reported with first-line chemoimmunotherapy.2 With the advent of active targeted agents such as ibrutinib, chemoimmunotherapy for patients with CLL with TP53 aberrations should be avoided. The role of allogeneic stem-cell transplantation in such patients is being re-assessed.13
Acknowledgments
This work was funded by the Intramural Research Program of the National Heart, Lung, and Blood Institute and the National Cancer Institute (National Institutes of Health). Pharmacyclics Inc provided the study drug and research support. We thank the patients who participated in this trial and their families; Ajunae Wells, Jennifer Gyamfi, Stephanie Housel, Adrian Byrnes, Milena Kirilyuk, and Allison Wise for protocol support; Judith Starling for Pharmacy support, and Francine Thomas for imaging analysis; Tatyana Sarkisova, Jocelyn Bailey, and Sindhuja Edara for data management; Olha Katynska, Inna Churbanova, Arman Sabet Kashani, and Jae Hyung Chang for clinical trial monitoring.
Footnotes
Contributors: MZHF and AW designed and supervised the clinical trial and correlative studies, collected, analyzed, and interpreted the data, designed the figures and wrote the report. JV, GA, GM, YL, SM, NS, and CUN contributed to clinical patient management, data and sample collection. SS coordinated clinic visits. TEH was involved in study design, and supervised drug administration. SEMH collected and analyzed data. LBP, and CHG performed and analyzed IGHV mutation analysis. SP analyzed the lymph node biopsies. IM and KRC evaluated bone marrow biopsies and supervised clinical laboratory analyses. MS and CY performed and analyzed diagnostic flow cytometry. DCA supervised FISH studies and interpreted data. AL and JJ collected and assembled data for analysis. XT performed statistical analysis. RC and NSY provided institutional support and supervised study conduct. All authors reviewed and approved the final manuscript.
Declaration of interests: AW and MZHF received research funding from Pharmacyclics Inc. CUN reports grants from Danish Cancer Society, during the conduct of the study; personal fees from Janssen, grants from Roche, outside the submitted work; and Principal investigator / national coordinator for upcoming clinical trials sponsored by Roche, the German CLL study group and Acerta Pharma.
Dr. Geisler reports grants from Novo Nordisk Foundation, during the conduct of the study; personal fees from Roche, personal fees from GSK, personal fees from Janssen, personal fees from Novartis, personal fees from Gilead, personal fees from Celgene, other from Norpharma, outside the submitted work; and Principal investigator, Roche. Principal investigator, GSK.
All other authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Xin Tian, Office of Biostatistics Research.
Thomas E Hughes, Clinical Center Pharmacy Department.
Jade Jones, Medical Scholars Research Program.
Stefania Pittaluga, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD, USA; Laboratory of Pathology, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Maryalice Stetler-Stevenson, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD, USA; Laboratory of Pathology, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Constance Yuan, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD, USA; Laboratory of Pathology, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Lone B Pedersen, Department of Hematology, Rigshospitalet, Copenhagen University Hospital, Copenhagen, Denmark.
Christian H Geisler, Department of Hematology, Rigshospitalet, Copenhagen University Hospital, Copenhagen, Denmark.
Katherine R Calvo, Department of Laboratory Medicine, Clinical Research Center, National Institutes of Health, Bethesda, MD, USA.
Diane C Arthur, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD, USA; Laboratory of Pathology, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Irina Maric, Department of Laboratory Medicine, Clinical Research Center, National Institutes of Health, Bethesda, MD, USA.
References
- 1.Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376:1164–74. doi: 10.1016/S0140-6736(10)61381-5. [DOI] [PubMed] [Google Scholar]
- 2.Strati P, Keating MJ, O'Brien SM, et al. Outcomes of first-line treatment for chronic lymphocytic leukemia with 17p deletion. Haematologica. 2014;99:1350–55. doi: 10.3324/haematol.2014.104661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schnaiter A, Stilgenbauer S. 17p deletion in chronic lymphocytic leukemia: risk stratification and therapeutic approach. Hematol Oncol Clin North Am. 2013;27:289–301. doi: 10.1016/j.hoc.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez D, Martinez P, Wade R, et al. Mutational status of the TP53 gene as a predictor of response and survival in patients with chronic lymphocytic leukemia: results from the LRF CLL4 trial. J Clin Oncol. 2011;29:2223–29. doi: 10.1200/JCO.2010.32.0838. [DOI] [PubMed] [Google Scholar]
- 5.Stilgenbauer S, Schnaiter A, Paschka P, et al. Gene mutations and treatment outcome in chronic lymphocytic leukemia: results from the CLL8 trial. Blood. 2014;123:3247–54. doi: 10.1182/blood-2014-01-546150. [DOI] [PubMed] [Google Scholar]
- 6.Badoux XC, Keating MJ, Wierda WG. What is the best frontline therapy for patients with CLL and 17p deletion? Curr Hematol Malig Rep. 2011;6:36–46. doi: 10.1007/s11899-010-0069-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dreger P, Dohner H, Ritgen M, et al. Allogeneic stem cell transplantation provides durable disease control in poor-risk chronic lymphocytic leukemia: long-term clinical and MRD results of the German CLL Study Group CLL3X trial. Blood. 2010;116:2438–47. doi: 10.1182/blood-2010-03-275420. [DOI] [PubMed] [Google Scholar]
- 8.Hillmen P, Skotnicki AB, Robak T, et al. Alemtuzumab compared with chlorambucil as first-line therapy for chronic lymphocytic leukemia. J Clin Oncol. 2007;25:5616–23. doi: 10.1200/JCO.2007.12.9098. [DOI] [PubMed] [Google Scholar]
- 9.Pettitt AR, Jackson R, Carruthers S, et al. Alemtuzumab in combination with methylprednisolone is a highly effective induction regimen for patients with chronic lymphocytic leukemia and deletion of TP53: final results of the national cancer research institute CLL206 trial. J Clin Oncol. 2012;30:1647–55. doi: 10.1200/JCO.2011.35.9695. [DOI] [PubMed] [Google Scholar]
- 10.Byrd JC, Brown JR, O'Brien S, et al. Ibrutinib versus Of atumumab in Previously Treated Chronic Lymphoid Leukemia. N Engl J Med. 2014;371:213–23. doi: 10.1056/NEJMoa1400376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369:32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370:997–1007. doi: 10.1056/NEJMoa1315226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dreger P, Schetelig J, Andersen N, et al. Managing high-risk chronic lymphocytic leukemia during transition to a new treatment era: stem cell transplantation or novel agents? Blood. 2014 doi: 10.1182/blood-2014-07-586826. published online Oct 9. http://dx.doi.org/10.1182/blood-2014-07-586826. [DOI] [PMC free article] [PubMed]
- 14.Buggy JJ, Elias L. Bruton Tyrosine Kinase (BTK) and Its Role in B-cell Malignancy. Int Rev Immunol. 2012;31:119–32. doi: 10.3109/08830185.2012.664797. [DOI] [PubMed] [Google Scholar]
- 15.Herman SE, Mustafa RZ, Gyamfi JA, et al. Ibrutinib inhibits BCR and NF-kappaB signaling and reduces tumor proliferation in tissue-resident cells of patients with CLL. Blood. 2014;123:3286–95. doi: 10.1182/blood-2014-02-548610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–56. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hallek M, Cheson BD, Catovsky D, et al. Response Assessment in Chronic Lymphocytic Leukemia Treated with Novel Agents Causing an Increase of Peripheral Blood Lymphocytes. [accessed Dec 18, 2014];Blood. 2012 Jun 4; http://www.bloodjournal.org/content/111/12/5446.e-letters.
- 18.Herman SE, Niemann CU, Farooqui M, et al. Ibrutinib-induced lymphocytosis in patients with chronic lymphocytic leukemia: correlative analyses from a phase II study. Leukemia. 2014;28:2188–96. doi: 10.1038/leu.2014.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Badoux XC, Keating MJ, Wen S. Lenalidomide as initial therapy of elderly patients with chronic lymphocytic leukemia. Blood. 2011;118:3489–98. doi: 10.1182/blood-2011-03-339077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin TS, Ruppert AS, Johnson AJ, et al. Phase II study of flavopiridol in relapsed chronic lymphocytic leukemia demonstrating high response rates in genetically high-risk disease. J Clin Oncol. 2009;27:6012–18. doi: 10.1200/JCO.2009.22.6944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tam CS, O'Brien S, Wierda W, et al. Long-term results of the fludarabine, cyclophosphamide, and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood. 2008;112:975–80. doi: 10.1182/blood-2008-02-140582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Brien S, Furman RR, Coutre SE, et al. Ibrutinib as initial therapy for elderly patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma: an open-label, multicentre, phase 1b/2 trial. Lancet Oncol. 2014;15:48–58. doi: 10.1016/S1470-2045(13)70513-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng S, Ma J, Guo A, et al. BTK inhibition targets in vivo CLL proliferation through its eff ects on B-cell receptor signaling activity. Leukemia. 2013;28:649–57. doi: 10.1038/leu.2013.358. [DOI] [PubMed] [Google Scholar]
- 24.Woyach JA, Smucker K, Smith LL, et al. Prolonged lymphocytosis during ibrutinib therapy is associated with distinct molecular characteristics and does not indicate a suboptimal response to therapy. Blood. 2014;123:1810–17. doi: 10.1182/blood-2013-09-527853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caligaris-Cappio F, Bertilaccio MT, Scielzo C. How the microenvironment wires the natural history of chronic lymphocytic leukemia. Semin Cancer Biol. 2012;24:43–48. doi: 10.1016/j.semcancer.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 26.Dal Bo M, Tissino E, Benedetti D, et al. Microenvironmental interactions in chronic lymphocytic leukemia: the master role of CD49d. Semin Hematol. 2014;51:4684–91. doi: 10.1053/j.seminhematol.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Wiestner A, et al. Emerging role of kinase-targeted strategies in chronic lymphocytic leukemia. Blood. 2012;120:4684–91. doi: 10.1182/blood-2012-05-423194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Furman RR, Cheng S, Lu P, et al. Ibrutinib resistance in chronic lymphocytic leukemia. N Engl J Med. 2014;370:2352–54. doi: 10.1056/NEJMc1402716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woyach JA, Furman RR, Liu TM, et al. Resistance mechanisms for the Bruton's tyrosine kinase inhibitor ibrutinib. N Engl J Med. 2014;370:2286–94. doi: 10.1056/NEJMoa1400029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burger JA, Keating MJ, Wierda WG, et al. Safety and activity of ibrutinib plus rituximab for patients with high-risk chronic lymphocytic leukaemia: a single-arm, phase 2 study. Lancet Oncol. 2014;15:1090–99. doi: 10.1016/S1470-2045(14)70335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.