Abstract
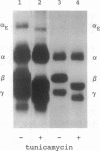
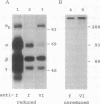
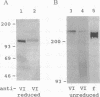
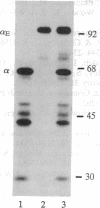
In fibrinogen, alpha E chains form a subpopulation of alpha subunits that are distinguished by a carboxyl extension homologous to the C termini of the other two constituent chains: beta and gamma. The molecular mass of alpha E is > 50% greater than that of the common alpha subunit, due in part to an extra 236 amino acids. These residues are encoded by exon VI, a recently discovered extension of the fibrinogen alpha gene. Additional mass is contributed by posttranslational processing, including N-glycosylation, which, based on experiments with the inhibitor tunicamycin, was found to account in large measure for alpha E migration on SDS/PAGE at approximately 110 kDa rather than at its calculated mass of 92,843 Da. An antibody specific for the exon VI-encoded domain of alpha E (anti-VI) and capable of recognizing alpha E-containing fibrinogen in both native and denatured form was generated using a recombinant protein as immunogen. Its use in Western blot analysis of fractions of normal human blood (plasma and preparations of fibrinogen) revealed a single, sharp, alpha E-containing band migrating behind the position of the broad, predominant fibrinogen band, (alpha beta gamma)2. Designation of the upper band as Fib420, an approximately 420-kDa homodimer of the formula (alpha E beta gamma)2, is based on the overwhelming proportion of alpha E subunits (> 80% of the total alpha chains) found in anti-VI-immunoprecipitable material from hepatoma cell medium. Several lines of evidence suggest that the alpha E subunit, alone or incorporated into fibrinogen, is more stable than the common alpha chain, a feature of potential clinical importance.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J. J., Bey E. M., Geddes E. W., Lecatsas G. Establishment of a continuously growing cell line from primary carcinoma of the liver. S Afr Med J. 1976 Dec 18;50(54):2124–2128. [PubMed] [Google Scholar]
- Blombäck B., Blombäck M., Henschen A., Hessel B., Iwanaga S., Woods K. R. N-terminal disulphide knot of human fibrinogen. Nature. 1968 Apr 13;218(5137):130–134. doi: 10.1038/218130a0. [DOI] [PubMed] [Google Scholar]
- Erickson H. P., Fowler W. E. Electron microscopy of fibrinogen, its plasmic fragments and small polymers. Ann N Y Acad Sci. 1983 Jun 27;408:146–163. doi: 10.1111/j.1749-6632.1983.tb23242.x. [DOI] [PubMed] [Google Scholar]
- Farrell D. H., Huang S., Davie E. W. Processing of the carboxyl 15-amino acid extension in the alpha-chain of fibrinogen. J Biol Chem. 1993 May 15;268(14):10351–10355. [PubMed] [Google Scholar]
- Fu Y., Weissbach L., Plant P. W., Oddoux C., Cao Y., Liang T. J., Roy S. N., Redman C. M., Grieninger G. Carboxy-terminal-extended variant of the human fibrinogen alpha subunit: a novel exon conferring marked homology to beta and gamma subunits. Biochemistry. 1992 Dec 8;31(48):11968–11972. doi: 10.1021/bi00163a002. [DOI] [PubMed] [Google Scholar]
- Gollwitzer R., Bode W., Schramm H. J., Typke D., Guckenberger R. Laser diffraction of oriented fibrinogen molecules. Ann N Y Acad Sci. 1983 Jun 27;408:214–225. doi: 10.1111/j.1749-6632.1983.tb23246.x. [DOI] [PubMed] [Google Scholar]
- Henschen A. H. Human fibrinogen--structural variants and functional sites. Thromb Haemost. 1993 Jul 1;70(1):42–47. [PubMed] [Google Scholar]
- Henschen A., Lottspeich F., Kehl M., Southan C. Covalent structure of fibrinogen. Ann N Y Acad Sci. 1983 Jun 27;408:28–43. doi: 10.1111/j.1749-6632.1983.tb23232.x. [DOI] [PubMed] [Google Scholar]
- Holm B., Nilsen D. W., Kierulf P., Godal H. C. Purification and characterization of 3 fibrinogens with different molecular weights obtained from normal human plasma. Thromb Res. 1985 Jan 1;37(1):165–176. doi: 10.1016/0049-3848(85)90043-x. [DOI] [PubMed] [Google Scholar]
- Kant J. A., Fornace A. J., Jr, Saxe D., Simon M. I., McBride O. W., Crabtree G. R. Evolution and organization of the fibrinogen locus on chromosome 4: gene duplication accompanied by transposition and inversion. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2344–2348. doi: 10.1073/pnas.82.8.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles B. B., Howe C. C., Aden D. P. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science. 1980 Jul 25;209(4455):497–499. doi: 10.1126/science.6248960. [DOI] [PubMed] [Google Scholar]
- Koopman J., Haverkate F., Grimbergen J., Egbring R., Lord S. T. Fibrinogen Marburg: a homozygous case of dysfibrinogenemia, lacking amino acids A alpha 461-610 (Lys 461 AAA-->stop TAA). Blood. 1992 Oct 15;80(8):1972–1979. [PubMed] [Google Scholar]
- Lee M. H., Kaczmarek E., Chin D. T., Oda A., McIntosh S., Bauer K. A., Clyne L. P., McDonagh J. Fibrinogen Ledyard (A alpha Arg16----Cys): biochemical and physiologic characterization. Blood. 1991 Oct 1;78(7):1744–1752. [PubMed] [Google Scholar]
- Lorand L. New approaches to old problems in the clotting of fibrinogen. Ann N Y Acad Sci. 1983 Jun 27;408:226–232. doi: 10.1111/j.1749-6632.1983.tb23247.x. [DOI] [PubMed] [Google Scholar]
- May L. T., Shaw J. E., Khanna A. K., Zabriskie J. B., Sehgal P. B. Marked cell-type-specific differences in glycosylation of human interleukin-6. Cytokine. 1991 May;3(3):204–211. doi: 10.1016/1043-4666(91)90018-9. [DOI] [PubMed] [Google Scholar]
- Medved L. V., Gorkun O. V., Privalov P. L. Structural organization of C-terminal parts of fibrinogen A alpha-chains. FEBS Lett. 1983 Aug 22;160(1-2):291–295. doi: 10.1016/0014-5793(83)80985-5. [DOI] [PubMed] [Google Scholar]
- Miyata T., Terukina S., Matsuda M., Kasamatsu A., Takeda Y., Murakami T., Iwanaga S. Fibrinogens Kawaguchi and Osaka: an amino acid substitution of A alpha arginine-16 to cysteine which forms an extra interchain disulfide bridge between the two A alpha chains. J Biochem. 1987 Jul;102(1):93–101. doi: 10.1093/oxfordjournals.jbchem.a122046. [DOI] [PubMed] [Google Scholar]
- Mosesson M. W. Fibrinogen heterogeneity. Ann N Y Acad Sci. 1983 Jun 27;408:97–113. doi: 10.1111/j.1749-6632.1983.tb23237.x. [DOI] [PubMed] [Google Scholar]
- Mosesson M. W., Finlayson J. S., Umfleet R. A. Human fibrinogen heterogeneities. 3. Identification of chain variants. J Biol Chem. 1972 Aug 25;247(16):5223–5227. [PubMed] [Google Scholar]
- Mosesson M. W., Hainfeld J., Wall J., Haschemeyer R. H. Identification and mass analysis of human fibrinogen molecules and their domains by scanning transmission electron microscopy. J Mol Biol. 1981 Dec 15;153(3):695–718. doi: 10.1016/0022-2836(81)90414-9. [DOI] [PubMed] [Google Scholar]
- Nakabayashi H., Taketa K., Miyano K., Yamane T., Sato J. Growth of human hepatoma cells lines with differentiated functions in chemically defined medium. Cancer Res. 1982 Sep;42(9):3858–3863. [PubMed] [Google Scholar]
- Pan Y., Doolittle R. F. cDNA sequence of a second fibrinogen alpha chain in lamprey: an archetypal version alignable with full-length beta and gamma chains. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2066–2070. doi: 10.1073/pnas.89.6.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price T. M., Strong D. D., Rudee M. L., Doolittle R. F. Shadow-cast electron microscopy of fibrinogen with antibody fragments bound to specific regions. Proc Natl Acad Sci U S A. 1981 Jan;78(1):200–204. doi: 10.1073/pnas.78.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S. N., Procyk R., Kudryk B. J., Redman C. M. Assembly and secretion of recombinant human fibrinogen. J Biol Chem. 1991 Mar 15;266(8):4758–4763. [PubMed] [Google Scholar]
- Rudee M. L., Price T. M. Observation of the alpha-chain extensions of fibrinogen through a new electron microscope specimen preparation technique. Ultramicroscopy. 1981;7(2):193–195. doi: 10.1016/0304-3991(81)90010-3. [DOI] [PubMed] [Google Scholar]
- Veklich Y. I., Gorkun O. V., Medved L. V., Nieuwenhuizen W., Weisel J. W. Carboxyl-terminal portions of the alpha chains of fibrinogen and fibrin. Localization by electron microscopy and the effects of isolated alpha C fragments on polymerization. J Biol Chem. 1993 Jun 25;268(18):13577–13585. [PubMed] [Google Scholar]
- Weisel J. W., Stauffacher C. V., Bullitt E., Cohen C. A model for fibrinogen: domains and sequence. Science. 1985 Dec 20;230(4732):1388–1391. doi: 10.1126/science.4071058. [DOI] [PubMed] [Google Scholar]
- Weissbach L., Grieninger G. Bipartite mRNA for chicken alpha-fibrinogen potentially encodes an amino acid sequence homologous to beta- and gamma-fibrinogens. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5198–5202. doi: 10.1073/pnas.87.13.5198. [DOI] [PMC free article] [PubMed] [Google Scholar]