Abstract
Changes in protein expression within a matched set of Candida albicans isolates representing the acquisition of azole resistance were examined by two-dimensional polyacrylamide gel electrophoresis and peptide mass fingerprinting. Proteins differentially expressed in association with azole resistance included Grp2p, Ifd1p, Ifd4p, Ifd5p, and Erg10p, a protein involved in the ergosterol biosynthesis pathway.
Candida albicans is a cause of mucosal, cutaneous, and systemic infections including oropharyngeal candidiasis (OPC), the most frequent opportunistic infection among AIDS patients (8, 12). The azole antifungal agents have proven effective for the management of OPC. The repetition and lengthy duration of therapy for OPC in this patient population has led to an increased incidence of treatment failures secondary to the emergence of azole resistance in this pathogenic fungus (11, 14, 18, 25). Several mechanisms of azole resistance have been described for C. albicans, including point mutations in the gene encoding lanosterol demethylase (ERG11), as well as increased expression of ERG11 and the genes encoding the multidrug efflux pumps, CaMDR1, CDR1, and CDR2 (9, 10, 13, 19-21, 23, 24). In the present study, we examine changes in the C. albicans proteome of a well-characterized matched set of clinical isolates representing the acquisition of azole antifungal resistance.
C. albicans isolates 2-79 (fluconazole MIC, 0.25 μg/ml) and 12-99 (fluconazole MIC, ≥64 μg/ml) were used in this study. Isolate 12-99 has been shown to overexpress ERG11, CaMDR1, CDR1, and CDR2 (16, 17, 23) and to have loss of allelic variation and a point mutation in ERG11 (24). For each of three independent experiments, an aliquot of glycerol stock from each isolate was diluted in YPD broth (1% yeast extract, 2% peptone, 1% dextrose) and grown overnight at 30°C in an environmental shaking incubator. Cultures were diluted to an optical density at 600 nm of 0.2 in 0.5 liters of fresh YPD and grown as before to logarithmic phase (4.5 h) to an equivalent optical density. Cytosolic proteins were extracted, subjected to isoelectric focusing, and separated by polyacrylamide gel electrophoresis. Coomassie blue-stained gels were scanned (300-dpi resolution), and gel images were analyzed with PDQuest version 7.0 (Bio-Rad Laboratories). Spots were considered to represent differentially expressed proteins if they were up- or down-regulated ≥1.5-fold in three independent experiments. Differentially expressed proteins were selected for identification.
Spots of interest were excised and subjected to trypsinization. Peptides were extracted and analyzed using matrix-assisted laser desorption ionization-time of flight mass spectrometry. PROWL software (formerly Proteometrics, Inc.) was used to search a custom database constructed from the CandidaDB database of C. albicans open reading frame DNA sequences (http://genolist.pasteur.fr/CandidaDB/). A probability score for the match was attained in PROWL, with an accompanying Z score that represents a goodness of fit of the probability score for the search result. A Z score of 1.65 ranks the search result in the 95th percentile of nonrandom matches of the mass data set to the specific open reading frame.
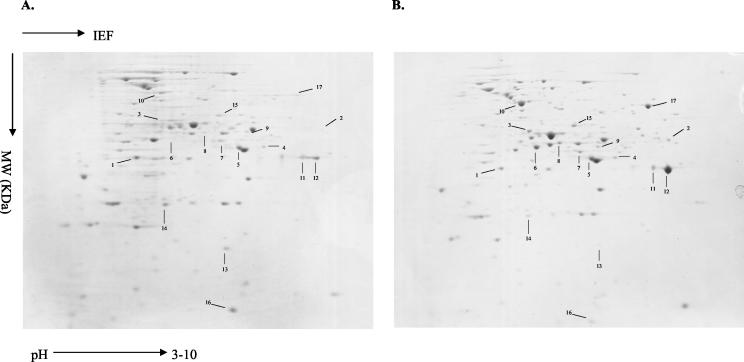
We identified 17 proteins that were reproducibly differentially expressed in isolate 12-99 compared to isolate 2-79. Among these were 13 up-regulated proteins and 4 down-regulated proteins in isolate 12-99 (Fig. 1, Table 1). Technical limitations complicate the accurate quantification of protein abundance from staining intensities. We therefore used a semiquantitative scoring system to represent changes in protein abundance.
FIG. 1.
Two-dimensional polyacrylamide gel electrophoresis separation of total protein extract from C. albicans isolates 2-79 (A) and 12-99 (B). The numbered spots represent up- or down-regulated proteins between these two isolates identified by peptide mass fingerprinting (Table 1). IEF, isoelectric focusing.
TABLE 1.
Proteins identified by two-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis and matrix-assisted laser desorption ionization-time of flight mass spectrometry as being differentially expressed between isolates 2-79 and 12-99
| Spot | Protein | pI | Molecular mass (kDa) | Estimated Z scoreb | Protein coverage (%) | Fold change (12-99/2-79)c |
|---|---|---|---|---|---|---|
| 1 | Ilv5p (ketol-acid reducto-isomerase) | 6.2 | 44.83 | 2.37 | 59 | − |
| 2 | Pot14p (Erg10p) (acetyl-coenzyme A acetyl transferase) | 6.5 | 41.92 | 1.51 | 24 | ++ |
| 3 | Sah1p (S-adenosyl-l-homocysteine hydrolase) | 5.4 | 49.05 | 2.23 | 31 | + |
| 4a | Grp2p (reductase) | 6.0 | 37.62 | 2.15 | 57 | + |
| 5a | Grp2p (reductase) | 6.0 | 37.62 | 2.32 | 43 | + |
| 6a | Ifd1p (aryl-alcohol dehydrogenase) | 5.6 | 39.14 | 2.39 | 24 | +++ |
| 7a | Ifd4p (aryl-alcohol dehydrogenase) | 6.0 | 38.26 | 2.38 | 41 | ++ |
| 8a | Ifd5p (aryl-alcohol dehydrogenase) | 5.4 | 39.20 | 2.27 | 39 | ++ |
| 9a | Ifd6p (aryl-alcohol dehydrogenase) | 5.9 | 39.06 | 2.35 | 29 | +++ |
| 10 | Pdc11p (pyruvate decarboxylase) | 5.4 | 62.42 | 2.38 | 40 | + |
| 11 | Gap1p (glyceraldehyde-3-phosphate dehydrogenase) | 6.6 | 35.81 | 2.4 | 72 | + |
| 12 | Gap1p (glyceraldehyde-3-phosphate dehydrogenase) | 6.6 | 35.81 | 2.4 | 72 | + |
| 13 | Aat1p (aspartate aminotransferase) | 8.7 | 48.87 | 2.36 | 33 | −− |
| 14 | Pmm1p (phosphomannomutase) | 5.5 | 29.00 | 2.24 | 29 | − |
| 15 | Gnd1p (6-phosphogluconate dehydrogenase) | 6.1 | 56.89 | 2.01 | 46 | ++ |
| 16 | Ynk1p (nucleoside diphosphate kinase) | 6.1 | 16.87 | 2.35 | 53 | − |
| 17 | Cdc19p (pyruvate kinase) | 6.5 | 55.43 | 2.38 | 32 | ++ |
Also found previously to be differentially expressed in DNA microarray analysis of this set of isolates (17). Homolog of a S. cerevisiae protein found to be differentially expressed in PDR1-3 gain-of-function mutants that exhibit azole resistance (15).
A probability score of 1.0 was determined for each spot.
+ or −, 1.5- to 3-fold; ++ or −−, 3.1- to 10-fold; +++, more than, 10-fold. The plus and minus represent increase and reduction, respectively.
The protein isolation technique employed in the present study is inclusive of cytosolic proteins but is deficient in water-insoluble proteins such as those found in cell and organelle membranes. It is therefore not surprising that we did not detect the membrane-associated multidrug transporters Cdr1p, Cdr2p, and Mdr1p in this study. Of particular interest, however, was the finding of up-regulation of Grp2p, Ifd1p, Ifd4p, Ifd5p, and Ifd6p in association with azole resistance. The genes encoding all but one of these have been shown to be differentially expressed in this and other matched series of isolates (4, 16, 17). Ifd1p, Ifd4p, Ifd5p, and Ifd6p are members of a family of homologs of the Saccharomyces cerevisiae YPL088W gene product, a putative alcohol dehydrogenase-oxidoreductase. The YPL088W gene and gene product have been shown to be up-regulated in microarray and proteomic studies of azole-resistant S. cerevisiae isolates with gain-of-function mutations of the transcription factor PDR3 (7, 15). A C. albicans homolog of YPL088W has been shown to contain a drug response element in its promoter which leads to induction of mRNA expression upon estradiol treatment (6). This drug response element was recently shown to be shared by other genes, including CDR1 and CDR2 (M. Karababa, A. Coste, B. Rognon, J. Bille, and D. Sanglard, Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. M-401, 2003). Likewise, Grp2p is a homolog of ScGre2p, which encodes a putative reductase with similarity to plant dihydroflavonol-4-reductases. It appears to have a role in protecting cells from the toxic effects of methylglyoxal (3). ScGre2p has also been observed to be up-regulated in microarray and proteomic studies of azole-resistant S. cerevisiae isolates with constitutive activation of the transcription factor PDR3 (7, 15). While ScGRE2 is responsive to osmotic, ionic, oxidative, and heat stresses, its function is unknown.
Sulfur amino acid biosynthesis is peripherally linked to ergosterol biosynthesis. Both aspartate and homocysteine influence the biosynthesis of S-adenosylmethionine, which is required for the ability of sterol C-24 methyltransferase to convert zymosterol to fecosterol (5). Up-regulation of S-adenosyl-l-homocysteine hydrolase (Sah1p) and down-regulation of aspartate aminotransferase (Aat1p) may therefore impact the ergosterol biosynthesis pathway in azole resistance. Also of interest is the up-regulation of acetyl-coenzyme A acetyltransferase (Pot14p or Erg10p). This enzyme represents the first step in ergosterol biosynthesis. Its expression may be regulated by membrane ergosterol content, as the ERG10 gene is responsive to ergosterol depletion by itraconazole treatment (5).
Other changes in protein abundance in association with azole resistance included the up-regulation of proteins involved in carbohydrate metabolism. These were glyceraldehyde-3-phosphate dehydrogenase (Gap1p), pyruvate decarboxylase (Pdc11p), pyruvate kinase (Cdc19p), and phosphogluconate dehydrogenase (Gnd1p). Phosphomannomutase (Pmm1p), an enzyme involved in mannose and GDP-mannose metabolism (22), was also up-regulated in the resistant isolate. Ketol-acid reducto-isomerase (Ilv5p), an enzyme central to leucine, isoleucine, and valine biosynthesis, as well as mitochondrial DNA stability (2), was down-regulated in the azole-resistant isolate, as was the nucleoside diphosphate kinase Ynk1p. In S. cerevisiae, Ynk1p plays an important role in cellular homeostasis of nucleoside triphosphates and nucleoside diphosphates and is also thought to function as a signaling molecule (1).
We have demonstrated the differential expression of proteins whose genes have been previously shown to be differentially expressed in both experimentally induced and clinically acquired azole resistance, as well as proteins whose differential expression was found for the first time to be associated with this process. Proteomic analysis of multiple matched sets of azole-susceptible and -resistant C. albicans isolates will further our understanding of the mechanisms underlying azole antifungal resistance.
Acknowledgments
We thank Spencer Redding for kindly providing the isolates used in this study. We thank Clive Slaughter, Michael Pabst, and Dominic Desiderio for helpful advice and generous assistance. We thank Russ Lewis for assistance with susceptibility testing.
REFERENCES
- 1.Amutha, B., and D. Pain. 2003. Nucleotide diphosphate kinase of Saccharomyces cerevisiae, Ynk1p: localization to the mitochondrial membrane space. Biochem. J. 370:805-815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bateman, J. M., M. Iacovino, P. S. Perlman, and R. A. Butow. 2002. Mitochondrial DNA instability mutants of the bifunctional protein Ilv5p have altered organization in mitochondria and are targeted for degradation by Hsp78 and the Pim1p protease. J. Biol. Chem. 277:47946-47953. [DOI] [PubMed] [Google Scholar]
- 3.Chen, C. N., L. Porubleva, G. Shearer, M. Svrakic, L. G. Holden, J. L. Dover, M. Johnston, P. R. Chitnis, and D. H. Kohl. 2003. Associating protein activities with their genes: rapid identification of a gene encoding a methylglyoxal reductase in the yeast Saccharomyces cerevisiae. Yeast 20:545-554. [DOI] [PubMed] [Google Scholar]
- 4.Cowen, L. E., A. Nantel, M. S. Whiteway, D. Y. Thomas, D. C. Tessier, L. M. Kohn, and J. B. Anderson. 2002. Population genomics of drug resistance in Candida albicans. Proc. Natl. Acad. Sci. USA 99:9284-9289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Backer, M. D., T. Ilyina, X. J. Ma, S. Vandoninck, W. H. Luyten, and H. Vanden Bossche. 2001. Genomic profiling of the response of Candida albicans to itraconazole treatment using a DNA microarray. Antimicrob. Agents Chemother. 45:1660-1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Micheli, M., J. Bille, C. Schueller, and D. Sanglard. 2002. A common drug-responsive element mediates the upregulation of the Candida albicans ABC transporters CDR1 and CDR2, two genes involved in antifungal drug resistance. Mol. Microbiol. 43:1197-1214. [DOI] [PubMed] [Google Scholar]
- 7.DeRisi, J., B. van den Hazel, P. Marc, E. Balzi, P. Brown, C. Jacq, and A. Goffeau. 2000. Genome microarray analysis of transcriptional activation in multidrug resistance yeast mutants. FEBS Lett. 470:156-160. [DOI] [PubMed] [Google Scholar]
- 8.Feigal, D. W., M. H. Katz, D. Greenspan, J. Westenhouse, W. Winkelstein, Jr., W. Lang, M. Samuel, S. P. Buchbinder, N. A. Hessol, and A. R. Lifson. 1991. The prevalence of oral lesions in HIV-infected homosexual and bisexual men: three San Francisco epidemiological cohorts. AIDS 5:519-525. [DOI] [PubMed] [Google Scholar]
- 9.Franz, R., M. Ruhnke, and J. Morschhäuser. 1999. Molecular aspects of fluconazole resistance development in Candida albicans. Mycoses 42:453-458. [DOI] [PubMed] [Google Scholar]
- 10.Franz, R., S. L. Kelly, D. C. Lamb, D. E. Kelly, M. Ruhnke, and J. Morschhäuser. 1998. Multiple molecular mechanisms contribute to a stepwise development of fluconazole resistance in clinical Candida albicans strains. Antimicrob. Agents Chemother. 42:3065-3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelly, S. L., D. C. Lamb, D. E. Kelly, J. Loeffler, and H. Einsele. 1996. Resistance to fluconazole and amphotericin in Candida albicans from AIDS patients. Lancet 348:1523-1524. [DOI] [PubMed] [Google Scholar]
- 12.Klein, R. S., C. A. Harris, C. B. Small, B. Moll, M. Lesser, and G. H. Friedland. 1984. Oral candidiasis in high-risk patients as the initial manifestation of the acquired immunodeficiency syndrome. N. Engl. J. Med. 311:354-358. [DOI] [PubMed] [Google Scholar]
- 13.Lopez-Ribot, J. L., E. K. McAtee, L. N. Lee, W. R. Kirkpatrick, T. C. White, D. Sanglard, and T. F. Patterson. 1998. Distinct patterns of gene expression associated with development of fluconazole resistance in serial Candida albicans isolates from human immunodeficiency virus-infected patients with oropharyngeal candidiasis. Antimicrob. Agents Chemother. 42:2932-2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morschhäuser, J. 2002. The genetic basis of fluconazole resistance development in Candida albicans. Biochim. Biophys. Acta 1587:240-248. [DOI] [PubMed] [Google Scholar]
- 15.Nawrocki, A., S. J. Fey, A. Goffeau, P. Roepstorff, and P. M. Larsen. 2001. The effects of transcription regulating genes PDR1, pdr1-3 and PDR3 in pleiotropic drug resistance. Proteomics 1:1022-1032. [DOI] [PubMed] [Google Scholar]
- 16.Rogers, P. D., and K. S. Barker. 2002. Evaluation of differential gene expression in fluconazole-susceptible and -resistant isolates of Candida albicans using cDNA microarray analysis. Antimicrob. Agents Chemother. 46:3412-3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rogers, P. D., and K. S. Barker. 2003. Genome-wide expression profile analysis reveals coordinately regulated genes associated with stepwise acquisition of azole resistance in Candida albicans clinical isolates. Antimicrob. Agents Chemother. 47:1220-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruhnke, M., A. Eigler, I. Tennagen, B. Geiseler, E. Engelmann, and M. Trautmann. 1994. Emergence of fluconazole-resistant strains of Candida albicans in patients with recurrent oropharyngeal candidosis and human immunodeficiency virus infection. J. Clin. Microbiol. 32:2092-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanglard, D. 2002. Resistance of human fungal pathogens to antifungal drugs. Curr. Opin. Microbiol. 5:379-385. [DOI] [PubMed] [Google Scholar]
- 20.Sanglard, D., F. Ischer, L. Koymans, and J. Bille. 1998. Amino acid substitutions in the cytochrome P-450 lanosterol 14α-demethylase (CYP51A1) from azole-resistant Candida albicans clinical isolates contribute to resistance to azole antifungal agents. Antimicrob. Agents Chemother. 42:241-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanglard, D., K. Kuchler, F. Ischer, J. L. Pagani, M. Monod, and J. Bille. 1995. Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob. Agents Chemother. 39:2378-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith, D. J., M. Cooper, M. DeTiani, C. Losberger, and M. A. Payton. 1992. The Candida albicans PMM1 gene encoding phosphomannomutase complements a Saccharomyces cerevisiae sec 53-6 mutation. Curr. Genet. 22:501-503. [DOI] [PubMed] [Google Scholar]
- 23.White, T. C. 1997. Increased mRNA levels of ERG16, CDR, and MDR1 correlate with increases in azole resistance in Candida albicans isolates from a patient infected with human immunodeficiency virus. Antimicrob. Agents Chemother. 41:1482-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White, T. C. 1997. The presence of an R467K amino acid substitution and loss of allelic variation correlate with an azole-resistant lanosterol 14α demethylase in Candida albicans. Antimicrob. Agents Chemother. 41:1488-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.White, T. C., K. A. Marr, and R. A. Bowden. 1998. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin. Microbiol. Rev. 11:382-402. [DOI] [PMC free article] [PubMed] [Google Scholar]