Abstract
The prophylactic potential of a single injection of sustained-release doxycycline hyclate (Atridox) was compared to that of a single oral dose of doxycycline hyclate in a murine model of Lyme borreliosis. Prophylaxis, as measured by the lack of cultivable spirochetes and demonstrable pathology, was noted for 43% of orally treated mice; in contrast, the sustained-release doxycycline hyclate completely protected mice from infection and resultant pathology.
Since Lyme borreliosis became a reportable disease in 1982, it has consistently been the most common vector-borne disease reported in the United States (3). Given the lack of an available vaccine, the prophylaxis of tick bite infection consists of personal protective measures directed against ticks in areas where Borrelia burgdorferi is endemic (4). Reports on the efficacy of post-tick exposure antimicrobial prophylaxis in animals and humans demonstrate a high degree of variability, and opinions vary greatly as to the necessity of such prophylactic treatment (5, 11, 16, 17, 19). The need for effective prophylaxis is underscored by the increase in cases of Lyme disease and the seriousness of untreatable sequelae in a subset of infected individuals (7, 8). This study compares the effectiveness of a single oral dose of doxycycline hyclate, designed to mimic levels in the plasma of humans given 200 mg orally (6, 14), to that of a subcutaneous injection of sustained-release doxycycline (Atridox; Atrix Laboratories, Fort Collins, Colo.) in a mouse model of Lyme borreliosis (22).
Laboratory-reared nymphal ticks (Ixodes scapularis) that were raised as previously described (13) and that have previously been shown to be free of Anaplasma phagocytophila and Babesia microti (2, 21) were infected with B. burgdorferi strain B31. Five infected ticks were placed on the heads and neck areas of specific-pathogen-free, 7-week-old female C3H/HeJ mice (Jackson Laboratories, Bar Harbor, Maine). Seventy-two hours after tick infestation, the partially engorged ticks were removed from all of the mice. Since three or four infected ticks (average, 3.4) fed on each mouse, the mice were then randomly assigned to receive 2 mg of oral doxycycline hyclate in water, 4.2 mg of a sustained-release doxycycline hyclate copolymer formulation (Atrix Laboratories), or a water or poly (dl-lactide) in N-methyl-2-pyrrolidone copolymer (Atrix Laboratories) control. The oral doxycycline or water vehicle was delivered by gavage in 0.1 ml of tissue-grade water. Sustained-release doxycycline hyclate was mixed according to the manufacturer's instructions and transferred to a 1-ml Luer-lock syringe (Becton Dickinson, Chicago, Ill.) fitted with a 25-gauge needle for subcutaneous injection. A total of 0.05 ml was delivered to each mouse. At 4 weeks posttreatment, an ear biopsy was obtained and cultured in Barbour-Stoenner-Kelly medium (15) to determine B. burgdorferi infection status (18). The mice were euthanized (by exposure to CO2) 8 weeks after tick infestation, and heart and bladder tissues were placed in culture medium for spirochete isolation and into tissue fixative (Streck Laboratories, La Vista, Nebr.) to determine pathology (22). By using the comparative rates formula, 1 − (number of infected treated mice/number of treated mice)/(number of infected control mice/number of control mice) × 100, a 95% confidence interval was determined for the treatment efficacy rates (10). A Fisher's test was utilized to determine significant differences (P < 0.05) between the treatment groups.
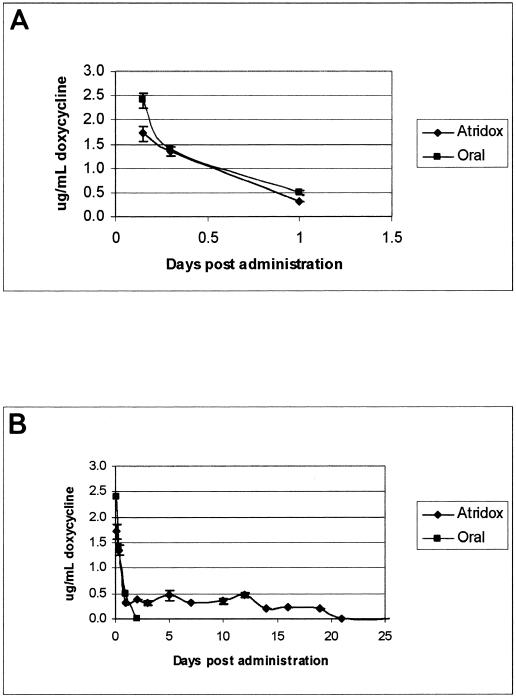
High-pressure liquid chromatography analysis of plasma doxycycline was determined as previously described (1) with the following modifications. A Beckman System Gold high-pressure liquid chromatograph (Beckman Coulter, Fullerton, Calif.) and associated 32 Karat version 5.0 software were used in combination with a C18 column (100- by 4.6-mm inside diameter; Alltech, Deerfield, Ill.). Two hundred microliters of plasma was extracted (Oasis HLB cartridge WAT094225; Waters Corp., Milford, Mass.) and eluted in 450 μl of the mobile phase. Doxycycline hyclate standards, ranging from 0.2 to 3.0 μg/ml, were generated with mouse plasma and run in parallel with experimental samples. Preliminary experiments determined that a 2-mg oral doxycycline hyclate dose and delivery of 4.2 mg of sustained-release doxycycline hyclate approximated the peak and trough pharmacokinetic values reported with a single 200-mg oral dose of doxycycline in humans (14; data not shown). We then assessed plasma pharmacokinetic values for C3H/HeJ mice by collecting blood in an EDTA microtainer (Becton Dickinson) at 3, 8, 24, and 48 h postadministration for both the oral and subcutaneous treatments and at periodic time points throughout a 25-day period for sustained-release doxycycline hyclate. Figure 1A demonstrates the pharmacokinetic curve for the first 24 h after dosing (five mice per time point) and illustrates that the pharmacokinetic curves and the maximum concentrations of the drug in serum for oral (2.43 ± 0.9 μg/ml; range, 1.2 to 4.3 μg/ml) and sustained-release (1.89 ± 0.5 μg/ml; range, 1.2 to 2.1 μg/ml) administration are similar (Welch's approximate t test, P = 0.15). The data in Fig. 1B illustrate that doxycycline delivered orally in water is absent from plasma by 48 h postadministration, but that doxycycline delivered via sustained-release doxycycline hyclate persists at levels ranging between 0.1 and 0.5 μg/ml for 19 days after subcutaneous delivery.
FIG. 1.
(A) Plasma doxycycline levels in mice over a 24-h period postadministration. Each data point is the mean per time point of results from five mice, with error bars representing the standard errors of the means. (B) Plasma doxycycline levels in mice over a 21-day period after a single oral dose of doxycycline hyclate or a single subcutaneous administration of sustained-release doxycycline hyclate (Atridox). Each data point is the mean value for three mice, and error bars represent the standard errors of the means.
In challenge experiments with B. burgdorferi-infected ticks, 43% of orally treated mice (7 of 13 infected mice) were protected (11 to 70% rate at a 95% confidence interval) while sustained-release doxycycline hyclate-treated mice demonstrated a significantly higher level of protection (0 of 12 infected mice; P = 0.005) (Table 1). These results represent two independent treatment trials where levels of infection and protection among groups did not vary significantly between treatment trials (Fisher's test; data not shown). The culture results of the bladder and heart taken 8 weeks after tick infestation correlate directly with the initial skin biopsy results (data not shown). Likewise, those mice that were not protected from infection after oral treatment developed nodular cystitis of the bladder, myocarditis, and vasculitis of the heart, as described previously (22). No histopathology of the bladder or heart was noted for mice treated with sustained-release doxycycline hyclate. The control mice receiving tissue-grade water demonstrated a 94% infection rate (15 of 16 mice), while those receiving an injection of the copolymer-vehicle control had an 83% infection rate (10 of 12 mice), results which are statistically indistinguishable from one another (Fisher's test, P = 0.56) and similar to results of previous infectivity experiments (12). No significant difference was noted in the numbers of ticks that fed on mice from the treatment groups and control groups (data not shown).
TABLE 1.
Treatment efficacy of sustained-release doxycycline hyclate by subcutaneous injection versus oral administration of doxycycline hyclatea
| Treatment or control | No. of mice infected/total no. testedb | Level of protection (%)c |
|---|---|---|
| Oral doxycycline hyclate | 7/13 | 43 |
| Water control | 15/16 | NA |
| Sustained-release doxycycline hyclate | 0/12 | 100 |
| Copolymer controld | 10/12 | NA |
Mice received either a single oral dose of 2 mg of doxycycline hyclate or a single subcutaneous injection of 4.2 mg of sustained-release doxycycline hyclate 72 h after tick infestation. Cultures of ear biopsies were taken 4 weeks postinfestation; bladder and heart tissues were cultured 8 weeks postinfestation.
Fisher's test results were as follows: for values for the oral doxycycline hyclate treatment compared with those for the water control treatment, P was 0.02; for values for the sustained-release doxycycline hyclate treatment compared with those for the copolymer control treatment, P was 0.0001; for values for the sustained-release doxycycline hyclate treatment compared with those for the oral doxycycline hyclate treatment, P was 0.005; and for values for the water control treatment compared with those for the copolymer control treatment, P was 0.56.
NA, not applicable.
Poly(dl-lactide) in N-methyl-2-pyrrolidone was used as the copolymer control.
Our data indicate that the pharmacokinetic curves for both doxycycline hyclate treatments are statistically indistinguishable for the first 24 h after administration. We conclude that the enhanced prophylactic efficacy of sustained-release doxycycline hyclate in this animal model is due to the sustained-release effect over a 19-day period after administration. Interestingly, concentrations of doxycycline in plasma after the administration of sustained-release doxycycline hyclate (0.1 to 0.5 μg/ml) were lower than those reported as the MIC in vitro for B. burgdorferi (1.6 μg/ml) (5). Whether this reflects a lack of correlation between in vitro and in vivo effects of doxycycline, the length of exposure to the drug, or the strain of B. burgdorferi used (B31 versus 297) remains to be tested.
In this study, we attempted to maximize the experimental challenge model by placing five infected nymphal ticks on a host (C3H mouse) known to be extremely sensitive to B. burgdorferi infection and resultant pathology (20). Despite this sensitivity, our protection data for a single oral administration of doxycycline compare favorably with those reported for humans by Nadelman et al. (11), in whose study the 95% confidence interval varied widely (from 25 to 98%) and true protection efficacy approached 50%. Although the mechanisms for enhanced antibacterial effect seen with sustained-release doxycycline hyclate were not explored in that study, parenteral biodegradable controlled-release systems have been shown to both (i) increase the bioavailability of short-lived antibiotics to tissues and (ii) completely bypass the first-pass effect of oral administration to enhance treatment efficacy (9). Thus, a single injection of a sustained-release-formulation antibiotic may offer a viable option for prophylactic treatment of Lyme borreliosis for patients presenting with B. burgdorferi-infected tick bites.
REFERENCES
- 1.Axisa, B., A. R. Naylor, P. R. F. Bell, and M. M. Thompson. 2000. Simple and reliable method of doxycycline determination in human plasma and biological tissues. J. Chromatogr. 744:359-365. [DOI] [PubMed] [Google Scholar]
- 2.Burkot, T. R., B. S. Schneider, N. Pieniazek, C. M. Happ, J. S. Rutherford, S. G. Slemenda, E. Hoffmeister, G. O. Maupin, and N. S. Zeidner. 2000. Babesia microti and Borrelia burgdorferi transmission by Ixodes spinipalpis ticks among prairie voles, Microtus ochrogaster, in Colorado. Parasitology 121:595-599. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 2002. Lyme disease—United States, 2000. Morb. Mortal. Wkly. Rep. 51:29-31. [PubMed] [Google Scholar]
- 4.Hayes, E. B., and J. Piesman. 2003. How can we prevent Lyme disease? N. Engl. J. Med. 348:2424-2430. [DOI] [PubMed] [Google Scholar]
- 5.Johnson, R. C., C. B. Kodner, P. J. Jurkovich, and J. J. Collins. 1990. Comparative in vitro and in vivo susceptibilities of the Lyme disease spirochete Borrelia burgdorferi to cefuroxime and other antimicrobial agents. Antimicrob. Agents Chemother. 34:2133-2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joshi, N., and D. Q. Miller. 1997. Doxycycline revisited. Arch. Intern. Med. 157:1421-1428. [PubMed] [Google Scholar]
- 7.Klempner, M. S. 2002. Controlled trials of antibiotic treatment in patients with post-treatment chronic Lyme disease. Vector Borne Zoonotic Dis. 2:255-263. [DOI] [PubMed] [Google Scholar]
- 8.Krupp, L. B., L. G. Hyman, R. Grimson, P. K. Coyle, P. Melville, S. Ahnn, R. Dattwyler, and B. Chandler. 2003. Study and treatment of post Lyme disease (STOP-LD). Neurology 60:1923-1930. [DOI] [PubMed] [Google Scholar]
- 9.Matschke, C., U. Isele, P. V. Hoogevest, and A. Fahr. 2002. Sustained-release injectables formed in situ and their potential use for veterinary products. J. Control. Release 85:1-15. [DOI] [PubMed] [Google Scholar]
- 10.Miettinen, O., and M. Nurminen. 1985. Comparative analysis of two rates. Stat. Med. 4:213-226. [DOI] [PubMed] [Google Scholar]
- 11.Nadelman, R. B., J. Nowakowski, D. Fish, R. C. Flaco, K. Freeman, D. McKenna, P. Welch, R. Marcus, M. E. Aguero-Rosenfeld, D. T. Dennis, and G. P. Wormser. 2001. Prophylaxis with single-dose doxycycline for the prevention of Lyme disease after an Ixodes scapularis tick bite. N. Engl. J. Med. 345:79-84. [DOI] [PubMed] [Google Scholar]
- 12.Piesman, J., T. N. Mather, R. J. Sinsky, and A. Spielman. 1987. Duration of tick attachment and Borrelia burgdorferi transmission. J. Clin. Microbiol. 25:557-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piesman, J. 1993. Standard system for infecting ticks (Acari: Ixodidae) with the Lyme disease spirochete Borrelia burgdorferi. J. Med. Entomol. 30:199-203. [DOI] [PubMed] [Google Scholar]
- 14.Saivin, S., and G. Gouin. 1988. Clinical pharmacokinetics of doxycycline and minocycline. Clin. Pharmacokinet. 15:355-366. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz, I., G. P. Wormser, J. J. Schwartz, D. Cooper, P. Weissensee, A. Gazumyan, E. Zimmermann, N. S. Goldberg, S. Bittker, G. L. Campbell, and C. S. Pavia. 1992. Diagnosis of early Lyme disease by polymerase chain reaction amplification and culture of skin biopsies from erythema migrans lesions. J. Clin. Microbiol. 30:3082-3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shapiro, E. D., M. A. Gerber, N. B. Holabird, A. T. Berg, H. M. Feder, G. L. Bell, P. N. Rys, and D. H. Persing. 1992. A controlled trial of antimicrobial prophylaxis for Lyme disease after deer-tick bites. N. Engl. J. Med. 327:1769-1773. [DOI] [PubMed] [Google Scholar]
- 17.Shih, C. M., and A. Spielman. 1993. Topical prophylaxis for Lyme disease after tick bite in a rodent model. J. Infect. Dis. 168:1042-1045. [DOI] [PubMed] [Google Scholar]
- 18.Sinsky, R. J., and J. Piesman. 1989. Ear punch biopsy method for detection and isolation of Borrelia burgdorferi from rodents. J. Clin. Microbiol. 27:1723-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Warshafsky, S., J. Nowakowski, R. B. Nadelman, R. S. Kamer, S. J. Peterson, and G. P. Wormser. 1996. Efficacy of antibiotic prophylaxis for prevention of Lyme disease. J. Gen. Intern. Med. 11:329-333. [DOI] [PubMed] [Google Scholar]
- 20.Yang, L., J. H. Weiss, E. Eichwald, C. P. Kolbert, D. H. Persing, and J. J. Weis. 1994. Heritable susceptibility to severe Borrelia burgdorferi-induced arthritis is dominant and is associated with persistence of large numbers of spirochetes in tissues. Infect. Immun. 62:492-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeidner, N. S., M. C. Dolan, R. Massung, J. Piesman, and D. Fish. 2000. Coinfection with Borrelia burgdorferi and the agent of human granulocytic ehrlichiosis suppresses IL-2 and IFNγ production and promotes an IL-4 response in C3H/HeJ mice. Parasite Immunol. 22:581-588. [DOI] [PubMed] [Google Scholar]
- 22.Zeidner, N. S., B. S. Schneider, M. C. Dolan, and J. Piesman. 2001. An analysis of spirochete load, strain, and pathology in a model of tick-transmitted Lyme borreliosis. Vector Borne Zoonotic Dis. 1:35-44. [DOI] [PubMed] [Google Scholar]