Abstract
Background
Schistosomiasis is the second-most widespread tropical parasitic disease after malaria. Various research strategies and treatment programs for achieving the objective of eradicating schistosomiasis within a decade have been recommended and supported by the World Health Organization. One of these approaches is based on the control of snail vectors in endemic areas. Previous field studies have shown that competitor or predator introduction can reduce snail numbers, but no systematic investigation has ever been conducted to identify snail microbial pathogens and evaluate their molluscicidal effects.
Methodology/Principal findings
In populations of Biomphalaria glabrata snails experiencing high mortalities, white nodules were visible on snail bodies. Infectious agents were isolated from such nodules. Only one type of bacteria, identified as a new species of Paenibacillus named Candidatus Paenibacillus glabratella, was found, and was shown to be closely related to P. alvei through 16S and Rpob DNA analysis. Histopathological examination showed extensive bacterial infiltration leading to overall tissue disorganization. Exposure of healthy snails to Paenibacillus-infected snails caused massive mortality. Moreover, eggs laid by infected snails were also infected, decreasing hatching but without apparent effects on spawning. Embryonic lethality was correlated with the presence of pathogenic bacteria in eggs.
Conclusions/Significance
This is the first account of a novel Paenibacillus strain, Ca. Paenibacillus glabratella, as a snail microbial pathogen. Since this strain affects both adult and embryonic stages and causes significant mortality, it may hold promise as a biocontrol agent to limit schistosomiasis transmission in the field.
Author Summary
The present paper reports the isolation and the characterization of a new microbial pathogen of the freshwater snail, Biomphalaria glabrata. Genetic analyses revealed that the species has not been previously described and could be classified into the Paenibacillus genus. These bacteria invade most snail tissues and proliferate, causing massive lethality. Moreover, the bacterial infection can be transmitted both vertically and horizontally to other snails, causing their death in 30 days. This discovery is potentially important because B. glabrata, as an intermediate host, plays an important role in transmitting schistosomiasis, the second-most widespread human parasitic disease. The World Health Organization’s objective of schistosomiasis eradication in a decade encourages the development of multiple approaches for countering the disease, one of which is vector population control. This new bacterial strain clearly could be a potential agent for such a strategy.
Introduction
Schistosomiasis, the second-most widespread human parasitic disease after malaria, is caused by flatworms of the genus Schistosoma (Platyhelminthes, Digenea), currently known to consist of 22 species, three of which Schistosoma haematobium, Schistosoma japonicum, and Schistosoma mansoni are the principal agents of human schistosomiasis. S. mansoni, the most common causative agent, has a complex life cycle that involves two hosts [1–3]. Adult worms mate in the venous system of a human host, producing eggs that are expelled with the infected person’s feces. If deposited in an aquatic environment, the eggs hatch and each releases a miracidium that can infect Biomphalaria sp. a freshwater snail. Inside snail tissues, the miracidium transforms into a primary sporocyst (Sp1) that multiplies asexually to produce secondary sporocysts (Sp2), which then produce cercariae. Cercariae leave the snail and actively infect the vertebrate definitive host.
Because of their medical and epidemiological importance as intermediate hosts for Schistosoma parasites, freshwater snails have attracted significant research attention. A number of studies have focused on the immunology of infections transmitted by snails like Biomphalaria sp., seeking to identify key genes associated with snail resistance or susceptibility to the parasite. Indeed, several comparative transcriptomic studies have been performed on snails infected by different parasites, such as Echinostoma or Schistosoma [4–12], or gram-positive or -negative bacteria, like E. coli [9,13]. Although host snails were shown to develop an immune response against these different potential pathogens, no lethal effects were observed with bacteria even at higher densities of inoculation. To our knowledge, there are no reports of the isolation of bacteria from field samples or under laboratory conditions that are pathogenic towards Biomphalaria. However, many efforts were made to find microbial pathogens for use as biocontrol agents to reduce snail populations and thereby control transmission of the parasite. For example, Bacillus thuringiensis, a gram-positive, spore-forming bacterium known to secrete many toxins, is widely used against different insect pests [14–16]. Its broad-spectrum action has also been tested against Schistosoma vector snails such Biomphalaria alexandrina. Although B. thuringiensis israelensis has no effect [17], B. thuringiensis kurtsaki has negative effects on snail populations by exerting molluscicidal activity and preventing egg hatching [18]. Brevibacillus laterosporus has also been reported to be pathogenic against juveniles of Biomphalaria glabrata [19]. A preliminary study suggested the potential pathogenicity of Bacillus brevis towards Biomphalaria pfeifferi and Bulinus truncatus [20], but this has never been tested in the field. Bacillus pinotti, isolated from the ovotestis of Australorbis glabratus (now re-named Biomphalaria glabrata), has also been examined as a potential biological snail control agent, but results were disappointing [21].
Few studies have examined snails for abnormal symptoms suggestive of infection by microorganisms, such as the presence of nodules. The presence of a mycobacterium (acid-alcohol–resistant microorganism) and a gram-negative bacterium growing as a tumor have been reported in B. glabrata and Bulinus jousseaumei, but neither was associated with signs of pathogenicity [22,23]. Thus, a better knowledge of the potential microbial pathogens of B. glabrata could contribute to the discovery of new means for preventing and/or controlling schistosomiasis by limiting the vector snail population in the field.
We report the identification of a new snail microbial pathogen named Ca. Paenibacillus glabratella. After isolation from infected snails and molecular characterization, we evaluated its pathogenicity against adult snails. The results suggest that this biological agent has potential for vector control of human schistosomiasis.
Methods
Ethics statement
For experiments on animals. Our laboratory holds permit #A66040 for experiments on animals from both the French Ministry of Agriculture and Fisheries, and the French Ministry of National Education, Research, and Technology. The housing, breeding, and care of animals utilized here followed the ethical requirements of our country. The experimenter also possesses an official certificate for animal experimentation from both French ministries (Decree #87–848, October 19, 1987). Animal experimentation followed the guidelines of the CNRS (Centre National de la Recherche Scientifique). The protocols used in this study have been approved by the French veterinary agency from the DRAAF Languedoc-Roussillon (Direction Régionale de l’Alimentation, de l’Agriculture et de la Forêt), Montpellier, France (Authorization #007083).
Biological material
The B. glabrata strains used in this study were the Venezuelan strain of pigmented B. glabrata (BgVEN) and the Brazilian strain of unpigmented (BgBRE). The new Paenibacillus species was discovered by virtue of its direct association with B. glabrata snails carrying atypical large, white nodules.
Bacterial genomic DNA extraction
Bacterial nodules of five BgBRE snails were collected with the aid of an optical microscope and were emulsified in 200 μL of sterile milliQ water. Genomic DNA was extracted from nodules using a PowerLyser UltraClean Microbial DNA Isolation Kit (MO BIO Laboratories), as described by the manufacturer. Briefly, 50 μL of emulsified nodule was resuspended in 300 μL of Microbead solution, which lyses cells through detergent and mechanical actions. After protein precipitation, genomic DNA was selectively bound to a silica-based membrane, washed with ethanol, and eluted with 50 μL of 10 mM Tris buffer at pH 7. DNA concentration was measured using an Epoch micro-volume spectrophotometer system.
Paenibacillus isolation
About three nodules were collected from each of five BgBRE snails with autoclaved dissecting forceps and transferred into 100 μL of sterile milliQ water. After vortexing for 10 minutes at maximum speed, suspended materials were heated for 20 minutes at 75°C to eliminate vegetative microbes. In order to encourage growth of surviving microbes (e.g. spores), inocula were incubated at 25°C or 37°C under aerobic or anaerobic conditions on different media, including LB (Luria Bertani); TSB (trypticase soy boy); brain heart and meat liver infusions; Mueller Hinton supplemented with yeast, phosphate, glucose and pyruvate (MYPGP); and Columbia agars. Only germinated bacteria were present on different media but no bacterial growth was observed under these different conditions. The nodules and the germinated bacteria were picked and investigated by Gram staining and genetic characterization.
Histopathological examination
Snails presenting symptoms of a bacterial infection were examined histologically. Five infected BgBRE snails were fixed in Halmi’s fixative (90% Heidenhain’s SuSa solution and 10% picric acid-saturated water solution) for 48 hours. Fixed mollusks were then dehydrated successively in two baths of absolute ethanol (24 hours each) and three baths of water-saturated butanol (24 hours each). Dehydrated snails were embedded in paraffin by impregnating for 8 hours. Transverse histological sections (10-μm thick) were cut, mounted on glass slides, then dipped sequentially in toluene (two times for 10 minutes), butanol (10 minutes), and 70% ethanol (5 minutes). After rehydration, slides were treated first with Lugol’s iodine for 30 seconds and then with 5% sodium thiosulfate until bleaching was complete, after which slides were rinsed in distilled water. Rehydrated slides were stained with 0.05% aqueous azocarmine G (Merck, Germany) supplemented with 1% glacial acetic acid for 45 minutes at 60°C, then transferred to a solution of 1% aniline blue in 70% ethanol for 15 minutes. Staining was stopped by addition of 1% acetic acid in 95% ethanol. After treatment with 5% phosphotungstic acid for 30 minutes, slides were rinsed in distilled water and stained with Heidenhain’s azan trichrome for 50 minutes. Preparations were then dehydrated in 95% ethanol for 10 minutes and absolute ethanol for 30 minutes, cleared by immersion in butanol and toluene (50:50 v/v), and mounted with Entellan prior to microscopic examination. Pictures were taken with a Nikon MICROPHOT-FX microscope and a Nikon digital sight DS-Fi1 camera.
Molecular characterization by polymerase chain reaction
The 16S rRNA gene was amplified by polymerase chain reaction (PCR) using the universal primers 27F (5’-AGAGTTTGATCMTGGCTCAG-3’) and 1492R (5’-ACCTTGTTACGACTT-3’) [24]. To support the identification of a new bacterial species, we also amplified the RNA polymerase beta subunit gene (Rpob) with the primer pair, Rpob1698f (5’-AACATCGGTTTGATCAAC-3’) and Rpob2041r (5’-CGTTGCATGTTGGTACCCAT-3’) [25–27], and performed PCR using the eukaryotic-specific primer pair, Unif-F-15 (5’-CTCCCAGTAGTCATATGC-3’) and Unif-R-1765 (5’-ACCTTGTTACGACTAC-3’), to amplify the 18S rRNA gene [28]. In each case, thermocycling conditions were 94°C for 8 minutes followed by 35 cycles of 94°C for 30 seconds, 54°C for 30 seconds, 72°C for 2 minutes and a final 5-minute extension step at 72°C. The PCR products were then cloned into the pCR4-TOPO vector according to the manufacturer’s instructions (Invitrogen). The accuracy of cloning steps was confirmed by sequencing of both strands of each clone (GATC Biotech, Germany).
Phylogenetic analysis
Sequences of ribosomal 16S rRNA and Rpob genes and proteins were retrieved from GenBank (S1 Table). The DNA dataset for the 16S sequences used for phylogenetic analyses contained 26 taxa and 1389 characters. The dataset for the Rpob partial coding sequences contained 22 taxa and 303 characters. The dataset for the Rpob amino-acid sequences contained 25 taxa and 101 characters. Two Clostridium species were used as an out-group.
Sequences were analyzed using MEGA version 5.2.2 [29]. Each set of sequences was aligned using Muscle software, and the best substitution model, that is, the one with the lowest Bayesian Information Criterion (BIC), was selected. Phylogenetic trees were constructed using the maximum likelihood (ML) algorithm and tested with the bootstrap method (500 replications). Bayesian analyses were performed using MrBayes 3.2 with four Markov chains for 106 generations. Every 1000th generation was sampled, and the first 25% of trees were discarded; we checked that the standard deviation of the split frequencies fell below 0.01 to ensure convergence of the tree search.
For 16S sequences, the ML tree was computed using the Kimura two parameter model [30] with a proportion of invariant sites (I) along with Gamma-distributed among-site rate heterogeneity (G). For Rpob sequences, the Tamura three parameter substitution model [31] with a gamma-distributed rate heterogeneity was used. The same topology was obtained using the translated amino-acid sequences instead of the DNA sequences. For Bayesian trees, Rpob protein sequences were analyzed using a mixed amino acid model, and 16S nucleotide sequences were analyzed using a general time reversible (GTR) model with gamma-distributed rate variation across sites and a proportion of invariant sites.
Assay of snail survival after Paenibacillus exposure
Sixty uninfected albino B. glabrata snails originating from Brazil (BgBRE) with shell diameters ranging from 7 to 11 mm were divided in two groups and placed in 3 L of Ca. Paenibacillus glabratella-free water. One group of 30 BgBRE snails was exposed to five Paenibacillus nov. sp.-infected pigmented (BgVEN) snails for 3 months and a second group of 30 BgBRE snails (controls) was exposed to five uninfected BgVEN snails for 3 months. Albino snails were chosen because they facilitated tracking of the spread of bacteria and could be easily separated from infected pigmented snails. Kaplan-Meier survival analyses followed by pairwise log-rank tests were used to compare survival data [32,33]. Infection was determined by direct observation using a binocular stereomicroscope, and mortality was assessed every 2 or 3 days. Dead snails were not removed during the course of the experiment. Snails were fed fresh lettuce twice a week, and half the aquarium water was changed every week. The number of egg masses and the juveniles were measured weekly during the first 28 days before the first observation of mortality.The presence or absence of Ca. Paenibacillus glabratella in the rearing water of snails was tested by filtering 1 L of tank water through a 0.22-μm pore-size membrane filter (Millipore). The membrane filter was placed into a 14 mL sterile tube containing 5 mL of sterile water. The tube was vortexed at maximum speed for 3 minutes and then centrifuged for 20 minutes at 4000 × g. The membrane and 4.5 mL of supernatant were removed, and the pellet was suspended in the remaining supernatant by vortexing. The resuspended pellet was 10-fold serially diluted for PCR analysis using universal primers for the 16S rRNA gene and specific primers for the 16S rRNA gene of infectious Ca. Paenibacillus glabratella (5’-ATCATCCTCGCATGAGGGAGAT-3’ and 5’-GAGCAGTTCTCTCCTTGTTC-3’). PCR was performed using a hybridization temperature of 43°C and an elongation time of 40 seconds. This pair of primers was also used to verify the absence of Paenibacillus nov. sp. in healthy snails and confirm its presence in infected eggs and snails.
Accession numbers
Nucleotide sequence data for Ca. Paenibacillus glabratella reported in this paper are available in the GenBank database under the accession numbers KF801672 and KF801673 for the partial 16S rDNA and Rpob genes, respectively.
Results
Isolation of a gram-positive pathogenic bacillus from B. glabrata.
For several years, our laboratory has been a recognized World Health Organization (WHO) collaborating center for the maintenance of the schistosomiasis parasite life cycle and for breeding field-collected snails. During a routine checkup, some snail strains presented with white nodules (Fig. 1A) located on the ovotestis, hepatopancreas, and mantle regions (Fig. 1B). No changes in behavior were observed for these snails, but there was high mortality in breeding tanks. Snails were dissected to isolate and analyze the nature of these white clusters. Under a light microscope, nodules appeared to contain a homogeneous population of microorganisms similar to Ascomyceta or Firmicute phyla (Fig. 2A). Gram staining indicated that all isolates were gram-variable, rod shaped organisms capable of forming subterminal endospores (Fig. 2B).
Fig 1. A. Infected Biomphalaria glabrata exhibits white nodules.
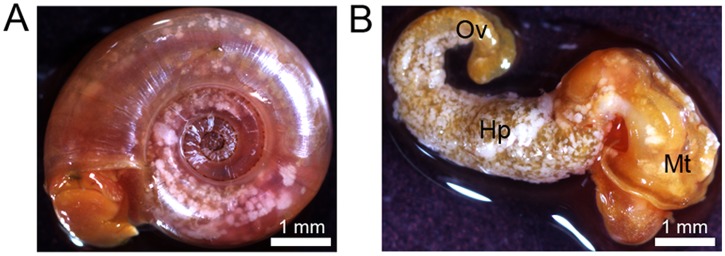
B. Dissected snail presents nodules on mantle (Mt), hepato-pancreas (Hp) and ovotestis (Ov) regions.
Fig 2. A. An exophytic nodule contains a homogenous population of bacillus- like bacteria.
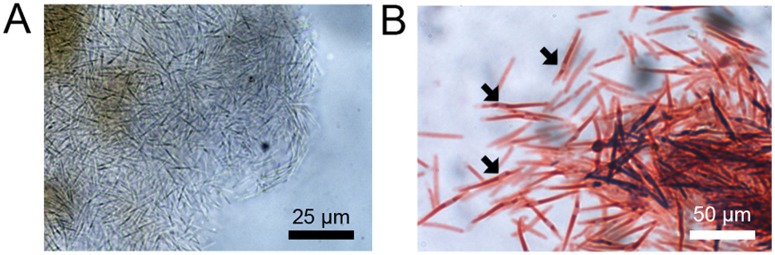
B. These rod-shaped bacteria present a mixed pattern of Gram staining and produce some endospores indicated by the black arrow.
Histological characterization of Paenibacillus infection
Histological examination of infected snails revealed that pathology was not limited to the external tegument. Indeed, bacterial masses were detected in almost all sections analyzed. Colonies were more or less spherical, depending on their location in the snail tissues. The bacterial colonies were surrounded by collagen-like fibers that formed cyst-shape structures without associated-granuloma or hemocytic nodules.
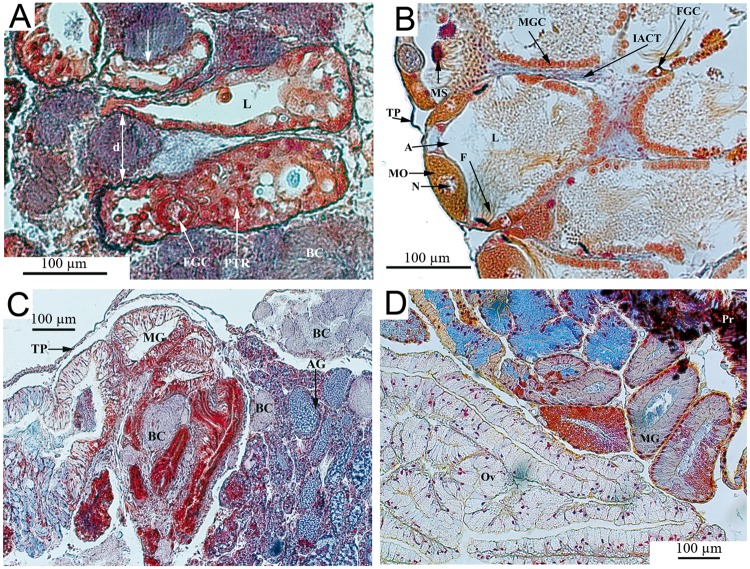
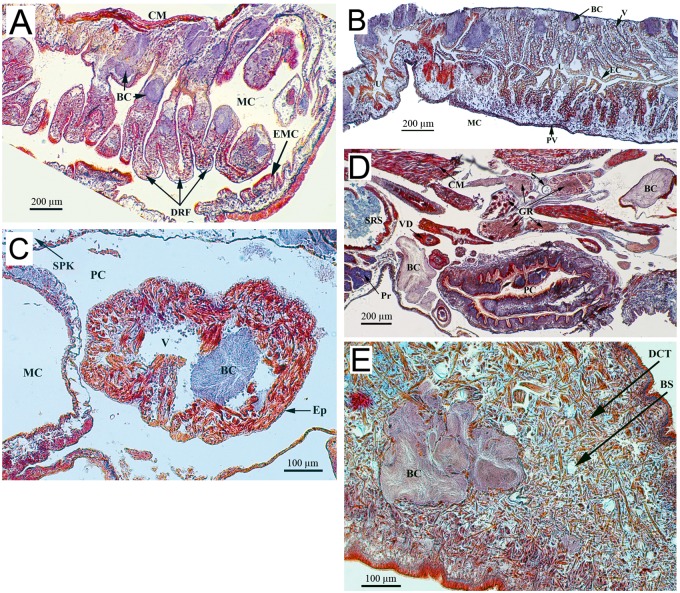
Organs with the most severe bacteria invasion were the massive hepatopancreas, the gastrointestinal tract (Fig. 3), the hermaphroditic reproductive gland (ovotestis) (Fig. 4), and the dorsal ridge of the mantle cavity (Fig. 5). The interlobular connective tissue of the hepatopancreas (Fig. 3A) and interacini connective tissue of the ovotestis (Fig. 4A) were densely occupied and extremely affected by the mechanical pressure imposed by the bacterial load. Some digestive lobules were severely damaged with visible cellular destruction. The thin layer of connective tissue enveloping the hepatopancreas was ruptured. The walls of reproductive acini showed increased thickness with physiological suppression of gamete production (gametogenesis); no spermatozoa were detected in the acinar lumen (Fig. 4A). The dorsal ridge of the mantle cavity (respiratory organ) showed many bacterial colonies in the epithelial tissue of different folds (Fig. 5). A large number of bacterial colonies of different sizes surrounded the intestinal tract and stomach (Fig. 3B). A few bacterial colonies were observed inside sex accessory organs, such as muciparous and albumin glands (Fig. 4C). Saccular and tubular portions of the kidney were moderately infected, with bacterial colonies mainly concentrating in the dorsal area of the kidney and developing towards the kidney lumen (Fig. 5A). Very few bacterial colonies were located on the ventral side of the kidney overlooking the mantle cavity (Fig. 5B). Hematopoietic tissue located between the saccular part of the kidney and the pericardium was also infected. No bacterial colonies were observed in the pericardial cavity; however, infection was detected in cardiac chambers, including a ventricle containing one colony that appeared to occupy a large volume (Fig. 5C). The headfoot sinus was moderately infected by bacterial colonies without any visible damage to male or female genitalia that end in this area (Fig. 5D). Irregularly shaped bacterial colonies were observed in the foot region (Fig. 5E). No necrosis of snail tissues was observed.
Fig 3. Histological sections of Biomphalaria glabrata digestive organs diseased by Paenibacillus (A and B) and control (C).
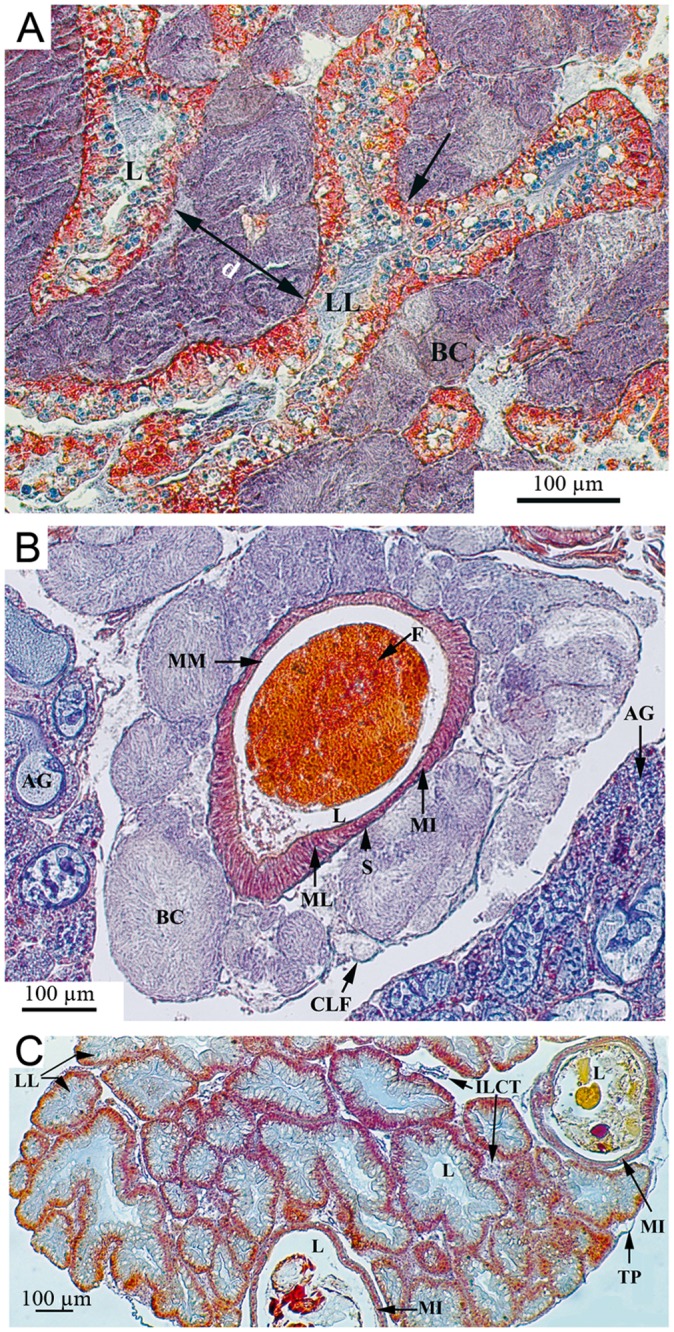
(A) The hepatic interlobular space is heavily invaded by the bacterial colonies which separate the hepatic lobules widely (d = 159 μm). The compression exerted (arrows) provoke mechanical damages with degeneration and atrophy of the digestive gland cells. (B) The intestine is densely surrounded by big bacterial colonies causing a slight compression. (C) The control shows normal liver tissue with numerous lobules separated by tight interlobular spaces and normal organization of the midintestine. AG: Albumin gland; BC: Bacterial colony; CLF: Collagen-like fibers; d: Interlobular distance; F: Feces; ILCT: Interlobular connective tissue; L: lumen; LL: Liver lobule; MI: Midintestine; ML: Muscle layer; MM: Mucous membrane; S: Serosa; TP: Tunica propria.
Fig 4. Histological sections of reproductive organs from Biomphalaria glabrata infected by Paenibacillus (A and C) and control (B and D).
(A) The ovotestis interacini space is heavily invaded by the bacterial colonies which exert compression (arrows), separating the acini widely (d = 81 μm). (B) The control shows normal acini organisation in the ovotestis with numerous spermatozoa and some ova. (C) Muciparous and albumin glands are moderately invaded. A: Acinus; AG: Albumin gland; BC: Bacterial colony; d: Interacini distance; F: Flagella; FGC: Female germinal cell; IACT: Interacini connective tissue; L: Lumen; MG: Muciparous gland; MGC: Male germinal cells; MO: Mature ova; N: Nucleus; Ov: Oviduct; Pr: Prostate; PTR: Proliferative tissue response; TP: Tunica propria.
Fig 5. Histological analysis of the bacterial invasion within other organs of Biomphalaria glabrata.
(A) The dorsal ridge of the mantle cavity with prominent folds invaded by Paenibacillus colonies. (B) The kidney tubular portion of Biomphalaria glabrata showing Paenibacillus colonies exerting compression on the epithelial tissue. The epithelium, constituted by one layer of cells, shows an irregular wavy appearance and a large vacuole in each cell. (C) Presence of Paenibacillus colony in the heart cavity. (D and E) Many bacterial colonies are present in the headfoot region with no visible damages on the genitalia organs. BC: Bacterial colony; CM: Columnar muscle; DRF: Dorsal ridge folds; EMC: Epithelium of the mantle cavity; MC: Mantle cavity; EC: Epithelial cells; L: Lumen; PV: Pulmonary vein; U: Ureter; V: Vacuole; Ep: Epicardium; PC: Pericardial cavity; SPK: Saccular portion of the kidney; GR: Ganglion ring; Pr: Prostate; PC: Penial complex; S: Statocyst; SRS: Seminal receptacle sac; VD: Vas deferens; BS: Blood space; DCT: Dense connective tissue.
Molecular identification of Paenibacillus and phylogenetic analysis
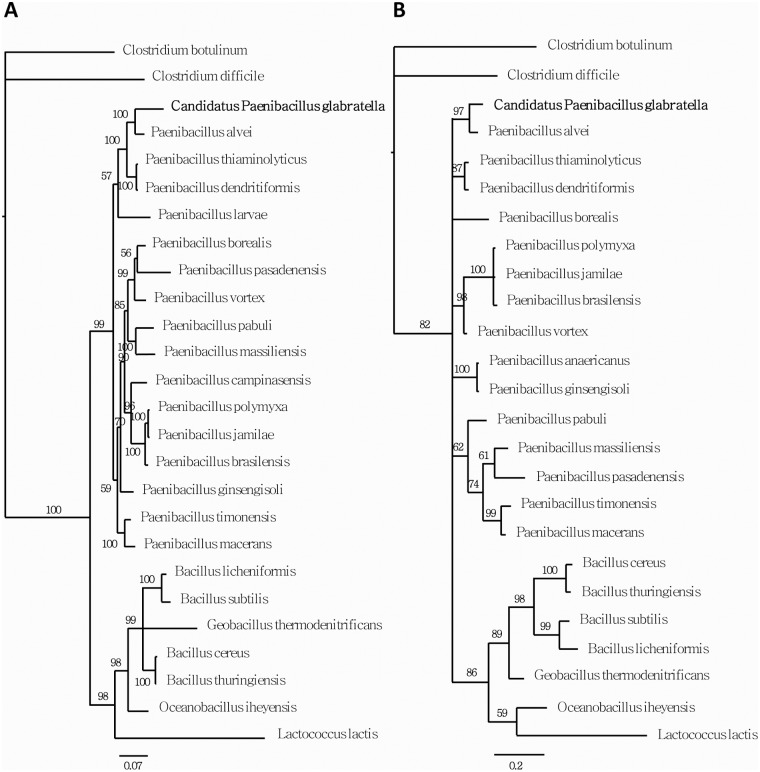
In order to identify the microbial pathogenic agent, we performed a PCR analysis of genomic DNA extracted from the white nodules using universal bacterial primers (27f and 1492r) and eukaryotic-specific primers (Unif-F-15 and Unif-R-1765). No amplification was obtained with eukaryotic-specific primers, whereas one ~1400-bp fragment with a single sequence was amplified using primers for the 16S rRNA gene. This sequence was compared against the NCBI database of 16S ribosomal RNA sequences using a BLASTN search. The best hits were for the 16S rRNA genes of Paenibacillus alvei (accession number NR_113577.1) and Paenibacillus apiarius (accession number NR_040890.1) with 95% and 94% identity, respectively. In order to confirm this identification, we partially amplified the RNA polymerase beta subunit gene (Rpob) using previously reported primers for the Rpob gene of different Paenibacillus species [26,27], and compared the predicted amino acid sequences against the NCBI protein database using a BLASTP search. The most closely related protein sequences were from Paenibacillus thiaminolyticus (accession number AY728285.1), with 94% similarity and 87% identity (E-value = 6.10–62), and P. alvei (accession number WP 005544566.1), with 96% similarity and 94% identity (E-value = 9.10–59).
The family relationships of the infectious agent described in this work were clarified by constructing phylogenetic trees. Similar topologies were obtained using different methods and datasets. The most terminal nodes were highly supported, whereas the relationships among the different families were less consistent, with lower concordance across the two 16S and Rpob datasets (Fig. 6). Nevertheless, these analyses established a new pathogenic bacteria clade within the Paenibacillus genus that is most closely related to P. alvei. With an identity rate of 94% between 16S rDNA sequences, the results of this phylogenetic analysis are indicative of a new species that we call Candidatus Paenibacillus glabratella. As recommended for noncultivable bacteria, the provisionnal Candidatus status was retained for this newly described Paenibacillus nov.sp. [34].
Fig 6. Molecular phylogenies using Bayesian analysis of (A) 16S nucleotide sequences and (B) Rpob aminoacid sequences.
Numbers above nodes represent posterior probabilities recovered by the Bayesian analysis.
Assay of bacterial pathogenicity against B. glabrata
As a first step toward testing the virulence of this new bacterial species, we attempted to develop an appropriate growth media. Various types of liquid growth media, including LB, TSB, MYPGP, brain heart or meat infusions, were tested. Before culturing, samples were heat-shock treated to eliminate most vegetative bacteria. No bacterial growth was obtained under aerobic or anaerobic conditions at 27°C or 37°C, even if media were supplemented with 10% filtered, crushed-snail extract. However, bacteria could be maintained on these different agar media with atypical colony formation, suggesting a swarming behavior characteristic of some gram-positive bacteria (S1 Fig.). Bacterial clumps on agar plates were characterized by PCR and found to be identical to those from nodules.
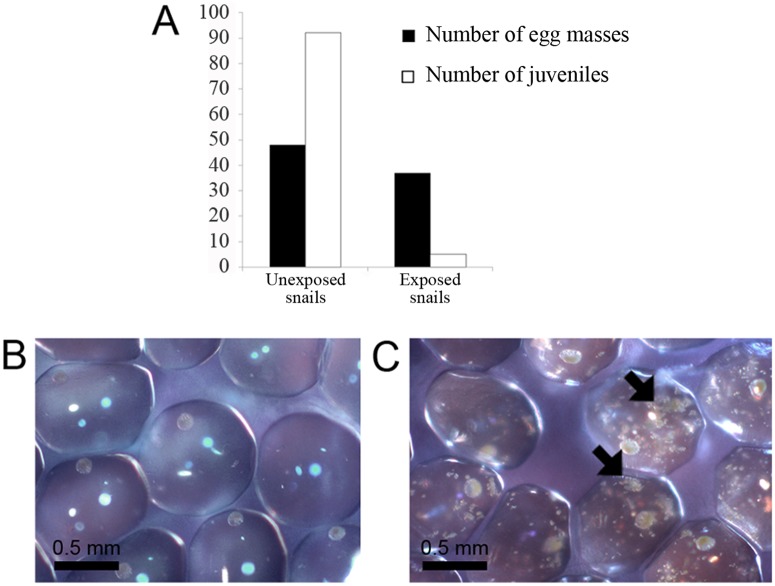
Because this novel strain exhibited no growth under our culture conditions, we decided to follow its pathogenicity by exposing healthy BgBRE albino snails to infected pigmented (BgVEN) snails. In our experimental conditions, infected snails had many modules, and exposed snails developed multiple detectable nodules after about 1 month. Fifteen days later, the number of dead snails increased sharply, reducing the original population approximately in half. By 30 days (60 days after the initial exposure), about 90% of the population had been eliminated. A Kaplan-Meier analysis confirmed the statistical significance of this result (p<0.0001) (Fig. 7). All dead snails exposed to infected snails presented with visible white nodules as clinical signs. Interestingly, before the period of high mortality (i.e., until 30 days post-exposure), a significantly lower number of juveniles was observed in containers of exposed snails, despite the fact that the number of egg masses was almost identical to that in the control group (Fig. 8A). Indeed, there were 20-times more juveniles in the control container than in the container containing bacteria. This lack of juveniles was correlated with the presence of Ca. Paenibacillus glabratella inside the snail eggs, as confirmed by PCR (Fig. 8B and C).
Fig 7. Survival time of B. glabrata (BgBRE) exposed to either uninfected (a) or bacteria-infected snails (BgVEN).
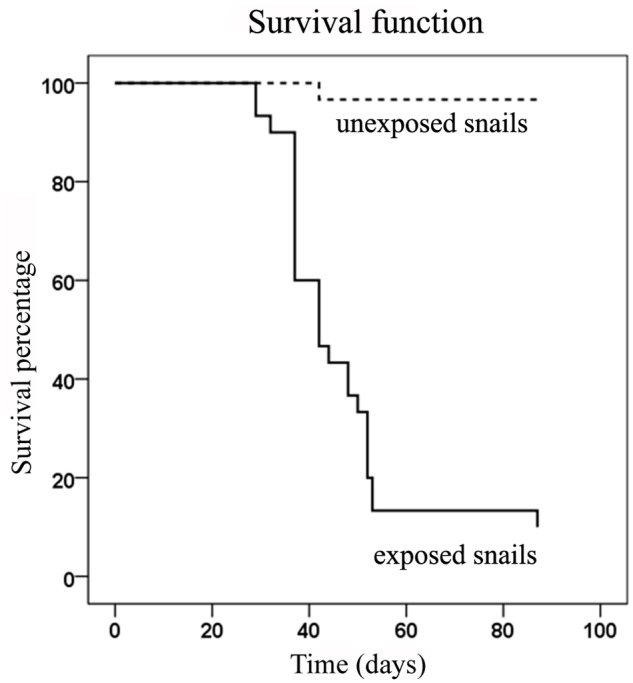
A Kaplan-Meier analysis was performed to analyze survival data.
Fig 8. A. Effect of Candidatus Paenibacillus glabratella exposure on egg masses and juvenile snails production.
B and C. Photomicrographs of egg masses from unexposed and exposed snails, respectively. The arrow indicates the presence of Ca. Paenibacillus glabratella inside each egg from exposed snail population.
Discussion
In May 2012, WHO called for the eradication of schistosomiasis and encouraged an integrated approach including chemotherapeutic control, hygiene and health education, and snail control [35]. To date, mass application of praziquantel has proven to be the most effective approach for reducing schistosomiasis prevalence and associated morbidity [36,37]. However, there is growing concern about the emergence of parasite resistance to praziquantel, and unfortunately, no vaccines against schistosomes are currently available [38,39]. A number of molluscicides have been developed in an attempt to decrease parasitic disease transmission. Chemical compounds, such as niclosamide and sodium pentachlorophenate, have been field tested and proven effective [40–43], although concerns about the emergence of a resistant snail population remain [44]. However, even if effective in decreasing the host snail population, these synthetic molluscicides can be toxic to other living organisms [45–47] and are transferred up the food web [48,49]. Consequently, increasing efforts have focused on identifying and characterizing plant-derived molluscicides as safer alternatives. Compounds belonging to saponin and terpenoid classes are highly effective against adult snails, but in most cases, the effects of these compounds on egg hatching are unknown; where they have been tested on egg masses, they have demonstrated lethal effects with higher concentrations than used against adult snails [50–59]. Other plant-derived molecules have effects on snail embryogenesis, but appear to be toxic to non-target animals [60,61]. Another “ecological” strategy for eliminating vector snails and preventing schistosomiasis transmission is the introduction of a competitor or predator into endemic sites. The Biomphalaria population is strongly suppressed in Guadeloupe, Puerto Rico and some parts of Brazil after invasion of freshwater snails like Melanoides tuberculata or Marisa cornuarietis [62–68]. The presence of predators, such as fish, insects or crustacean species, has also proved effective in limiting snail populations, but their effects are generally tested in the laboratory; thus, predator-prey population dynamics under environmental conditions are not known [69–73]. Another snail control strategy that has been considered is the reduction of reproductive capacity. The nematode Angiostrongylus cantonensis and trematode parasites belonging to the genus Plagiorchis or Echinostoma are known to affect growth as well as fecundity and fertility of B. glabrata, and have thus been proposed as biological control agents [74–76]. However, their use is not recommended since they may also cause severe human or animal infections. Efforts to control other parasitic diseases, such as malaria, and arthropod pests and vectors have focused on micropathogen identification [77–84]. Although WHO proposed studies on snail host microbial pathogens as part of its research proposal guidelines in 1984 [85], few such studies have been conducted [17–19].
In the current study, we report the isolation of a new pathogenic bacterial species for Biomphalaria, the snail intermediate host of S. mansoni. 16S rDNA and Rpob genes sequence analyses positioned this bacterium within the genus Paenibacillus. The highest identity rates for 16S rDNA and Rpob genes were only 95% and 94%, respectively, compared with Paenibacillus alvei genes. Since these identity levels are well below the threshold for genomic definition of a bacterial species based on the 16S rDNA gene (i.e., 97%), which is commonly used for molecular systematics [86,87], we propose to call this new pathogenic bacterium, Ca. Paenibacillus glabratella.
Paenibacillaceae family members are widely distributed in the environment, including in soil, air, the rhizosphere, food products, and aquatic environments [88–90]. Up to date, the exact origin of this new microbial pathogen named candidatus Paenibacillus glabratella is unknown. Screening of healthy snails for bacterial agents using a molecular approach excluded the possibility of a snail microbiota origin for the isolated bacterium. Moreover, all bacterial communities characterized and cultivated from B. glabrata were gram-negatives [91], and everything that came in contact with the mollusk (e.g., water, food) tested negative for Paenibacillus, strongly suggesting contamination of animals collected in the field and horizontal transmission between laboratory snails. Indeed as WHO collaborating center we recovered in the field and reared in our laboratory a great number of snail strains from different localities (mainly South America and Africa). One of these snail isolates could have been the point of entry of the disease in our laboratory, but we were not able to identify the strain of origin. In our experimental conditions, the duration to the first signs of mortality is longer than the time period reported in other studies, in which lethal effects appeared after 1 or 2 weeks [18,19]. However, instead of using massive exposure, as was done in these previous studies, we enabled bacterial infection by dissemination from snail to snail using a few initially infected individuals. This horizontal transmission was surprisingly effective, resulting in the death of almost all exposed snails.
Among bacteria belonging to the family Paenibacillaceae, only P. larvae and P. popilliae are described as invertebrate pathogens [92–94]. The first is a spore-forming bacterium that is a widespread larval pathogen of the honey bee, identified as the ethological agent for American foulbrood [95,96]. After being ingested in the form of a spore by honeybee larvae, this bacterium grows in the midgut of the insect. Then, the vegetative form secretes proteases that facilitate tissues invasion and contribute to liquefaction of the host [97,98]. The most marked histopathological feature of Paenibacillus interference with B. glabrata is its development in a large number of tissues, mainly in the hepatopancreas, that compromises the ability of the snail to nourish itself. The presence of Paenibacillus in the circulatory system, mainly in the heart, indicates that these bacteria may follow the path of the hemolymph to reach different organs involved in diverse functions, including digestion, respiration, excretion, and reproduction. In keeping with this, Paenibacillus was observed in the ovotestis, leading to the suppression of gametogenesis and partial destruction of ovotestis acini. So, the snail reproductive capacity could be affected by compromising the number of eggs laid in the advanced stage of the infection. A castration-like phenomenon similar to that observed in heavily infected snails has also been reported during parasitic infestation by S. mansoni [99,100], Plagiorchis elegans [74], and the nematode Angiostrongylus cantonensis [76]. This late infertility appears to be related to competition for energy resources between the host and pathogen [76,100]. Paenibacillus was also observed in the secondary reproductive organs, namely muciparous and albumin glands, which could be a sign of vertically transmitted infection from parent to offspring. Indeed, this pathogenic agent was also found in snail eggs, affecting their development and hatching. Collectively, these histological observations suggest that the main pathogenic effect of Ca. Paenibacillus glabratella was strong compression of tissues, which caused significant damage to soft tissue organs like the liver and ovotestis. However, we do not exclude the possibility of tissue lysis owing to bacterial excreta, as it has been observed for Brevibacillus laterosporus towards nematodes [101], or gametogenesis inhibitory bacterial factors, as has been reported for the digenean trematode Zoogonus lasius on the snail, Nassarius obsoletus snail [102].
In conclusion, a newly recognized pathogenic bacterium that is closely related to members of the genus Paenibacillus was isolated from abnormal nodules of the snail B. glabrata and characterized. Although this microbial pathogen could only be cultivated in vivo (in vitro cultivation conditions have not yet been established), we demonstrated that it is infective through aquatic dispersal and contact between snails. Upon infection, this bacterium is highly lethal for adult snails and severely reduces the number of offspring. Our current report describes the pathogenic effects of this bacterium on the neotropical snail B. glabrata, but the African snail B. pfeifferi and the genus Bulinus, other Schistosoma vectors, can also be affected (personal data). Among important future studies are tests of the spectrum of the molluscicidal properties of this bacterium against all freshwater snails that can serve as vector for schistosomiasis. However the high pathogenicity of this bacterium could be a limit for using it as biological control agent. Indeed its safety for non target species has also to be tested to avoid deleterious impact on endemic species. Finally, it would also be interesting to study the pathogenicity of other species that are closely related phylogenetically to Ca. Paenibacillus glabratella, like P. alvei [103]. Clearly, data obtained in the laboratory must be confirmed under field conditions including non-target species before proposing this pathogen as a biocontrol agent against schistosomiasis. Nevertheless, this study highlights the value of systematic surveys of snail pathology in providing potential tools to support the announced WHO goal of schistosomiasis eradication by 2025 [35,104].
Supporting Information
Sequence accession numbers of the species used for the phylogenetic analysis. The table provides the GenBank accession number of nucleotide sequences from 16S rDNA and RpoB genes and Rpob protein sequence.
(DOCX)
Individual cells and clump of bacteria interconnected by long strings forming some hyphae like structure were observed. The arrow shows an interconnection between two bacteria clumps.
(TIF)
Acknowledgments
The authors would like to thank Jean Pierre Pointier for many helpful and interesting discussions. Also, we would like to thank the two anonymous reviewers for their helpful comments.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by funds from the Centre National de la Recherche (CNRS) and the Universite de Perpignan Via Domitia (UPVD), and by a grant from the ANR JCJC INVIMORY number ANR-13-JSV7-0009. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Chitsulo L, Engels D, Montresor A, Savioli L (2000) The global status of schistosomiasis and its control. Acta Tropica 77: 41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chitsulo L, Loverde P, Engels D (2004) Schistosomiasis. Nat Rev Microbiol 2: 12–13. [DOI] [PubMed] [Google Scholar]
- 3. Gryseels B, Polman K, Clerinx J, Kestens L (2006) Human schistosomiasis. Lancet 368: 1106–1118. [DOI] [PubMed] [Google Scholar]
- 4. Guillou F, Mitta G, Galinier R, Coustau C (2007) Identification and expression of gene transcripts generated during an anti-parasitic response in Biomphalaria glabrata. Dev Comp Immunol 31: 657–671. [DOI] [PubMed] [Google Scholar]
- 5. Bouchut A, Coustau C, Gourbal B, Mitta G (2007) Compatibility in the Biomphalaria glabrata/Echinostoma caproni model: new candidate genes evidenced by a suppressive subtractive hybridization approach. Parasitology 134: 575–588. [DOI] [PubMed] [Google Scholar]
- 6. Adema CM, Hanington PC, Lun C-M, Rosenberg GH, Aragon AD, et al. (2010) Differential transcriptomic responses of Biomphalaria glabrata (Gastropoda, Mollusca) to bacteria and metazoan parasites, Schistosoma mansoni and Echinostoma paraensei (Digenea, Platyhelminthes). Molecular Immunology 47: 849–860. 10.1016/j.molimm.2009.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nowak TS, Woodards AC, Jung Y, Adema CM, Loker ES (2004) Identification of transcripts generated during the response of resistant Biomphalaria glabrata to Schistosoma mansoni infection using suppression subtractive hybridization. J Parasitol 90: 1034–1040. [DOI] [PubMed] [Google Scholar]
- 8. Lockyer AE, Spinks JN, Walker AJ, Kane RA, Noble LR, et al. (2007) Biomphalaria glabrata transcriptome: identification of cell-signalling, transcriptional control and immune-related genes from open reading frame expressed sequence tags (ORESTES). Dev Comp Immunol 31: 763–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hanelt B, Lun CM, Adema CM (2008) Comparative ORESTES-sampling of transcriptomes of immune-challenged Biomphalaria glabrata snails. J Invertebr Pathol 99: 192–203. 10.1016/j.jip.2008.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lockyer AE, Spinks J, Kane RA, Hoffmann KF, Fitzpatrick JM, et al. (2008) Biomphalaria glabrata transcriptome: cDNA microarray profiling identifies resistant- and susceptible-specific gene expression in haemocytes from snail strains exposed to Schistosoma mansoni. BMC Genomics 9: 634 10.1186/1471-2164-9-634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hanington PC, Forys MA, Dragoo JW, Zhang SM, Adema CM, et al. (2010) Role for a somatically diversified lectin in resistance of an invertebrate to parasite infection. Proc Natl Acad Sci U S A 107: 21087–21092. 10.1073/pnas.1011242107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lockyer AE, Emery AM, Kane RA, Walker AJ, Mayer CD, et al. (2012) Early differential gene expression in haemocytes from resistant and susceptible Biomphalaria glabrata strains in response to Schistosoma mansoni. PLoS One 7: e51102 10.1371/journal.pone.0051102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Deleury E, Dubreuil G, Elangovan N, Wajnberg E, Reichhart JM, et al. (2012) Specific versus non-specific immune responses in an invertebrate species evidenced by a comparative de novo sequencing study. PLoS One 7: e32512 10.1371/journal.pone.0032512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bravo A, Likitvivatanavong S, Gill SS, Soberon M (2011) Bacillus thuringiensis: A story of a successful bioinsecticide. Insect Biochemistry and Molecular Biology 41: 423–431. 10.1016/j.ibmb.2011.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Soberon M, Lopez-Diaz JA, Bravo A (2013) Cyt toxins produced by Bacillus thuringiensis: A protein fold conserved in several pathogenic microorganisms. Peptides 41: 87–93. 10.1016/j.peptides.2012.05.023 [DOI] [PubMed] [Google Scholar]
- 16. Boyce R, Lenhart A, Kroeger A, Velayudhan R, Roberts B, et al. (2013) Bacillus thuringiensis israelensis (Bti) for the control of dengue vectors: systematic literature review. Trop Med Int Health 18: 564–577. 10.1111/tmi.12087 [DOI] [PubMed] [Google Scholar]
- 17. Larget I, de Barjac H (1981) Specificity and active principle of Bacillus thuringiensis var. israelensis. Bull Soc Pathol Exot Filiales 74: 216–227. [PubMed] [Google Scholar]
- 18. Gamalat YO, Ahmed MM, Ahmed AK, Asmaa AM (2011) Biological studies on Biomphalaria alexandrina snails treated with Furcraea selloa marginata plant (family: Agavaceae) and Bacillus thuringiensis kurstaki (Dipel-2x). Journal of Applied Pharmaceutical Science 01: 47–55. [Google Scholar]
- 19. de Oliveira EJ, Rabinovitch L, Monnerat R, Passos L, Zahner V (2004) Molecular characterization of Brevibacillus laterosporus and its potential use in biological control. Appl Environ Microbiol 70: 6657–6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Singer S, Bair T, Hammill TB, Berte AM, Correa-Ochoa MM, et al. (1994) Fermentation and toxin studies of the molluscicidal strains of Bacillus brevis. J Ind Microbiol 13: 112–119. [DOI] [PubMed] [Google Scholar]
- 21. Texera D, Vicente-Scorza J (1954) Studies on a bacterial type resembling bacillus pinotti found in Venezuela with pathogenic action on Australorbis glabratus. Arch Venez Pat Trop Parasitol Med 2: 235–243. [Google Scholar]
- 22. Bean-Knudsen DE, Uhazy LS, Wagner JE, Young BM (1988) Systemic infection of laboratory-reared Biomphalaria glabrata (Mollusca: Gastropoda) with an acid-fast bacillus. J Invertebr Pathol 51: 291–293. [DOI] [PubMed] [Google Scholar]
- 23. Cole RM, Richards CS, Popkin TJ (1977) Novel bacterium infecting an African snail. J Bacteriol 132: 950–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weisburg WG, Barns SM, Pelletier DA, Lane DJ (1991) 16S ribosomal DNA amplification for phylogenetic study. 173: 697–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dahlloff I, Baillie H, Kjelleberg S (2000) rpoB-Based Microbial Community Analysis Avoids Limitations Inherent in 16S rRNA Gene Intraspecies Heterogeneity. 66: 3376–3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Da Mota FF, Gomes EA, Paiva E, Rosado AS, Seldin L (2004) Use of rpoB gene analysis for identification of nitrogen-fixing Paenibacillus species as an alternative to the 16S rRNA gene. Letters in Applied Microbiology 39: 34–40. [DOI] [PubMed] [Google Scholar]
- 27. Da Mota FF, Gomes EA, Paiva E, Seldin L (2005) Assessment of the diversity of Paenibacillus species in environmental samples by a novel rpoB-based PCR-DGGE method. FEMS Microbiology Ecology 53: 317–328. [DOI] [PubMed] [Google Scholar]
- 28. Tomas G, Marc EF, Michael S, Monika N, Andreas NM, et al. (2002) Development of an 18S rRNA gene targeted PCR based diagnostic for the blue crab parasite Hematodinium sp. 49: 61–70. [DOI] [PubMed] [Google Scholar]
- 29. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, et al. (2011) MEGA5: Molecular Evolutionary Genetics Analysis Using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. 28: 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16: 111–120. [DOI] [PubMed] [Google Scholar]
- 31. Tamura K (1992) The rate and pattern of nucleotide substitution in Drosophila mitochondrial DNA. Mol Biol Evol 9: 814–825. [DOI] [PubMed] [Google Scholar]
- 32. Mattos AC, Kusel JR, Pimenta PF, Coelho PM (2006) Activity of praziquantel on in vitro transformed Schistosoma mansoni sporocysts. Mem Inst Oswaldo Cruz 101 Suppl 1: 283–287. [DOI] [PubMed] [Google Scholar]
- 33. Galinier R, Portela J, Mone Y, Allienne JF, Henri H, et al. (2013) Biomphalysin, a new beta pore-forming toxin involved in Biomphalaria glabrata immune defense against Schistosoma mansoni. PLoS Pathog 9: e1003216 10.1371/journal.ppat.1003216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Murray RG, Stackebrandt E (1995) Taxonomic note: implementation of the provisional status Candidatus for incompletely described procaryotes. Int J Syst Bacteriol 45: 186–187. [DOI] [PubMed] [Google Scholar]
- 35.WHO (2012) Accelerating work to overcome the global impact of neglected tropical diseases: a roadmap for implementation
- 36. Chai J-Y (2013) Praziquantel Treatment in Trematode and Cestode Infections: An Update. Infect Chemother 45: 32–43. 10.3947/ic.2013.45.1.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cioli D, Pica-Mattoccia L, Basso A, Guidi A (2014) Schistosomiasis control: praziquantel forever? Molecular and Biochemical Parasitology 195: 23–29. 10.1016/j.molbiopara.2014.06.002 [DOI] [PubMed] [Google Scholar]
- 38. Doenhoff MJ, Kusel JR, Coles GC, Cioli D (2002) Resistance of Schistosoma mansoni to praziquantel: is there a problem? Trans R Soc Trop Med Hyg 96: 465–469. [DOI] [PubMed] [Google Scholar]
- 39. Melman SD, Steinauer ML, Cunningham C, Kubatko LS, Mwangi IN, et al. (2009) Reduced susceptibility to praziquantel among naturally occurring Kenyan isolates of Schistosoma mansoni. PLoS Negl Trop Dis 3: e504 10.1371/journal.pntd.0000504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jobin WR, Ferguson FF, Palmer JR (1970) Control of schistosomiasis in Guayama and Arroyo, Puerto Rico. Bull World Health Organ 42: 151–156. [PMC free article] [PubMed] [Google Scholar]
- 41. Saladin B, Saladin K, Holzer B, Dennis E, Hanson A, et al. (1983) A pilot control trial of schistosomiasis in central Liberia by mass chemotherapy of target populations, combined with focal application of molluscicide. Acta Trop 1983 40: 271–295. [PubMed] [Google Scholar]
- 42. Coura-Filho P, Mendes NM, de Souza CP, Pereira JP (1992) The prolonged use of niclosamide as a molluscicide for the control of Schistosoma mansoni. Rev Inst Med Trop Sao Paulo 34: 427–431. [DOI] [PubMed] [Google Scholar]
- 43. Kariuki H, Madsen H, Ouma JH, Butterworth AE, Dunne DW, et al. (2013) Long term study on the effect of mollusciciding with niclosamide in stream habitats on the transmission of schistosomiasis mansoni after community-based chemotherapy in Makueni District, Kenya. Parasit Vectors 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dai J-R, Li Y-Z, Wang WEI, Xing Y-T, Qu G-L, et al. (2014) Resistance to niclosamide in Oncomelania hupensis, the intermediate host of Schistosoma japonicum: should we be worried? FirstView: 1–9. [DOI] [PubMed] [Google Scholar]
- 45. Harrison AD (1966) The effects of Bayluscid on gastropod snails and other Aquatic Fauna in Rhodesia. Hydrobiologia 28: 371–384. [Google Scholar]
- 46. Souza CP (1995) Molluscicide control of snail vectors of schistosomiasis. Mem Inst Oswaldo Cruz 90: 165–168. [DOI] [PubMed] [Google Scholar]
- 47. Oliveira-Filho EC, Paumgartten FJR (2000) Toxicity of Euphorbia milii Latex and Niclosamide to Snails and Nontarget Aquatic Species. Ecotoxicology and Environmental Safety 46: 342–350. [DOI] [PubMed] [Google Scholar]
- 48. Zinada OA (2000) Effect of niclosamide on the marketable fish Liza ramada (Risso, 1826) concerning accumulation in muscles and activities of three metabolic liver enzymes. J Egypt Soc Parasitol 30: 791–797. [PubMed] [Google Scholar]
- 49. Xiao K, Zhao X, Liu Z, Zhang B, Fang L, et al. (2010) Polychlorinated dibenzo-p-dioxins and dibenzofurans in blood and breast milk samples from residents of a schistosomiasis area with Na-PCP application in China. Chemosphere 79: 740–744. 10.1016/j.chemosphere.2010.02.042 [DOI] [PubMed] [Google Scholar]
- 50. Shoeb HA, Hassan AA, el-Sayed MM, Refahy L (1984) The molluscicidal properties of Agave decepiens and Agave americana (var. marginata). J Egypt Soc Parasitol 14: 265–273. [PubMed] [Google Scholar]
- 51. Chifundera K, Baluku B, Mashimango B (1993) Phytochemical Screening and Molluscicidal Potency of Some Zairean Medicinal Plants. Pharmacological Research 28: 333–340. [DOI] [PubMed] [Google Scholar]
- 52. Schall VT, de Vasconcellos MC, de Souza CP, Baptista DF (1998) The molluscicidal activity of Crown of Christ (Euphorbia splendens var. hislopii) latex on snails acting as intermediate hosts of Schistosoma mansoni and Schistosoma haematobium. Am J Trop Med Hyg 58: 7–10. [DOI] [PubMed] [Google Scholar]
- 53. Nihei K, Ying BP, Murakami T, Matsuda H, Hashimoto M, et al. (2005) Pachyelasides A-D, novel molluscicidal triterpene saponins from Pachyelasma tessmannii. J Agric Food Chem 53: 608–613. [DOI] [PubMed] [Google Scholar]
- 54. Radwan MA, El-Zemity SR, Mohamed SA, Sherby SM (2008) Potential of some monoterpenoids and their new N-methyl carbamate derivatives against Schistosomiasis snail vector, Biomphalaria alexandrina. Ecotoxicology and Environmental Safety 71: 889–894. [DOI] [PubMed] [Google Scholar]
- 55. Tadros MM, Ghaly NS, Moharib MN (2008) Molluscicidal and schistosomicidal activities of a steroidal saponin containing fraction from Dracaena fragrans (L.). J Egypt Soc Parasitol 38: 585–598. [PubMed] [Google Scholar]
- 56. Zhang H, Xu H-H, Song Z-J, Chen L-Y, Wen H-J (2012) Molluscicidal activity of Aglaia duperreana and the constituents of its twigs and leaves. Fitoterapia 83: 1081–1086. 10.1016/j.fitote.2012.05.003 [DOI] [PubMed] [Google Scholar]
- 57. Singh KL, Singh DK, Singh VK (2012) Characterization of the molluscicidal activity of Bauhinia variegata and Mimusops elengi plant extracts against the fasciola vector Lymnaea acuminata. Rev Inst Med Trop Sao Paulo 54: 135–140. [DOI] [PubMed] [Google Scholar]
- 58. Diab Y, Ioannou E, Emam A, Vagias C, Roussis V (2012) Desmettianosides A and B, bisdesmosidic furostanol saponins with molluscicidal activity from Yucca desmettiana. Steroids 77: 686–690. 10.1016/j.steroids.2012.02.014 [DOI] [PubMed] [Google Scholar]
- 59. Dias CN, Rodrigues KA, Carvalho FA, Carneiro SM, Maia JG, et al. (2013) Molluscicidal and leishmanicidal activity of the leaf essential oil of Syzygium cumini (L.) SKEELS from Brazil. Chem Biodivers 10: 1133–1141. 10.1002/cbdv.201200292 [DOI] [PubMed] [Google Scholar]
- 60. Bagalwa J-JM, Voutquenne-Nazabadioko L, Sayagh C, Bashwira AS (2010) Evaluation of the biological activity of the molluscicidal fraction of Solanum sisymbriifolium against non target organisms. Fitoterapia 81: 767–771. 10.1016/j.fitote.2010.04.003 [DOI] [PubMed] [Google Scholar]
- 61. Rapado LN, Pinheiro AS, Lopes PO, Fokoue HH, Scotti MT, et al. (2013) Schistosomiasis control using piplartine against Biomphalaria glabrata at different developmental stages. PLoS Negl Trop Dis 7: e2251 10.1371/journal.pntd.0002251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jobin WR, Ferguson FF, Berrios-Duran LA (1973) Effect of Marisa cornuarietis on populations of Biomphalaria glabrata in farm ponds of Puerto Rico. Am J Trop Med Hyg 1973: 2. [DOI] [PubMed] [Google Scholar]
- 63. Perera de Puga G, Cong MY, Ferrer JR, Gutierrez A, Sanchez J (1994) Importance of Tarebia granifera in the control of a population of Biomphalaria peregrina introduced in Cuba. Rev Cubana Med Trop 46: 20–24. [PubMed] [Google Scholar]
- 64. Giboda M, Malek EF, Correa R (1997) Human schistosomiasis in Puerto Rico: reduced prevalence rate and absence of Biomphalaria glabrata. Am J Trop Med Hyg 57: 564–568. [DOI] [PubMed] [Google Scholar]
- 65. Pointier JP, Jourdane J (2000) Biological control of the snail hosts of schistosomiasis in areas of low transmission: the example of the Caribbean area. Acta Tropica 77: 53–60. [DOI] [PubMed] [Google Scholar]
- 66. Guimaraes CT, Souza CP, Soares D (2001) Possible competitive displacement of planorbids by Melanoides tuberculata in Minas Gerais, Brazil. Mem Inst Oswaldo Cruz 96: 173–176. [DOI] [PubMed] [Google Scholar]
- 67. Pointier JP (2001) Invading freshwater snails and biological control in Martinique Island, French West Indies. Mem Inst Oswaldo Cruz 96: 67–74. [DOI] [PubMed] [Google Scholar]
- 68. Giovanelli A, da Silva CL, Leal GB, Baptista DF (2005) Habitat preference of freshwater snails in relation to environmental factors and the presence of the competitor snail Melanoides tuberculatus (Muller, 1774). Mem Inst Oswaldo Cruz 100: 169–176. [DOI] [PubMed] [Google Scholar]
- 69. Sohn IG, Kornicker LS (1972) Predation of schistosomiasis vector snails by Ostracoda (Crustacea). Science 175: 1258–1259. [DOI] [PubMed] [Google Scholar]
- 70. Sodeman WA, Rodrick GE, Vincent AL (1980) Lampyridae larva: a natural predator of schistosome vector snails in Liberia. Am J Trop Med Hyg 29: 319 [DOI] [PubMed] [Google Scholar]
- 71. Consoli RA, Guimaraes CT, do Carmo JA, Soares DM, dos Santos JS (1991) Astronotus ocellatus (Cichlidae:Pisces) and Macropodus opercularis (Anabatidae:Pisces) as predators of immature Aedes fluviatilis (Diptera:Culicidae) and Biomphalaria glabrata (Mollusca:Planorbidae). Mem Inst Oswaldo Cruz 86: 419–424. [DOI] [PubMed] [Google Scholar]
- 72. Hofkin BV, Hofinger DM, Koech DK, Loker ES (1992) Predation of Biomphalaria and non-target molluscs by the crayfish Procambarus clarkii: implications for the biological control of schistosomiasis. Ann Trop Med Parasitol 86: 663–670. [DOI] [PubMed] [Google Scholar]
- 73. Sokolow SH, Lafferty KD, Kuris AM (2014) Regulation of laboratory populations of snails (Biomphalaria and Bulinus spp.) by river prawns, Macrobrachium spp. (Decapoda, Palaemonidae): Implications for control of schistosomiasis. Acta Tropica 132: 64–74. 10.1016/j.actatropica.2013.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zakikhani M, Rau ME (1998) Effects of Plagiorchis elegans (Digenea: Plagiorchiidae) infection on the reproduction of Biomphalaria glabrata (Pulmonata: Planorbidae). J Parasitol 84: 927–930. [PubMed] [Google Scholar]
- 75. Munoz-Antoli C, Marin A, Toledo R, Esteban J-G (2007) Effect of Echinostoma friedi (Trematoda: Echinostomatidae) experimental infection on longevity, growth and fecundity of juvenile Radix peregra (Gastropoda: Lymnaeidae) and Biomphalaria glabrata (Gastropoda: Planorbidae) snails. Parasitology Research 101: 1663–1670. [DOI] [PubMed] [Google Scholar]
- 76. Tunholi-Alves VM, Tunholi VM, Lustrino D, Amaral LS, Thiengo SC, et al. (2011) Changes in the reproductive biology of Biomphalaria glabrata experimentally infected with the nematode Angiostrongylus cantonensis. Journal of Invertebrate Pathology 108: 220–223. 10.1016/j.jip.2011.08.009 [DOI] [PubMed] [Google Scholar]
- 77. van den Hurk AF, Hall-Mendelin SF, Pyke AT, Frentiu FD, McElroy K, et al. (2012) Impact of Wolbachia on infection with chikungunya and yellow fever viruses in the mosquito vector Aedes aegypti. PLoS Negl Trop Dis 6: e1892 Epub 0002012 Nov 0001891. 10.1371/journal.pntd.0001892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Baton LA, Pacidonio EC, Goncalves DS, Moreira LA (2013) wFlu: characterization and evaluation of a native Wolbachia from the mosquito Aedes fluviatilis as a potential vector control agent. PLoS One 8: e59619 10.1371/journal.pone.0059619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Burt A (2014) Heritable strategies for controlling insect vectors of disease. Phil Trans R Soc B 369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. St. Leger R, Wang C (2010) Genetic engineering of fungal biocontrol agents to achieve greater efficacy against insect pests. Applied Microbiology and Biotechnology 85: 901–907. 10.1007/s00253-009-2306-z [DOI] [PubMed] [Google Scholar]
- 81. Swamy HM, Asokan RF, Rajasekaran PE, Mahmood R, Nagesha SN, et al. (2012) Analysis of opportunities and challenges in patenting of Bacillus thuringiensis insecticidal crystal protein genes. Recent Pat DNA Gene Seq 6: 64–71. [DOI] [PubMed] [Google Scholar]
- 82. Zhang CR, Zhang S, Xia J, Li FF, Xia WQ, et al. (2014) The immune strategy and stress response of the Mediterranean species of the Bemisia tabaci complex to an orally delivered bacterial pathogen. PLoS One 9: e94477 10.1371/journal.pone.0094477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Contreras J, Mendoza J, Martinez-Aguirre M, Garcia-Vidal L, Izquierdo J, et al. (2014) Efficacy of enthomopathogenic fungus Metarhizium anisopliae against Tuta absoluta. J Econ Entomol 107: 121–124. [DOI] [PubMed] [Google Scholar]
- 84. Fischbein D, Corley JC (2014) Classical biological control of an invasive forest pest: a world perspective of the management of Sirex noctilio using the parasitoid Ibalia leucospoides (Hymenoptera: Ibaliidae). Bull Entomol Res FirstView: 1–12. [DOI] [PubMed] [Google Scholar]
- 85.WHO (1984) Report of an Informal Consultation on Research on the Biological Control of Snail Intermediate Hosts, Geneva, 25–27 January 1984.
- 86. Stackebrandt E, Frederiksen WF, Garrity GM, Grimont PA, Kampfer P, et al. (2002) Report of the ad hoc committee for the re-evaluation of the species definition in bacteriology. Int J Syst Evol Microbiol 52: 1043–1047. [DOI] [PubMed] [Google Scholar]
- 87. Gevers D, Cohan FM, Lawrence JG, Spratt BG, Coenye T, et al. (2005) Re-evaluating prokaryotic species. Nat Rev Micro 3: 733–739. [DOI] [PubMed] [Google Scholar]
- 88. McSpadden Gardener BB (2004) Ecology of Bacillus and Paenibacillus spp. in Agricultural Systems. Phytopathology 94: 1252–1258. 10.1094/PHYTO.2004.94.11.1252 [DOI] [PubMed] [Google Scholar]
- 89. Lal S, Tabacchioni S (2009) Ecology and biotechnological potential of Paenibacillus polymyxa: a minireview. Indian J Microbiol 49: 2–10. 10.1007/s12088-009-0008-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ivy RA, Ranieri MI, Martin NH, den Bakker HC, Xavier BM, et al. (2012) Identification and characterization of psychrotolerant sporeformers associated with fluid milk production and processing. Appl Environ Microbiol 78: 1853–1864. 10.1128/AEM.06536-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Silva TM, Melo ES, Lopes ACS, Veras DL, Duarte CR, et al. (2013) Characterization of the bacterial microbiota of Biomphalaria glabrata (Say, 1818) (Mollusca: Gastropoda) from Brazil. Letters in Applied Microbiology 57: 19–25. 10.1111/lam.12068 [DOI] [PubMed] [Google Scholar]
- 92. Heyndrickx M, Vandemeulebroecke K, Hoste B, Janssen P, Kersters K, et al. (1996) Reclassification of Paenibacillus (formerly Bacillus) pulvifaciens (Nakamura 1984) Ash et al. 1994, a Later Subjective Synonym of Paenibacillus (formerly Bacillus) larvae (White 1906) Ash et al. 1994, as a Subspecies of P. larvae, with Emended Descriptions of P. larvae as P. larvae subsp. larvae and P. larvae subsp. pulvifaciens. 46: 270–279. [DOI] [PubMed] [Google Scholar]
- 93. Harrison H, Patel R, Yousten AA (2000) Paenibacillus Associated with Milky Disease in Central and South American Scarabs. Journal of Invertebrate Pathology 76: 169–175. [DOI] [PubMed] [Google Scholar]
- 94. Genersch E (2010) American Foulbrood in honeybees and its causative agent, Paenibacillus larvae. Journal of Invertebrate Pathology 103, Supplement: S10–S19. 10.1016/j.jip.2009.06.015 [DOI] [PubMed] [Google Scholar]
- 95. Fries I, Lindström A, Korpela S (2006) Vertical transmission of American foulbrood (Paenibacillus larvae) in honey bees (Apis mellifera). Veterinary Microbiology 114: 269–274. [DOI] [PubMed] [Google Scholar]
- 96. Lindstrom A, Korpela S, Fries I (2008) The distribution of Paenibacillus larvae spores in adult bees and honey and larval mortality, following the addition of American foulbrood diseased brood or spore-contaminated honey in honey bee (Apis mellifera) colonies. Journal of Invertebrate Pathology 99: 82–86. 10.1016/j.jip.2008.06.010 [DOI] [PubMed] [Google Scholar]
- 97. Genersch E, Evans JD, Fries I (2010) Honey bee disease overview. Journal of Invertebrate Pathology 103, Supplement: S2–S4. 10.1016/j.jip.2009.07.015 [DOI] [PubMed] [Google Scholar]
- 98. Garcia-Gonzalez E, Poppinga L, Funfhaus A, Hertlein G, Hedtke K, et al. (2014) Paenibacillus larvae Chitin-Degrading Protein PlCBP49 Is a Key Virulence Factor in American Foulbrood of Honey Bees. PLoS Pathog 10: e1004284 10.1371/journal.ppat.1004284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Crews AE, Yoshino TP (1989) Schistosoma mansoni: effect of infection on reproduction and gonadal growth in Biomphalaria glabrata. Exp Parasitol 68: 326–334. [DOI] [PubMed] [Google Scholar]
- 100. Faro MJ, Perazzini M, Correa LdR, Mello-Silva CC, Pinheiro J, et al. (2013) Biological, biochemical and histopathological features related to parasitic castration of Biomphalaria glabrata infected by Schistosoma mansoni. Experimental Parasitology 134: 228–234. 10.1016/j.exppara.2013.03.020 [DOI] [PubMed] [Google Scholar]
- 101. Huang X, Tian B, Niu Q, Yang J, Zhang L, et al. (2005) An extracellular protease from Brevibacillus laterosporus G4 without parasporal crystals can serve as a pathogenic factor in infection of nematodes. Research in Microbiology 156: 719–727. [DOI] [PubMed] [Google Scholar]
- 102. Cheng TC, Sullivan JT, Harris KR (1973) Parasitic castration of the marine prosobranch gastropod Nassarius obsoletus by sporocysts of Zoogonus rubellus (Trematoda): Histopathology. Journal of Invertebrate Pathology 21: 183–190. [DOI] [PubMed] [Google Scholar]
- 103. Djordjevic SP, Forbes WA, Smith LA, Hornitzky MA (2000) Genetic and biochemical diversity among isolates of Paenibacillus alvei cultured from Australian honeybee (Apis mellifera) colonies. Appl Environ Microbiol 66: 1098–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Adema CM, Bayne CJ, Bridger JM, Knight M, Loker ES, et al. (2012) Will all scientists working on snails and the diseases they transmit please stand up? [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sequence accession numbers of the species used for the phylogenetic analysis. The table provides the GenBank accession number of nucleotide sequences from 16S rDNA and RpoB genes and Rpob protein sequence.
(DOCX)
Individual cells and clump of bacteria interconnected by long strings forming some hyphae like structure were observed. The arrow shows an interconnection between two bacteria clumps.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.