Abstract
This work reports the functional analysis of Saccharomyces cerevisiae open reading frame YIL121w, encoding a member of a family of drug:H+ antiporters with 12 predicted membrane-spanning segments (DHA12 family). Like its close homologue Qdr1p, Yil121wp was localized at the plasma membrane, and its increased expression also led to increased tolerance to the antiarrhythmia and antimalarial drug quinidine. The quinidine resistance phenotype was confirmed for different yeast strains and growth media, including a prototrophic strain, and YIL121w was named the QDR2 gene. Both QDR1 and QDR2 were also implicated in yeast resistance to the herbicide barban (4-chloro-2-butynyl [3-chlorophenyl] carbamate), and the genes are functionally interchangeable with respect to both resistance phenotypes. The average intracellular pH of a yeast population challenged with quinidine added to the acidic growth medium was significantly below the intracellular pH of the unstressed population, suggesting plasma membrane permeabilization by quinidine with consequent increase of the H+ influx rate. For the same extracellular quinidine concentration, internal acidification was more intense for the Δqdr2 deletant compared with the parental strain. Although QDR2 transcription was not enhanced in response to quinidine, the results confirmed that Qdr2p is involved in the active export of quinidine out of the cell, thus conferring resistance to the drug.
The therapeutic potential of drugs is seriously limited by the manifestation of cellular drug resistance (11). The yeast Saccharomyces cerevisiae is an easy-to-manipulate experimental eukaryotic model useful to unveil novel determinants and mechanisms underlying the apparently conserved multidrug resistance (MDR) phenomenon in more complex and less genetically accessible eukaryotes. The present knowledge on the poorly characterized families of putative yeast drug:H+ antiporters belonging to the major facilitator superfamily (MFS) was driven by postgenomic yeast research (21). The great majority of the approximately 23 yeast genes encoding proteins of the MFS (15, 18, 21), putatively or proven to be involved in multidrug resistance, escaped characterization before the release of the genome sequence. These proteins comprise 12 and 14 predicted membrane-spanning segments and were included in the so-called DHA12 and DHA14 drug efflux families (18).
In the present work, we carried out a functional analysis of S. cerevisiae open reading frame (ORF) YIL121w, encoding a member of the DHA12 family and a close homologue to the previously characterized QDR1 gene (ORF YIL120w), required for yeast resistance to quinidine and ketoconazole (17). Qdr1p and Yil121wp, with 64% sequence identity, belong to the same cluster I in the classification of Nelissen (15), who distinguished two clusters in the DHA12 drug efflux family. Like Qdr1p, Yil121wp was localized at the plasma membrane, and its increased expression led to increased tolerance to quinidine. ORF YIL121w was thus named the QDR2 gene. Other proteins of the same cluster I of the DHA12 family confer resistance to quinidine, specifically AQR1 (YNL065w) (26) and DTR1 (YBR180w) (5). Although DTR1 was proved to be an MDR determinant, a biological role in spore wall synthesis was attributed to this transporter because it facilitated the transport of bisformyl dityrosine through the prospore membrane (5).
Two other proteins belonging to cluster II of the DHA12 family and encoded by the TPO1/YLL028w and TPO4/YOR273c genes (3, 4) are additional quinidine determinants, being required for yeast tolerance to polyamines. In the course of this work, both QDR1 and QDR2 were found to be involved in yeast resistance to the selective postemergence herbicide barban (4-chloro-2-butynyl [3-chlorophenyl] carbamate). As with Qdr1p, Qdr2p could not alleviate the deleterious effects of quinine, a stereoisomer of quinidine (17), and its deletion led to a slight decrease of ketoconazole tolerance.
The quinoline ring-containing drug quinidine is used as an antiarrhythmic and antimalarial drug (10, 23, 28). Although S. cerevisiae adaptation to growth in the presence of quinidine involves induction of the active efflux of quinidine out of the cell, clear experimental evidence supporting the direct involvement of Qdr1p in this inducible export were not obtained before (17). Similarly, QDR2 transcription was not enhanced in response to quinidine or to barban despite its role in conferring resistance to these compounds. Nevertheless, Qdr2p expression was implicated in the active export of quinidine out of the cell, even though this drug is not present in the natural S. cerevisiae environment.
MATERIALS AND METHODS
Strains, growth media, and general methods.
The Sacharomyces cerevisiae strains W303.1b (MATα ura3-1 leu2-3,112 his3-11,15,15 trp1-1 ade1-2 can1-100), extensively used in our laboratory to assess drug susceptibility phenotypes (2, 17, 26); W303.1bΔyil121w (MATα ura3-1 leu2-3,112 his3-11,15,15 trp1-1 ade1-2 can1-100 YIL121w::kanMX4) (this work); BY4741 (MATa ura3Δ0 leu2Δ0 his3Δ1 met15Δ0) (Euroscarf collection); BY4741Δyil121w (MATa ura3Δ0 leu2Δ0 his3Δ1 met15Δ0 YIL121w::kanMX4) (Euroscarf collection); and 23344c (MATα ura3) (14), kindly provided by B. André (Université Libre de Bruxelles, Brussels, Belgium), were used in this work.
Cells were batch-cultured at 30°C with orbital agitation (250 rpm) in differently supplemented basal growth media with the following composition (per liter): 1.7 g of yeast nitrogen base without amino acids or NH4+ (Difco), 20 g of glucose, and 2.65 g of (NH4)2SO4. For strain BY4741, growth medium MM4 (basal medium supplemented with 20 mg of methionine, 20 mg of histidine, 60 mg of leucine, and 20 mg of uracil [all from Sigma]) was used. For strain W303.1b, growth medium MM2 (basal medium supplemented with 80 mg of adenine, 10 mg of histidine, 10 mg of leucine, 20 mg of tryptophan, and 20 mg of uracil) was used. For strain 23344c, the growth medium MM5 (basal medium supplemented with 20 mg of uracil) was used. Agar-treated solid media contained 20 g of agar (Iberagar) per liter.
Escherichia coli strain XL1-blue and the growth medium used were described before (2). Cloning procedures were carried out by standard methods (22). Transformation of S. cerevisiae cells was performed by the method of Gietz (7).
Disruption and cloning of ORF YIL121w.
Disruption of ORF YIL121w in S. cerevisiae FY73 was performed with a disruption cassette, consisting of the dominant resistance marker loxP-kanMX4-loxP flanked by short flanking homology regions to the target ORF. This was prepared by DNA amplification by PCR with plasmid pUG6 (9, 27) as a template and the following primers: 5′-ATGGCAGGAGCAACATCAAGTATAATTCGGGAAAATGATTTTGAGGACGCGGCCGCTTCGTACGCTGCAGGTCGAC-3′ and 5′-AGCGATCAAAAGGAACATTTTCCTTTGATTCAAGAAGCTTTACTTGCGGCCGCGCATAGGCCACTAGTGGATCTG-3′, where the sequence complementary to pUG6 is italic. These primers included 48 and 45 nucleotides homologous to the flanking region of the ORF at the 5′ end, followed by the NotI site, and 20 and 22 nucleotides homologous to pUG6 at the 3′ end. The PCR product of 1,690 bp, generated with the amplification conditions described before (2), was purified and used to transform strain FY73. Transformants were selected on YPD plates with 200 mg of geneticin per liter, and the correct replacement of the gene was confirmed by two independent PCRs (2). A replacement cassette was prepared to be used for the systematic inactivation of ORF YIL121w in any S. cerevisiae strain, in particular strain W303.1b, by creating longer homologous sequences on both sides of the loxP-kanMX4-loxP module. This YIL121w replacement cassette was obtained by PCR amplification with Pwo and genomic DNA isolated from the FY73Δyil121w deletant strain and the following primers: 5′-GGGGTACCCCCTGTCATTTTGGATTCAGTC-3′ and 5′-GCGGCATGCGTCTCTTCATGAGCTATCTG-3′, designed to be located 765 and 872 bp upstream and downstream of the start and stop codons, respectively.
ORF YIL121w was cloned by the gap repair technique (20) in the SphI site of pFL38, giving rise to plasmid pYCG_YIL121w, as described before (2).
Drug susceptibility assays.
The drug susceptibility of the various parental strains and the deletion mutants was compared by spot assays, as described before (2). Whenever a consistent phenotype was detected, these tests were followed by complementation studies. Cells were grown on minimal (with or without uracil for plasmid maintenance) agar plates supplemented with suitable concentrations of the different compounds. The cell suspensions used to inoculate the agar plates were mid-exponential-phase cells, prepared by cell cultivation in the same liquid medium until the standardized culture optimal density at 600 nm (OD600) of 0.2 ± 0.02 was reached, followed by dilution to a standardized OD600 of 0.05. These cell suspensions and subsequent dilutions (1:5 and 1:10) were applied as spots (4 μl) onto the surface of the agar medium supplemented with adequate concentrations of the compound. The range of drug concentrations tested in agar plates were 0.5 to 1.5 μg of nistatine per ml; 100 to 160 μg of ethidium bromide per ml; 100 to 250 μg of rodamine 6G per ml; 3.5 to 4.5 μg of amphotericin B per ml; 250 to 700 μg of menadione per ml; 100 to 400 μg of spermine per ml; 0.15 to 0.20 μg of cycloheximide per ml; 15 to 20 μg of benomyl per ml; 10 to 15 μg of methotrexate per ml; and 0.15 to 0.25 μg of 4-nitroquinoline oxide per ml (all obtained from Sigma), and the stock was dissolved in dimethyl sulfoxide (DMSO; Sigma); 7 to 10 μg of fluconazole (diflucan, in saline solution) per ml; 1 to 3 μg of itraconazole per ml; and 2 to 5 μg of ketoconazole per ml, kindly supplied by Janssen Research Foundation and dissolved in DMSO; 0.03 to 0.05 μg of miconazole (Sigma, stock solution in DMSO) per ml; 0.07 to 0.1 mM barban (Riedel-de-Haën, stock solution in acetone), 1.0 to 1.5 g of quinine per liter and 2.5 to 4.2 g of quinidine (sulfate salt dehydrate) per liter, both obtained from Sigma and dissolved in 70% ethanol. DMSO and ethanol concentrations in the growth media (including the control medium lacking the growth inhibitors) were kept below 0.1% (wt/vol) or 1.4% (vol/vol), respectively, to avoid growth inhibition due to the solvents. Plates were incubated at 30°C for 3 to 8 days, depending on the severity of growth inhibition.
Susceptibility assays were also carried out by comparing the growth curves in liquid minimal medium, supplemented with the drugs, at 30°C with orbital agitation (250 rpm) by measuring culture OD600.
Subcellular localization of Yil121w-GFP fusion protein.
The subcellular localization of Yil121wp was based on the observation, by fluorescence microscopy, of the distribution of Yil121w-green fluorescent protein (GFP) fusion protein in S. cerevisiae living cells. The YIL121w-GFP fusion plasmid was prepared by using the multicopy expression vector pMET25_GFP, kindly provided by E. Boles (University of Düsseldorf) and the protocol described before (24).
The two primers used in the present work (5′-GATACATAGATACAATTCTATTACCCCCATCCATACTCTAGAAAATGGCAGGAGCAACATCAAG-3′ and 5′-GTGAAAAGTTCTTCTCCTTTACTCATACCAGCACCAGCGGCCGCATTTACTCCCAGTTCTTGCTTTT-3′ were designed to have the first 44 bp complementary to the end of the MET25 promoter (italic) followed by the first 20 bp of ORF YIL121w, starting at the ATG. The 3′ primer was designed to have the first 44 bp complementary to the beginning of the GFP gene (italic) followed by the last 23 bp of ORF YIL121w before the stop codon, which was not included.
Northern blot analysis.
The effect on YIL121w transcription of S. cerevisiae exposure to quinidine and barban was examined by Northern blot analysis. RNA extraction from S. cerevisiae cells and Northern experiments were carried out as described before (25). The total RNA in each sample used for Northern blotting was approximately constant, 20 μg (RNA quantification was based on the measurement of A260). The specific DNA probe for YIL121w transcripts was prepared by PCR amplification with the following primers: 5′-AGCTCCCATTCTTGTTTTGC-3′ and 5′-GCAACCTCGCCTTGAAGATAT-3′. This probe comprises 486 bp of the YIL121w coding region and shows no significant homology to the rest of the genome. The specificity of the probe was tested with RNA extracts of the Δyil121w deletion mutant, and ethidium bromide-stained rRNA was considered an internal control.
Assessment of intracellular pHs.
Intracellular pH (pHi) was estimated by fluorescence intensity measurements, as reported before (6). Yeast cells were harvested during growth in the absence or presence of quinidine by centrifugation, washed twice with CF buffer, consisting of 50 mM glycine, 10 mM NaCl, 5 mM KCl, and 1 mM MgCl2 in 40 mM Tris-100 mM morpholineethanesulfonic acid (MES, pH 5.8) and resuspended in 2 ml of CF buffer to an OD600 of 10; 4.0 μl of cFDA,SE (45 mM carboxyfluorescein diacetate succinimidyl ester in DMSO; C-1157, Molecular Probes) was added to the cell suspension for pHi staining. The mixture was vortexed in two bursts of 1 min each, interspersed with 15 min on ice. After being washed twice with cold CF buffer, CF-loaded cells were resuspended in 2 ml of CF buffer and examined immediately with a Zeiss Axioplan microscope equipped with adequate epifluorescence interference filters (Zeiss BP450-490 and Zeiss LP520).
Fluorescent emission was collected with a cooled charge-coupled device camera (Cool SNAPFX; Roper Scientific Photometrics). Bright-field images for determinations of pHi were obtained concurrently. Images were recorded at 1-min intervals, and each experiment was finished within 15 min. The images were stored on a computer with MetaMorph 3.5 and analyzed later. The fluorescence images were background corrected with dark-current images. The pHi values were calculated for a minimum of 80 cells/experiment. Individual cells were selected with regions of interest obtained from bright-field images recorded before or after the experiment. The value of fluorescence intensity emitted by each cell was obtained pixel by pixel in the region of interest. To estimate average pHi, an in vivo calibration curve was prepared with cell suspensions grown in the absence of toxic compounds, loaded with CF as described above, and incubated at 30°C with 0.5 mM carbonyl cyanide m-chlorophenylhydrazone (CCCP) to dissipate the plasma membrane pH gradient before adjustment of external pH (in the range of 3.0 to 7.0) by the addition of HCl or NaOH at 2 M.
Accumulation assays and energy-dependent efflux of [9-3H]quinidine.
To estimate the passive accumulation of quinidine (intracellular/extracellular [9-3H]quinidine) and its eventual active export (extracellular [9-3H]quinidine/OD600) from yeast cells, the parental strain BY4741 and the Δyil121w mutant were grown in MM4 medium and harvested in mid-exponential phase (at the standardized OD600 of 0.4 ± 0.1). The cells were washed and resuspended in TM buffer (0.1 M MES and 41 mM Tris adjusted to pH 5.5 with HCl) to obtain dense cell suspensions (OD600 of 5.0 ± 0.2, equivalent to approximately 2.2 mg ml−1). After 30 to 40 min of incubation at 30°C with agitation (150 rpm) to deenergize the cells, 0.1 μM [9-3H]quinidine (ICN; 37 MBq/ml) was added to the cell suspensions. Incubation proceeded for an additional period of 15 min, found to be enough for reaching equilibrium. During this period of incubation, the intracellular accumulation of labeled quinidine was monitored by filtering 200 μl of cell suspension, at adequate time intervals, through prewetted glass microfiber filters (Whatman GF/C). The filters were washed with ice-cold TM, and the radioactivity was measured in a Beckman LS 5000TD scintillation counter. Extracellular [9-3H]quinidine was estimated, by radioactivity assessment of 100 μl of the supernatant.
The eventual active efflux of the labeled quinidine, accumulated beforehand, was examined by transferring the preloaded cells to TM buffer supplemented or not with 20 g of glucose per liter. Extracellular [9-3H]quinidine was estimated by radioactivity assessment of 100 μl of the supernatant during 10 min of incubation at 30°C. Nonspecific [9-3H]quinidine adsorption to the filters and to the cells (less than 5% of the total radioactivity) was assessed and taken into consideration. To calculate the intracellular concentration of labeled quinidine, the internal cell volume (Vi) of the exponential-phase cells grown in the absence of drug and used for accumulation assays was considered constant and equal to 2.5 μl (mg [dry weight])−1 (19).
RESULTS
ORF YIL121w is a determinant of S. cerevisiae resistance to quinidine and barban.
The comparison of the susceptibility to different drugs of cells of S. cerevisiae W303.1b and the mutant deleted for ORF YIL121w, harboring either recombinant plasmid pYCG_YIL121w, with ORF YIL121w inserted into pFL38, or the the cloning vector as a control, was performed by spot assays in agar minimal medium MM2 without uracil for plasmid maintenance and supplemented with inhibitory concentrations of several compounds. The results clearly indicate the involvement of ORF YIL121w in S. cerevisiae resistance to quinidine (Fig. 1) but not its stereoisomer quinine. Also, as observed with its close homologue QDR1 (17), QDR2 expression confers a slight increase in S. cerevisiae resistance to ketoconazole (Fig. 1). ORF YIL121w was thus named QDR2, for quinidine resistance.
FIG. 1.
ORF YIL121w is a determinant of yeast resistance to quinidine and provides slight protection against ketoconazole. The susceptibility to the drugs, at the indicated concentrations, of the S. cerevisiae W303.1b parental strain and the deletion mutant Δyil121w harboring either recombinant plasmid pYCG_YIL121w, with ORF YIL121w inserted into pFL38, or the cloning vector was compared by spot assays in MM2 (without uracil) agar plates. The cell suspensions used to prepare the spots in b and c were 1:2 and 1:4 dilutions of the cell suspension used in a, respectively.
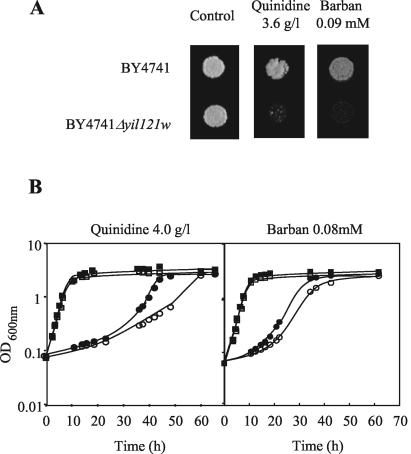
The phenotype associated with QDR2 expression was confirmed in another genetic background (strain BY4741, from which the Euroscarf collection of deletion mutants was derived), in both solid and liquid MM4 medium supplemented with 4.0 g of quinidine per liter (Fig. 2). The comparison of the growth curves of parental strain and Δqdr2 mutant under this quinidine stress indicated that QDR2 expression implicates the alteration in the level of inhibition of the specific growth rate, from 0.029 to 0.072 h−1 (Fig. 2B).
FIG. 2.
Comparison of the susceptibility to quinidine and barban of the S. cerevisiae BY4741 parental strain (▪, •) and the deletion mutant Δyil121w (□, ○), (A) by spot assays (3.6 g of quinidine per liter or 0.09 mM barban) or (B) by cultivation in MM4 liquid medium (▪, □) or in this medium supplemented with 4.0 g of quinidine per liter or 0.08 mM barban (•, ○). Cell suspensions used as inocula were exponential-phase cells cultivated in the absence of chemical stress.
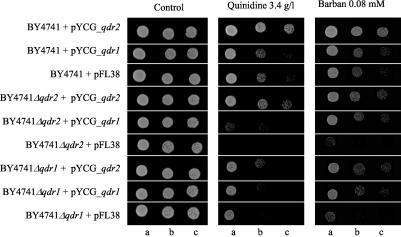
No clear evidence was obtained for the involvement of Qdr2p in S. cerevisiae resistance to the other azoles tested (itraconazole, miconazole, and fluconazole) or to menadione, spermine, benomyl, nistatine, amphotericin B, rodamine 6G, methotrexate, ethidium bromide, 4-nitroquinoline-N-oxide, and cycloheximide (results not shown). However, QDR2 was proved to be a determinant of S. cerevisiae resistance to the herbicide barban (4-chloro-2-butynyl [3-chlorophenyl] carbamate), by both spot assays and growth in liquid medium supplemented with this carbinilate (Fig. 2 and 3). Qdr1p also confers resistance to barban (Fig. 3). Remarkably, although in the W303.1b background and MM2 growth medium, the effect of QDR1 expression on quinidine resistance was significant (17) and stronger than the effect of QDR2 expression, in the BY4741 background and MM4 medium, the effect of QDR1 on quinidine resistance was very slight and only observed for highly deleterious drug concentrations (result not shown). Interestingly, QDR1 expression is capable of increasing the tolerance of the Δqdr2 mutant to both quinidine and barban (Fig. 3). Identically, QDR2 expression partially complements the referred Δqdr1 susceptibility phenotypes (Fig. 3).
FIG. 3.
Comparison of the susceptibility to barban and quinidine, assessed by spot assays in MM4-U medium, of the S. cerevisiae BY4741 parent strain and the deletion mutants Δqdr1 and Δqdr2 harboring either recombinant plasmid pYCG_YIL120w or pYCG_YIL121w, which have the QDR1 and QDR2 gene inserted into pFL38, respectively, or the cloning vector. The cell suspensions used to prepare the spots in b and c were 1:2 and 1:4 dilutions of the cell suspension used in a, respectively.
In the light of the results of Bauer et al. (1) and of the significant differences observed in the effect of QDR1 and QDR2 on S. cerevisiae resistance to quinidine depending on the S. cerevisiae strain and growth medium used, it could be speculated if the phenotypes observed for QDR2 with the two strains examined, with specific auxotrophies for some amino acids, are maintained in a prototrophic background. However, the phenotypes previously observed for quinidine and barban were confirmed by increasing the expression of QDR2 from plasmid pYCG_QDR2 in the prototrophic strain 23344c, grown in a medium without amino acid supplementation (Fig. 4).
FIG. 4.
Comparison of the susceptibility, in minimal medium MM5, to 2.5 g of quinidine per liter or 0.09 mM barban of the prototrophic strain 23344c harboring either recombinant plasmid pYCG_QDR2, with the QDR2 gene inserted into pFL38, or the cloning vector. The cell suspensions used to prepare the spots in b and c were 1:2 and 1:4 dilutions of the cell suspension used in a, respectively.
QDR2 transcription is not stimulated by the presence of quinidine or barban.
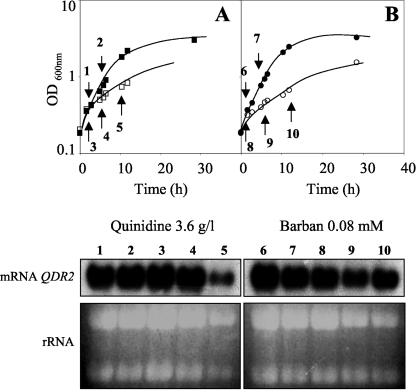
In view of the results indicating that Qdr2p is required for S. cerevisiae resistance to quinidine and barban, we compared QDR2 transcription levels in cells harvested during the exponential phase of growth with inhibitory concentrations of these drugs and in unstressed cells cultivated in the same basal medium. However, QDR2 mRNA levels in exponential-phase cells grown with these chemical stress agents were not above those detected in unstressed cells (Fig. 5A and B).
FIG. 5.
Northern blot hybridization experiments with a DNA fragment of QDR2 as the probe. Ethidium bromide-stained rRNA was used as a loading control. Total RNA was extracted from (A) cells of BY4741 grown in MM4 medium (with 1.4% ethanol) (1 and 2) or in this medium supplemented with 3.6 g of quinidine per liter (3 to 5) or (B) cells of BY4741 grown in MM4 medium (with 0.2% acetone), in the absence (6 and 7) or presence of 0.08 mM of barban (8 to 10).
Localization of Qdr2p-GFP fusion protein in the plasma membrane.
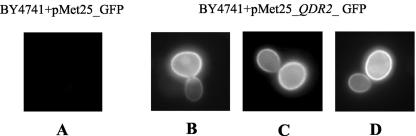
The fluorescence of exponential-phase cells of S. cerevisiae BY4741 expressing Qdr2-GFP fusion protein from plasmid pMET25_YIL121w_GFP was essentially localized to the cell periphery, while control cells expressing GFP alone from plasmid pMET25_GFP exhibited a slight and uniform distribution of green fluorescence throughout the cell, similar to the autofluorescence of the host cells (Fig. 6). The strong ring-like fluorescence staining around the cell was observed for the majority of the cells from an exponential-phase culture of transformants expressing Qdr2p-GFP protein (Fig. 6B, C, and D) but was absent from the control cells (Fig. 6A). As observed before (24), some heterogeneity in the signal intensity in the periphery of cells expressing Qdr2p-GFP protein was detected, possibly due to plasmid copy number differences. Since QDR2 was predicted to code for an integral membrane protein (15), these results strongly suggest that Qdr2p is a plasma membrane protein.
FIG. 6.
Fluorescence of exponential-phase cells of S. cerevisiae BY4741 harboring (A) expression vector pMET25_GFP (control cells) or the multicopy plasmid pMET25_QDR2-GFP (B, C, and D), indicating that the Qdr2-Gfp fusion protein is localized at the plasma membrane.
QDR2 expression reduces the level of intracellular acidification induced by quinidine supplementation of the acidic growth medium.
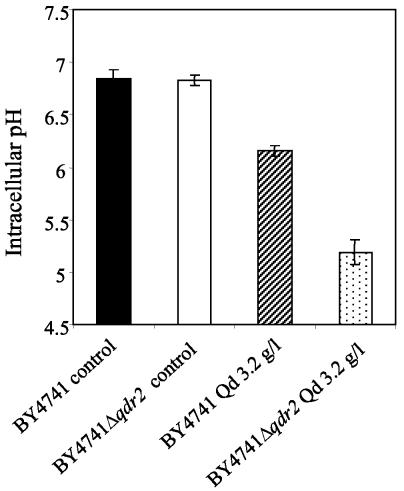
Even though the accumulation of the weak base quinidine into the S. cerevisiae cells is more compatible with the alkalinization of the cell interior, quinidine supplementation of MM4 medium at pH 5.5 led to intracellular acidification. Indeed, the average pHi values for the quinidine-stressed cell populations of BY4741 and BY4741Δqdr2 grown to the mid-exponential phase were significantly below the pHi value of the unstressed populations. The pHi for both unstressed populations was estimated by a fluorescence microscopic image processing technique to be around neutrality (pHi of 6.8) (Fig. 7). The lowest pHi was estimated for the quinidine-stressed Δqdr2 population (pHi of 5.2) compared to the wild-type population cultivated in the presence of an identical concentration of quinidine (pHi of 6.2). Since the quinidine-supplemented growth medium is acidic (pH 5.5), these results strongly suggest that the drug may lead to plasma membrane permeabilization with a consequent increase in the H+ influx rate, the internal acidification being more intense for the S. cerevisiae population not expressing a functional QDR2 gene.
FIG. 7.
Comparison of the average pHi of cell populations of the BY4741 parental strain (solid and striped symbols) or the Δqdr2 mutant (open and dotted symbols) grown to the exponential phase (at the standardized culture OD600 of 0.6) in the presence (striped and dotted symbols) or the absence (solid and open symbols) of 3.2 g of quinidine (qd) per liter.
QDR2 is involved in the active efflux of [9-3H]quinidine from preloaded cells.
Following Qdr2p localization at the plasma membrane, we examined its possible involvement in the reduction of quinidine accumulation in energized cells by active export of the drug out of the cell. To compare the active export of labeled quinidine from preloaded cells, assays were performed with cells of the parental strain S. cerevisiae BY4741 and the Δqdr2 deletion mutant harvested in the mid-exponential phase of growth in the absence of the drug. The decision to use these cells instead of cells adapted to the drug was based on the fact that considerable differences were registered between the average pHi of the parental strain and that of the deletion mutant Δqdr2 cell populations grown under quinidine stress (Fig. 7). The observed differences would lead to differential passive accumulation of the radiolabeled drug into the more acidic interior of Δqdr2 cells merely by a consequence of the weak-base properties of the drug.
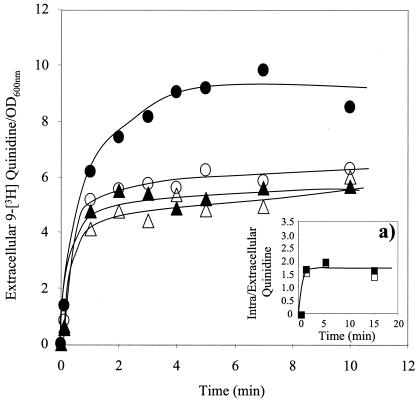
When deenergized yeast cells were incubated with [9-3H]quinidine for 15 min in buffer (at pH 5.5), the passive uptake of the labeled drug into the yeast cells was extremely rapid, as shown in Fig. 8a); the accumulation ratio reached a value of 1.7 times the concentration in the surrounding medium after 1 min of incubation. No significant differences in the accumulation ratio were detected for wild-type and Δqdr2 cells with no previous exposure to quinidine.
FIG. 8.
Time course of the expulsion of [9-3H]quinidine accumulated beforehand in cells of BY4741 (▴, •) or the Δqdr2 mutant (▵, ○) (as shown in a) after transfer to TM buffer (pH 5.5) (▴, ▵) or to the same buffer supplemented with glucose (•, ○). Extracellular quinidine concentrations are average values from at least two independent transport assays with a standard deviation below 0.8. In the inset(a) is shown the time course of [9-3H]quinidine accumulation in deenergized cells of parental strain BY4741 (▪) and deletion mutant Δqdr2 (□) grown in the absence of quinidine and resuspended in TM buffer without glucose. The values shown in the inset are also average values for at least two independent experiments used to obtain a standard deviation below 0.4.
Following the resuspension and incubation of the yeast cells previously loaded with the labeled quinidine in TM buffer (pH 5.5) supplemented or not with 20 g of glucose per liter, efflux of the drug occurred (Fig. 8). The drug concentrations in the external medium of the parental strain and the Δqdr2 mutant support the idea that Qdr2p is involved in the active export of the labeled drug. Indeed, in the presence of glucose, the quinidine-exporting activity of preloaded cells of the wild type was significantly above that of the mutant, while in the absence of glucose, the differences observed were negligible (Fig. 8). The transmembrane proton gradient imposed during the assays with an external medium pH of 5.5 is expected to increase as a result of the in vivo activation by glucose of plasma membrane H+-ATPase, which is consistent with the observed increase of quinidine export mediated by the putative drug antiporter Qdr2p in glucose-supplemented buffer.
DISCUSSION
As predicted by structural considerations (15), the ORF YIL121w/QDR2 gene confers resistance to structurally dissimilar hydrophobic growth-inhibitory compounds, in particular quinidine. Also, in agreement with the conclusions of two previous studies on the global analysis of protein localization in S. cerevisiae (12, 13), Qdr2p was localized at the plasma membrane. To expand our understanding of the mechanisms of action and resistance associated with quinidine, we examined its effects in S. cerevisiae as a model eukaryotic system, using cells expressing different levels of QDR2. As first observed (17), the uptake of labeled quinidine into the yeast cell was extremely rapid. At the acidic incubation medium pH (5.5), the weak base quinidine, with a high partition coefficient of octanol-water (logP of 2.79 to 2.84) and the two protonation sites with a pKa1 of 8.58 and a pKa2 of 4.42 (28), has a calculated distribution coefficient between the lipid and the aqueous phases (based on the equation proposed earlier [28]) of 0.47 to 0.53. The anticipated disturbing effect exerted by this hydrophobic drug on plasma membrane spatial organization and the consequent increase in H+ passive influx are in agreement with the observed internal acidification resulting from the addition of quinidine to the characteristic acidic growth medium used for fungi growth.
Consistent with the damage caused by quinidine in the yeast plasma membrane suggested by internal acidification, the antimalarial drug chloroquine, containing the same quinoline ring present in quinidine, also has a negative effect on Plasmodium membrane stability, the protonated form putatively interacting with the various phospholipids by both hydrophobic and electrostatic forces (8). The average pHi of the yeast cell population under stressed exponential-phase growth in the presence of 3.2 g of quinidine per liter was estimated to be 6.2 or 5.2, depending on the presence of a functional QDR2 gene. This is consistent with a role of QDR2 in protection against the deleterious effects of quinidine. The proper functioning of yeast cells relies on maintenance of their intracellular pH within relatively narrow limits, as large deviations from the normal neutral pH severely inhibit metabolism as the result of suboptimal activities of cytosolic enzymes. Additionally, maintenance of the transmembrane proton gradient is crucial to the secondary active transport of nutrients across the plasma membrane.
The resistance phenotypes of QDR2 are similar to those observed with the close homologue QDR1. In particular, both genes confer resistance to quinidine and barban in S. cerevisiae and are functionally interchangeable. Indeed, each gene is capable of complementing, at least partially, the susceptibility phenotype resulting from deletion of the other gene, as assessed by spot assays. However, while QDR1 expression is essential during the period of adaptation of yeast cells to sudden exposure to the drug, having apparently no effect during quinidine-inhibited exponential-phase growth (17), QDR2 function in protecting the cells from the drug was also observed in quinidine-adapted exponential-phase cells.
Remarkably, at least three other members of the DHA12 family of S. cerevisiae H+-drug antiporters belonging to the same cluster I or to cluster II are required for quinidine resistance. The presence of all these transporters in providing resistance to quinidine in yeast cells, together with the fact that neither of the compounds to which QDR2 and QDR1 confer resistance induces the expression of these genes, suggests that their physiological function may have nothing to do with broad chemoprotection but rather with the transport of a still unidentified specific substrate. This is apparently the case of the DHA12 multidrug resistance transporter DTR1, whose primary function appears to be the translocation of bisformyl dityrosine through the prospore membrane, thus playing an essential role in spore wall maturation (5). This raises the possibility that, as hypothesized before (16), many MDR transporters may have been selected for specific purposes but also transport drugs and other hydrophobic compounds that can be accommodated in their binding sites merely opportunistically.
Based on this hypothesis, MDR transporters can be used by the cell for broad chemoprotection, presumably because these transporters have never been selected not to transport molecules that are not usually present in their natural environment (16). Although the expression of Qdr2p, Qdr1p, or other MFS transporters confers resistance (at least) to quinidine in the yeast cell, their expression patterns differ significantly and are also distinct from the expression pattern described for DTR1 (5). This is suggested by our more recent results and by the published expression datasets provided by the on-line Yeast Microarray Global Viewer database (http://www.transcriptome.ens.fr/ymgv.html). Although the present study still leaves a number of open questions concerning the biological role of QDR2, experimental evidence strongly suggests its involvement in active export of quinidine out of the cell. Recent structural studies of MDR transporters suggest that they possess large hydrophobic binding sites that may bind to substrates through a combination of a hydrophobic effect and electrostatic attraction, this principle of substrate recognition being compatible with their remarkably broad specificity (16).
Acknowledgments
This research was supported by FEDER and Fundação para a Ciência e a Tecnologia contracts POCTI/BIO/38115/2001 and POCTI/BME/46526/2002 and Ph.D. grants to R. C. Vargas (BD/924/00) and M. C. Teixeira (BD/21650/99) and postdoctoral grants to S. Tenreiro (BPD/5649/01) and A. R. Fernandes (BPD/1541/00)).
REFERENCES
- 1.Bauer, B. E., D. Rossington, M. Mollapour, Y. Mamnun, K. Kuchler, and P. W. Piper. 2003. Weak organic acid stress inhibits aromatic amino acid uptake in yeast, causing a strong influence of amino acid auxotrophies in phenotypes of membrane transporter mutants. Eur. J. Biochem. 270:3189-3195. [DOI] [PubMed] [Google Scholar]
- 2.Brôco, N., S. Tenreiro, C. A. Viegas, and I. Sá-Correia. 1999. FLR1 gene (ORF YBR008c) is required for benomyl and methotrexate resistance in Saccharomyces cerevisiae and its benomyl-induced expression is dependent on Pdr3 transcriptional regulator. Yeast 15:1595-1608. [DOI] [PubMed] [Google Scholar]
- 3.Delling, U., M. Raymond, and E. Schurr. 1998. Identification of Saccharomyces cerevisiae genes conferring resistance to quinoline ring-containing antimalarial drugs. Antimicrob. Agents Chemother. 42:1034-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.do Valle Matta, M. A., J. L. Jonniaux, E. Balzi, A. Goffeau, and B. van den Hazel. 2001. Novel target genes of the yeast regulator Pdr1p: a contribution of the TPO1 gene in resistance to quinidine and other drugs. Gene. 272:111-119. [DOI] [PubMed] [Google Scholar]
- 5.Felder, T., E. Bogengruber, S. Tenreiro, A. Ellinger, I. Sá-Correia, and P. Briza. 2002. Dtr1p, a multidrug resistance transporter of the Major Facilitator Superfamily, plays an essential role in spore wall maturation in Saccharomyces cerevisiae. Eukaryot. Cell 1:799-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernandes, A. R., P. J. Durão, P. M. Santos, and I. Sá-Correia. 2003. Activation and significance of vacuolar H+-ATPase in Saccharomyces cerevisiae adaptation and resistance to the herbicide 2,4-dichlorophenoxyacetic acid (2,4-D). Biochem. Biophys. Res. Commun. 312:1317-1324. [DOI] [PubMed] [Google Scholar]
- 7.Gietz, D., A. St Jean, R. A. Woods, and R. H. Schiestl. 1992. Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res. 20:1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ginsburg, H., and T. G. Geary. 1987. Current concepts and new ideas on the mechanism of action of quinoline-containing antimalarials. Biochem. Pharmacol. 36:1567-1576. [DOI] [PubMed] [Google Scholar]
- 9.Güldener, U., S. Heccks, T. Fiedler, J. Beinhauer, and J. Hegemann. 1996. A new efficient gene disruption cassette for repeated use in budding yeast. Nucleic Acids Res. 24:2519-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hawley, S. R., P. G. Bray, M. Mungthin, J. D. Atkinson, P. M. O'Neill, and S. A. Ward. 1998. Relationship between antimalarial drug activity, accumulation and inhibition of heme polymerization in Plasmodium falciparum in vitro. Antimicrob. Agents Chemother. 42:682-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayes, J. D., and C. R. Wolf. 1997. Molecular genetics of drug resistance (Modern Genetics, vol. 3). Harwood Academic Publishers, London, United Kingdom.
- 12.Huh, W.-K., J. V. Falvo, L C. Gerke, A. S. Carroll, R. W. Howson, J. S. Weissman, and E. K. O′Shea. 2003. Global analysis of protein localization in bidding yeast. Nature 425:686-691. [DOI] [PubMed] [Google Scholar]
- 13.Kumar, A., S. Agarwal, J. A. Heyman, S. Matson, M. Heidtman, S. Piccirillo, L. Umansky, A. David, R. Jansen, Y. Liu, K.-H. Cheung, P. Miller, M. Gerstein, G. S. Roeder, and M. Snyder 2002. Subcellular localization of the yeast proteome. Genes Dev. 16:707-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marini, A., S. Soussi-Boudekou, S. Vissers, and B. André. 1997. A family of ammonium transporters in Saccharomyces cerevisiae. Mol. Cell. Biol. 17:4282-4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nelissen, B., R. De Wachter, and A. Goffeau. 1997. Classification of all putative permeases and other membrane plurispanners of the major facilitator superfamily encoded by the complete genome of Saccharomyces cerevisiae. FEMS Microbiol. Rev. 21:113-134. [DOI] [PubMed] [Google Scholar]
- 16.Neyfakh, A. A. 2002. Mystery of multidrug transporters: the answer can be simple. Mol. Microbiol. 44:1123-1130. [DOI] [PubMed] [Google Scholar]
- 17.Nunes, P. A., S. Tenreiro, and I. Sá-Correia 2001. Resistance and adaptation to quinidine in Saccharomyces cerevisiae: role of QDR1 (YIL120w), encoding a plasma membrane transporter of the major facilitator superfamily required for multidrug resistance. Antimicrob. Agents Chemother. 45:1528-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paulsen, I. T., M. K. Sliminski, B. Nelissen, A. Goffeau, and M. R. Saier Jr. 1998. Unified inventory of established and putative transporters encoded within the complete genome of Saccharomyces cerevisiae. FEBS Lett. 430:116-125. [DOI] [PubMed] [Google Scholar]
- 19.Rosa, M. F., and I. Sá-Correia 1996. Intracellular acidification does not account for inhibition of Saccharomyces cerevisiae growth in the presence of ethanol. FEMS Microbiol. Lett. 135:271-274. [DOI] [PubMed] [Google Scholar]
- 20.Rothstein, R. 1991. Targeting, disruption, replacement, and allele rescue: integrative DNA transformation in yeast. Methods Enzymol. 194:281-301. [DOI] [PubMed] [Google Scholar]
- 21.Sá-Correia, I., and S. Tenreiro. 2002. The multidrug-resistance transporters of the major facilitator superfamily, five years after disclosure of Saccharomyces cerevisiae genome sequence. Biotech. J. 98:215-226. [DOI] [PubMed] [Google Scholar]
- 22.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 23.Snyders, D. J., and S. W. Yeola. 1995. Determinants of Antiarrythmic drug action. Electrostatic and hydrophobic components of block of the human cardiac hKv1.5 channel. Circ. Res. 77:575-583. [DOI] [PubMed] [Google Scholar]
- 24.Tenreiro, S., P. C. Rosa, C. A. Viegas, and I. Sá-Correia. 2000. Expression of the AZR1 gene (ORF YGR224w), encoding a plasma membrane transporter of the major facilitator superfamily, is required for adaptation to acetic acid and resistance to azoles in Saccharomyces cerevisiae. Yeast 16:1469-1481. [DOI] [PubMed] [Google Scholar]
- 25.Tenreiro, S., A. R. Fernandes, and I. Sá-Correia. 2001. Transcriptional activation of FLR1 gene during Saccharomyces cerevisiae adaptation to growth in benomyl: role of Yap1p and Pdr3p. Biochem. Biophys. Res. Commun. 280:216-222. [DOI] [PubMed] [Google Scholar]
- 26.Tenreiro, S., P. A. Nunes, C. A. Viegas, M. S. Neves, M. C. Teixeira, M. G. Cabral, and I. Sá-Correia. 2002. AQR1 gene (ORF YNL065w) encodes a plasma membrane transporter of the major facilitator superfamily that confers resistance to short-chain monocarboxylic acids and quinidine in Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 292:741-748. [DOI] [PubMed] [Google Scholar]
- 27.Wach, A., A. Brachat, R. Pohlmann, and P. Philippsen. 1994. New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast 10:1793-1808. [DOI] [PubMed] [Google Scholar]
- 28.Warhurst, D. C., J. C. Craig, I. S. Adagu, D. J. Meyer, and S. Y. Lee. 2003. The relationship of physicochemical properties and structure to the differential antiplasmodial activity of the cinchona alkaloids. Malar J. 2:26-40. [DOI] [PMC free article] [PubMed] [Google Scholar]