Abstract
BACKGROUND
The pathophysiology and time course of coagulopathy after major burns are inadequately understood. Our study objectives were to determine whether acute traumatic coagulopathy (ATC) is seen in burn patients on admission and to determine the changes in international normalized ratio (INR), activated partial thromboplastin time (aPTT), platelet count (PLT), and hemoglobin (Hgb) in the first 7 days after injury.
METHODS
We conducted a retrospective study of patients with at least 15% total body surface area (TBSA) burn who presented to the University of North Carolina. Data on patient demographics, injury characteristics, and laboratory data (INR, aPTT, PLT, and Hgb) on admission and within the first 7 days after injury were recorded. We defined ATC as INR ≥ 1.3, aPTT ≥ 1.5 times the mean normal limit, and normal PLT on admission.
RESULTS
We studied the hematologic profile of 102 patients with 15–100% TBSA burn, but did not identify a single patient with ATC on admission. The screening hematologic profile on admission was not influenced by burn severity. In the first 7 days after injury, the INR and aPTT were relatively preserved, while the PLT quickly recovered to baseline after an early decline and the Hgb remained stable at around 10 g/dL; all these changes occurred during the time when the burn patients had received large amounts of fluid resuscitation.
CONCLUSION
The screening hematologic profile of burn patients on admission is normal and the standard screening assays do not suggest the existence of ATC on admission. While this is a relatively small study, it provides evidence to suggest that ATC is unique to trauma patients.
LEVEL OF EVIDENCE
III, Prognostic and Epidemiological
Keywords: Acute traumatic coagulopathy, burn injury, trauma, coagulation disorder
Recent observational studies in trauma have described a characteristic coagulopathy referred to as acute traumatic coagulopathy (ATC). ATC is an endogenous impairment of hemostasis that begins at the moment of injury and is seen in 20–30% of severely injured trauma patients upon arrival to the emergency room prior to the onset of significant hypothermia, hemodilution or acidosis. ATC is associated with a 4- to 5-fold increase in mortality and transfusion requirement compared to patients without evidence of ATC. It is characterized by systemic anticoagulation and hyperfibrinolysis that manifests as a bleeding phenotype. Accompanying biomarkers include a prolonged prothrombin time (PT)/international normalized ratio (INR) and activated partial thromboplastin time (aPTT) with relative preservation of platelet count (PLT) and fibrinogen on screening coagulation assays.1–7
In the early post-burn period, the presence of an abnormal coagulation profile has been reported to be a risk factor for increased morbidity and mortality.8–9 Given that burn patients, who are sometimes considered a subset of trauma patients,10 have been inconsistently reported to manifest perturbed hemostasis early after injury, the purpose of this study was to evaluate whether a coagulopathy analogous to ATC exists after major burn injury. If present, we wished to determine whether affected burn victims experience excessive bleeding and/or excess mortality.
PATIENTS AND METHODS
Study design and objectives
At a single large volume American Burn Association (ABA)-verified Burn Center, we conducted a retrospective cohort study in which we evaluated the hematologic profile of burn patients on admission and for 7 days after injury. Patients were admitted between January 1, 2008 and December 31, 2009. Patient demographics such as age, gender, medical history, and injury characteristics were obtained from electronic medical records. Information regarding laboratory test results in addition to blood utilization and mortality were extracted also from electronic medical records.
The primary study objective was to determine whether a clinically important acute coagulopathy analogous to ATC exists in burn patients immediately or soon after injury, and if present, to determine its association with blood utilization and mortality within the first 7 days after injury. The secondary study objectives were (1) to determine the association between total body surface area (TBSA) burn and admission INR, aPTT, PLT, and hemoglobin (Hgb); (2) to determine any changes in INR, aPTT, PLT, and Hgb values from the time of admission until day 7 after injury; and (3) to estimate the effect of fluid resuscitation that is given to mitigate burn shock on the INR, aPTT, PLT, and Hgb at hospital day 2.
Patients
Management of burn patients is guided by well-defined principles of fluid resuscitation in an effort to mitigate burn shock and multi-system organ failure as dictated by the Parkland formula. Patients with large burns predictably develop burn shock that usually resolves within the first 48–72 hours after injury. It is during this time period that burn patients receive large amounts of fluid resuscitation.11–13 As part of resuscitation and acute management of burn injuries, many patients underwent escharatomies and fasciotomies to decompress areas in danger of developing compartment syndrome. These interventions were often done at bedside and did not require transfusion of blood products. In this study, we recorded surgical procedures performed in the first 7 days after injury that included debridement, excision, and grafting. For those patients that had these procedures performed, the administration of blood products, if any, was also recorded, since these patients may be at risk of excessive perioperative bleeding requiring blood transfusions.14–18
Eligibility criteria
Subjects included adult patients (≥ 18 years old) with 15–100% TBSA burn. All mechanisms of burn injury were included except electrical injury. Patients with and without coexisting inhalation injury were included, while patients with associated traumatic injuries who required medical attention, patients receiving therapeutic anticoagulation before injury, and patients with known bleeding disorders were excluded. Burns exceeding 15% TBSA were selected as the lower limit of burn size because burns of this magnitude are often associated with increased activation of inflammatory and coagulation mediators that may contribute to adverse clinical outcomes.8 Patients with elapsed time from injury of >12 hours to initial tests for INR, aPTT, and PLT were excluded. An interval of 12 hours was chosen as the cutoff to capture the earliest possible hematologic changes directly induced by burn injury, if any, and possibly reduce the iatrogenic effect of fluid resuscitation on these baseline laboratory tests. Patients who subsequently received therapeutic anticoagulation during the first 7 days were excluded due to possible effect of the medication on INR and/or aPTT. This study was conducted after approval of the Institutional Review Board of the University of North Carolina at Chapel Hill.
Standardization of aPTT test results
While the standard definition of ATC in trauma literature is lacking, an association between injury severity score, base deficit, and development of ATC has been observed. ATC presents as slightly prolonged screening coagulation times (INR and aPTT) with preservation of PLT and fibrinogen.4–7 It was proposed that an INR > 1.2 be adopted as part of its definition,6 but the degree of aPTT prolongation is yet to be defined. For the purpose of this study, we defined ATC as INR of ≥ 1.3, aPTT ≥ 1.5 times mean normal, and normal PLT (reference range: 150–440 × 109/L) on admission. As a major burn center, several patients were transferred from other medical facilities and many had laboratory tests that were performed prior to transfer to our institution. While the INR is comparable between laboratories, the aPTT is not, because of inter-laboratory variability of test reagents used to perform the test; hence, we expressed the aPTT as an aPTT ratio by dividing the patient’s actual aPTT result by the mean value of the reference range. In determining the hematologic profile of burn patients in the first 7 days after injury, we recorded the INR, aPTT, PLT, and Hgb values on a daily basis. For patients in whom laboratory studies were drawn more than once per day, we chose the first set of test results.
Statistical analysis
The admission laboratory tests were summarized as median with range or inter-percentile range due to skewness of the variables. The data distribution was shown by histograms and compared to the reference ranges of healthy population. To test an observed proportion of subjects against a pre-specified value, two-sided binomial tests against 95% were conducted using the proportion of burn patients whose laboratory test results fell within the reference range. Linear regression analyses were used to determine the association between admission laboratory data and TBSA with adjustment for time from injury to the initial laboratory test. The laboratory data for the first 7 days were plotted using boxplots to visualize the individual trends over time. Wilcoxon signed-rank test was used to compare the laboratory data on admission and those at day 2. The incidence of ATC on admission, i.e. the proportion of burn patients who developed the predefined abnormal laboratory tests within 12 hours after injury was compared to 20%, which is the incidence of ATC in trauma patients using a two-sided binomial test. Blood utilization was described as percentage of patients who received blood products and as total number of units transfused. P values smaller than 0.05 were considered statistically significant. Statistical analyses were implemented using R 2.14.0.19
RESULTS
Patient characteristics
A total of 102 patients with 15–100% TBSA burn were identified and grouped arbitrarily according to burn severity as 15–30%, 31–50%, 51–65%, and 66–100% TBSA burn. There were 89 patients with ≤ 65% TBSA burn and 13 patients with 66–100% TBSA burn. The majority of patients were younger than 65 years old (81.4%) and male (69.6%). The median age of the cohort was 43.2 years (range: 18–90 years). The mechanism of injury was categorized as flame-related injuries and others. Flame-related injuries included thermal (67 cases), explosion (14), motor vehicle collision (5), and grease burn (9), while others included scald (3), steam (1), and chemical (3) burns. The majority of patients experienced flame-related injuries (93.1%). Coexisting inhalation injury was present in about a third of patients. The median time to obtain the initial set of laboratory tests from the time of injury was 150 minutes (2 hours and 30 minutes) (range: 30–672 minutes) (Table 1). A total of 15 patients (14.7%) died within the first 7 days after injury; 10 of these patients had > 65% TBSA burn and after evaluation of the extent of injury and discussion with family, were treated with comfort measures within 24 to 48 hours of admission rather than active resuscitation due to poor estimated survivability.
Table 1.
Patient characteristics on admission
| Variables | n (%) |
|---|---|
| Total # of patients | 102 (100) |
| Total body surface area burn (%) | |
| 15–30 | 63 (61.8) |
| 31–50 | 19 (18.6) |
| 51–65 | 7 (6.9) |
| 66–100 | 13 (12.7) |
| Age (years) | |
| 18–40 | 37 (36.3) |
| 41–65 | 46 (45.1) |
| >65 | 19 (18.6) |
| Gender | |
| Male | 71 (69.6) |
| Female | 31 (30.4) |
| Mechanism of injury | |
| Flame-related | 95 (93.1) |
| Others | 7 (6.9) |
| Inhalation injury | |
| Yes | 31 (30.4) |
| No | 71 (69.6) |
| Median elapsed time from injury to admission sample | 150 minutes (2 hours and 30 minutes) |
Is ATC present after a major burn injury?
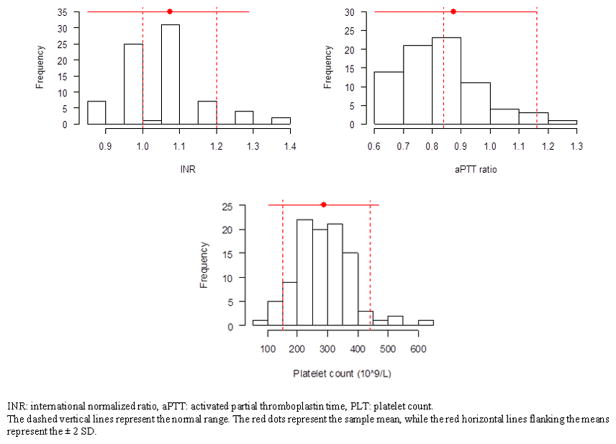
On admission, we did not identify a single patient who met our criteria for ATC. While the majority of patients had normal PLT and a few patients had a slightly prolonged INR, there were no patients with aPTT ratio ≥ 1.5 times the mean normal on admission (Figure 1). A two-sided binomial test against the 20% incidence of ATC in trauma patients was highly significant (p value < 0.0001 and 95% confidence interval of 0 to 0.047), suggesting that the incidence of ATC in burn patients is much less than in trauma patients. Given that an analogous coagulopathy was not seen in our cohort, there is no evidence to suggest an association with increased blood utilization and mortality in the initial 7 days.
Figure 1.
Histograms of admission laboratory data
Association of TBSA burn and admission INR, aPTT ratio, PLT, and Hgb
In evaluating the association between TBSA burn and admission laboratory data, we did not find any significant correlation between TBSA and aPTT ratio, PLT, and Hgb, but found a marginal association between TBSA and INR (r=0.22, p value=0.0518), which became significant after controlling for elapsed time from the time of injury to the time of initial INR (p value=0.0403). These findings suggest that the screening hematologic profile on admission is not influenced by burn severity, except the INR, which becomes prolonged as elapsed time increases (Figure 2). Patients had a median INR of 1.1, aPTT ratio of 0.9, and PLT of 280 × 109/L on admission. The median Hgb on admission for males and females were 14.5 g/dL and 13.7 g/dL, respectively. All the admission median values were within the reference ranges.
Figure 2.
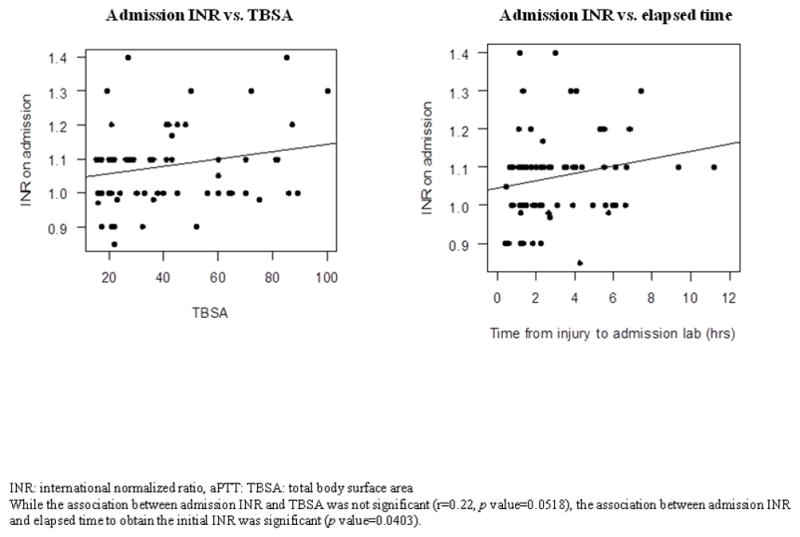
Association between admission INR, total body surface area burn, and elapsed time to obtain the admission INR
Hematologic profile in the first 7 days after a major burn injury
During the first 7 days after injury, the INR slightly increased on the first day and remained minimally elevated throughout at 1.2–1.3. While the aPTT ratio similarly increased on the first day and stayed slightly elevated, it was generally < 1.5, except in three patients. PLT showed a sharp decline in the first two days and gradually increased to almost baseline level by day 7. Hgb showed a steady decrease and was approximately 10.5 g/dL by day 7. In comparing the laboratory test results of INR, aPTT ratio, PLT, and Hgb on admission to those at day 2, we found significant differences in all test results (p <0.0001). The INR and aPTT ratio increased by + 0.27 (from 1.07 to 1.34) and + 0.34 (0.88 to 1.22), respectively, while the PLT decreased by about −120 × 109/L (from 287 to 167 × 109/L) and Hgb decreased by − 2.2 g/dL for males (from 14.6 to 12.4 g/dL) and − 2.5 g/dL for females (from 13.4 to 10.9 g/dL) (Figure 3).
Figure 3.
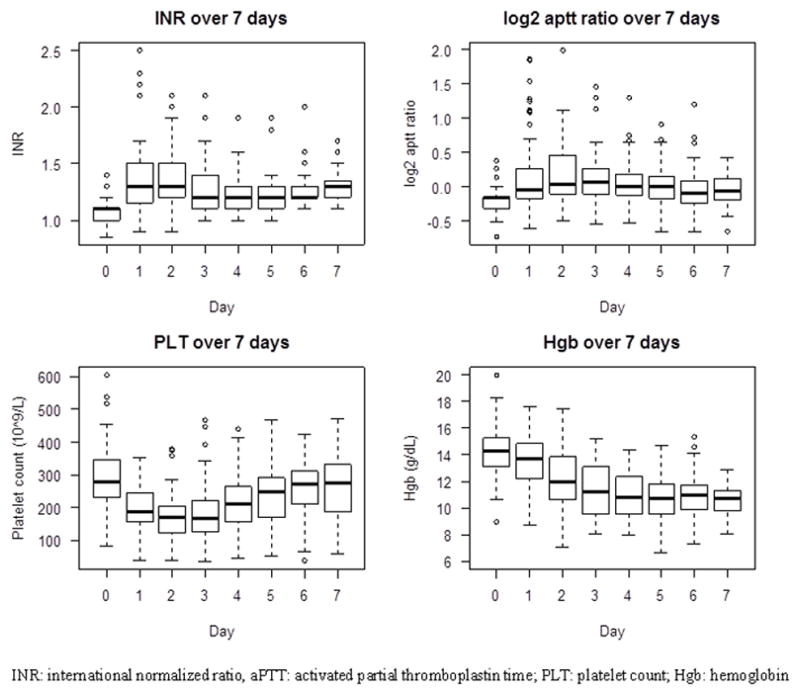
Trends of individual laboratory data in the first 7 days after injury
Blood utilization in the first 7 days after a major burn injury
Fifty-eight of 102 patients (56.8%) received a surgical debridement within the first 7 days after injury, with the majority receiving at least one surgical debridement (± grafting) during this time period. Amongst these 58 patients, a total of 60 procedures were performed. Many patients received escharatomies and fasciotomies as part of their initial surgical management. Forty four percent (26 patients) of those that had a surgical intervention received blood products during their interventions. Of these, 46.2% (12 patients) received packed red blood cells (PRBCs) alone, 42.3% (11 patients) received PRBCs and plasma, and 11.5% (3 patients) received PRBCs, plasma, and platelets. A total of 271 units of blood products were transfused within 7 days— 192 units of PRBC, 65 units of plasma, and 14 doses of apheresed platelets.
DISCUSSION
There is limited literature describing the incidence, risk factors, and clinical outcomes of abnormal coagulation profiles in burn patients. Current evidence suggests that the presence of early coagulopathy after major burn injury is a risk factor for increased morbidity and mortality.8–9 It has been hypothesized that burn injuries initiate an early coagulopathy via activation of coagulation, fibrinolysis, and contact factors; and the presence of early coagulopathy is proportional to burn severity.8,12,20
Recognizing that burn patients are often considered to be a subset of trauma patients, we hypothesized that burn patients develop an acute coagulopathy analogous to ATC that is present on admission. In a recent study of 99 burn patients, investigators identified that an INR > 1.5 and aPTT > 60 seconds was present in 3 patients upon initial presentation, but these criteria identified a total of 37 patients when the observation period was extended to 24 hours from the time of injury. The investigators concluded that a very low proportion of patients present with a coagulopathy but a substantial proportion develop coagulopathy within 24 hours of injury, which was attributed to hemodilution from fluid resuscitation.9 In contrast to this study, we did not find a single case of coagulopathy on admission based on our criteria. This difference may not only be due to differences in the definition of coagulopathy but also the elapsed time until obtaining the initial sample. Since ATC occurs within a few hours after injury, we set our time limit for admission samples to 12 hours from the time of injury. In fact, the median time elapsed was 2 hours and 30 minutes, which is similar to the timing of obtaining samples in articles discussing ATC in trauma patients. In using our criteria to define ATC, we found that the incidence of ATC in burn patients is significantly less than that in trauma patients, if present at all. We also found that TBSA is not associated with any abnormalities of the screening hematologic profile on admission, despite severity of injury.
While tissue trauma and systemic shock causing widespread activation of protein C have been associated with the development of ATC in trauma,4–7 it is unclear whether these factors are sufficient to result in early coagulopathy in burn patients. However, excessive bleeding following immediate or early grafting is a well-recognized complication after burn injury.21–22 In our cohort, 56.8% of patients received a surgical intervention within the first 7 days after injury, almost half of which required blood products. As we have previously shown, the predictors for increased PRBC and plasma transfusions in burn patients are large TBSA burn and the use of systemic anticoagulation.18
In our study, the INR increased slightly throughout the 7-day period. In contrast, the aPTT ratios were normal throughout the study period, and were actually on the lower end of normal. It is possible that an acutely elevated post-injury factor VIII level serves to shorten the aPTT.22,23 It is surprising to find that the INR and aPTT ratio did not appear to be appreciably affected by the large volume of fluid resuscitation received in the first 7 days. The Hgb level gradually decreased over 7 days, suggesting that the decline might not be only due to burn shock -- which should resolve within 48–72 hours after initiation of burn resuscitation -- but additional factors that may contribute to anemia such as wound bleeding, iatrogenic blood loss from repeated phlebotomies, operative blood loss from wound excision and debridement, and possibly anemia of inflammation.24 PLT decreased until day 2, with near return to baseline by day 7. This PLT trend suggests that the PLT might have also been hemodiluted due to fluid resuscitation within the first 48 hours after injury. This PLT trend was similarly shown in a previous study, which also demonstrated that the results of platelet function studies were abnormally decreased when PLT was low and that platelets may actually increase to abnormally high levels beyond the first week of injury.25 Other possible factors contributing to transient thrombocytopenia include increased platelet consumption and decreased platelet production.9,22
Our study has several limitations. This is a single-center, retrospective cohort study with a small sample size. While an association between injury severity score, base deficit, and development of ATC has been observed,1–7 we categorized our study patients based solely on TBSA without regard to burn severity score or inflammatory biomarkers, which could potentially improve categorization. While time elapsed from the time of injury until the initial sample was less than 12 hours, we were unable to provide information about the amount of fluid resuscitation, which could be an important variable modifying the results of these hematologic parameters. We were also unable to determine the effect of surgery especially in patients with larger burns who do not only require large amounts of fluids for resuscitation but also are at risk for excessive perioperative bleeding requiring blood transfusions.14–18 Lastly, while we were able to evaluate the laboratory tests of all patients on admission to determine whether ATC is detectable within 12 hours after injury, not all patients had daily laboratory studies to determine the hematologic profile of patients within the first 7 days; therefore, the observed trends may only be reflective of the laboratory values of patients who survived and had less severe injury rather than being a true representation of the hematologic response to burn injury.
We conclude that the screening hematologic profile of burn patients on admission is normal and the standard screening assays do not suggest the existence of an admission coagulopathy analogous to ATC. However, the definition of ATC requires standardization, and screening coagulation assays may not be appropriate to detect aberrations in the coagulation system of burn patients. While this is a relatively small study, it provides a step towards a better understanding of the hematologic profile of burn patients and further evidence to suggest that ATC is unique to trauma patients.
Acknowledgments
RPL acknowledges funding support from the NIH T32HL007149. FCL acknowledges support from the NIH UL1 TR000083. We thank Ms. Valorie A. Buchholz, Ms. Paula L. Steele, and Ms. Carrie A. Nielsen of the Department of Surgery in providing support in data collection and study coordination.
Source of Funding:
RPL had received financial support provided by the NIH T32 Hematology Research Training Grant (T32HL007149) for the University of North Carolina at Chapel Hill. FCL acknowledges support from the NIH UL1 TR000083.
Footnotes
AUTHORSHIP: RPL conceptualized the study, collected clinical data, analyzed data, wrote the manuscript, and performed all coordination efforts. AN and FCL performed the statistical analysis. SOP, SDA, DMM, HCW, BAC, and NSK conceptualized the study, analyzed data, edited the manuscript, and provided critical revisions.
Conflict of Interest:
DMM reported no conflict of interest with this submission but has relevant financial activities outside of this work. For the remaining authors, no conflicts were declared.
Part of this article was presented as a poster, entitled “Acute traumatic coagulopathy: is it also present after thermal burn injury?” at the Annual Scientific Symposium of Hemostasis and Thrombosis Research Society at Northwestern University in Chicago, IL on April 28–30, 2011.
Contributor Information
Rommel P. Lu, Email: rlu@med.unc.edu, rommelperillolu@yahoo.com.
Ai Ni, Email: andyni@live.unc.edu.
Feng-Chang Lin, Email: flin33@email.unc.edu.
Shiara M. Ortiz-Pujols, Email: shiara_ortiz-pujols@med.unc.edu.
Sasha D. Adams, Email: sasha_adams@med.unc.edu.
Dougald M. Monroe, III, Email: dougald_monroe@med.unc.edu.
Herbert C. Whinna, Email: whinna@med.unc.edu.
Bruce A. Cairns, Email: bruce_cairns@med.unc.edu.
Nigel S. Key, Email: nigel_key@med.unc.edu.
References
- 1.MacLeod JB, Lynn M, McKenney MG, Cohn SM, Murtha M. Early coagulopathy predicts mortality in trauma. J Trauma. 2003;55(1):39–44. doi: 10.1097/01.TA.0000075338.21177.EF. [DOI] [PubMed] [Google Scholar]
- 2.Brohi K, Singh J, Heron M, Coats T. Acute Traumatic Coagulopathy. J Trauma. 2003;54(6):1127–30. doi: 10.1097/01.TA.0000069184.82147.06. [DOI] [PubMed] [Google Scholar]
- 3.Maegele M, Lefering R, Yucel N, Tjardes T, Rixen D, Paffrath T, Simanski C, Neugebauer E, Bouillon B AG Polytrauma of the German Trauma Society (DGU) Early coagulopathy in multiple injury: an analysis from the German Trauma Registry on 8724 patients. Injury. 2007;38(3):298–304. doi: 10.1016/j.injury.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Brohi K, Cohen MJ, Ganter MT, Matthay MA, Mackersie RC, Pittet JF. Acute traumatic coagulopathy: initiated by hypoperfusion: modulated through the protein C pathway? Ann Surg. 2007;245(5):812–8. doi: 10.1097/01.sla.0000256862.79374.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brohi K, Cohen MJ, Ganter MT, Schultz MJ, Levi M, Mackersie RC, Pittet JF. Acute coagulopathy of trauma: hypoperfusion induces systemic anticoagulation and hyperfibrinolysis. J Trauma. 2008;64(5):1211–1217. doi: 10.1097/TA.0b013e318169cd3c. [DOI] [PubMed] [Google Scholar]
- 6.Frith D, Goslings JC, Gaarder C, Maegele M, Cohen MJ, Allard S, Johansson PI, Stanworth S, Thiemermann C, Brohi K. Definition and drivers of acute traumatic coagulopathy: clinical and experimental investigations. J Thromb Haemost. 2010;8(9):1919–25. doi: 10.1111/j.1538-7836.2010.03945.x. [DOI] [PubMed] [Google Scholar]
- 7.Frith D, Davenport R, Brohi K. Acute traumatic coagulopathy. Curr Opin Anaesthesiol. 2012;25(2):229–34. doi: 10.1097/ACO.0b013e3283509675. [DOI] [PubMed] [Google Scholar]
- 8.Lavrentieva A, Kontakiotis T, Bitzani M, Lavrentieva A, Kontakiotis T, Bitzani M, Papaioannou-Gaki G, Parlapani A, Thomareis O, Tsotsolis N, Giala MA. Early coagulation disorders after severe burn injury: impact on mortality. Intensive Care Med. 2008;34(4):700–6. doi: 10.1007/s00134-007-0976-5. [DOI] [PubMed] [Google Scholar]
- 9.Mitra B, Wasiak J, Cameron PA, O’Reilly G, Dobson H, Cleland H. Early coagulopathy of major burns. Injury. 2013;44(1):40–3. doi: 10.1016/j.injury.2012.05.010. Epub 2012 Jun 5. [DOI] [PubMed] [Google Scholar]
- 10.Pruitt BA., Jr Forces and factors influencing trauma care: 1983 A.A.S.T.(American Association for the Surgery of Trauma) Presidential address. J Trauma. 1984;24(6):463–70. [PubMed] [Google Scholar]
- 11.Demling RH. The burn edema process: current concepts. J Burn Care Rehabil. 2005;26(3):207–27. [PubMed] [Google Scholar]
- 12.Pham TN, Cancio LC, Gibran NS American Burn Association. American Burn Association practice guidelines burn shock resuscitation. J Burn Care Res. 2008;29(1):257–66. doi: 10.1097/BCR.0b013e31815f3876. [DOI] [PubMed] [Google Scholar]
- 13.Latenser BA. Critical care of the burn patient: the first 48 hours. Crit Care Med. 2009;37(10):2819–26. doi: 10.1097/CCM.0b013e3181b3a08f. [DOI] [PubMed] [Google Scholar]
- 14.Palmieri TL, Caruso DM, Foster KN, Cairns BA, Peck MD, Gamelli RL, Mozingo DW, Kagan RJ, Wahl W, Kemalyan NA, Fish JS, Gomez M, Sheridan RL, Faucher LD, Latenser BA, Gibran NS, Klein RL, Solem LD, Saffle JR, Morris SE, Jeng JC, Voigt D, Howard PA, Molitor F, Greenhalgh DG American Burn Association Burn Multicenter Trials Group. Effect of blood transfusion on outcome after major burn injury: a multicenter study. Crit Care Med. 2006;34:1602–7. doi: 10.1097/01.CCM.0000217472.97524.0E. [DOI] [PubMed] [Google Scholar]
- 15.Kwan P, Gomez M, Cartotto R. Safe and successful restriction of transfusion in burn patients. J Burn Care Res. 2006;27(6):826–34. doi: 10.1097/01.BCR.0000245494.45125.3E. [DOI] [PubMed] [Google Scholar]
- 16.Boral L, Kowal-Vern A, Yogore M, 3rd, Patel H, Latenser BA. Transfusions in burn patients with/without comorbidities. J Burn Care Res. 2009;30(2):268–73. doi: 10.1097/BCR.0b013e318198a22f. [DOI] [PubMed] [Google Scholar]
- 17.Curinga G, Jain A, Feldman M, Prosciak M, Phillips B, Milner S. Red blood cell transfusion following burn. Burns. 2011;37(5):742–52. doi: 10.1016/j.burns.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 18.Lu RP, Lin FC, Ortiz-Pujols SM, Adams SD, Whinna HC, Cairns BA, Key NS. Blood utilization in patients with burn injury and association with clinical outcomes. Transfusion. doi: 10.1111/trf.12057. Epub 2012 Dec 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2011. [Accessed December 5, 2012]. URL http://www.R-project.org/ [Google Scholar]
- 20.Kowal-Vern A, Gamelli RL, Walenga JM, Hoppensteadt D, Sharp-Pucci M, Schumacher HR. The effect of burn wound size on hemostasis: a correlation of the hemostatic changes to the clinical state. J Trauma. 1992;33(1):50–6. doi: 10.1097/00005373-199207000-00011. discussion 56–7. [DOI] [PubMed] [Google Scholar]
- 21.Irving GA, Butt AD. Anesthesia for burns in children: a review of procedures practiced at Red Cross Memorial Children’s Hospital, Cape Town. Burns. 1994;20:241–243. doi: 10.1016/0305-4179(94)90190-2. [DOI] [PubMed] [Google Scholar]
- 22.Niemi T, Svartling N, Niemi T, Svartling N, Syrjälä M, Asko-Seljavaara S, Rosenberg P. Haemostatic disturbances in burned patients during early excision and skin grafting. Blood Coagul Fibrinolysis. 1998;9:19–28. doi: 10.1097/00001721-199801000-00003. [DOI] [PubMed] [Google Scholar]
- 23.King DR, Namias N, Andrews DM. Coagulation abnormalities following thermal injury. Blood Coagul Fibrinolysis. 2010;21(7):666–9. doi: 10.1097/MBC.0b013e32833ceb08. [DOI] [PubMed] [Google Scholar]
- 24.Prakash D. Anemia of Chronic Disease Versus Anemia of Acute Illness. Crit Care Clinic. 2012;28(3):333–343. doi: 10.1016/j.ccc.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 25.Bartlett RH, Fong SW, Marrujo G, Hardeman T, Anderson W. Coagulation and platelets changes after thermal injury in man. Burns. 1981;7:370–377. [Google Scholar]