Abstract
The polymorphic fungus Candida albicans is one of the most versatile opportunistic pathogens in humans. Many organs of the human body are potential targets for infection by this pathogen, but infection is commonly localized in the gastrointestinal tract, an environment providing anaerobic growth conditions. We describe a chemically defined anaerobic growth medium for four strains of Candida albicans (A72, SC5314, MEN, and 10261). It is a defined liquid glucose-phosphate-proline growth medium supplemented with oleic acid, nicotinic acid, and ammonium chloride. The cells did not require or respond to added ergosterol. Oleic acid and nicotinic acid are growth factors which are required only for the anaerobic growth of C. albicans. An important technical feature of this study was the use of anaerobically grown inocula to study anaerobic growth. Anaerobically, the cells grew exclusively as mycelia at 25, 30, and 37°C. The doubling time at 30°C was ca. 20 h. The cells did not produce farnesol and did not respond to exogenous farnesol, and they were resistant to the highest tested levels of amphotericin B and four of the azole antifungals. We suggest that the anaerobic growth of C. albicans may contribute to the trailing end point phenomenon and the resistance of C. albicans biofilms to antifungal drugs.
The dimorphic fungus Candida albicans is one of the most commonly isolated fungal pathogens in humans. Infections with C. albicans, called candidoses, occur predominantly in immunosuppressed patients (AIDS, cancer treatment, and transplant patients). For AIDS patients, candidosis has been shown to be the most common human immunodeficiency virus-associated opportunistic infection (19). C. albicans is also associated with 78% of fungal nosocomial infections and over 10% of all nosocomial infections documented (19). The annual cost of treating candidal infections in the United States has been estimated at 1 billion dollars (22).
The aerobic growth of C. albicans has been studied extensively, but even though C. albicans has often been referred to as a facultative anaerobe (25), papers describing anaerobic growth are rare. Our literature search recovered only eight articles (10, 16, 17, 33, 35-38), two of which (16, 33) describe survival under anaerobic conditions rather than actual anaerobic growth and three more of which (10, 17, 38) merely confirm that anaerobic growth is possible. However, Szawatkowski and Hamilton-Miller (35) reported that 20 strains of C. albicans could grow anaerobically on either Sabouraud agar or brucella agar supplemented with 10% whole horse blood, and Webster and Odds (37) showed that seven Candida species, including C. albicans, could grow anaerobically on four media: Eagle's minimal medium with 10% horse serum, a peptone-glucose broth, and two defined but complicated media (yeast nitrogen base-glucose broth and yeast nitrogen base-asparagine-glucose-phosphate [YAGP] broth). However, in all cases the experiments were conducted with aerobically grown inocula and GasPak anaerobic systems. Thus, the experiments studied both steady-state anaerobic conditions and an aerobic-to-anaerobic adaptation of indeterminate length. To gauge the likely duration of an aerobic-to-anaerobic adaptive period, we note that when grown anaerobically, the yeast Saccharomyces cerevisiae requires ergosterol as an added growth factor (1). However, when an aerobically grown culture of S. cerevisiae is inoculated into anaerobic medium that does not contain ergosterol, growth stops only after five to seven generations (27).
This comparative lack of interest in anaerobic growth is surprising for two reasons. First, in many cases C. albicans infections spread into the body from the anaerobic gastrointestinal tract (19, 25), and second, the interiors of many biofilms are anaerobic (9). Furthermore, the accurate assessment of an organism's nutritional requirements requires the use of chemically defined media, and reproducible anaerobically grown inocula require liquid media. We now describe a minimal defined liquid medium for the anaerobic growth of C. albicans, consisting of a glucose-phosphate-proline (GPP) medium (13, 18) supplemented with oleic acid, nicotinic acid, and ammonium chloride.
Effective antifungal antibiotics are rare due to few fungus-specific targets and the rapid development of drug resistance. Research has been done to test the sensitivity and resistance of C. albicans cells grown aerobically (21, 32) and anaerobically (35, 36, 38) to several antifungal agents. We have tested five classes of antifungal agents on cells grown anaerobically: polyenes, allylamines, azoles, zaragozic acid B, and cerulenin. Polyenes, such as amphotericin B, bind to ergosterol present in the cell membrane, causing damage to the cell membrane and leakage of intracellular ions (31). Azoles such as fluconazole, ketoconazole, voriconazole, and itraconazole block ergosterol biosynthesis by inhibiting the cytochrome P450 responsible for 14α demethylation of lanosterol (31). Additionally, the allylamine terbinafine inhibits squalene epoxidase (31), zaragozic acid B inhibits squalene synthase (14), and cerulenin inhibits fatty acid synthesis (26). We found that anaerobically grown cells were at least fourfold more resistant than aerobically grown cells to all eight of the antifungals tested. In particular, they were resistant to the highest levels tested for amphotericin B and the four azole antifungals. Our results suggest that the anaerobic growth of C. albicans may contribute to the trailing end point phenomenon (21) and the marked resistance of C. albicans biofilms to antifungal drugs (3, 20, 23). The twin purposes of this study were to define the basic biology of anaerobic growth in C. albicans and to show how anaerobic conditions greatly enhance resistance to common antifungals. It was not to describe a better method of antifungal susceptibility testing.
MATERIALS AND METHODS
Strains.
C. albicans A72 and 10261 were obtained from Patrick Sullivan, University of Otago, Dunedin, New Zealand; strain MEN was obtained from Richard Cannon, University of Otago; and strain SC5314 was obtained from Burk Braun, University of California at San Francisco.
Media.
GPP is the growth medium for fungi described by Kulkarni and Nickerson (18). Normal GPP medium contains the following (per 900 ml of distilled water): 4.0 g of KH2PO4, 3.2 g of NaH2PO4, 1.2 g of l-proline, and 0.7 g of MgSO4 · 7H2O. After the medium was autoclaved, 100 ml of 20% (wt/vol) glucose, 1 ml of a vitamin mix, and 0.25 ml of a mineral mix were added. The vitamin mix contains the following (per 100 ml of 20% ethanol): 2 mg of biotin, 20 mg of thiamine-HCl, and 20 mg of pyridoxine-HCl. The mineral mix contains the following (per 100 ml of 0.1 N HCl): 0.5 g of CuSO4 · 5H2O, 0.5 g of ZnSO4 · 7H2O, 0.8 g of MnCl2 · 4H2O, and 0.5 g of FeSO4. The vitamin mix and mineral mix were filter sterilized through 0.2-μm-pore-size Whatman (Maidstone, United Kingdom) cellulose nitrate filters and stored at 4°C. For anaerobic growth of C. albicans, regular GPP medium (10 ml) was supplemented with 200 μl of 1 mM oleic acid in 100% methanol, 200 μl of 4 mM nicotinic acid, and 1 ml of 500 mM NH4Cl. The pH of this complete defined growth medium was 5.
Aerobic inoculum preparation.
The modified glucose-salt-biotin growth medium (13) used for preparing aerobic C. albicans cell stocks contains the following (per liter of distilled water): 1 g of (NH4)2SO4, 2 g of KH2PO4, 50 mg of MgSO4 · 7H2O, 50 mg of CaCl2 · 2H2O, 1 g of peptone, and 5 g of yeast nitrogen base. After this medium was autoclaved, 10.25 ml of 50% (wt/vol) glucose and 0.4 ml of the GPP vitamin stock were added aseptically. This vitamin mix contains biotin, pyridoxine, and thiamine (18). Cells were harvested after 26 to 28 h of growth, washed three times in 50 mM potassium phosphate buffer (pH 6.5), and stored at 4°C in the same buffer at a cell density of ca. 2 × 109 cells/ml.
Anaerobic-medium preparation.
The Hungate technique for growing stringent anaerobes (7) was employed. The anaerobic medium was prepared in 25-ml anaerobic tubes (Bellco). To the complete defined medium were added increasing concentrations of resazurin (0.001 to 0.005%) to verify anaerobiosis of the medium and 10 mg of solid cysteine to decrease the redox potential of the medium. The medium was bubbled with ultrahigh-purity nitrogen for 15 min at room temperature and then autoclaved for 30 min at 121°C. The color of the medium changed from purple to pale yellow, confirming that the tubes were anaerobic.
Anaerobic inoculum preparation.
An aerobic inoculum of 2 × 106 cells/ml was used to inoculate 10 ml of complete defined anaerobic medium. The culture was incubated at 30°C, and growth was monitored by measuring the optical density (OD) at 660 nm (Spectronic 20; Milton Roy) every 4 h and by examining cellular morphology by phase-contrast microscopy. When the cells were in the logarithmic phase at an OD of 0.8, a 500-μl portion was used to inoculate another 10 ml of anaerobic medium. This second tube was designated the anaerobic culture of C. albicans. During growth, the pH decreased from a pH of 5 to a pH of 4.
Antifungal susceptibility.
The following antifungal drugs were used in this study: amphotericin B, fluconazole, ketoconazole, clotrimazole, miconazole, zaragozic acid B, terbinafine, and cerulenin. All were prepared as stock solutions at 1 mg of drug/ml of sterile water and stored frozen, except for cerulenin, with which the stock solution was in ethanol. C. albicans A72 cells were inoculated at 106 cells/ml into our complete defined anaerobic growth medium at 30°C, and growth was monitored by OD for either 48 h (aerobic) or 5 days (anaerobic). The aerobic and anaerobic susceptibility measurements differed only in that the aerobic cultures were transferred to 25-ml Erlenmeyer flasks and shaken at 200 rpm. Fluconazole and zaragozic acid B were gifts from Pfizer and Merck, respectively. The terbinafine was purchased from ChemPacific, Inc., Baltimore, Md., and the other antifungals were purchased from Sigma, St. Louis, Mo.
RESULTS
Anaerobic growth.
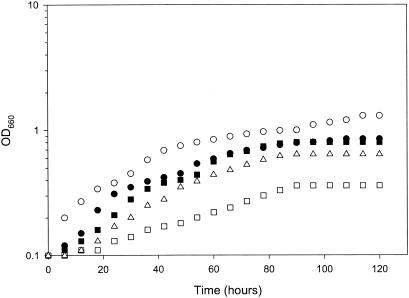
C. albicans A72 did not grow anaerobically in unsupplemented GPP liquid medium. However, it did grow when the medium was supplemented with either 1% yeast extract (data not shown) or a combination of oleic acid, nicotinic acid, and ammonium chloride (Fig. 1). All of our subsequent work focused on the complete defined medium containing oleic acid, nicotinic acid, and ammonium chloride. Each of the supplements was necessary; removal of either ammonium, proline, nicotinic acid, or oleic acid individually gave much-reduced growth (Fig. 1). However, these were the only supplements identified as necessary. Added l-arginine, betaine, bile salts, ergosterol, fumarate, or potassium nitrate did not improve growth at either 30 or 37°C. With an anaerobically grown inoculum of ca. 106 cells per ml, it took ca. 110 h to reach the stationary phase at 30°C (Fig. 1). Calculated graphically, this indicates an anaerobic doubling time of 20 h. This value is in contrast to the aerobic doubling time for C. albicans A72 of ca. 2 h when grown with shaking in either GPP medium or our complete defined medium. The cultures were completely anaerobic throughout growth, as measured by the resazurin indicator dyes. We have conducted six successive transfers with this complete defined medium, and the growth parameters of the sixth were identical to those of the first. Equivalent anaerobic growth has been obtained on complete defined medium with C. albicans strains A72, MEN, SC5314, and 10261. All of our subsequent work used C. albicans A72.
FIG. 1.
Anaerobic growth of C. albicans cells at 30°C. ○, growth in the complete minimal medium; •, growth in medium without proline; ▪, growth in medium without NH4Cl; ▵, growth in medium without oleic acid; □, growth in medium without nicotinic acid.
Morphology of anaerobically grown cells.
The dominant morphology for anaerobically grown cells was mycelia. With an aerobically grown inoculum, anaerobic cultures at 25, 30, and 37°C grew initially as budding yeasts and then gradually shifted to the hyphal morphology and stayed as mycelia. When examined by phase-contrast microscopy, the 30°C culture had reached ca. 50% mycelia by 48 h and 90% mycelia by 72 h, and by the stationary phase (4 to 5 days), dense mycelial mats were prevalent (Fig. 2). No spiral hyphae were observed (16). The conversion to mycelia occurred more slowly at 25°C. With an anaerobically grown inoculum, the cells remained ca. 95% mycelial throughout growth at 25, 30, and 37°C.
FIG. 2.
Morphology of C. albicans cells grown under anaerobic conditions after 72 h. Bars = 200 μm (A) and 50 μm (B).
It is well known that under aerobic conditions germ tubes or hyphae are triggered at 37°C by either l-proline, serum, or N-acetyl-d-glucosamine (13, 25). Consequently, we wondered whether the l-proline in our complete defined anaerobic medium was necessary for the production of mycelia. It was not. Equivalent percentages of mycelia were observed when l-proline was replaced with either l-arginine or l-phenylalanine, although in both cases the growth rates were somewhat lower (data not shown).
Farnesol.
We next investigated whether anaerobically grown C. albicans cells either produced farnesol or responded to exogenous farnesol. They did neither. When examined by our standard gas chromatography-mass spectrometry detection system (13), no extracellular farnesol was detected in supernatants from cells which had been grown anaerobically at 30°C for 4 to 5 days. That is, there was less than 0.001 μg of farnesol per mg (dry weight) of cells. In contrast, aerobically grown cells produce ca. 0.18 to 0.22 μg of farnesol/mg (15). Furthermore, during anaerobic growth, C. albicans did not respond to farnesol. In complete defined medium with farnesol present from time zero, the cell growth rates and morphologies remained unchanged, with farnesol concentrations ranging from 0 to 1.2 mM. The same results occurred at both 30 and 37°C and with both aerobically and anaerobically grown inocula. In particular, the kinetics for the yeast-to-mycelium conversion of an aerobically grown inoculum were the same with and without farnesol. This concentration of farnesol (1.2 mM) represents the maximum solubility of farnesol in water. In contrast, aerobic C. albicans exhibits a 50% conversion of mycelia to yeast cells at only 1.2 μM farnesol (34).
Antifungals.
Our studies revealed that anaerobically grown cells were much more resistant than aerobically grown cells to eight antifungals. Anaerobically grown C. albicans was resistant to all concentrations of amphotericin B, clotrimazole, fluconazole, miconazole, and ketoconazole (Table 1). Also, anaerobically grown cells were 4, 8, and 16 times more resistant than aerobically grown cells to cerulenin, terbinafine, and zaragozic acid B, respectively (Table 1). The experimental design whose results are shown in Table 1 used an aerobic inoculum for aerobic growth and an anaerobic inoculum for anaerobic growth. We also employed a crossover design in which both aerobic and anaerobic inocula were used for both aerobic and anaerobic growth. The crossover design was used to test for miconazole sensitivity. Since the same medium was used for all these experiments, the presence or absence of oxygen was the only variable. With both inocula, the MIC of miconazole was 4 μg/ml during aerobic growth and >80 μg/ml during anaerobic growth.
TABLE 1.
Inhibitory concentrations of cell inhibitors and antifungal compounds for aerobically and anaerobically grown C. albicans A72
| Compound | Inhibitory concn (μg/ml)a
|
|
|---|---|---|
| Aerobic growth | Anaerobic growth | |
| Amphotericin B | 0.5 | >20 (not reached) |
| Cerulenin | 1 | 4 |
| Terbinafine | 4 | 32 |
| Zaragozic acid B | 0.5 | 8 |
| Clotrimazole | 2 | >16 (not reached) |
| Fluconazole | 8 | >40 (not reached) |
| Miconazole | 4 | >16 (not reached) |
| Ketoconazole | 4 | >4 (not reached) |
Concentration at which no growth was evident after 48 h (aerobic) or 5 days (anaerobic). C. albicans A72 grown anaerobically appeared to be resistant to amphotericin B and the four azole antifungals, in that the cells still grew at the highest concentrations tested.
The morphology of the cells growing anaerobically in the presence of 4 to 16 μg of amphotericin B, fluconazole, ketoconazole, zaragozic acid B, and cerulenin per ml was the same as that of cells growing in the absence of these agents. Mycelia were present continuously. This observation is in contrast to what was observed for aerobic C. albicans, with which subinhibitory concentrations of the azole antifungals inhibit mycelial development and maintain the cells in the yeast morphology (12, 25). This morphological conversion is likely due to the secretion of excess farnesol by azole-treated C. albicans (15). However, for sublethal levels of terbinafine, the anaerobic cells grew as yeast cells; the mycelial form was inhibited.
Physiological versus genetic.
Stationary-phase anaerobically grown cells were diluted and counted as CFU on yeast extract-peptone-dextrose agar. Ten colonies were picked at random and grown aerobically in unsupplemented GPP medium. All 10 strains were equally as sensitive to fluconazole as the starting C. albicans strain, which had never been grown anaerobically. Therefore, the antibiotic resistance shown in Table 1 is not due to selection for antibiotic-resistant mutants.
DISCUSSION
We have devised a defined liquid medium for the anaerobic growth of C. albicans. It consists of the GPP medium that we use for the aerobic growth of C. albicans (13) supplemented with oleic acid, nicotinic acid, and ammonium chloride. Ergosterol is not required. All anaerobic protocols used the Hungate technique for stringently growing anaerobes (7). Anaerobic growth was much slower than aerobic growth, and the cell morphology during anaerobic growth was filamentous at all temperatures tested, i.e., 25, 30, and 37°C. The mycelial growth is a consequence, direct or indirect, of anaerobiosis rather than the stringent conditions of the Hungate procedure for achieving anaerobiosis (7). The Hungate procedure (7) includes l-cysteine (1 mg/ml, 8.3 mM). However, it is unlikely that this cysteine contributes to mycelial growth, because Nickerson and van Rij (24) showed that 1 to 10 mM l-cysteine shifted the morphology of aerobic C. albicans towards that of yeast cells, not towards that of mycelia. Our observation of anaerobic mycelia agrees with the previous findings of Webster and Odds (37). Additionally, Szawatkowski and Hamilton-Miller (35) reported that stellate (more filamentous) colonies were formed anaerobically, unlike the creamy colonies formed aerobically (35).
Remembering that C. albicans forms farnesol from farnesyl pyrophosphate (14), three observations regarding the anaerobic growth of C. albicans are consistent with one another. First, anaerobically grown cells do not excrete farnesol. Second, during growth the morphological progression is from yeast cells to mycelia rather than vice versa as it is in air, where farnesol would be produced (13). Third, anaerobic cells appear to be insensitive to the azole and polyene antibiotics. These three observations could all result from reduced, altered, or absent carbon flow through the sterol pathway under anaerobic growth conditions. We conclude that anaerobically grown C. albicans does not use the same quorum-sensing system that the organism uses when it is grown aerobically (13). Two previous reports also examined the effectiveness of antifungals during the anaerobic growth of C. albicans. Using yeast morphology agar and the paper disk method in GasPak jars, Uno et al. (36) showed that ketoconazole inhibited growth aerobically but not anaerobically. In contrast, Szawatkowski and Hamilton-Miller (35) incorporated various levels of amphotericin B and clotrimazole into Sabouraud agar. They reported that the MICs were little changed for aerobically versus anaerobically grown cells. However, in comparing anaerobic drug sensitivity data, a key technical feature is the use of an anaerobically grown inoculum. With an anaerobic inoculum, the cells have already lost their characteristic aerobic sterol composition. Otherwise, drug sensitivity would be measured while the aerobic sterol composition was in the process of being diluted out. As an example of the time frame needed for sterol dilution, when an aerobically grown culture of S. cerevisiae is inoculated into an anaerobic medium in the absence of sterols, growth stops only after five to seven generations (27).
Added nutritional requirements for the anaerobic growth of fungi are well known. Anaerobically, S. cerevisiae requires both ergosterol (1) and an unsaturated fatty acid, usually oleic acid (2), while Mucor rouxii requires nicotinic acid (6). We have added to this list by finding that C. albicans requires both oleic acid and nicotinic acid. Recognition of these added nutritional requirements for C. albicans provides further confirmation that the conditions employed were indeed anaerobic.
An understanding of nutritional requirements should also prove useful in interpreting physiological aspects of anaerobic growth. For instance, Webster and Odds (37) noted marked differences in the cell yields for anaerobically grown C. albicans. For the four media they studied (Eagle's minimal medium with 10% horse serum, YAGP broth, yeast nitrogen base-glucose broth, and peptone-glucose broth), the cell yields were in the ratio of ca. 1:2:3:4, respectively. These differences could be due to altered glucose content, as suggested by the authors (37), or they could be due to differences in available oleic or nicotinic acid.
Significantly, C. albicans did not require ergosterol for growth. This finding has ample precedent. (i) S. cerevisiae requires ergosterol for anaerobic growth (1), but Schizosaccharomyces japonicus does not (8). (ii) In two cases (35, 38), the anaerobic growth of C. albicans was achieved without added ergosterol, although in both cases the media contained peptone and yeast extract. (iii) The erg11 gene is an essential gene in S. cerevisiae but not in C. albicans (5, 32). Erg11p is the target for the azole antifungals (31). (iv) D10 is a slowly growing cytochrome P450-deficient mutant of C. albicans which does not produce ergosterol but also does not require ergosterol (4). Thus, there are similarities between the anaerobic growth of C. albicans A72 and the aerobic growth of C. albicans D10 (4). (v) If we assume that C. albicans is well adapted for anaerobic growth in the gastrointestinal tract, where ergosterol is not available, then it is also reasonable to assume that it is able to cope with the absence of ergosterol. In this light, the suggestion has been made (11) that a two-step reaction of dehydrogenation and hydration could accomplish single-oxygen hydroxylation, as in ergosterol biosynthesis, with the inserted oxygen coming from water, not O2. The exact physiological and molecular mechanisms which allow anaerobically grown C. albicans to be resistant to the azole antifungals is being investigated in our laboratory.
The results presented here demonstrate that C. albicans is able to grow anaerobically in vitro and that other targets must be taken into consideration when this pathogen grows under anaerobiosis. Clinical implications of this study are apparent. If patients with intestinal candidosis fail to respond to azoles and polyenes, which are the most used antifungals today, other antifungals must be taken into consideration.
Exact parallels cannot be drawn between our in vitro results and clinical practice. However, the recognition that anaerobically grown C. albicans cells are more resistant to some antifungals than aerobically grown ones provides an alternate explanation for two aspects of antifungal testing. First, the trailing end point phenotype in antifungal susceptibility testing (21) describes incomplete growth inhibition, even at very high concentrations of the antifungal. Thus, an in vitro-in vivo discrepancy is created; for instance, mice infected with a certain strain of C. albicans responded to fluconazole therapy despite the fact that in vitro testing indicated that the strain was not susceptible to fluconazole (30). One cause of the trailing growth phenotype may be anaerobic growth in the automated microtiter plates in which the in vitro susceptibility measurements were made. The geometry of the individual wells would make it difficult to achieve adequate aeration. Repeating the in vitro-in vivo comparison with fully aerated cultures should resolve this dilemma. Second, C. albicans cells exhibit greater resistance to fluconazole and other antifungal drugs when they are in biofilms than when they are not (3, 20, 23). However, the interiors of many microbial biofilms are highly anaerobic (9), and at least three features of C. albicans biofilms could be explained if their interiors were anaerobic or partially anaerobic. (i) Mature biofilms contain more than 95% true hyphae (29). (ii) Although it is known that drug efflux determinants are expressed within biofilms (23, 28), the mechanism that gives rise to the expression of these genes is unknown (20). It could be due to anaerobiosis. (iii) Biofilms appear to have an intrinsic drug resistance. Ramage et al. (28) demonstrated that C. albicans cells within a biofilm express genes encoding drug resistance determinants but that biofilms formed from mutants lacking these genes still exhibit antifungal resistance. Our laboratory is currently investigating the potential mechanisms for the exceptional drug resistance shown by anaerobically grown C. albicans.
Acknowledgments
We thank Sara Basiaga for her assistance with GC-MS and Razvan Dumitru for his assistance with strict anaerobic culture techniques. We also thank Pfizer, Sandwich, United Kingdom, and Merck, Rahway, N.J., for providing the fluconazole and zaragozic acid B, respectively.
This work was supported by grants from the National Science Foundation (MCB-0110999) and the University of Nebraska Tobacco Settlement Biomedical Research Enhancement Fund.
REFERENCES
- 1.Andreasen, A. A., and T. J. B. Stier. 1953. Anaerobic nutrition of Saccharomyces cerevisiae. I. Ergosterol requirement for growth in a defined medium. J. Cell. Physiol. 41:23-36. [DOI] [PubMed] [Google Scholar]
- 2.Andreasen, A. A., and T. J. B. Stier. 1954. Anaerobic nutrition of Saccharomyces cerevisiae. II. Unsaturated fatty acid requirement for growth in a defined medium. J. Cell. Physiol. 43:271-281. [DOI] [PubMed] [Google Scholar]
- 3.Baillie, G. S., and L. J. Douglas. 1999. Candida biofilms and their susceptibility to antifungal agents. Methods Enzymol. 310:644-656. [DOI] [PubMed] [Google Scholar]
- 4.Bard, M., N. D. Lees, R. J. Barbuch, and D. Sanglard. 1987. Characterization of a cytochrome P450 deficient mutant of Candida albicans. Biochem. Biophys. Res. Commun. 147:794-800. [DOI] [PubMed] [Google Scholar]
- 5.Bard, M., N. D. Lees, T. Turi, D. Craft, L. Cofrin, R. Barbuch, C. Koegel, and J. C. Loper. 1993. Sterol synthesis and viability of erg11 (cytochrome P450 lanosterol demethylase) mutations in Saccharomyces cerevisiae and Candida albicans. Lipids 28:963-967. [DOI] [PubMed] [Google Scholar]
- 6.Bartnicki-Garcia, S., and W. J. Nickerson. 1961. Thiamine and nicotinic acid: anaerobic growth factors for Mucor rouxii. J. Bacteriol. 82:142-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breznak, J. A., and R. N. Costilow. 1994. Physicochemical factors in growth, p. 137-154. In P. Gerhardt (ed.), Methods for general and molecular bacteriology. ASM Press, Washington, D.C.
- 8.Bulder, C. J. E. A. 1971. Anaerobic growth, ergosterol content and sensitivity to a polyene antibiotic of the yeast Schizosaccharomyces japonicus. Antonie Leeuwenhoek 37:353-358. [DOI] [PubMed] [Google Scholar]
- 9.Davey, M. E., and G. A. O'Toole. 2000. Microbial biofilms: from ecology to molecular genetics. Microbiol. Mol. Biol. Rev. 64:847-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eklund, T., and T. Jarmund. 1983. Microculture model studies on the effect of various gas atmospheres on microbial growth at different temperatures. J. Appl. Bacteriol. 55:119-125. [DOI] [PubMed] [Google Scholar]
- 11.Griffin, D. H. 1994. Fungal physiology, 2nd ed. Wiley-Liss, New York, N.Y.
- 12.Ha, K. C., and T. C. White. 1999. Effects of azole antifungal drugs on the transition from yeast cells to hyphae in susceptible and resistant isolates of the pathogenic yeast Candida albicans. Antimicrob. Agents Chemother. 43:763-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hornby, J. M., E. C. Jensen, A. D. Lisec, J. J. Tasto, B. Jahnke, R. Shoemaker, P. Dussault, and K. W. Nickerson. 2001. Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl. Environ. Microbiol. 67:2982-2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hornby, J. M., B. W. Kebaara, and K. W. Nickerson. 2003. Farnesol biosynthesis in Candida albicans: cellular response to sterol inhibition by zaragozic acid B. Antimicrob. Agents Chemother. 47:2366-2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hornby, J. M., and K. W. Nickerson. 2004. Enhanced production of farnesol by Candida albicans treated with four azoles. Antimicrob. Agents Chemother. 48:2305-2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaminishi, H., A. Iwata, T. Tamaki, T. Cho, and Y. Hagihara. 1994. Spiral hyphae of Candida albicans formed in anaerobic culture. Mycoses 37:349-352. [DOI] [PubMed] [Google Scholar]
- 17.Kot, E. J., V. L. Olson, L. J. Rolewic, and D. O. McClary. 1976. An alternate respiratory pathway in Candida albicans. Antonie Leeuwenhoek 42:33-48. [DOI] [PubMed] [Google Scholar]
- 18.Kulkarni, R. K., and K. W. Nickerson. 1981. Nutritional control of dimorphism in Ceratocystis ulmi. Exp. Mycol. 5:148-154. [Google Scholar]
- 19.Kullberg, B. J., and S. G. Filler. 2002. Candidemia, p. 327-340. In R. A. Calderone (ed.), Candida and candidiasis. ASM Press, Washington, D.C.
- 20.Kumamoto, C. A. 2002. Candida biofilms. Curr. Opin. Microbiol. 5:608-611. [DOI] [PubMed] [Google Scholar]
- 21.Marr, K. A., T. R. Rustad, J. H. Rex, and T. C. White. 1999. The trailing end point phenotype in antifungal susceptibility testing is pH dependent. Antimicrob. Agents Chemother. 43:1383-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller, L. G., R. A. Hajjeh, and J. E. Edwards, Jr. 2001. Estimating the cost of nosocomial candidemia in the United States. Clin. Infect. Dis. 32:1110. [DOI] [PubMed] [Google Scholar]
- 23.Mukherjee, P. K., J. Chandra, D. M. Kuhn, and M. A. Ghannoum. 2003. Mechanism of fluconazole resistance in Candida albicans biofilms: phase-specific role of efflux pumps and membrane sterols. Infect. Immun. 71:4333-4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nickerson, W. J., and N. J. W. van Rij. 1949. The effect of sulfhydryl compounds, penicillin, and cobalt on the cell division mechanism of yeasts. Biochim. Biophys. Acta 3:461-475. [Google Scholar]
- 25.Odds, F. C. 1988. Candida and candidosis, 2nd ed. Bailliere Tindall, London, England.
- 26.Ōmura, S. 1976. The antibiotic cerulenin, a novel tool for biochemistry as an inhibitor of fatty acid synthesis. Bacteriol. Rev. 40:681-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parks, L. W., C. D. K. Bottema, R. J. Rodriguez, and T. A. Lewis. 1985. Yeast sterols: yeast mutants as tools for the study of sterol metabolism. Methods Enzymol. 111:333-346. [DOI] [PubMed] [Google Scholar]
- 28.Ramage, G., S. Bachmann, T. F. Patterson, B. L. Wickes, and J. L. Lopez-Ribot. 2002. Investigation of multidrug efflux pumps in relation to fluconazole resistance in Candida albicans biofilms. J. Antimicrob. Chemother. 49:973-980. [DOI] [PubMed] [Google Scholar]
- 29.Ramage, G., S. P. Saville, B. L. Wickes, and J. L. López-Ribot. 2002. Inhibition of Candida albicans biofilm formation by farnesol, a quorum-sensing molecule. Appl. Environ. Microbiol. 68:5459-5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rex, J. H., P. W. Nelson, V. L. Paetznick, M. Lozano-Chiu, A. Espinel-Ingroff, and E. J. Anaissie. 1998. Optimizing the correlation between results of testing in vitro and therapeutic outcome in vivo for fluconazole by testing critical isolates in a murine model of invasive candidiasis. Antimicrob. Agents Chemother. 42:129-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanglard, D., and J. Bille. 2002. Current understanding of the modes of action of and resistance mechanisms to conventional and emerging antifungal agents for treatment of Candida infections, p. 349-383. In R. A. Calderone (ed.), Candida and candidiasis. ASM Press, Washington, D.C.
- 32.Sanglard, D., F. Ischer, T. Parkinson, D. Falconer, and J. Bille. 2003. Candida albicans mutations in the ergosterol biosynthetic pathway and resistance to several antifungal agents. Antimicrob. Agents Chemother. 47:2404-2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sarachek, A. 1989. Anaerobically induced production of hybrid monokaryons by heterokaryons of Candida albicans. Mycopathologia 105:39-43. [DOI] [PubMed] [Google Scholar]
- 34.Shchepin, R., J. M. Hornby, E. Burger, T. Niessen, P. Dussault, and K. W. Nickerson. 2003. Quorum sensing in Candida albicans: probing farnesol's mode of action with 40 natural and synthetic farnesol analogs. Chem. Biol. 10:743-750. [DOI] [PubMed] [Google Scholar]
- 35.Szawatkowski, M., and J. M. T. Hamilton-Miller. 1978. Anaerobic growth and sensitivity of Candida albicans. Microbios Lett. 5:61-66. [Google Scholar]
- 36.Uno, J., M. L. Shigematsu, and T. Arai. 1982. Primary site of action of ketoconazole on Candida albicans. Antimicrob. Agents Chemother. 21:912-918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Webster, C. E., and F. C. Odds. 1986. Growth of pathogenic Candida isolates anaerobically and under elevated concentrations of CO2 in air. J. Med. Vet. Mycol. 25:47-53. [PubMed] [Google Scholar]
- 38.Zimmermann, K., J. Bernhardt, M. Knoke, and H. Bernhardt. 2002. Influence of voriconazole and fluconazole on Candida albicans in long-time continuous flow culture. Mycoses 45:41-46. [DOI] [PubMed] [Google Scholar]