Abstract
Background
Understanding the dynamics of the gut-brain axis has clinical implications for physical and mental health conditions, including obesity and anxiety. As such disorders have early life antecedents, it is of value to determine if associations between the gut microbiome and behavior are present in early life in humans.
Methods
We used next generation pyrosequencing to examine associations between the community structure of the gut microbiome and maternal ratings of child temperament in 77 children at 18-27 months of age. It was hypothesized that children would differ in their gut microbial structure, as indicated by measures of alpha and beta diversity, based on their temperamental characteristics.
Results
Among both boys and girls, greater Surgency/Extraversion was associated greater phylogenetic diversity. In addition, among boys only, subscales loading on this composite scale were associated with differences in phylogenetic diversity, the Shannon Diversity index (SDI), beta diversity, and differences in abundances of Dialister, Rikenellaceae, Ruminococcaceae, and Parabacteroides. In girls only, higher Effortful Control was associated with a lower SDI score and differences in both beta diversity and Rikenellaceae were observed in relation to Fear. Some differences in dietary patterns were observed in relation to temperament, but these did not account for the observed differences in the microbiome.
Conclusions
Differences in gut microbiome composition, including alpha diversity, beta diversity, and abundances of specific bacterial species, were observed in association with temperament in toddlers. This study was cross-sectional and observational and, therefore, does not permit determination of the causal direction of effects. However, if bidirectional brain-gut relationships are present in humans in early life, this may represent an opportunity for intervention relevant to physical as well as mental health disorders.
Introduction
Our bodies are colonized by trillions of bacteria known as the microbiome which reside in many niches of the human body including the gut, skin, vagina, and oral cavity. There are remarkable differences in microbial communities across individuals (Huttenhower et al., 2012). The role of the gut microbiome in health is rapidly gaining attention; overall bacterial diversity as well as specific bacterial abundances in the gut have been implicated in not only obesity, but also allergy, asthma, and inflammatory bowel disease among other conditions (Kinross et al., 2011). In addition to affecting physical health, a central role of the gut microbiome in regulating mood and behavior is emerging. Via communication along the gut-brain axis, bacterial communities may affect both the hypothalamic-pituitary-adrenal (HPA) axis and central nervous system via cytokine and neurotransmitter production among other mediators (for review see Collins and Bercik, 2009; Forsythe et al., 2010; Foster and McVey Neufeld, 2013). Relatedly, there is interest in the possibility of intervening on the gut microbiome to affect mental health disorders (Dinan and Cryan, 2012; Foster and McVey Neufeld, 2013).
Conversely, a causal direction from behavior to gut is also now clearly established. Stressor-induced activation of the autonomic nervous system affects gastric acid, bile, and mucus secretion as well as gut motility (Beckh and Arnold, 1991; Shigeshiro et al., 2012; Soderholm and Perdue, 2001), all factors that impact gut microbes (Boesjes and Brufau, 2014; Drasar et al., 1969; Santos et al., 1999; Saunders et al., 2002; Sommer et al., 2014; Sommer and Backhed, 2013; Tache and Perdue, 2004). Moreover, in vivo and in vitro studies demonstrate that microbial composition can be altered through a direct recognition of stress hormones, including norepinephrine and epinephrine (Freestone et al., 1999; Freestone et al., 2002; Lyte, 2004; Lyte and Bailey, 1997; Lyte et al., 2003; Lyte et al., 2011).
Determining the dynamics of the behavior-gut associations in early life is important because many physical and mental health conditions (e.g., obesity, anxiety) have early life antecedents (Caspi et al., 1996; Parsons et al., 1999) and the gut microbiome may be more malleable in early versus later life (Clarke et al., 2013). Considerable changes in the structure of the gut microbiota occur during the first year of life in response to changing diet (i.e., introduction of solid foods) and environmental exposures (Dominguez-Bello et al., 2010; Favier et al., 2003). However, by approximately two years of age, profiles of gut microbiota resemble profiles found in adults (Koenig et al., 2011; Palmer et al., 2007). Once established, these profiles are relatively stable; although the gut microbiome changes in response to illness, diet, and exposures such as antibiotics, overall profiles and the majority of dominant microbes tend to revert back to the pre-exposure state after a given disruption has passed (David et al., 2014; De La Cochetiere et al., 2005; Dethlefsen et al., 2008). Thus, assessment of the gut microbiome as early as two years of age may provide insight as to long-term functioning.
In order to link gut microbiome composition to behavior in early life, behavior must be captured in a valid and relevant manner. Reflecting affective-motivational and attentional style, temperament is a central construct in behavioral measurement in early childhood. Parental as well as direct observational ratings of temperament in early childhood predict personality, behavior, and risk for psychopathology in later childhood, adolescence, and adulthood (Rothbart and Posner, 2006). In addition, temperament has been linked to differences in functioning of the HPA axis (Dougherty et al., 2013; Mackrell et al., 2014) as well as autonomic nervous system (Brooker and Buss, 2010; Huffman et al., 1998; Stifter and Fox, 1990), providing a plausible basis by which individual differences in temperament may be mechanistically linked to the gut microbiome.
In this study, we examined the association between the community structure of the gut microbiome, using next generation pyrosequencing, and maternal ratings of child temperament in 77 children assessed at approximately two years of age. In this exploratory investigation, we hypothesized that children would differ in their gut microbial structure, as indicated by diversity, richness, and evenness of communities, based on their temperamental characteristics. Consistent with the literature reviewed, we postulate direct physiological pathways linking temperament and gut microbiome composition. However, the role of diet must also be considered, as diet appreciably affects gut microbiome composition (David et al., 2014; Wu et al., 2011). Thus, we examined dietary patterns in relation to temperament and the gut microbiome in this cohort.
Methods
Study Design
This study included 79 mother-toddler pairs. Mothers of toddler-aged children were recruited from the general community of Columbus, Ohio. Children were excluded if their mother reported the child had a major health condition or developmental delay. Children were also excluded if they were already toilet trained, as this hindered collection of stool samples. Each mother completed an online questionnaire that included assessment of her child's temperament and feeding behaviors, as detailed below.
Stool samples were collected by the mother from the child within 7 days of questionnaire completion by the mother, as per the protocol detailed below. A final sample of 77 mother-toddler pairs were used after removing two samples due to low sequence count (<5108). This study was approved by the Ohio State University Biomedical Institutional Review Board. All women completed written informed consent for themselves and provided written consent on behalf of their children. Women received modest compensation for their participation. Data collection occurred from May 2011 to December 2012.
Demographic Characteristics and Child Diet
Women provided their age, race (self and child's father), marital status, and child's sex. Women also reported the occurrence and duration of breastfeeding and the age at which formula (if applicable), cereals/grains, fruits/vegetables, and meats were introduced as part of the child's diet. The current frequency of each food type was also reported, from less than once per month to two or more times per day.
Child Temperament
Temperament was assessed with the Early Childhood Behavior Questionnaire (ECBQ), a widely used and well-validated instrument appropriate for children 18-36 months. This is a finely differentiated measure providing 18 dimensions of temperament that load onto three composite scales: Negative Affectivity, Surgency/Extraversion, and Effortful Control (Putnam et al., 2006). Subscales are detailed and defined in Table 1.
Table 1. Scale Definitions from the Early Childhood Behavior Questionnaire (ECBQ).
| Negative Affectivity | |
| Discomfort | Negative affect in response to stimulation |
| Fear | Negative affect related to anticipated pain, distress, sudden events and/or potentially threatening situations |
| Motor Activation | Repetitive small-motor movements; fidgeting |
| Sadness | Tearfulness or lowered mood related to suffering, disappointment, or loss |
| Perceptual Sensitivity | Detection of slight, low intensity stimuli from the external environment |
| Shyness | Slow or inhibited approach and/or discomfort in social situations involving novelty or uncertainty |
| Soothability | Rate of recovery from peak distress, excitement, or general arousal |
| Frustration | Negative affect related to interruption of ongoing tasks or goal blocking |
| Surgency/Extraversion | |
| Impulsivity | Speed of response initiation |
| Activity Level | Level (rate and intensity) of gross motor activity, including rate and extent of locomotion |
| High-Intensity Pleasure | Pleasure or enjoyment related to situations involving high intensity, rate, complexity, novelty and incongruity |
| Sociability | Seeking and taking pleasure in interactions with others |
| Positive Anticipation | Excitement about expected pleasurable activities |
| Effortful Control | |
| Inhibitory Control | The capacity to stop, moderate, or refrain from a behavior under instruction |
| Attentional Shifting | The ability to transfer attentional focus from one activity/task to another |
| Low-Intensity Pleasure | Pleasure or enjoyment related to situation involving low intensity, rate, complexity, novelty and incongruity |
| Cuddliness | Child's expression of enjoyment in and molding of the body to being held by a caregiver |
| Attentional Focusing | Sustained duration of orienting on an object of attention; resisting distraction |
Stool Sample Collection and Storage
Stool samples were used for analysis of the child gut microbiome in lieu of tissue collection due to the advantages of non-invasive collection and the common use of stool in human microbiome analysis (Qin et al., 2014; Raman et al., 2013; Stiverson et al., 2014; Xiao et al., 2014). Women were provided with sterile wooden applicators and 50-milliliter plastic conical collection tubes for collection. The stool was sterilely collected from the child's soiled diaper with the wooden applicator and placed in the collection tube. Samples were stored at 4° Celsius (i.e., refrigerated) for up to 24 hours until collection by study personnel from the participant's home or delivery by the participant to the Ohio State University Wexner Medical Center (OSUWMC). In the latter case, women were instructed to transport samples in a cooler with ice. While at OSUWMC, samples were stored at -80°C until pyrosequencing was conducted.
bTEFAP
Bacterial tag-encoded FLX-Amplicon Pyrosequencing (bTEFAP) was performed as previously described (Dowd et al., 2008a; Dowd et al., 2008b). The 16s rrn universal primers 27f (AGA GTT TGA TCM TGG CTC AG) and 519r (GWATTACCGCGGCKGCTG) were used in a single-step 30 cycle PCR with the following thermoprofile: a single cycle of 94°C for 3 minutes, then 28 cycles of: 30 seconds at 94°C ; 40 seconds at 53°C, 1 minute at 72°C, with a single 5 minute cycle at 72°C for 5 minutes for elongation. Amplicons were pooled at equivalent concentrations and purified (Agencourt Bioscience Corporation, MA, USA). Sequencing was performed with the Roche 454 FLX Titanium system using manufacturer's guidelines.
Sequencing Analysis
The software package, Quantitative Insights Into Microbial Ecology (QIIME), v.1.8.0. (Caporaso et al., 2010b) was used for filtering and analysis of attained sequences. Quality filtering and demultiplexing were performed using the provided sequence file (.fasta) and sequence quality file (.qual). Filtering was completed with the following parameters: quality score >25, sequence length between 200bp-1000bp, 6 allowed ambiguous bases, maximum of 6 homopolymer run, and zero allowed primer mismatches. On average, 14862 sequences passed filtering per sample.
UClust (Edgar, 2010) clustered sequences at 0.97 similarity into operational taxonomic units (OTUs). After representative sequence selection for each OTU, Greengenes v.13_8 was used for taxonomic assignment (McDonald et al., 2012). PyNAST was used for sequence alignment (Caporaso et al., 2010a) with the Greengenes core reference alignment database (DeSantis et al., 2006). A phylogenetic tree was constructed from these alignments with FastTree for downstream statistical analysis. (Price et al., 2010). Sequences from boys and girls were filtered and de-multiplexed using the above method together, but were separated before OTU-picking.
Statistical Analyses
Because prior data show that temperament ratings differ by child sex (e.g., Casalin et al., 2012), we compared temperament ratings between boys and girls. Temperament ratings among boys versus girls were compared via t-tests. As temperament ratings differed by child sex, analyses related to associations between temperament and gut microbiome composition were conducted separately for boys and girls. Cases in which temperament measures were ≥ 3 standard deviations from the mean were considered outliers and excluded from analyses.
Alpha diversity was measured with a phylogenetic diversity measurement, PD_Whole_tree, and the Shannon Diversity Index (SDI), a non-phylogenetic measurement of bacterial abundance (richness) and how equal these abundances are (evenness), using QIIME (Faith and Baker, 2006; Shannon, 1997). Depths of 5780 sequences for boys and 4838 sequences for girls were used in statistical analyses related to the SDI. Two samples were below the threshold for SDI, resulting in a sample of 75 for analyses of SDI. Parametric t-tests were used to compare SDI and PD whole tree values. In order to detect changes in beta-diversity defined as the overall microbiota community composition, weighted (accounts for abundances of OTUs) and unweighted (presence/absence of OTUs only) UniFrac distances were used (Lozupone and Knight, 2005). For beta-diversity, a depth of 5108 sequences/sample was used for boys, and 6022 sequences/sample for girls. UniFrac distance variances were measured and beta diversity compared through permutational multivariate analysis of variance through the vegan package on the open-source statistical software R, and implemented in QIIME (Oksanen J. et al., 2012; R Core Team Development, 2013). Temperament characteristics, Surgency/Extraversion, Sociability, High intensity pleasure and activity level were measured and analyzed as continuous covariates in their relationship with beta diversity.
Pearson's correlations (denoted by r) and regression analyses were used to examine associations between temperament ratings as continuous measures with the SDI. Spearman's correlation coefficient (denoted by rs) was used to estimate associations between the temperament ratings and genus abundances given the skewed distribution of the bacterial abundances. Associations between genus abundances and diet were assessed by the rank based Kruskal-Wallis test and rank based linear regression was used to explore the relationship between several covariates (predictors) on genus abundances. To examine the potential mediating role of diet in the relationship between temperament and the gut microbiome, we examined eating behavior in association with those temperament characteristics that had previously shown significant associations with microbiome parameters. All presented p-values are two-sided and are unadjusted for multiple hypothesis tests. These analyses were performed using SPSS v.21 (IBM, Chicago, IL) and Stata Statistical Software version 13 (StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP.).
Results
Participant characteristics
This study included 77 children, 41 boys and 36 girls. Children were 18-27 months at the time of assessment (Mean = 23.14 SD = 2.00), with 91% falling between 21-26 months. In this sample, 87.0% (n = 67) of mothers were White, 9.1% (n = 7) were Black and 3.9% (n = 3) were Asian. The mean maternal age at the time of delivery was 31.1 (SD=5.43) and 87.0% of mothers (n = 67) were married.
Temperament ratings and gut microbiome indicators by child sex
Significant sex differences in temperament ratings were found for 4 of 18 individual scales. Boys received higher ratings for Motor Activation, (t(75) = 2.31, 95% CI [0.056, 0.77], p = 0.024), and High-Intensity Pleasure, (t(75) = 2.57, 95% CI [0.13, 1.01], p = 0.012), while girls were rated as having greater Inhibitory Control, (t(75) = -2.49, 95% CI [-0.95, -0.11], p = 0.015), and Soothability, (t(75) = -2.12, 95% CI [-0.69, -0.02], p = 0.037). Correspondingly, significant differences were observed for the 2 composite scales on which these 4 individual scales loaded. Specifically, compared to girls, boys were rated more highly on Surgency/Extraversion, (t(75) = 2.28, 95% CI [0.04, 0.61], p = 0.026), and lower on the Effortful Control scale (t(75) = -2.37, 95% CI [-0.46, -0.40], p = 0.02).
In relation to microbiome measures, boys and girls did not differ substantially in alpha diversity as indicated by comparison of the Shannon Diversity Index (t(73) = -0.92, 95% CI [-0.76, 0.26], p = 0.36) or through the phylogenetic diversity measure, (i.e., PD_whole_tree; t(73) = -0.69, p = 0.49). In addition, community distance matrices were compared in boys versus girls directly, showing no significant difference in the community structure of the fecal microbiota between boys and girls using an unweighted UniFrac distance matrix (p = 0.776) or a weighted UniFrac distances (p = 0.68).
Thus, sex differences in temperament ratings were not paralleled in differences in the microbiome. Indeed, sex differences in temperament have been established (Else-Quest et al., 2006). Moreover, sex differences in the associations between HPA axis functioning and child behavior (Kryski et al., 2013), and the associations between child temperament, HPA axis functioning, and child behavior (Hastings et al., 2011), have been reported. As such, subsequent analyses were conducted separately for girls versus boys. For similar reasons, others studying the associations of temperament with food consumption and related constructs have also analyzed data for boys and girls separately (e.g., Faith and Hittner, 2010; Vollrath et al., 2012).
Child temperament and alpha and beta diversity in the gut microbiome
We first examined the association between temperament ratings and both the Shannon Diversity Index (SDI) and phylogenetic diversity measurement provided by QIIME (PD_Whole_tree). Among boys, higher scores on the composite scale of Surgency/Extraversion were associated with greater phylogenetic diversity (r = .414, p = 0.009; Fig 1a), but were not significantly associated with the SDI (r = .249, p =0.126; Supp Fig 1c). Also among boys, two subscales that load on the composite scale of Surgency/Extraversion were associated with microbiome measures. Specifically, greater Sociability was associated with greater phylogenetic diversity (r = 0.55, p < 0.001; Fig 1b) and higher SDI scores (r = 0.45, p = 0.004; Supp. Fig 1A). In addition, an association between greater High-Intensity Pleasure and higher phylogenetic diversity (r = 0.35, p = 0.029; Fig 1c) and SDI scores (r = 0.31, p = 0.052; Supp Fig 1a) were also observed.
Figure 1. a-c. Temperament and Phylogenetic Diversity.
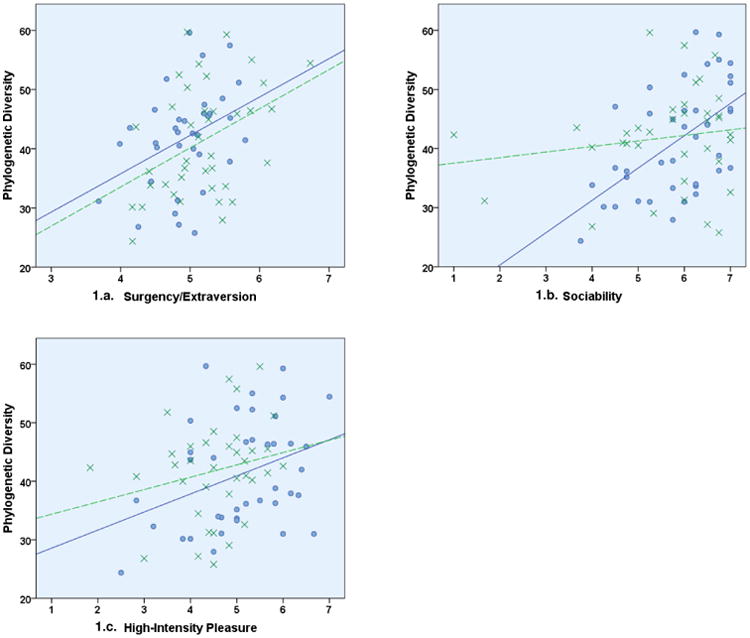
Associations between temperament characteristics and phylogenetic diversity among boys (circles; solid lines) and girls (X's; dashed lines). Significant associations were observed among both boys and girls for the composite scale of Surgency/Extraversion (ps ≤ 0.03). Among boys only, phylogenetic diversity was significantly correlated with the subscales of Sociability (r = 0.55, p < 0.001) and High-Intensity Pleasure (r = 0.35, p = 0.029).
Paralleling results in boys, among girls, higher scores on the Surgency/Extraversion composite scale were associated with greater phylogenetic diversity (r = .375, p = 0.027; Fig 1a) but not the SDI (r = .249, p = .126; Supp Fig 1c). Also among girls, lower scores on the composite scale of Effortful Control were significantly associated with higher SDI scores (r = -0.38, p = 0.023; Supp Fig 1d) but there was no association of this scale with phylogenetic diversity (r = -2.1, p = 0.22).
No temperament variables were significantly associated with age in boys or girls. However, both the SDI and phylogenetic diversity were associated with age at sampling among boys, (r = 0.42, p = 0.009 and r = .49, p = 0.001, respectively), but not in girls (r = 0.11, p = 0.512 and r = .073, p = 0.673, respectively). When age was included in the model, associations of High-Intensity pleasure with the SDI and phylogenetic diversity among boys were attenuated (p = 0.166 and p = 0.117, respectively). However, associations of Sociability with SDI and phylogenetic diversity among boys were not meaningfully affected (p = 0.033 and p = 0.005, respectively). In addition, the association between the Surgency/Extraversion composite scale and phylogenetic diversity remained after controlling for age (p = .052).
To support these analyses, unweighted UniFrac distance matrices, based upon the presence and absence of bacterial OTUs, and weighted UniFrac distance matrices which accounted for the actual abundances of the OTUs, were used to assess differences between overall microbiota community structures, known as beta diversity, in children based on temperament ratings. The Adonis statistic showed that, among boys, Surgency/Extraversion was associated with a unique microbiota community structure using unweighted UniFrac (p = 0.002; Fig 2a), but not weighted UniFrac (p = 0.061; Supp. Fig 2a). Analyses of subscales demonstrated that 3 subscales loading on this composite scale drove the effect seen with unweighted UniFrac distances. Specifically, Sociability (p = 0.025; Fig 2b), High-Intensity Pleasure (p < 0.008; Fig 2c), and Activity Level (p = 0.039; Fig 2d) were all significantly associated with differences in microbiota structures among boys. Only High-Intensity Pleasure was significantly associated with a different microbiota community structure when OTU abundances were included via weighted UniFrac (p = 0.035; Supp Fig 2b-d).
Figure 2. Beta Diversity and Temperament in Boys.
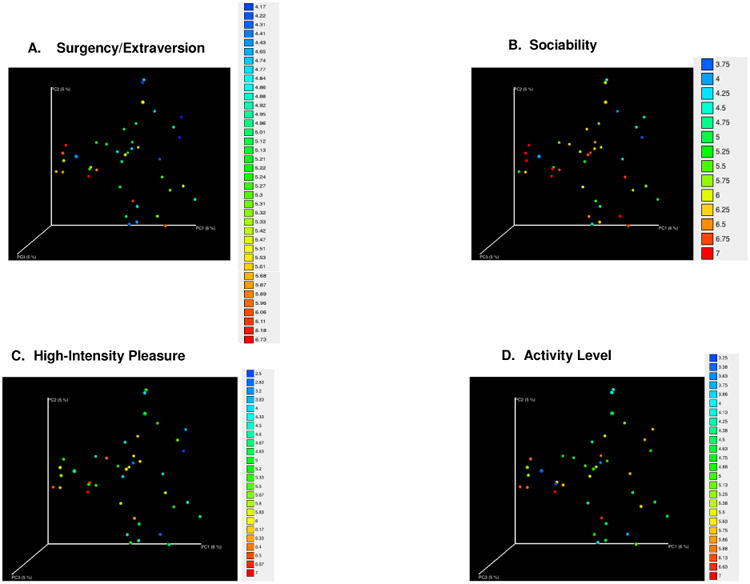
Permutational multivariate analysis of variance indicated that microbial community structure was significantly different in boys with different levels of Surgency/Extraversion (p < .05). This was reflected in Principal Coordinate Analyses (PCoA) in which samples were plotted continuously based on ratings in A) Sociability/Extraversion and its subscales, including B) Sociability, C) High-Intensity Pleasure, and D) Activity subscales of the Surgency/Extraversion composite scale. Overall, samples were distributed on a gradient based on increasing temperament level. PCoAs were based on Unweighted UniFrac distances calculated by QIIME.
In contrast, among girls only one significant association was observed; the subscale for Fear, which loads on the composite scale of Negative Affectivity, was associated with a unique microbiome using unweighted UniFrac distances (p = 0.03; Fig 3). This was not significant using weighted UniFrac distances (p = 0.095; Supp Fig 3).
Figure 3. Beta Diversity and Temperament in Girls.
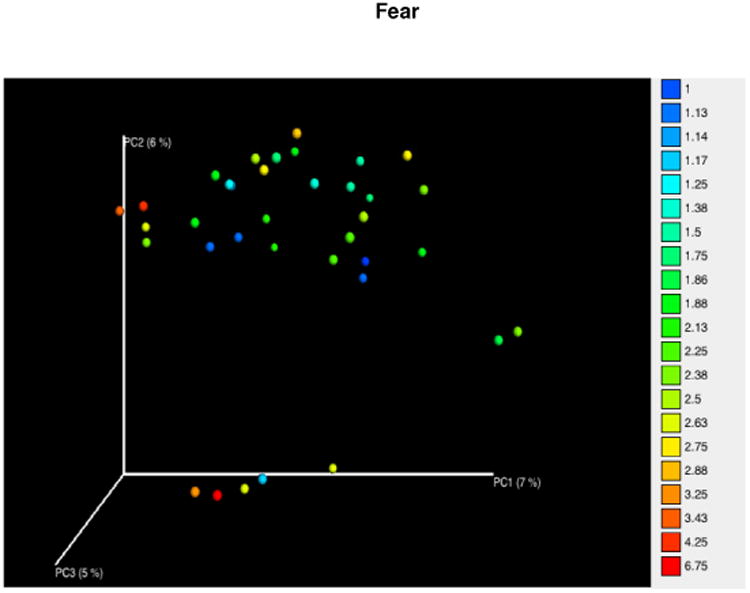
Microbial populations were different in girls with different Fear ratings, which loads on the Negative Affectivity composite scale, as indicated by a significant difference in Permutational multivariate analysis of variance (p = .03). Samples were distributed on a gradient based on increasing Fear rating. Unweighted UniFrac distances were calculated in QIIME.
Child temperament and phylogenetic differences in the gut microbiome
We next examined phylogenetic differences in the fecal microbiome of the children to determine if differences in abundances of given genera were evident in relation to temperamental characteristics. Analyses were limited to the genera that made up at least 1% of the total sample by relative abundance, in order to focus on the association between the dominant, highly abundant genera and temperament (Kong et al., 2013), as lesser abundant genera may have reduced functional input (Bajaj et al., 2012). This encompassed the top 20 genera for boys (92% of total male sample sequences) and the top 18 genera for girls (92% of total female sample sequences).
In boys, three subscales that load onto the composite scale of Surgency/Extraversion were related to differences in abundances. Sociability was positively associated with the abundances of an undefined genus in the family Ruminococcaceae (rs = 0.37, p = 0.019) and the genus Parabacteroides (rs = 0.44, p = 0.004). High-Intensity pleasure was positively associated with the genus Dialister (rs = 0.37, p = 0.019) and an undefined genus in the family Rikenellaceae (rs = 0.43, p = 0.005), while Activity Level was positively associated with abundances of the genus Dialister (rs = 0.48, p = 0.001) and an undefined genus in the family Rikenellaceae (rs = 0.35, p = 0.026). In girls, Fear was positively associated with an undefined genus in the family Rikenellaceae (rs = 0.37, p = 0.028).
The potential association of these genera with age at the time of sampling was examined. Among boys, age was significant associated with abundances of Ruminococcaceae ((rs = 0.43, p = 0.005), but no other markers. Analyses including age in the model demonstrated that the observed associated of Ruminococcaceae with Sociability among boys was attenuated (F(1,38) = 2.51, p = 0.121).
Associations of temperament with diet
Among boys, temperament characteristics associated with microbiome differences (Surgency/Extraversion, Activity Level, High-Intensity Pleasure, Sociability) were not associated with breastfeeding duration (<6 mos vs ≥ 6 mo; t(39) ≤ 1.7, ps ≥ 0.09), age at which grains/cereals were introduced into the diet (< 6 mos vs ≥ 6 mos; t(39) ≤ 1.46, ps ≥ 0.15), or age at which non-cereal foods (vegetable, fruits, and/or meats) were introduced (< 6 mos vs ≥ 6 mos; t(39) ≤ 0.86, ps ≥ 0.43).
In relation to current feeding patterns in boys, frequency of meat consumption (< once per day, once per day and > once per day) was related to High-Intensity Pleasure (F(2, 38) = 3.38, p = 0.045), with higher scores on both scales associated with less meat consumption. Frequency of vegetable consumption (< once per day, once per day, > once per day) was also significantly associated with High-Intensity Pleasure (F(2, 38) = 4.54, p = 0.017), with higher scores among those with less vegetable consumption.
In girls, temperament characteristics associated with differences in the microbiome (Fear and Effortful Control) were not associated with breastfeeding duration (t(34) ≤ .28, ps ≥ 0.78), age at which grains/cereals were introduced into the diet (t(34) ≤ 1.6, ps ≥ 0.13), or age at which non-cereal foods (vegetable, fruits, and/or meats) were introduced (t(34) ≤ 0.18, ps ≥ 0.46). In relation to current feeding patterns in girls, neither Fear nor Effortful Control were associated with the frequency of vegetable consumption (Fear: F(2, 33) = 1.01, p = 0.374), Effortful control: F(2, 33) = 0.17, p = 0.845) or meat consumption (Fear: F(2, 33) = 0.51, p = 0.604), Effortful control: F(2, 33) = 0.17, p = 0.843).
Child temperament, diet and the gut microbiome
To examine a potential mediating role for diet, we further examined eating behaviors and indicators of gut microbiome composition associated with High-Intensity pleasure in boys, as this temperament characteristic showed associations with both. Kruskal-Wallis rank test revealed no associations of abundances of either Dialister with either meat (p = 0.974) or vegetable consumption (p = 0.331) in boys. In relation to Rikenellaceae, significant associations were seen with vegetable consumption (p = 0.039), but not meat consumption (p = 0.117). Rank based regression models demonstrated that when both vegetable consumption and High-Intensity Pleasure were included in the model with Rikenellaceae as the outcome, effects of vegetable consumption were considerably reduced (F(2, 37) = 1.47, p = 0.244), while effects of High-Intensity Pleasure were only marginally attenuated (F(1, 37) = 3.28, p = 0.078). These relationships were not meaningfully affected by the addition of child age to the model.
Discussion
In the current investigation, we found differences in alpha and beta diversity as well as the structure and specific bacterial taxa of the gut microbiome in association with maternal ratings of temperament in toddlers, particularly among boys. Some associations between temperament and dietary patterns were observed. However, these did not appear to explain the observed differences in the microbiome.
The most consistent associations were observed in relation to Surgency/Extraversion. Higher scores on this composite scale were associated with greater phylogenetic diversity in boys as well as girls. In addition, among boys only, subscales loading on the composite scale of Surgency/Extraversion were associated with differences in beta diversity, the SDI, and differences in the relative abundances of Dialister, Rikenellaceae, Ruminococcaceae, and Parabacteroides although some of these relationships were attenuated by the inclusion of age at sampling in the model. The Surgency/Extraversion scale reflects a trait aspect of emotional reactivity characterized by a tendency towards high levels of positive affect, engagement with the environment, and activity. In children, higher scores are associated with lower depressive symptoms (Rothbart and Posner, 2006). Some data suggest that greater Surgency/Extraversion assessed as early as 3 months of age may be associated with growth trajectories in infants (Burton et al., 2011). Similarly, low sociability in 6-12 year olds has been linked with higher BMI at ages 24-30 years (Pulkki-Raback et al., 2005).
Overall, associations of temperament with the gut microbiome in girls were fewer and less consistent in terms of their clustering with particular temperament scales. In addition to the noted association of higher Surgency/Extraversion with greater phylogenetic diversity, higher Effortful Control was associated with a lower SDI score and differences in both beta diversity and Rikenellaceae were observed in relation to Fear. Greater Effortful Control reflects better executive attention and regulation of emotional responses and can buffer from risk of depression and anxiety (Rothbart and Posner, 2006). In addition, greater inhibitory control in 2-year-olds has been associated with reduced risk of being classified as overweight or at-risk in later childhood (Graziano et al., 2010). It is unknown if such relationships between temperament and body composition may be mediated by differences in the gut microbiome.
Analyses were conducted separately for boys versus girls because, as expected, temperament ratings differed based on child sex. Specifically, girls were rated higher in Effortful Control and boys had higher scores for Surgency/Extraversion. Both findings are highly consistent with prior studies (Casalin et al., 2012; Gartstein and Rothbart, 2003; Parade and Leerkes, 2008; Putnam et al., 2006). Sex differences are also seen in experimenter ratings of children's behavioral responses to standardized laboratory tasks (Kochanska et al., 2000). Thus, although parental gender bias may play a role, objective behavioral differences likely underlie these ratings. In contrast, there were no sex differences in microbiome indicators. Because sex differences in temperament did not correspond to differences in the gut microbiome, associations of microbial profiles with temperament characteristics may be most readily interpreted in the context of same sex comparisons. Notably, emerging data from animal studies also suggests that associations between the gut and behavior are sex dependent (Clarke et al., 2013).
The significant associations between microbiota community structure and temperament ratings observed using unweighted UniFrac distances were not duplicated using weighted UniFrac distances. This indicates that it is the presence and absence of bacterial OTUs, not the relative abundance of the OTUs, that is directing this association with overall community structure. Despite this, there were significant correlations between the relative abundance of bacteria in the families Rickenellaceae and Ruminococcaceae, and the Parabacteroides and Dialister genera and temperament (i.e., Fear in girls and Activity Level and High-Intensity Pleasure in boys). This is not the first report of an association between these groups and host behavior. For example, Alistipes, a member of Rikenellaceae, has been associated with depression in humans and is increased in stressor-exposed mice using the grid-floor stress model (Bendtsen et al., 2012; Naseribafrouei et al., 2014). Likewise, members of the family Ruminococcaceae have been associated with differences in behavior in mice in the grid-floor stress model, as assessed using anxiety-like behavior tests such as open-field testing and the elevated plus maze (Bendtsen et al., 2012), and in humans, Parabacteroides has been associated with autism (Finegold et al., 2010). Though not previously associated with host behavior, Dialister has been linked to lower levels of IL-6 after exposure to a whole gratin diet in humans (Martinez et al., 2013). However, higher Dialister in rectal samples from children with appendicitis, an inflammatory condition, versus controls have been reported. (Jackson et al., 2014). Thus, associations of Dialster with host inflammatory function are suggested, although the nature of this relationship is poorly defined. The presence of the gut microbiota is key in the gut-brain axis and in behavior, as shown in mouse studies, but more work must be done to delineate how individual bacterial groups impact host behavior, as well as the extent to which these associations evolve over time (e.g., with chronic exposure to a given behavior pattern or bacterial group).
In order for direct physiological pathways to plausibly underlie the observed associations, key behavioral influences must be ruled out. A primary behavioral pathway by which the gut and temperament may be linked is diet. As noted earlier, although parents control what foods are offered, children with certain temperamental characteristics may accept different quantities, varieties, or types of food (Faith and Hittner, 2010; Haycraft et al., 2011). In addition, parental feeding behavior may be influenced by child temperament; for example, parents may use food to soothe or reward fussy children (Stifter et al., 2011). As described, in the current study, we observed some associations between temperament and child dietary patterns, as reported by mothers. However, these differences did not appear to be a central contributor to the observed temperamental differences in the microbiome, supporting a role for postulated direct physiological links between the gut and brain. However, in this pilot investigation, diet was measured in a relatively simple manner. Comprehensive and detailed assessment of diet, ideally in a longitudinal manner would greatly strengthen future research.
If diet is truly not a central contributor to the observed associations, direct physiological pathways may be implicated. As described, animal studies demonstrate bi-directional connections between the gut and brain. For example, in infant Rhesus monkeys, the stress of maternal separation causes significant disruptions in the composition of the gut microbiome (Bailey and Coe, 1999). Thus, temperament may affect the microbiome via differences in novelty seeking, stressor exposure, and responses to stressors. Conversely, the gut affects behavior/stress-responses. For example, germ-free mice exhibit an exaggerated HPA response compared to conventional mice (Sudo et al., 2004). Therefore, a causal pathway from gut to temperament is also possible. In the current study, due to the correlational nature of the analyses, we are unable to determine if observed associations are a function of effects of temperament on the gut, effects of gut on temperament, or a combination thereof.
In addition to dietary and direct physiological pathways linking gut microbiome composition and behavior in early life, the potential role of the prenatal environment must be considered. Most dimensions of temperament show moderate genetic influences (Saudino, 2005) and prenatal stress has been shown to alter bacterial colonization of the gut in infant monkeys (Bailey et al., 2004). Thus, as child temperament may reflect genetic factors shared with the mother, it is possible that differences in the composition of the gut microbiome in relation to child temperament may actually reflect differences in the prenatal environment.
Studies in both human subjects and animal samples have highlighted major differences in the communities that comprise the stool and mucosa-associated microbiomes of the host GI tract (Carroll et al., 2011; Hong et al., 2011; Zoetendal et al., 2002). Those microbes that adhere to the mucosal layer nearer the epithelium are believed to interact with host immunity, guiding immunoregulation, while bacteria associated with the luminal/fecal niche are involved in nutrition and metabolism (Van den Abbeele et al., 2011). However, it should be noted that true stratification of the luminal and mucosal populations does not exist. Microbes that can adhere to colonic mucus originate from the lumen of the intestines, and over time, tissue-associated microbes are shed into the lumen. Thus, there is substantial crossover between luminal and tissue-associated microbial populations. To obtain a complete picture of the GI microbiome, it is important to analyze both luminal and stool microbiota samples. However, colonic tissue sample collection in humans is particularly invasive and the lone use of stool in human microbiome studies is common (Qin et al., 2014; Raman et al., 2013; Stiverson et al., 2014; Xiao et al., 2014).
This study was cross-sectional and observational in approach and, therefore, does not permit determination of the causal direction of effects. However, if the gut microbiome influences human behavior in a meaningful and relatively stable manner, this may represent an opportunity for early life intervention. Psychiatric disorders account for a larger portion of disability in developed countries than any other group of illnesses including cancer and heart disease (Reeves et al., 2011). As with physical health disorders, behavior problems in childhood and mental health disorders in adulthood are commonly preceded by indicators earlier in life (Caspi et al., 1996; Lahey et al., 2008). Thus, identification of modifiable early life antecedents may be key to addressing this global health burden.
Temperament in this study was defined based on maternal report. Reports from primary caregivers are desirable because they have the greatest opportunity to observe the child's behavior across a wide variety of situations and contexts (Gartstein and Rothbart, 2003). Moreover, parental reports have excellent predictive validity in relation to future child behavior problems (Gartstein et al., 2012; van Aken et al., 2007). However, maternal and paternal reports of child temperament tend to differ (Casalin et al., 2012; Parade and Leerkes, 2008), which may be attributable to biases as well as interaction styles (e.g., more rough play of fathers with boys) which elicit different behaviors from the child. In addition, parental reports often do not correspond strongly with observers' ratings of the child's behavior in standardized settings (Mangelsdorf et al., 2000). Thus, while each provides insight, maternal ratings, paternal ratings, and objective observations tap into different aspects of the construct of temperament. Inclusion of observational as well as both maternal and paternal reports would greatly strengthen future studies on this topic.
In the current investigation, we used next generation 454 pyrosequencing which allows for wider study of microbial communities than permitted by earlier methods, including denaturing gradient gel electrophoresis (DGGE) and polymerase chain reaction (PCR). This technology permits the analyses of entire bacterial communities rather than examination of smaller classification subsets selected by a priori hypotheses. Thus, our ability to examine the gut microbiome in a comprehensive manner is greatly enhanced by technological advancements. The 454 pyrosequencing approach was chosen over competing technologies, particularly Illumina, due to the greater average amplicon length at the time the sequencing was conducted. In addition, although it provides less coverage (sequences per sample), 454 pyrosequencing has a lower error rate. Thus, while greater sequence depth might have allowed for the detection of more bacterial groups and OTUs, the greater read length provided by 454 pyrosequencing granted classification at lower taxonomic levels. The average sequence depth was ∼14k per sample and a rarefaction curve indicated that increasing depth further would not substantially increase observed OTUs, supporting the use of 454 in this dataset. However, we did not assess microbial function, through the use of metagenomic or metatranscriptomic methodologies, or microbial metabolites, through metabolomics-based analyses. Such technologies will be utilized in future studies.
In conclusion, this study contributes to a growing literature that links gut microbiota to host behavior and physiology, by demonstrating that microbial populations are associated with host behavior, operationalized as maternal ratings of temperament. These associations were evident in this relatively small exploratory study in young children, suggesting that microbiota-behavior interactions are already evident at an early age and are relatively robust. Although this study was not designed to address causal pathways between the microbiota and host behavior, future studies involving larger cohorts and incorporating metagenomics, or even metabolomics, will help begin to elucidate mechanisms by which the bidirectional communication between the host and its microbiota occur.
Supplementary Material
Highlights.
This study examined associations between temperament and gut microbiome composition in toddlers
Among boys and girls, greater Surgency/Extraversion was associated greater phylogenetic diversity
Additional sex-specific associations were observed in relation to phylogenetic diversity, the SDI, beta diversity, and abundances of specific bacteria
The observed associations did not appear to be accounted for by differences in diet
Bidirectional brain-gut relationships may exert measurable effects in humans in early life
Acknowledgments
We would like to thank Clinical Research Assistants Kelly Marceau Wohleb and Rebecca Long for their contributions to data collection as well as our study participants.
Funding Sources: This study was supported by an Innovative Initiative Award to MTB and LMC from the Food Innovation Center at The Ohio State University. This study was also supported by awards from NINR (R01 NR01366) and NICHD (R21 HD067670) to LMC, NCCAM (R01 AT006552) to MTB and NIDCR (T32 DE014320). This study was supported by the Ohio State University Center for Clinical and Translational Sciences (CCTS), funded by UL1TR001070 from the National Center for Advancing Translational Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bailey MT, Coe CL. Maternal separation disrupts the integrity of the intestinal microflora in infant rhesus monkeys. Dev Psychobiol. 1999;35:146–155. [PubMed] [Google Scholar]
- Bailey MT, Lubach GR, Coe CL. Prenatal stress alters bacterial colonization of the gut in infant monkeys. J Pediatr Gastroenterol Nutr. 2004;38:414–421. doi: 10.1097/00005176-200404000-00009. [DOI] [PubMed] [Google Scholar]
- Bajaj JS, Hylemon PB, Ridlon JM, Heuman DM, Daita K, White MB, Monteith P, Noble NA, Sikaroodi M, Gillevet PM. Colonic mucosal microbiome differs from stool microbiome in cirrhosis and hepatic encephalopathy and is linked to cognition and inflammation. American journal of physiology Gastrointestinal and liver physiology. 2012;303:G675–685. doi: 10.1152/ajpgi.00152.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckh K, Arnold R. Regulation of bile secretion by sympathetic nerves in perfused rat liver. The American journal of physiology. 1991;261:G775–780. doi: 10.1152/ajpgi.1991.261.5.G775. [DOI] [PubMed] [Google Scholar]
- Bendtsen KMB, Krych L, Sorensen DB, Pang WY, Nielsen DS, Josefsen K, Hansen LH, Sorensen SJ, Hansen AK. Gut Microbiota Composition Is Correlated to Grid Floor Induced Stress and Behavior in the BALB/c Mouse. Plos One. 2012;7 doi: 10.1371/journal.pone.0046231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boesjes M, Brufau G. Metabolic Effects of Bile Acids in the Gut in Health and Disease. Current medicinal chemistry. 2014 doi: 10.2174/0929867321666140303142053. [DOI] [PubMed] [Google Scholar]
- Brooker RJ, Buss KA. Dynamic Measures of RSA Predict Distress and Regulation in Toddlers. Dev Psychobiol. 2010;52:372–382. doi: 10.1002/dev.20432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton P, Wells JCK, Kennedy K, Nicholl R, Khakoo A, Fewtrell MS. Association between infant correlates of impulsivity - surgency (extraversion) - and early infant growth. Appetite. 2011;57:504–509. doi: 10.1016/j.appet.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Caporaso JG, Bittinger K, Bushman FD, DeSantis TZ, Andersen GL, Knight R. PyNAST: a flexible tool for aligning sequences to a template alignment. Bioinformatics. 2010a;26:266–267. doi: 10.1093/bioinformatics/btp636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, et al. QIIME allows analysis of high-throughput community sequencing data. Nature methods. 2010b;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll IM, Ringel-Kulka T, Keku TO, Chang YH, Packey CD, Sartor RB, Ringel Y. Molecular analysis of the luminal- and mucosal-associated intestinal microbiota in diarrhea-predominant irritable bowel syndrome. American journal of physiology Gastrointestinal and liver physiology. 2011;301:G799–807. doi: 10.1152/ajpgi.00154.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casalin S, Luyten P, Vliegen N, Meurs P. The structure and stability of temperament from infancy to toddlerhood: a one-year prospective study. Infant Behav Dev. 2012;35:94–108. doi: 10.1016/j.infbeh.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE, Newman DL, Silva PA. Behavioral observations at age 3 years predict adult psychiatric disorders - Longitudinal evidence from a birth cohort. Arch Gen Psychiatry. 1996;53:1033–1039. doi: 10.1001/archpsyc.1996.01830110071009. [DOI] [PubMed] [Google Scholar]
- Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, Dinan TG, Cryan JF. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry. 2013;18:666–673. doi: 10.1038/mp.2012.77. [DOI] [PubMed] [Google Scholar]
- Collins SM, Bercik P. The Relationship Between Intestinal Microbiota and the Central Nervous System in Normal Gastrointestinal Function and Disease. Gastroenterology. 2009;136:2003–2014. doi: 10.1053/j.gastro.2009.01.075. [DOI] [PubMed] [Google Scholar]
- David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Cochetiere MF, Durand T, Lepage P, Bourreille A, Galmiche JP, Dore J. Resilience of the dominant human fecal microbiota upon short-course antibiotic challenge. J Clin Microbiol. 2005;43:5588–5592. doi: 10.1128/JCM.43.11.5588-5592.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Huber T, Dalevi D, Hu P, Andersen GL. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol. 2006;72:5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLOS Biology. 2008;6 doi: 10.1371/journal.pbio.0060280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinan TG, Cryan JF. Regulation of the stress response by the gut microbiota: implications for psychoneuroendocrinology. Psychoneuroendocrinology. 2012;37:1369–1378. doi: 10.1016/j.psyneuen.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, Knight R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107:11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty LR, Smith VC, Olino TM, Dyson MW, Bufferd SJ, Rose SA, Klein DN. Maternal psychopathology and early child temperament predict young children's salivary cortisol 3 years later. J Abnorm Child Psychol. 2013;41:531–542. doi: 10.1007/s10802-012-9703-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd SE, Callaway TR, Wolcott RD, Sun Y, McKeehan T, Hagevoort RG, Edrington TS. Evaluation of the bacterial diversity in the feces of cattle using 16S rDNA bacterial tag-encoded FLX amplicon pyrosequencing (bTEFAP) BMC Microbiol. 2008a;8 doi: 10.1186/1471-2180-8-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd SE, Wolcott RD, Sun Y, McKeehan T, Smith E, Rhoads D. Polymicrobial Nature of Chronic Diabetic Foot Ulcer Biofilm Infections Determined Using Bacterial Tag Encoded FLX Amplicon Pyrosequencing (bTEFAP) Plos One. 2008b;3 doi: 10.1371/journal.pone.0003326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drasar BS, Shiner M, McLeod GM. Studies on the intestinal flora. I. The bacterial flora of the gastrointestinal tract in healthy and achlorhydric persons. Gastroenterology. 1969;56:71–79. [PubMed] [Google Scholar]
- Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- Else-Quest NM, Hyde JS, Goldsmith HH, Van Hulle CA. Gender differences in temperament: a meta-analysis. Psychol Bull. 2006;132:33–72. doi: 10.1037/0033-2909.132.1.33. [DOI] [PubMed] [Google Scholar]
- Faith DP, Baker AM. Phylogenetic diversity (PD) and biodiversity conservation: some bioinformatics challenges. Evolutionary bioinformatics online. 2006;2:121–128. [PMC free article] [PubMed] [Google Scholar]
- Faith MS, Hittner JB. Infant temperament and eating style predict change in standardized weight status and obesity risk at 6 years of age. Int J Obesity. 2010;34:1515–1523. doi: 10.1038/ijo.2010.156. [DOI] [PubMed] [Google Scholar]
- Favier CF, de Vos WM, Akkermans ADL. Development of bacterial and bifidobacterial communities in feces of newborn babies. Anaerobe. 2003;9:219–229. doi: 10.1016/j.anaerobe.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Finegold SM, Dowd SE, Gontcharova V, Liu C, Henley KE, Wolcott RD, Youn E, Summanen PH, Granpeesheh D, Dixon D, et al. Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe. 2010;16:444–453. doi: 10.1016/j.anaerobe.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Forsythe P, Sudo N, Dinan T, Taylor VH, Bienenstock J. Mood and gut feelings. Brain Behav Immun. 2010;24:9–16. doi: 10.1016/j.bbi.2009.05.058. [DOI] [PubMed] [Google Scholar]
- Foster JA, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36:305–312. doi: 10.1016/j.tins.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Freestone PP, Haigh RD, Williams PH, Lyte M. Stimulation of bacterial growth by heat-stable, norepinephrine-induced autoinducers. FEMS microbiology letters. 1999;172:53–60. doi: 10.1111/j.1574-6968.1999.tb13449.x. [DOI] [PubMed] [Google Scholar]
- Freestone PP, Williams PH, Haigh RD, Maggs AF, Neal CP, Lyte M. Growth stimulation of intestinal commensal Escherichia coli by catecholamines: a possible contributory factor in trauma-induced sepsis. Shock. 2002;18:465–470. doi: 10.1097/00024382-200211000-00014. [DOI] [PubMed] [Google Scholar]
- Gartstein MA, Putnam SP, Rothbart MK. Etiology of Preschool Behavior Problems: Contributions of Temperament Attributes in Early Childhood. Infant Ment Health J. 2012;33:197–211. doi: 10.1002/imhj.21312. [DOI] [PubMed] [Google Scholar]
- Gartstein MA, Rothbart MK. Studying infant temperament via the Revised Infant Behavior Questionnaire. Infant Behav Dev. 2003;26:64–86. [Google Scholar]
- Graziano PA, Calkins SD, Keane SP. Toddler self-regulation skills predict risk for pediatric obesity. Int J Obesity. 2010;34:633–641. doi: 10.1038/ijo.2009.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings PD, Ruttle PL, Serbin LA, Mills RS, Stack DM, Schwartzman AE. Adrenocortical responses to strangers in preschoolers: relations with parenting, temperament, and psychopathology. Dev Psychobiol. 2011;53:694–710. doi: 10.1002/dev.20545. [DOI] [PubMed] [Google Scholar]
- Haycraft E, Farrow C, Meyer C, Powell F, Blissett J. Relationships between temperament and eating behaviours in young children. Appetite. 2011;56:689–692. doi: 10.1016/j.appet.2011.02.005. [DOI] [PubMed] [Google Scholar]
- Hong PY, Croix JA, Greenberg E, Gaskins HR, Mackie RI. Pyrosequencing-based analysis of the mucosal microbiota in healthy individuals reveals ubiquitous bacterial groups and micro-heterogeneity. PloS one. 2011;6:e25042. doi: 10.1371/journal.pone.0025042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman LC, Bryan YE, del Carmen R, Pedersen FA, Doussard-Roosevelt JA, Porges SW. Infant temperament and cardiac vagal tone: Assessments at twelve weeks of age. Child Dev. 1998;69:624–635. [PubMed] [Google Scholar]
- Huttenhower C, Gevers D, Knight R, Abubucker S, Badger JH, Chinwalla AT, Creasy HH, Earl AM, FitzGerald MG, Fulton RS, et al. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson HT, Mongodin EF, Davenport KP, Fraser CM, Sandler AD, Zeichner SL. Culture-independent evaluation of the appendix and rectum microbiomes in children with and without appendicitis. PloS one. 2014;9:e95414. doi: 10.1371/journal.pone.0095414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinross JM, Darzi AW, Nicholson JK. Gut microbiome-host interactions in health and disease. Genome Med. 2011;3 doi: 10.1186/gm228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanska G, Murray KT, Harlan ET. Effortful control in early childhood: Continuity and change, antecedents, and implications for social development. Dev Psychol. 2000;36:220–232. [PubMed] [Google Scholar]
- Koenig JE, Spor A, Scalfone N, Fricker AD, Stombaugh J, Knight R, Angenent LT, Ley RE. Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci U S A. 2011;108:4578–4585. doi: 10.1073/pnas.1000081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong LC, Tap J, Aron-Wisnewsky J, Pelloux V, Basdevant A, Bouillot JL, Zucker JD, Dore J, Clement K. Gut microbiota after gastric bypass in human obesity: increased richness and associations of bacterial genera with adipose tissue genes. The American journal of clinical nutrition. 2013;98:16–24. doi: 10.3945/ajcn.113.058743. [DOI] [PubMed] [Google Scholar]
- Kryski KR, Smith HJ, Sheikh HI, Singh SM, Hayden EP. HPA axis reactivity in early childhood: associations with symptoms and moderation by sex. Psychoneuroendocrinology. 2013;38:2327–2336. doi: 10.1016/j.psyneuen.2013.05.002. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Van Hulle CA, Keenan K, Rathouz PJ, D'Onofrio BM, Rodgers JL, Waldman ID. Temperament and Parenting during the First Year of Life Predict Future Child Conduct Problems. J Abnorm Child Psychol. 2008;36:1139–1158. doi: 10.1007/s10802-008-9247-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone C, Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol. 2005;71:8228–8235. doi: 10.1128/AEM.71.12.8228-8235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyte M. Microbial endocrinology and infectious disease in the 21st century. Trends in microbiology. 2004;12:14–20. doi: 10.1016/j.tim.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Lyte M, Bailey MT. Neuroendocrine-bacterial interactions in a neurotoxin-induced model of trauma. The Journal of surgical research. 1997;70:195–201. doi: 10.1006/jsre.1997.5130. [DOI] [PubMed] [Google Scholar]
- Lyte M, Freestone PP, Neal CP, Olson BA, Haigh RD, Bayston R, Williams PH. Stimulation of Staphylococcus epidermidis growth and biofilm formation by catecholamine inotropes. Lancet. 2003;361:130–135. doi: 10.1016/S0140-6736(03)12231-3. [DOI] [PubMed] [Google Scholar]
- Lyte M, Vulchanova L, Brown DR. Stress at the intestinal surface: catecholamines and mucosa-bacteria interactions. Cell and tissue research. 2011;343:23–32. doi: 10.1007/s00441-010-1050-0. [DOI] [PubMed] [Google Scholar]
- Mackrell SV, Sheikh HI, Kotelnikova Y, Kryski KR, Jordan PL, Singh SM, Hayden EP. Child temperament and parental depression predict cortisol reactivity to stress in middle childhood. J Abnorm Psychol. 2014;123:106–116. doi: 10.1037/a0035612. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf SC, Schoppe SJ, Buur H. The meaning of parental reports: A contextual approach to the study of temperament and behavior problems in childhood. In: Molfese VJ, M DL, editors. Temperament and personality development across the lifespan. Erlbaum; New Jersey: 2000. pp. 121–140. [Google Scholar]
- Martinez I, Lattimer JM, Hubach KL, Case JA, Yang J, Weber CG, Louk JA, Rose DJ, Kyureghian G, Peterson DA, et al. Gut microbiome composition is linked to whole grain-induced immunological improvements. The ISME journal. 2013;7:269–280. doi: 10.1038/ismej.2012.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald D, Price MN, Goodrich J, Nawrocki EP, DeSantis TZ, Probst A, Andersen GL, Knight R, Hugenholtz P. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. The ISME journal. 2012;6:610–618. doi: 10.1038/ismej.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naseribafrouei A, Hestad K, Avershina E, Sekelja M, Linlokken A, Wilson R, Rudi K. Correlation between the human fecal microbiota and depression. Neurogastroenterol Motil. 2014;26:1155–1162. doi: 10.1111/nmo.12378. [DOI] [PubMed] [Google Scholar]
- Oksanen J, Blanchet FG, Kindt R, Legendre R, M PR, O'Hara RB, Simpson GL, Solymos P, Stevens HH, Wagner H. R package version 2.0-3. 2012. Vegan: community ecology package. [Google Scholar]
- Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS Biol. 2007;5:1556–1573. doi: 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parade SH, Leerkes EM. The reliability and validity of the Infant Behavior Questionnaire-Revised. Infant Behav Dev. 2008;31:637–646. doi: 10.1016/j.infbeh.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons TJ, Power C, Logan S, Summerbell CD. Childhood predictors of adult obesity: a systematic review. Int J Obesity. 1999;23:S1–S107. [PubMed] [Google Scholar]
- Price MN, Dehal PS, Arkin AP. FastTree 2-Approximately Maximum-Likelihood Trees for Large Alignments. Plos One. 2010;5 doi: 10.1371/journal.pone.0009490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulkki-Raback L, Elovainio M, Kivimaki M, Raitakari OT, Keltikangasjarvinen L. Temperament in childhood predicts body mass in adulthood: The Cardiovascular Risk in Young Finns Study. Health Psychol. 2005;24:307–315. doi: 10.1037/0278-6133.24.3.307. [DOI] [PubMed] [Google Scholar]
- Putnam SP, Gartstein MA, Rothbart MK. Measurement of fine-grained aspects of toddler temperament: The early childhood behavior questionnaire. Infant Behav Dev. 2006;29:386–401. doi: 10.1016/j.infbeh.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin N, Yang F, Li A, Prifti E, Chen Y, Shao L, Guo J, Le Chatelier E, Yao J, Wu L, et al. Alterations of the human gut microbiome in liver cirrhosis. Nature. 2014;513:59–64. doi: 10.1038/nature13568. [DOI] [PubMed] [Google Scholar]
- R Core Team Development. R: a language and environment for statistical computing. 2.14.1 R Foundation for Statistical Computing; Coventry, United Kingdom: 2013. [Google Scholar]
- Raman M, Ahmed I, Gillevet PM, Probert CS, Ratcliffe NM, Smith S, Greenwood R, Sikaroodi M, Lam V, Crotty P, et al. Fecal microbiome and volatile organic compound metabolome in obese humans with nonalcoholic fatty liver disease. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2013;11:868–875 e861-863. doi: 10.1016/j.cgh.2013.02.015. [DOI] [PubMed] [Google Scholar]
- Reeves WC, Strine TW, Pratt LA, Thompson W, Ahluwalia I, Dhingra PS, McKnight-Eily LR, Harrison L, D'Angelo DV, Williams L, et al. Mental Illness Surveillance Among Adults in the United States. Morbidity and Mortality Weekly Report (MMWR) 2011;60:1–30. [PubMed] [Google Scholar]
- Rothbart MK, Posner MI. Temperament, attention, and psychopathology. In: Cohen DCaDJ., editor. Developmental Psychopathology. Wiley & Sons; New Jersey: 2006. pp. 465–501. [Google Scholar]
- Santos J, Saunders PR, Hanssen NP, Yang PC, Yates D, Groot JA, Perdue MH. Corticotropin-releasing hormone mimics stress-induced colonic epithelial pathophysiology in the rat. The American journal of physiology. 1999;277:G391–399. doi: 10.1152/ajpgi.1999.277.2.G391. [DOI] [PubMed] [Google Scholar]
- Saudino KJ. Behavioral genetics and child temperament. J Dev Behav Pediatr. 2005;26:214–223. doi: 10.1097/00004703-200506000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders PR, Santos J, Hanssen NP, Yates D, Groot JA, Perdue MH. Physical and psychological stress in rats enhances colonic epithelial permeability via peripheral CRH. Digestive diseases and sciences. 2002;47:208–215. doi: 10.1023/a:1013204612762. [DOI] [PubMed] [Google Scholar]
- Shannon CE. The mathematical theory of communication (Reprinted) M D Comput. 1997;14:306–317. [PubMed] [Google Scholar]
- Shigeshiro M, Tanabe S, Suzuki T. Repeated exposure to water immersion stress reduces the Muc2 gene level in the rat colon via two distinct mechanisms. Brain, behavior, and immunity. 2012;26:1061–1065. doi: 10.1016/j.bbi.2012.05.016. [DOI] [PubMed] [Google Scholar]
- Soderholm JD, Perdue MH. Stress and gastrointestinal tract. II. Stress and intestinal barrier function American journal of physiology. Gastrointestinal and liver physiology. 2001;280:G7–G13. doi: 10.1152/ajpgi.2001.280.1.G7. [DOI] [PubMed] [Google Scholar]
- Sommer F, Adam N, Johansson ME, Xia L, Hansson GC, Backhed F. Altered mucus glycosylation in core 1 O-glycan-deficient mice affects microbiota composition and intestinal architecture. PloS one. 2014;9:e85254. doi: 10.1371/journal.pone.0085254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer F, Backhed F. The gut microbiota--masters of host development and physiology. Nature reviews Microbiology. 2013;11:227–238. doi: 10.1038/nrmicro2974. [DOI] [PubMed] [Google Scholar]
- Stifter CA, Anzman-Frasca S, Birch LL, Voegtline K. Parent use of food to soothe infant/toddler distress and child weight status. An exploratory study Appetite. 2011;57:693–699. doi: 10.1016/j.appet.2011.08.013. [DOI] [PubMed] [Google Scholar]
- Stifter CA, Fox NA. Infant Reactivity - Physiological Correlates of Newborn and 5-Month Temperament. Dev Psychol. 1990;26:582–588. [Google Scholar]
- Stiverson J, Williams T, Chen J, Adams S, Hustead D, Price P, Guerrieri J, Deacon J, Yu Z. A comparative evaluation of prebiotic oligosaccharides using in vitro cultures of infant fecal microbiome. Applied and environmental microbiology. 2014 doi: 10.1128/AEM.02200-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, Kubo C, Koga Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. The Journal of physiology. 2004;558:263–275. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tache Y, Perdue MH. Role of peripheral CRF signalling pathways in stress-related alterations of gut motility and mucosal function. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2004;16(Suppl 1):137–142. doi: 10.1111/j.1743-3150.2004.00490.x. [DOI] [PubMed] [Google Scholar]
- van Aken C, Junger M, Verhoeven M, van Aken MAG, Dekovic M. Externalizing behaviors and minor unintentional injuries in toddlers: Common risk factors? J Pediatr Psychol. 2007;32:230–244. doi: 10.1093/jpepsy/jsj118. [DOI] [PubMed] [Google Scholar]
- Van den Abbeele P, Van de Wiele T, Verstraete W, Possemiers S. The host selects mucosal and luminal associations of coevolved gut microorganisms: a novel concept. FEMS microbiology reviews. 2011;35:681–704. doi: 10.1111/j.1574-6976.2011.00270.x. [DOI] [PubMed] [Google Scholar]
- Vollrath ME, Hampson SE, Juliusson PB. Children and eating. Personality and gender are associated with obesogenic food consumption and overweight in 6-to 12-year-olds. Appetite. 2012;58:1113–1117. doi: 10.1016/j.appet.2012.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S, Fei N, Pang X, Shen J, Wang L, Zhang B, Zhang M, Zhang X, Zhang C, Li M, et al. A gut microbiota-targeted dietary intervention for amelioration of chronic inflammation underlying metabolic syndrome. FEMS microbiology ecology. 2014;87:357–367. doi: 10.1111/1574-6941.12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoetendal EG, von Wright A, Vilpponen-Salmela T, Ben-Amor K, Akkermans AD, de Vos WM. Mucosa-associated bacteria in the human gastrointestinal tract are uniformly distributed along the colon and differ from the community recovered from feces. Applied and environmental microbiology. 2002;68:3401–3407. doi: 10.1128/AEM.68.7.3401-3407.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


