Abstract
Background
Regular gallbladder contraction reduces bile stasis and prevents gallstone formation. Intraduodenal administration of exogenous pancreatic secretory trypsin inhibitor (PSTI-I, also known as monitor peptide) causes cholecystokinin (CCK) secretion.
Design
We proposed that stimulation of CCK release by PSTI would produce gallbladder contraction and prevent gallstones in mice fed a lithogenic diet. Therefore, we tested the effect of overexpression of rat PSTI-I in pancreatic acinar cells on plasma CCK levels and gallbladder function in a transgenic mouse line (TgN[Psti1]; known hereafter as PSTI-I tg).
Results
Importantly, PSTI tg mice had elevated fasting and fed plasma CCK levels compared to wild-type (WT) mice. Only mice fed the lithogenic diet developed gallstones. Both fasting and stimulated plasma CCK levels were substantially reduced in both WT and PSTI-I tg mice on the lithogenic diet. Moreover, despite higher CCK levels PSTI-I tg animals developed more gallstones than WT animals.
Conclusions
Together with the previously observed decrease in CCK-stimulated gallbladder emptying in mice fed a lithogenic diet, our findings suggest that a lithogenic diet causes gallstone formation by impaired CCK secretion in addition to reduced gallbladder sensitivity to CCK.
Keywords: bile salts, cholesterol crystals, gallbladder motility, intestinal hormone, lithogenic bile, mucin gel
Introduction
It is well-established that the small intestinal hormone cholecystokinin (CCK) is the main stimulant of gallbladder contraction after eating a meal [1]. The control of CCK secretion is complex involving multiple intraluminal factors including proteins, fats, and pancreatic secretory trypsin inhibitor-I (PSTI-1, also known as monitor peptide)[2]. PSTI-I is a 61-amino acid, trypsin-sensitive peptide purified from rat pancreatic juice [3, 4]. It stimulates intestinal CCK release resulting in increased pancreatic enzyme secretion in response to dietary protein and may be partially responsible for physiological feedback regulation of pancreatic enzyme secretion [5]. PSTI-I also inhibits intrapancreatic trypsin activity. The trypsin inhibiting activity has been shown to be effective in reducing pancreatic inflammation in animal models of both acute [6] and chronic [7] pancreatitis when PSTI-I is overexpressed in the pancreas of a transgenic mouse line. However, it is not known if transgenic overexpression of PSTI-I affects plasma CCK levels in the fasted or fed state or whether elevated plasma CCK concentrations affect gallbladder function or gallstone formation.
Decreased gallbladder motility can lead to gallstone formation by nucleation of cholesterol into solid cholesterol monohydrate crystals when the cholesterol saturation index in gallbladder bile exceeds solubility limits [8]. In addition, abnormal gallbladder motility has been observed in cholesterol gallstone patients [9]. These observations have led to speculation about whether decreased plasma CCK levels may predispose to gallstone disease [10, 11]. Feeding mice a lithogenic diet has also been shown to decrease gallbladder expression of the CCK1 receptor [12] as has the presence of gallstones in human gallbladders [13]. Genetic deletion of the cholecystokinin-1 receptor (CCK1) in mice resulted in cholesterol gallstone formation secondary to gallbladder stasis due to the absence of CCK-induced gallbladder contraction [11, 13, 14]. Furthermore, genetic deletion of CCK itself also caused gallbladder hypomotility and rapid precipitation of solid cholesterol gallstones in mice fed a lithogenic diet [10]. It has also recently been demonstrated that cholesterol-rich lithogenic diets reduce gallbladder contraction in response to CCK by (i) causing CCK1 receptors to be sequestered in gallbladder smooth muscle cell caveolae [15, 16], and (ii) reducing the intracellular Ca++ stores that are required for CCK-induced contractions [17]. These studies provide experimental support for the concept that decreased stimulation of gallbladder motility caused by either decreased blood CCK levels or by decreased gallbladder CCK1 receptor levels can lead to susceptibility to gallstone formation. These studies are supported by the efficacy of CCK in preventing biliary sludge in patients receiving parenteral nutrition [18, 19]. These observations led us to question whether chronic CCK stimulation of gallbladder motility may prevent or protect against the development of gallstone disease. If so, then it is conceivable that strategies could be developed to chronically stimulate CCK secretion and prevent gallstone formation in humans.
We have developed a transgenic mouse line in which the rat pancreatic secretory trypsin inhibitor I (PSTI-I) gene is overexpressed in mouse pancreatic acinar cells [6, 20, 21]. Among other actions, PSTI-I stimulates the secretion of CCK from I cells in the mucosal epithelium of the small intestine and increased plasma concentrations of CCK in turn cause gallbladder contraction. We therefore hypothesized that the PSTI-I transgenic mice would exhibit elevated plasma levels of CCK resulting in continuous stimulation of gallbladder contraction, and we assessed whether these responses would protect the mice against gallstone formation when fed a lithogenic diet. We discovered that expression of PSTI-I in transgenic mice did indeed lead to chronic elevation of CCK. However, this elevation did not prevent gallstone formation. We also observed that the lithogenic diet inhibited CCK secretion thus reducing gallbladder contraction and therefore contributing to gallstone formation.
Methods
Mice
Two strains of mice were used in these studies. Wild-type and C57BL/6J mice were purchased from Jackson Laboratory (Bar Harbor, Maine, USA). Transgenic mice on the genetic C57BL/6J background and expressing the rat pancreatic secretory trypsin inhibitor I gene in pancreatic acinar cells (TgN[Psti1]; PSTI-I tg) were generated in our laboratory as previously described [6]. The transgene was comprised of a chimeric construct of the mouse elastase I enhancer/promoter cloned upstream of a 4.6 kb minigene of rat PSTI-I and the rat PSTI-I mRNA and protein were expressed exclusively in pancreas. Mice were housed in climate-controlled rooms with a 12:12 hour light-dark cycle, and permitted water and chow ad libitum. All animal experiments were performed with approval of the Duke University Institutional Animal Care and Use Committee.
Diet
At the age of 6 weeks, male wild-type and PSTI-I tg mice were placed on one of two ad libitum diets: (1) normal mouse chow (Purina) or (2) a lithogenic diet consisting of 1% cholesterol and 0.5% sodium taurocholate (Harlan-Teklad mouse diet #5015 CB, Indianapolis, IN). At the end of eight weeks of continuous diet, animals were euthanized for the collection of blood and tissues.
Plasma CCK assay
Plasma CCK concentrations were measured by a specific bioassay as described [22]. This assay is based on the ability of CCK to stimulate amylase release from isolated rat pancreatic acini. Briefly, plasma is extracted and concentrated by adsorption onto octadecylsilylsilica C-18 cartridges (Waters Associates, Millipore Corp., Milford, MA). To obtain adequate sensitivity, plasma was pooled from 3 mice [23].
Gallbladder histopathology
Gallbladders were fixed in 10% formalin, paraffin-embedded, and sections were cut at 5 μm and mounted on glass slides. Chronic cholecystitis was graded by a pathologist (blinded to the group designation) by assessing chronic inflammation in the lamina propria, presence of Rokitansky-Aschoff sinuses, and degree of thickening of the muscularis externa. Other inflammatory changes including number of neutrophils in the epithelium, number of neutrophils and eosinophils in the lamina propria, reactive epithelial changes such as eosinophilic cytoplasmic inclusions, papillary mucosal architecture, and absence of inflammatory cells versus papillary edema were also examined; each category was scored 0, 1 or 2 (maximum possible score = 16).
Gallbladder volume
Gallbladder volume was measured as previously described [10, 24]. Briefly, mice fasted overnight with free access to drinking water were anesthetized, a laparotomy was performed under sterile conditions, the gallbladder was exposed and its size measured using a micro-caliper. Gallbladder volumes were calculated using the following formula, assuming an ellipsoid shape (which has been shown to be accurate in the absence of major differences in gallbladder shape) [24]:
To determine gallbladder emptying and plasma CCK concentrations in response to intraduodenal fat, a PE-10 polyethylene catheter was inserted into the duodenum during laparotomy. The duodenal catheter was externalized through the left abdominal wall and connected to an infusion pump. After surgery, gallbladder size was measured with a micro-caliper and gallbladder volume was calculated using the formula above. Mice were then infused intraduodenally with corn oil at 40 μl/min for 5 minutes. Controls were infused identically with 0.9% NaCl. After 30 minutes, gallbladder volume was measured again as before and mixed arteriovenous blood was collected for plasma CCK assay. The thirty minute interval was chosen because this has been shown to correspond to peak plasma CCK levels and gallbladder contraction in previous studies in people [1, 25] and rats [26] and to be appropriate in mice [27]. Gallbladder emptying was defined as the difference in gallbladder volume before and after the duodenal infusions.
Microscopic studies of gallbladder bile and gallstones
After fasting overnight, a cholecystectomy was performed in PSTI-I transgenic and wild-type mice (N = 18–20 per group) at 8 weeks on the lithogenic or the chow diets. Gallbladder bile was examined by polarizing light microscopy for mucin gel, liquid crystals, anhydrous cholesterol crystals, plate-like cholesterol monohydrate crystals, sandy stones, and real gallstones, all of which were defined according to previously established criteria [28].
Lipid analysis
Biliary cholesterol, total and individual bile salts, and phospholipids were determined as described previously [29]. Cholesterol saturation index (CSI) values of pooled gallbladder bile were calculated from the critical tables [30]. Relative lipid compositions of pooled gallbladder bile samples were plotted on a triangular phase diagram according to their total lipid concentrations. Phase boundaries and crystallization pathways were extrapolated from model bile systems based on sodium taurocholate at 37°C [31]. Hydrophobicity indexes of gallbladder bile were calculated according to Heuman’s method [32].
Statistical Analysis
Results are expressed as mean ± SEM. The Gaussian distribution of the data was assured by using the test of Kolgorov and Smirnov. Mean differences between 2 groups were analyzed by the Student t test, and mean differences among several groups were analyzed by one-way analysis of variance followed by the Tukey-Kramer posttest, using GraphPad Instat version 3.10 for Windows (GraphPad Software, San Diego, CA). P values < 0.05 were considered significant.
Results
Plasma CCK levels
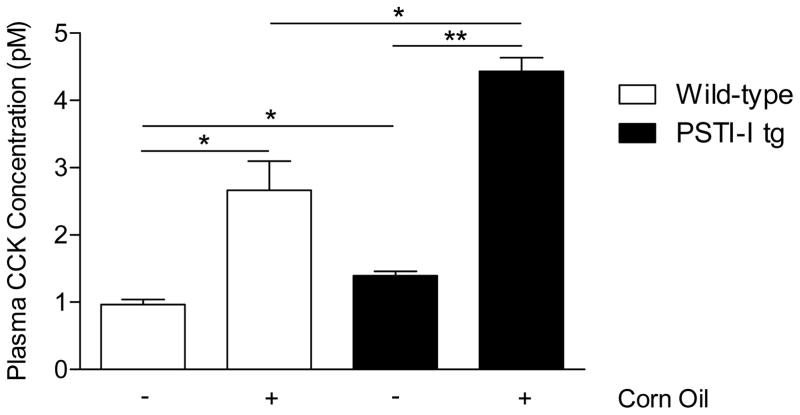
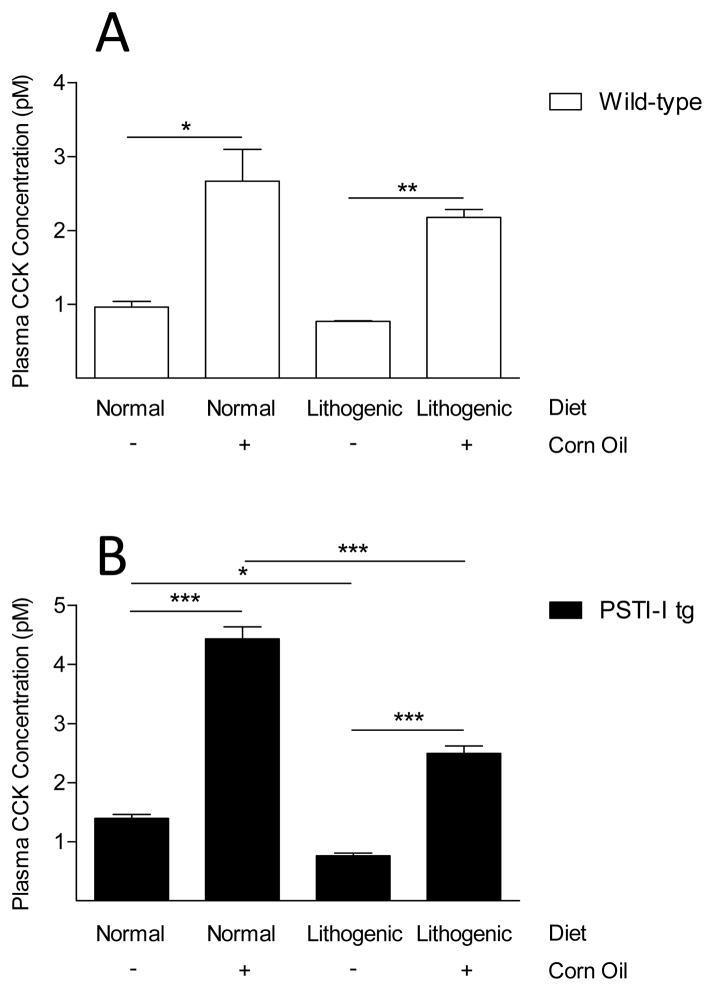
Exogenously administered PSTI-I has been shown to stimulate CCK release in vivo and it has been classified as a CCK-releasing factor. However, the effect of over-expression of PSTI-I on CCK secretion has not been characterized. We tested the hypothesis that PSTI-I secreted from pancreatic acinar cells in mice over-expressing the rat PSTI-I transgene would result in elevated CCK levels in the blood. Fasting CCK levels in wild-type mice averaged 1.0 ± 0.1 pM. In contrast, CCK levels in PSTI-I tg mice were significantly elevated (1.4 ± 0.1 pM; P < 0.05; Fig. 1). Not only did PSTI-I tg mice have elevated fasting plasma CCK levels, they also exhibited significantly higher plasma CCK responses to intraduodenal administration of the lipid, corn oil, a potent stimulant of CCK secretion, compared to wild-type mice (Fig. 1).
Figure 1.
Comparison of fasting and lipid-fed (corn oil) plasma CCK concentrations in wild-type mice (open bars) and in PSTI-I tg mice (filled bars) fed a normal diet (N = 3 pools of 3 mice each). Over-expression of PSTI-I significantly increased fasting and lipid-fed CCK concentrations. (*P < 0.05; **P < 0.01)
Previous work has demonstrated that feeding a lithogenic diet contributes to gallstone formation through reduced gallbladder contraction. We wondered if the elevated fasting and fed plasma CCK levels resulting from over-expression of PSTI could prevent gallstone formation. Therefore, we placed wild-type and PSTI-I tg mice on normal or lithogenic diets for 8 weeks and assessed gallbladder inflammation, gallbladder contraction, gallstone formation, and plasma CCK concentrations.
Gallbladder Inflammation
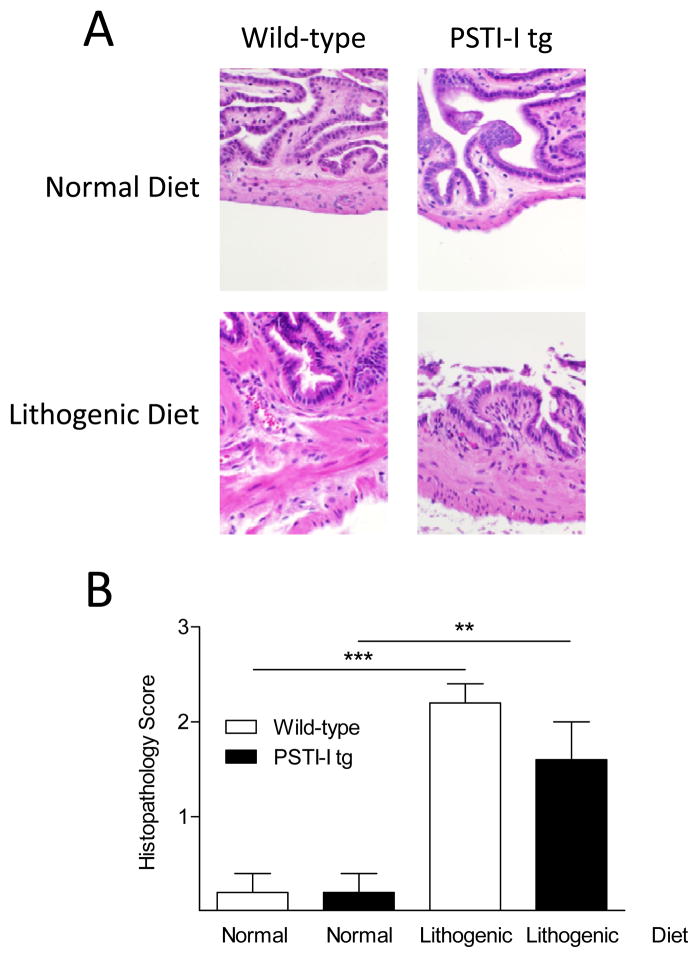
The histology of the gallbladders of both wild-type and PSTI-I tg mice appeared normal in animals fed the normal diet, but exhibited several abnormalities in animals fed the lithogenic diet (Fig. 2A). In both mouse strains, the lithogenic diet caused significant histopathological changes including increased inflammatory cells, thickened muscular walls, reactive epithelial changes, and altered mucosal papillary architecture. However, there was no significant difference between wild-type and PSTI-I tg mice fed a lithogenic diet when the histopathology scores were quantitated (Fig. 2B).
Figure 2.
Effect of the lithogenic diet on gallbladder histology in wild-type and PSTI-I tg mice. (A) Gallbladder histology was normal in both wild-type and PSTI-I tg mice on the normal diet. The lithogenic diet caused histopathological changes of inflammation, thickened muscular wall, increased numbers of neutrophils in the epithelium and lamina propria, reactive epithelial changes, and altered papillary mucosal architecture. (B) Semiquantitative histological score (N = 5). Wild-type, open bars; PSTI-I tg, filled bars; (**P < 0.01; ***P < 0.001)
Gallbladder Emptying
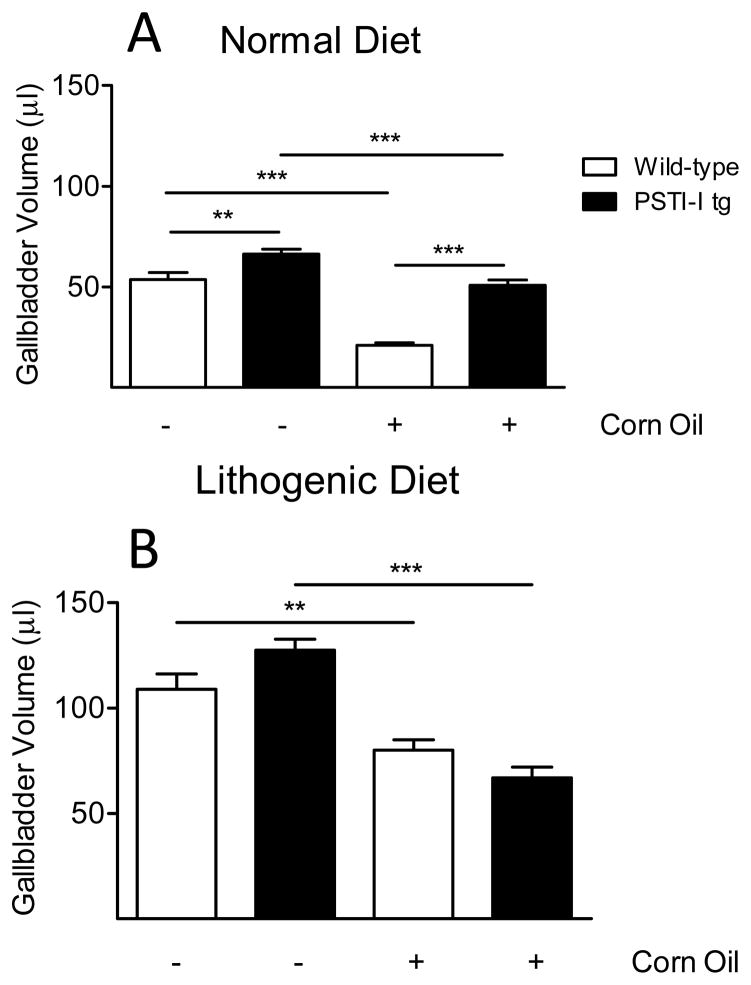
To assess the effects of PSTI-I overexpression on the gallbladder, we examined gallbladder contraction in the fasted and lipid-fed (corn oil) states. Interestingly, despite PSTI-I tg mice having higher fasting CCK levels, their fasting gallbladder volumes were larger, not smaller, than wild-type mice on both normal and lithogenic diets although this effect was only significant in the mice on the normal diet (Fig. 3). We then administered the CCK-releasing lipid, corn oil, to mice intraduodenally to stimulate gallbladder contraction. Under these conditions, PSTI-I tg mice on a normal diet had less gallbladder emptying (23 ± 4%) than did wild-type mice (61 ± 2%; P < 0.0001), suggesting that the elevated serum CCK levels in PSTI-I tg mice caused a decrease in gallbladder sensitivity to CCK-stimulated contraction (Fig. 3A).
Figure 3.
Gallbladder volumes in wild-type and PSTI-I tg mice eating the normal diet (A) or the lithogenic diet (B) and responses to acute intraduodenal injection of lipid (corn oil). Note that all gallbladder volumes are larger in the mice eating the lithogenic diet than in mice eating the normal diet. Administering corn oil decreased gallbladder volume in both wild-type (open bars) and PSTI-I tg mice (filled bars) eating both diets. However, there were differences in the degree of gallbladder emptying among the various groups. In the wild-type mice, eating the lithogenic diet significantly inhibited gallbladder emptying (27 ± 4%) compared to mice eating the normal diet (61 ± 2%; P < 0.0001; N = 9–11). In contrast, chronic overexpression of PSTI-I almost restored gallbladder emptying to normal (48 ± 4%) in mice eating the lithogenic diet compared to those eating the normal diet (23 ± 4%; P < 0.001; N = 10–12). (**P < 0.01; ***P < 0.001)
Both strains of mice eating the lithogenic diet exhibited markedly enlarged fasting and lipid-fed gallbladder volumes (Fig. 3B) compared to mice eating the normal diet (Fig. 3A). Administration of corn oil to the wild-type mice eating the lithogenic diet (Fig. 3B) caused a significantly smaller decrease in gallbladder volume than occurred in wild-type mice eating the normal diet (27 ± 4% vs 61 ± 2%; P < 0.0001; Fig. 3). When the effects of corn oil administration were examined in PSTI-I tg mice on a lithogenic diet, there was a significantly greater decrease in gallbladder volume compared to PSTI-I tg eating a normal diet (48 ± 4% vs 23 ± 4%; P < 0.001; Fig. 3).
Gallstone Formation
The crystallization, growth, and agglomeration of cholesterol monohydrate crystals, as well as the development of gallstones, were significantly accelerated in PSTI-I tg mice compared to the wild-type mice. After 8 weeks on the lithogenic diet, gallstones were markedly larger in the former than in the later (Fig. 4A).
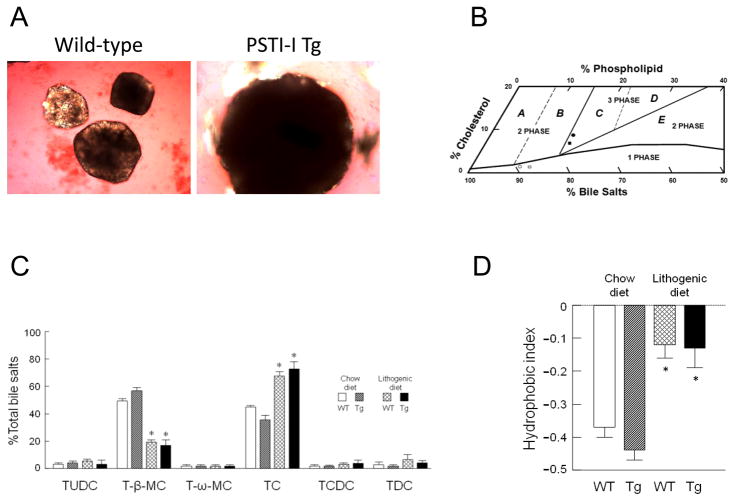
Figure 4.
(A) Representative photomicrographs of cholesterol gallstones as observed in gallbladder bile samples after 8 weeks on the lithogenic diet. Gallstones exhibited rounded contours and black centers from light scattering/absorption by polarizing light microscopy (×400). (B) The relative lipid compositions (moles per 100 moles) of pooled gallbladder bile samples plotted on a condensed phase diagram according to average total lipid concentration (12.50 g/dL; see Table 1). The one-phase micellar zone is enclosed by a solid curved line. Above it, two solid and two dashed lines divide the phase diagram into regions A–E with different crystallization sequences. The relative lipid compositions of pooled gallbladder bile samples from PSTI-I transgenic and wild-type mice fed 8 weeks with the chow diet are located in the one-phase micellar zone. By phase analysis, these samples were composed of unsaturated micelles at equilibrium and are unsaturated with cholesterol. In contrast, feeding the lithogenic diet for 8 weeks resulted in the relative biliary lipid compositions of pooled gallbladder bile samples plotted in the central three-phase zone, where they were composed of solid cholesterol monohydrate crystals, liquid crystals, and saturated micelles at equilibrium. The symbols ◀ and □ represent the relative lipid compositions of pooled gallbladder bile samples at 8 weeks on the normal chow diet, and ● and ■ represent the lithogenic diet in PSTI-I transgenic and wild-type mice, respectively. (C) Percent bile salt species in gallbladder bile samples from PSTI-I tg and wild-type mice fed the normal chow or the lithogenic diets for 8 weeks (N = 10). With normal chow, taurocholate (TC) and tauro-β-muricholate (T-β-MC) were the predominant bile salts. In mice fed the lithogenic diet, TC became the major bile salt replacing most T-β-MC. Other bile salts present in low concentrations include tauroursodeoxycholate (TUDC), tauro-ω-muricholate (T-ω-MC), taurochenodeoxycholate (TCDC), and taurodeoxycholate (TDC). (*P < 0.01, compared to the corresponding strain of mice on the chow diet) (D) Feeding the lithogenic diet significantly increased the hydrophobic indices in both PSTI-I tg and wild-type mice compared to the chow diet (N = 10). (* P < 0.01, compared to the corresponding strain of mice on the chow diet)
Figure 4B shows the relative lipid compositions of pooled gallbladder bile samples with micellar phase boundaries and cholesterol crystallization pathways for the appropriate mean total lipid concentrations in taurocholate-rich bile. On the normal diet, the relative lipid compositions of pooled gallbladder bile samples in both PSTI-I tg and wild-type mice were located in the micellar zone, which are consistent with their bile being unsaturated with cholesterol. Thus, their cholesterol saturation index (CSI) values were lower than 1 (Table 1). However, after 8 weeks on the lithogenic diet, the relative lipid compositions of pooled gallbladder bile samples in both strains of mice were above the micellar zone and fell in the central three-phase area denoted region C. By phase analysis, these samples were predicted to be composed of solid cholesterol monohydrate crystals, liquid crystals, and saturated micelles, exactly as was observed experimentally. Of note, although gallbladder bile was saturated with cholesterol, the CSI value was markedly higher in PSTI-I tg mice compared to wild-type mice (Table 1). This explains why overexpression of the Psti1gene enhances susceptibility to cholesterol gallstone formation in mice.
Table 1.
Biliary lipid composition of gallbladder bile samples after 8 weeks on the normal diet or on the lithogenic diet
| Mice | Cholesterol, mole% | Phospholipid, mole% | Bile salts, mole% | Cholesterol/phospholipid ratio | Cholesterol/bile salts ratio | Total lipid concentration, g/dL | CSI |
|---|---|---|---|---|---|---|---|
| Normal diet | |||||||
| Wild-type | 2.19 | 10.39 | 87.41 | 0.21 | 0.03 | 12.09 | 0.50 |
| PSTI-I tg | 2.38 | 8.49 | 89.13 | 0.28 | 0.03 | 13.43 | 0.59 |
| Lithogenic diet | |||||||
| Wild-type | 7.05 | 15.66 | 77.29 | 0.45 | 0.09 | 12.39 | 1.18 |
| PSTI-I tg | 8.46 | 16.29 | 75.26 | 0.52 | 0.11 | 11.28 | 1.39 |
Values were determined from pooled gallbladder bile samples (n=18–20 per group).
CSI, cholesterol saturation index
Figure 4C shows the distributions of bile salt species in pooled gallbladder bile samples for PSTI-I tg and wild-type mice after 8 weeks on the normal or the lithogenic diets. As we found in previous studies, all bile salts were taurine conjugated. In mice on normal chow, the predominant bile salt species were taurocholate (TC; range 35.9–45.0%) and tauro-β-muricholate (T-β-MC; range 48.6–56.4%). Tauro-ω-muricholate (T-ω-MC; 0.2–1.2%), tauroursodeoxycholate (TUDC; 2.1–3.6%), taurochenodeoxycholate (TCDC; 0.8–1.9%), and taurodeoxycholate (TDC; 2.0–5.3%) were present in appreciably lower concentrations. Mice fed the lithogenic diet displayed a significant (P < 0.01) decrease in concentration of the hydrophilic bile salt T-β-MC (16.5–19.1%) and a significant (P < 0.01) increase in TC (67.4–72.6%). Figure 4D shows that hydrophobic indices of gallbladder bile samples were significantly (P < 0.01) increased in mice on the lithogenic diet compared to the normal diet. The bile salt species and the hydrophobic indices were similar between PSTI-I tg and wild-type mice regardless of whether the normal or the lithogenic diets were fed.
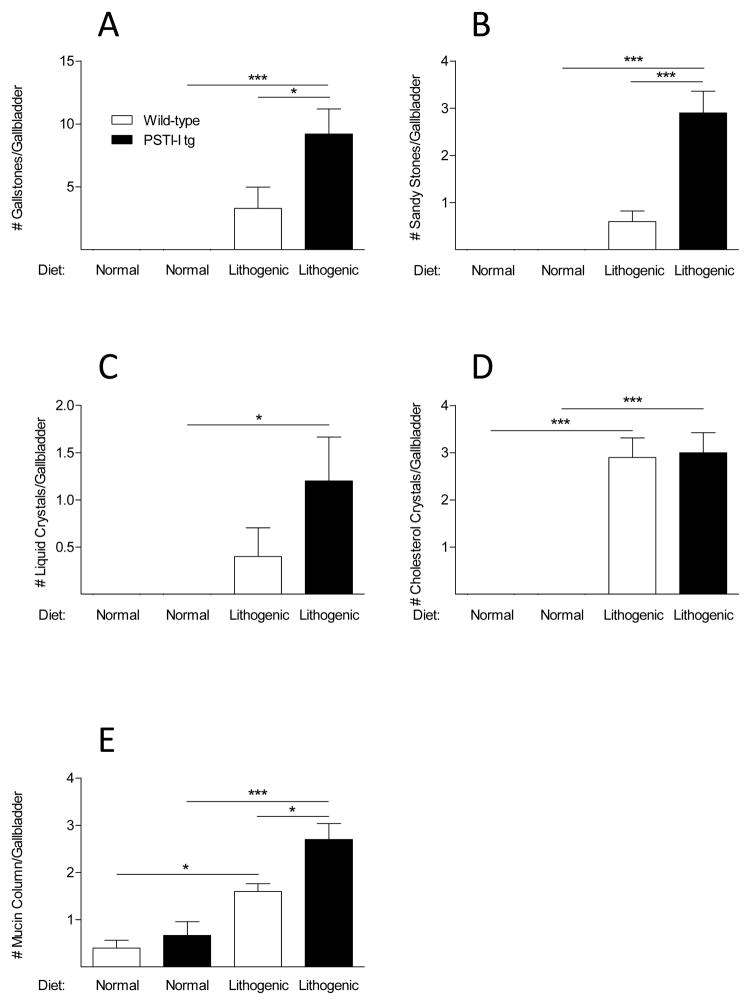
To determine if the elevated blood CCK levels we observed prevented gallstone formation, mice were fed a lithogenic diet because mice do not develop gallstones when they are fed a normal diet. Lithogenic diets have previously been shown to alter bile composition and induce gallstones within eight weeks. Interestingly, the lithogenic diet caused gallstones in both the wild-type and transgenic mice and contrary to initial expectations, the PSTI-I tg mice with elevated plasma CCK levels fed the lithogenic diet formed more rather than fewer gallstones than did the wild-type mice on the same diet (Fig. 5).
Figure 5.
Effect of a lithogenic diet on bile composition and gallstone formation in wild-type and PSTI-I tg mice (N = 10). Gallstones, sandy stones, liquid crystals, and cholesterol crystals were only found in mice eating the lithogenic diet, and there were significantly more gallstones, sandy stones, liquid crystals, and mucin columns in the PSTI-I tg mice than in the wild-type mice eating that diet. Wild-type, open bars; PSTI-I tg, filled bars; (*P < 0.05; ***P < 0.001)
Effect of a Lithogenic Diet on CCK Secretion
To determine whether the lithogenic diet affected CCK secretion, we measured plasma CCK levels in fasting and corn oil-fed mice. Fasting plasma CCK concentrations were lower in the wild-type mice fed a lithogenic diet than in those fed the normal diet, but this difference was not statistically significant (Fig. 6A). Corn oil administration significantly increased plasma CCK concentrations in the wild-type mice fed both diets, but this effect was lower in mice fed the lithogenic diet than in mice fed the normal diet (Fig. 6A; NS). The PSTI-I tg mice exhibited similar patterns of plasma CCK levels, but the reductions in both fasted and fed plasma CCK concentrations in response to the lithogenic diet in these mice were statistically significant (Fig. 6B). Most notably, the plasma CCK response to corn oil administration was inhibited much more in the PSTI-I tg mice than in the WT mice eating the lithogenic diet compared to the normal diet (43.7 ± 2.8% vs 18.3 ± 4.0; P < 0.01) demonstrating that the lithogenic diet blunts stimulated CCK secretion disproportionately more when plasma CCK levels are chronically elevated.
Figure 6.
Comparison of fasted and fed plasma CCK concentrations in wild-type mice (A; open bars) and PSTI-I tg mice (B; filled bars) eating a normal or a lithogenic diet. The fasted and fed results for the mice eating the normal diet are reproduced from Figure 1 for comparison to the results for the mice eating the lithogenic diet. Eating a lithogenic diet reduced both fasting and fed plasma CCK concentrations in wild-type mice (A) and in PSTI-I tg mice (B). However, the reductions of CCK levels were not only greater in the PSTI-I tg mice than in the wild-type mice but were also statistically significant in the PSTI-I tg mice but not in the wild-type mice. (* P < 0.05; **P < 0.01; ***P < 0.001)
Discussion
We show herein that continuous over-expression of PSTI-I in the pancreas results in significantly elevated fasting and fed plasma CCK concentrations. We also observed that the chronically elevated plasma CCK levels did not protect mice from developing gallstones when fed a lithogenic diet. In fact, PSTI-I tg mice developed significantly more gallstones that did wild-type mice when both mouse strains were fed the lithogenic diet. In order to understand this unexpected finding, we analyzed the effects of PSTI-I expression on bile and gallstone chemistry, gallbladder histopathology, gallbladder volume, and fasting and lipid-stimulated CCK secretion. These studies unveiled an intriguing interaction of diet on CCK secretion and consequent effects on gallbladder function.
As expected, mice fed the lithogenic diet exhibited increases in all parameters of gallstone formation, including the numbers of gallstones, sandy stones, liquid crystals, cholesterol crystals, and mucin gels compared to mice fed the normal diet. In the lithogenic diet groups, the PSTI-I tg mice formed significantly more gallstones (and larger ones), sandy stones, and mucin gels than the wild-type mice. The lithogenic diet also induced significant changes in bile salt composition, but there were no changes in composition between wild-type and PSTI-I tg mice. The explanation for the increased incidence of gallstones in the PSTI-I tg mice fed the lithogenic diet may be related to the increased cholesterol saturation index of the bile in this group relative to wild-type mice fed the same diet. In order to understand the basis for this difference in bile composition that resulted in increased gallstone formation in the PSTI-I tg mice, we turned to analysis of gallbladder contraction in the various groups.
Initially, we made the important observation that PSTI-I overexpression caused a persistent elevation in plasma CCK levels. Our discovery that the lithogenic diet caused the same amount of gallbladder histopathology in both wild-type and PSTI-I tg mice and that the PSTI-I tg mouse gallbladders were bigger than the wild-type mouse gallbladders made us question whether chronically elevated CCK levels actually promoted gallbladder emptying or, alternatively, PSTI-I-induced excessive CCK release may actually impair gallbladder emptying. Under normal conditions, plasma CCK levels are low in the basal condition and increase after ingestion of nutrients such as fat. Elevated plasma CCK acts directly on CCK1 receptors on gallbladder smooth muscle to cause gallbladder emptying. However, under the condition of PSTI-I overexpression, CCK release is chronically stimulated and persistently high CCK levels may desensitize or down-regulate the gallbladder CCK1 receptor and gallbladder contraction is impaired. Gallbladder stasis, due to decreased CCK-induced contraction, is known to facilitate nucleation, growth, and agglomeration of cholesterol monohydrate crystals resulting ultimately in gallstone formation [11]. These observations are consistent with previous results demonstrating that impaired CCK-induced gallbladder emptying in susceptible mice fed the lithogenic diet is associated with gallstone formation [33]. In addition, when CCK-null [10] or CCK1 receptor-null [11, 13, 14] mice were fed the lithogenic diet, they also exhibited gallbladder hypomotility resulting in greater gallstone formation compared to wild-type mice.
Mice do not develop gallstones spontaneously unless they are fed a lithogenic diet. Prior to this study, we had no reason to expect that feeding the lithogenic diet would result in a decrease in plasma CCK levels in both fasted and fed states in both wild-type and PSTI-I tg mice as described here. Thus, one mechanism whereby the lithogenic diet may cause gallstones in wild-type mice is through gallbladder stasis caused by reduced CCK secretion. Impaired gallbladder contraction resulting in gallbladder stasis is also a potential mechanism for gallstone formation in people who are not eating or who have reduced food consumption. However, the lithogenic diet also appears to alter gallbladder contraction directly in response to exogenous CCK since it has been shown that the lithogenic diet causes gallstone formation associated with decreased CCK-stimulated gallbladder emptying in mice [9, 12].
Thus, both PSTI-I transgenic overexpression and feeding the lithogenic diet resulted in decreased gallbladder contraction in response to administering corn oil intraduodenally. The reduced gallbladder contraction in response to feeding the lithogenic diet may be explained by the deleterious effects of the lithogenic diet on CCK secretion by unknown mechanisms that await future investigation. One result that is difficult to explain within this framework is the greater decrease in gallbladder volume caused by corn oil administration observed in the PSTI-I tg mice versus the wild-type mice (Fig. 3B). This finding may be related to the presumably different mechanisms by which intraluminal lipids and PSTI-I stimulate CCK release. We have recently shown that lipids in the lumen of the small intestine stimulate CCK release via the immunoglobulin-like domain containing receptor 1 (ILDR1) and that this pathway is distinct from the mechanism by which trypsin inhibitors such as PSTI-I stimulate CCK secretion [27].
Overall, it appears that anything that reduces CCK signaling to gallbladder smooth muscle results in reduced gallbladder emptying and promotes gallstone formation. Earlier evidence in favor of this concept came from genetic deletion of either the CCK1 receptor [11, 13, 14] or of CCK itself [10]. There is also evidence that polymorphisms in the CCK1 receptor gene (CCK-AR) are correlated with increased gallstone disease in people [13, 34]. We show here that feeding the lithogenic diet has the same effect due to impairment of CCK secretion. Our findings are also consistent with the previous demonstration that feeding mice a lithogenic diet slows small intestinal motility due to excessive incorporation of absorbed cholesterol into intestinal smooth muscle sarcolemmal membranes [35]. The authors inferred that this excessive cholesterol enrichment of intestinal smooth muscle cells produced hypomotility by uncoupling CCK signal transduction, a physiological mechanism of stimulated small intestinal propulsion. Our current findings suggest that another mechanism by which lithogenic diets decrease CCK signaling to both gallbladder smooth muscle and small intestinal smooth muscle is by inhibiting CCK secretion, either directly by acting on CCK-secreting I cells in the mucosal epithelium or indirectly by decreasing intestinal motility.
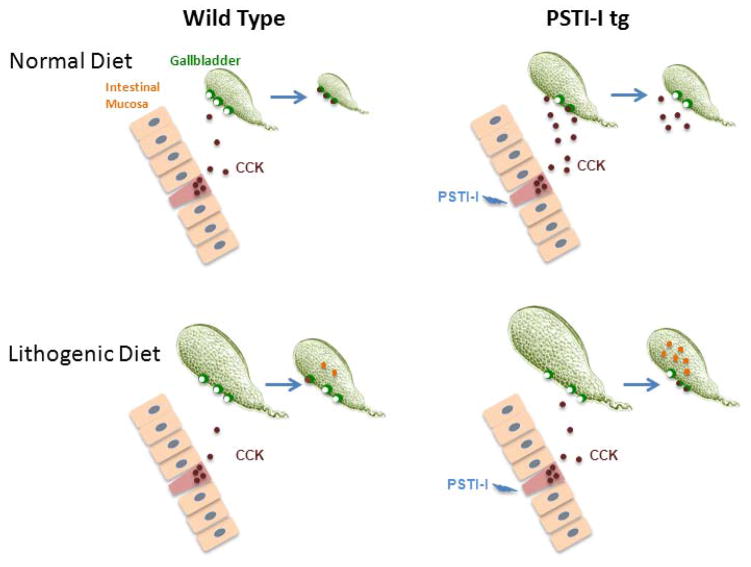
Figure 7.
Model depicting the effect of the lithogenic diet on CCK secretion and gallbladder contraction. In wild-type mice, ingestion of the normal diet stimulates CCK secretion which in turn causes gallbladder contraction (top left). However, PSTI-I overexpression persistently stimulates CCK secretion causing gallbladder desensitization to CCK possibly due to CCK1 receptor downregulation and thus impaired gallbladder contraction (top right). Gallbladder volumes are larger in mice on the lithogenic diet. In wild-type mice the lithogenic diet impairs CCK secretion and gallbladder contraction is incomplete, resulting in gallstone formation (lower left). In PSTI-I tg mice, impaired CCK secretion in mice on the lithogenic diet lowers blood CCK to levels that do not cause gallbladder desensitization and the gallbladder response to dietary stimulus is partially restored, but this effect is apparently not great enough to prevent gallstone formation (lower right).
Acknowledgments
This work was supported by NIH grants DK064213 and DK091946 (R. A. L.) and DK73917 and DK101793 (D. Q.-H. W.).
Abbreviations
- CCK
cholecystokinin
- CSI
cholesterol saturation index
- PSTI-I
pancreatic secretory trypsin inhibitor-I
- TCDC
taurochenodeoxycholate
- TDC
taurodeoxycholate
- T-ω-MC
tauro-ω-muricholate
- TUDC
tauroursodeoxycholate
References
- 1.Liddle RA, Goldfine ID, Rosen MS, Taplitz RA, Williams JA. Cholecystokinin bioactivity in human plasma. Molecular forms, responses to feeding, and relationship to gallbladder contraction. J Clin Invest. 1985;75:1144. doi: 10.1172/JCI111809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liddle RA. Regulation of cholecystokinin secretion by intraluminal releasing factors. Am J Physiol. 1995;269:G319. doi: 10.1152/ajpgi.1995.269.3.G319. [DOI] [PubMed] [Google Scholar]
- 3.Fushiki T, Fukuoka SI, Iwai K. Stimulatory effect of an endogenous peptide in rat pancreatic juice on pancreatic enzyme secretion in the presence of atropine: Evidence for different mode of action of stimulation from exogenous trypsin inhibitors. Biochem Biophys Res Comm. 1984;118:532. doi: 10.1016/0006-291x(84)91335-4. [DOI] [PubMed] [Google Scholar]
- 4.Iwai K, Fukuoka SI, Fushiki T, Tsujikawa M, Hirose M, Tsunasawa S, et al. Purification and sequencing of a trypsin-sensitive cholecystokinin-releasing peptide from rat pancreatic juice. Its homology with pancreatic secretory trypsin inhibitor. J Biol Chem. 1987;262:8956. [PubMed] [Google Scholar]
- 5.Miyasaka K, Guan D, Liddle RA, Green GM. Feedback regulation by trypsin: evidence for intraluminal CCK-releasing peptide. Am J Physiol. 1989;257:G175. doi: 10.1152/ajpgi.1989.257.2.G175. [DOI] [PubMed] [Google Scholar]
- 6.Nathan JD, Romac J, Peng RY, Peyton M, Macdonald RJ, Liddle RA. Transgenic expression of pancreatic secretory trypsin inhibitor-I ameliorates secretagogue-induced pancreatitis in mice. Gastroenterology. 2005;128:717–27. doi: 10.1053/j.gastro.2004.11.052. [DOI] [PubMed] [Google Scholar]
- 7.Romac JM, Shahid RA, Choi SS, Karaca GF, Westphalen CB, Wang TC, et al. Pancreatic secretory trypsin inhibitor I reduces the severity of chronic pancreatitis in mice overexpressing interleukin-1beta in the pancreas. Am J Physiol. 2012;302:G535–41. doi: 10.1152/ajpgi.00287.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Portincasa P, Di Ciaula A, Wang HH, Palasciano G, van Erpecum KJ, Moschetta A, et al. Coordinate regulation of gallbladder motor function in the gut-liver axis. Hepatology. 2008;47:2112–26. doi: 10.1002/hep.22204. [DOI] [PubMed] [Google Scholar]
- 9.Portincasa P, Di Ciaula A, Baldassarre G, Palmieri V, Gentile A, Cimmino A, et al. Gallbladder motor function in gallstone patients: sonographic and in vitro studies on the role of gallstones, smooth muscle function and gallbladder wall inflammation. J Hepatol. 1994;21:430–40. doi: 10.1016/s0168-8278(05)80324-1. [DOI] [PubMed] [Google Scholar]
- 10.Wang HH, Portincasa P, Liu M, Tso P, Samuelson LC, Wang DQ. Effect of gallbladder hypomotility on cholesterol crystallization and growth in CCK-deficient mice. Biochim Biophys Acta. 2010;1801:138–46. doi: 10.1016/j.bbalip.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang DQ, Schmitz F, Kopin AS, Carey MC. Targeted disruption of the murine cholecystokinin-1 receptor promotes intestinal cholesterol absorption and susceptibility to cholesterol cholelithiasis. J Clin Invest. 2004;114:521–8. doi: 10.1172/JCI16801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu GQ, Xu CF, Chen HT, Liu S, Teng XD, Xu GY, et al. Association of caveolin-3 and cholecystokinin A receptor with cholesterol gallstone disease in mice. World journal of gastroenterology : World J Gastroenterol. 2014;20:9513–8. doi: 10.3748/wjg.v20.i28.9513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyasaka K, Takata Y, Funakoshi A. Association of cholecystokinin A receptor gene polymorphism with cholelithiasis and the molecular mechanisms of this polymorphism. J Gastroenterol. 2002;37 (Suppl 14):102–6. doi: 10.1007/BF03326426. [DOI] [PubMed] [Google Scholar]
- 14.Sato N, Miyasaka K, Suzuki S, Kanai S, Ohta M, Kawanami T, et al. Lack of cholecystokinin-A receptor enhanced gallstone formation: a study in CCK-A receptor gene knockout mice. Dig Dis Sci. 2003;48:1944–7. doi: 10.1023/a:1026110002713. [DOI] [PubMed] [Google Scholar]
- 15.Xiao Z, Schmitz F, Pricolo VE, Biancani P, Behar J. Role of caveolae in the pathogenesis of cholesterol-induced gallbladder muscle hypomotility. Am J Physiol. 2007;292:G1641–9. doi: 10.1152/ajpgi.00495.2006. [DOI] [PubMed] [Google Scholar]
- 16.Cong P, Pricolo V, Biancani P, Behar J. Effects of cholesterol on CCK-1 receptors and caveolin-3 proteins recycling in human gallbladder muscle. Am J Physiol. 2010;299:G742–50. doi: 10.1152/ajpgi.00064.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lavoie B, Nausch B, Zane EA, Leonard MR, Balemba OB, Bartoo AC, et al. Disruption of gallbladder smooth muscle function is an early feature in the development of cholesterol gallstone disease. Neurogastroenterol Motil. 2012;24:e313–24. doi: 10.1111/j.1365-2982.2012.01935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sitzmann JV, Pitt HA, Steinborn PA, Pasha ZR, Sanders RC. Cholecystokinin prevents parenteral nutrition induced biliary sludge in humans. Surg Gynecol Obs. 1990;170:25–31. [PubMed] [Google Scholar]
- 19.Doty JE, Pitt HA, Porter-Fink V, Denbesten L. Cholecystokinin prophylaxis of parenteral nutrition-induced gallbladder disease. Annals Surg. 1985;201:76–80. [PMC free article] [PubMed] [Google Scholar]
- 20.Nathan JD, Romac J, Peng RY, Peyton M, Rockey DC, Liddle RA. Protection against chronic pancreatitis and pancreatic fibrosis in mice overexpressing pancreatic secretory trypsin inhibitor. Pancreas. 2010;39:e24–30. doi: 10.1097/MPA.0b013e3181bc45e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romac JM, Shahid RA, Choi SS, Karaca GF, Westphalen CB, Wang TC, et al. Pancreatic secretory trypsin inhibitor I reduces the severity of chronic pancreatitis in mice overexpressing interleukin-1beta in the pancreas. Am J Physiol. 2012;302:G535–41. doi: 10.1152/ajpgi.00287.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liddle RA, Goldfine ID, Williams JA. Bioassay of plasma cholecystokinin in rats: effects of food, trypsin inhibitor, and alcohol. Gastroenterology. 1984;87:542. [PubMed] [Google Scholar]
- 23.Tashiro M, Samuelson LC, Liddle RA, Williams JA. Calcineurin mediates pancreatic growth in protease inhibitor-treated mice. Am J Physiol. 2004;286:G784–90. doi: 10.1152/ajpgi.00446.2003. [DOI] [PubMed] [Google Scholar]
- 24.Everson GT, Braverman DZ, Johnson ML, Kern F., Jr A critical evaluation of real-time ultrasonography for the study of gallbladder volume and contraction. Gastroenterology. 1980;79:40–6. [PubMed] [Google Scholar]
- 25.Zhu J, Han TQ, Chen S, Jiang Y, Zhang SD. Gallbladder motor function, plasma cholecystokinin and cholecystokinin receptor of gallbladder in cholesterol stone patients. World journal of gastroenterology : World J Gastroenterol. 2005;11:1685–9. doi: 10.3748/wjg.v11.i11.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Green GM, Taguchi S, Friestman J, Chey WY, Liddle RA. Plasma secretin, CCK, and pancreatic secretion in response to dietary fat in the rat. Am J Physiol. 1989;256:G1016–21. doi: 10.1152/ajpgi.1989.256.6.G1016. [DOI] [PubMed] [Google Scholar]
- 27.Chandra R, Wang Y, Shahid RA, Vigna SR, Freedman NJ, Liddle RA. Immunoglobulin-like domain containing receptor 1 mediates fat-stimulated cholecystokinin secretion. J Clin Invest. 2013;123:3343–52. doi: 10.1172/JCI68587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang DQ, Paigen B, Carey MC. Phenotypic characterization of Lith genes that determine susceptibility to cholesterol cholelithiasis in inbred mice: physical-chemistry of gallbladder bile. J Lipid Res. 1997;38:1395–411. [PubMed] [Google Scholar]
- 29.Wang DQ, Lammert F, Cohen DE, Paigen B, Carey MC. Cholic acid aids absorption, biliary secretion, and phase transitions of cholesterol in murine cholelithogenesis. Am J Physiol. 1999;276:G751–60. doi: 10.1152/ajpgi.1999.276.3.G751. [DOI] [PubMed] [Google Scholar]
- 30.Carey MC. Critical tables for calculating the cholesterol saturation of native bile. J Lipid Res. 1978;19:945–55. [PubMed] [Google Scholar]
- 31.Wang DQ, Carey MC. Complete mapping of crystallization pathways during cholesterol precipitation from model bile: influence of physical-chemical variables of pathophysiologic relevance and identification of a stable liquid crystalline state in cold, dilute and hydrophilic bile salt-containing systems. J Lipid Res. 1996;37:606–30. [PubMed] [Google Scholar]
- 32.Heuman DM. Quantitative estimation of the hydrophilic-hydrophobic balance of mixed bile salt solutions. J Lipid Res. 1989;30:719–30. [PubMed] [Google Scholar]
- 33.van Erpecum KJ, Wang DQ, Moschetta A, Ferri D, Svelto M, Portincasa P, et al. Gallbladder histopathology during murine gallstone formation: relation to motility and concentrating function. J Lipid Res. 2006;47:32–41. doi: 10.1194/jlr.M500180-JLR200. [DOI] [PubMed] [Google Scholar]
- 34.Srivastava A, Pandey SN, Dixit M, Choudhuri G, Mittal B. Cholecystokinin receptor A gene polymorphism in gallstone disease and gallbladder cancer. J Gastroenterol Hepatol. 2008;23:970–5. doi: 10.1111/j.1440-1746.2007.05170.x. [DOI] [PubMed] [Google Scholar]
- 35.Xie M, Kotecha VR, Andrade JD, Fox JG, Carey MC. Augmented cholesterol absorption and sarcolemmal sterol enrichment slow small intestinal transit in mice, contributing to cholesterol cholelithogenesis. J Physiol. 2012;590:1811–24. doi: 10.1113/jphysiol.2011.224717. [DOI] [PMC free article] [PubMed] [Google Scholar]