Abstract
Negative feedback regulation of glucocorticoid (GC) synthesis and secretion occurs through the function of glucocorticoid receptor (GR) at sites in the hypothalamic-pituitary-adrenal (HPA) axis, as well as in brain regions such as the hippocampus, prefrontal cortex, and sympathetic nervous system. This function of GRs in negative feedback coordinates basal glucocorticoid secretion and stress-induced increases in secretion that integrate GC production with the magnitude and duration of the stressor. This review describes the effects of GR loss along major sites of negative feedback including the entire brain, the paraventricular nucleus of the hypothalamus (PVN), and the pituitary. In genetic mouse models, we evaluate circadian regulation of the HPA axis, stress-stimulated neuroendocrine response and behavioral activity, as well as the integrated response of organism metabolism. Our analysis provides information on contributions of region-specific GR-mediated negative feedback to provide insight in understanding HPA axis dysregulation and the pathogenesis of psychiatric and metabolic disorders.
Keywords: Glucocorticoid Receptor, Corticotropin-Releasing Hormone, Adrenocorticotropic Hormone, Corticosterone, Hypothalamic-Pituitary-Adrenal Axis, Paraventricular Nucleus of the Hypothalamus, Pituitary, Circadian Regulation, Stress, Metabolism
1. Introduction
Glucocorticoids (GCs) are endogenous steroid hormones that were first identified circa 1929 when studies in adrenalectomized animals demonstrated that adrenal cortex extracts ameliorated the effects of adrenal insufficiency (DeFremery et al., 1937; Hartman and Brownell, 1930; Reichstein et al., 1937). Two decades later, GCs isolated from the adrenal cortex were shown to treat symptoms of Addison’s disease and rheumatoid arthritis leading to the Nobel Prize in Physiology for Edward Kendall, Tadeus Reichstein, and Philip Hench in 1950 (Hench and Kendall, 1949; Kendall, 1951; Raju, 1999). Since then, GC function has been demonstrated as important in the stress response for promoting coping and adaptation to stress as well as recovery from the stressor (De Kloet et al., 2005; Herman and Cullinan, 1997; Joëls et al., 2012; McEwen, 2007; Munck et al., 1984; Sapolsky et al., 2000). In addition, GC function has been demonstrated in metabolism, reproduction, and inflammatory and immune responses (McEwen, 2007; Spies et al., 2011). As a steroid hormone, GC synthesis begins in the adrenal gland from cholesterol. Specifically, in the adrenal cortex, cholesterol is transported into the mitochondria by the steroidogenic acute regulatory (StAR) transport protein (Miller, 2007). In the mitochondria, a series of enzymatic reactions ultimately results in the conversion of 11-deoxycortisol by 11β-hydroxylase (CYP11B1) into cortisol (humans) or corticosterone (rodents) (Payne and Hales, 2004). Embryonic GC synthesis begins as early as E14.5 in mice (Michelsohn and Anderson, 1992) and at approximately 50 days post-conception in humans (Goto et al., 2006). Secretion of GCs shows ultradian and circadian regulation as well as modulation by stress. Circadian regulation of GC results in a diurnal rhythm of GC secretion that has a 24-hour period and is governed by function of the suprachiasmatic nucleus of the hypothalamus (SCN) (Lightman, 2008; Young et al., 2004).
The SCN is a located in the anterior hypothalamus just dorsal to the optic chiasm. It receives light input from the optic region through the retinohypothalamic tract. This light input synchronizes the SCN to cause the generation of the 24-hour circadian cycle of biological processes that regulates mammalian physiological homeostasis. One of the most robust endocrine processes governed in a circadian fashion is the activity of the hypothalamic-pituitary-adrenal (HPA) axis, the major output of which is GC production. Neuronal tracing has provided evidence showing that arginine vasopressin (AVP) neurons from the SCN project into the paraventricular nucleus of the hypothalamus (PVN) through the dorsal hypothalamus and regulates the circadian rhythm of corticotropin-releasing hormone (CRH) (Kalsbeek et al., 1996; Tonsfeldt and Chappell, 2012). The circadian release of CRH then regulates adrenocorticotropic hormone (ACTH) secretion from the anterior pituitary, which subsequently triggers the secretion of corticosterone in rodents and cortisol in humans (CORT) from the adrenal cortex. This circadian rhythmicity is confirmed in SCN lesion studies that result in abolished CORT rhythms (Krieger et al., 1977; Moore and Eichler, 1972; Raisman and Brown-Grant, 1977) as well as disrupting secretion of CRH and ACTH (Cascio et al., 1987; Szafarczyk et al., 1983). CORT secretion however does not occur only through the HPA axis drive. Studies suggest that the adrenal cortex contains its own endogenous clock system that regulates circadian GC secretion that is independent of HPA axis activity but is dependent upon the SCN (Bittman et al., 2003; Ishida et al., 2005; Oster et al., 2006). This adrenal modulation may be mediated by multisynaptic projections from the SCN through the autonomic nervous system to the adrenal cortex (Buijs et al., 1999). The result is a diurnal rhythm of CORT secretion over a 24-hour period that provides a basis for an organism’s daily internal homeostasis. Underlying this circadian regulation is an ultradian rhythm of pulsatile CORT secretion throughout the day that is independent of SCN or stress-inputs. This pulsatility is crucial for stress responsiveness and can vary in frequency and amplitude as a consequence of feedback latency (Waite et al., 2012; Walker et al., 2010).
The circadian rise in GCs occurs immediately prior to the onset of the active period in mammals. In humans, this increase occurs in the morning before awakening, while in nocturnal animals CORT rise occurs in the evening before their active period begins (Clow et al., 2010; Pruessner et al., 1997; Schulz et al., 1998). This awakening rise is CORT may promote mobilization of necessary energy to begin the activity necessary to carry out daily function (Munck et al., 1984). In order to mediate its functions, GCs act on two major receptors, type 1 high affinity receptors, mineralocorticoid receptors (MR) and type 2 low affinity receptors, GC receptors (GR) (Kellendonk et al., 2002b). These receptors have different spatial distribution in the brain and periphery, with GR being more broadly distributed and serving as the main receptor in times of stress (Reul and de Kloet, 1985). Stress activation of the HPA axis serves to prepare an organism to respond to a perceived threat and then to permit adaption that reinstates homeostasis (De Kloet et al., 2005). This latter function is especially important because chronic HPA axis activation leads to constantly elevated GC levels, which is detrimental to an organism. Negative feedback of GCs on the HPA axis is therefore essential to inhibit the damaging effects of CORT.
Since steroid hormones such as CORT are highly lipophilic, they are able to cross the plasma membrane and enter the cytoplasm of a cell through simple diffusion. Once CORT is inside a cell, it binds to GRs that are initially inactively bound to heat shock protein 90 (HSP90). The binding of CORT to GR results in a conformational change that releases GR from inactivation and causes two major modes of GR action. One mode of GR action occurs through non-genomic signaling pathways, that produce fast-acting effects in the order of seconds to a few minutes (de Kloet et al., 2008; Groeneweg et al., 2011a). This occurs through GC action on membrane bound receptors or cytoplasmic GR that activate rapid signaling of second messenger pathways (Bartholome et al., 2004; Cato et al., 2002; Gametchu et al., 1991; Spies et al., 2011; Zhang et al., 2012). The other mode is the classical GR function which occurs when activated GR translocates into the nucleus and functions as a transcription factor to modulate genomic signaling pathways, which can take a period of 15 minutes to several hours.
As a transcription factor, GR can act as a homodimer, monomer, or heterodimer (with MR or other transcription factors) to regulate gene transcription. Direct binding of GR to positive or negative GC-response elements (GRE) on target genes serves to activate or repress transcription respectively (Oakley and Cidlowski, 2013). In certain cases some genes require DNA-bound GR to physically interact with another transcription factor adjacently bound to the gene of interest for transcriptional activity to occur. Indirect DNA binding by GR monomers through tethering in a protein-protein interaction to DNA-bound transcription factors, such as AP-1 and NF-KB also regulates gene expression (Heck et al., 1994; Jonat et al., 1990; Nissen and Yamamoto, 2000; Scheinman et al., 1995). In general, dimerization of GR and its binding to GRE results in transactivation of GR-responsive genes to control of energy metabolism and executive cognitive functions in the face of stress, while monomeric GR interacts with other transcription factors and leads to transrepression of genes and limits activation of signaling pathways induced by transmitters, neuropeptides and other messengers induced by stress. (Kumar and Thompson, 2005). Indeed, mutations in the DNA-binding domain of GR that prevent its ability to dimerize, disrupts GR’s transactivation function but not its transrepressive function (Reichardt et al., 1998). There is also evidence of a GR trimer bound to POMC that results in transrepression of the POMC gene (Drouin et al., 1993). Heterodimers of GR with MR have been identified to bind GRE and regulate gene transactivation with different kinetics than GR homodimers (Liu et al., 1995; T Trapp et al., 1994; Thorsten Trapp et al., 1994). Elevated levels of GC have been shown to increase the likelihood of GR-MR heterodimerization (Nishi et al., 2004). These data imply that variable levels of GC may result in differences in homodimer and heterodimer formation to contribute to the fine-tuning of regulation of GC-responsive genes (Nishi, 2011). Moreover, as indicated by recent hippocampal chromatin immunoprecipitation and sequencing studies, low versus high GC concentrations can activate distinct sets of genes through differential interaction with GR binding sites and GR-associated transcription factors (Polman et al., 2013). The variety in GR signaling, as a monomer, homodimer, heterodimer, or trimer, or due to concentration of GCs, is demonstrative of the ability of GC signaling to modulate diverse functions in an organism (Trapp and Holsboer, 1996).
While GCs are important for the treatment of many disorders, their chronic use, especially systemic administration, can result in mood disorders, osteoporosis, obesity, metabolic syndrome, and reduced immune system function (Biddie et al., 2012; Goldstein, 1999; Marques et al., 2009; Wolkowitz et al., 2009).Therefore, regulation of the HPA axis by CORT is extremely important in preventing maladaptive phenotypes and maintaining normal mammalian function. Disrupted GC regulation is observed in a number of psychiatric and metabolic disorders. Major sites of negative feedback regulation are the PVN and anterior pituitary, although the hippocampus and prefrontal cortex also mediate GC feedback. This review will explore genetic models of GR targeting along the major negative feedback regions of the HPA axis and explore the function of GR in these regions on baseline, stimulated, and integrated responses of activity.
2. Whole brain and Forebrain GR targeting
The GR gene, NRC31, has as its protein product many isoforms due to alternative splicing, alternate translation start sites, and post-translational modifications. These processing events result in the formation of at least five alternatively spliced isoforms and eight translational isoforms of GR. Post-translation modifications such as phosphorylation, sumoylation and acetylation also produce a variety of tissue-specific localizations and differential GC sensitivities that allow for the plethora of GR functions (Review (Oakley and Cidlowski, 2013; Zanchi et al., 2010)).
The effects of genetic GR disruption throughout the brain was first studied by Tronche et. al using Nestin-Cre mice, expressing Cre-recombinase in neural progenitor cells, to excise the exon 3 region of GR in GR floxed mice (GRNesCre) (Tronche et al., 1999). These mice display GR deletion during embryonic development in the entire central nervous system including the PVN (Fig. 1B). As a result, the GRNesCre mice display extreme hyperactivity of the HPA axis that causes Cushing-like symptoms and decreased anxiety and despair-like behaviors (Tronche et al., 1999).
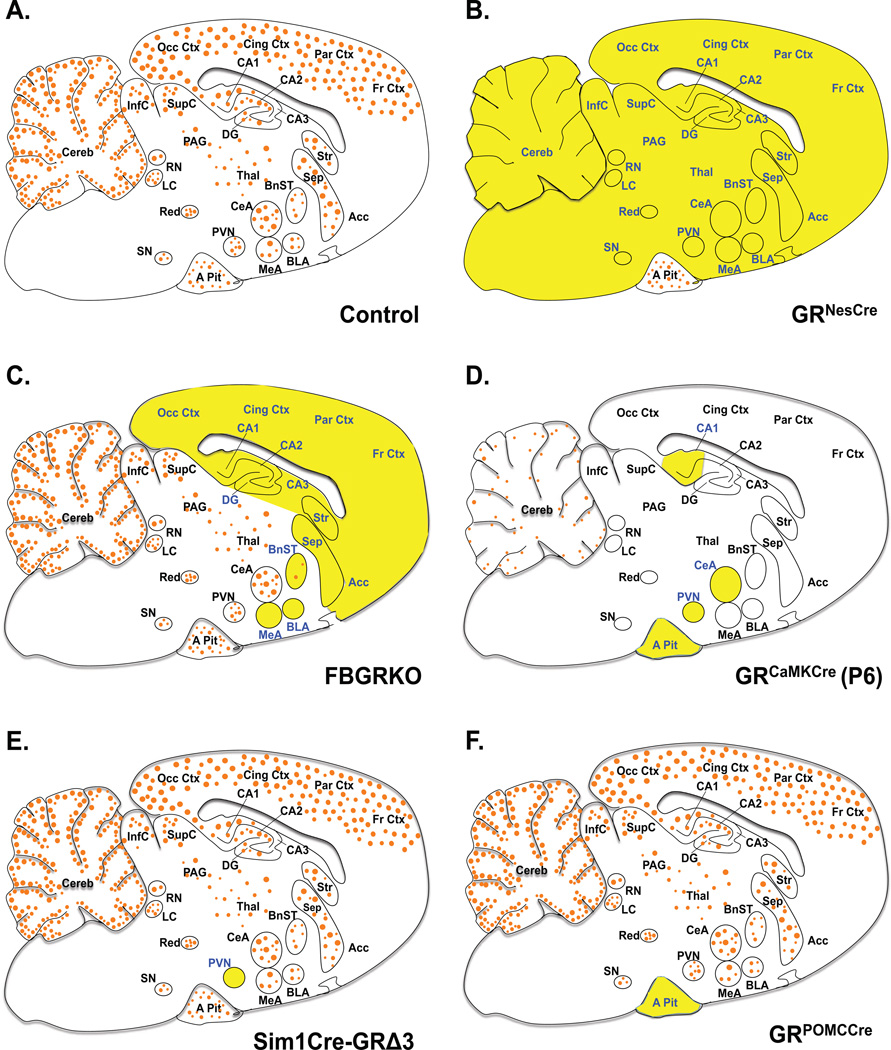
Figure 1. Glucocorticoid Receptor Expression in Genetic Mouse Models.
Sagittal brain sections depicting areas of GR mRNA expression and deletion in mice with targeted GR deletion. Orange circles (●) represent GR and targeted regions are in yellow highlight and blue font. A. Adult control mice ubiquitously express GR mRNA in the entire brain and pituitary, with higher expression in limbic regions. B. Adult GRNesCre mice have GR loss in the entire brain, neurons and glial cells. C. FBGRKO mice have GR loss in forebrain neurons during adulthood. D. At postnatal day 6 GR expression is low and limited to few brain region (blue font). GRCaMKCre mice have GR loss in most of the neurons in brain and cells in the pituitary at P6. E. Adult Sim1Cre-GRe3Δ mice have GR loss primarily in PVN neurons. F. GRPOMCCre mice have GR loss in the anterior pituitary cells throughout life. Abbreviations: Anterior pituitary, A Pit; Basolateral nucleus of the amygdala, BLA; Bed nucleus of the stria terminalis, BnST; Central nucleus of the amygdala, CeA; Hippocampal areas, CA1, CA2, CA3; Cerebellum, Cereb; Cingulate cortex, Cing Ctx; Dentate gyrus, DG; Frontal cortex, Fr Ctx; Inferior colliculus, InfC; Locus coeruleus, LC; Medial nucleus of the amygdala, MeA; Occipital cortex, Occ Ctx; Periaqueductal gray, PAG; Parietal cortex, Par Ctx; Paraventricular hypothalamic nucleus, PVN; Raphe nucleus, RN; Septum, Sep; Supraoptic nucleus, SN; Superior colliculus, SupC; Thalamus, Thal;
Limiting GR disruption specifically in forebrain neurons during adulthood (FBGRKO) (Fig 1C) in male mice, on the other hand, demonstrated increased basal and stress-stimulated CORT and despair behavior in male mice, although there are differences between studies (Table 1) (Boyle et al., 2006, 2005; Furay et al., 2008). For instance, whereas Boyle et al revealed increases in plasma CORT at nadir and peak in FBGRKO mice, Furay and colleagues do not observe changes in peak CORT between control and FBGRKO mice. Moreover, whereas stress increases CORT levels of FBGRKO in both studies, the time line of this increase differs between studies. Given ultradian fluctuations in glucocorticoid secretion, the differences in these studies may be due to the time when blood samples were collected. However, both studies implicate a role for forebrain GR in negative feedback regulation in adult male mice (Boyle et al., 2006, 2005; Furay et al., 2008). The fact that male FBGRKO mice displayed elevated levels of CORT despite intact GR in the PVN and pituitary confirmed the existence of extrahypothalamic negative feedback sites. Indeed, lesion studies as well as GR inhibition studies in the hippocampus and prefrontal cortex both disrupt HPA axis activity, identifying these regions as sites of GC feedback regulation in the CNS (Diorio et al., 1993; Feldman and Conforti, 1985; Herman et al., 1998, 1992; Jacobson and Sapolsky, 1991; McKlveen et al., 2013; Radley et al., 2011; van Haarst et al., 1997). The despair behavior observed in male FBGRKO mice was reversed after chronic antidepressant treatment, with consequent increases in hippocampal MR expression(Boyle et al., 2005). There are overlapping regions of MR and GR expression in limbic forebrain regions such as the hippocampus, prefrontal cortex, and amygdala, where MR has a role in appraisal processes and response selection (De Kloet et al., 2005; Joëls et al., 2012). The data in FBGRKO mice supports a role for MR in compensating for GR loss in the hippocampus to mediate therapeutic responses to antidepressants.
Table 1.
Effects of glucocorticoid receptor targeting on HPA axis activity, behavior, and metabolic phenotypes
| Phenotype | GRNesCre | FBGRKO Male |
FBGRKO Female |
GRCaMKCre | Sim1Cre- GRe3Δ |
Sim1Cre- GRe2Δ |
GRflox+/+; Sim1Cre+ |
GRflox+/−; Sim1Cre+ |
GRPOMCCre |
|---|---|---|---|---|---|---|---|---|---|
| GR floxed | Exon 3 | Exon 1C-2 | Exon 1C-2 | Exon 3 | Exon 3 | Exon 1C-2 | Exon 2 | Exon 2 | Exon 3 |
| Primary region | CNS | Forebrain | Forebrain | CNS + PIT | PVN | PVN | PVN/Lung | PVN | PIT |
| GR loss | 75–100% | 75–100% | 87% | 43% | 70% | 90% | |||
| Lethality | None | None | None | Postnatal | None | None | Embryonic | None | None |
| Postnatal Day 6 | |||||||||
| PVN CRH | ↑ | ↑ | ↑ | ↓ | |||||
| PVN AVP | ↑ | ||||||||
| POMC mRNA | ↑ | ↑ N | |||||||
| Plasma ACTH | ↑N | ↑ N | |||||||
| Plasma CORT | ↑ | ↑ | ↑ N | ||||||
| Weight | ↓ | ↓ | ↓pw | ↓ | |||||
| Skin pigm. | ⬄ | ↓ | |||||||
| Skin fat | ⬄ | ↓sc | |||||||
| Thymus weight | ⬄ | ↓ | ⬄ | ||||||
| Spleen weight | ⬄ | ↓ | |||||||
| Adult | |||||||||
| PVN CRH | ↑ | ⬄B, ↑S | ⬄ | ↑ | ⬄ | ↑ | ⬄ | ||
| PVN AVP | ⬄ | ↑ | ⬄ | ||||||
| Plasma ACTH | ↑PIT, ➘N | ↑P/ S | ⬄ | ↑N/P, ⬄S | ⬄ N/P/S | ↑B | ⬄ | ||
| Plasma CORT | ↑N/P | ➚N, ↑P/S | ⬄ | ↑N/P/S | ⬄ N/P/S | ↑B | ⬄ | ||
| ↑N, ⬄P | |||||||||
| DST | Impaired | Impaired | ⬄ | Impaired | |||||
| Weight | ↓ | ⬄ | ↑ | ⬄ | ↓ | ⬄ | |||
| Fat Mass | ↓ | ↑ | ⬄ | ||||||
| Anxiety | ↓ | ↓ | ⬄ | ⬄ | ↑ | ||||
| Despair | ↓ | ↑ | ⬄ | ⬄ | ⬄ | ↓ | |||
| Stress-coping | ↑ | ↓ | ⬄ | ⬄ | ⬄ | ↑ | |||
| Locomotor activity | ⬄ | ↑EZM | ↓OF | ⬄ | ⬄ | ||||
| References | (Tronche et al., 1999) (Kellendonk et al., 2002) (Erdmann et al., 2008) |
(Boyle et al., 2005) (Boyle et al., 2006) (Furay et al., 2008) |
(Solomon et al., 2012) | (Erdmann et al., 2008) | (Laryea et al., 2013) | (Jeanneteau et al., 2012) | (Schmidt et al., 2009) (Wagner et al., 2011) |
||
PIT, pituitary; Prot, protein; N, nadir; P, peak; S, stress; B, Basal; sc, subcutaneous; pw, pre-weaning; EZM, elevated zero maze; OF, open field; ↑, increase; decrease; ➚, trend towards increase; ➘, trend towards decrease; ⬄, similar to controls; DST, dexamethasone suppression test.
In contrast to male mice, female FBGRKO mice do not show any changes in HPA axis activity compared to controls despite the fact that they had the same level of GR deletion observed in males (Solomon et al., 2012). Moreover, female FBGRKO mice do not exhibit the despair-like behaviors in the forced swim or sucrose preference tests that were observed in the males. These gender differences may be due to the influence of gonadal hormones on the HPA axis and stress sensitivity, or the possibility that the GR sites targeted in the forebrain are differentially regulated in males and females. Differences in gender-specific glucocorticoid actions have been exemplified in the evaluation of GC effects on gene expression in the rat liver (Duma et al., 2010). The authors found unique sets of genes regulated by GR that were male-specific or female-specific genes that were induced to a higher degree in males than females or vice versa, and genes that were repressed to a higher degree in males than females or vice versa. When the pathways regulating some of these genes were evaluated, it was determined that GC-mediated anti-inflammatory actions were more effective in males than females, and this was caused by ovarian hormones suppressing GC-mediated anti-inflammatory faction. The data from this paper presents evidence for differential GC signaling in males and females that results in distinct gene expression patterns (Duma et al., 2010). Although this data explored gender differences in liver GR-mediated gene regulation, it is conceivable that the underlying concept can be extrapolated to other tissues as well.
The effects of global GR loss, as well as disruption in the CNS and forebrain, have been reviewed in previous articles (Arnett et al., 2011; Finotto et al., 1999; Kellendonk et al., 2002b; Kolber and Muglia, 2009; Kolber et al., 2008). The next sections of this review will focus more on recent comparisons of phenotypes between the aforementioned transgenic mice and those in which GR is targeted along major regulatory sites of the HPA axis.
3. GR Disruption in all Sites of Negative Feedback
3.1. Circadian Regulation
Transgenic mice generated by Erdmann and colleagues used a CamKIIα transgene capable of driving Cre-recombinase expression during embryonic development in the whole brain as well as the pituitary to delete GR (GRCamKCre) (Erdmann et al., 2008). Thus, in contrast to GRNesCre mice that retain pituitary GR feedback regulation (Fig 1D), GRCamKCre mice have GR disrupted along all sites of HPA axis regulation. GRCamKCre mice die between day 6 and 10 of postnatal life (P6, P10). Endocrine data from these mice indicate dramatically elevated levels of plasma CORT (Fig. 2) and ACTH at P6 with concomitant increases in pituitary proopiomelanocortin (POMC) mRNA and PVN CRH protein levels (Erdmann et al., 2008). The postnatal demise of these mice is likely due to constitutive loss of CORT negative feedback inducing a pathological excess of GC activity at peripheral sites where GR remains intact. Stress regulation was not studied due to the early postnatal death of GRCamKCre mice.
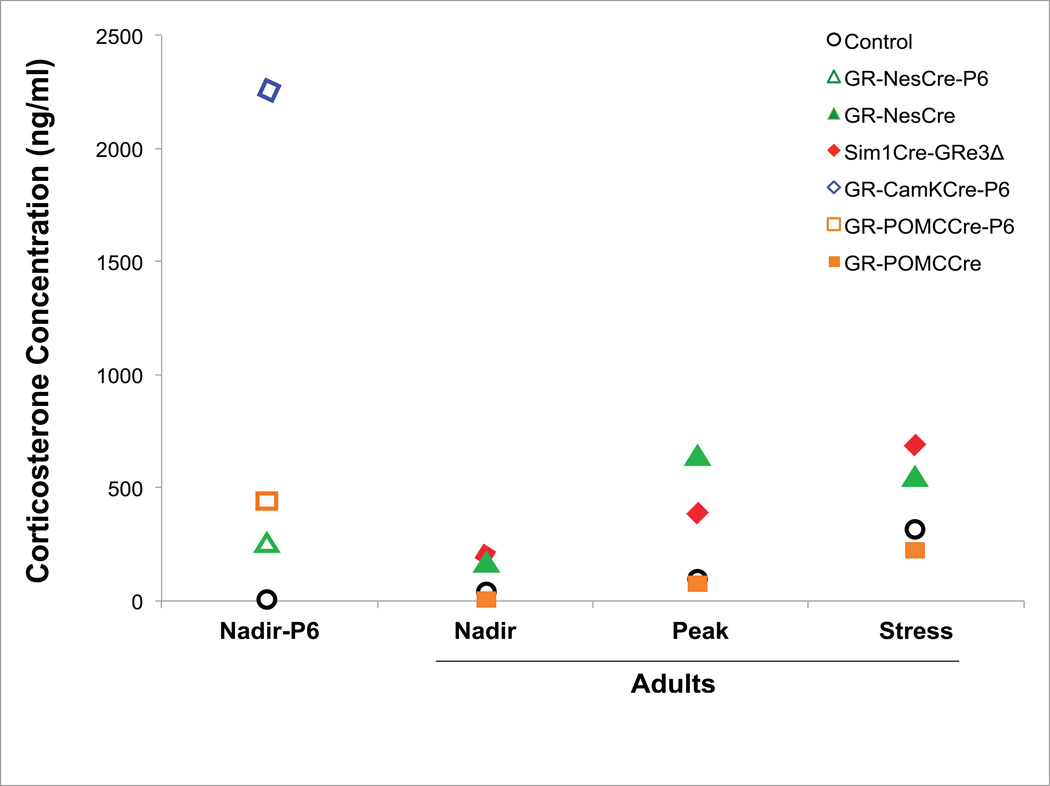
Figure 2. Schematic of CORT levels in GR-targeted Mice.
This figure depicts the relative increases in plasma CORT levels in GRNesCre, GRCaMKCre, GRPOMCCre, and Sim1Cre-GRe3Δ compared to controls at postnatal day 6 and in adulthood. Values on the y-axis represent estimates of plasma CORT values provided from respective articles. Controls are averaged values from individual studies.
3.2. Metabolic Regulation
GRCamKCre mice did not phenotypically differ from control mice at the time of birth, but at P6, they exhibited a 50% reduction in body weight compared to controls. Reduced weight in GRNesCre mice is also observed from P2 throughout adulthood (Kellendonk et al., 2002a). The growth deficit in GRNesCre mice was attributed to decreased food intake due to metabolic effects of hypercorticosteronism. This is a likely a mechanism for the observed deficits in GRCamKCre mice as well. ACTH serves as a precursor for the α-melanocyte-stimulating hormone (MSH) that regulates melanin synthesis (Stevens and White, 2010). The excess ACTH observed in GRCamKCre mice might therefore disrupt MSH signaling to cause the lack of skin pigmentation in these mice (Erdmann et al., 2008). In contrast to GRNesCre mice, GRCamKCre mice also display pathological abnormalities including a thin epidermis, reduced thymus weight, and a small and under developed spleen. It is likely that the excess GCs acting on peripheral GR mediates these metabolic phenotypes in these mutant mice. The differences between the GRNesCre and GRCamKCre mice is likely a function of the extent of GR involving the brain versus brain and pituitary, respectively, leading to drastically higher plasma CORT and ACTH levels in GRCaMKCre mice (Fig. 2) (Table 1). In contrast with GRCamKCre mice, the CamKIIα-Cre transgenic line used in FBGRKO mice begins its expression of Cre-recombinase during the third week of postnatal life (Boyle et al., 2005; Tsien et al., 1996) and does not express Cre-recombinase in the pituitary. No metabolic effects are observed in FBGRKO mice.
4. GR Disruption in the Hypothalamus
4.1. Circadian Regulation
The PVN of the hypothalamus serves as a major integration center for stress input from limbic regions. In the HPA axis, the PVN is the site of CRH synthesis that drives GC synthesis. Circadian regulation of PVN CRH activity is mediated by SCN synchronization. Neuroanatomical studies have provided evidence for SCN neuropeptide input to the PVN. For instance, AVP neurons originating from the SCN project into the dorsal hypothalamic nuclei which then relay information to CRH neurons in the PVN (Kalsbeek et al., 1996). Physiological data has also demonstrated that the SCN evokes both inhibitory and excitatory postsynaptic potentials in PVN neurons and in this way directly targets PVN CRH neurons (Hermes et al., 1996). This SCN control would therefore drive the canonical HPA axis pathway of GC synthesis and secretion by synchronizing PVN CRH to induce the secretion of pituitary ACTH that leads to the synthesis and secretion of CORT. It is of note, however, that both the pituitary and adrenal cortex have endogenous clock pathways that can be activated independently of HPA axis rhythmicity. Furthermore, GC action via GR does not have a direct effect on SCN clock gene expression, but does cause phase shifts in peripheral clock gene-driven circadian functions peripherally, indicating a reciprocal relationship between SCN and GC, in which diurnal GC is able to coordinate SCN driven circadian functions in peripheral oscillators (Balsalobre et al., 2000).
To specifically investigate the impact of GC feedback at the level of the hypothalamus, we generated mutant mice using a Sim1Cre transgene that expresses Cre-recombinase in Sim-1 neurons, primarily in the PVN, as well as sparsely in other nuclei (Balthasar et al., 2005). The Sim1Cre transgene was used to remove exon1C-2 (Sim1Cre-GRe2Δ) or exon 3 (Sim1Cre-GRe3Δ) (Fig. 1E) in floxed GR mice. We demonstrated in the resulting mutant mice that loss of 50%–90% of PVN GR protein does not disrupt circadian CORT or ACTH release (Laryea, 2013). In control mice, Sim1Cre-GRe2Δ and Sim1Cre-GRe3Δ mice, basal evening CORT and ACTH levels were elevated significantly above morning levels. This retention of diurnal CORT and ACTH rhythms occurred in spite of significantly increased CORT and ACTH secretion in Sim1Cre-GRe3Δ mice. This is evidence that PVN GR is not involved in regulating the qualitative pattern of diurnal secretion of GCs. A different PVN GR knockout mouse generated by Jeanneatteau and colleagues showed a more drastic phenotype in that the homozygous mutants (GRflox/flox;Sim1Cre+) are embryonic lethal due to possible residual Cre-recombinase expression in the lung (Jeanneteau et al., 2012). The heterozygous mice (GRflox/+;Sim1Cre+), which are hypomorphic for GR in the PVN however, also demonstrate increased plasma CORT ACTH levels. The elevated plasma CORT and ACTH levels are a reflection of increased CRH transcripts in the PVN of GRflox/+;Sim1Cre+ and Sim1Cre-GRe3Δ mice (Jeanneteau et al., 2012; Laryea et al., 2013). Interestingly, none of these HPA axis changes were observed in Sim1Cre-GRe2Δ mice. The level of GR loss, 47% in Sim1Cre-GRe2Δ mice vs. 87% in Sim1Cre-GRe3Δ mice, is likely a contributing factor to the difference in phenotype. This is supported by data in GR null heterozygous mice which have 50% loss of GR and display no changes in glucocorticoid secretion under basal conditions (Ridder et al., 2005) as well as in FBGRKO mice, which do not show HPA axis abnormalities when less than 60% GR is loss (Boyle et al., 2005). An alternate explanation for the differences between Sim1Cre-GRe2Δ and Sim1Cre-GRe3Δ mice might be the influence of the distinct exons deleted and the potential for residual production of portions of GR (Table 1). The exon 3 of GR contains the zinc-finger of the DNA binding domain while the exon 2 contains the transcriptional activation domain. Genetically altered mice with site specific mutations along the GR gene, such as in the GR dimerization-deficient mice (GRdim) (Reichardt et al., 1998), may provide more intricate analysis of importance of exon/domain functions. In fact, GRdim mice, have elevated CORT levels at nadir and peak compared to control mice (Oitzl et al., 2001), indicating that the dimerization and/or DNA binding function of GR is necessary for negative feedback regulation of HPA axis activity.
Negative feedback in the hypothalamus occurs when CORT binds to GR in the PVN. Activated GR translocates to the nucleus of parvocellular neurons where it may bind to a negative GRE in the CRH promoter gene, causing repression of CRH transcription (Malkoski et al., 1997; Webster and Cidlowski, 1999). Specifically, Malkoski and colleagues identified a GC-responsive cis-regulatory element on the CRH promoter, which is stimulated by cAMP. GR was shown to mediate greater than a 50% reduction in CRH promoter activity through direct binding to a negative GRE on the CRH promoter in a region that is highly conserved (Malkoski et al., 1997). However, many other studies have found a lack of direct GR interaction with the CRH gene. For instance, studies of GRdim mice, which have a mutation in the DNA-binding domain, demonstrate no changes in CRH expression (Reichardt et al., 1998). Moreover, hypothalamic chromatin immunoprecipitation of the CRH promoter demonstrated no GR interaction or GRE detected near the promoter (Evans et al., 2013). Further exploration of the CRH gene indicated that GCs act on GR to promote DNA methylation and histone acetylation of the CRH promoter to repress CRH expression (Elliott et al., 2010; Sharma et al., 2013). In vitro studies show that GR repression of CRH can occur through its disruption of second messenger signaling pathways (Yamamori et al., 2007). Studies also indicate that GCs act on G-protein coupled membrane receptors in PVN neurons to increase endocannabinoid signaling. The activated endocannabinoids cause retrograde suppression of glutamate secretion onto PVN neurons, thereby reducing the frequency of miniature excitatory post-synaptic currents (Di et al., 2003; Malcher-Lopes et al., 2006; Tasker, 2006). This results in GC-mediated fast-feedback inhibition of CRH neurons in the PVN and thus inhibition of ACTH and CORT secretion (Dallman, 2005; Evanson et al., 2010; Groeneweg et al., 2011a, 2011b; Myers et al., 2012). These data collectively indicate the existence of multiple mechanisms for GC-mediated repression of PVN CRH activity that does not depend on direct GR interaction with the CRH gene.
Data from our lab demonstrates that loss of ~50% of GR in the PVN does not affect HPA axis activity or behavior. When levels of GR are reduced below 20% deleterious effects due to CORT excess are observed (Laryea et al., 2013). Indeed, Sim1Cre-GRe3Δ mice show a 3-fold increase in CRH mRNA levels in the PVN as a result of 87% GR loss, whereas Sim1Cre-GRe2Δ mice have only 43% of GR PVN loss. Thus, the level of GR loss in the PVN directly influences alterations in CRH transcripts in the PVN and subsequent plasma ACTH and CORT levels. While GCs act on the CRH gene in the PVN to inhibit CRH expression in order to mediate negative feedback regulation of the HPA axis, GC actions in other tissues has the effect of increasing CRH. Examples are the effects of GC on increasing amygdala CRH (Makino et al., 1994a, 1994b) or placental CRH (Korebrits et al., 1998; Marinoni et al., 1998; Robinson et al., 1988). This indicates that there are different mechanisms of GR – CRH interaction depending on their tissue-specific location.
4.2. Stress-mediated stimulation
In addition to having elevated basal nadir and peak levels of CORT, Sim1Cre-GRe3Δ mice respond to restraint stress with further exacerbations in CORT release than controls (Laryea et al., 2013). In contrast to CORT, stress-induced ACTH levels in Sim1Cre-GRe3Δ mice did not differ from that of control mice. This dissociation of elevated stress-induced CORT with normal stress-induced ACTH expression implies a possible increase in adrenal cortex sensitivity to ACTH under stressful conditions. Indeed, increased adrenal sensitivity to ACTH is observed in GRNesCre mice, but not in FBGRKO, resulting in elevated CORT secretion in response to exogenous ACTH injections (Table 1). A role for PVN GR mediating this increased adrenal sensitivity during stress is therefore possible.
4.3. Integrated consequences: Metabolism (adiposity; metabolism)
At the onset of a stress response, GCs are known to mediate metabolic activity by enhancing glycogenolysis, lipolysis, and proteolysis to produce substrates of glucose, fatty acids, and amino acids, respectively, that can be diverted for use in specific tissues depending on need (Sapolsky et al., 2000). Given the involvement of GR in metabolic activity as well as the hypothalamus being a center for regulation of energy metabolism, (Kellendonk 2002, endocrinology), Sim1Cre-GRe3Δ mice present an opportunity to evaluate the influence of loss of PVN GR on the metabolic phenotype.
At birth, the Sim1Cre-GRe3Δ mutants appear similar to control littermates. During postnatal development, Sim1Cre-GRe3Δ mice show stunted growth (Laryea et al., 2013). Phenotypic differences start manifest at P20 and there is significantly reduced body weight. In contrast to pituitary GR loss (Section 5.3), Sim1Cre-GRe3Δ male and female mice begin to catch up to their control littermates beginning around P70 and surpass controls in weight from P100 onward. This increased weight included the accumulation of adipose tissue in the gonadal region of the mutants, and a truncal obesity phenotype. Given the chronically high levels of GCs in the mutants, this result is clearly consistent with Cushing’s syndrome in these mice. Compared to Sim1Cre-GRe3Δ mice, the excess GCs in GRNesCre and GRflox/+; Sim1Cre+ mice do not result in increased adiposity; in fact these mice have reduced weight in adulthood. GCs regulate expression of hypothalamic peptides, such as leptin, insulin, and neuropeptide Y, which govern growth and energy homeostasis (Kellendonk et al., 2002a). Leptin, which signals the hypothalamus to decrease hunger and therefore food intake as well as fat storage, is elevated in young GRNesCre mice. Adult GRNesCre mice have reduced levels of Insulin-growth factor 1 (IGF-1) which is involved in stimulating growth in many bodily organs (Kellendonk et al., 2002a). Thus, excess GCs are inducing decreased body weight by regulation of leptin and IGF-1. Since Sim1CreGRe3Δ mice become obese and have increased adiposity, this finding is suggestive of a difference in CORT function during early development compared to adulthood in mediating growth signaling. This ramification is supported by rat studies showing that prenatal dexamethasone application causes low birth weight (Drake et al., 2010), but dexamethasone application into adult rats causes an increase in enzymes responsible for synthesis of lipids, and thus increased visceral adiposity (Chimin et al., 2014). However evidence in most studies demonstrates catabolic effects of dexamethasone in adult rodents that lead to weight loss (Franco-Colín et al., 2006; Minet-Quinard et al., n.d.; Savary et al., 1998). Thus the cause of discordance in weight between adult Sim1Cre-GRe3Δ and GRflox/+;Sim1Cre+ mice is unclear, given that excess GCs also occurs in these lines. The influence of increased PVN CRH in mediating some of the growth phenotypes in these mutants cannot be discounted, as CRH signaling also regulates feeding and energy balance (Stengel and Taché, 2014; Stengel et al., 2009). In a number of transgenic mice with CRH overexpressed, constitutively or only in the forebrain, the Cushing’s phenotypes (obesity, hair loss, thin skin) driven by chronically elevated GC are observed (Bentley et al., 2014; Kolber et al., 2010; Stenzel-Poore et al., 1992). The most recent of these studies by Bentley et al, performed detailed characterization of mice with a gain of function mutation in the CRH gene (CRH-120) that had Cushing’s syndrome. These data indicate that disruption of CRH or GR gene that result in chronic GC excess, leads to a Cushing’s phenotype. However, there is a deviation in the role of CRH and GR in the glucose regulatory aspect of this metabolic phenotype. Whereas CRH gene disruption in the CRH-120 mice leads to hyperglycemia that is typically observed in Cushing’s syndrome (Bentley et al., 2014), GR disruption in Sim1Cre-GRe3Δ mice does not lead to hyperglycemia under basal conditions, and is unaffected after stress, compared with stress-induced elevation in control and Sim1Cre-GRe2Δ mice (Laryea et al., 2013).
5. GR Disruption in the Pituitary
5.1. Circadian Regulation
The pituitary, located ventrally to the brain, consists of an anterior, intermediate (adenohypophysis) and posterior lobe (neurohypophysis) in rodents. The anterior lobe is the target of PVN CRH and arginine vasopressin input that stimulates the synthesis and secretion of ACTH. CRH activates the synthesis of ACTH through the function of an orphan nuclear receptor, Nur77 (Webster and Cidlowski, 1999). When activated by CRH signaling through its receptors, Nur77 induces the transcription of POMC by binding to DNA-response elements on the POMC gene. Prohormone convertase 1 and 2 enzymes process the POMC precursor polypeptide to generate ACTH and β-lipoprotein, which can be further processed into α-melanocyte-stimulating hormone, β-endorphin, corticotrophin-like intermediate peptide and γ-lipotrophins (Papadimitriou and Priftis, 2009). It is important to note that Nur77 deficient mice display normal HPA axis signaling, and there is evidence that closely-related members of the Nur77 nuclear receptor family can compensate in its absence (Crawford et al., 1995; Fernandez et al., 2000). Diurnally secreted ACTH binds to type 2 melanocortin receptors (MCR2) on the surface of the adrenal cortex. Activation of MCR2, a G-protein coupled receptors, triggers cAMP second messenger activity that results in immediate and chronic synthesis of StAR and other steroidogenic enzymes (Papadimitriou and Priftis, 2009) to cause CORT synthesis and secretion (Liu et al., 2013).
GC negative feedback at the level of the pituitary begins at E16.5 (Reichardt and Schütz, 1996) when CORT acts on pituitary GR. Rapid and delayed feedback inhibition of pituitary ACTH by GC have been demonstrated to occur through both genomic and non-genomic pathways in a number of studies (Dayanithi and Antoni, 1989; Hinz and Hirschelmann, 2000; John et al., 2004; Keller-Wood and Dallman, 1984; Widmaier and Dallman, 1984). A negative GRE has been previously identified proximal to the POMC gene responsive to dexamethasone suppression in vitro, indicating direct GR genomic effects (Drouin et al., 1993). Indirect effects have also been suggested in that activated GR homodimer can represses Nur77 activity, thereby decreasing POMC mRNA expression and inhibiting ACTH production (Webster and Cidlowski, 1999). To evaluate negative feedback regulation at the level of the pituitary, Schmidt and colleagues generated mice with pituitary GR deletion. This was accomplished by the crossing of GR floxed exon 3 mice to a POMC-Cre transgenic line (GRPOMCCre). GR expression was lost in the POMC-expressing cells of the pituitary and arcuate nucleus of these mice (Fig. 1F) (Schmidt et al., 2009). Morning levels of plasma CORT and ACTH under basal conditions were extremely elevated by P6. This excess GC is particularly interesting because it occurred despite reduced PVN CRH. It could be argued that this excess CORT is actually mediating negative feedback at the level of the PVN, hence the reduced CRH mRNA. However this is unlikely due to the fact that GR mRNA in these mice is also reduced at that age and levels of plasma ACTH are increased. While PVN CRH mRNA is decreased, expression of AVP transcripts in the PVN is increased and may contribute to the increase in plasma ACTH levels. These effects of pituitary GR deletion only happen developmentally, because by P30, the HPA dysregulation phenotypes were normalize in GRPOMCCre mice (Fig. 2) (Table 1), to levels similar to control mice (Schmidt et al., 2009). Development of the HPA axis in mice includes a stress hyporesponsive period (SHRP) established from postnatal day 1 (P1) through P12 (Schmidt et al., 2005). During this time, neonates have low basal CORT, and high PVN CRH transcript levels. The alterations in GRPOMCCre mice during SHRP but not in adulthood, confirm as previously identified that pituitary GR function is indispensable for HPA axis control during the SHRP (Schmidt et al., 2005).
5.2. Stress-mediated stimulation
Developmental GC excess in GRPOMCCre mice impaired GR-mediated negative feedback in adulthood, as GRPOMCCre mice took longer to decrease their CORT levels after restraint stress and in the dexamethasone suppression test (DST) (Schmidt et al., 2009). While the initial study of GRPOMCCre mice demonstrated no basal anxiety phenotype (Schmidt et al., 2009), a subsequent study revealed a more anxious phenotype of GRPOMCCre mice in the elevated zero maze test (Wagner et al., 2011). This anxious phenotype was abolished with a chronic social defeat stress, leading the authors to infer that in the initial study, the mice were inadvertently exposed to a stressor that masks their basal anxiety phenotype. Adult GRPOMCCre mice display decreased despair in the forced swim test compared to control mice. The reduced despair behavior was inhibited if GRPOMCCre mice were treated with a GR antagonist during the developmental period when CORT levels are high. This finding indicates that transient increases in GR activation early in development have differing effects on anxiety and despair behaviors later in adulthood. This data also suggests that when increased GR activation is persistent, as observed in Sim1Cre-GRe3Δ mice (Laryea et al., 2013), it results in habituation to anxiety and despair behavior under basal circumstances. The data reviewed here is part of a larger body of evidence on the programming effects of early life experience (Buwalda et al., 2013; Champagne et al., 2008; Daskalakis et al., 2012; Francis et al., 1999; Liu, 1997; Santarelli et al., 2014; Zhang et al., 2005). The observations described in these studies have strong implications for the role of MR and GR in the predictive adaptive capacity, match/mismatch and cumulative stress exposure theories (Champagne et al., 2009; Daskalakis et al., 2013; Nederhof and Schmidt, 2012; Schmidt, 2011).
FBGRKO and Sim1Cre-GRe3Δ both fail to suppress CORT in the DST test, and GRPOMCCre mice suppress CORT but in a less efficient manner than observed in controls (Table 1). This suggests differential levels of contributions to negative feedback regulation from these regions, with the PVN and forebrain GR contributing more heavily in adulthood and pituitary GR more during early postnatal life. From this data, we conclude that pituitary GR mediates basal HPA activity in early life (young GRPOMCCre mice have disrupted PVN CRH, normal plasma CORT and ACTH levels) but not in adulthood (Adult GRPOMCCre have normal PVN CRH, normal plasma CORT and ACTH levels). On the other hand, PVN GR is involved in mediating basal HPA activity in adulthood, (adult Sim1Cre-GRe3Δ have disrupted PVN CRH, normal plasma CORT and ACTH levels).
The data in GRPOMCCre and GRCamKCre (Section 3) mice demonstrate the importance of pituitary GR function in early postnatal development. The retention of negative feedback sites in the brain and PVN of GRPOMCCre mice prevents the postnatal death that occurs in GRCamKCre mice (Erdmann et al., 2008). This finding suggests that CORT elevations in excess of a certain threshold are lethal. At least one site of negative feedback regulation must be retained for life. This conclusion is supported by the fact that when pituitary GR is the only negative feedback site retained in GRNesCre mice, the mice survive similar to controls. While GRPOMCCre mice do show decreases in PVN GR early in life, the level of this GR deletion may not be enough to completely avert negative feedback, and furthermore PVN GR mRNA levels normalize in adulthood while pituitary GR loss is retained (Schmidt et al., 2009). Given data in from our group that indicates that 50% loss of PVN GR does not affect HPA axis activity or behavioral phenotypes (Laryea et al., 2013), it seems unlikely that the level of GR loss in GRPOMCCre mice is influential in the observed phenotypes. Thus, one question that arises is, when PVN and pituitary GRs are both lost, is GR feedback in the forebrain sufficient to maintain viability?
After the SHRP, basal plasma CORT levels increase and PVN CRH mRNA levels decrease beginning from P12, while plasma ACTH levels remain constant (Schmidt et al., 2003). Moreover, before P9, the stress of a novel environment has no effect on plasma CORT or ACTH levels, but after P12 levels of both hormones increase in response to novelty stress in control mice. The data in GRPOMCCre mice indicate that developmentally, pituitary GR is sensitive to stimuli. Generally, GR is known as being responsive to circadian peak or stress-induced but not nadir changes in HPA axis activity (De Kloet and Reul, 1987; De Kloet et al., 1998). However the studies described in GRPOMCCre and Sim1Cre-GRe3Δ mice suggest that in early postnatal development, pituitary GR is sensitive to basal HPA axis changes and along development there is a switch to PVN GR becoming sensitive to basal changes in HPA axis activity. A comparison of GRPOMCCre and Sim1Cre-GRe3Δ mice also shows that whereas loss of pituitary GR increases anxiety in the EZM and causes stress coping in the FST during adulthood, loss of PVN GR had no effect. This outcome is possibly due to lower plasma CORT concentrations early in development of Sim1Cre-GRe3Δ mice than GRPOMCCre mice. A closer evaluation of the ontogeny of the HPA axis in Sim1Cre-GRe3Δ mice would better enable this to be resolved.
Chronic social defeat stress in GRPOMCCre mice not only prevented anxiety phenotypes but also abolished increases in basal CORT that occurred in control stressed mice. There is much evidence of early life stress being protective later in life. The data in GRPOMCCre mice supports the notion that the resilience to social defeat stress occurs due to the transient GC excess that mimics the neuroendocrine effects of early life stress (Wagner et al., 2011). It appears though, that while genetic defects that produce a brief period of GC excess postnatally have no effect on HPA axis dysregulation later in adulthood, genetic defects that produce long-term GC excess completely disrupt HPA axis activity.
5.3. Integrated consequences: Metabolism
GRPOMCCre mice have stunted growth until during the first two weeks of life (Schmidt et al., 2009). In GRPOMCCre mice, GR is also deleted in the arcuate nucleus, a region of the brain involved in regulating feeding behavior (Cone et al., 2001; Li et al., 2012; Schwartz et al., 2000; Trivedi, 2014). It is therefore possible that GR loss in the arcuate is mediating the growth impairments in GRPOMCCre mice. However, this seems unlikely, given that early postnatal growth deficits also occur in GRNesCre, GRCamKCre and Sim1Cre-GRe3Δ mice, all of which have elevated levels of CORT during that developmental period. Moreover, in GRPOMCCre mice, weight returns to similar levels as that of controls from P28 onwards, and coincides with normalization of HPA axis dysregulation, suggesting that disruption of the HPA axis, independent of arcuate nucleus GR, is primarily responsible for the growth impairments. However, more specific targeting of GR in the arcuate nucleus would provide more definitive conclusions on its role in feeding behavior.
In contrast to normalized HPA axis in adult GRPOMCCre mice, the HPA axis is still hyperactive in GRNesCre and Sim1Cre-GRe3Δ mice in adulthood and leads to a differential impact on growth (Section 4.3). These data suggest that GR in the brain and pituitary are required to maintain normal growth processes in mice. Loss of GR early in development leading to GC excess reduces growth, while in adulthood GC influence on growth is region dependent. Pituitary GR, however, does not have a major influence in mediating growth pathways in adulthood. Taken together, these data suggest differential involvement of GR in regulating growth during early development versus during adulthood, as well as differential involvement of GR in specific brain regions in terms of the level of involvement in growth regulation.
6. GR in Sympathetic NS- dorsal raphe, DA neurons
GCs and GR regulate the HPA axis, and the HPA axis is responsive to stress as well as to circadian signals. Various abnormalities of the HPA axis (e.g. loss of negative feedback) are seen as biomarkers of psychiatric diseases. Recent analyses of the interactions of GCs with monoamine neurotransmitter signaling during stress have indicated that the interaction of GCs with GR in serotonergic and dopaminergic pathways is critical to normal as well as abnormal responses to stressors.
6.1. GR Disruption in Serotoninergic Pathways
Serotonergic pathways from the dorsal raphé nuclei (DRN) innervate numerous forebrain regions involved in mood and stress processing (Waselus et al., 2011). Serotonin (5-HT) abnormalities have been implicated in the symptoms of various mood disorders, and serotonin-reuptake inhibitors are frequently used for the treatment of depression and anxiety. As discussed previously, GR in forebrain neurons plays an important role in stress-induced anxiety and despair. But recent findings show that GR in DR serotonergic neurons also is critical for modulating the effects of stress. Vincent and Jacobson have shown that deletion of GR in DR neurons (DRNGRKO) does not affect basal HPA axis activity, but decreases anxiety and behavior and caused increased stress coping behavior (Vincent and Jacobson, 2014). Upon application of an acute stressor, plasma CORT is increased in DRNGRKO mice, demonstrating stress reactivity. Thus the DRN appears to be another site of HPA negative feedback, which is impaired in the GRKOs. DRN GR deletion in chronically stressed mice (from repeated aggressions, a paradigm which results in social avoidance) is anxiolytic only in certain behavioral tests. Thus chronic stress can alter the effects of GR deletion in some of the DRN acute stress behavior pathways.
6.2. GR Disruption in Dopaminergic Pathways
Dopamine (DA) is a catecholamine neurotransmitter functioning in 4 major neuronal pathways (Zhang et al., 2007): mesocortical (ventrotegmental area (VTA) projecting to the frontal cortex (Fc)), mesolimbic (VTA projecting to the nucleus accumbens (NAc) and hippocampus), nigrostriatal (substantia nigra projecting to the striatum) and tuberoinfundibular (arcuate nucleus projecting to the median eminence). Neural processing of emotional, motor, motivational, and stress stimuli, and the behavioral sequelae, involves DA signaling. Certain neuropathologies (e.g. Parkinson’s disease, drug addiction and schizophrenia) have been linked to alterations in DA signaling, or loss of DA-containing neurons. GR is present on dopaminergic neurons in the VTA as well as on dopaminoceptive neurons in the NAc and frontal cortex. Site-specific effects of GC/GR on DA neurons have recently been shown to be critical in behavioral responses to addictive drugs as well as to stress. Specifically, GR in dopaminoceptive neurons in the NAc and striatum, but not dopaminergic VTA neurons, increases cell firing and motivation of mice to self-administer cocaine (Ambroggi et al., 2009).
Adult mice subjected to repeated aggression (defeat) develop persistent social aversion. GR present on postsynaptic (NAc) DA neurons is necessary for the behavioral change – if this GR site is inactivated, social interactions of the defeated mice remain the same as undefeated controls (no social aversion) (Barik et al., 2013). In contrast, mice with inactivated GR on pre-synaptic (VTA) neurons develop social aversion normally. It is thought that the aversion behavior develops due to a positive feedback loop linking NAc GC/GR to DA-release from the VTA and this is interrupted when GR is inactivated in NAc dopaminoceptive neurons. Treatment with RU486, a GR antagonist, before the daily aggressions, also blocks the development of social aversion (Barik et al., 2013).
In a different stress paradigm, adolescent mice experiencing 3 weeks of social isolation develop behavioral changes such as an altered startle response, increased immobility in the forced swim test, and abnormal locomotor activity. Neurochemical alterations (increased plasma corticosterone, decreased DA & tyrosine hydroxylase (TH, the rate-limiting enzyme necessary in DA synthesis)), and an increase in D2 DA receptors) are seen in the frontal cortex. In the presence of a genetic risk factor, a DISC1 (disrupted in schizophrenia) transgene these abnormalities were demonstrated to require GR in the mesocortical projections of DA neurons (VA to frontal cortex) (Niwa et al., 2013). These mice showed a significant increase in methylation of the TH gene (which contains a GC response element), only in cells projecting from the VTA to the Fc, which is dependent on the excess GCs found in the GxE mice: GR antagonist treatment normalized TH methylation as well as the neurochemical and behavioral abnormalities (Niwa et al., 2013).
The complex interactions of GR with neurotransmitter pathways, chaperone proteins, and other genes such as DISC1 appear critical to the development of stress-related psychiatric diseases. These results give hope that careful dissection of the multiple layers of regulation of GR present in neurotransmitter pathways will lead to new pharmacologic interventions for prevention and better treatment of psychiatric disorders.
7. Clinical Effects of Impaired GC Signaling
The human GR gene, NR3C1, is located on the short arm of chromosome 5, 5q31–32 and undergoes alternative RNA splicing and translation initiation to yield multiple functionally distinct isoforms. The two most abundant isoforms generated are GRα and GRβ, with GRα being expressed at higher levels in most tissues (Oakley and Cidlowski, 2013). Alternative translation start sites of the human GR mRNA produce translational isoforms of GRα, GR-A, -B, -C1, -C2, -C3, -D1, -D2, and -D3, also each have their own unique distribution patterns and gene targets (Oakley and Cidlowski, 2013; Oakley et al., 1997). This selectivity may underlie the varied GC responses amongst tissues and patients. The multiple GR isoforms, alternative processing, tissue and cell-specific expression patterns and post-translational modifications demonstrate how the GR gene can be modified to produce a maximum range of functions. In the rodent studies described in prior sections, GR disruption along negative feedback sites reduces GR expression in specific brain regions and is associated with abnormal HPA axis regulation that affects anxiety, despair, and metabolic phenotypes. In this section, we briefly explore studies demonstrating that GR disruption and its effects are translatable to human psychiatric and metabolic diseases.
7.1. Psychiatric Disorders
As the end products of the HPA axis, GCs bind to GRs at the level of the hypothalamus and anterior pituitary to exert negative feedback effects and maintain homeostasis in response to stress. Increased cortisol levels and an inability to suppress cortisol secretion in the DST are known hallmarks of a number of psychiatric disorders including schizophrenia, bipolar disorder, generalized anxiety, and major depressive disorders (Beck-Friis et al., 1985; Carroll et al., 1980; Gibbons, 1964; Lesch et al., 1988; López et al., 1998; Owens and Nemeroff, 1993; Rubin et al., 1996). Individuals with major psychiatric illness often display hypercortisolemia (Linkowski et al., 1994; Mück-Seler et al., 1999) and a down-regulation in GR mRNA in a network of regions including the hippocampus, amygdala, and temporal cortex (Perlman et al., 2004; Sinclair et al., 2011; Webster et al., 2002).
Major depressive disorder (MDD) is characterized by increased release of GCs and hyperactivity of the HPA axis (Holsboer, 2000; Pariante and Lightman, 2008). A primary feature of this hyperactivity is non-suppression of cortisol secretion in response to administration of the synthetic glucocorticoid, dexamethasone. Patients with MDD display a reduction in the number and function of GR (Gormley et al., 1985; Lowy et al., 1988; Sallee et al., 1995; Webster et al., 2002; Whalley et al., 1986; Yehuda et al., 1993). Many studies have addressed the role of GR abnormalities in the pathophysiology of MDD by examining the effects of antidepressants on GR expression and function and HPA axis activity (Pariante and Miller, 2001). Indeed, impaired GR function has been suggested to be causal for HPA axis hyperactivity in depression, as GCs typically regulate the HPA axis through negative feedback inhibition and thereby reduce the production of GCs themselves. This effect is thought to be mediated in part by GR. Therefore, hyperactivity of the HPA axis has been explained by an impaired feedback inhibition of GCs, possibly due to an impaired or dysfunctional GR (“glucocorticoid resistance”) (Klengel et al., 2013). In support of this, data in numerous studies of antidepressant treatment on rodents have demonstrated increased GR expression in the hypothalamic and hippocampal brain regions, which may indicate a mode for antidepressant mediated HPA axis normalization (Heydendael and Jacobson, 2008; Reul et al., 1994, 1993; Seckl and Fink, 1992).
The non-coding exon 1 of GR contains a 5’ untranslated region (UTR) that determines tissue specific production of GR. Epigenetic modifications such reduced DNA methylation of the GR promoter in this 5’ UTR have been observed in rodent models of low maternal care and are associated with increased risk for addiction, depression, diabetes and obesity (Weaver et al., 2004; Zhang et al., 2013). The mechanism of action due to increased methylation of NGF1-A disrupts its binding to hGR17 promoter and thus causes a reduction of GR transcription (McGowan et al., 2009; Weaver and D’Alessio, 2007). Moreover, expression of other variants, decreased hGR1B and hGR1C but increased hGR1H, due to increased methylation have also been identified (Labonte et al., 2012; Zhang et al., 2013). Specifically, these GR variants are reduced and correlate with decreased total GR in the hippocampus of suicide victims only when they have a history of childhood abuse. The idea that GC excess early in life can reprogram HPA axis reactivity in part through decreasing GR expression in specific brain regions that are responsible for mediating negative feedback. In support of this concept, fetal GC excess in humans has been found to increase the risk for schizophrenia in adulthood (Koenig et al., 2002). Moreover increased fetal GCs due to maternal obesity or malnutrition also increases the risk of schizophrenia when the child grows into an adult (Khandaker et al., 2012; Lesage et al., 2001; Reynolds et al., 2013; Sasaki et al., 2013). Post mortem analysis shows GR mRNA is decreased in the brain of patients with schizophrenia and depression (Knable et al., 2001). These data represent a convergence between psychiatric and metabolic diseases both associated with increased GC levels, potentially the result of disrupted GR expression hindering negative GC feedback.
A number of single nucleotide polymorphisms (SNPs) have been identified in GR, namely Bcl1, N363S, and ER22/23EK, that cause changes in GC sensitivity (Review (DeRijk, R., de Kloet, 2005; Zobel et al., 2008)). Bcl1 and N363S are associated with increased GC sensitivity, while ER22/23EK associates with reduced GC sensitivity. Moreover some of these SNPs are associated with HPA axis disruption, including altered cortisol and ACTH secretion after stress, and may modify the risk of depression (Zobel et al., 2008). SNPs in genes that mediate GR function have also been observed to associate with psychiatric disorders. For instance, FKBP5, is a GR chaperone protein that prevents nuclear translocation and reduced GR affinity to GC (Binder, 2009). An increase in FKBP5 due to a SNP results in impaired GR-mediated negative feedback and GC resistance and is found to be associated with PTSD and MDD (Binder et al., 2008, 2004; Tatro et al., 2009). In patients with MDD, decreased hippocampal volume and increased inflammatory cytokines are associated with decreased expression of genes targeted by GR activation (Frodl et al., 2012). These data point to a prime role of regulation of GR function in mediating neuroendocrine and histopathology that affect the development of psychiatric disorders.
7.2. Metabolic Disorders
GC activation of GR regulates a variety of important cardiovascular, metabolic and immunologic functions. Synthetic GCs, including prednisone/prednisolone and dexamethasone, are widely prescribed anti-inflammatory drugs used to treat a number of immune and inflammatory diseases including allergies, asthma, lupus and rheumatoid arthritis. Although therapeutic benefits of GCs are vast, long-term use of GCs results in negative side effects including muscle wasting, abdominal fat deposition, and insulin resistance. In addition, some patients do not respond to GC therapy and those that do may develop GC resistance. Moreover, discontinuation of long-term GC therapy has been shown to result in increased risk of delirium and depression in 10% of users (Fardet et al., 2013). Tissue-specific GC resistance has been associated with rheumatoid arthritis, osteoarthritis, Crohn's disease, ulcerative colitis and asthma (Kino and Chrousos, 2001). Impaired negative feedback of GC along the HPA axis that leads to excess circulating GCs may contribute to the development of metabolic disorders. Excess GCs have been observed in metabolic syndrome cluster diseases including Cushing’s syndrome, obesity, type II diabetes, and insulin resistance.
Bioactive concentrations of plasma CORT are regulated, not only by negative feedback, but also by the expression of the corticosteroid binding globulin (CBG) and 11β-hydroxysteroid dehydrogenase type 1 and type 2 (11β-HSD 1/2) (Wang, 2005). These proteins have tissue-specific expression that govern tissue-specific activity of GCs. CBG binding to plasma CORT and sequester its diffusion into cells to facilitate transport through circulation, thus governing the bioavailability of CORT. Low levels of CBG have been identified in stress states as well as adipose tissue of obese rats (Bernier et al., 1998; Grasa et al., 1998; Tinnikov et al., 1996). 11β-HSD1 is the enzyme that converts the precursor 11β-deoxycortisol or 11β-deoxycorticosterone to cortisol and corticosterone, respectively. 11β-HSD2 on the other hand, mediates the reverse reaction of CORT to inactive metabolites (Wang, 2005). Disruption of the availability of these regulatory proteins have been linked to obesity and insulin resistance (Atalar et al., 2012; Barat et al., 2005; Fernandez-Real et al., 2005; Kannisto et al., 2004; Rask et al., 2002; Stimson et al., 2009). To understand how disruption of GR along the negative feedback sites contribute to metabolic disorders, future studies in the previously described genetic models will be needed to evaluate CBG, 11β-HSD1/2, and proteins involved in metabolic activity in more detail.
8. Conclusion
Numerous studies in animal models and humans clarify the importance of glucocorticoids and the HPA axis in mediating stress reactivity in terms of endocrine output, behavioral activity, and energy balance. In this review, we primarily focus on studies in sites of negative feedback in the brain and pituitary of genetically targeted mice. In all of the genetic models addressed, diurnal rhythmicity of CORT secretion is maintained indicating that GR in negative feedback sites are not important for circadian regulation of GC synthesis and secretion. During periods of GC excess, increased PVN CRH and/or AVP lead to increased plasma ACTH and CORT. In terms of physical development, it seems that excess GCs during early life cause growth deficits that normalize if GC levels normalize. Early postnatal GC excess impairs growth and may reprogram HPA axis reactivity to stress later in life. Moreover, the effects of this GC excess are dependent on the site where GR is lost. Brief GC elevations during postnatal development, due to pituitary GR loss, slows dexamethasone suppression while increasing anxiety and stress-coping behavior. GC excess due to PVN GR loss throughout development impairs negative suppression of CORT and causes a Cushing’s phenotype but does not affect basal anxiety or despair behavior. GR loss in both PVN and pituitary as well as in the brain is incompatible with sustained life, primarily due to the necessity for pituitary GR function early in life. These data imply that in various human psychiatric and metabolic disorders, evaluating the root of HPA axis dysfunction may aide in identifying more effective treatment strategies.
There are a number of questions that are key to understanding the diversity of GR function. (1) The role of different GR isoforms in regulating various aspects of HPA axis function. (2) The differences in GC signaling mediated by membrane bound versus intracellular GR activity. (3) The consequences of gender-specific GC signaling on HPA axis function. Gender specific analysis and targeting of distinct isoforms would be critical in revealing the intricacies of GR function and further elucidate mechanisms for individual differences in stress responsivity.
Highlights.
-
➢
Feedback via GR maintains cellular homeostasis and promotes behavioral adaptation
-
➢
GR loss in both brain and pituitary results in neonatal death
-
➢
Pituitary GR is important in early postnatal development of HPA axis function
-
➢
Hypothalamic GR is more essential for maintaining HPA axis activity in adulthood
-
➢
Impaired GR-mediated feedback is observed in psychiatric and metabolic disorders
Acknowledgements
Investigations described from our laboratory were supported by NIH grants T32 MH064913 (Laryea) and R01AMH079010 (Muglia). We would also like to thank the reviewers for their suggestions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Gloria Laryea, Email: Gloria.n.laryea@vanderbilt.edu.
Lisa Muglia, Email: lisa.muglia@cchmc.org.
Melinda Arnett, Email: melinda.arnett@cchmc.org.
Louis J. Muglia, Email: Louis.muglia@cchmc.org.
References
- Ambroggi F, Turiault M, Milet A, Deroche-Gamonet V, Parnaudeau S, Balado E, Barik J, van der Veen R, Maroteaux G, Lemberger T, Schütz G, Lazar M, Marinelli M, Piazza PV, Tronche F. Stress and addiction: glucocorticoid receptor in dopaminoceptive neurons facilitates cocaine seeking. Nat. Neurosci. 2009;12:247–249. doi: 10.1038/nn.2282. [DOI] [PubMed] [Google Scholar]
- Arnett MG, Kolber BJ, Boyle MP, Muglia LJ. Behavioral insights from mouse models of forebrain--and amygdala-specific glucocorticoid receptor genetic disruption. Mol. Cell. Endocrinol. 2011;336:2–5. doi: 10.1016/j.mce.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atalar F, Gormez S, Caynak B, Akan G, Tanriverdi G, Bilgic-Gazioglu S, Gunay D, Duran C, Akpinar B, Ozbek U, Buyukdevrim AS, Yazici Z. The role of mediastinal adipose tissue 11β-hydroxysteroid d ehydrogenase type 1 and glucocorticoid expression in the development of coronary atherosclerosis in obese patients with ischemic heart disease. Cardiovasc. Diabetol. 2012;11:115. doi: 10.1186/1475-2840-11-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schütz G, Schibler U. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289:2344–2347. doi: 10.1126/science.289.5488.2344. [DOI] [PubMed] [Google Scholar]
- Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, Christiansen LM, Edelstein E, Choi B, Boss O, Aschkenasi C, Zhang C, Mountjoy K, Kishi T, Elmquist JK, Lowell BB. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Barat P, Duclos M, Gatta B, Roger P, Mormede P, Moisan MP. Corticosteroid binding globulin gene polymorphism influences cortisol driven fat distribution in obese women. Obes. Res. 2005;13:1485–1490. doi: 10.1038/oby.2005.179. [DOI] [PubMed] [Google Scholar]
- Barik J, Marti F, Morel C, Fernandez SP, Lanteri C, Godeheu G, Tassin J-P, Mombereau C, Faure P, Tronche F. Chronic stress triggers social aversion via glucocorticoid receptor in dopaminoceptive neurons. Science. 2013;339:332–335. doi: 10.1126/science.1226767. [DOI] [PubMed] [Google Scholar]
- Bartholome B, Spies CM, Gaber T, Schuchmann S, Berki T, Kunkel D, Bienert M, Radbruch A, Burmester G-R, Lauster R, Scheffold A, Buttgereit F. Membrane glucocorticoid receptors (mGCR) are expressed in normal human peripheral blood mononuclear cells and up-regulated after in vitro stimulation and in patients with rheumatoid arthritis. FASEB. J. 2004;18:70–80. doi: 10.1096/fj.03-0328com. [DOI] [PubMed] [Google Scholar]
- Beck-Friis J, Ljunggren JG, Thorén M, von Rosen D, Kjellman BF, Wetterberg L. Melatonin, cortisol and ACTH in patients with major depressive disorder and healthy humans with special reference to the outcome of the dexamethasone suppression test. Psychoneuroendocrinology. 1985;10:173–186. doi: 10.1016/0306-4530(85)90055-1. [DOI] [PubMed] [Google Scholar]
- Bentley L, Esapa CT, Nesbit MA, Head RA, Evans H, Lath D, Scudamore CL, Hough TA, Podrini C, Hannan FM, Fraser WD, Croucher PI, Brown MA, Brown SDM, Cox RD, Thakker RV. An N-ethyl-N-nitrosourea induced corticotropin-releasing hormone promoter mutation provides a mouse model for endogenous glucocorticoid excess. Endocrinology. 2014;155:908–922. doi: 10.1210/en.2013-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier J, Jobin N, Emptoz-Bonneton A, Pugeat MM, Garrel DR. Decreased corticosteroid-binding globulin in burn patients: relationship with interleukin-6 and fat in nutritional support. Crit. Care Med. 1998;26:452–460. doi: 10.1097/00003246-199803000-00014. [DOI] [PubMed] [Google Scholar]
- Biddie SC, Conway-Campbell BL, Lightman SL. Dynamic regulation of glucocorticoid signalling in health and disease. Rheumatology (Oxford) 2012;51:403–412. doi: 10.1093/rheumatology/ker215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder EB. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology. 2009;34(Suppl 1):S186–S195. doi: 10.1016/j.psyneuen.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, Tang Y, Gillespie CF, Heim CM, Nemeroff CB, Schwartz AC, Cubells JF, Ressler KJ. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder EB, Salyakina D, Lichtner P, Wochnik GM, Ising M, Pütz B, Papiol S, Seaman S, Lucae S, Kohli MA, Nickel T, Künzel HE, Fuchs B, Majer M, Pfennig A, Kern N, Brunner J, Modell S, Baghai T, Deiml T, Zill P, Bondy B, Rupprecht R, Messer T, Köhnlein O, Dabitz H, Brückl T, Müller N, Pfister H, Lieb R, Mueller JC, Lõhmussaar E, Strom TM, Bettecken T, Meitinger T, Uhr M, Rein T, Holsboer F, Muller-Myhsok B. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat. Genet. 2004;36:1319–1325. doi: 10.1038/ng1479. [DOI] [PubMed] [Google Scholar]
- Bittman EL, Doherty L, Huang L, Paroskie A. Period gene expression in mouse endocrine tissues. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;285:R561–R569. doi: 10.1152/ajpregu.00783.2002. [DOI] [PubMed] [Google Scholar]
- Boyle MP, Brewer Ja, Funatsu M, Wozniak DF, Tsien JZ, Izumi Y, Muglia LJ. Acquired deficit of forebrain glucocorticoid receptor produces depression-like changes in adrenal axis regulation and behavior. Proc. Natl. Acad. Sci. U. S. A. 2005;102:473–478. doi: 10.1073/pnas.0406458102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle MP, Kolber BJ, Vogt SK, Wozniak DF, Muglia LJ. Forebrain glucocorticoid receptors modulate anxiety-associated locomotor activation and adrenal responsiveness. J. Neurosci. 2006;26:1971–1978. doi: 10.1523/JNEUROSCI.2173-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buijs RM, Wortel J, Van Heerikhuize JJ, Feenstra MG, Ter Horst GJ, Romijn HJ, Kalsbeek A. Anatomical and functional demonstration of a multisynaptic suprachiasmatic nucleus adrenal (cortex) pathway. Eur. J. Neurosci. 1999;11:1535–1544. doi: 10.1046/j.1460-9568.1999.00575.x. [DOI] [PubMed] [Google Scholar]
- Buwalda B, Stubbendorff C, Zickert N, Koolhaas JM. Adolescent social stress does not necessarily lead to a compromised adaptive capacity during adulthood: a study on the consequences of social stress in rats. Neuroscience. 2013;249:258–270. doi: 10.1016/j.neuroscience.2012.12.050. [DOI] [PubMed] [Google Scholar]
- Carroll BJ, Feinberg M, Greden JF, Haskett RF, James NM, Steiner M, Tarika J. Diagnosis of endogenous depression. Comparison of clinical, research and neuroendocrine criteria. J. Affect. Disord. 1980;2:177–194. doi: 10.1016/0165-0327(80)90004-x. [DOI] [PubMed] [Google Scholar]
- Cascio CS, Shinsako J, Dallman MF. The suprachiasmatic nuclei stimulate evening ACTH secretion in the rat. Brain Res. 1987;423:173–178. doi: 10.1016/0006-8993(87)90837-7. [DOI] [PubMed] [Google Scholar]
- Cato ACB, Nestl A, Mink S. Rapid actions of steroid receptors in cellular signaling pathways. Sci. STKE. 2002 doi: 10.1126/stke.2002.138.re9. 2002, re9. [DOI] [PubMed] [Google Scholar]
- Champagne DL, Bagot RC, van Hasselt F, Ramakers G, Meaney MJ, de Kloet ER, Joëls M, Krugers H. Maternal care and hippocampal plasticity: evidence for experience-dependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress. J. Neurosci. 2008;28:6037–6045. doi: 10.1523/JNEUROSCI.0526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne DL, de Kloet ER, Joëls M. Fundamental aspects of the impact of glucocorticoids on the (immature) brain. Semin. Fetal Neonatal Med. 2009;14:136–142. doi: 10.1016/j.siny.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Chimin P, Farias T, da SM, Torres-Leal FL, Bolsoni-Lopes A, Campaña AB, Andreotti S, Lima FB. Chronic glucocorticoid treatment enhances lipogenic activity in visceral adipocytes of male Wistar rats. Acta Physiol. (Oxf) 2014 doi: 10.1111/apha.12226. [DOI] [PubMed] [Google Scholar]
- Clow A, Hucklebridge F, Stalder T, Evans P, Thorn L. The cortisol awakening response: more than a measure of HPA axis function. Neurosci. Biobehav. Rev. 2010;35:97–103. doi: 10.1016/j.neubiorev.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Cone RD, Cowley MA, Butler AA, Fan W, Marks DL, Low MJ. The arcuate nucleus as a conduit for diverse signals relevant to energy homeostasis. Int. J. Obes. Relat. Metab. Disord. 2001;25(Suppl 5):S63–S67. doi: 10.1038/sj.ijo.0801913. [DOI] [PubMed] [Google Scholar]
- Crawford PA, Sadovsky Y, Woodson K, Lee SL, Milbrandt J. Adrenocortical function and regulation of the steroid 21-hydroxylase gene in NGFI-B-deficient mice. Mol. Cell. Biol. 1995;15:4331–4316. doi: 10.1128/mcb.15.8.4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman MF. Fast glucocorticoid actions on brain: back to the future. Front. Neuroendocrinol. 2005;26:103–108. doi: 10.1016/j.yfrne.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Daskalakis NP, Bagot RC, Parker KJ, Vinkers CH, de Kloet ER. The three-hit concept of vulnerability and resilience: toward understanding adaptation to early-life adversity outcome. Psychoneuroendocrinology. 2013;38:1858–1873. doi: 10.1016/j.psyneuen.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalakis NP, Oitzl MS, Schächinger H, Champagne DL, de Kloet ER. Testing the cumulative stress and mismatch hypotheses of psychopathology in a rat model of early-life adversity. Physiol. Behav. 2012;106:707–721. doi: 10.1016/j.physbeh.2012.01.015. [DOI] [PubMed] [Google Scholar]
- Dayanithi G, Antoni FA. Rapid as well as delayed inhibitory effects of glucocorticoid hormones on pituitary adrenocorticotropic hormone release are mediated by type II glucocorticoid receptors and require newly synthesized messenger ribonucleic acid as well as protein. Endocrinology. 1989;125:308–313. doi: 10.1210/endo-125-1-308. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nat. Rev. Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, Karst H, Joëls M. Corticosteroid hormones in the central stress response: quick-and-slow. Front. Neuroendocrinol. 2008;29:268–272. doi: 10.1016/j.yfrne.2007.10.002. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, Reul JM. Feedback action and tonic influence of corticosteroids on brain function: a concept arising from the heterogeneity of brain receptor systems. Psychoneuroendocrinology. 1987;12:83–105. doi: 10.1016/0306-4530(87)90040-0. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, Vreugdenhil E, Oitzl MS, Joëls M. Brain corticosteroid receptor balance in health and disease. Endocr. Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- DeFremery P, Laqueur E, Reichstein T. Corticosteron, a crystallized compound with the biological activity of the adrenalcortical hormone. Nature. 1937;139:26. [Google Scholar]
- DeRijk R, de Kloet ER. Corticosteroid receptor genetic polymorphisms and stress responsivity. Endocrine. 2005;28:263–270. doi: 10.1385/ENDO:28:3:263. [DOI] [PubMed] [Google Scholar]
- Di S, Malcher-Lopes R, Halmos KC, Tasker JG. Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: a fast feedback mechanism. J. Neurosci. 2003;23:4850–4857. doi: 10.1523/JNEUROSCI.23-12-04850.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J. Neurosci. 1993;13:3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake AJ, Raubenheimer PJ, Kerrigan D, McInnes KJ, Seckl JR, Walker BR. Prenatal dexamethasone programs expression of genes in liver and adipose tissue and increased hepatic lipid accumulation but not obesity on a high-fat diet. Endocrinology. 2010;151:1581–1587. doi: 10.1210/en.2009-1088. [DOI] [PubMed] [Google Scholar]
- Drouin J, Sun YL, Chamberland M, Gauthier Y, De Léan a, Nemer M, Schmidt TJ. Novel glucocorticoid receptor complex with DNA element of the hormone-repressed POMC gene. EMBO. J. 1993;12:145–156. doi: 10.1002/j.1460-2075.1993.tb05640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duma D, Collins JB, Chou JW, Cidlowski JA. Sexually dimorphic actions of glucocorticoids provide a link to inflammatory diseases with gender differences in prevalence. Sci. Signal. 2010;3:ra74. doi: 10.1126/scisignal.2001077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott E, Ezra-Nevo G, Regev L, Neufeld-Cohen A, Chen A. Resilience to social stress coincides with functional DNA methylation of the Crf gene in adult mice. Nat. Neurosci. 2010;13:1351–1353. doi: 10.1038/nn.2642. [DOI] [PubMed] [Google Scholar]
- Erdmann G, Schütz G, Berger S. Loss of glucocorticoid receptor function in the pituitary results in early postnatal lethality. Endocrinology. 2008;149:3446–3451. doi: 10.1210/en.2007-1786. [DOI] [PubMed] [Google Scholar]
- Evans AN, Liu Y, Macgregor R, Huang V, Aguilera G. Regulation of hypothalamic corticotropin-releasing hormone transcription by elevated glucocorticoids. Mol. Endocrinol. 2013;27:1796–1807. doi: 10.1210/me.2013-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evanson NK, Tasker JG, Hill MN, Hillard CJ, Herman JP. Fast feedback inhibition of the HPA axis by glucocorticoids is mediated by endocannabinoid signaling. Endocrinology. 2010;151:4811–4819. doi: 10.1210/en.2010-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fardet L, Nazareth I, Whitaker HJ, Petersen I. Severe neuropsychiatric outcomes following discontinuation of long-term glucocorticoid therapy: a cohort study. J. Clin. Psychiatry. 2013;74:e281–e286. doi: 10.4088/JCP.12m08034. [DOI] [PubMed] [Google Scholar]
- Feldman S, Conforti N. Modifications of adrenocortical responses following frontal cortex simulation in rats with hypothalamic deafferentations and medial forebrain bundle lesions. Neuroscience. 1985;15:1045–1047. doi: 10.1016/0306-4522(85)90253-2. [DOI] [PubMed] [Google Scholar]
- Fernandez PM, Brunel F, Jimenez MA, Saez JM, Cereghini S, Zakin MM. Nuclear receptors Nor1 and NGFI-B/Nur77 play similar, albeit distinct, roles in the hypothalamo-pituitary-adrenal axis. Endocrinology. 2000;141:2392–2400. doi: 10.1210/endo.141.7.7562. [DOI] [PubMed] [Google Scholar]
- Fernandez-Real J-M, Pugeat M, López-Bermejo A, Bornet H, Ricart W. Corticosteroid-binding globulin affects the relationship between circulating adiponectin and cortisol in men and women. Metabolism. 2005;54:584–589. doi: 10.1016/j.metabol.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Finotto S, Krieglstein K, Schober A, Deimling F, Lindner K, Brühl B, Beier K, Metz J, Garcia-Arraras JE, Roig-Lopez JL, Monaghan P, Schmid W, Cole TJ, Kellendonk C, Tronche F, Schütz G, Unsicker K. Analysis of mice carrying targeted mutations of the glucocorticoid receptor gene argues against an essential role of glucocorticoid signalling for generating adrenal chromaffin cells. Development. 1999;126:2935–2944. doi: 10.1242/dev.126.13.2935. [DOI] [PubMed] [Google Scholar]
- Francis DD, Champagne FA, Liu D, Meaney MJ. Maternal care, gene expression, and the development of individual differences in stress reactivity. Ann. N. Y. Acad. Sci. 1999;896:66–84. doi: 10.1111/j.1749-6632.1999.tb08106.x. [DOI] [PubMed] [Google Scholar]
- Franco-Colín M, Villanueva I, Piñón M, Racotta R. The effects of sympathectomy and dexamethasone in rats ingesting sucrose. Int. J. Biol. Sci. 2006;2:17–22. doi: 10.7150/ijbs.2.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T, Carballedo a, Hughes MM, Saleh K, Fagan a, Skokauskas N, McLoughlin DM, Meaney J, O’Keane V, Connor TJ. Reduced expression of glucocorticoidinducible genes GILZ and SGK-1: high IL-6 levels are associated with reduced hippocampal volumes in major depressive disorder. Transl. Psychiatry. 2012;2:e88. doi: 10.1038/tp.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furay AR, Bruestle AE, Herman JP. The role of the forebrain glucocorticoid receptor in acute and chronic stress. Endocrinology. 2008;149:5482–5490. doi: 10.1210/en.2008-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gametchu B, Watson CS, Pasko D. Size and steroid-binding characterization of membrane-associated glucocorticoid receptor in S-49 lymphoma cells. Steroids. 1991;56:402–410. doi: 10.1016/0039-128x(91)90028-t. [DOI] [PubMed] [Google Scholar]
- Gibbons JL. Cortisol secretion rate in depressive illness. Arch. Gen. Psychiatry. 1964;10:572–575. doi: 10.1001/archpsyc.1964.01720240026004. [DOI] [PubMed] [Google Scholar]
- Goldstein MF. Chronic Glucocorticoid Therapy-Induced Osteoporosis in Patients With Obstructive Lung Disease*. Chest. 1999;116:1733–1749. doi: 10.1378/chest.116.6.1733. [DOI] [PubMed] [Google Scholar]
- Gormley GJ, Lowy MT, Reder AT, Hospelhorn VD, Antel JP, Meltzer HY. Glucocorticoid receptors in depression: relationship to the dexamethasone suppression test. Am. J. Psychiatry. 1985;142:1278–1284. doi: 10.1176/ajp.142.11.1278. [DOI] [PubMed] [Google Scholar]
- Goto M, Piper Hanley K, Marcos J, Wood PJ, Wright S, Postle AD, Cameron IT, Mason JI, Wilson DI, Hanley NA. In humans, early cortisol biosynthesis provides a mechanism to safeguard female sexual development. J. Clin. Invest. 2006;116:953–960. doi: 10.1172/JCI25091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasa MM, Cabot C, Balada F, Virgili J, Sanchis D, Monserrat C, Fernández-López JA, Remesar X, Alemany M. Corticosterone binding to tissues of adrenalectomized lean and obese Zucker rats. Horm. Metab. Res. 1998;30:699–704. doi: 10.1055/s-2007-978962. [DOI] [PubMed] [Google Scholar]
- Groeneweg FL, Karst H, de Kloet ER, Joëls M. Rapid non-genomic effects of corticosteroids and their role in the central stress response. J. Endocrinol. 2011a;209:153–167. doi: 10.1530/JOE-10-0472. [DOI] [PubMed] [Google Scholar]
- Groeneweg FL, Karst H, de Kloet ER, Joëls M. Mineralocorticoid and glucocorticoid receptors at the neuronal membrane, regulators of nongenomic corticosteroid signalling. Mol. Cell. Endocrinol. 2011b doi: 10.1016/j.mce.2011.06.020. [DOI] [PubMed] [Google Scholar]
- Hartman FA, Brownell KA. The hormone of the adrenal cortex. Science. 1930;72:76. doi: 10.1126/science.72.1855.76. [DOI] [PubMed] [Google Scholar]
- Heck S, Kullmann M, Gast A, Ponta H, Rahmsdorf HJ, Herrlich P, Cato AC. A distinct modulating domain in glucocorticoid receptor monomers in the repression of activity of the transcription factor AP-1. EMBO. J. 1994;13:4087–4095. doi: 10.1002/j.1460-2075.1994.tb06726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hench PS, Kendall EC. The effect of a hormone of the adrenal cortex (17-hydroxy-11- dehydrocorticosterone; compound E) and of pituitary adrenocorticotropic hormone on rheumatoid arthritis. Proc. Staff Meet. Mayo Clin. 1949;24:181–197. [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamopituitary- adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE, Young Ea, Akil H, Watson SJ. Selective forebrain fiber tract lesions implicate ventral hippocampal structures in tonic regulation of paraventricular nucleus corticotropin-releasing hormone (CRH) and arginine vasopressin (AVP) mRNA expression. Brain Res. 1992;592:228–238. doi: 10.1016/0006-8993(92)91680-d. [DOI] [PubMed] [Google Scholar]
- Herman JP, Dolgas CM, Carlson SL. Ventral subiculum regulates hypothalamopituitary- adrenocortical and behavioural responses to cognitive stressors. Neuroscience. 1998;86:449–459. doi: 10.1016/s0306-4522(98)00055-4. [DOI] [PubMed] [Google Scholar]
- Hermes ML, Coderre EM, Buijs RM, Renaud LP. GABA and glutamate mediate rapid neurotransmission from suprachiasmatic nucleus to hypothalamic paraventricular nucleus in rat. J. Physiol. 1996;496(Pt 3):749–757. doi: 10.1113/jphysiol.1996.sp021724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydendael W, Jacobson L. Differential effects of imipramine and phenelzine on corticosteroid receptor gene expression in mouse brain: potential relevance to antidepressant response. Brain Res. 2008;1238:93–107. doi: 10.1016/j.brainres.2008.08.018. [DOI] [PubMed] [Google Scholar]
- Hinz B, Hirschelmann R. Rapid non-genomic feedback effects of glucocorticoids on CRF-induced ACTH secretion in rats. Pharm. Res. 2000;17:1273–1277. doi: 10.1023/a:1026499604848. [DOI] [PubMed] [Google Scholar]
- Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23:477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- Ishida A, Mutoh T, Ueyama T, Bando H, Masubuchi S, Nakahara D, Tsujimoto G, Okamura H. Light activates the adrenal gland: timing of gene expression and glucocorticoid release. Cell Metab. 2005;2:297–307. doi: 10.1016/j.cmet.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr. Rev. 1991;12:118–134. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- Jeanneteau FD, Lambert WM, Ismaili N, Bath KG, Lee FS, Garabedian MJ, Chao MV. BDNF and glucocorticoids regulate corticotrophin-releasing hormone (CRH) homeostasis in the hypothalamus. Proc. Natl. Acad. Sci. U. S. A. 2012;109:1305–1310. doi: 10.1073/pnas.1114122109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joëls M, Sarabdjitsingh RA, Karst H. Unraveling the time domains of corticosteroid hormone influences on brain activity: rapid, slow, and chronic modes. Pharmacol. Rev. 2012;64:901–938. doi: 10.1124/pr.112.005892. [DOI] [PubMed] [Google Scholar]
- John CD, Christian HC, Morris JF, Flower RJ, Solito E, Buckingham JC. Annexin 1 and the regulation of endocrine function. Trends Endocrinol. Metab. 2004;15:103–109. doi: 10.1016/j.tem.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Jonat C, Rahmsdorf HJ, Park KK, Cato AC, Gebel S, Ponta H, Herrlich P. Antitumor promotion and antiinflammation: down-modulation of AP-1 (Fos/Jun) activity by glucocorticoid hormone. Cell. 1990;62:1189–1204. doi: 10.1016/0092-8674(90)90395-u. [DOI] [PubMed] [Google Scholar]
- Kalsbeek a, van Heerikhuize JJ, Wortel J, Buijs RM. A diurnal rhythm of stimulatory input to the hypothalamo-pituitary-adrenal system as revealed by timed intrahypothalamic administration of the vasopressin V1 antagonist. J. Neurosci. 1996;16:5555–5565. doi: 10.1523/JNEUROSCI.16-17-05555.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannisto K, Pietiläinen KH, Ehrenborg E, Rissanen A, Kaprio J, Hamsten A, Yki-Järvinen H. Overexpression of 11beta-hydroxysteroid dehydrogenase-1 in adipose tissue is associated with acquired obesity and features of insulin resistance: studies in young adult monozygotic twins. J. Clin. Endocrinol. Metab. 2004;89:4414–4421. doi: 10.1210/jc.2004-0153. [DOI] [PubMed] [Google Scholar]
- Kellendonk C, Eiden S, Kretz O, Schütz G, Schmidt I, Tronche F, Simon E. Inactivation of the GR in the nervous system affects energy accumulation. Endocrinology. 2002a;143:2333–2340. doi: 10.1210/endo.143.6.8853. [DOI] [PubMed] [Google Scholar]
- Kellendonk C, Gass P, Kretz O, Schütz G, Tronche F. Corticosteroid receptors in the brain: gene targeting studies. Brain Res. Bull. 2002b;57:73–83. doi: 10.1016/s0361-9230(01)00638-4. [DOI] [PubMed] [Google Scholar]
- Keller-Wood ME, Dallman MF. Corticosteroid inhibition of ACTH secretion. Endocr. Rev. 1984;5:1–24. doi: 10.1210/edrv-5-1-1. [DOI] [PubMed] [Google Scholar]
- Kendall EC. The development of cortisone as a therapeutic agent. Indian Med. J. 1951;45:239–241. [PubMed] [Google Scholar]
- Khandaker GM, Dibben CRM, Jones PB. Does maternal body mass index during pregnancy influence risk of schizophrenia in the adult offspring? Obes. Rev. 2012;13:518–527. doi: 10.1111/j.1467-789X.2011.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kino T, Chrousos G. Glucocorticoid and mineralocorticoid resistance / hypersensitivity syndromes. J. Endocrinol. 2001:437–445. doi: 10.1677/joe.0.1690437. [DOI] [PubMed] [Google Scholar]
- Klengel T, Mehta D, Anacker C, Rex-Haffner M, Pruessner JC, Pariante CM, Pace TWW, Mercer KB, Mayberg HS, Bradley B, Nemeroff CB, Holsboer F, Heim CM, Ressler KJ, Rein T, Binder EB. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat. Neurosci. 2013;16:33–41. doi: 10.1038/nn.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knable MB, Torrey EF, Webster MJ, Bartko JJ. Multivariate analysis of prefrontal cortical data from the Stanley Foundation Neuropathology Consortium. Brain Res. Bull. 2001;55:651–659. doi: 10.1016/s0361-9230(01)00521-4. [DOI] [PubMed] [Google Scholar]
- Koenig JI, Kirkpatrick B, Lee P. Glucocorticoid hormones and early brain development in schizophrenia. Neuropsychopharmacology. 2002;27:309–318. doi: 10.1016/S0893-133X(01)00396-7. [DOI] [PubMed] [Google Scholar]
- Kolber BJ, Boyle MP, Wieczorek L, Kelley CL, Onwuzurike CC, Nettles Sa, Vogt SK, Muglia LJ. Transient early-life forebrain corticotropin-releasing hormone elevation causes long-lasting anxiogenic and despair-like changes in mice. J. Neurosci. 2010;30:2571–2581. doi: 10.1523/JNEUROSCI.4470-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolber BJ, Muglia LJ. Defining brain region-specific glucocorticoid action during stress by conditional gene disruption in mice. Brain Res. 2009;1293:85–90. doi: 10.1016/j.brainres.2009.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolber BJ, Wieczorek L, Muglia LJ. Hypothalamic-pituitary-adrenal axis dysregulation and behavioral analysis of mouse mutants with altered glucocorticoid or mineralocorticoid receptor function. Stress. 2008;11:321–338. doi: 10.1080/10253890701821081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korebrits C, Yu DH, Ramirez MM, Marinoni E, Bocking AD, Challis JR. Antenatal glucocorticoid administration increases corticotrophin-releasing hormone in maternal plasma. Br. J. Obstet. Gynaecol. 1998;105:556–561. doi: 10.1111/j.1471-0528.1998.tb10158.x. [DOI] [PubMed] [Google Scholar]
- Krieger DT, Hauser H, Krey LC. Suprachiasmatic nuclear lesions do not abolish food-shifted circadian adrenal and temperature rhythmicity. Science. 1977;197:398–399. doi: 10.1126/science.877566. [DOI] [PubMed] [Google Scholar]
- Kumar R, Thompson EB. Gene regulation by the glucocorticoid receptor: structure:function relationship. J. Steroid Biochem. Mol. Biol. 2005;94:383–394. doi: 10.1016/j.jsbmb.2004.12.046. [DOI] [PubMed] [Google Scholar]
- Labonte B, Yerko V, Gross J, Mechawar N, Meaney MJ, Szyf M, Turecki G. Differential glucocorticoid receptor exon 1(B), 1(C), and 1(H) expression and methylation in suicide completers with a history of childhood abuse. Biol. Psychiatry. 2012;72:41–48. doi: 10.1016/j.biopsych.2012.01.034. [DOI] [PubMed] [Google Scholar]
- Laryea G, Schütz G, Muglia LJ. Disrupting hypothalamic glucocorticoid receptors causes HPA axis hyperactivity and excess adiposity. Mol. Endocrinol. 2013;27:1655–1665. doi: 10.1210/me.2013-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesage J, Blondeau B, Grino M, Bréant B, Dupouy JP. Maternal undernutrition during late gestation induces fetal overexposure to glucocorticoids and intrauterine growth retardation, and disturbs the hypothalamo-pituitary adrenal axis in the newborn rat. Endocrinology. 2001;142:1692–1702. doi: 10.1210/endo.142.5.8139. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Laux G, Schulte HM, Pfüller H, Beckmann H. Corticotropin and cortisol response to human CRH as a probe for HPA system integrity in major depressive disorder. Psychiatry Res. 1988;24:25–34. doi: 10.1016/0165-1781(88)90136-9. [DOI] [PubMed] [Google Scholar]
- Li A-J, Wiater MF, Oostrom MT, Smith BR, Wang Q, Dinh TT, Roberts BL, Jansen HT, Ritter S. Leptin-sensitive neurons in the arcuate nuclei contribute to endogenous feeding rhythms. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012;302:R1313–R1326. doi: 10.1152/ajpregu.00086.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightman SL. The neuroendocrinology of stress: a never ending story. J. Neuroendocrinol. 2008;20:880–884. doi: 10.1111/j.1365-2826.2008.01711.x. [DOI] [PubMed] [Google Scholar]
- Linkowski P, Kerkhofs M, Van Onderbergen A, Hubain P, Copinschi G, L’Hermite- Balériaux M, Leclercq R, Brasseur M, Mendlewicz J, Van Cauter E. The 24- hour profiles of cortisol, prolactin, and growth hormone secretion in mania. Arch. Gen. Psychiatry. 1994;51:616–624. doi: 10.1001/archpsyc.1994.03950080028004. [DOI] [PubMed] [Google Scholar]
- Liu D. Maternal Care, Hippocampal Glucocorticoid Receptors, and Hypothalamic- Pituitary-Adrenal Responses to Stress. Science (80-.) 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Liu W, Wang J, Sauter NK, Pearce D. Steroid receptor heterodimerization demonstrated in vitro and in vivo. Proc. Natl. Acad. Sci. U. S. A. 1995;92:12480–12484. doi: 10.1073/pnas.92.26.12480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Smith LI, Huang V, Poon V, Coello A, Olah M, Spiga F, Lightman SL, Aguilera G. Transcriptional regulation of episodic glucocorticoid secretion. Mol. Cell. Endocrinol. 2013;371:62–70. doi: 10.1016/j.mce.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López JF, Chalmers DT, Little KY, Watson SJ. A.E. Bennett Research Award. Regulation of serotonin1A, glucocorticoid, and mineralocorticoid receptor in rat and human hippocampus: implications for the neurobiology of depression. Biol. Psychiatry. 1998;43:547–573. doi: 10.1016/s0006-3223(97)00484-8. [DOI] [PubMed] [Google Scholar]
- Lowy MT, Reder AT, Gormley GJ, Meltzer HY. Comparison of in vivo and in vitro glucocorticoid sensitivity in depression: relationship to the dexamethasone suppression test. Biol. Psychiatry. 1988;24:619–630. doi: 10.1016/0006-3223(88)90136-9. [DOI] [PubMed] [Google Scholar]
- Makino S, Gold PW, Schulkin J. Corticosterone effects on corticotropin-releasing hormone mRNA in the central nucleus of the amygdala and the parvocellular region of the paraventricular nucleus of the hypothalamus. Exeter Software. 1994a doi: 10.1016/0006-8993(94)91862-7. [DOI] [PubMed] [Google Scholar]
- Makino S, Gold PW, Schulkin J. Effects of corticosterone on CRH mRNA and content in the bed nucleus of the stria terminalis; comparison with the effects in the central nucleus of the amygdala and the paraventricular nucleus of the hypothalamus. Brain Res. 1994b;657:141–149. doi: 10.1016/0006-8993(94)90961-x. [DOI] [PubMed] [Google Scholar]
- Malcher-Lopes R, Di S, Marcheselli VS, Weng F-J, Stuart CT, Bazan NG, Tasker JG. Opposing crosstalk between leptin and glucocorticoids rapidly modulates synaptic excitation via endocannabinoid release. J. Neurosci. 2006;26:6643–6650. doi: 10.1523/JNEUROSCI.5126-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkoski SP, Handanos CM, Dorin RI. Localization of a negative glucocorticoid response element of the human corticotropin releasing hormone gene. Mol. Cell. Endocrinol. 1997;127:189–199. doi: 10.1016/s0303-7207(96)04004-x. [DOI] [PubMed] [Google Scholar]
- Marinoni E, Korebrits C, Di Iorio R, Cosmi EV, Challis JR. Effect of betamethasone in vivo on placental corticotropin-releasing hormone in human pregnancy. Am. J. Obstet. Gynecol. 1998;178:770–778. doi: 10.1016/s0002-9378(98)70490-9. [DOI] [PubMed] [Google Scholar]
- Marques AH, Silverman MN, Sternberg EM. Glucocorticoid dysregulations and their clinical correlates. From receptors to therapeutics. Ann. N. Y. Acad. Sci. 2009;1179:1–18. doi: 10.1111/j.1749-6632.2009.04987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol. Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonté B, Szyf M, Turecki G, Meaney MJ. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat. Neurosci. 2009;12:342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKlveen JM, Myers B, Flak JN, Bundzikova J, Solomon MB, Seroogy KB, Herman JP. Role of prefrontal cortex glucocorticoid receptors in stress and emotion. Biol. Psychiatry. 2013;74:672–679. doi: 10.1016/j.biopsych.2013.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelsohn aM, Anderson DJ. Changes in competence determine the timing of two sequential glucocorticoid effects on sympathoadrenal progenitors. Neuron. 1992;8:589–604. doi: 10.1016/0896-6273(92)90285-l. [DOI] [PubMed] [Google Scholar]
- Miller WL. StAR search--what we know about how the steroidogenic acute regulatory protein mediates mitochondrial cholesterol import. Mol. Endocrinol. 2007;21:589–601. doi: 10.1210/me.2006-0303. [DOI] [PubMed] [Google Scholar]
- Minet-Quinard R, Moinard C, Walrand S, Villié F, Normand B, Vasson MP, Chopineau J, Cynober L. Induction of a catabolic state in rats by dexamethasone: dose or time dependency? JPEN. J. Parenter. Enteral Nutr. 24:30–36. doi: 10.1177/014860710002400130. n.d. [DOI] [PubMed] [Google Scholar]
- Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972;42:201–206. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- Mück-Seler D, Pivac N, Jakovljevic M, Brzovic Z. Platelet serotonin, plasma cortisol, and dexamethasone suppression test in schizophrenic patients. Biol. Psychiatry. 1999;45:1433–1439. doi: 10.1016/s0006-3223(98)00174-7. [DOI] [PubMed] [Google Scholar]
- Munck A, Guyre PM, Holbrook NJ. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr. Rev. 1984;5:25–44. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- Myers B, McKlveen JM, Herman JP. Neural Regulation of the Stress Response: The Many Faces of Feedback. Cell. Mol. Neurobiol. 2012 doi: 10.1007/s10571-012-9801-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nederhof E, Schmidt MV. Mismatch or cumulative stress: toward an integrated hypothesis of programming effects. Physiol. Behav. 2012;106:691–700. doi: 10.1016/j.physbeh.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Nishi M. Dynamics of corticosteroid receptors: lessons from live cell imaging. Acta Histochem. Cytochem. 2011;44:1–7. doi: 10.1267/ahc.10028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi M, Tanaka M, Matsuda K, Sunaguchi M, Kawata M. Visualization of glucocorticoid receptor and mineralocorticoid receptor interactions in living cells with GFP-based fluorescence resonance energy transfer. J. Neurosci. 2004;24:4918–27. doi: 10.1523/JNEUROSCI.5495-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen RM, Yamamoto KR. The glucocorticoid receptor inhibits NFkappaB by interfering with serine-2 phosphorylation of the RNA polymerase II carboxy-terminal domain. Genes Dev. 2000;14:2314–29. doi: 10.1101/gad.827900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa M, Jaaro-Peled H, Tankou S, Seshadri S, Hikida T, Matsumoto Y, Cascella NG, Kano S, Ozaki N, Nabeshima T, Sawa A. Adolescent stress-induced epigenetic control of dopaminergic neurons via glucocorticoids. Science. 2013;339:335–339. doi: 10.1126/science.1226931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley RH, Cidlowski Ja. The biology of the glucocorticoid receptor: new signaling mechanisms in health and disease. J. Allergy Clin. Immunol. 2013;132:1033–1044. doi: 10.1016/j.jaci.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley RH, Webster JC, Sar M, Parker CR, Cidlowski JA. Expression and subcellular distribution of the beta-isoform of the human glucocorticoid receptor. Endocrinology. 1997;138:5028–5038. doi: 10.1210/endo.138.11.5501. [DOI] [PubMed] [Google Scholar]
- Oitzl MS, Reichardt HM, Joëls M, de Kloet ER. Point mutation in the mouse glucocorticoid receptor preventing DNA binding impairs spatial memory. Proc. Natl. Acad. Sci. U. S. A. 2001;98:12790–12795. doi: 10.1073/pnas.231313998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oster H, Damerow S, Kiessling S, Jakubcakova V, Abraham D, Tian J, Hoffmann MW, Eichele G. The circadian rhythm of glucocorticoids is regulated by a gating mechanism residing in the adrenal cortical clock. Cell Metab. 2006;4:163–173. doi: 10.1016/j.cmet.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Owens MJ, Nemeroff CB. The role of corticotropin-releasing factor in the pathophysiology of affective and anxiety disorders: laboratory and clinical studies. Ciba Found. Symp. 1993;172:296–308. doi: 10.1002/9780470514368.ch15. discussion 308-16. [DOI] [PubMed] [Google Scholar]
- Papadimitriou A, Priftis KN. Regulation of the hypothalamic-pituitary-adrenal axis. Neuroimmunomodulation. 2009;16:265–271. doi: 10.1159/000216184. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 2008;31:464–468. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Miller AH. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol. Psychiatry. 2001;49:391–404. doi: 10.1016/s0006-3223(00)01088-x. [DOI] [PubMed] [Google Scholar]
- Payne AH, Hales DB. Overview of Steroidogenic Enzymes in the Pathway from Cholesterol to Active Steroid Hormones. Endocr. Rev. 2004;25:947–970. doi: 10.1210/er.2003-0030. [DOI] [PubMed] [Google Scholar]
- Perlman WR, Webster MJ, Kleinman JE, Weickert CS. Reduced glucocorticoid and estrogen receptor alpha messenger ribonucleic acid levels in the amygdala of patients with major mental illness. Biol. Psychiatry. 2004;56:844–852. doi: 10.1016/j.biopsych.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Polman JAE, Kloet ER, de Datson NA. Two Populations of Glucocorticoid Receptor-Binding Sites in the Male Rat Hippocampal Genome. 2013 doi: 10.1210/en.2012-2187. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Wolf OT, Hellhammer DH, Buske-Kirschbaum A, von Auer K, Jobst S, Kaspers F, Kirschbaum C. Free Cortisol Levels after Awakening: A Reliable Biological Marker for the Assessment of Adrenocortical Activity. Life Sci. 1997;61:2539–2549. doi: 10.1016/s0024-3205(97)01008-4. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Kabbaj M, Jacobson L, Heydendael W, Yehuda R, Herman JP. Stress risk factors and stress-related pathology: neuroplasticity, epigenetics and endophenotypes. Stress. 2011;14:481–497. doi: 10.3109/10253890.2011.604751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raisman G, Brown-Grant K. The “suprachiasmatic syndrome”: endocrine and behavioural abnormalities following lesions of the suprachiasmatic nuclei in the female rat. Proc. R. Soc. Lond. B. Biol. Sci. 1977;198:297–314. doi: 10.1098/rspb.1977.0099. [DOI] [PubMed] [Google Scholar]
- Raju TN. The Nobel Chronicles. Lancet. 1999;353:1370. doi: 10.1016/s0140-6736(05)74374-9. [DOI] [PubMed] [Google Scholar]
- Rask E, Walker BR, Söderberg S, Livingstone DEW, Eliasson M, Johnson O, Andrew R, Olsson T. Tissue-specific changes in peripheral cortisol metabolism in obese women: increased adipose 11beta-hydroxysteroid dehydrogenase type 1 activity. J. Clin. Endocrinol. Metab. 2002;87:3330–3336. doi: 10.1210/jcem.87.7.8661. [DOI] [PubMed] [Google Scholar]
- Reichardt HM, Kaestner KH, Tuckermann J, Kretz O, Wessely O, Bock R, Gass P, Schmid W, Herrlich P, Angel P, Schütz G. DNA binding of the glucocorticoid receptor is not essential for survival. Cell. 1998;93:531–541. doi: 10.1016/s0092-8674(00)81183-6. [DOI] [PubMed] [Google Scholar]
- Reichardt HM, Schütz G. Feedback control of glucocorticoid production is established during fetal development. Mol. Med. 1996;2:735–744. [PMC free article] [PubMed] [Google Scholar]
- Reichstein T, Verzar F, Laszt L. Activity of Corticosteron in the Glucose Test in Rats. Nature. 1937;139:331. [Google Scholar]
- Reul JM, de Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117:2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- Reul JM, Labeur MS, Grigoriadis DE, De Souza EB, Holsboer F. Hypothalamicpituitary- adrenocortical axis changes in the rat after long-term treatment with the reversible monoamine oxidase-A inhibitor moclobemide. Neuroendocrinology. 1994;60:509–519. doi: 10.1159/000126788. [DOI] [PubMed] [Google Scholar]
- Reul JM, Stec I, Söder M, Holsboer F. Chronic treatment of rats with the antidepressant amitriptyline attenuates the activity of the hypothalamic-pituitaryadrenocortical system. Endocrinology. 1993;133:312–320. doi: 10.1210/endo.133.1.8391426. [DOI] [PubMed] [Google Scholar]
- Reynolds RM, Labad J, Buss C, Ghaemmaghami P, Räikkönen K. Transmitting biological effects of stress in utero: implications for mother and offspring. Psychoneuroendocrinology. 2013;38:1843–1849. doi: 10.1016/j.psyneuen.2013.05.018. [DOI] [PubMed] [Google Scholar]
- Ridder S, Chourbaji S, Hellweg R, Urani A, Zacher C, Schmid W, Zink M, Hörtnagl H, Flor H, Henn Fa, Schütz G, Gass P. Mice with genetically altered glucocorticoid receptor expression show altered sensitivity for stress-induced depressive reactions. J. Neurosci. 2005;25:6243–6250. doi: 10.1523/JNEUROSCI.0736-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson BG, Emanuel RL, Frim DM, Majzoub JA. Glucocorticoid stimulates expression of corticotropin-releasing hormone gene in human placenta. Proc. Natl. Acad. Sci. U. S. A. 1988;85:5244–5248. doi: 10.1073/pnas.85.14.5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin RT, Phillips JJ, McCracken JT, Sadow TF. Adrenal gland volume in major depression: relationship to basal and stimulated pituitary-adrenal cortical axis function. Biol. Psychiatry. 1996;40:89–97. doi: 10.1016/0006-3223(95)00358-4. [DOI] [PubMed] [Google Scholar]
- Sallee FR, Nesbitt L, Dougherty D, Hilal R, Nandagopal VS, Sethuraman G. Lymphocyte glucocorticoid receptor: predictor of sertraline response in adolescent major depressive disorder (MDD) Psychopharmacol. Bull. 1995;31:339–345. [PubMed] [Google Scholar]
- Santarelli S, Lesuis SL, Wang X-D, Wagner KV, Hartmann J, Labermaier C, Scharf SH, Müller MB, Holsboer F, Schmidt MV. Evidence supporting the match/mismatch hypothesis of psychiatric disorders. Eur. Neuropsychopharmacol. 2014;24:907–918. doi: 10.1016/j.euroneuro.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Sasaki A, de Vega WC, St-Cyr S, Pan P, McGowan PO. Perinatal high fat diet alters glucocorticoid signaling and anxiety behavior in adulthood. Neuroscience. 2013;240:1–12. doi: 10.1016/j.neuroscience.2013.02.044. [DOI] [PubMed] [Google Scholar]
- Savary I, Debras E, Dardevet D, Sornet C, Capitan P, Prugnaud J, Mirand PP, Grizard J. Effect of glucocorticoid excess on skeletal muscle and heart protein synthesis in adult and old rats. Br. J. Nutr. 1998;79:297–304. doi: 10.1079/bjn19980047. [DOI] [PubMed] [Google Scholar]
- Scheinman RI, Gualberto A, Jewell CM, Cidlowski JA, Baldwin AS. Characterization of mechanisms involved in transrepression of NF-kappa B by activated glucocorticoid receptors. Mol. Cell. Biol. 1995;15:943–953. doi: 10.1128/mcb.15.2.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt MV. Animal models for depression and the mismatch hypothesis of disease. Psychoneuroendocrinology. 2011;36:330–338. doi: 10.1016/j.psyneuen.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Schmidt MV, Enthoven L, van der Mark M, Levine S, de Kloet ER, Oitzl MS. The postnatal development of the hypothalamic–pituitary–adrenal axis in the mouse. Int. J. Dev. Neurosci. 2003;21:125–132. doi: 10.1016/s0736-5748(03)00030-3. [DOI] [PubMed] [Google Scholar]
- Schmidt MV, Schmidt M, Levine S, Oitzl MS, van der Mark M, Müller MB, Holsboer F, de Kloet ER. Glucocorticoid receptor blockade disinhibits pituitary-adrenal activity during the stress hyporesponsive period of the mouse. Endocrinology. 2005;146:1458–1464. doi: 10.1210/en.2004-1042. [DOI] [PubMed] [Google Scholar]
- Schmidt MV, Sterlemann V, Wagner K, Niederleitner B, Ganea K, Liebl C, Deussing JM, Berger S, Schütz G, Holsboer F, Müller MB. Postnatal glucocorticoid excess due to pituitary glucocorticoid receptor deficiency: differential short- and long-term consequences. Endocrinology. 2009;150:2709–2716. doi: 10.1210/en.2008-1211. [DOI] [PubMed] [Google Scholar]
- Schulz P, Kirschbaum C, Pruessner J, Hellhammer D. Increased free cortisol secretion after awakening in chronically stressed individuals due to work overload. Stress Med. 1998;14:91–97. [Google Scholar]
- Schwartz MW, Woods SC, Porte D, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- Seckl JR, Fink G. Antidepressants increase glucocorticoid and mineralocorticoid receptor mRNA expression in rat hippocampus in vivo. Neuroendocrinology. 1992;55:621–626. doi: 10.1159/000126180. [DOI] [PubMed] [Google Scholar]
- Sharma D, Bhave S, Gregg E, Uht R. Dexamethasone induces a putative repressor complex and chromatin modifications in the CRH promoter. Mol. Endocrinol. 2013;27:1142–1152. doi: 10.1210/me.2013-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair D, Tsai SY, Woon HG, Weickert CS. Abnormal glucocorticoid receptor mRNA and protein isoform expression in the prefrontal cortex in psychiatric illness. Neuropsychopharmacology. 2011;36:2698–2709. doi: 10.1038/npp.2011.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon MB, Furay aR, Jones K, Packard aEB, Packard Ba, Wulsin aC, Herman JP. Deletion of forebrain glucocorticoid receptors impairs neuroendocrine stress responses and induces depression-like behavior in males but not females. Neuroscience. 2012;203:135–143. doi: 10.1016/j.neuroscience.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spies CM, Strehl C, van der Goes MC, Bijlsma JWJ, Buttgereit F. Glucocorticoids. Best Pract. Res. Clin. Rheumatol. 2011;25:891–900. doi: 10.1016/j.berh.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Stengel A, Goebel M, Million M, Stenzel-Poore MP, Kobelt P, Mönnikes H, Taché Y, Wang L. Corticotropin-releasing factor-overexpressing mice exhibit reduced neuronal activation in the arcuate nucleus and food intake in response to fasting. Endocrinology. 2009;150:153–160. doi: 10.1210/en.2008-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stengel A, Taché Y. CRF and urocortin peptides as modulators of energy balance and feeding behavior during stress. Front. Neurosci. 2014;8:52. doi: 10.3389/fnins.2014.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenzel-Poore MP, Cameron VA, Vaughan J, Sawchenko PE, Vale W. Development of Cushing’s syndrome in corticotropin-releasing factor transgenic mice. Endocrinology. 1992;130:3378–3386. doi: 10.1210/endo.130.6.1597149. [DOI] [PubMed] [Google Scholar]
- Stevens A, White A. ACTH: cellular peptide hormone synthesis and secretory pathways. Results Probl. Cell Differ. 2010;50:63–84. doi: 10.1007/400_2009_30. [DOI] [PubMed] [Google Scholar]
- Stimson RH, Andersson J, Andrew R, Redhead DN, Karpe F, Hayes PC, Olsson T, Walker BR. Cortisol release from adipose tissue by 11beta-hydroxysteroid dehydrogenase type 1 in humans. Diabetes. 2009;58:46–53. doi: 10.2337/db08-0969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szafarczyk a, Ixart G, Alonso G, Malaval F, Nouguier-Soulé J, Assenmacher I. CNS control of the circadian adrenocortical rhythm. J. Steroid Biochem. 1983;19:1009–1015. doi: 10.1016/0022-4731(83)90047-x. [DOI] [PubMed] [Google Scholar]
- Tasker JG. Rapid glucocorticoid actions in the hypothalamus as a mechanism of homeostatic integration. Obesity (Silver Spring) 2006;14(Suppl 5):259S–265S. doi: 10.1038/oby.2006.320. [DOI] [PubMed] [Google Scholar]
- Tatro ET, Everall IP, Masliah E, Hult BJ, Lucero G, Chana G, Soontornniyomkij V, Achim CL. Differential expression of immunophilins FKBP51 and FKBP52 in the frontal cortex of HIV-infected patients with major depressive disorder. J. Neuroimmune Pharmacol. 2009;4:218–226. doi: 10.1007/s11481-009-9146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinnikov AA, Legan MV, Sheveluk NA, Cvetovskaya GA, Naumenko SE, Sidelnikov SG. Corticosteroid and immune responses to cardiac surgery. Steroids. 1996;61:411–415. doi: 10.1016/0039-128x(96)00060-8. [DOI] [PubMed] [Google Scholar]
- Tonsfeldt KJ, Chappell PE. Clocks on top: the role of the circadian clock in the hypothalamic and pituitary regulation of endocrine physiology. Mol. Cell. Endocrinol. 2012;349:3–12. doi: 10.1016/j.mce.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp T, Holsboer F. Heterodimerization between mineralocorticoid and glucocorticoid receptors increases the functional diversity of corticosteroid action. Trends Pharmacol. Sci. 1996;17:145–149. doi: 10.1016/0165-6147(96)81590-2. [DOI] [PubMed] [Google Scholar]
- Trapp T, Rupprecht R, Castrén M, Reul JM, Holsboer F. Heterodimerization between mineralocorticoid and glucocorticoid receptor: a new principle of glucocorticoid action in the CNS. Neuron. 1994;13:1457–1462. doi: 10.1016/0896-6273(94)90431-6. [DOI] [PubMed] [Google Scholar]
- Trapp T, Rupprecht R, Castrén M, Reul JMHM, Holsboer F. Heterodimerization between mineralocorticoid and glucocorticoid receptor: A new principle of glucocorticoid action in the CNS. Neuron. 1994;13:1457–1462. doi: 10.1016/0896-6273(94)90431-6. [DOI] [PubMed] [Google Scholar]
- Trivedi BP. Neuroscience: Dissecting appetite. Nature. 2014;508:S64–S65. doi: 10.1038/508S64a. [DOI] [PubMed] [Google Scholar]
- Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, Bock R, Klein R, Schütz G. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat. Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- Tsien JZ, Chen DF, Gerber D, Tom C, Mercer EH, Anderson DJ, Mayford M, Kandel ER, Tonegawa S. Subregion- and Cell Type–Restricted Gene Knockout in Mouse Brain. Cell. 1996;87:1317–1326. doi: 10.1016/s0092-8674(00)81826-7. [DOI] [PubMed] [Google Scholar]
- Van Haarst AD, Oitzl MS, de Kloet ER. Facilitation of feedback inhibition through blockade of glucocorticoid receptors in the hippocampus. Neurochem. Res. 1997;22:1323–1328. doi: 10.1023/a:1022010904600. [DOI] [PubMed] [Google Scholar]
- Vincent MY, Jacobson L. Glucocorticoid receptor deletion from the dorsal raphé nucleus of mice reduces dysphoria-like behavior and impairs hypothalamic-pituitaryadrenocortical axis feedback inhibition. Eur. J. Neurosci. 2014 doi: 10.1111/ejn.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner KV, Wang X-D, Liebl C, Scharf SH, Müller MB, Schmidt MV. Pituitary glucocorticoid receptor deletion reduces vulnerability to chronic stress. Psychoneuroendocrinology. 2011;36:579–587. doi: 10.1016/j.psyneuen.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Waite EJ, McKenna M, Kershaw Y, Walker JJ, Cho K, Piggins HD, Lightman SL. Ultradian corticosterone secretion is maintained in the absence of circadian cues. Eur. J. Neurosci. 2012;36:3142–3150. doi: 10.1111/j.1460-9568.2012.08213.x. [DOI] [PubMed] [Google Scholar]
- Walker JJ, Terry JR, Lightman SL. Origin of ultradian pulsatility in the hypothalamicpituitary- adrenal axis. Proc. Biol. Sci. 2010;277:1627–1633. doi: 10.1098/rspb.2009.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M. The role of glucocorticoid action in the pathophysiology of the Metabolic Syndrome. Nutr. Metab. (Lond) 2005;2:3. doi: 10.1186/1743-7075-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waselus M, Valentino RJ, Van Bockstaele EJ. Collateralized dorsal raphe nucleus projections: a mechanism for the integration of diverse functions during stress. J. Chem. Neuroanat. 2011;41:266–280. doi: 10.1016/j.jchemneu.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat. Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Weaver IC, D’Alessio A. The Transcription Factor Nerve Growth Factor-Inducible Protein A Mediates Epigenetic Programming: Altering Epigenetic Marks by Immediate- Early Genes. J. Neurosci. 2007;27:1756–1768. doi: 10.1523/JNEUROSCI.4164-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Webster JC, Cidlowski JA. Mechanisms of Glucocorticoid-receptor-mediated Repression of Gene Expression. Trends Endocrinol. Metab. 1999;10:396–402. doi: 10.1016/s1043-2760(99)00186-1. [DOI] [PubMed] [Google Scholar]
- Webster MJ, Knable MB, O’Grady J, Orthmann J, Weickert CS. Regional specificity of brain glucocorticoid receptor mRNA alterations in subjects with schizophrenia and mood disorders. Mol. Psychiatry. 2002;7:985–994. 924. doi: 10.1038/sj.mp.4001139. [DOI] [PubMed] [Google Scholar]
- Whalley LJ, Borthwick N, Copolov D, Dick H, Christie JE, Fink G. Glucocorticoid receptors and depression. Br. Med. J. (Clin. Res. Ed) 1986;292:859–861. doi: 10.1136/bmj.292.6524.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmaier EP, Dallman MF. The effects of corticotropin-releasing factor on adrenocorticotropin secretion from perifused pituitaries in vitro: rapid inhibition by glucocorticoids. Endocrinology. 1984;115:2368–2374. doi: 10.1210/endo-115-6-2368. [DOI] [PubMed] [Google Scholar]
- Wolkowitz OM, Burke H, Epel ES, Reus VI. Glucocorticoids. Mood, memory, and mechanisms. Ann. N. Y. Acad. Sci. 2009;1179:19–40. doi: 10.1111/j.1749-6632.2009.04980.x. [DOI] [PubMed] [Google Scholar]
- Yamamori E, Iwasaki Y, Taguchi T, Nishiyama M, Yoshida M, Asai M, Oiso Y, Itoi K, Kambayashi M, Hashimoto K. Molecular mechanisms for corticotropin-releasing hormone gene repression by glucocorticoid in BE(2)C neuronal cell line. Mol. Cell. Endocrinol. 2007;264:142–148. doi: 10.1016/j.mce.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Boisoneau D, Mason JW, Giller EL. Glucocorticoid receptor number and cortisol excretion in mood, anxiety, and psychotic disorders. Biol. Psychiatry. 1993;34:18–25. doi: 10.1016/0006-3223(93)90252-9. [DOI] [PubMed] [Google Scholar]
- Young EA, Abelson J, Lightman SL. Cortisol pulsatility and its role in stress regulation and health. Front. Neuroendocrinol. 2004;25:69–76. doi: 10.1016/j.yfrne.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Zanchi NE, Filho MADS, Felitti V, Nicastro H, Lorenzeti FM, Lancha AH. Glucocorticoids: extensive physiological actions modulated through multiple mechanisms of gene regulation. J. Cell. Physiol. 2010;224:311–315. doi: 10.1002/jcp.22141. [DOI] [PubMed] [Google Scholar]
- Zhang A, Neumeyer JL, Baldessarini RJ. Recent progress in development of dopamine receptor subtype-selective agents: potential therapeutics for neurological and psychiatric disorders. Chem. Rev. 2007;107:274–302. doi: 10.1021/cr050263h. [DOI] [PubMed] [Google Scholar]
- Zhang TY, Chrétien P, Meaney MJ, Gratton A. Influence of naturally occurring variations in maternal care on prepulse inhibition of acoustic startle and the medial prefrontal cortical dopamine response to stress in adult rats. J. Neurosci. 2005;25:1493–1502. doi: 10.1523/JNEUROSCI.3293-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang TY, Labonté B, Wen XL, Turecki G, Meaney MJ. Epigenetic mechanisms for the early environmental regulation of hippocampal glucocorticoid receptor gene expression in rodents and humans. Neuropsychopharmacology. 2013;38:111–123. doi: 10.1038/npp.2012.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Sheng H, Qi J, Ma B, Sun J, Li S, Ni X. Glucocorticoid acts on a putative G protein-coupled receptor to rapidly regulate the activity of NMDA receptors in hippocampal neurons. Am. J. Physiol. Endocrinol. Metab. 2012;302:E747–E758. doi: 10.1152/ajpendo.00302.2011. [DOI] [PubMed] [Google Scholar]
- Zobel A, Jessen F, von Widdern O, Schuhmacher A, Höfels S, Metten M, Rietschel M, Scheef L, Block W, Becker T, Schild HH, Maier W, Schwab SG. Unipolar depression and hippocampal volume: impact of DNA sequence variants of the glucocorticoid receptor gene. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2008;147B:836–843. doi: 10.1002/ajmg.b.30709. [DOI] [PubMed] [Google Scholar]