Abstract
Disorders and diseases of the gastrointestinal system encompass a wide array of pathogenic mechanisms as a result of genetic, infectious, neoplastic, and inflammatory conditions. Inflammatory diseases in general are rising in incidence and are emerging clinical problems in gastroenterology and hepatology. Hemeoxygenase-1 (HO-1) is a stress-inducible enzyme that has been shown to confer protection in various organ-system models. Its downstream effectors, carbon monoxide and biliverdin have also been shown to offer these beneficial effects. Many studies suggest that induction of HO-1 expression in gastrointestinal tissues and cells plays a critical role in cytoprotection and resolving inflammation as well as tissue injury. In this review, we examine the protective role of HO-1 and its downstream effectors in modulating inflammatory diseases of the upper (esophagus and stomach) and lower (small and large intestine) gastrointestinal tract, the liver, and the pancreas. Cytoprotective, anti-inflammatory, anti-proliferative, antioxidant, and anti-apoptotic activities of HO-1 make it a promising if not ideal therapeutic target for inflammatory diseases of the gastrointestinal system.
Keywords: Hemeoxygenase-1, Carbon monoxide, Biliverdin
Introduction
The biological importance of hemeoxygenase (HO) originates from its function as the rate-limiting enzyme in heme catabolism. Heme is oxidatively cleaved by the HO system into equimolar quantities of carbon monoxide (CO), biliverdin, and Fe2+ [1]; and in a coupled reaction biliverdin is rapidly converted into bilirubin via biliverdin reductase [2]. Three distinctive HO isoforms have been identified and although they catalyze the same biochemical reaction they are the products of different genes with different expression patterns in cells and tissues [3]. HO-3 (33 kDa) is a poor heme catalyst that has been found only in rat brain with no activity reported in humans [4, 5]; HO-2 (36 kDa) which contributes to cell homeostasis is constitutively expressed in many tissues including neuronal and testicular tissues [6, 7]; whereas, HO-1 (32 kDa), also known as heat shock protein-32 (Hsp32), is stress-inducible and expressed at a relatively low level in most tissues. In addition to its substrate heme, HO-1 is upregulated by heavy metals [8] and stimuli that cause oxidative stress such as heat shock [9], ischemia, hemorrhagic shock [10], reactive oxygen species (ROS) [11], radiation, and hypoxia [12]. Many reports have also shown that inflammatory mediators such IL-1, TNF-α, LPS, ROS and reactive nitrogen species (RNS) are able to upregulate HO-1 in vitro [13, 14].
HO-1 induction is usually associated with a protective response [15]; classically the beneficial nature of HO is attributed to its ability of removing free heme, which has cytotoxic effects [5]. However, new evidence indicates that although HO-1 as such does not directly catalyze an antioxidant reaction, its upregulation, and the production of CO and biliverdin, influences many biological events linked to a cytoprotective and anti-inflammatory response against oxidative stress [16–18]. For instance CO is believed to act as a signaling molecule in a similar manner as nitric oxide (NO), with anti-inflammatory and anti-apoptotic properties [19, 20]. The anti-inflammatory actions of CO have been proposed to be mediated through p38 mitogen-activated protein kinase (MAPK) pathway [19, 21]. Biliverdin and bilirubin are reducing agents with antioxidant properties and have the ability to efficiently scavenge proxyl radicals and inhibit lipid peroxidation [22]. Although biliverdin is rapidly converted to bilirubin and has a short half-life, in a recycling process bilirubin as a potent antioxidant oxidizes itself back to biliverdin [23, 24]. Treatment with biliverdin decrease mRNA expression of inducible nitric oxide synthase (iNOS), cyclooxygenase 2, and the inflammatory cytokines IL-6 and IL-1β, as well as decrease neutrophil infiltration into the jejunal muscularis in rat model of small intestinal transplants [25]. Moreover, biliverdin is an endogenous ligand of the aryl hydrocarbon receptor (AhR), which upon activation protects against experimental acute pancreatitis by induction of IL-22 [26]. Similarly, Fe2+ is involved in gene regulation including that of NO synthase (NOS). Although potentially toxic, Fe2+ leads to the opening of channels that export Fe2+ from the cells inducing the upregulation of ferritin, an iron storing protein which protects cells against oxidant damage by oxidation of low-density lipoproteins [27, 28].
The beneficial effects of HO-1 have been demonstrated in HO-1-deficient mice models which have atypical innate and adaptive immune responses with a general pro-inflammatory response displaying a T helper 1 (Th1)-type cytokine profile (IL-1, IFN-γ, TNF-α, IL-6) [29]. HO-1-deficient mice develop chronic inflammation, and are vulnerable to endotoxin sepsis [30] and have defective expression of interferon-β [31]. In addition, ablation of the HO-1 gene results in alteration of the endothelial monolayer making it more susceptible to apoptosis and denudation from the extracellular matrix [32, 33]. On the other hand, HO-2-deficient mice have intact immune regulation but have defects in their central and autonomic nervous system [34]. Many of the clinical hallmarks observed in HO-1-deficient mice are consistent with those found in HO-1-deficient patients. HO-1 deficiency in both in human and mice is either lethal before birth or results in death at early age; survivors present with anemia, erythrocyte fragmentation and iron deposition [35]. In humans HO-1 deficiency is characterized by hemolysis, disseminated intravascular coagulation, nephritis and asplenia [35, 36]. In the first human reported case of HO-1 deficiency an infant, born to parents without clinical manifestation each of whom had a partial deletion of the HO-1 gene in different alleles, had severe inadequate HO-1 expression with affected phenotype that proved to be fatal [35]. The afflicted patient had marked growth and developmental retardation associated with erythrocyte fragmentation and abnormal coagulation/fibrinolysis system since early age [35]. In a different case of HO-1 deficiency, the characteristic symptoms manifested later in life, but the clinical outcome was the same as in the first patient [36].
HO-1 regulation
Currently the transcriptional regulation of the HO-1 gene is attributed to two transcription factors: nuclear erythroid 2-related factor-2 (Nrf2) and the heme-binding protein Bach1 [37, 38]. Nrf2 plays a crucial role in cytoprotection and contains an activation domain that initiates HO-1 transcription, whereas Bach1 represses HO-1 transcription by competing with Nrf2 [37, 38]. Other molecules are also associated with HO-1 upregulation; for instance, MAPK signaling is involved in HO-1 induction [39] and the phosphatidylinositol-3 kinase (PI3K)/Akt signaling modulates HO-1 activity by phosphorylation of Ser-188 [40]. It was also shown that the anti-inflammatory cytokine IL-10 induces HO-1 expression via a p38 MAPK-dependent pathway [21]. Upregulation of HO-1 in turn amplifies the effects of IL-10. In activated macrophages, HO-1 and CO mediate IL-10 inhibition of iNOS expression and NO production [21]. In addition, HO-1 and IL-10 work in conjunction to suppress the expression of the pro-inflammatory cytokine TNF-α [21]. The suppressive effect of IL-10 on TNF-α production was reversible with zinc protoporphyrin (ZnPP), an HO-1 inhibitor [21].
The regulation of HO-1 response to an inflammatory stimuli is further demonstrated via its association with an HO-1 gene promoter microsatellite (GT) (n) dinucleotide repeat polymorphism [41] and a single nucleotide polymorphism (SNP) A(-413)T in the promoter [42, 43]. In humans the GT length polymorphism in the HO-1 promoter is associated with the strength of HO-1 response to a given stimulus. Individuals with short GT repeats are reported to have a lower risk of necrotizing acute pancreatitis [44], chronic pulmonary emphysema [45], rheumatoid arthritis [46], restenosis after balloon angioplasty [47], coronary artery [48], and other diseases [41]. In addition to the (GT) (n) polymorphism, the A(-413)T SNP has been identified as a functionally relevant variation of the HO-1 gene; even more important for HO-1 promoter activity than the (GT)n polymorphism [42, 43]. The A(-413)T genotype of HO-1 is associated with an increased incidence of hypertension in women [42] and reduced the incidence of ischemic heart disease [43], possibly due to the high expression of HO-1.
HO-1 expression
At the cellular level, HO-1 is highly expressed in Kupffer cells in the liver [49], spleen macrophages [50] and dendritic cells [51]. HO-1 upregulation in these cells in response to LPS attenuates the expression of various pro-inflammatory genes [52, 53]. Likewise, pharmacological induction of HO-1 in dendritic cells alters their maturation state and interaction with other cells [51, 54]. For instance HO-1 expression in dendritic cells is essential for the function of T cells influencing the proliferation of both effector and regulatory CD4 T cells [55, 56]. Accumulating evidence shows that HO-1 is involved in multiple functions of macrophages including infiltration, differentiation and polarization. HO-1 induction is essential for monocyte/macrophage early infiltration to damaged tissues. Induction of HO-1 by hemin is associated with upregulation of chemokines (MCP-1/MIP-1α) and recruitment of HO-1+ monocyte/macrophages into the pancreas [57]. HO-1 expression is increased during myeloid progenitor cell differentiation, and is critical for myeloid cells differentiation into macrophages [58]. In addition, upregulation of HO-1 contributes to alternative activation of macrophage that plays a critical role in anti-inflammation and tissue repair [59]. At a tissue level HO-1 is constitutively expressed in the liver, gastric, intestinal, and colonic mucosa [60–62]. Expression of HO-1 is increased during inflammation and gastrointestinal injury such as in gastric ulcers [63], radiation enteritis [64], inflammatory bowel disease [65–67], and liver fibrosis [68].
In this review, we provide an overview of the protective roles of HO-1 in diseases of the upper (stomach and esophagus) and lower (small and large intestine) gastrointestinal tract, the pancreas and the liver.
HO-1 and diseases of the esophagus and stomach
It is suggested that HO-1 and CO may play a role in the maintenance of proper function of the lower esophageal sphincter (LES) [69, 70]. Whenever the LES is dysfunctional due to weakness or improper relaxation, the contents of the stomach rise up into the esophagus, leading to reflux of gastroduodenal contents and subsequent gastroduodenal reflux disease and/or esophagitis [71]. CO is believed to play a role as a peripheral messenger due to its relaxant effect on the circular LES muscle [70]. The expression of CO in the LES [69, 70] is linked to HO-1 activity since CO production was blocked with the use of HO-1 inhibitor ZnPP [70]. Reflux of duodenal juice was found to be necessary to induce the oxidative damage leading to HO-1 upregulation in rats that underwent esophagoduodenal anastomosis (EDA); a model for esophagitis due to duodenoesophageal reflux into the distal esophagus [72].
Users of non-steroidal anti-inflammatory drugs (NSAID) have a greater risk of developing esophagitis, esophageal and gastric ulcers [73]. The beneficial effect of HO-1 expression on esophageal and gastric ulcers has also been demonstrated in models of NSAIDs such as indomethacin- and ketoprofen-mediated injuries [74–76]. In an indomethacin-induced gastric ulcer model there was increased tissue expression of IL-6 and TNF-α [74]. HO-1 induction with cobalt protoporphyrin (CoPP) ameliorated the disease by reducing gastric inflammation, tissue neutrophil activation and the expression of pro-inflammatory cytokines [74]. Furthermore, in this model HO-1 upregulation, via activation of Nrf2, was found to protect the gastric mucosa by inhibiting apoptosis [75]. Conversely, HO inhibitor tin mesoporphyrin (SnMP) resulted in exacerbation of gastric lesions and increased apoptosis [75]. In ketoprofen-induced peptic ulcer catechin, a dietary polyphenol antioxidant, was protective against epithelial cell injury by inducing the expression of HO-1 via modulation of Nfr2 and inhibition of oxidative damage [76].
Numerous studies have shown that the cytoprotective effects observed by many pharmacological agents used in upper gastric diseases such as, polaprezinc [77], eupatilin [78] and lansoprazole [79] may be in part mediated via HO-1 upregulation. In cultured esophageal epithelial cells, there was a dose- and time-dependent expression of HO-1 in response to eupatilin in vitro and in esophageal epithelium of rats in vivo [80]. Eupatilin-induced HO-1 expression in the cultured esophageal epithelial cells was mediated by nuclear translocation of Nrf2 and by ERKs and PI3K/Akt signaling [80]. In addition, eupatilin prevented cytotoxic action of indomethacin.
HO-1 induction has also been reported to have a beneficial effect in diabetic gastroparesis, a condition with delayed gastric emptying due to complications of diabetes mellitus. In diabetic gastroparesis there was a decrease in HO-1+ CD206+ macrophages and an increase in oxidative stress that was associated with low expression of the receptor tyrosine kinase KIT in interstitial cells of Cajal (ICC) resulting in delayed gastric emptying [78, 81]. In a non-obese diabetic (NOD) model of diabetic gastroparesis, hemin treatment protected ICC by decreasing reactive oxygen species and restoring KIT expression [82]. In addition, hemin treatment led to repopulation of HO-1+ gastric CD206+ macrophages and a phenotypic switch from pro-inflammatory M1 macrophages to wound healing inducer M2 macrophages [59]. The increased number of HO-1+ expressing gastric macrophages was associated with normalization of gastric emptying [59]. Thus, HO-1 expressing CD206+ macrophages appear to play an important role in preventing delayed gastric emptying in diabetic mice.
HO-1 and diseases of the small and large intestine
The expression of HO-1 in intestinal tissue is localized in mononuclear cells of the submucosal layer [83] and epithelial cells in the human duodenal mucosa [60, 61]. Although HO-1 is constitutively expressed in intestinal epithelial cells, HO-1 inducers appear to ameliorate mucosal injury by decreasing infiltrating inflammatory cells such as neutrophils and lymphocytes [65]. HO-1 induction has been shown to be beneficial in models of small intestinal injury. For instance, in indomethacin-mediated injury, pharmacologic induction of HO-1 by lansoprazole [84, 85] and sulforafane [86] resulted in inhibition of intestinal injury, which was reversed by HO-1 inhibition with tin protoporphyrin (SnPP) [84].
CO mediates many of the biological actions of HO-1; CO releasing molecules (CO-RMs) were shown to lessen intestinal injury during postoperative ileus [39], indomethacin injury [85] and sepsis [87]. Postoperative Ileus is the transient impairment of bowel motility usually as a result of a major abdominal surgery. Pretreatment with CO-RMs reduced the development of postoperative ileus in mice [39]. The protective effects were mediated in part via induction of HO-1 expression and activity through modulation of the MAPK signaling pathway (p38 and ERK1/2) [39]. In addition, CO-RMs reduced oxidative stress and suppressed the inflammatory response associated with intestinal manipulation [39]. The beneficial effect was abrogated by chromium mesoporphyrin (CrMP), an HO-1 inhibitor, which aggravated the intestinal injury [39]. Similarly in an indomethacin-induced small intestinal ulceration, pretreatment with CO-RMs reduced the severity of injury by inhibition of iNOS expression through upregulation of HO-1/CO in the mucosa [85]. Likewise, this protective effect was reversible with the use of HO-1 inhibitor SnPP [85]. Furthermore, the beneficial role of HO-1 and CO during sepsis, a complex syndrome characterized by both infection and a systemic inflammatory response, was demonstrated with the use of CO-RMs [87]. Administration of CO-RMs 6 h after sepsis onset decreased bacterial counts, increased bacterial phagocytosis and reduced mortality in HO-1-deficient mice highlighting the importance of CO as a protective downstream effector of HO-1 [87].
Ischemia/reperfusion injury
Ischemia/reperfusion (I/R) injury of the gut occurs frequently due to interruption and reintroduction of blood supply; it has been shown that induction of HO-1 has anti-inflammatory and cytoprotective effects in I/R-mediated small intestinal injuries [88–90]. Administration of CoPP before intestinal I/R induces HO-1 and reduces I/R injury [91]. Other agents such as glutamine, a major fuel for enterocytes, protect the intestine from I/R injury by inducing the expression of HO-1 in the intestinal mucosa of villous epithelial cells, crypts, and muscular layers and by inhibiting inflammatory cytokines [92]. In a similar manner, pyrrolidine dithiocarbamate improves the outcome of I/R injury by inducing HO-1 production and enhancing perfusion in the microvasculature [93]. Ablation of the HO-1 gene in mice exacerbates I/R injury [94, 95], conversely mice that overexpress HO-1 due to Bach1 deficiency have a damped response to I/R-mediated injury [96]. In addition, the products of HO-1 activity have also been found to ameliorate intestinal I/R injury. CO inhalation at a low concentration attenuates the remote intestinal inflammatory response caused by hindlimb I/R [97, 98]; and protects against I/R injury of intestinal grafts during prolonged cold preservation [99]. Similarly, biliverdin and bilirubin act as potent cytoprotective agents during I/R [98, 100].
HO-1 expression has also been found to be increased in patients with ischemic colitis a condition in which there is inadequate blood flow to the large intestine due to narrowed or blocked blood vessels leading to colonic inflammation [101].
Inflammatory bowel disease
Inflammatory bowel disease (IBD) comprises Crohn’s disease and ulcerative colitis. While ulcerative colitis is limited to the colon and rectum, Crohn’s disease can affect any part of the gastrointestinal tract from the mouth to the anus, although over a third of Crohn’s disease patients have disease involving the terminal ileum [102]. HO-1 mRNA and protein are increased in inflamed colonic mucosa of IBD patients [65, 67] and in colitis mouse models [66, 67, 103]. Upregulation of HO-1 in the large intestine by hemin [66, 104], heme [105] and CoPP [65, 106] results in a better outcome in experimental colitis. HO-1 expression and activity were increased in the damaged colonic tissue following the acute model of 2,4,6-trinitrobenzene sulfonic acid (TNBS) colitis and it is believed that the protective effect of HO-1 could be the result of radical scavenging and inhibition of NO production and iNOS expression since HO-1 induction by hemin lowered iNOs mRNA and protein expression in the affected colon [66]. Similarly, HO-1 induction with CoPP was protective in the dextran sulfate sodium (DSS) mouse model of colitis [65]. On the contrary, the use of HO-1 inhibitors reversed the beneficial effects of HO-1 induction. For instance, in the TNBS colitis model, administration of the HO inhibitor SnMP resulted in reduction of HO-1 activity and increased colonic damage [66]. In the same way, treatment with the HO inhibitor Znpp enhanced intestinal inflammation and increased the disease severity score in the DSS colitis model [103]. Furthermore, mice deficient of the HO-1 transcription factor Nrf2, have low HO-1 expression and are more susceptible to developing colitis in the DSS model [107]. In contrast, mice deficient of the Bach1 transcription factor have higher expression of HO-1 mRNA and protein in colonic mucosa and have significantly attenuated colonic inflammation after induction of TNBS colitis [108]. The expression of HO-1 from Bach1-deficient mice was localized mainly in F4/80+ and CD11b+ macrophages which presented M2-type markers such as, Fizz-1, Ym1, and MRC1 [108]. Further experiments indicated that transfer of these macrophages into wild-type mice, inhibited TNBS-induced colitis [108].
The therapeutic benefits of HO-1 in colitis can be attributed to the effect of HO-1 activation: biliverdin [106] and CO [109, 110]. In a DSS-induced acute colitis model, administration of biliverdin has a protective effect comparable with the one seen with treatment of HO-1 inducer CoPP [106]. In addition, in the chronic Th1-mediated IL-10-deficient mice model of colitis, CO at a low concentration diminishes chronic intestinal inflammation by inducing HO-1 expression [109]. It was shown that CO acts upon macrophages by altering IFN-γ signaling and by inhibiting activation of IL-12 p40 and iNOS after LPS/IFN-γ stimulation through selective inhibition of IRF-8 [109]. Similarly, the beneficial effects of CO and HO-1 induction were also demonstrated in a Th2-mediated TCRα-deficient mice model of colitis by increasing the expression levels of the anti-inflammatory cytokines IL-10 and IL-22 [110]. HO-1 upregulation has also been attributed to the efficacy of current IBD therapies such as 5-amino salicylic acid (5-ASA) [111]. Intracolonic administration of 5-ASA in a TNBS model of colitis increased HO-1 expression levels, reduced colonic injury, decreased myeloperoxidase activity and TNF-α levels and these effects were abolished with HO-1 inhibitor ZnPP [111].
Necrotizing enterocolitis
HO-1 activity has also been linked to the development of necrotizing enterocolitis (NEC) [112], a condition in premature or low weight infants that mostly affects the distal ileum and less commonly the right colon or upper small bowel [113]. Mice heterozygous for the HO-1 gene (HO-1Het) have abnormal intestinal morphology, are predisposed to intestinal injury and enhanced apoptosis [112]. Due to the fact that HO-1Het mice have deficient upregulation of HO-1, they have exaggerated inflammatory response observed by increased expression of IL-1β, P-selectin, and MMP2, resulting in higher incidence and onset of NEC [112].
Radiation enteritis
Radiation enteritis is a condition in which there is mucosal damage to the lining of the intestine with infiltration of activated inflammatory cells during or after ionizing radiotherapy to the abdomen, pelvis, or rectum [114]. HO-1 induction by glutamine protects against abdominal radiation-induced damage to the intestinal tissues in a rat model by decreasing myeloperoxidase (MPO) and caspase-3 activity, and malondialdehyde (MDA) levels which leads to reduction of tissue inflammation and suppression of apoptosis [115]. HO-1 inhibition by ZnPP in irradiated rats worsened the intestinal damage [115]. In addition, in a rat model of abdominal radiation it was proposed that the protective effect of octreotide (OCT), the synthetic analogue of somatostatin, against radiation-induced intestinal damage [116] was in part mediated by modification of the inflammatory response and induction of HO-1 expression [117].
HO-1 and diseases of the pancreas
Pancreatitis is an inflammatory disease that is clinically categorized into acute and chronic. Acute pancreatitis (AP) is one of the most frequent gastrointestinal causes of hospital admission in the United States. AP ranges from mild transient self-limited inflammatory reaction to severe high-mortality disease state with multiple organ dysfunction syndrome (MODS) [118]. Chronic pancreatitis (CP), although lower in incidence, significantly reduces patients’ quality of life [119]. CP is characterized by inflammation, fibrosis, exocrine and/or endocrine insufficiency as well as chronic abdominal pain [120]. CP can be driven by recurrent acute pancreatitis and is a risk factor for the development of pancreatic ductal adenocarcinoma (PDAC) [121]. Gallstone and excessive alcohol use are the most common causes of AP. In addition to alcohol, genetics, obesity and smoking have been identified as important independent risk factors for pancreatitis [119]. However, the current therapeutic options in the treatment of both AP and CP are limited to supportive care and active therapies that can alter outcome and natural cause of these diseases are urgently needed. Several independent groups have shown that HO-1 activation and its downstream effectors (CO and biliverdin) have the ability to ameliorate experimental AP [26, 122, 123].
Hemeoxygenase-1 is upregulated both in animal models of AP [124] and hospitalized AP patients [125]. HO-1 is induced in both mild and severe experimental AP induced by cerulein and feeding a choline-deficient diet (CDD), respectively. Intraperitoneal hemin administration dramatically increases peritoneal and pancreas macrophages that overexpress HO-1 and was responsible for the beneficial therapeutic effect of hemin [126]. Pilot and proof-of-concept study in patients hospitalized with AP demonstrated that HO-1 was upregulated in peripheral blood mononuclear cells (PBMCs), primarily in monocytes, during the course of pancreatitis and decreased significantly upon recovery [125]. These findings showed an association between reversible monocyte HO-1 upregulation and clinical improvement of AP. Furthermore, the beneficial effect of Panhematin (FDA-approved hemin formulation) was demonstrated in established experimental AP, supporting a potential use of hemin in clinical trial for pancreatitis in the future [127]. More recently, HO-1-based gene therapy (adenoviral transfer of HO-1) showed beneficial effects in experimental severe AP [128]. Most of the studies demonstrate that HO-1 based therapy to be mediated mainly via macrophages. HO-1 is induced in macrophages as early as 2 h after intraperitoneal and intravenous hemin or Panhematin administration. Recruitment of monocytes/macrophages into the inflamed pancreas likely starts resolution phase of pancreatitis by decreasing pro-inflammatory cytokines (TNFα, IL-6 and IL-1β) and by increasing anti-inflammatory cytokines such as IL-10 [126, 127]. In this manner, HO-1 contributes to a shift of macrophage polarization from classically (M1) to alternative activated (M2) phenotype, which plays a critical role in anti-inflammation and tissue repair [129]. Interestingly, recently it was reported that the length of the GT repeats in the HO-1 gene promoter polymorphism is associated with the development of severe and necrotizing AP [44]. Patients with SS (GT) (n) genotype have higher HO-1 expression and may be protected against the development of severe pancreatic injury and fatal outcomes once the inflammatory response is initiated [44].
A wide array of evidence suggests that protective properties of HO-1 can also be mediated via its effector molecules—carbon monoxide (CO) and biliverdin (BV) [26, 122, 123]. CO deliverance is achieved mainly through inhalation and CO-RMs. CO or CO-RMs have been shown to protect against experimental AP via inhibition of NF-κB signaling [130]. Recently, our group showed that CO-RMs ameliorate experimental AP via inhibition of macrophage TLR4 expression and activation. To avoid potential side effects of systemic CO or CO-RMs, a novel therapeutic approach was taken by showing therapeutic potential of CORM-primed monocytes adoptive transfer in experimental AP [123]. Biliverdin is another product of HO-1 activation with promising future clinical use in AP. In a recent report, biliverdin attenuated experimental AP through activation of the aryl hydrocarbon receptor (AhR)/IL-22 signaling pathway [26].
Furthermore, specific induction of HO-1 might also be applicable for treatment of chronic pancreatitis. Pancreatic stellate cells (PSCs) play a cardinal role during pancreatic fibrosis development [131]. Therefore, the suppression of PSCs growth represents a therapeutic option for the treatment of pancreatic fibrosis. In addition, it was shown that HO-1 induction by curcumin inhibited pancreatic stellate cell proliferation [132]. These finding have been extended in a more recent report, in which PSCs proliferation was inhibited by CORM2 via activation of p38 MAPK/HO-1 pathway, indicating a therapeutic potential of CO carriers in the treatment of pancreatic fibrosis [133]. Although in vitro data implicate a potential influence of HO-1 on pathogenesis of CP, the function of HO-1 or its therapeutic use in development of CP still needs to be further confirmed by in vivo experimental and clinical studies, as the role of HO-1 + macrophages in CP remains unknown.
HO-1 and diseases of the liver
Liver damage due to I/R injury occurs often during shock and surgical procedures such as liver resection or transplantation. The use of CoPP, an HO-1 inducer, or gene therapy with a recombinant adenovirus encoding HO-1 cDNA can prevent severe I/R in a steatotic rat liver model of ex vivo cold I/R injury [134]. The protective effect of HO-1 was abrogated after administration of the HO-1 inhibitor ZnPP [134]. Similarly, in a mouse model of segmental hepatic I/R the administration of CoPP prior to the I/R protects the liver from hepatic parenchymal and apoptotic injury via a NF-κB pathway and induction of endoplasmic reticulum stress transcriptional C/EBP homologous protein (CHOP) [135]. It was also found that remote ischemic preconditioning (RIPC) protects against warm liver I/R injury via HO-1 upregulation [136]. In this model HO-1 induction by hemin increased autophagy via a p38-MAPK-mediated pathway, whereas HO-1 inhibition by ZnPP resulted in hepatic damage or cell death [136]. Similarly, HO-1 upregulation in the liver in response to sepsis or LPS stimulation limits hepatocyte death and protects against liver damage via autophagy [137]. This was confirmed with HO-1-deficient mice or pharmacologic inhibition of HO-1 by SnPP in which HO-1 deficiency resulted in interruption of autophagic signaling resulting in hepatic injury and apoptosis [137].
Orthotopic liver transplantation (OLT) is used as treatment for end-stage liver diseases [138]; however, I/R to the graft leads to allograft rejection a condition mediated by apoptosis via the CD95/FasL (CD95L) pathway [139]. In a rat model of OLT it was demonstrated that HO-1 overexpression after local adenoviral gene therapy impedes liver graft rejection by prevention of CD95L-mediated apoptosis, prolongs the allogeneic OLT survival and inhibits Th-1 type cytokines via the downstream CO signaling pathway [140]. Likewise, the expression of HO-1 in human liver transplants was correlated to I/R injury and graft function [141]. In a study that investigated the levels of HO-1 in human livers before and after transplantation, human livers that initially had low HO-1 levels and increased HO-1 expression further had less injury and better hepatobiliary function than those with initially high HO-1 expression. Thus an increase in HO-1 during transplantation is more protective than high HO-1 expression pre-transplantation [141]. In a clinical study of OLT, graft survival at 1 year was significantly better when the donor had the single nucleotide polymorphism A(-413)T genotype compared to TT-genotype corresponding to high and low HO-1 expression, respectively [142]. Graft loss due to primary dysfunction occurred more frequently in TT-genotype compared to A-receivers [142]. Recipients of a liver with TT-genotype had significantly higher serum transaminases after transplantation and lower hepatic HO-1 mRNA levels compared to the A-allele livers [142].
Hemeoxygenase-1 is also beneficial in liver fibrosis, a condition caused by activation of hepatic stellate cells (HSCs) resulting in a continuous wound healing response accompanied by inflammation and oxidative stress [143]. The severity of carbon tetrachloride (CCl4)-induced micronodular fibrosis was reduced using adenovirus-mediated gene transfer where HSCs were targeted to overexpress HO-1 [68]. HO-1 overexpression in HSCs controls the fibrogenic activities associated with CCl4-induced recurrent liver injury by impairing the proliferative ability of HSCs and reducing the transcript levels of type 1 collagen and transforming growth factor β1 [68].
HO-1 is also protective in models of immune- and alcohol-mediated liver injury [139, 144]. The immunologic cascade leading to liver damage can be studied with apoptotic liver damage models [145]. Upregulation of HO-1 by CoPP or adenoviral gene transfer protected mice from apoptotic liver damage induced by anti-CD95 antibody (ab) or d-galactosamine in combination with either anti-CD3 ab, LPS or TNF-α; whereas, HO-1 inhibition by SnPP abrogated the protective effect [144]. HO-1 mediated liver protection by reducing caspase-3 activation [144]. In addition, it was demonstrated that CO provided either as a gas, methylene chloride, or CO-RMs, contributed to prevention of apoptotic liver injury in vivo [144]. CO in cooperation with biliverdin prolongs survival and reduces the expression of Th-1 inflammatory cytokines TNF-α and IFN-γ in immune-mediated liver injury [139]. Alcoholic liver disease (ALD) is characterized by a wide spectrum of liver pathology including steatosis and cirrhosis [146]. HO-1 induction in human primary hepatocytes by hemin and quercetin, a flavonoid with potent hepatoprotective effects, protects from ethanol-induced oxidative damage via the MAPK/Nrf2 pathway [147]. HO-1 inhibition by ZnPP or MAPK signaling transduction inhibitor(s) not only significantly blocked the protection of quercetin, but also promoted ethanol-induced cytotoxicity [147]. Furthermore, it was determined that heme cleavage and CO release contributed to the protective effect through dose-dependent inhibition of ethanol-induced cytochrome P450 2E1 (CYP 2E1) activity and hepatotoxicity [148].
Potential therapeutic challenges
Despite the array of evidence supporting the beneficial effects of HO-1 upregulation in diverse animal models of diseases of the gastrointestinal tract; the inability to induce or block HO-1 upregulation and activity in humans by genetic or pharmacological means under similar conditions still remains a challenge. The beneficial effect of HO-1 upregulation has been proven in animal models with HO-1 inhibitors such as the metalloporphyrins (SnPP, SnMP and ZnPP), which exacerbate disease state. Nevertheless, there is not enough data to indicate that this is the case in humans. The data available on the use of metalloporphyrins in humans is on clinical studies that sought to inhibit HO-1 activity to treat excessive neonatal hyperbilirubinemia [149]. A comprehensive review on metalloporphyrins clinical studies by Schulz et al. [149] reports transient erythema and photosensitization as common side effects; however, there are no reports suggesting that gastrointestinal disorders manifest after HO-1 inhibition. The lack of attestation, by means of HO-1 inhibitors, that HO-1 upregulation is beneficial in the outcome of human disorders of the GI tract remains an area of further investigation. However, recent data showing HO-1 polymorphism association with disease or disease severity lend support to the potential protective roles of HO-1 in humans. The upregulation of HO-1 protein and activity in humans has been demonstrated in a clinical study using intravenous hemin [150]. In the first reported human study, hemin which is normally used for the treatment of diseases involving porphyrin metabolism was able to induce HO-1 protein concentration and activity in healthy volunteers [150]. The increase in HO-1 expression did not increase venous carboxyhemoglobin concentrations or induce significant changes in hematological, biochemical or coagulation parameters and resulted in minimal adverse side effects such a headaches and vomiting reported in two out of the ten subjects [150]. This study suggests that HO-1 upregulation in humans is possible, yet a more detailed study assessing a dose response followed by hemin treatment is necessary due to the involvement of HO-1 in a myriad of pathways. The modulation of HO-1 expression offers potential as a therapy for various human diseases but at the same time there are many impending side effects due to acute or chronic induction of HO-1, heme and the products of heme degradation. For instance, free heme can activate the inflammatory cascade [151] and biliverdin/bilirubin and CO can be toxic at high levels in tissues. Biliverdin which is rapidly converted to bilirubin is neurotoxic in newborns resulting in kernicterus [152] and CO can affect hemostasis by having both anticoagulant and procoagulant functions [153]. Thus, CO’s potential effects on coagulation are an important consideration for gastrointestinal diseases that are associated with vascular complication; for example, venous or arterial thromboembolism are both well-recognized extraintestinal complications in IBD [154]. Thus, an extensive assessment of the possible adverse effects should be carefully evaluated in order to determine the beneficial effects of HO-1 upregulation or its downstream effectors as therapy.
Conclusion
The HO-1 system plays an important role in many different pathways and its upregulation on different cell types is associated with the modulation of inflammatory diseases of the gastrointestinal tract (Fig. 1). It is well established that HO-1 prevents oxidative stress by removal of excess heme; in addition, HO-1 and heme degradation products work synergistically or in conjunction to modulate and dampen inflammatory and cytotoxic responses. The data obtained in recent years have started to elucidate the mechanisms by which HO-1 protects the gastrointestinal tract from inflammation and oxidative injury. Therefore, the regulation of HO-1 by pharmacological means, at the gene or cellular level offers a potential new therapeutic target for inflammatory gastrointestinal diseases. Although the data in favor of this idea is compelling, HO-1 mode of action requires further investigation particularly in humans. Most of the studies supporting the protective role of HO-1 have been performed in animal models, thus a better understanding of HO-1 activity and regulation in human studies will likely allow efficient translation of the protective roles of HO-1 and bring forth potential HO-1 therapeutic targets for clinical use in gastrointestinal diseases.
Fig. 1.
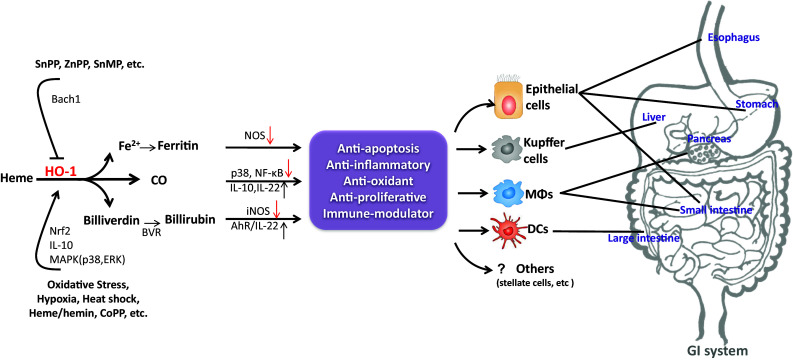
Hemeoxygenase-1 (HO-1) induction has a protective role in the gastrointestinal (GI) system. Upregulation of HO-1 and/or its downstream effectors in different cells and tissues is associated with protection against oxidative stress and tissue injury. AhR aryl hydrocarbon receptor, Bach1 heme-binding protein, BVR biliverdin reductase, CoPP cobalt protoporphyrin, DC dendritic cells, HO-1 hemeoxygenase-1, iNOS inducible nitric oxide synthase, MΦs macrophages, NOS nitric oxide synthase, Nrf2 nuclear erythroid 2-related factor-2, SnMP tin mesoporphyrin, SnPP tin protoporphyrin, ZnPP zinc protoporphyrin
Acknowledgments
This work was supported in part by the Robert Wood Johnson Foundation grant (to A.H.), the National Institutes of Health Grant DK092421 (to A.H.) and Digestive Disease Center grant DK56339 (to Stanford University).
References
- 1.Tenhunen R, Marver HS, Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci USA. 1968;61(2):748. doi: 10.1073/pnas.61.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kapitulnik J, Maines MD. Pleiotropic functions of biliverdin reductase: cellular signaling and generation of cytoprotective and cytotoxic bilirubin. Trends Pharmacol Sci. 2009;30(3):129–137. doi: 10.1016/j.tips.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997;37(1):517–554. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- 4.Mccoubrey WK, Huang T, Maines MD. Isolation and characterization of a cDNA from the rat brain that encodes hemoprotein heme oxygenase-3. Eur J Biochem. 1997;247(2):725–732. doi: 10.1111/j.1432-1033.1997.00725.x. [DOI] [PubMed] [Google Scholar]
- 5.Elbirt KK, Bonkovsky HL. Heme oxygenase: recent advances in understanding its regulation and role. Proc Assoc Am Phys. 1998;111(5):438–447. [PubMed] [Google Scholar]
- 6.Trakshel G, Kutty R, Maines M. Purification and characterization of the major constitutive form of testicular heme oxygenase. The noninducible isoform. J Biol Chem. 1986;261(24):11131–11137. [PubMed] [Google Scholar]
- 7.Maines MD (1992) Heme oxygenase: clinical applications and functions. CRC Press
- 8.Maines MD, Snyder R. New developments in the regulation of heme metabolism and their implications. CRC Crit Rev Toxicol. 1984;12(3):241–314. doi: 10.3109/10408448409021604. [DOI] [PubMed] [Google Scholar]
- 9.Tsuji T, Kato A, Yasuda H, Miyaji T, Luo J, Sakao Y, Ito H, Fujigaki Y, Hishida A. The dimethylthiourea-induced attenuation of cisplatin nephrotoxicity is associated with the augmented induction of heat shock proteins. Toxicol Appl Pharmacol. 2009;234(2):202–208. doi: 10.1016/j.taap.2008.09.031. [DOI] [PubMed] [Google Scholar]
- 10.Umeda K, Takahashi T, Inoue K, Shimizu H, Maeda S, Morimatsu H, Omori E, Akagi R, Katayama H, Morita K. Prevention of hemorrhagic shock-induced intestinal tissue injury by glutamine via heme oxygenase-1 induction. Shock. 2009;31(1):40–49. doi: 10.1097/SHK.0b013e318177823a. [DOI] [PubMed] [Google Scholar]
- 11.Cooper KL, Liu KJ, Hudson LG. Enhanced ROS production and redox signaling with combined arsenite and UVA exposure: contribution of NADPH oxidase. Free Radic Biol Med. 2009;47(4):381–388. doi: 10.1016/j.freeradbiomed.2009.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang AY, Chan JY, Cheng H-L, Tsai C-Y, Chan SH. Hypoxia-inducible factor 1/heme oxygenase 1 cascade as upstream signals in the prolife role of heat shock protein 70 at rostral ventrolateral medulla during experimental brain stem death. Shock. 2009;32(6):651–658. doi: 10.1097/SHK.0b013e3181a71027. [DOI] [PubMed] [Google Scholar]
- 13.Terry CM, Clikeman JA, Hoidal JR, Callahan KS. Effect of tumor necrosis factor-α and interleukin-1α on heme oxygenase-1 expression in human endothelial cells. Am J Physiol Heart Circ Physiol. 1998;274(3):H883–H891. doi: 10.1152/ajpheart.1998.274.3.H883. [DOI] [PubMed] [Google Scholar]
- 14.Niess AM, Passek F, Lorenz I, Schneider EM, Dickhuth H-H, Northoff H, Fehrenbach E. Expression of the antioxidant stress protein heme oxygenase-1 (HO-1) in human leukocytes: acute and adaptational responses to endurance exercise. Free Radic Biol Med. 1999;26(1):184–192. doi: 10.1016/s0891-5849(98)00192-0. [DOI] [PubMed] [Google Scholar]
- 15.Nath KA, Balla G, Vercellotti GM, Balla J, Jacob HS, Levitt M, Rosenberg ME. Induction of heme oxygenase is a rapid, protective response in rhabdomyolysis in the rat. J Clin Investig. 1992;90(1):267. doi: 10.1172/JCI115847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keyse SM, Applegate L, Tromvoukis Y, Tyrrell R. Oxidant stress leads to transcriptional activation of the human heme oxygenase gene in cultured skin fibroblasts. Mol Cell Biol. 1990;10(9):4967–4969. doi: 10.1128/mcb.10.9.4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Applegate LA, Luscher P, Tyrrell RM. Induction of heme oxygenase: a general response to oxidant stress in cultured mammalian cells. Cancer Res. 1991;51(3):974–978. [PubMed] [Google Scholar]
- 18.Abraham NG, Rezzani R, Rodella L, Kruger A, Taller D, Volti GL, Goodman AI, Kappas A. Overexpression of human heme oxygenase-1 attenuates endothelial cell sloughing in experimental diabetes. Am J Physiol Heart Circ Physiol. 2004;287(6):H2468–H2477. doi: 10.1152/ajpheart.01187.2003. [DOI] [PubMed] [Google Scholar]
- 19.Otterbein LE, Bach FH, Alam J, Soares M, Lu HT, Wysk M, Davis RJ, Flavell RA, Choi AM. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000;6(4):422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- 20.Petrache I, Otterbein LE, Alam J, Wiegand GW, Choi AM. Heme oxygenase-1 inhibits TNF-α-induced apoptosis in cultured fibroblasts. Am J Physiol-Lung Cell Mol Physiol. 2000;278(2):L312–L319. doi: 10.1152/ajplung.2000.278.2.L312. [DOI] [PubMed] [Google Scholar]
- 21.Lee T-S, Chau L-Y. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat Med. 2002;8(3):240–246. doi: 10.1038/nm0302-240. [DOI] [PubMed] [Google Scholar]
- 22.Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235(4792):1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- 23.Barañano DE, Rao M, Ferris CD, Snyder SH. Biliverdin reductase: a major physiologic cytoprotectant. Proc Natl Acad Sci. 2002;99(25):16093–16098. doi: 10.1073/pnas.252626999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sedlak TW, Snyder SH. Bilirubin benefits: cellular protection by a biliverdin reductase antioxidant cycle. Pediatrics. 2004;113(6):1776–1782. doi: 10.1542/peds.113.6.1776. [DOI] [PubMed] [Google Scholar]
- 25.Nakao A, Otterbein LE, Overhaus M, Sarady JK, Tsung A, Kimizuka K, Nalesnik MA, Kaizu T, Uchiyama T, Liu F. Biliverdin protects the functional integrity of a transplanted syngeneic small bowel. Gastroenterology. 2004;127(2):595–606. doi: 10.1053/j.gastro.2004.05.059. [DOI] [PubMed] [Google Scholar]
- 26.Xue J, Nguyen DT, Habtezion A. Aryl hydrocarbon receptor regulates pancreatic IL-22 production and protects mice from acute pancreatitis. Gastroenterology. 2012;143(6):1670–1680. doi: 10.1053/j.gastro.2012.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balla G, Jacob HS, Balla J, Rosenberg M, Nath K, Apple F, Eaton J, Vercellotti G. Ferritin: a cytoprotective antioxidant stratagem of endothelium. J Biol Chem. 1992;267(25):18148–18153. [PubMed] [Google Scholar]
- 28.Juckett MB, Balla J, Balla G, Jessurun J, Jacob HS, Vercellotti GM. Ferritin protects endothelial cells from oxidized low density lipoprotein in vitro. Am J Pathol. 1995;147(3):782. [PMC free article] [PubMed] [Google Scholar]
- 29.Kapturczak MH, Wasserfall C, Brusko T, Campbell-Thompson M, Ellis TM, Atkinson MA, Agarwal A. Heme oxygenase-1 modulates early inflammatory responses: evidence from the heme oxygenase-1-deficient mouse. Am J Pathol. 2004;165(3):1045–1053. doi: 10.1016/S0002-9440(10)63365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poss KD, Tonegawa S. Reduced stress defense in heme oxygenase 1-deficient cells. Proc Natl Acad Sci. 1997;94(20):10925–10930. doi: 10.1073/pnas.94.20.10925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tzima S, Victoratos P, Kranidioti K, Alexiou M, Kollias G. Myeloid heme oxygenase–1 regulates innate immunity and autoimmunity by modulating IFN-β production. J Exp Med. 2009;206(5):1167–1179. doi: 10.1084/jem.20081582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.True AL, Olive M, Boehm M, San H, Westrick RJ, Raghavachari N, Xu X, Lynn EG, Sack MN, Munson PJ. Heme oxygenase-1 deficiency accelerates formation of arterial thrombosis through oxidative damage to the endothelium, which is rescued by inhaled carbon monoxide. Circ Res. 2007;101(9):893–901. doi: 10.1161/CIRCRESAHA.107.158998. [DOI] [PubMed] [Google Scholar]
- 33.Vachharajani TJ, Work J, Issekutz AC, Granger DN. Heme oxygenase modulates selectin expression in different regional vascular beds. Am J Physiol Heart Circ Physiol. 2000;278(5):H1613–H1617. doi: 10.1152/ajpheart.2000.278.5.H1613. [DOI] [PubMed] [Google Scholar]
- 34.Burnett AL, Johns DG, Kriegsfeld LJ, Klein SL, Calvin DC, Demas GE, Schramm LP, Tonegawa S, Nelson RJ, Snyder SH. Ejaculatory abnormalities in mice with targeted disruption of the gene for heme oxygenase-2. Nat Med. 1998;4(1):84–87. doi: 10.1038/nm0198-084. [DOI] [PubMed] [Google Scholar]
- 35.Yachie A, Niida Y, Wada T, Igarashi N, Kaneda H, Toma T, Ohta K, Kasahara Y, Koizumi S. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J Clin Investig. 1999;103(1):129–135. doi: 10.1172/JCI4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Radhakrishnan N, Yadav SP, Sachdeva A, Pruthi PK, Sawhney S, Piplani T, Wada T, Yachie A. Human heme oxygenase-1 deficiency presenting with hemolysis, nephritis, and asplenia. J Pediatr Hematol Oncol. 2011;33(1):74–78. doi: 10.1097/MPH.0b013e3181fd2aae. [DOI] [PubMed] [Google Scholar]
- 37.Igarashi K, Sun J. The heme-Bach1 pathway in the regulation of oxidative stress response and erythroid differentiation. Antioxid Redox Signal. 2006;8(1–2):107–118. doi: 10.1089/ars.2006.8.107. [DOI] [PubMed] [Google Scholar]
- 38.Jang JS, Piao S, Cha Y-N, Kim C. Taurine chloramine activates Nrf2, increases HO-1 expression and protects cells from death caused by hydrogen peroxide. J Clin Biochem Nutr. 2009;45(1):37. doi: 10.3164/jcbn.08-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Backer O, Elinck E, Blanckaert B, Leybaert L, Motterlini R, Lefebvre RA. Water-soluble CO-releasing molecules reduce the development of postoperative ileus via modulation of MAPK/HO-1 signalling and reduction of oxidative stress. Gut. 2009;58(3):347–356. doi: 10.1136/gut.2008.155481. [DOI] [PubMed] [Google Scholar]
- 40.Salinas M, Wang J, Rosa de Sagarra M, Martín D, Rojo AI, Martin-Perez J, Ortiz de Montellano PR, Cuadrado A. Protein kinase Akt/PKB phosphorylates heme oxygenase-1 in vitro and in vivo. FEBS Lett. 2004;578(1):90–94. doi: 10.1016/j.febslet.2004.10.077. [DOI] [PubMed] [Google Scholar]
- 41.Exner M, Minar E, Wagner O, Schillinger M. The role of heme oxygenase-1 promoter polymorphisms in human disease. Free Radic Biol Med. 2004;37(8):1097–1104. doi: 10.1016/j.freeradbiomed.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 42.Ono K, Mannami T, Iwai N. Association of a promoter variant of the haeme oxygenase-1 gene with hypertension in women. J Hypertens. 2003;21(8):1497–1503. doi: 10.1097/00004872-200308000-00013. [DOI] [PubMed] [Google Scholar]
- 43.Ono K, Goto Y, Takagi S, Baba S, Tago N, Nonogi H, Iwai N. A promoter variant of the heme oxygenase-1 gene may reduce the incidence of ischemic heart disease in Japanese. Atherosclerosis. 2004;173(2):313–317. doi: 10.1016/j.atherosclerosis.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 44.Gulla A, Evans BJ, Navenot JM, Pundzius J, Barauskas G, Gulbinas A, Dambrauskas Z, Arafat H, Wang Z-X. Heme Oxygenase-1 Gene Promoter Polymorphism Is Associated With the Development of Necrotizing Acute Pancreatitis. Pancreas. 2014;43(8):1271–1276. doi: 10.1097/MPA.0000000000000171. [DOI] [PubMed] [Google Scholar]
- 45.Yamada N, Yamaya M, Okinaga S, Nakayama K, Sekizawa K, Shibahara S, Sasaki H. Microsatellite polymorphism in the heme oxygenase-1 gene promoter is associated with susceptibility to emphysema. Am J Human Genet. 2000;66(1):187–195. doi: 10.1086/302729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rueda B, Oliver J, Robledo G, López-Nevot MA, Balsa A, Pascual-Salcedo D, González-Gay MA, González-Escribano MF, Martín J. HO-1 promoter polymorphism associated with rheumatoid arthritis. Arthritis Rheum. 2007;56(12):3953–3958. doi: 10.1002/art.23048. [DOI] [PubMed] [Google Scholar]
- 47.Schillinger M, Exner M, Minar E, Mlekusch W, Müllner M, Mannhalter C, Bach FH, Wagner O. Heme oxygenase-1 genotype and restenosis after balloon angioplasty: a novel vascular protective factor. J Am Coll Cardiol. 2004;43(6):950–957. doi: 10.1016/j.jacc.2003.09.058. [DOI] [PubMed] [Google Scholar]
- 48.Chen Y-H, Lin S-J, Lin M-W, Tsai H-L, Kuo S-S, Chen J-W, Charng M-J, Wu T-C, Chen L-C, Ding P. Microsatellite polymorphism in promoter of heme oxygenase-1 gene is associated with susceptibility to coronary artery disease in type 2 diabetic patients. Hum Genet. 2002;111(1):1–8. doi: 10.1007/s00439-002-0769-4. [DOI] [PubMed] [Google Scholar]
- 49.Immenschuh S, Tan M, Ramadori G. Nitric oxide mediates the lipopolysaccharide dependent upregulation of the heme oxygenase-1 gene expression in cultured rat Kupffer cells. J Hepatol. 1999;30(1):61–69. doi: 10.1016/s0168-8278(99)80008-7. [DOI] [PubMed] [Google Scholar]
- 50.Bissell DM, Hammaker L, Schmid R. Liver sinusoidal cells Identification of a subpopulation for erythrocyte catabolism. J Cell Biol. 1972;54(1):107–119. doi: 10.1083/jcb.54.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chauveau C, Rémy S, Royer PJ, Hill M, Tanguy-Royer S, Hubert F-X, Tesson L, Brion R, Beriou G, Gregoire M. Heme oxygenase-1 expression inhibits dendritic cell maturation and proinflammatory function but conserves IL-10 expression. Blood. 2005;106(5):1694–1702. doi: 10.1182/blood-2005-02-0494. [DOI] [PubMed] [Google Scholar]
- 52.Wijayanti N, Huber S, Samoylenko A, Kietzmann T, Immenschuh S. Role of NF-kB and p38 MAP kinase signaling pathways in the lipopolysaccharide-dependent activation of heme oxygenase-1 gene expression. Antioxid Redox Signal. 2004;6(5):802–810. doi: 10.1089/ars.2004.6.802. [DOI] [PubMed] [Google Scholar]
- 53.Lin H-Y, Juan S-H, Shen S-C, Hsu F-L, Chen Y-C. Inhibition of lipopolysaccharide-induced nitric oxide production by flavonoids in RAW264. 7 macrophages involves heme oxygenase-1. Biochem Pharmacol. 2003;66(9):1821–1832. doi: 10.1016/s0006-2952(03)00422-2. [DOI] [PubMed] [Google Scholar]
- 54.Rémy S, Blancou P, Tesson L, Tardif V, Brion R, Royer PJ, Motterlini R, Foresti R, Painchaut M, Pogu S. Carbon monoxide inhibits TLR-induced dendritic cell immunogenicity. J Immunol. 2009;182(4):1877–1884. doi: 10.4049/jimmunol.0802436. [DOI] [PubMed] [Google Scholar]
- 55.George JF, Braun A, Brusko TM, Joseph R, Bolisetty S, Wasserfall CH, Atkinson MA, Agarwal A, Kapturczak MH. Suppression by CD4+ CD25+ regulatory t cells is dependent on expression of heme oxygenase-1 in antigen-presenting cells. Am J Pathol. 2008;173(1):154–160. doi: 10.2353/ajpath.2008.070963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moreau A, Hill M, Thebault P, Deschamps J, Chiffoleau E, Chauveau C, Moullier P, Anegon I, Alliot-Licht B, Cuturi M. Tolerogenic dendritic cells actively inhibit T cells through heme oxygenase-1 in rodents and in nonhuman primates. FASEB J. 2009;23(9):3070–3077. doi: 10.1096/fj.08-128173. [DOI] [PubMed] [Google Scholar]
- 57.Nakamichi I, Habtezion A, Zhong B, Contag CH, Butcher EC, Omary MB. Hemin-activated macrophages home to the pancreas and protect from acute pancreatitis via heme oxygenase-1 induction. J Clin Investig. 2005;115(11):3007–3014. doi: 10.1172/JCI24912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wegiel B, Hedblom A, Li M, Gallo D, Csizmadia E, Harris C, Nemeth Z, Zuckerbraun B, Soares M, Persson JL. Heme oxygenase-1 derived carbon monoxide permits maturation of myeloid cells. Cell Death Dis. 2014;5(3):e1139. doi: 10.1038/cddis.2014.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Choi KM, Kashyap PC, Dutta N, Stoltz GJ, Ordog T, Shea Donohue T, Bauer AJ, Linden DR, Szurszewski JH, Gibbons SJ. CD206-positive M2 macrophages that express heme oxygenase-1 protect against diabetic gastroparesis in mice. Gastroenterology. 2010;138(7):2399–2409. doi: 10.1053/j.gastro.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Coëffier M, Le Pessot F, Leplingard A, Marion R, Lerebours E, Ducrotté P, Déchelotte P. Acute enteral glutamine infusion enhances heme oxygenase-1 expression in human duodenal mucosa. J Nutr. 2002;132(9):2570–2573. doi: 10.1093/jn/132.9.2570. [DOI] [PubMed] [Google Scholar]
- 61.Barton S, Rampton D, Winrow V, Domizio P, Feakins R. Expression of heat shock protein 32 (hemoxygenase-1) in the normal and inflamed human stomach and colon: an immunohistochemical study. Cell Stress Chaperones. 2003;8(4):329. doi: 10.1379/1466-1268(2003)008<0329:eohsph>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goda N, Suzuki K, Naito M, Takeoka S, Tsuchida E, Ishimura Y, Tamatani T, Suematsu M. Distribution of heme oxygenase isoforms in rat liver. Topographic basis for carbon monoxide-mediated microvascular relaxation. J Clin Investig. 1998;101(3):604. doi: 10.1172/JCI1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guo J, Cho C, Wang J, Koo M. Expression and immunolocalization of heat shock proteins in the healing of gastric ulcers in rats. Scand J Gastroenterol. 2002;37(1):17–22. doi: 10.1080/003655202753387293. [DOI] [PubMed] [Google Scholar]
- 64.Giriş M, Erbil Y, Doğru-Abbasoğlu S, Yanık BT, Alış H, Olgaç V, Toker GA. The effect of heme oxygenase-1 induction by glutamine on TNBS-induced colitis. Int J Colorectal Dis. 2007;22(6):591–599. doi: 10.1007/s00384-006-0238-y. [DOI] [PubMed] [Google Scholar]
- 65.Paul G, Bataille F, Obermeier F, Bock J, Klebl F, Strauch U, Lochbaum D, Rümmele P, Farkas S, Schölmerich J. Analysis of intestinal haem-oxygenase-1 (HO-1) in clinical and experimental colitis. Clin Exp Immunol. 2005;140(3):547–555. doi: 10.1111/j.1365-2249.2005.02775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang W, Guo X, Koo M, Wong B, Lam S, Ye Y, Cho C. Protective role of heme oxygenase-1 on trinitrobenzene sulfonic acid-induced colitis in rats. American Journal of Physiology-Gastrointestinal and Liver. Physiology. 2001;281(2):G586–G594. doi: 10.1152/ajpgi.2001.281.2.G586. [DOI] [PubMed] [Google Scholar]
- 67.Takagi T, Naito Y, Mizushima K, Nukigi Y, Okada H, Suzuki T, Hirata I, Omatsu T, Okayama T, Handa O. Increased intestinal expression of heme oxygenase-1 and its localization in patients with ulcerative colitis. J Gastroenterol Hepatol. 2008;23:S229–S233. doi: 10.1111/j.1440-1746.2008.05443.x. [DOI] [PubMed] [Google Scholar]
- 68.Tsui TY, Lau CK, Ma J, Wu X, Wang YQ, Farkas S, Xu R, Schlitt HJ, Fan ST. rAAV-mediated stable expression of heme oxygenase-1 in stellate cells: a new approach to attenuate liver fibrosis in rats. Hepatology. 2005;42(2):335–342. doi: 10.1002/hep.20803. [DOI] [PubMed] [Google Scholar]
- 69.Werkström V, Ny L, Persson K, Andersson KE. Carbon monoxide-induced relaxation and distribution of haem oxygenase isoenzymes in the pig urethra and lower oesophagogastric junction. Br J Pharmacol. 1997;120(2):312–318. doi: 10.1038/sj.bjp.0700893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ny L, Alm P, Ekström P, Larsson B, Grundemar L, Andersson KE. Localization and activity of haem oxygenase and functional effects of carbon monoxide in the feline lower oesophageal sphincter. Br J Pharmacol. 1996;118(2):392–399. doi: 10.1111/j.1476-5381.1996.tb15415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lillemoe K, Johnson L, Harmon J. Role of the components of the gastroduodenal contents in experimental acid esophagitis. Surgery. 1982;92(2):276–284. [PubMed] [Google Scholar]
- 72.Kruel CRP, Pinto LFR, Blanco TCM, Barja-Fidalgo TC, Melo LL, Kruel CDP. Evaluation of the heme oxygenase-1 expression in esophagitis and esophageal cancer induced by different reflux experimental models and diethylnitrosamine. Acta Cirurgica Brasileira. 2010;25(3):304–310. doi: 10.1590/s0102-86502010000300015. [DOI] [PubMed] [Google Scholar]
- 73.Bjorkman D. Nonsteroidal anti-inflammatory drug-associated toxicity of the liver, lower gastrointestinal tract, and esophagus. Am J Med. 1998;105(5):17S–21S. doi: 10.1016/s0002-9343(98)00276-9. [DOI] [PubMed] [Google Scholar]
- 74.Uc A, Zhu X, Wagner BA, Buettner GR, Berg DJ. Heme oxygenase-1 is protective against nonsteroidal anti-inflammatory drug–induced gastric ulcers. J Pediatr Gastroenterol Nutr. 2012;54(4):471–476. doi: 10.1097/MPG.0b013e3182334fdf. [DOI] [PubMed] [Google Scholar]
- 75.Aburaya M, Tanaka K-I, Hoshino T, Tsutsumi S, Suzuki K, Makise M, Akagi R, Mizushima T. Heme oxygenase-1 protects gastric mucosal cells against non-steroidal anti-inflammatory drugs. J Biol Chem. 2006;281(44):33422–33432. doi: 10.1074/jbc.M602074200. [DOI] [PubMed] [Google Scholar]
- 76.Cheng Y-T, Wu C-H, Ho C-Y, Yen G-C. Catechin protects against ketoprofen-induced oxidative damage of the gastric mucosa by up-regulating Nrf2 in vitro and in vivo . J Nutr Biochem. 2013;24(2):475–483. doi: 10.1016/j.jnutbio.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 77.Ueda K, Ueyama T, Oka M, Ito T, Tsuruo Y, Ichinose M. Polaprezinc (Zinc L-carnosine) is a potent inducer of anti-oxidative stress enzyme, heme oxygenase (HO)-1-a new mechanism of gastric mucosal protection. J Pharmacol Sci. 2009;110(3):285–294. doi: 10.1254/jphs.09056fp. [DOI] [PubMed] [Google Scholar]
- 78.Choi E-J, Oh H-M, Na B-R, Ramesh T, Lee H-J, Choi C-S, Choi S-C, Oh T-Y, Choi S-J, Chae J-R. Eupatilin protects gastric epithelial cells from oxidative damage and down-regulates genes responsible for the cellular oxidative stress. Pharm Res. 2008;25(6):1355–1364. doi: 10.1007/s11095-008-9531-5. [DOI] [PubMed] [Google Scholar]
- 79.Takagi T, Naito Y, Okada H, Ishii T, Mizushima K, Akagiri S, Adachi S, Handa O, Kokura S, Ichikawa H. Lansoprazole, a proton pump inhibitor, mediates anti-inflammatory effect in gastric mucosal cells through the induction of heme oxygenase-1 via activation of NF-E2-related factor 2 and oxidation of kelch-like ECH-associating protein 1. J Pharmacol Exp Ther. 2009;331(1):255–264. doi: 10.1124/jpet.109.152702. [DOI] [PubMed] [Google Scholar]
- 80.Song HJ, Shin CY, Oh TY, Min YS, Park ES, Sohn UD. Eupatilin with heme oxygenase-1-inducing ability protects cultured feline esophageal epithelial cells from cell damage caused by indomethacin. Biol Pharm Bull. 2009;32(4):589–596. doi: 10.1248/bpb.32.589. [DOI] [PubMed] [Google Scholar]
- 81.Kadinov B, Itzev D, Gagov H, Christova T, Bolton T, Duridanova D. Induction of heme oxygenase in guinea-pig stomach: roles in contraction and in single muscle cell ionic currents. Acta Physiol Scand. 2002;175(4):297–313. doi: 10.1046/j.1365-201X.2002.00995.x. [DOI] [PubMed] [Google Scholar]
- 82.Choi KM, Gibbons SJ, Nguyen TV, Stoltz GJ, Lurken MS, Ordog T, Szurszewski JH, Farrugia G. Heme oxygenase-1 protects interstitial cells of Cajal from oxidative stress and reverses diabetic gastroparesis. Gastroenterology. 2008;135(6):2055–2064. doi: 10.1053/j.gastro.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Takagi T, Naito Y, Mizushima K, Nukigi Y, Okada H, Suzuki T, Hirata I, Omatsu T, Okayama T, Handa O. Increased intestinal expression of heme oxygenase-1 and its localization in patients with ulcerative colitis. J Gastroenterol Hepatol. 2008;23(s2):S229–S233. doi: 10.1111/j.1440-1746.2008.05443.x. [DOI] [PubMed] [Google Scholar]
- 84.Higuchi K, Yoda Y, Amagase K, Kato S, Tokioka S, Murano M, Takeuchi K, Umegaki E. Prevention of NSAID-induced small intestinal mucosal injury: prophylactic potential of lansoprazole. J Clin Biochem Nutr. 2009;45(2):125. doi: 10.3164/jcbn.SR09-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yoda Y, Amagase K, Kato S, Tokioka S, Murano M, Kakimoto K, Nishio H, Umegaki E, Takeuchi K, Higuchi K. Prevention by lansoprazole, a proton pump inhibitor, of indomethacin-induced small intestinal ulceration in rats through induction of heme oxygenase-1. J Physiol Pharmacol. 2010;61(3):287. [PubMed] [Google Scholar]
- 86.Yeh CT, Chiu HF, Yen GC. Protective effect of sulforaphane on indomethacin-induced cytotoxicity via heme oxygenase-1 expression in human intestinal Int 407 cells. Mol Nutr Food Res. 2009;53(9):1166–1176. doi: 10.1002/mnfr.200800558. [DOI] [PubMed] [Google Scholar]
- 87.Chung SW, Liu X, Macias AA, Baron RM, Perrella MA. Heme oxygenase-1–derived carbon monoxide enhances the host defense response to microbial sepsis in mice. J Clin Investig. 2008;118(1):239–247. doi: 10.1172/JCI32730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tamion F, Richard V, Lacoume Y, Thuillez C. Intestinal preconditioning prevents systemic inflammatory response in hemorrhagic shock. Role of HO-1. Am J Physiol-Gastrointest Liver Physiol. 2002;283(2):G408–G414. doi: 10.1152/ajpgi.00348.2001. [DOI] [PubMed] [Google Scholar]
- 89.Attuwaybi B, Kozar R, Moore-Olufemi S, Sato N, Hassoun H, Weisbrodt N, Moore F. Heme oxygenase-1 induction by hemin protects against gut ischemia/reperfusion injury. J Surg Res. 2004;118(1):53–57. doi: 10.1016/j.jss.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 90.Mallick IH, Winslet MC, Seifalian AM. Ischemic preconditioning of small bowel mitigates the late phase of reperfusion injury: heme oxygenase mediates cytoprotection. Am J Surg. 2010;199(2):223–231. doi: 10.1016/j.amjsurg.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 91.Wasserberg N, Pileggi A, Salgar SK, Ruiz P, Ricordi C, Inverardi L, Tzakis AG. Heme oxygenase-1 upregulation protects against intestinal ischemia/reperfusion injury: a laboratory based study. Int J Surg. 2007;5(4):216–224. doi: 10.1016/j.ijsu.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 92.Tamaki T, Konoeda Y, Yasuhara M, Tanaka M, Yokota N, Hayashi T, Katori M, Uchida Y, Kawamura A (1999) Glutamine-induced heme oxygenase-1 protects intestines and hearts from warm ischemic injury. In: Transplantation proceedings, vol 1. Elsevier, pp 1018–1019 [DOI] [PubMed]
- 93.Mallick IH, Yang W-X, Winslet MC, Seifalian AM. Pyrrolidine dithiocarbamate reduces ischemia-reperfusion injury of the small intestine. World J Gastroenterol. 2005;11(46):7308. doi: 10.3748/wjg.v11.i46.7308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yoshida T, Maulik N, Ho Y-S, Alam J, Das DK. Hmox-1 constitutes an adaptive response to effect antioxidant cardio protection a study with transgenic mice heterozygous for targeted disruption of the heme oxygenase-1 gene. Circulation. 2001;103(12):1695–1701. doi: 10.1161/01.cir.103.12.1695. [DOI] [PubMed] [Google Scholar]
- 95.Liu X, Wei J, Peng DH, Layne MD, Yet S-F. Absence of heme oxygenase-1 exacerbates myocardial ischemia/reperfusion injury in diabetic mice. Diabetes. 2005;54(3):778–784. doi: 10.2337/diabetes.54.3.778. [DOI] [PubMed] [Google Scholar]
- 96.Yano Y, Ozono R, Oishi Y, Kambe M, Yoshizumi M, Ishida T, Omura S, Oshima T, Igarashi K. Genetic ablation of the transcription repressor Bach1 leads to myocardial protection against ischemia/reperfusion in mice. Genes Cells. 2006;11(7):791–803. doi: 10.1111/j.1365-2443.2006.00979.x. [DOI] [PubMed] [Google Scholar]
- 97.Scott JR, Cukiernik MA, Ott MC, Bihari A, Badhwar A, Gray DK, Harris KA, Parry NG, Potter RF. Low-dose inhaled carbon monoxide attenuates the remote intestinal inflammatory response elicited by hindlimb ischemia–reperfusion. Am J Physiol-Gastrointest Liver Physiol. 2009;296(1):G9–G14. doi: 10.1152/ajpgi.90243.2008. [DOI] [PubMed] [Google Scholar]
- 98.Nakao A, Kaczorowski DJ, Sugimoto R, Billiar TR, McCurry KR. Application of heme oxygenase-1, carbon monoxide and biliverdin for the prevention of intestinal ischemia/reperfusion injury. J Clin Biochem Nutr. 2008;42(2):78. doi: 10.3164/jcbn.2008013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nakao A, Kimizuka K, Stolz DB, Neto JS, Kaizu T, Choi AM, Uchiyama T, Zuckerbraun BS, Nalesnik MA, Otterbein LE. Carbon monoxide inhalation protects rat intestinal grafts from ischemia/reperfusion injury. Am J Pathol. 2003;163(4):1587–1598. doi: 10.1016/S0002-9440(10)63515-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fondevila C, Shen XD, Tsuchiyashi S, Yamashita K, Csizmadia E, Lassman C, Busuttil RW, Kupiec-Weglinski JW, Bach FH. Biliverdin therapy protects rat livers from ischemia and reperfusion injury. Hepatology. 2004;40(6):1333–1341. doi: 10.1002/hep.20480. [DOI] [PubMed] [Google Scholar]
- 101.Yun KJ, Choi SC, Oh JM. Expression of heme oxygenase-1 in ischemic colitis. Korean J Gastroenterol (Taehan Sohwagi Hakhoe chi) 2005;45(5):335–339. [PubMed] [Google Scholar]
- 102.Gasche C, Scholmerich J, Brynskov J, D’Haens G, Hanauer SB, Irvine EJ, Jewell DP, Rachmilewitz D, Sachar DB, Sandborn WJ. A simple classification of Crohn’s disease: report of the working party for the World Congresses of Gastroenterology, Vienna 1998. Inflamm Bowel Dis. 2000;6(1):8–15. doi: 10.1097/00054725-200002000-00002. [DOI] [PubMed] [Google Scholar]
- 103.Naito Y, Takagi T, Yoshikawa T. Heme oxygenase-1: a new therapeutic target for inflammatory bowel disease. Aliment Pharmacol Ther. 2004;20:177–184. doi: 10.1111/j.1365-2036.2004.01992.x. [DOI] [PubMed] [Google Scholar]
- 104.Zhong W, Xia Z, Hinrichs D, Rosenbaum JT, Wegmann KW, Meyrowitz J, Zhang Z. Hemin Exerts Multiple Protective Mechanisms and Attenuates Dextran Sulfate Sodium–induced Colitis. J Pediatr Gastroenterol Nutr. 2010;50(2):132–139. doi: 10.1097/MPG.0b013e3181c61591. [DOI] [PubMed] [Google Scholar]
- 105.Varga C, Laszlo F, Fritz P, Cavicchi M, Lamarque D, Horvath K, Posa A, Berko A, Whittle BJ. Modulation by heme and zinc protoporphyrin of colonic heme oxygenase-1 and experimental inflammatory bowel disease in the rat. Eur J Pharmacol. 2007;561(1):164–171. doi: 10.1016/j.ejphar.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 106.Berberat PO, Yamashita K, Warny MM, Csizmadia E, Robson SC, Bach FH. Heme oxygenase-1-generated biliverdin ameliorates experimental murine colitis. Inflamm Bowel Dis. 2005;11(4):350–359. doi: 10.1097/01.mib.0000164017.06538.8a. [DOI] [PubMed] [Google Scholar]
- 107.Khor TO, Huang M-T, Kwon KH, Chan JY, Reddy BS, Kong A-N. Nrf2-deficient mice have an increased susceptibility to dextran sulfate sodium–induced colitis. Cancer Res. 2006;66(24):11580–11584. doi: 10.1158/0008-5472.CAN-06-3562. [DOI] [PubMed] [Google Scholar]
- 108.Harusato A, Naito Y, Takagi T, Uchiyama K, Mizushima K, Hirai Y, Higashimura Y, Katada K, Handa O, Ishikawa T. BTB and CNC homolog 1 (Bach1) deficiency ameliorates TNBS colitis in mice: role of M2 macrophages and heme oxygenase-1. Inflamm Bowel Dis. 2013;19(4):740–753. doi: 10.1097/MIB.0b013e3182802968. [DOI] [PubMed] [Google Scholar]
- 109.Hegazi RA, Rao KN, Mayle A, Sepulveda AR, Otterbein LE, Plevy SE. Carbon monoxide ameliorates chronic murine colitis through a heme oxygenase 1–dependent pathway. J Exp Med. 2005;202(12):1703–1713. doi: 10.1084/jem.20051047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sheikh SZ, Hegazi RA, Kobayashi T, Onyiah JC, Russo SM, Matsuoka K, Sepulveda AR, Li F, Otterbein LE, Plevy SE. An anti-inflammatory role for carbon monoxide and heme oxygenase-1 in chronic Th2-mediated murine colitis. J Immunol. 2011;186(9):5506. doi: 10.4049/jimmunol.1002433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Horváth K, Varga C, Berkó A, Pósa A, László F, Whittle BJ. The involvement of heme oxygenase-1 activity in the therapeutic actions of 5-aminosalicylic acid in rat colitis. Eur J Pharmacol. 2008;581(3):315–323. doi: 10.1016/j.ejphar.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 112.Schulz S, Wong RJ, Jang KY, Kalish F, Chisholm KM, Zhao H, Vreman HJ, Sylvester KG, Stevenson DK. Heme oxygenase-1 deficiency promotes the development of necrotizing enterocolitis-like intestinal injury in a newborn mouse model. Am J Physiol-Gastrointest Liver Physiol. 2013;304(11):G991–G1001. doi: 10.1152/ajpgi.00363.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Santulli TV, Schullinger JN, Heird WC, Gongaware RD, Wigger J, Barlow B, Blanc WA, Berdon WE. Acute necrotizing enterocolitis in infancy: a review of 64 cases. Pediatrics. 1975;55(3):376–387. [PubMed] [Google Scholar]
- 114.Yeoh E, Horowitz M. Radiation enteritis. Surg Gynecol Obstet. 1987;165(4):373–379. [PubMed] [Google Scholar]
- 115.Giriş M, Erbil Y, Öztezcan S, Olgaç V, Barbaros U, Deveci U, Kirgiz B, Uysal M, Toker GA. The effect of heme oxygenase-1 induction by glutamine on radiation-induced intestinal damage: the effect of heme oxygenase-1 on radiation enteritis. Am J Surg. 2006;191(4):503–509. doi: 10.1016/j.amjsurg.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 116.Wang J, Zheng H, Sung C-C, Hauer-Jensen M. The synthetic somatostatin analogue, octreotide, ameliorates acute and delayed intestinal radiation injury. Int J Radiat Oncol Biol Phys. 1999;45(5):1289–1296. doi: 10.1016/s0360-3016(99)00293-x. [DOI] [PubMed] [Google Scholar]
- 117.Abbasoğlu SD, Erbil Y, Eren T, Giriş M, Barbaros U, Yücel R, Olgaç V, Uysal M, Toker G. The effect of heme oxygenase-1 induction by octreotide on radiation enteritis. Peptides. 2006;27(6):1570–1576. doi: 10.1016/j.peptides.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 118.Xue J, Sharma V, Habtezion A. Immune cells and immune-based therapy in pancreatitis. Immunol Res. 2014;58(2–3):378–386. doi: 10.1007/s12026-014-8504-5. [DOI] [PubMed] [Google Scholar]
- 119.Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144(6):1252–1261. doi: 10.1053/j.gastro.2013.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sarles H. Etiopathogenesis and definition of chronic pancreatitis. Dig Dis Sci. 1986;31(9 Suppl):91S–107S. doi: 10.1007/BF01295992. [DOI] [PubMed] [Google Scholar]
- 121.Zheng L, Xue J, Jaffee EM, Habtezion A. Role of immune cells and immune-based therapies in pancreatitis and pancreatic ductal adenocarcinoma. Gastroenterology. 2013;144(6):1230–1240. doi: 10.1053/j.gastro.2012.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nuhn P, Mitkus T, Ceyhan GO, Kunzli BM, Bergmann F, Fischer L, Giese N, Friess H, Berberat PO. Heme oxygenase 1-generated carbon monoxide and biliverdin attenuate the course of experimental necrotizing pancreatitis. Pancreas. 2013;42(2):265–271. doi: 10.1097/MPA.0b013e318264cc8b. [DOI] [PubMed] [Google Scholar]
- 123.Xue J, Habtezion A. Carbon monoxide-based therapy ameliorates acute pancreatitis via TLR4 inhibition. J Clin Invest. 2014;124(1):437–447. doi: 10.1172/JCI71362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sato H, Siow RC, Bartlett S, Taketani S, Ishii T, Bannai S, Mann GE. Expression of stress proteins heme oxygenase-1 and -2 in acute pancreatitis and pancreatic islet betaTC3 and acinar AR42J cells. FEBS Lett. 1997;405(2):219–223. doi: 10.1016/s0014-5793(97)00191-9. [DOI] [PubMed] [Google Scholar]
- 125.Habtezion A, Kwan R, Yang AL, Morgan ME, Akhtar E, Wanaski SP, Collins SD, Butcher EC, Kamal A, Omary MB. Heme oxygenase-1 is induced in peripheral blood mononuclear cells of patients with acute pancreatitis: a potential therapeutic target. Am J Physiol Gastrointest Liver Physiol. 2011;300(1):G12–G20. doi: 10.1152/ajpgi.00231.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nakamichi I, Habtezion A, Zhong B, Contag CH, Butcher EC, Omary MB. Hemin-activated macrophages home to the pancreas and protect from acute pancreatitis via heme oxygenase-1 induction. J Clin Invest. 2005;115(11):3007–3014. doi: 10.1172/JCI24912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Habtezion A, Kwan R, Akhtar E, Wanaski SP, Collins SD, Wong RJ, Stevenson DK, Butcher EC, Omary MB. Panhematin provides a therapeutic benefit in experimental pancreatitis. Gut. 2011;60(5):671–679. doi: 10.1136/gut.2010.217208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang F, Fei J, Zhao B, Chen E, Mao E. Protective effect of adenoviral transfer of heme oxygenase-1 gene on rats with severe acute pancreatitis. Am J Med Sci. 2014 doi: 10.1097/MAJ.0000000000000225. [DOI] [PubMed] [Google Scholar]
- 129.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3(1):23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 130.Chen P, Sun B, Chen H, Wang G, Pan S, Kong R, Bai X, Wang S. Effects of carbon monoxide releasing molecule-liberated CO on severe acute pancreatitis in rats. Cytokine. 2010;49(1):15–23. doi: 10.1016/j.cyto.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 131.Omary MB, Lugea A, Lowe AW, Pandol SJ. The pancreatic stellate cell: a star on the rise in pancreatic diseases. J Clin Invest. 2007;117(1):50–59. doi: 10.1172/JCI30082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Schwer CI, Guerrero AM, Humar M, Roesslein M, Goebel U, Stoll P, Geiger KK, Pannen BH, Hoetzel A, Schmidt R. Heme oxygenase-1 inhibits the proliferation of pancreatic stellate cells by repression of the extracellular signal-regulated kinase1/2 pathway. J Pharmacol Exp Ther. 2008;327(3):863–871. doi: 10.1124/jpet.108.136549. [DOI] [PubMed] [Google Scholar]
- 133.Schwer CI, Mutschler M, Stoll P, Goebel U, Humar M, Hoetzel A, Schmidt R. Carbon monoxide releasing molecule-2 inhibits pancreatic stellate cell proliferation by activating p38 mitogen-activated protein kinase/heme oxygenase-1 signaling. Mol Pharmacol. 2010;77(4):660–669. doi: 10.1124/mol.109.059519. [DOI] [PubMed] [Google Scholar]
- 134.Amersi F, Buelow R, Kato H, Ke B, Coito AJ, Shen X-D, Zhao D, Zaky J, Melinek J, Lassman CR. Upregulation of heme oxygenase-1 protects genetically fat Zucker rat livers from ischemia/reperfusion injury. J Clin Investig. 1999;104(11):1631–1639. doi: 10.1172/JCI7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ben-Ari Z, Issan Y, Katz Y, Sultan M, Safran M, Michal L-S, Nader GA, Kornowski R, Grief F, Pappo O. Induction of heme oxygenase-1 protects mouse liver from apoptotic ischemia/reperfusion injury. Apoptosis. 2013;18(5):547–555. doi: 10.1007/s10495-013-0814-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang Y, Shen J, Xiong X, Xu Y, Zhang H, Huang C, Tian Y, Jiao C, Wang X, Li X. Remote Ischemic Preconditioning Protects against Liver Ischemia-Reperfusion Injury via Heme Oxygenase-1-Induced Autophagy. PLoS ONE. 2014;9(6):e98834. doi: 10.1371/journal.pone.0098834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Carchman EH, Rao J, Loughran PA, Rosengart MR, Zuckerbraun BS. Heme oxygenase-1–mediated autophagy protects against hepatocyte cell death and hepatic injury from infection/sepsis in mice. Hepatology. 2011;53(6):2053–2062. doi: 10.1002/hep.24324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Starzl TE, Demetris AJ. Liver transplantation: a 31-year perspective part III. Curr Probl Surg. 1990;27(4):187–240. doi: 10.1016/0011-3840(90)90017-y. [DOI] [PubMed] [Google Scholar]
- 139.Sass G, Seyfried S, Parreira Soares M, Yamashita K, Kaczmarek E, Neuhuber WL, Tiegs G. Cooperative effect of biliverdin and carbon monoxide on survival of mice in immune-mediated liver injury. Hepatology. 2004;40(5):1128–1135. doi: 10.1002/hep.20450. [DOI] [PubMed] [Google Scholar]
- 140.Ke B, Buelow R, Shen X-D, Melinek J, Amersi F, Gao F, Ritter T, Volk H-D, Busuttil RW, Kupiec-Weglinski JW. Heme oxygenase 1 gene transfer prevents CD95/Fas ligand-mediated apoptosis and improves liver allograft survival via carbon monoxide signaling pathway. Hum Gene Ther. 2002;13(10):1189–1199. doi: 10.1089/104303402320138970. [DOI] [PubMed] [Google Scholar]
- 141.Geuken E, Buis CI, Visser DS, Blokzijl H, Moshage H, Nemes B, Leuvenink HG, De Jong KP, Peeters PM, Slooff MJ. Expression of Heme Oxygenase-1 in Human Livers Before Transplantation Correlates with Graft Injury and Function After Transplantation. Am J Transplant. 2005;5(8):1875–1885. doi: 10.1111/j.1600-6143.2005.00960.x. [DOI] [PubMed] [Google Scholar]
- 142.Buis CI, van der Steege G, Visser DS, Nolte IM, Hepkema BG, Nijsten M, Slooff MJ, Porte RJ. Heme Oxygenase-1 Genotype of the Donor Is Associated With Graft Survival After Liver Transplantation. Am J Transplant. 2008;8(2):377–385. doi: 10.1111/j.1600-6143.2007.02048.x. [DOI] [PubMed] [Google Scholar]
- 143.Flier JS, Underhill LH, Friedman SL. The Cellular Basis of Hepatic Fibrosis-Mechanisms and Treatment Strategies. N Engl J Med. 1993;328(25):1828–1835. doi: 10.1056/NEJM199306243282508. [DOI] [PubMed] [Google Scholar]
- 144.Sass G, Soares MCP, Yamashita K, Seyfried S, Zimmermann WH, Eschenhagen T, Kaczmarek E, Ritter T, Volk HD, Tiegs G. Heme oxygenase-1 and its reaction product, carbon monoxide, prevent inflammation-related apoptotic liver damage in mice. Hepatology. 2003;38(4):909–918. doi: 10.1053/jhep.2003.50386. [DOI] [PubMed] [Google Scholar]
- 145.Wendela A ICE-protease inhibitors block murine liver injury and apoptosis caused by CD95 or by TNF-a [DOI] [PubMed]
- 146.Dey A, Cederbaum AI. Alcohol and oxidative liver injury. Hepatology. 2006;43(S1):S63–S74. doi: 10.1002/hep.20957. [DOI] [PubMed] [Google Scholar]
- 147.Yao P, Nussler A, Liu L, Hao L, Song F, Schirmeier A, Nussler N. Quercetin protects human hepatocytes from ethanol-derived oxidative stress by inducing heme oxygenase-1 via the MAPK/Nrf2 pathways. J Hepatol. 2007;47(2):253–261. doi: 10.1016/j.jhep.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 148.Yao P, Hao L, Nussler N, Lehmann A, Song F, Zhao J, Neuhaus P, Liu L, Nussler A. The protective role of HO-1 and its generated products (CO, bilirubin, and Fe) in ethanol-induced human hepatocyte damage. Am J Physiol-Gastrointest Liver Physiol. 2009;296(6):G1318–G1323. doi: 10.1152/ajpgi.00555.2007. [DOI] [PubMed] [Google Scholar]
- 149.Schulz S, Wong RJ, Vreman HJ, Stevenson DK (2012) Metalloporphyrins—an update. Front Pharmacol 3 [DOI] [PMC free article] [PubMed]
- 150.Bharucha AE, Kulkarni A, Choi KM, Camilleri M, Lempke M, Brunn GJ, Gibbons SJ, Zinsmeister AR, Farrugia G. First-in-human study demonstrating pharmacological activation of heme oxygenase-1 in humans. Clin Pharmacol Ther. 2009;87(2):187–190. doi: 10.1038/clpt.2009.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Abraham NG, Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev. 2008;60(1):79–127. doi: 10.1124/pr.107.07104. [DOI] [PubMed] [Google Scholar]
- 152.Kappas A. A method for interdicting the development of severe jaundice in newborns by inhibiting the production of bilirubin. Pediatrics. 2004;113(1):119–123. doi: 10.1542/peds.113.1.119. [DOI] [PubMed] [Google Scholar]
- 153.Nielsen VG, Pretorius E. Carbon monoxide: anticoagulant or procoagulant? Thromb Res. 2014;133(3):315–321. doi: 10.1016/j.thromres.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 154.Bargen JA, Barker NW. Extensive arterial and venous thrombosis complicating chronic ulcerative colitis. Arch Intern Med. 1936;58(1):17–31. [Google Scholar]


