Abstract
Objective
It is known that osteoarthritis (OA) increases risk of sleep disturbance, and that both pain and sleep problems may trigger functional disability and depression. However, studies examining all four variables simultaneously are rare. This research therefore examined cross-sectional and longitudinal associations of self-reported sleep disturbance with OA-related pain and disability, and depressive symptoms.
Methods
At baseline, 367 persons with physician-diagnosed knee OA reported sleep disturbances, pain, functional limitations, and depressive symptoms. All measures were repeated a year later (N = 288). Baseline analyses examined the independent and interactive associations of sleep disturbance with pain, disability and depression, net of demographics and general health. Longitudinal analyses used baseline sleep disturbance to predict one-year change in pain, disability and depression.
Results
At baseline, sleep was independently associated with pain and depression, but not disability. The sleep-pain relationship was mediated by depressive symptoms; sleep interacted with pain to exacerbate depression among persons with high levels of pain. Baseline sleep disturbance predicted increased depression and disability, but not pain, at follow-up.
Conclusions
These data confirm known cross-sectional relationships of sleep disturbance with pain and depression, and provide new insights on longitudinal associations among those variables. Depression appears to play a strong role in the sleep-pain linkage, particularly where pain is severe. The unique predictive role of sleep in progression of disability requires further study, but may be an important point of intervention to prevent OA-related functional decline among persons whose sleep is disrupted by OA pain.
In the U.S., arthritis ranks among the top three health conditions causing disability (1). Osteoarthritis (OA), the most common form of arthritis, is pervasive and costly, affecting at least 26.9 million Americans and fueling a $185.5 billion increase in healthcare expenditures between 1996 and 2005 (2,3). Of joints commonly affected by OA, the knee ranks high, with symptomatic prevalence rates reaching 16.7% (4). The multifaceted, hyperalgesic nature of OA creates potentially debilitating physical and psychological burdens, making individuals particularly susceptible to comorbid disorders that may exacerbate OA-associated symptoms. Sleep disturbance is one such comorbidity. Among persons with knee OA, up to 31% report significant disturbances initiating sleep, 81% have difficulties maintaining nighttime sleep, and up to 77% report any sleep problem (5,6). Sleep disruption and pain frequently co-occur; both have uniquely been linked with depressed mood (7–9) and various forms of functional disability (10–12). The aim of the current study was therefore to disentangle the complex relationships among these conditions by examining cross-sectional and longitudinal associations of sleep disturbances with pain, depression, and disability among adults with knee OA.
The relationship between sleep and pain is robust and likely bidirectional (6,9,13). According to Spielman’s model of chronic insomnia (14), pain may serve as a precipitating factor that interacts with certain predisposing factors (e.g., tendency toward physiological hyperarousal) to fuel onset and maintenance of insomnia. Sleep problems (e.g., sleep fragmentation, difficulty initiating sleep) may disrupt various physiological processes that influence pain perception. A similar, cyclical relationship is proposed by Smith and colleagues (9), whereby disrupted sleep may contribute directly to increased central pain processing, exacerbating daily pain—which may then perpetuate sleep disturbances.
An evidence-based review of the general literature on chronic pain and sleep concluded that pain may be etiologically related to disordered sleep (15). Analyses from a cross-sectional, nationally representative survey of adults supported this conclusion, indicating that self-reported insomnia symptoms and dissatisfaction with sleep were correlated with pain severity in individuals with and without arthritis (16). Further, pain partially mediated the relationship of an arthritis diagnosis with sleep outcomes, even when accounting for sociodemographic and lifestyle factors, other chronic conditions, and mental health. Sleep quantity predicted presence of painful conditions in a cross-sectional, national survey in Spain: persons who reported fewer than 6 hours sleep per day were at increased risk for experiencing a painful condition (17). Wilcox and colleagues (6) similarly demonstrated that OA-related knee pain was related to sleep disturbance. Interestingly, the unique variance in sleep disturbance explained by knee pain was rather small (IR2 = .01); this contrasts with other findings that suggest a stronger relationship between sleep and pain (e.g., [16,18]).
Sleep difficulties are also closely linked with depressed mood. Using 14 days of subjective (daily diaries) and objective (actigraph) sleep measures, McCrae and colleagues (19) examined the sleep-affect association in healthy older adults. Subjective sleep measures were linked with both positive and negative affect, such that lower reported sleep quality or greater wake time was associated with less positive and greater negative affect the next day. In persons with OA, Hawker et al. (20) similarly identified a negative correlation between sleep quality and depressed mood. Interestingly, in contrast to subjective measures, McCrae et al.’s (19) actigraphic measures were not significantly associated with affect, reflecting the strong influence of perceptions of sleep on mood outcomes. In fact, objective and subjective sleep measures have often been found to be discordant (e.g., [21]) suggesting that they may be assessing different components of the sleep experience.
Sleep problems may also be associated with functional decline in osteoarthritis. Conceptually, sleep difficulties may function as a predisposing risk factor for developing impairments or, alternatively, as an intra-individual intervening factor that increases the extent and pace of functional decline (22). This relationship may be based in part on linkages among sleep, pain and depression just discussed, and particularly on the mediating role of depression and pain in the sleep–disability association (23). Empirically, poor sleep and diagnosed OA are separately associated with functional impairment in otherwise healthy adults (10–12). However, the influence of poor sleep on functional limitations in individuals with osteoarthritis has not been extensively explored.
In contract with these cross-sectional findings, far fewer studies have examined longitudinal associations among sleep disturbances, pain, depression, and disability in persons with OA; what evidence does exist is contradictory. For example, in a one-year, longitudinal examination in the general adult population, baseline insomnia predicted persistence of pain already present at baseline, but not incident pain (24). Pain also predicted development of insomnia over the one-year interval. In contrast, Morphy and colleagues (25) found, in individuals with knee OA, that insomnia symptoms increased risk of developing pain over a one-year period, but not persistence of baseline pain.
A number of longitudinal analyses have identified insomnia as a risk factor for the onset of depression net of the diagnostic association of the two disorders (e.g., [26]). A recent prospective study of community dwelling older women indicated that shorter total sleep time and lower sleep efficiency (measured actigraphically) increased risk of self-reported IADL impairment at 5-year follow-up (27). Although approximately 27% of the sample reported having OA, generalizability to osteoarthritic and male populations is limited.
Although intriguing, extant literature underscores the need for further investigation. The current research therefore examined cross-sectional and one-year longitudinal relationships of sleep difficulties with pain, depression, and functional disability in older adults with physician-diagnosed knee OA. Because variables of interest are known to vary with both demographic characteristics (28–30) and health status (31), those factors were examined as covariates, and their effects controlled as needed in all analyses.
Method
Sample and procedure
The sample comprised 367 individuals with physician-diagnosed OA of the knee. We purposely aimed to recruit a broad general sample of OA sufferers, including those who do and do not seek specialty care. We therefore recruited through diverse sources including university (N = 53, 14.6% of total sample) and Veterans Affairs Medical Center (44, 12.2%) rheumatology clinics, a general geriatric outpatient clinic (44, 12.2%), a previous psychoeducational study of osteoarthritis (47, 13.0%) and public service announcements in local news media (174, 48.1%). Recruitment information was missing for 5 participants. All participants were able to respond to questions in English, had no other life-threatening or severely disabling medical conditions, and provided signed consent for physician confirmation of the knee OA diagnosis. Persons for whom physician confirmation was unavailable were excluded from the sample.
Route of initial contact with participants varied by site. At investigator-affiliated clinics and for previous research participants, knee OA patients were sent introductory letters from attending physicians; a follow-up telephone call from project staff ascertained interest and eligibility. Respondents to PSAs telephoned the research office directly. Regardless of recruitment method, all individuals who assented during the screening call were mailed questionnaires and consent forms for independent completion. These were retrieved 1–2 weeks later during an in-person interview to collect additional data. All respondents received $25 at baseline (Time 1; T1) and one-year follow-up (Time 2; T2). All methods were approved by the institutional review boards of the Philadelphia Geriatric Center and the University of Pennsylvania.
Measures
Demographic characteristics include age, sex, race (White vs. other; 94% of non-White respondents were African American), relationship status (married/cohabiting vs. not), education (8-point scale, less than high school to completed graduate degree), and income (7-point scale coded in $10,000 increments). For the 22% of cases missing income information, values were imputed using SPSS v.22. Because data were assumed to be missing at random, we used stochastic regression methods with all T1 variables as predictors and a random residual term (32). Comparison of analyses using these imputed values with findings for only the subset of participants who provided full data yielded no differential patterns of significance for primary variables of interest (results available on request from the first author).
Health was measured objectively by a count of health conditions endorsed on a 31-item checklist adapted from Lawton et al. (33). Subjective health was represented by a 3-item composite: health overall (4-point scale, excellent to poor), now vs. 5 years ago, and vs. others the same age (both rated better, the same or worse). Higher scores indicate poorer perceived health.
Sleep disturbance was evaluated during a research diagnostic interview designed to capture significant mood disorders and other psychiatric symptomatology. Responses to a semi-structured question regarding trouble sleeping were coded to indicate insomnia as 1 = not present, 2 = possibly present, but doubtful clinical significance, 3 = present and probably significant, or 4 = definitely present and significant for at least two weeks. Follow-up probes captured presence/absence of initial (“trouble falling asleep”), middle (“waking up in the middle of the night”) and terminal insomnia (“waking up early in the morning before you want to get up”).
Depressive symptoms were assessed with the Center for Epidemiologic Studies Depression scale (34). To avoid confounding, the sleep disturbance item was omitted; the remaining 19 items were summed (possible range 0–57).
Pain was measured with the 6-item Philadelphia Geriatric Center Pain Scale (35); α=.84. Possible range is 0.71, 5.71; higher scores denote greater pain severity. Although this measure did not tap OA pain specifically, it correlated r = .90 with a parallel measure of OA pain, and yielded identical findings to the OA-specific measure. We therefore used the validated general measure for current analyses.
Functional disability was represented by Arthritis Impact Measurement Scales, 2nd edition (AIMS2; [34]) subscales tapping mobility, walking and bending, hand and finger function, arm function, self-care and household tasks. The 28 5-point items were averaged to yield a single composite (α = .78).
Analytic plan
Sample characteristics were examined using analysis of variance (ANOVA) and chi square statistics. Ordinary least squares (OLS) regression analyses identified demographic and health characteristics associated with primary variables (sleep, pain, disability, depression) for statistical control in primary analysis. In those primary analyses, OLS regression equations used sleep disturbance to predict T1 depression, disability and pain. Block entry of other primary variables pinpointed independent associations of sleep disturbance with each outcome, and interactions of sleep with other key predictors (e.g., does the interaction of sleep with pain significantly improve prediction of depression?). Longitudinal analyses examined T1–T2 change in depression, pain and disability as a function of T1 sleep disturbance. Again, block entry of other outcome variables elucidated direct and indirect effects.
Results
Sample characteristics
Table 1 presents baseline (T1) characteristics of the 367 T1 participants and the 288 T2 completers. Persons who failed to complete the one-year follow-up were disproportionately non-White and more poorly educated; they also reported poorer subjective health, greater disability and slightly greater pain at T1. Although there were no differences in overall prevalence of sleep disturbance, T2 completers were slightly more likely to report terminal insomnia. Because of these differences, we reran all T1 analyses using only the 288 T2 completers (results available from the first author). Minimal differences reflected minor shifts in coefficients for covariates (e.g., from significance to marginal significance, or from marginal to nonsignificance) but not for primary variables. We therefore used the full sample in T1 analyses.
Table 1.
Baseline Characteristics of Time 1 and Time 2 Participants
| Time 1 N=367 |
Time 2 N= 288 |
|||
|---|---|---|---|---|
| Variable (range in this sample) | Mean (SD) or N (%) |
Mean (SD) or N (%) |
F or χ2 | P |
| Age in years (48–91) | 67.9 (9.7) | 68.3 (9.5) | 2.46 | .117 |
| Female | 234 (63.8%) | 190 (66.0%) | 2.83 | .092 |
| Nonwhite | 99 (27.0%) | 68 (23.6%) | 7.69 | .006 |
| Married/cohabiting | 196 (53.4%) | 149 (51.7%) | 1.50 | .221 |
| Some college | 185 (50.4%) | 157 (54.5%) | 9.02 | .003 |
| Income (1, 7)a | 3.11 (2) | 3.16 (2) | 2.32 | .128 |
| Health problems (0, 11) | 2.65 (2.08) | 2.56 (1.95) | 2.68 | .103 |
| Subjective health (1, 3.63)b | 1.92 (0.41) | 1.89 (0.39) | 7.20 | .008 |
| Any sleep disturbancec | 255 (69.5%) | 205 (71.2%) | 1.82 | .177 |
| Initial insomnia | 114 (31.1%) | 88 (30.6%) | < 1 | .689 |
| Middle insomnia | 183 (50.0%) | 149 (51.9%) | 1.95 | .162 |
| Terminal insomnia | 145 (39.7%) | 122 (42.7%) | 4.74 | .029 |
| Pain (0.83, 5.33) | 2.77 (1.03) | 2.70 (1.01) | 5.11 | .024 |
| Disability (6, 25.3) | 9.93 (3.17) | 9.68 (3.05) | 8.45 | .004 |
| Depressive symptoms (0, 46) | 9.70 (9.28) | 9.31 (9.01) | 2.47 | .117 |
Note: Numbers in parentheses following variable names indicate observed range of scores on that scale or variable. All significance tests compared T1 subjects who completed T2 (N = 288) against those dropped out of the project (N = 79). For all χ2 values, df = 1. For all F values, df = 1, 365 except as noted.
Figures are median and interquartile range
df = 1, 364
Rated 2 or higher on a 4-point scale; see text for details.
Demographic and health covariates
Separate OLS regression analyses identified demographic and general health correlates of sleep, pain, disability and depressive symptoms, with block entry of demographics and then health status variables. Results (available from first author) closely parallel those reported below for primary analyses and, hence, are not described in detail here. Briefly, sex, race, education, and subjective and objective health were each associated with one or more of the four key variables; they were used as covariates in all primary analyses. Because age, income and marital status did not uniquely predict any primary variables, they were dropped from analysis.
Cross-sectional associations of sleep disturbance with pain, disability and depression
Separate OLS regressions examined how sleep disturbance is associated with pain, disability and depressive symptoms. For each outcome, demographics (sex, education, race) and objective and subjective health were entered at Step 1, followed by sleep disturbance (Step 2; summarized in Table 2). Where sleep disturbance was a significant predictor, we reanalyzed substituting initial, middle and terminal insomnia for the global sleep disturbance variable.
Table 2.
Pain, Disability and Depressive Symptoms as a Function of Demographic Characteristics, Health Status, and Sleep Disturbance
| Pain1 | Disability2 | Depressive Symptoms3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| r | β | 95% CI (β) | r | β | 95% CI (β) | r | β | 95% CI (β) | |
| Demographics | |||||||||
| Sex | .153 | .195d | .101, .289 | .173 | .198d | .116, .279 | .099 | .114a | .018, .209 |
| Race | −.018 | .056 | −.039, .151 | .135 | .026 | −.057, .108 | −.047 | −.083e | .180, .013 |
| Education | −.154 | −.073 | −.169, .023 | −.201 | −.096a | −.179, −.013 | −.145 | −.095e | −.193, .002 |
| Health status | |||||||||
| Health conditions | .185 | −.028 | .136, .080 | .443 | .207d | .114, .301 | .214 | .057 | −.052, .165 |
| Perceived health | .373 | .375d | .266, .484 | .557 | .463d | .368, .557 | .313 | .259d | .149, .370 |
| Sleep disturbance | .182 | .112a | .018, .206 | .106 | −.015 | −.098, .067 | .271 | .206d | .110, .302 |
Note: All coefficients are final step of hierarchical regression model. See text for details. The coefficients reported as “r” are Pearson product-moment coefficients. CI = confidence interval..
Adjusted R2 = .188, F (6, 359) = 15.10, p < .001
Adjusted R2 = .397, F (6, 359) = 39.33, p < .001
Adjusted R2 = .164, F (6, 359) = 1.2.95 p < .001
p < .05
p < .01
p < .005
p < .001
.05 < p < .10
For pain, a significant Step 1 equation, adjusted R2 = .178, F (5, 360) = 16.82, p < .001, is attributable to effects of sex and subjective health. Addition of sleep disturbance at Step 2 significantly improved prediction, Δ F (1,359) = 5.44, p < .02, reflecting a positive association of sleep disturbance with pain (Table 2). However, when Step 2 was repeated using the 3 types of insomnia, the effect was not significant, Δ F (3, 354) = 1.73.
For disability, a significant Step 1 equation, adjusted R2 = .388, F (5, 360) = 47.28, p < .001, was driven by sex, education, and objective and subjective health. Sleep disturbance did not increase explained variance, Δ F < 1.
Depressive symptoms were significantly predicted by sex, education and subjective health, and marginally by race, Step 1 F (5, 360) = 8.68, p < .001. Sleep disturbance significantly improved prediction of depression, Δ F (1, 359) = 17.71, p < .001. Here, reanalysis using the three types of insomnia yielded a significant effect, Δ F (3, 354) = 4.18, p < .006, driven solely by initial insomnia, β = .106, p < .04.
To explore interrelationships of sleep problems, pain and depression, we expanded pain and depression analyses, adding the other outcome (Step 3) and the interaction of sleep disturbance with that outcome (Step 4), using centered crossproducts as interaction terms. Thus, for pain we added depressive symptoms at Step 3, and the depression × sleep disturbance interaction at Step 4. Pain and sleep were similarly examined singly and interactively as predictors of depression. Because disability was not independently associated with sleep disturbance, it was not examined further.
For pain, addition of depression to the Step 2 equation increased variance explained, β = .201, p < .001, Δ F (1, 358) = 15.56, p < .001, and reduced the beta for sleep disturbance to nonsignificance, β = .063, p > .19. The sleep × depression interaction did not improve prediction, F < 1.
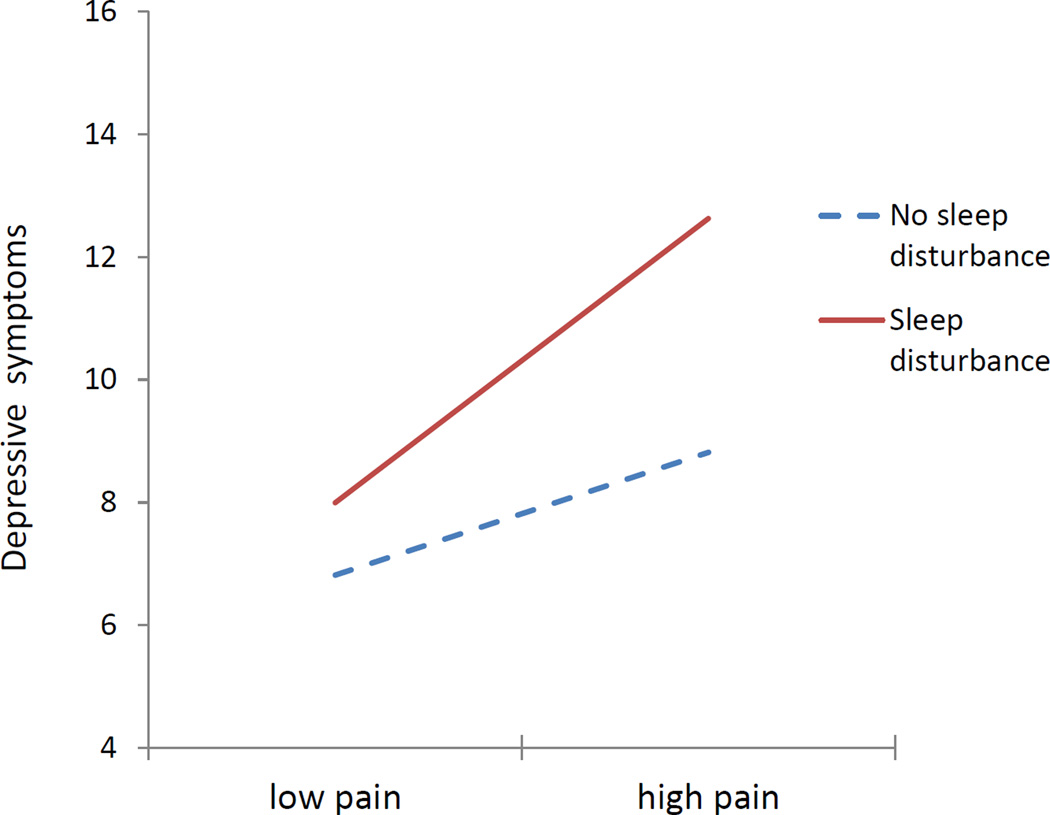
For depression, adding pain to the equation already containing sleep disturbance similarly increased explained variance, Δ F (1, 358) = 15.56, p < .001. Here, both pain (β = .207) and sleep disturbance (.183) were independently associated with depression, both ps < .001. The sleep × pain interaction further improved prediction, F (1, 357) = 5.86, p < .02. To elucidate this effect, we dichotomized sleep disturbance (presence/absence of clinically significant disturbance: 1,2 vs. 3,4) and pain (median split). Means for the interaction of these variables, adjusted for covariates, indicate that sleep disturbance exacerbates the known association of pain with depression (Figure 1). For the low pain group, sleep disturbance was unrelated to depression; but at high levels of pain, depression was greater among those who also reported sleep problems.
Figure 1.
Interactive effects of sleep disturbance and pain on depressive symptoms
Longitudinal associations of sleep disturbance with pain, disability and depression
Sleep disturbance at T1 was used to predict one-year change in pain, disability and depression by residualizing the T2 outcome on its T1 counterpart (Step 1) before entering covariates (Step 2) and then T1 sleep disturbance (Step 3). Results at this step appear as Table 3. Where a significant effect of T1 sleep disturbance emerged, we then added the other well-being indicators, e.g., for T2 pain, we entered T1 depression and disability (Step 4).
Table 3.
One-Year Change in Pain, Disability and Depressive Symptoms as a Function of Demographic Characteristics, Health Status, and Sleep Disturbance
| Pain1 | Disability2 | Depressive Symptoms3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| r | β | 95% CI (β) | r | β | 95% CI (β) | r | β | 95% CI (β) | |
| Time 1 status | .664 | .603d | .510, .696 | .768 | .633d | .541, .724 | .682 | .611d | .521, .701 |
| Demographics | |||||||||
| Sex | .151 | .029 | −.056, .114 | .216 | .079a | .005, .152 | .082 | .028 | −.055, .112 |
| Race | .007 | −.048 | .133, .037 | .029 | −.038 | −.109, .032 | −.022 | −.012 | −.096, .072 |
| Education | −.136 | −.042 | −.128, .044 | −.286 | −.144c | −.216, .072 | −.177 | −.065 | −.149, .020 |
| Health status | |||||||||
| Health conditions | .318 | .198d | .102, .294 | .448 | .098a | .015, .182 | .207 | .052 | −.043, .148 |
| Perceived health | .369 | .042 | −.060, .144 | .501 | .074 | .017, .165 | .297 | .043 | −.056, .143 |
| Sleep disturbance | .179 | .054 | −.031, .139 | .203 | .114c | .043, .185 | .315 | .158d | .072, .243 |
Note: All coefficients are Step 3 of hierarchical regression model. See text for details. The coefficients reported as “r” are Pearson product-moment coefficients. CI = confidence interval.
Adjusted R2 = .485, F (7, 279) = 39.50, p < .001
Adjusted R2 = .639, F (7, 279) = 73.20, p < .001
Adjusted R2 = .492, F (7, 279) = 40.54, p < .001
p < .05
p < .01
p < .005
p < .001
.05 < p < .10
For 1-year change in pain, a significant Step 2 effect, Δ F (5, 280) = 6.06, p < 001, was attributable entirely to objective health, β = .203, p < .001. Adding T1 sleep disturbance did not improve prediction, Δ F (1, 279) = 1.57, p > .21
For disability, a significant Step 2 effect, Δ F (5, 280) = 6.83, p < .001, was driven by sex (β = .088, p < .03), education (−.150, .001), and objective health (.109, .02); perceived health was marginal, β = .087, p < .07. Addition of T1 sleep disturbance increased explained variance, β = .114, Δ F (1, 279) = 9.34, p < .002 (Table 3). The effect of T1 sleep disturbance remained significant even after entry of both T1 and T2 pain and depressive symptoms, Step 5 sleep disturbance β = .088, p < .02, overall F (9,277) = 61.14, p < .001. In addition, change in disability was significantly associated with T1 depressive symptoms, β = .092, p < .02, and T1 pain, β = .114, p < .01.
Change in depression was not significantly associated with T1 demographic and health covariates, Δ F (5, 280) = 1.66, p > .14. Baseline sleep disturbance significantly increased explained variance, Δ F (1, 279) = 13.01, p < .001 (Table 3). Addition of T1 pain and disability variables did not further improve prediction, nor reduce the effect of sleep disturbance, final β = .154, p < .001.
Discussion
The data reported here illustrate the complex interrelationships among osteoarthritis-related pain and disability, sleep disturbances, and depressive symptoms. Cross-sectional analyses revealed a significant association of sleep disturbance with pain and depression, but not functional disability. The sleep–pain relationship was wholly explained by depressive symptoms; in contrast, depression was significantly, independently associated with both pain and sleep problems. Furthermore, sleep disturbance exacerbated effects of pain on depression, such that depressive symptoms were greatest among those with both significant sleep problems and higher-than-average pain. In one-year longitudinal analyses, sleep problems predicted increases in depression and disability, but not pain.
Before discussing findings in depth, it is important to note that our sample differed from that used in many previous studies. We purposely cast a broad net to attract a demographically and clinically diverse sample. Thus, although all participants had diagnosed knee OA, they reported a broad range of symptoms and had not all sought treatment specifically for OA. While this may have introduced some noise into analyses, it enhances generalizability and depicts associations as they may occur in a broader cross-section of OA sufferers than is typical for purely clinical samples.
We should also point out some limitations of this research. Most notable is our very global and inexact measure of sleep disturbance. Use of a single, semi-structured probe did capture the nature and extent of subjective sleep problems. However, it does not provide the rigor of standardized sleep quality measures and certainly not of objective monitoring techniques such as actigraphy and polysomnography. Although it is heartening that even our relatively gross measure of sleep quality produced clearly interpretable effects, further work with more sensitive measures is in order. This is especially important given known discrepancies among objective and subjective measures of sleep (21).
Limitations of the sleep measure, in turn, drove our decision to treat sleep as a predictor and not an outcome. Existing evidence implies that the associations of sleep with both pain (6,9,13) and depression (35,36) are bidirectional; though fewer hard data are available, it is also likely that the three variables interact complexly over time. Patterns of longitudinal associations in the current data illustrate this complexity, as well as the added role of disability in the mix. However, both our rudimentary measurement of sleep quality and single-follow-up design preclude addressing definitively the long-range, possibly cyclical linkages among sleep, OA-related pain and disability, and emotional well-being.
Current findings nonetheless confirm the previously documented associations of sleep with pain (7,13,17,20,39,40) and depression (20,37,38). In our sample, however, depressive symptoms entirely accounted for the association of sleep with pain; nor was there any association of baseline sleep disturbance with one-year change in pain. This contradicts current thinking about the mutual influence of pain and sleep disturbance, and adds yet another layer of complexity to existing, conflicting research on long-term associations of pain and sleep. In contrast to previous, population-based studies examining incidence and persistence of pain (24,25), the current analyses addressed change in OA-related pain severity. The chronic nature of OA pain, as well as its close linkage with disability, is likely to shape its association with both sleep and emotional well-being beyond dynamics seen in the general population.
Interestingly, the current sample of older OA sufferers showed no cross-sectional association of sleep with functional disability. However, baseline sleep disturbance did predict functional decline over a year’s time. Furthermore, the effect of sleep problems was independent of the contributions of baseline depressive symptoms and increased pain from T1–T2 (assessed as T1–T2 residual). Thus, it appears that sleep problems are a robust risk factor for functional decline net of pain or mood. Of course, causal mechanisms underlying this association remain unclear, and potential influences of some unmeasured third factor must be considered. One intriguing possibility on which further research is needed is fatigue, which recent work suggests is a frequent concomitant of both chronic pain and sleep disturbance (20,41,42). A recent intervention conducted with older adults with comorbid osteoarthritis and insomnia showed that short-term improvements in sleep predicted long-term (18 month) improvements in chronic pain and fatigue (43). Given the important role of daytime activity in managing chronic pain, further research is needed to investigate the associations among sleep, fatigue, and pain in osteoarthritis.
Our findings also enhance understanding of the linkage between sleep disturbance and depression among persons with osteoarthritis. Of particular interest here is the obtained interaction of sleep and pain on depressive symptoms at baseline. At low levels of pain, sleep disturbance is unrelated to mood; but where pain is more severe, sleep problems exacerbate depression. This has important implications for treatment, suggesting that the documented efficacy of analgesic treatment of OA-related pain in reducing sleep disturbance (44,45) may help relieve pain-related emotional distress as well. At the same time, our analyses suggest that the effects of sleep disturbance on depression may be independent of pain and disability, inasmuch as only sleep was independently associated with increased depression over time. Similarly, changes in pain and disability did not track with change in depression. This suggests that sleep may be an important mediator of known relationships among pain, disability and depression.
In sum, this analysis sheds new light on the interrelationships among sleep, pain, disability and depressive symptoms among older adults with osteoarthritis. Findings highlight the unique role of sleep problems as immediate drivers of pain and depression, and as long-range influences on functional disability and depressed mood. In particular, it appears that poor sleep may interact with severe OA pain to place persons at increased risk of depression and, long range, of functional limitations. Further, depression represents an important possible mediator of the pain – sleep linkage, highlighting the importance of assessing and treating emotional distress in this very common chronic disorder. Future research should continue examining these linkages, with an eye toward pinpointing specific mechanisms and, hence, the types of clinical interventions most likely to interrupt the cycle of OA-related distress.
Significance and Innovations.
Although osteoarthritis is known to produce sleep problems, investigation of the dynamics of that association is in its early stages. This research uniquely examines the association of sleep with OA-related pain, disability and depressed mood simultaneously in a single study.
In particular, there has previously been very little longitudinal analysis of how sleep disturbance may fuel changes in pain, disability and depressive symptoms among persons with OA.
Acknowledgments
This research was supported by MH51800 to the first author.
Footnotes
The authors received no other financial support or benefits for this research and have no financial interests relevant to this research.
We are indebted to Tina Harralson, Lori Smith, Ralph Schumacher, and others who helped with recruitment and data collection.
References
- 1.Centers for Disease Control and Prevention. Morbidity and Mortality Weekly Report Update: Prevalence and Most Common Causes of Disability Among Adults — Department of Health and Human Services. 2009;58:421–452. [Google Scholar]
- 2.Kotlarz H, Gunnarsson CL, Fang H, Rizzo JA. Insurer and out-of-pocket costs of osteoarthritis in the US: evidence from national survey data. Arthritis Rheum. 2009;60:3546–3553. doi: 10.1002/art.24984. [DOI] [PubMed] [Google Scholar]
- 3.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo R, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jordan JM, Helmick CG, Renner JB, Luta G, Dragomir AD, Woodard J, et al. Prevalence of knee symptoms and radiographic and symptomatic knee osteoarthritis in African Americans and Caucasians: the Johnston County Osteoarthritis Project. J Rheumatol. 2007;34:172–180. [PubMed] [Google Scholar]
- 5.Allen KD, Renner JB, Devellis B, Helmick CG, Jordan JM. Osteoarthritis and sleep: the Johnston County Osteoarthritis Project. J Rheumatol. 2008;35:1102–1107. [PMC free article] [PubMed] [Google Scholar]
- 6.Wilcox S, Brenes GA, Levine D, Sevick MA, Shumaker SA, Craven T. Factors related to sleep disturbance in older adults experiencing knee pain or knee pain with radiographic evidence of knee osteoarthritis. J Am Geriatr Soc. 2000;48:1241–1251. doi: 10.1111/j.1532-5415.2000.tb02597.x. [DOI] [PubMed] [Google Scholar]
- 7.Chen Q, Hayman LL, Shmerling RH, Bean JF, Leveille SG. Characteristics of chronic pain associated with sleep difficulty in the older population: The MOBILIZE Boston study. J Am Geriatr Soc. 2011;59:1385–1392. doi: 10.1111/j.1532-5415.2011.03544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCurry SM, Von Korff M, Vitiello MV, Saunders K, Balderson BH, Moore AL, et al. Frequency of comorbid insomnia, pain, and depression in older adults with osteoarthritis: predictors of enrollment in a randomized treatment trial. J Psychosom Res. 2011;71:296–299. doi: 10.1016/j.jpsychores.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith MT, Quartana PJ, Okonkwo RM, Nasir A. Mechanisms by which sleep disturbance contributes to osteoarthritis pain: A conceptual model. Curr Pain Headache Reports. 2009;13:447–454. doi: 10.1007/s11916-009-0073-2. [DOI] [PubMed] [Google Scholar]
- 10.Goldman SE, Stone KL, Ancoli-Israel S, Blackwell T, Ewing SK, Boudreau R, et al. Poor sleep is associated with poorer physical performance and greater functional limitations in older women. Sleep. 2007;30:1317–1324. doi: 10.1093/sleep/30.10.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guccione AA, Felson DT, Anderson JJ, Anthony JM, Zhang Y, Wilson PW, et al. The effects of specific medical conditions on the functional limitations of elders in the Framingham Study. Am J Public Health. 1994;84:351–358. doi: 10.2105/ajph.84.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCracken LM, Iverson GL. Disrupted sleep patterns and daily functioning in patients with chronic pain. Pain Res Manag. 2002;7:75–79. doi: 10.1155/2002/579425. [DOI] [PubMed] [Google Scholar]
- 13.Dzierzewski JM, Williams JM, Roditi D, Marsiske M, McCoy K, McNamara J, et al. Daily variations in objective nighttime sleep and subjective morning pain in older adults with insomnia: evidence of covariation over time. J Am Geriatr Soc. 2010;58:925–930. doi: 10.1111/j.1532-5415.2010.02803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spielman AJ, Caruso LS, Glovinsky PB. A behavioral perspective on insomnia treatment. Psychiatr Clin North Am. 1987;10:541–553. [PubMed] [Google Scholar]
- 15.Fishbain DA, Cole B, Lewis JE, Gao J. What is the evidence for chronic pain being etiologically associated with the DSM-IV category of sleep disorder due to a general medical condition? A structured evidence-based review. Pain Med. 2010;11:158–179. doi: 10.1111/j.1526-4637.2009.00706.x. [DOI] [PubMed] [Google Scholar]
- 16.Power JD, Perruccio AV, Badley EM. Pain as a mediator of sleep problems in arthritis and other chronic conditions. Arthritis Rheum. 2005;53:911–919. doi: 10.1002/art.21584. [DOI] [PubMed] [Google Scholar]
- 17.Salazar A, Dueñas M, Ojeda B, Failde I. Association of painful musculoskeletal conditions and migraine headache with mental and sleep Disorders among adults with disabilities, Spain, 2007 – 2008. Prev Chronic Dis. 2014;11:1–11. doi: 10.5888/pcd11.130144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Irwin MR, Olmstead R, Carrillo C, Sadeghi N, Fitzgerald JD, Ranganath VK, et al. Sleep loss exacerbates fatigue, depression, and pain in rheumatoid arthritis. Sleep. 2012;35:537–543. doi: 10.5665/sleep.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCrae CS, McNamara JPH, Rowe MA, Dzierzewski JM, Dirk J, Marsiske M, et al. Sleep and affect in older adults: using multilevel modeling to examine daily associations. J Sleep Res. 2008;17:42–53. doi: 10.1111/j.1365-2869.2008.00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hawker GA, French MR, Waugh EJ, Gignac MAM, Cheung C, Murray BJ. The multidimensionality of sleep quality and its relationship to fatigue in older adults with painful osteoarthritis. Osteoarthritis Cartilage. 2010;18:1365–1371. doi: 10.1016/j.joca.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 21.Dautovich ND, Kay DB, Perlis ML, Dzierzewski JM, Rowe MA, McCrae CS. Day-to-day variability in nap duration predicts medical morbidity in older adults. Health Psychol. 2012;31:671–676. doi: 10.1037/a0027374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McDonough CM, Jette AM. The contribution of osteoarthritis to functional limitations and disability. Clin Geriatr Med. 2010;26:387–399. doi: 10.1016/j.cger.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naughton F, Ashworth P, Skevington SM. Does sleep quality predict pain-related disability in chronic pain patients? The mediating roles of depression and pain severity. Pain. 2007;127:243–252. doi: 10.1016/j.pain.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 24.Jansson-Fröjmark M, Boersma K. Bidirectionality between pain and insomnia symptoms: a prospective study. Br J Health Psychol. 2012;17:420–431. doi: 10.1111/j.2044-8287.2011.02045.x. [DOI] [PubMed] [Google Scholar]
- 25.Morphy H, Dunn KM, Lewis M, Boardman HF, Croft PR. Epidemiology of insomnia: a longitudinal study in a UK population. Sleep. 2007;30:274–280. [PubMed] [Google Scholar]
- 26.Ohayon MM, Roth T. Place of chronic insomnia in the course of depressive and anxiety disorders. J Psychiatr Res. 2003;37:9–15. doi: 10.1016/s0022-3956(02)00052-3. [DOI] [PubMed] [Google Scholar]
- 27.Spira AP, Covinsky K, Rebok GW, Punjabi NM, Stone KL, Hillier TA, et al. Poor sleep quality and functional decline in older women. J Am Geriatr Soc. 2012;60:1092–1098. doi: 10.1111/j.1532-5415.2012.03968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Affleck G, Tennen H, Keefe FJ, Lefebvre JC, Kashikar-Zuck S, Wright K, et al. Everyday life with osteoarthritis or rheumatoid arthritis: independent effects of disease and gender on daily pain, mood, and coping. Pain. 1999;83:601–609. doi: 10.1016/S0304-3959(99)00167-0. [DOI] [PubMed] [Google Scholar]
- 29.Thomas E, Peat G, Harris L, Wilkie R, Croft PR. The prevalence of pain and pain interference in a general population of older adults: cross-sectional findings from the North Staffordshire Osteoarthritis Project (NorStOP) Pain. 2004;110:361–368. doi: 10.1016/j.pain.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 30.Knight JB, Callahan LF, Luong ML, Shreffler J, Schoster B, Renner JB, et al. The association of disability and pain with individual and community socioeconomic status in people with hip osteoarthritis. Open Rheumatol J. 2011;5:51–58. doi: 10.2174/1874312901105010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riddle DL, Dumenci L. Self-rated health and symptomatic knee osteoarthritis over three years: data from a multicenter observational cohort study. Arthritis Care Res. 2013;65:169–176. doi: 10.1002/acr.21661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sinharay S, Stern HS, Russell D. The use of multiple imputation for the analysis of missing data. Psychol Methods. 2001;6:317–329. [PubMed] [Google Scholar]
- 33.Lawton MP, Moss M, Fulcomer M, Kleban MH. A research and service oriented multilevel assessment instrument. J Gerontol. 1982;37:91–99. doi: 10.1093/geronj/37.1.91. [DOI] [PubMed] [Google Scholar]
- 34.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 35.Parmelee PA. Assessment of pain in the elderly. In: Lawton MP, Teresi J, editors. Annual Review of Gerontology and Geriatrics. New York: Springer; 1994. pp. 281–301. [Google Scholar]
- 36.Meenan RF, Mason JH, Anderson JJ, Guccione AA, Kazis LE. AIMS2. The content and properties of a revised and expanded Arthritis Impact Measurement Scales Health Status Questionnaire. Arthritis Rheum. 1992;35:1–10. doi: 10.1002/art.1780350102. [DOI] [PubMed] [Google Scholar]
- 37.Staner L. Comorbidity of insomnia and depression. Sleep Med Rev. 2010;14:35–46. doi: 10.1016/j.smrv.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 38.Baglioni C, Battagliese G, Feige B, Spiegelhalder K, Nissen C, Voderholzer U, et al. Insomnia as a predictor of depression: A meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011;135:10–19. doi: 10.1016/j.jad.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 39.Eakman AM. Measurement characteristics of the engagement in meaningful activities survey in an age-diverse sample. Am J Occup Ther. 2012;66:e20–e29. doi: 10.5014/ajot.2012.001867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Asih S, Neblett R, Mayer TG, Brede E, Gatchel RJ. Insomnia in a chronic musculoskeletal pain with disability population is independent of pain and depression. Spine J. 2013 doi: 10.1016/j.spinee.2013.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murphy SL, Lyden AK, Phillips K, Clauw DJ, Williams DA. Association between pain, radiographic severity, and centrally-mediated symptoms in women with knee osteoarthritis. Arthritis Care Res. 2011;63:1543–1549. doi: 10.1002/acr.20583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stebbings S, Herbison P, Doyle TCH, Treharne GJ, Highton J. A comparison of fatigue correlates in rheumatoid arthritis and osteoarthritis: disparity in associations with disability, anxiety and sleep disturbance. Rheumatology. 2010;49:361–367. doi: 10.1093/rheumatology/kep367. [DOI] [PubMed] [Google Scholar]
- 43.Vitiello MV, McCurry SM, Shortreed SM, Baker LD, Rybarczyk BD, Keefe FJ, et al. Short-term improvement in insomnia symptoms predicts long-term improvements in sleep, pain, and fatigue in older adults with comorbid osteoarthritis and insomnia. Pain. 2014 doi: 10.1016/j.pain.2014.04.032. (epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kosinski M, Janagap C, Gajria K, Schein J, Freedman J. Pain relief and pain-related sleep disturbance with extended-release tramadol in patients with osteoarthritis. Curr Med Res Opin. 2007;23:1615–1626. doi: 10.1185/030079907x199493. [DOI] [PubMed] [Google Scholar]
- 45.Turk DC, Cohen MJM. Sleep as a marker in the effective management of chronic osteoarthritis pain with opioid analgesics. Semin Arthritis Rheum. 2010;39:477–490. doi: 10.1016/j.semarthrit.2008.10.006. [DOI] [PubMed] [Google Scholar]