Abstract
Ultrasound imaging is a powerful tool in medicine because of the millisecond temporal resolution and sub-millimeter spatial resolution of acoustic imaging. However, the current generation of acoustic contrast agents is primarily limited to vascular targets due to their large size. Nano-size particles have the potential to be used as a contrast agent for ultrasound molecular imaging. Silica-based nanoparticles have shown promise here, however their slow degradation rate may limit their applications as a contrast agent. Phosphate-based glasses are an attractive alternative with controllable degradation rate and easily metabolized degradation components in the body. In this study, biodegradable P2O5-CaO-Na2O phosphate-based glass nanospheres (PGNs) were synthesized and characterized as contrast agents for ultrasound imaging. The structure of the PGNs was characterised using scanning electron microscopy (SEM), energy dispersive X-ray spectroscopy (EDX), X-ray diffraction (XRD), 31P nuclear magnetic resonance (31P MAS-NMR), and Fourier transform infrared (FTIR) spectroscopy. The SEM images indicated a spherical shape with a diameter size range of 200-500 nm. The XRD, 31P NMR and FTIR results revealed the amorphous and glassy nature of PGNs that consisted of mainly Q1 and Q2 phosphate units. We used this contrast to label mesenchymal stem cells and determined in vitro and in vivo detection limits of 5 and 9 μg/mL, respectively. Cell counts down to 4000 could be measured with ultrasound imaging with no cytoxicity at doses needed for imaging. Importantly, ion release studies confirmed these PGNs biodegrade into aqueous media with degradation products that can be easily metabolized in the body.
Keywords: Sol-gel, Electrospraying, Phosphate-based glasses, Ultrasound imaging
Ultrasound imaging is a real time, low cost, non-ionizing and effective imaging tool, yet remains primarily an anatomic tool versus positron emission tomography (PET) or bioluminescence. While ultrasound contrast agents can increase the signal specificity for molecular imaging by increasing backscattered echoes from the target site, the current generation of microbubbles are too large to be used outside of the vasculature or inside specific cells of interest.1-4 Microbubbles were first introduced as a contrast agent in 1968 by injecting agitated saline into the ascending aorta and cardiac chambers during echocardiographic examination.5 Strong echoes were produced within the heart, due to the acoustic mismatch between free air microbubbles in the saline and the surrounding blood. However, microbubbles produced by agitation are both large and unstable and diffusing into solution in a short period of time.
To overcome these limitations encapsulated gas bubbles were invented, however these microbubbles are unstable in circulation even with polyethylene glycol surface treatment.6-8 Furthermore, microbubbles require a careful choice of ultrasound frequency because microbubbles burst at low frequencies that can result in local microvasculature rupture and hemolysis.9 While nanobubbles have made important improvements in this area, a complete solution has remained elusive.10-12
One alternative is solid nanoparticles that have utility as backscatter contrast agents for ultrasound molecular imaging.13-15 These solid particles have been used to image stem cells in cardiac regenerative medicine or to image cell surface proteins in cancer cells.13, 14 However, the material properties of these agents are critical. They must have acoustic impedance capable of strongly and stably backscattering incident acoustic energy, but also be metabolized and removed safely from the body shortly thereafter. Finally, the particles must be small enough to extravasate from tumor vasculature due to the enhanced permeation and retention effect or be phagocytosed by cells when growing in culture. While silica-based nanoparticles have shown promise here,16-18 phosphate-based glasses are an attractive alternative because they offer a controllable degradation rate by varying their chemical compositions and which give rise to easily metabolized degradation components in the body.19-21
Previous approaches to preparing ternary phosphate-based glasses in the P2O5-CaO-Na2O system via a sol-gel synthesis method have focused on preparing bulk sol-gel glasses.22, 23 However, because of the slow reaction of the phosphate precursors, the reaction times were long. In addition, subsequent high drying temperatures made it difficult to prepare bulk glasses suitable for biomedical applications.
Here we report a new simplified sol-gel synthesis method to prepare a ternary P2O5-CaO-Na2O glass system at low temperature with subsequent electrospraying to obtain nano- spherical glasses. The PGNs were characterized by SEM, EDX, XRD, 31P MAS-NMR, and FTIR spectroscopy as well as in vitro and in vivo ultrasound imaging. Biodegradation was then monitored via inductively coupled plasma mass spectroscopy (ICP-MS) to measure phosphorus, calcium, and sodium ion release into aqueous media. We then used these tools to labelled mesenchymal stem cells (MSCs) and studied the effect the contrast agents had on cell viability as well as the signal increases generated by the PGNs. To the best of our knowledge, this is the first report of sol-gel synthesis and electrospraying to prepare biodegradable PGNs at low temperature and the first use of such a system for imaging purposes.
RESULTS
SEM and EDX
We successfully synthesized the nanoparticles with electrospraying technique (Fig. 1A). The shape and particle size of the particles were identified by SEM images. Figure 1B,C show nano-spherical particles and measurement of the particle size for more than 100 particles confirmed more than 80% of the particles had a diameter size distribution of 200-500 nm with a mean diameter size of 320 nm. The EDX results were also converted to mol% to allow the comparison to be made with the theoretical composition and reported in Table 1. The results show a reduction of around 12.5 mol% P2O5 content from the theoretical values, with a concomitant increase in the percentage content of the other oxides to compensate. This reduction can be related to the unreacted phosphorus that is lost during heating stages.
Figure 1.
(A) Schematic of the electrospraying setup. The high voltage between the nozzle and the ground created nanospheres that flew toward the heated silicone oil as a substrate to obtain dried gel nanospheres. SEM image of (B) washed and heat-treated PGNs (the scale bar is 2 μm) and (C) dispersed PGNs in ethanol solution after heat-treatment (the scale bar is 5 μm). The PGNs have an approximate size range of between 200-500 nm with mean diameter size of 320 nm (N=100 particles).
Table 1.
Intended compositions and measured values determined by EDX (in brackets).
| Composition (mol%) | P2O5 | CaO | Na2O |
|---|---|---|---|
| (P2O5) 55-(CaO) 30-(Na2O)15 | 55.0(42.5±1.0) | 30.0 (37.9±0.9) | 15.0 (19.6±0.8) |
XRD and 31P MAS-NMR
The XRD pattern of the PGNs was free of any detectable crystalline phase (Fig. 2A). Only two broad peaks at 2θ values between 20-50° were observed. This confirmed the amorphous nature of PGNs.
Figure 2.
(A) XRD pattern of the PGNs indicate that it is free from any detectable crystalline phase and (B) 31P NMR spectrum (black line) and the fitting (red line) that correspond to mainly Q1 and Q2 phosphate units.
Figure 2B shows the solid-state 31P MAS-NMR spectrum from PGNs. The peak at 1.7 ppm corresponds to the presence of Q0 phosphate units that can be related to unreacted phosphorus in the structure 23. The peaks with the chemical shifts at -8.3 and -21.9 ppm are assigned to Q1 and Q2 units, respectively (Table 2).23, 24
Table 2.
The 31P NMR peak parameters of the PGNs.
| Position (ppm) (±1.0) | Qi species | Line width (ppm) (±2.0) | Abundance(%) (±1.0) |
|---|---|---|---|
| 1.7 | 0 | 6.7 | 1.9 |
| −8.3 | 1 | 10.1 | 59.5 |
| −21.9 | 2 | 12.5 | 38.6 |
FTIR
Figure 3 presents FTIR data for PGNs that allow assignment according to the previous FTIR studies on phosphate-based glasses.22, 25-29 The peak at 730 cm−1 can be assigned to symmetrical stretching νs ( P–O– P) mode, while the peak at 900 −1cm can be assigned to asymmetrical stretching νas (P – O – P) mode (Q2 phosphate units). The peaks at 1100 and 1235 cm−1 are also assigned to asymmetrical ν (PO3)−2 and νas (PO2) modes that can be related to Q1 and Q2 phosphate units respectively.
Figure 3.
FTIR spectrum and band assignment for PGNs (ν, stretching; s, symmetric; as, asymmetric; δ, deformation)
Ion release study
The concentration of phosphorus, calcium, and sodium released into solution at different time points are presented in Figure 4. It can be seen that the highest rate of ion release occurs within the first 4 hours over the entire study period for all the elements. It should be noted that there is a small difference between the obtained data from EDX and ICP that can be related to the dissolution behavior of phosphorus that requires the hydrolysis of P-O-P bonds prior to release, whereas other elements may be released via diffusion.30
Figure 4.
Cumulative release of phosphorus, calcium, and sodium in deionized water as a function of time for the investigated PGNs. Error bars represent the standard deviation of 3 samples.
Cytotoxicity study
We measured the cell viability with MTS and studied whether reactive oxygen species were generated with DCFDA (Fig. 5). MSCs were used for both applications because of the utility of ultrasound in cardiac stem cell therapy.14, 31 The cells were positive for CD105, CD166, CD29, CD44, CD14, CD34, and CD45 and were below passage number 5.
Figure 5. Cytotoxicty data.
Increasing concentrations of PGNs were used to label MSCs for 4 hours followed by treatment with different markers of cytotoxicity. All experiments were validated with a positive control (POS). A) Cells were plated, tagged with DCHCF, and then perturbed with PGNs. Any ROS generation resulted in fluorescence from the DCHCF probe. Only the 1000 μg/mL sample significantly upregulated ROS. A hydrogen peroxide positive control validated the assay. The negative control was wells with no cells. B) The MTS reagent measures cell metabolism and showed no decrease at any concentration studied. Here, we used both freshly dissolved PGNs (Fresh) and PGNs that had been in solution for 24 hours (Degraded). No concentration decreased metabolism. Error bars represent the standard deviation of 6 wells.
The DCFDA assay was first validated with both positive and negative controls. The negative control used wells with no cells plated, and the positive control was wells with MSCs stimulated by 10% hydrogen peroxide. The negative control had a fluorescence value of 297 ± 76 which is attributed to auto-fluorescence from the plastic well plate. The MSCs in media with neither PGNs nor H2O2 had a value of 869 ± 118. The H2O2 positive control validated the assay and was 2.2-fold higher than the control cells. The experiments with PGNs added to the MSCs showed no increase in DCFDA signal except at 1000 μg/mL. Here, the signal increased 1.6-fold to 1372 ± 41—a value that was significant at p<0.0001 versus the 0 μg/mL control. All other PGNs concentrations had p values above 0.05 indicating no generation of ROS (Fig. 5A).
We also used the MTS assay to measure any effects on cell viability and used two fractions of PGNs; freshly dissolved PGNs in PBS (Fig. 5B, Fresh) and PGNs that had been in PBS overnight (Fig. 5B, Degraded). In both cases, cells were treated with increasing concentrations of PGNs. The positive control was the known cytotoxic agent CTAB. Cells without stimulation had an optical density at 490 nm of 0.85 ± 0.06 for the fresh sample and 0.90 ± 0.05 for the degraded sample. No concentrations had a statistically significant change to baseline except the positive control, which suppressed the signal 4.3-fold and 4.1-fold for the fresh and degraded samples, respectively.
In vitro ultrasound imaging
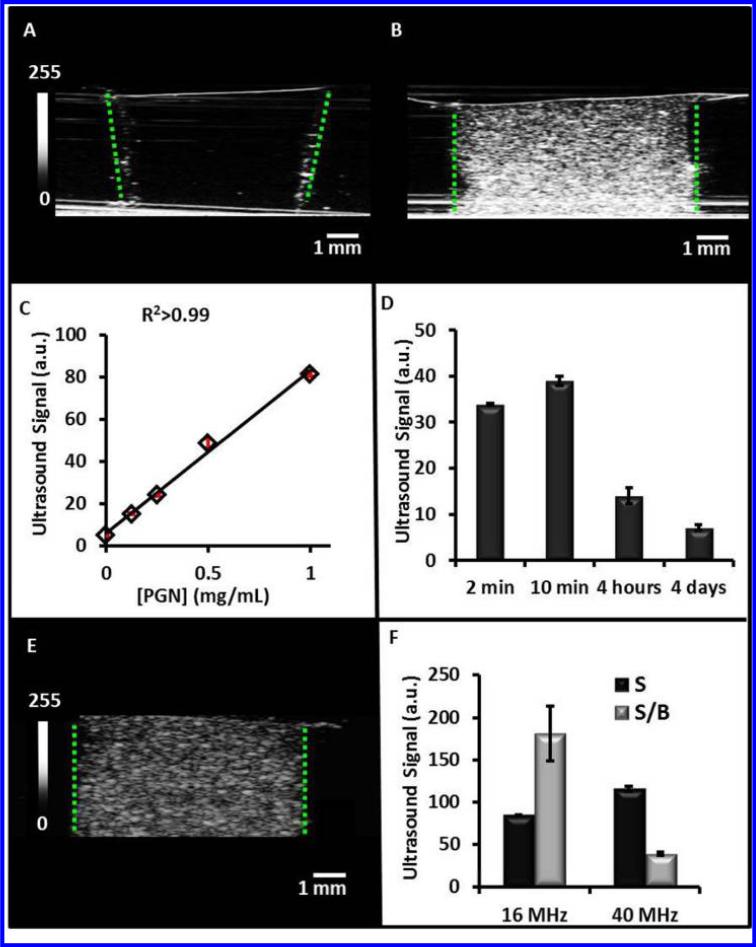
We tested the ability of the PGNs to backscatter acoustic energy with phantoms. Inclusions contain increasing concentrations of PGNs were added to the agarose support and imaged at 40 MHz (Fig. 6). In Figure 6A, the echogenicity of a 0 mg/mL water control is seen. The outline of the well can be seen clearly due to the presence of air bubbles that became trapped on the side of the well (green dashed lines). A much more intense signal is seen when an identical well is loaded with 1 mg/mL PGNs (Fig. 6B). The mean intensity of this image and at least three replicate images was measured to be 81.5 ± 1.3 a.u. This value and the value determined for the other concentrations of PGNs is plotted in Figure 6C. Using the best fit line (R2>0.99) and the background value of 5.3 ± 0.4, we estimated the limit of detection to be 5 μg/mL at three standard deviations above the mean of the background.
Figure 6. Ultrasound Signal Performance of the PGNs.
Ultrasound images in the z-axis for inclusions with 0 mg/mL PGNs (A) and 1 mg/mL PGNs at 40 MHz (B). The increased backscatter results from increasing number of particles that was further quantitated for multiple fields of view in (C). PGNs (0.25 mg/mL) at various time points were imaged with ultrasound to understand how signal changes as a function of time (D). These data indicate that ultrasound signal decreases as the nanoparticle degrades because of decreased acoustic impedance. The imaging window is within the first four hours of dissolution. Panel E is an ultrasound image in the z-axis for 1 mg/mL PGNs at 16 MHz, and F is signal and signal-to-background for both 16 and 40 MHz. Here, signal was defined as echogenicity of the sample and background was adjacent agar. While the signal was higher at 40 MHz, the signal-to-background at 16 MHz was 4.6-fold higher than 40 MHz because of a lower background signal. These data indicate that the PGNs are suitable for imaging at both clinical and pre-clinical frequencies.
To understand how biodegradation affects ultrasound signal we also imaged samples after different periods of dissolution in standard PBS (Fig. 6D). We found that the signal has a maximum near 10 minutes, but remains elevated at least 4 hours after wetting. Interestingly, the increase from 2 minutes to 10 minutes was significant (p<0.05) and suggests that the wetting of the PGNs may induce hydration that increases backscatter.
The frequency of imaging is also an important parameter. Higher frequencies have better resolution, but lower depth of penetration and the echogencity of the material may change as a function of frequency. To understand how the PGNs behave at both clinical and pre clinical frequencies, we also imaged at 16 MHz (Fig. 6E). We measured the signal of a 1 mg/mL sample of nanoparticles as well as the background signal of agar. We then plotted both the signal and signal-to-background (S/B) in Figure 6F. While the signal was 36% higher at 40 MHz, the signal-to-background at 16 MHz was 4.6-fold higher than 40 MHz because of a lower background signal. These data indicate that the PGNs are suitable for imaging at a variety of both clinical and pre-clinical frequencies.
In vivo and cellular ultrasound imaging
To understand the capability of PGNs for in vivo imaging and biodegradation, we implanted 100 μL boluses of increasing concentrations of PGNs onto the rear flake of nude mice (Fig. 7A). These samples were in a 50% matrigel carrier and this vehicle served as the 0 mg/mL or negative control. Each implant was imaged at 40 MHz and the backscatter quantitated for various concentrations in Figure 7C. The calculated limit of detection in vivo is 9 μg/mL of PGNs—a value nearly two-fold higher than in vitro analysis. This is because of the increased background signal in in vivo imaging.
Figure 7. In vivo imaging with PGNs.
Nu/nu mice received 100 μL boluses of 2 mg/mL PGNs in 1:1 matrigel:PBS and imaged at 40 MHz immediately after (A) and 1 day after (B). Red outlines in A and B indicate the region of implantation and the scale bar and intensity bar in A apply to both panels. (C) Decreasing concentrations of PGNs were also implanted to calculate the limit of detection and variance between animals. Error bars represent the standard error of measurement. (D) Changes in ultrasound backscatter for PGNs indicates a 3.5-fold decrease in signal-to-background ratio (SBR). Error bars represent the standard deviation.
We also studied the capability for in vivo biodegradation. Mice implanted with 2 mg/mL PGNs were imaged after implantation (Fig. 7A) and one day later (Fig. 7B). As expected, the matrigel and saline carriers were largely resorbed into circulation and extracellular matrix. Concurrently, the PGNs implanted began to degrade—we observed a 3.5-fold decrease in signal-to-background after one day (Fig. 7D).
A final study labelled MSCs with the PGNs. We first optimized the labelling protocol using the cell fluorescence to monitor labelling. There was a linear relationship (R2=0.98) between the starting concentration and the resulting cell fluorescence from 30 to 1000 μg/mL of PGNs at 2 hours of incubation. When the incubation time was optimized from 1 to 360 minutes at 500 μg/mL, a marked plateau effect was noted after 1 hour of incubation. Further incubation with cells did not significantly increase cell signal (p>0.05). Even 30 minutes of incubation gave cells signal that was 70% of maximum. We used these conditions to label MSCs and then imaged them with ultrasound (Fig. 8C) and fluorescence microscopy (Fig. 8D).
Figure 8. PGNs label MSCs.
The optimal labeling conditions were tested empirically including starting concentration of PGNs (A) and incubation time (B). We found a nearly linear relationship between starting concentration and cell fluorescence (A). These concentrations used 2 hours of labeling. In B, we studied the effect of time and found that incubation times beyond 2 hours offered no additional signal. (C) MSCs labelled with these conditions (500 μg/mL and 2 hours) were imaged with 40 MHz ultrasound—the limit of detection is 4000 MSCs. Phase contrast/fluorescence microscopy of naive MSCs (D) and MSCs labeled with PGNs shows that fluorescence only corresponds to the cells (low non-specific binding) (E). Panel F is ultrasound image of agarose phantom only (no cells), panel G is the ultrasound backscatter from 225,000 MSCs, and panel H is the same number MSCs labeled with PGNs in an agar phantom. The PGNs increased the backscatter two-fold versus unlabeled cells. Panel K is a confocal image through the medial slice of adherent cells labelled with fluorescently tagged PGNs. The image shows that the nanoparticles are located throughout the cell and are localized in the cytoplasm and are not simply on the cell surface.
We next used microscopy to understand the interaction of the PGNs with the MSC. Epifluorescence microscopy indicated low non-specific binding of PGNs to the culture flaks. Indeed, <5% of the fluorescent pixels do not correspond to a cell in Fig. 8E with many regions of increased signal throughout the MSC.
We also used confocal microscopy to study the distribution of the PGNs. Our goal was to determine whether the nanoparticles were on the cells or inside the cells. This is important because nanoparticles that were only on the surface could become detached after implant and incorrectly be considered a cell. The confocal data in Figure 8K shows that the nanoparticles are indeed located throughout the cell in the cytoplasm and are not simply on the cell surface.
Finally, we placed different numbers of cells in a 100 μL inclusion in an agar phantom and imaged at 40 MHz. We used this cell volume because it is similar to the injection volume used in trials of non-human primates and would likely be used in man. We noted a a linear relationship between the number of cells and the ultrasound backscatter (R2>0.99; Fig. 8C), which is important for studies in which unknown numbers of cells may need to be quantitated. The calculated limit of detection for ultrasound imaging was 4000 MSCs at three standard deviations above the mean of the background.
DISCUSSION
In the present study, we take advantage of the sol-gel preparation method to synthesise controlled degradation materials and the subsequent utility of electrospraying to prepare ternary phosphate-based sol-gel derived glass nanospheres in the diameter size range of 200-500 nm. One limitation of this approach is throughput versus “bottom-up” nanoparticles synthetic approaches, however we were able to produce 60 mg of nanoparticles per hour which is sufficient for imaging experiments. Previous approaches to preparing these glasses have been focused on preparing bulk sol-gel glasses. However, the slow sol-gel reaction and difficulty to make bulk glasses have limited their biomedical applications. In our work, faster reactive precursors and lower heat-treatment temperature were applied that allowed us to process these sol-gel derived glasses via electrospraying and also incorporate fluorescein dye whilst processing (due to the relatively benign processing temperatures).
The amorphous nature of these PGNs was confirmed by XRD analysis. The 31P NMR and FTIR results also show the structure of these PGNs consists of mainly Q1 and Q2 phosphate units. These findings confirm the amorphous and glassy structure of the PGNs that are more desirable as a biodegradable material because they do not fragment during the degradation, which may cause inflammation.32
The PGNs have strong ultrasound backscattering capabilities both in vivo and in vitro. Phantom experiments indicated a limit of detection of 5 μg/mL with linear behaviour below 1000 μg/mL (Fig. 6A). The imaging window of this material is below 4 hours. The ICP data show the highest rate of dissolution within the first 12 hours of immersion in distilled water (Fig. 4), and our time course study shows that by 4 hours the signal has decreased more than 2-fold with even further decreases the next day (Fig. 6D). This is particularly important because it bridges a gap between the minutes of stability of microbubbles and the months of stability of Stober silica particles.33, 34
One potential concern is that the high local ionic concentrations could perturb cellular function. For 0.1 mg/mL of PGNs, over the 48 hours of dissolution study and assuming no diffusion, cells would be exposed to 41 ppm (PO4)3− , 32 ppm Ca2+, and 16 ppm Na+ (Fig. 4). These values are well within the standard physiological range of serum chemistries 35. For example, the reference range of Ca2+ is 8.5-10 mg/dL or 85 – 100 ppm. The additional calcium due to the contrast agents is within the reference range and will not perturb cell metabolism. Furthermore, diffusion and normal circulation will equilibrate any locally disturbed ionic concentrations.
To further confirm this, we performed a series of cell toxicity assays. While we did find that very high doses (1000 μg/mL) increased ROS (hydroxyl, peroxyl, etc.) activity within the cell, this is much higher than the concentrations needed for imaging: 250 μg/mL for MSC imaging and >9 μg/mL for subcutaneous imaging (Fig. 5A). All materials—even water—are toxic at some concentration, our goal here was to discover what concentration began to dysregulates metabolism and work below that concentration. After PGNs implantation into animals, we noticed no changes in posture, behaviour, gait, or feeding with observation up to two weeks.
We also used the MTS assay to study cell metabolism and viability via mitochondrial reductase activity (Fig. 5). MTS is a colorimetric, water-soluble tetrazolium dye that indicates the activity of oxidoreductase enzymes. One concern was that the degradation products may increase the sodium, calcium, and phosphorus concentration to levels inconsistent with cell survival. Thus, it was important for us to study both fresh and degraded PGNs in this assay. Fortunately, we noticed no cytotoxicity in either sample group at concentrations below 500 μg/mL (Fig. 5).
Although we did not investigate tumor imaging here, the PGNs implantation experiments (Fig. 7) indicated that concentrations down to 9 μg/mL could be imaged in vivo. This is important because nanoparticle contrast agents must overcome significant competition from the reticuloendothelial system to reach target tissue. Values near 4%ID/g are common for nanoparticle contrast agents. Thus, a 1 g tumor would need at least 225 μg of material injected into the 2.5 mL murine blood pool to achieve 4%ID/g accumulation. Importantly, this concentration is well below the concentration that increased ROS generation.
Our primary goal was cell imaging, which is critical to understand the location of implanted stem cells in regenerative medicine. We achieved cell counting below 5000 cells in 100 μL volumes (Fig. 8). While we do not achieve the single cell counts possible with some MRI techniques,36 the cell counts described here are more than sufficient for clinical trials that will likely use millions of cells,37,38, 39 These low detection limits would be useful because it would allow one to not label all cells in a given population. The 4 hour imaging window would be useful for imaging the immediate implantation event in cells. Long term imaging could utilize a reporter gene.40
The detection limits reported here are higher than that achieved with perfluorocarbon microbubbles. Indeed, these can be detected even at the single microbubble level.41 The PGNs will definitely not be detectable at the single particle level, but did have detection levels down to 50 μg/mL, which is on par with the 20 μg/mL detection limits we have reported previously with silica nanoparticles.14 These materials also have lower overall signal intensity than perfluorocarbon microbubbles. However, perfluorocarbon microbubbles are approximately the same size as cells and using them for ultrasound cell imaging is difficult. Importantly, the PGNs are approximately 10-fold smaller in diameter (and 1000-fold smaller in volume) and thus have a significant advantage for ultrasound cell imaging. Also, the time stability of the PGNs (~4 hours), is much longer than the minutes of in vivo stability for perfluorocarbon microbubbles.
CONCLUSION
In this study, for the first time, according to the authors knowledge, ternary phosphate-based sol-gel glass nanospheres in the diameter size range of 200-500 nm has been successfully prepared. The amorphous and glassy nature of these PGNs was confirmed via XRD, 31P NMR, and FTIR analysis. This probe is useful for both in vivo and in vitro ultrasound imaging and has utility for cell tracking applications in regenerative medicine. Future work may utilize these contrast agents for tasks beyond regenerative medicine including targeted tumor imaging after i.v injection and tumor imaging.
MATERIALS AND METHODS
Synthesis of the sol for subsequent electrospraying
The following chemical precursors were used without further purification: triethyl phosphate (Aldrich, 99%), calcium methoxyethoxide (Ca-methoxyethoxide, ABCR, 20% in methoxyethanol), sodium methoxide solution (NaOMe, Aldrich, 30 wt% in methanol), 2-methoxyethanol (MeO-EtOH, Aldrich, 99.8%), n-dimethyl formamide (nDMF, Alfa-Aesar, 99.8%), acetone (Alfa-Aesar, 99.5%), fluorescein (Aldrich), and silicone oil (Aldrich).
The sol-gel synthesis was started by diluting triethyl phosphate in MeO-EtOH at a molar ratio of 1:3 and allowing them to react for about 10 minutes while it was magnetically stirring (the whole reaction being carried out in a dried vessel). The vessel was then cooled in an ice-bath before adding Ca-methoxyethoxide and NaOMe dropwise to the vessel. Finally, in the last stage n-DMF (a molar ratio of 0.25:1) was added to the solution and a homogeneous solution was obtained after 10 min of stirring. For labelling experiments, 1 wt% fluorescein dye was incorporated to a subset of the mixture.
Electrospraying
The mixtures were aged overnight at 60 °C before being electrosprayed over the heated silicone oil. The electrospraying technique used a stainless steel capillary tube (18 gauge), syringe pump, high voltage source, and silicone oil as a substrate. The distance between the nozzle and substrate was 10 cm and the temperature of the substrate was controlled and kept at 150 °C. The flow rate was 0.20 mL h−1 and high voltage of 20 kV was applied between the nozzle and the ground. The particles were separated from silicone oil via centrifugation at 4600 rpm for 30 min. The collected particles were washed three times with acetone and then dried at 180 °C for 2 h to evaporate the acetone and other remaining solvents.
Characterization
SEM and EDX
SEM images were obtained on a Philips-XL30 instrument (Philips, Netherland) at an accelerating voltage of 5 kV and working distance of 10 mm. The PGNs were mounted onto a stub using araldite and sputter-coated with gold alloys. The diameter was measured for more than 100 particles using Image-pro plus software (Media Cybernetics, USA). To determine the exact composition of the PGNs, EDX-Inca 300 (Oxford instrument, UK) was used. Scanning electron microscopy (SEM)-XL30 was operated at 20 kV, spot size 5, and working distance of 10 mm to identify the particular elements and their relative proportions from the scanned area.
XRD and 31P MAS-NMR
The XRD on PGNs was obtained on a Bruker-D8 Advance Diffractometer (Brüker, UK) in a flat plate geometry using Ni filtered Cu Kα radiation. Data were collected using a Lynx Eye detector with a step size of 0.02° over an angular range of 2Θ=10-100° and a count time of 12 sec.
The solid state 31P MAS-NMR spectrum was recorded at 161.87 MHz using a Varian VNMRS-400 spectrometer. The sample was loaded into a 4 mm (rotor o.d.) magic angle spinning probe and the spectrum was obtained using direct excitation (with a 90° pulse) with a 60 sec recycle delay at ambient probe temperature (~25 °C) at a sample spin rate of 10 kHz and between 20 and 88 repetitions were accumulated. The deconvolution of the spectrum was carried out using DMFit software and was referenced to the resonance of the ammonium dihydrogen phosphate (NH4H2PO4) at 0.9 ppm (relative to 85% H3PO4 solution at 0 ppm).42
FTIR
The FTIR spectrum of PGNs was collected using a Perkin Elmer spectrometer 2000 (USA). The sample was scanned at room temperature in the absorbance mode in the range 1500-600 cm−1 and the data were analysed by Perkin Elmer software.
Tissue Culture, Labelling, and Cytotoxicity
Mesenchymal stem cells (MSCs) and culture media were purchased from Lonza (p/n 2501 and 3001, respectively). Cells were seeded at 5000 cells/cm2, and the media was changed every 2-3 days. Cell labeling used MSCs plated at 5000 cells/cm2 in a 225 cm2 flask and grown to 80% confluence. For labeling optimization studies we used 12 well plates and the same seeding density. The day of labeling, we prepared the PGN contrast agent in PBS at 25 mg/mL. For optimizing the labeling experiments, decreasing volumes of this sample were then added to MSCs in media. The cells were exposed to PGN concentrations of 30 – 1000 μg/mL in the media; the NPs were then incubated with the cells for 2 hours and then washed thrice with PBS. To study the effect of time, we used 500 μg/mL of PGNs from 1 – 360 minutes. For labelling experiments, we used fluorescently-tagged PGNs. Cell fluorescence was measured with a plate reader (Bio-TEK), and fluorescence microscopy used a EVOS Fl (Invitrogen). Confocal microscopy used a Leica confocal microscope (TCS SP5) with a 20x APO objective (p/n 506147) seated on a Virbraplane air table. For z-stacking experiments, 20 slices were collected over ~ 50 μm. Video was made by compiling the individual TIFF files into an .avi file.
For the cell counting experiments, we used the optimized protocol of 2 hours and 250 μg/mL. After labeling, the cells were washed three times with saline. We monitored the washes with absorbance spectroscopy to confirm that the third wash containined no more nanoparticles. The cells were then removed from the flask with TrypLE Express (Invitrogen), washed with complete media to deactivate the trypsin, centrifuged at 300g at 4 °C for 5 minutes and resuspended in saline. The cells were counted with an automated hemocytometer before being placed in a phantom and imaged.
For cell viability, cells were plated in a 96 well plate and allowed to adhere overnight. The next day, increasing concentrations of PGNs were added and allowed to incubate for 4 hours. For a positive control, we added 10 μL of 1 mg/mL cetyl trimethylammonium bromide (CTAB) in phosphate buffered saline. We assessed cell viability using the tetrazolium-based metabolic reagent compound 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-(4-sulfophenyl) -2H-tetrazolium (MTS; Promega). The solution was used as received—20 μL of MTS solution was added to each well in a 96 well plate containing cells and allowed to incubate for 3 hours. Absorbance at 490 nm was measured to estimate cell viability. Each condition was tested with at least four replicates.
To determine whether the particles generated any reactive oxygen species, we utilized 2’,7’ –dichlorofluorescin diacetate (DCFDA; Biotium). We first made a 1 mg/mL working solution of DCFDA in dimethyl sulfoxide and then diluted 125 μL of the DMSO solution to 10 mL of Hanks Buffered Saline Solution (HBSS). Cells were washed with fresh HBSS and then 100 μL of the DCFDA solution in HBSS was added; the plate was incubated at 37 °C for 1 hour. Next, the labelling solution was aspirated and the cells again washed with fresh HBSS. Increasing concentrations of particles in 100 μL were then added. Controls include 10% H2O2 and wells with no cells. The fluorescence of each well was measured 1 hour later.
Biodegradation Monitoring
Biodegradation was monitored via inductively coupled plasma mass spectroscopy (ICP-MS). The experiment used 0.1 mg/mL samples in deionized water at 37 °C with three replicates at the following time points: 0, 0.2, 0.5, 1, 3, 6, 12, 24, and 48 hours. The tubes were secured to a tube rotation device set to 5 rotations per minute. This device was in a 37 °C incubator under ambient humidity. After each time point, the resulting suspension was centrifuged at 4600 rpm for 30 min to separate the PGNs from the solution. The liquid phase was then extracted and the PGNs were dried in the oven at 60 °C before they were resuspended in fresh deionized water. The release of calcium, phosphorus, and sodium in solution was measured by using a Spectro Mass 2000 Analytical System calibrated across the predicted concentrations range 0 ppb – 4000 ppb by dilution of 100 ppm elemental standards. Calcium standard was obtained as part of a pre-made standard solution (Fluka) whereas phosphorus and sodium standards were created from analytical grade K2HPO4 (Sigma) and NaNO3 (Sigma) salts respectively. Standards were first diluted in deionized water to result in the desired concentration range. Both samples and standards were diluted in 1:1 in 4% HNO3 (Fluka) and analysed in reference to a blank (2% HNO3) solution under standard operating conditions (Power: 1350 W; Coolant Flow: 15 L/min: Auxiliary Flow: 1 L/min). Results were expressed as a cumulative ion release over the full period of the degradation study.
In vitro ultrasound imaging
To measure the echogenicity of the nanoparticles, we placed increasing concentrations in a 1% agarose phantom. Ultrasound backscatter mode (B-mode) images were collected with a Visualsonics 2100 scanner with a MS-550 transducer (Fuji) for 40 MHz centre frequency imaging or a MS-250 transducer for 16 MHz. Other imaging parameters included 100% power, 35 dB gain and a 60 dB dynamic range. At least three frames were collected for each sample and saved as TIFF files and were quantitated with ImageJ software using region of interest analysis and 8-bit depth as the the dependant variable (0-255) 43. To calculate the limit of detection, we used three standard deviations above the mean of the blank.
In vivo ultrasound imaging
All animal studies were approved by the Administrative Panel for Laboratory Animal Care (IACUC) at Stanford University. Nude mice aged 10-14 weeks were anaesthetized with 2% isofluorane in oxygen at 2 L/min. The PGNs were dissolved in 50:50 matrigel:PBS at 2.0, 0.25, and 0.1 mg/mL. The injection volume was 100 μL that was subcutaneously implanted on the rear flank of the mice. The animals were imaged 10 minutes after implantation after the matrigel had solidified using 40 MHz centre frequency, 100% power, 35 dB gain, and a 60 dB dynamic range. At least three frames were collected for each sample and saved as TIFF files and images were quantitated with ImageJ. For longitudinal imaging the injection site was marked and the animal returned to the same position the next day for identical imaging conditions.
ACKNOWLEDGEMENTS
JVJ acknowledges support from NHLBI (K99-HL117048) and the Burroughs Wellcome Fund (1011172). Instrumentation used in this work was supported by NIH grant S10-OD010344. This work was supported in part by the Priority Research Centers Program (No. 2009-0093829) through the National Research Foundation, Republic of Korea. The authors would like to thank to the EPSRC NMR Service at Chemistry Department-Durham University for the MAS-NMR measurement.
REFERENCES
- 1.Goldberg BB, Liu JB. Contrast Agents in Abdominal Ultrasound. Front Gastroint Res. 1997;24:323–341. [Google Scholar]
- 2.Benchimol MJ, Hsu MJ, Schutt CE, Hall DJ, Mattrey RF, Esener SC. Phospholipid/Carbocyanine Dye Shelled Microbubbles as Ultrasound-Modulated Fluorescent Contrast Agents. Soft Matter. 2013;9:2384–2388. doi: 10.1039/C2SM26900G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakatsuka MA, Hsu MJ, Esener SC, Cha JN, Goodwin AP. DNA-Coated Microbubbles with Biochemically Tunable Ultrasound Contrast Activity. Adv. Mater. 2011;23:4908–4912. doi: 10.1002/adma.201102677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Willmann JK, Paulmurugan R, Chen K, Gheysens O, Rodriguez-Porcel M, Lutz AM, Chen IY, Chen X, Gambhir SS. Us Imaging of Tumor Angiogenesis with Microbubbles Targeted to Vascular Endothelial Growth Factor Receptor Type 2 in Mice. Radiology. 2008;246:508–518. doi: 10.1148/radiol.2462070536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gramiak R, Shah PM. Echocardiography of the Aortic Root. Invest Radiol. 1968;3:356–366. doi: 10.1097/00004424-196809000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Klibanov AL. Targeted Delivery of Gas-Filled Microspheres, Contrast Agents for Ultrasound Imaging. Advanced Drug Delivery Reviews. 1999;37:139–157. doi: 10.1016/s0169-409x(98)00104-5. [DOI] [PubMed] [Google Scholar]
- 7.Schutt EG, Klein DH, Mattrey RM, Riess JG. Injectable Microbubbles as Contrast Agents for Diagnostic Ultrasound Imaging: The Key Role of Perfluorochemicals. Angew Chem Int Edit. 2003;42:3218–3235. doi: 10.1002/anie.200200550. [DOI] [PubMed] [Google Scholar]
- 8.Kono Y, Holscher T, Mattrey RF. Use of Ultrasound Microbubbles for Vascular Imaging. Fund Biomed Technol. 2008:311–325. [Google Scholar]
- 9.Klibanov AL. Ligand-Carrying Gas-Filled Microbubbles: Ultrasound Contrast Agents for Targeted Molecular Imaging. Bioconjugate Chem. 2005;16:9–17. doi: 10.1021/bc049898y. [DOI] [PubMed] [Google Scholar]
- 10.Xing ZW, Wang JR, Ke HT, Zhao B, Yue XL, Dai ZF, Liu JB. The Fabrication of Novel Nanobubble Ultrasound Contrast Agent for Potential Tumor Imaging. Nanotechnology. 2010:21. doi: 10.1088/0957-4484/21/14/145607. [DOI] [PubMed] [Google Scholar]
- 11.Shapiro MG, Goodwill PW, Neogy A, Yin M, Foster FS, Schaffer DV, Conolly SM. Biogenic Gas Nanostructures as Ultrasonic Molecular Reporters. Nat Nanotechnol. 2014;9:311–316. doi: 10.1038/nnano.2014.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krupka TM, Solorio L, Wilson RE, Wu HP, Azar N, Exner AA. Formulation and Characterization of Echogenic Lipid-Pluronic Nanobubbles. Mol Pharmaceut. 2010;7:49–59. doi: 10.1021/mp9001816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casciaro S, Conversano F, Ragusa A, Malvindi MA, Franchini R, Greco A, Pellegrino T, Gigli G. Optimal Enhancement Configuration of Silica Nanoparticles for Ultrasound Imaging and Automatic Detection at Conventional Diagnostic Frequencies. Invest Radiol. 2010;45:715–724. doi: 10.1097/RLI.0b013e3181e6f42f. [DOI] [PubMed] [Google Scholar]
- 14.Jokerst JV, Khademi C, Gambhir SS. Intracellular Aggregation of Multimodal Silica Nanoparticles for Ultrasound-Guided Stem Cell Implantation. Sci Transl Med. 2013:5. doi: 10.1126/scitranslmed.3005228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liberman A, Wu Z, Barback CV, Viveros R, Blair SL, Ellies LG, Vera DR, Mattrey RF, Kummel AC, Trogler WC. Color Doppler Ultrasound and Gamma Imaging of Intratumorally Injected 500 Nm Iron Silica Nanoshells. Acs Nano. 2013;7:6367–6377. doi: 10.1021/nn402507d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ta CN, Liberman A, Martinez HP, Barback CV, Mattrey RF, Blair SL, Trogler WC, Kummel AC, Wu Z. Integrated Processing of Contrast Pulse Sequencing Ultrasound Imaging for Enhanced Active Contrast of Hollow Gas Filled Silica Nanoshells and Microshells. J Vac Sci Technol B. 2012:30. doi: 10.1116/1.3694835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinez HP, Kono Y, Blair SL, Sandoval S, Wang-Rodriguez J, Mattrey RF, Kummel AC, Trogler WC. Hard Shell Gas-Filled Contrast Enhancement Particles for Colour Doppler Ultrasound Imaging of Tumors. Medchemcomm. 2010;1:266–270. doi: 10.1039/c0md00139b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malvindi MA, Greco A, Conversano F, Figuerola A, Corti M, Bonora M, Lascialfari A, Doumari HA, Moscardini M, Cingolani R, et al. Magnetic/Silica Nanocomposites as Dual-Mode Contrast Agents for Combined Magnetic Resonance Imaging and Ultrasonography. Adv Funct Mater. 2011;21:2548–2555. [Google Scholar]
- 19.Knowles JC. Phosphate Based Glasses for Biomedical Applications. Journal of Materials Chemistry. 2003;13:2395. [Google Scholar]
- 20.Hench LL, Polak JM. Third-Generation Biomedical Materials. Science. 2002;295:1014–+. doi: 10.1126/science.1067404. [DOI] [PubMed] [Google Scholar]
- 21.Franks K, Abrahams I, Georgiou G, Knowles JC. Investigation of Thermal Parameters and Crystallisation in a Ternary Cao-Na(2)O-P(2)O(5)-Based Glass System. Biomaterials. 2001;22:497–501. doi: 10.1016/s0142-9612(00)00207-6. [DOI] [PubMed] [Google Scholar]
- 22.Pickup DM, Guerry P, Moss RM, Knowles JC, Smith ME, Newport RJ. New Sol–Gel Synthesis of a (Cao)0.3(Na2o)0.2(P2o5)0.5 Bioresorbable Glass and Its Structural Characterisation. Journal of Materials Chemistry. 2007;17:4777. [Google Scholar]
- 23.Lee IH, Foroutan F, Lakhkar NJ, Gong MS, Knowles JC. Sol-Gel Synthesis and Structural Characterization of P2o5-Cao-Na2o Glasses. Phys. Chem. Glasses-Eur. J. Glass Sci. Technol. Part B. 2013;54:115–120. [Google Scholar]
- 24.Carta D, Knowles JC, Smith ME, Newport RJ. Synthesis and Structural Characterization of P(2)O(5)-Cao Na(2)O Sol-Gel Materials. J. Non-Cryst. Solids. 2007;353:1141–1149. [Google Scholar]
- 25.Baia L, Muresan D, Baia M, Popp J, Simon S. Structural Properties of Silver Nanoclusters-Phosphate Glass Composites. Vibrational Spectroscopy. 2007;43:313–318. [Google Scholar]
- 26.Byun JO, Kim BH, Hong KS, Jung HJ, Lee SW, Izyneev AA. Properties and Structure of Ro-Na2o-Al2o3-P2o5 (R=Mg, Ca, Sr, Ba) Glasses. J. Non-Cryst. Solids. 1995;190:288–295. [Google Scholar]
- 27.Ilieva D, Jivov B, Kovacheva D, Tsacheva T, Dimitriev Y, Bogachev G, Petkov C. Ft-Ir and Raman Spectra of Gd Phosphate Crystals and Glasses. J. Non-Cryst. Solids. 2001;293:562–568. [Google Scholar]
- 28.Moustafa YM, El-Egili K. Infrared Spectra of Sodium Phosphate Glasses. Journal of Non-Crystalline Solids. 1998;240:144–153. [Google Scholar]
- 29.Sene FF, Martinelli JR, Gomes L. Synthesis and Characterization of Niobium Phosphate Glasses Containing Barium and Potassium. J. Non-Cryst. Solids. 2004;348:30–37. [Google Scholar]
- 30.Bunker BC, Arnold GW, Wilder JA. Phosphate-Glass Dissolution in Aqueous-Solutions. Journal of Non-Crystalline Solids. 1984;64:291–316. [Google Scholar]
- 31.Hare JM, Fishman JE, Gerstenblith G, Velazquez DLD, Zambrano JP, Suncion VY, Tracy M, Ghersin E, Johnston PV, Brinker JA, et al. Comparison of Allogeneic Vs Autologous Bone Marrow-Derived Mesenchymal Stem Cells Delivered by Transendocardial Injection in Patients with Ischemic Cardiomyopathy the Poseidon Randomized Trial. Jama-J Am Med Assoc. 2012;308:2369–2379. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bostman OM, Pihlajamaki HK. Adverse Tissue Reactions to Bioabsorbable Fixation Devices. Clin Orthop Relat R. 2000:216–227. [PubMed] [Google Scholar]
- 33.Hudson SP, Padera RF, Langer R, Kohane DS. The Biocompatibility of Mesoporous Silicates. Biomaterials. 2008;29:4045–4055. doi: 10.1016/j.biomaterials.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He QJ, Zhang ZW, Gao F, Li YP, Shi JL. In Vivo Biodistribution and Urinary Excretion of Mesoporous Silica Nanoparticles: Effects of Particle Size and Pegylation. Small. 2011;7:271–280. doi: 10.1002/smll.201001459. [DOI] [PubMed] [Google Scholar]
- 35.Bishop ML. Clinical Chemistry. 6th ed. 2010.
- 36.Shapiro EM, Sharer K, Skrtic S, Koretsky AP. In Vivo Detection of Single Cells by Mri. Magn Reson Med. 2006;55:242–249. doi: 10.1002/mrm.20718. [DOI] [PubMed] [Google Scholar]
- 37.Chong JJH, Yang XL, Don CW, Minami E, Liu YW, Weyers JJ, Mahoney WM, Van Biber B, Cook SM, Palpant NJ, et al. Human Embryonic-Stem-Cell-Derived Cardiomyocytesregenerate Non-Humanprimate Hearts. Nature. 2014;510:273–+. doi: 10.1038/nature13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hare J, Fishman J, Gerstenblith G. The Poseidon Randomized Trial. JAMA. 2012;308:2369–2379. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, Czer LS, Marbán L, Mendizabal A, Johnston PV. Intracoronary Cardiosphere-Derived Cells for Heart Regeneration after Myocardial Infarction (Caduceus): A Prospective, Randomised Phase 1 Trial. The Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yaghoubi SS, Couto MA, Chen C-C, Polavaram L, Cui G, Sen L, Gambhir SS. Preclinical Safety Evaluation of 18f-Fhbg: A Pet Reporter Probe for Imaging Herpes Simplex Virus Type 1 Thymidine Kinase (Hsv1-Tk) or Mutant Hsv1-Sr39tk's Expression. J. Nucl. Med. 2006;47:706–715. [PubMed] [Google Scholar]
- 41.Klibanov AL, Rasche PT, Hughes MS, Wojdyla JK, Galen KP, Wible JH, Brandenburger GH. Detection of Individual Microbubbles of Ultrasound Contrast Agents - Imaging of Free-Floating and Targeted Bubbles. Invest Radiol. 2004;39:187–195. doi: 10.1097/01.rli.0000115926.96796.75. [DOI] [PubMed] [Google Scholar]
- 42.Massiot D, Fayon F, Capron M, King I, Le Calv S. p., Alonso B, Durand J-O, Bujoli B, Gan Z, Hoatson G. Modelling One- and Two-Dimensional Solid-State Nmr Spectra. Magn. Reson. Chem. 2002;40:70–76. [Google Scholar]
- 43.Schneider CA, Rasband WS, Eliceiri KW. Nih Image to Imagej: 25 Years of Image Analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]