Abstract
Objective
The purpose of this study was to estimate the effectiveness of glucosamine and chondroitin in relieving knee symptoms and slowing disease progression among patients with knee osteoarthritis (OA).
Methods
The 4-year follow-up data from Osteoarthritis Initiative were analyzed. We used a “new-user” design, for which only participants who were not using glucosamine/chondroitin at baseline were included in analyses (n=1,625). Cumulative exposure was calculated as the number of visits when participants reported use of glucosamine/chondroitin. Knee symptoms were measured with WOMAC scale and structural progression was measured with joint space width (JSW). To control for the time-varying confounders that might be influenced by prior treatments, we used marginal structural models to estimate the effects of using glucosamine/chondroitin for three years, two years and one year on treating OA.
Results
During the study period, 18% of the participants initiated treatment with glucosamine/chondroitin. After adjustment for potential confounders with marginal structural models, we found no clinically significant differences between users at all assessments and never-users of glucosamine/chondroitin in WOMAC Pain: 0.68 (95% CI: -0.16 to 1.53); WOMAC Stiffness: 0.41 (95% CI: 0 to 0.82); WOMAC Function: 1.28 (95% CI: -1.23 to 3.79); or JSW: 0.11 (95% CI: -0.21 to 0.44).
Conclusions
Use of glucosamine/chondroitin did not appear to relieve symptoms or modify disease progression among patients with radiographically confirmed OA. Our findings, which are consistent with meta-analyses of clinical trials, extend the results to a more general population with knee OA.
Osteoarthritis (OA) is the most common type of arthritis and a leading cause of pain and physical disability in older adults (1). Although currently no effective remedies for OA exist, clinical guidelines recommend both pharmacological and non-pharmacological therapies to relieve symptoms (2). In the United States, glucosamine and chondroitin are two dietary supplements that are commonly used among patients with OA (3). Both glucosamine and chondroitin are essential components of the proteoglycans in normal cartilage and were purported to provide substrate for the biosynthesis of proteoglycans (4). In vitro and animal studies suggest that glucosamine and chondroitin simulate the synthesis of proteoglycans and inhibit the synthesis of proteolytic enzymes that lead to the premature breakdown of cartilage (5,6).
Despite the biologic plausibility, evidence regarding the efficacy of glucosamine and chondroitin in relieving OA symptoms and modifying structural progression is not established. Several meta-analyses which pooled results from existent randomized clinical trials that assessed symptomatic benefits reported substantial heterogeneity in findings across studies (7-10). Differences in study quality, preparation of the interventions, industry involvement and study size may have explained the observed heterogeneity (7-10). Large-scale trials with high quality and little connection to industry often reported a much smaller effect size of symptoms relief than earlier small industry-funded studies (8,9). Regarding the efficacy of glucosamine and chondroitin in modifying disease progression, several meta-analyses (11,12) reported small to moderate effect sizes and found that studies with longer intervention periods demonstrated a stronger effect of glucosamine on slowing joint space narrowing than studies with a shorter treatment period, whereas another meta-analysis concluded no benefits from glucosamine or chondroitin treatment (13).
The purpose of this study was to quantify the effectiveness of glucosamine and chondroitin in relieving OA symptoms and modifying structural progression. This study is warranted for several reasons. First, in the United States, glucosamine and chondroitin are almost always sold in a combination pill (14). Despite the extensive research on single treatment with glucosamine or chondroitin, studies of the combined treatment are sparse (13). Second, to our knowledge, the longest studies were three-year trials conducted more than a decade ago in Europe and supported by one pharmaceutical company (15,16). The Osteoarthritis Initiative (OAI) provides a unique opportunity to examine the long-term effectiveness of glucosamine and chondroitin on treating OA, because it administered comprehensive measurements on treatment use and changes in knee symptoms and joint structure for up to four years (17). Third, efficacy evidence of a treatment derived from clinical trials is often limited in generalizability because they often use strict study protocols and highly selected patients and are typically conducted at large medical centers (18). Non-experimental studies, on the other hand, can provide clinicians and patients with a more realistic expectation for treatment benefits in real-world environments (18). We are aware of one non-experimental study which assessed the impact of glucosamine and chondroitin on slowing structural progression (19). Our study used different designs and analytic methods and extended their work by assessing both symptoms relief and reduction in structural progression.
Patients and Methods
The Institutional Review Boards of the University of Massachusetts Medical School and the Memorial Hospital of Rhode Island approved this study.
Data source and study sample
This study used publicly available data from the OAI (http://oai.epi-ucsf.org/). From 2004 to 2006, four study sites (i.e., Baltimore, MD; Columbus, OH; Pittsburgh, PA; and Pawtucket, RI) enrolled 4,796 residents who had established or were at high risk for developing knee OA (17). The detailed OAI protocol can be found elsewhere (17). Follow-up information for up to four years was used (the dataset version numbers are 0.2.2, 1.2.1, 3.2.1, 5.2.1, and 6.2.2). Inclusion/exclusion criteria are shown in Figure 1. We included OAI participants with radiographic knee OA at enrollment, i.e., those with a Kellgren-Lawrence (K-L) grade of 2 or greater in at least one knee (n=2,539).
Figure 1. Flow-chart of identifying study samples.
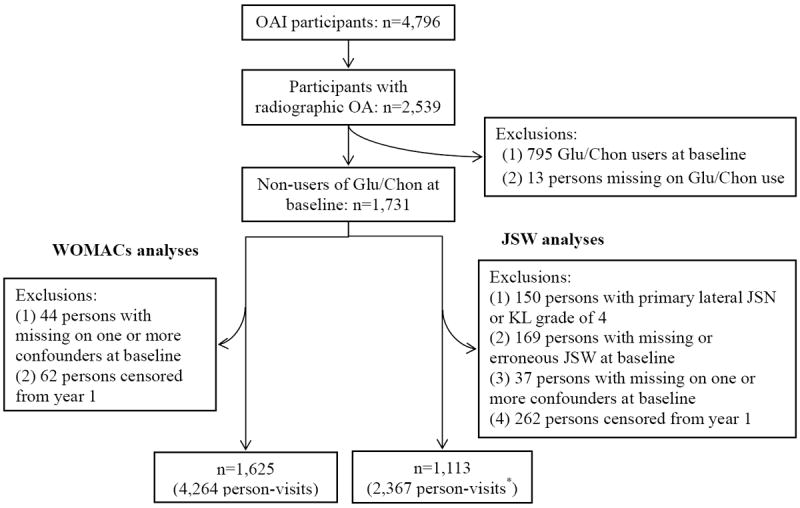
Glu/Chon: glucosamine/chondroitin; JSN: joint space narrowing.
*295 persons were further censored at visits from year 2 to year 4 because the JSW measures were missing or invalid due to poor knee positioning.
To improve study validity, we used a “new-user” design (20), for which only participants not reporting use of glucosamine or chondroitin at baseline were included in analyses (n=1,731). From this group, we then identified two samples: 1) for analysis of symptoms and 2) for the analysis of structural changes. For the analysis of symptoms, we also excluded participants with missing data on key confounders at baseline (n=44) and those missing exposure or outcome data at year 1 of the study (n=62). When analyzing structural progression, we excluded persons with following characteristics: 1) end-stage OA (i.e., K-L grade 4) or primary joint space narrowing in the lateral tibiofemoral compartment at baseline (n=150); 2) missing measures of joint space width (JSW) or JSW measures with a poor alignment of the tibial plateau at baseline (n=169); or 3) missing key confounders at baseline (n=37) or exposure or outcome at year 1 (n=262). The remaining 1,625 participants with 4,264 person-visits contributed to the analyses of symptoms, and 1,113 participants with 2,367 person-visits were included in analyses of structural changes.
Exposure definition
Use of glucosamine and chondroitin was defined based on self-reported information. At baseline and annual follow-up visits, participants were asked “During the past 6 months, did you use the following health supplements for joint pain or arthritis?” with separate questions for glucosamine and chondroitin sulfate use. The frequency of using the supplements was further inquired among those who reported ever-use in the past 6 months. We considered a participant taking glucosamine or chondroitin if he/she reported using it for at least 4 days per week, and not taking the supplement if they reported not using it or using it for less than 4 days per week. Throughout the study period, ~90% of the participants taking either one of the supplements were taking both concurrently. So at each visit we defined use of glucosamine/chondroitin as taking either of these supplements. To estimate the overall treatment effects, we calculated the cumulative exposure by summing the number of visits when participants reported using glucosamine/chondroitin.
Assessment of OA symptoms
If both knees had radiographic OA, we used measurements from the knee with more severe pain at baseline. OA symptoms and function were assessed annually with Western Ontario and McMaster Universities Arthritis Index (WOMAC) (the Likert version 3.1). WOMAC measures three separate domains: Pain (5 items), Stiffness (2 items), and Physical Function (17 items) (21). Each scale item uses a range of 5 Likert responses, ranging from ‘0=none’ to ‘4=extreme’. Responses to items in each domain were summed to produce subscale score, which ranges from 0~20 for Pain, 0~8 for Stiffness and 0~68 for Physical Function. Larger WOMAC scores represent worse symptoms or knee-related function.
Assessment of JSW
If both knees had radiographic OA, we used measures from the knee with narrower space width in the medial tibiofemural joint at baseline. Joint structural progression was measured with changes in medial JSW during follow-up from baseline. All participants at baseline and annual visits had bilateral standing knee x-rays obtained in posterior anterior projection with knees flexed to 20-30 degrees and feet internally rotated 10 degrees (17). Longitudinal measurements of JSW from serial knee x-rays were conducted through a customized software tool, which automatically delineated the margin of the femoral condyle and the tibial plateau (22). Multiple JSWs were measured at fixed locations along the joint. An anatomical coordinate system, which extended from the medial end (x=0) to the lateral end (x=1) in the joint space, was defined to facilitate an objective determination of measurement location. We chose the JSW measure at x=0.25 (in the medial compartment) because it was demonstrated to have best responsiveness to changes (23).
At each assessment of JSW, the distance from tibial plateau to tibial rim closest to femoral condyle was measured to indicate knee positioning (24). To take into account the potential error in JSW measurement due to poor knee positioning at a single visit or inconsistent positioning between visits, we did not use the JSW measures (i.e., considered them missing) if the plateau to rim distance was larger than 6.5 mm or change in this distance between visits was greater than 2 mm (24). Among the 431 persons who were excluded at baseline (n=169) and year 1 (n=262) due to not having a valid measure of exposure or JSW (shown in Figure 1), 355 (82%) were excluded due to a poor or inconsistent knee positioning at JSW measurement. An additional 181 persons had inconsistent knee positioning for ≥1 assessment at year 2 to year 4. The probability of having a potentially erroneous JSW measure at following assessments was comparable among users (10.7%) and non-users (11.4%) of glucosamine/chondroitin.
Measurement of potential confounders
The following variables were considered potential confounders: sociodemographics, clinical characteristics of OA, indices of general health status, body mass index (BMI), and use of treatments other than glucosamine/chondroitin. Our previous work has shown that use of glucosamine and chondroitin was more prevalent among older adults, women, non-Hispanic Whites, individuals with higher education or higher income (3). Income was defined as personal family income for the last year, including all sources such as wages, salaries, social security and retirement benefits.
OAI administered comprehensive measurements on participants’ clinical characteristics, including knee alignment, multi-joint symptoms, K-L grade, and history of having a knee injury or surgery (17). Knee alignment was measured with a goniometer, and varus or valgus deformity was recorded if malalignment was found. We considered the participants had multi-joint symptoms if they reported frequent pain, aching, or stiffness in at least two joints other than knee (25). Information was also collected on history of having a knee injury that limited ability to walk for at least two days, and history of having knee surgery including arthroscopy, ligament repair or meniscectomy.
The 12-item Short-Form Health Survey (SF-12) provided an assessment of general health status (26). Answers to the 12 questions were combined to generate Physical and Mental Component Summary scores, which range from 0 to 100, with higher scores indicating better health status. BMI has been reported as a risk factor for OA progression due to its potential local biomechanical effect and systemic metabolic effect (27). We calculated BMI from measured height and weight [weight (kg)/height (m2)]. Participants with a BMI less than 25 were defined as having normal weight, 25 and less than 30 as overweight, and 30 and over obese.
Trained interviewers obtained information about use of other arthritis treatments, including conventional medications and complementary and alternative medicine (CAM). At each visit, separate dummy variables were generated to indicate use of acetaminophen, non-steroidal anti-inflammatory agents (NSAIDs) and opioids in the past 30 days. Use of acetaminophen and NSAIDs included use of prescriptions and/or over-the-counter medications. Use of CAM, which was surveyed at baseline and year 2, covered therapies commonly used in the United States, including alternative medical systems, mind-body interventions, manipulation and body-based methods, energy therapies and biologically based therapies (28).
Sociodemographics and history of a knee surgery were considered invariant and all other potential confounders were considered time-varying during the study period. For participants missing information on the time-varying variables, we imputed missing values with the last observation carried forward (29).
Statistical analyses
We first described baseline sociodemographic and clinical characteristics of study participants by status of glucosamine/chondroitin use at year 1. No departures from normality were observed in the outcome variables - defined as the changes in from baseline. When estimating the overall effects of glucosamine/chondroitin use, we chose marginal structural models (MSMs) as the primary analytic method because we hypothesized that the data structure involved time-varying confounders that were influenced by previous treatments (30). Figure 2 depicts the hypothesized relationships between glucosamine/chondroitin use, study outcomes, and potential time-varying confounders. Previously measured study outcomes and time-varying confounders may be simultaneously confounders and intermediate variables. For instance, when studying WOMAC Pain as the outcome, the Pain score measured at the previous visit can be a potential confounder because it correlates with Pain score measured at current visit and patients with more severe pain are more likely to use glucosamine/chondroitin (3). Furthermore, if glucosamine/chondroitin is effective in relieving pain (which is a hypothesis tested in our study), the previously measured Pain score lies on the causal path from prior treatment use and currently measured WOMAC Pain. If so, standard regression models adjusting for previous pain severity will produce biased estimates of the overall treatment effects (31).
Figure 2. Hypothesized causal relationships between glucosamine/chondroitin treatment, study outcomes and potential time-varying confounders.
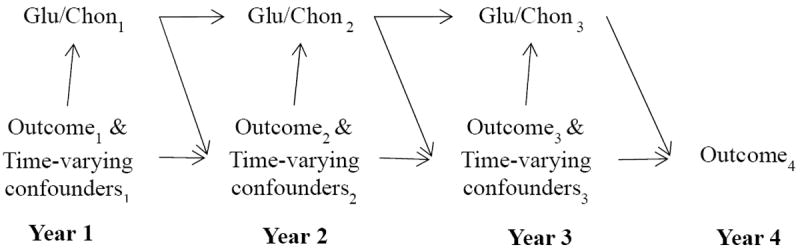
Glu/Chon denotes treatment with glucosamine/chondroitin and the subscript number denotes the follow-up time (year) when the information was measured.
MSMs rely on inverse probability weighting to adjust for time-varying confounding (30). At each visit, we estimated the conditional probability of receiving observed treatment with glucosamine/chondroitin given baseline characteristics and time-varying confounders (including WOMAC subscale, K-L grade, SF-12 subscales, BMI, knee alignment, prior incidence of knee injury, use of analgesics and CAMs) that were measured at the same visit as use of glucosamine/chondroitin. For each specific WOMAC outcome, we adjusted for only the same previously-measured subscale as potential confounder. When analyzing JSW, we adjusted for previously measured WOMAC Pain because we found it a stronger correlate with treatment use than Stiffness and Physical Function (3).
The inverse of the conditional probability was stabilized with the conditional probability of receiving observed treatment given baseline covariates. Conditional probabilities in numerator and denominator were estimated with logistic regression models (i.e., treatment models) (30). To take into account that associations of confounders to treatment initiation may be different from their associations to treatment continuation, we fit treatment models stratified by previous treatment status (32). Specifically, the treatment models estimated the probability of initiating treatment among those not using treatment at previous visit and the probability of continuing the treatment among those reporting use at previous visit.
Patients were excluded from analyses at the first occurrence of loss-to-follow-up, undergoing total knee replacement, or missing information on glucosamine/chondroitin use or outcome, whichever came first. To address the potential bias from informative dropout, we incorporated inverse-probability-of-censoring weighting in analyses (30). At each visit from year 2 to year 4, “censoring” status was categorized as follows: 1) not censored; 2) censored due to illness/death/total knee replacement; 3) censored due to refusal to participate/loss of contact/missing exposure or outcome. Censoring weights were calculated in the same way as treatment weights, except that multinomial logistic models were used to estimate the probability of having observed censoring status and that current treatment use was added in the censoring models (30). The final weights were the products of visit-specific treatment weights and censoring weights (30). In addition to checking the distributions of final weights, we truncated the final weights at the 99th percentile to ameliorate the impact of potential positivity violations (33).
After weights were constructed, weighted linear models (i.e., outcome models) were fit to estimate the relationships between cumulative exposure to glucosamine/chondroitin up to previous visit and changes in WOMAC scores and JSW measured at current visit (30). In addition to the cumulative treatment use, baseline variables were also included in these outcome models (30). We fit the outcome models using the GENMOD procedure in SAS (with an “independent” correlation structure and using “robust” standard errors) (34). Under the assumptions of no unmeasured confounding and correct specifications of the treatment and outcome models, the MSM estimates represent the treatment effects of using glucosamine/chondroitin for 1, 2 and 3 years on WOMACs and JSW among the study population (30). Previous validation studies (35-39) suggest the minimal clinically-important changes ranged from -4.6 to -1.2 for WOMAC Pain, -1.5 to -0.5 to for WOMAC Stiffness, -9.9 to -4.1 to for WOMAC Physical Function, and 0.2 to 0.5 mm for JSW.
We also compared the MSM estimates with the estimates derived from analyses with generalized estimating equations (GEE). In GEE analyses, we adjusted for baseline and time-varying confounders in the model and chose the working correlation structure that maximized the quasi-likelihood information criterion (40). We hypothesized that, if there is treatment effect that is mediated by the time-varying confounders, GEE estimates would be smaller in magnitude than the MSM estimates because GEE analyses cannot correctly estimate such mediated effect (30).
Results
Characteristics of study sample
Table 1 shows the baseline characteristics of the 1,625 participants included in analyses of WOMACs by status of glucosamine/chondroitin use at year 1. Overall, 43.6% were aged ≥65 years, 58.0% were women, 72.9% were non-Hispanic White and 37.8% had K-L grade 3 or 4. Ten percent of non-users at baseline initiated glucosamine/chondroitin at year 1. Compared to non-initiators of glucosamine/chondroitin at year 1, initiators tended to be younger, have higher education attainment and higher income, and were more likely to use other CAM and have a BMI ≥25 kg/m2 and valgus deformity at baseline. Similar trends were found in the study sample for the JSW analyses.
Table 1. Baseline characteristics by use of glucosamine/chondroitin at year one among persons with radiographic knee OA (n=1,625).
| Characteristics | Glucosamine/chondroitin initiators (n=165) | Non-initiators of glucosamine/chondroitin (n=1,460) | Total |
|---|---|---|---|
| Age (years) | Percentage | ||
| <65 | 60.0 | 56.0 | 56.4 |
| 65-74 | 32.7 | 31.6 | 31.7 |
| ≥75 | 7.3 | 12.4 | 11.9 |
| Women | 57.0 | 58.1 | 58.0 |
| Ethnicity/Race | |||
| Non-Hispanic White | 72.1 | 73.0 | 72.9 |
| Non-Hispanic Black | 23.0 | 24.1 | 24.0 |
| Other | 4.9 | 3.0 | 3.1 |
| Education | |||
| High school or less | 19.4 | 20.8 | 20.6 |
| Some college | 20.6 | 27.0 | 26.3 |
| College graduate | 17.0 | 20.8 | 20.4 |
| Graduate school | 43.0 | 31.4 | 32.6 |
| Income ($) | |||
| <25,000 | 17.6 | 17.5 | 17.5 |
| 25,000 - 50,000 | 23.0 | 29.5 | 28.9 |
| >50,000 | 59.4 | 53.0 | 53.7 |
| KL grade 3 or 4 | 37.6 | 37.8 | 37.8 |
| Multi-joint symptoms | 49.1 | 49.4 | 49.4 |
| Use of non-steroidal anti-inflammatory agents | 42.4 | 35.4 | 36.1 |
| Use of acetaminophen | 12.1 | 13.9 | 13.7 |
| Use of opioids | 6.1 | 6.1 | 6.1 |
| Use of complementary and alternative medicine | 35.8 | 24.4 | 25.5 |
| History of knee injury | 37.0 | 37.7 | 37.6 |
| History of knee surgery | 73.3 | 70.8 | 29.0 |
| Body Mass Index (kg/m2) | |||
| <25 | 8.5 | 15.7 | 15.0 |
| 25 - <30 | 42.4 | 37.5 | 38.0 |
| ≥30 | 49.1 | 46.8 | 47.0 |
| Knee alignment | |||
| Normal | 24.9 | 26.5 | 26.3 |
| Varus | 24.2 | 28.0 | 27.6 |
| Valgus | 50.9 | 45.5 | 46.0 |
| Mean (Standard Deviation) | |||
| WOMAC Pain | 4.2 (3.9) | 3.8 (4.1) | 3.9 (4.1) |
| WOMAC Stiffness | 2.2 (1.8) | 2 (1.8) | 2 (1.8) |
| WOMAC Physical Function | 12 (11.8) | 12 (13) | 12 (12.9) |
| SF-12 Physical Component Score | 48.1 (8.8) | 47.6 (9.6) | 47.7 (9.5) |
| SF-12 Mental Component Score | 54.6 (7.9) | 53.3 (8.5) | 53.4 (8.4) |
| Medial joint space width (mm)* | 5.2 (1.3) | 5.2 (1.2) | 5.2 (1.2) |
Based on information on 1,113 participants included in JSW analyses, among which 107 reported initiating glucosamine/chondroitin at year 1.
Predictors of glucosamine/chondroitin use
Around 18% of participants initiated glucosamine/chondroitin during the study period. Among these initiators, 22.8% reported treatment use at all assessments (other than baseline) and 38.4% discontinued the treatment at a later assessment. Table 2 shows the correlates of initiating and continuing glucosamine/chondroitin treatment. Older adults were less likely to initiate treatment, but more likely to stay on treatment once they initiated it. Longitudinally, participants were less likely to initiate treatment (comparing year 3 and year 2 with year 1), but more likely to continue the treatment (comparing year 3 with year 2). Being overweight, having K-L grade 3/4 and using NSAIDs were correlates of treatment initiation, while use of other CAM methods and acetaminophen was associated with both initiating and continuing treatment.
Table 2. Correlates* of glucosamine/chondroitin use in the three-year follow-up period among persons with radiographic knee OA.
| Correlates of treatment use | Adjusted odds ratios§ (95% CI) of “initiating” treatment | Adjusted odds ratios§ (95% CI) of “continuing” treatment |
|---|---|---|
| Baseline characteristics | ||
| Age: 75 vs <65 years | 0.63 (0.40-0.99) | 2.31 (0.69-7.74) |
| Age: 65-74 vs <65 years | 0.71 (0.53-0.95) | 2.27 (1.17-4.40) |
| Women vs men | 0.89 (0.68-1.17) | 0.65 (0.33-1.27) |
| Black vs White | 0.74 (0.53-1.03) | 0.67 (0.34-1.32) |
| Other race vs White | 1.02 (0.53-1.97) | 0.28 (0.08-1.02) |
| Graduate education vs High school | 1.60 (1.07-2.38) | 1.39 (0.59-3.26) |
| College graduate vs High school | 1.21 (0.78-1.86) | 0.57 (0.21-1.53) |
| Some college vs High school | 0.91 (0.61-1.36) | 1.05 (0.43-2.59) |
| Income ($): >50 k vs <25k | 0.99 (0.65-1.51) | 1.60 (0.66-3.92) |
| Income ($): 25-50k vs <25k | 0.93 (0.62-1.41) | 0.79 (0.33-1.89) |
| History of knee surgery | 0.78 (0.58-1.05) | 0.42 (0.22-0.83) |
| Time-varying confounders (concurrent) | ||
| Year 3 | (vs Year 1) 0.44 (0.32-0.60) |
(vs Year 2) 1.71 (1.01-2.88) |
| Year 2 | (vs Year 1) 0.51 (0.39-0.69) |
-- |
| Obese vs Normal weight | 1.20 (0.82-1.76) | 0.85 (0.37-1.93) |
| Overweight vs Normal weight | 1.49 (1.02-2.16) | 1.21 (0.55-2.69) |
| Alignment: Valgus vs Normal | 1.02 (0.74-1.40) | 0.58 (0.27-1.25) |
| Alignment: Varus vs Normal | 0.86 (0.61-1.21) | 0.70 (0.31-1.59) |
| K-L: 3/4 vs 2 | 1.37 (1.06-1.78) | 1.07 (0.59-1.93) |
| Multi-joint symptoms | 1.15 (0.89-1.50) | 0.94 (0.53-1.67) |
| History of knee injury | 0.99 (0.76-1.30) | 1.69 (0.92-3.10) |
| Use of non-steroidal anti-inflammatory agents | 1.46 (1.12-1.90) | 1.06 (0.57-1.94) |
| Use of acetaminophen | 1.45 (1.00-2.11) | 1.42 (0.58-3.50) |
| Use of opioids | 0.67 (0.40-1.13) | 0.61 (0.21-1.75) |
| Use of complementary/alternative medicine | 2.20 (1.69-2.89) | 2.90 (1.64-5.12) |
| WOMAC Pain # | 1.13 (0.98-1.32) | 1.23 (0.86-1.75) |
| SF-12 Physical Component Score # | 1.20 (1.02-1.41) | 1.32 (0.94-1.84) |
| SF-12 Mental Component Score # | 1.15 (1.00-1.32) | 0.99 (0.75-1.30) |
Correlates in this table were included in treatment models when analyzing WOMAC Pain as the outcome.
The outcome was glucosamine/chondroitin use (yes/no) at year 1, year 2 and year 3. The odds ratios were adjusted for other variables in this table.
Odds ratios are per one standard deviation changes in WOMAC Pain or SF-12 subscales.
Effects of glucosamine/chondroitin on treating knee OA
As shown in the top section in Table 3, after adjustment for potential confounders with MSMs, compared to participants who never reported previous use of glucosamine/chondroitin, those reporting use for three, two and one assessments had on average 0.68 points increase (95% CI: -0.16 to 1.53), 0.12 points decrease (95% CI: -0.71 to 0.48) and 0.28 points increase (95% CI: -0.08 to 0.65) in WOMAC Pain, respectively. In terms of WOMAC Stiffness and Function, the average differences in changes from baseline between participants using the treatment at all assessments and never-users were 0.41 (95% CI: 0 to 0.82) and 1.28 (95% CI: -1.23 to 3.79), respectively. The bottom section in Table 3 shows the estimates of treatment effects on JSW. After adjustment for confounders with MSMs, compared to never-users, those who reported previous use for three, two and one assessments had on average 0.11mm wider (95% CI: -0.21 to 0.44), 0.14mm wider (95% CI: -0.07 to 0.35) and 0.03mm narrower (95% CI: -0.16 to 0.10) in medial JSW, respectively. GEE analyses adjusting for baseline and time-varying confounders yielded similar results as MSMs.
Table 3. Estimated effects of glucosamine/chondroitin on treating OA among persons with radiographic knee OA, beta coefficients (95% CI)*.
| Models | Cumulative exposureǂ to glucosamine/chondroitin#
|
||
|---|---|---|---|
| Reported on all 3 annual assessments | Reported on 2 of 3 annual assessments | Reported on 1 of 3 annual assessments | |
| WOMAC Pain (Minimally important improvement: -4.6 to -1.2) | |||
|
| |||
| GEE: Crude § | 0.86 (0.10 to 1.61) | 0.14 (-0.33 to 0.60) | 0 (-0.36 to 0.37) |
| GEE: Full-adjusted § | 0.81 (0.16 to 1.45) | 0.07 (-0.30 to 0.45) | 0.20 (-0.04 to 0.44) |
| MSM with truncated weights | 0.68 (-0.16 to 1.53) | -0.12 (-0.71 to 0.48) | 0.28 (-0.08 to 0.65) |
|
| |||
| WOMAC Stiffness (Minimally important improvement: -1.5 to -0.5) | |||
|
| |||
| GEE: Crude § | 0.48 (0.08 to 0.89) | 0.09 (-0.17 to 0.34) | 0.14 (-0.04 to 0.31) |
| GEE: Full-adjusted § | 0.41 (0.04 to 0.79) | 0.13 (-0.08 to 0.34) | 0.17 (0.05 to 0.30) |
| MSM with truncated weights | 0.41 (0 to 0.82) | 0.10 (-0.18 to 0.37) | 0.25 (0.06 to 0.43) |
|
| |||
|
WOMAC Function (Minimally important improvement: -9.9 to -4.1)
| |||
| GEE: Crude § | 2.56 (0.64 to 4.48) | 1.23 (-0.17 to 2.64) | -0.06 (-1.15 to 1.02) |
| GEE: Full-adjusted § | 1.74 (0.03 to 3.46) | 0.94 (-0.19 to 2.07) | 0.31 (-0.41 to 1.03) |
| MSM with truncated weights | 1.28 (-1.23 to 3.79) | 0.24 (-1.45 to 1.94) | 0.66 (-0.50 to 1.82) |
|
| |||
| Joint space width (Minimally important change: 0.2 to 0.5) | |||
|
| |||
| GEE: Crude § | -0.35 (-0.58 to -0.12) | -0.25 (-0.45 to -0.06) | -0.12 (-0.23 to -0.01) |
| GEE: Full-adjusted § | 0.05 (-0.13 to 0.22) | 0.04 (-0.08 to 0.15) | -0.03 (-0.09 to 0.03) |
| MSM with truncated weights | 0.11 (-0.21 to 0.44) | 0.14 (-0.07 to 0.35) | -0.03 (-0.16 to 0.10) |
The reference group includes persons never using glucosamine/chondroitin up to “previous visit”.
Generalized estimating equations (GEE) analyses assumed an unstructured correlation matrix. The full-adjusted GEE estimates adjusted for baseline characteristics including age, gender, race/ethnicity, education, income and history of knee surgery and time-varying confounders (follow-up time, obesity status, knee malalignment, Kellgren-Lawrence grade, multi-joint symptoms, history of knee injuries, use of other complementary/alternative medicine, use of other analgesic medications, WOMAC subscale score, SF-12 physical and mental health scores) that were measured at the same visit as glucosamine/chondroitin use.
“Cumulative exposure” was operationally defined as the number of assessments for which participants reported use of glucosamine/chondroitin up to the visit before the study outcomes were measured.
Analyses of WOMAC outcomes and JSW were based on 1,625 persons (4,264 person-visits) and 1,113 persons (2,367 person-visits), respectively.
Discussion
Following a large sample of participants with knee OA who were “naïve” to treatment with glucosamine/chondroitin, we found that around 18% initiated the treatment and 4% reported use at all assessments during the study period. Age, BMI levels, K-L grade and use of other treatments were important correlates of initiating and/or continuing glucosamine/chondroitin treatment. After adjustment for potential confounders with MSMs, we found that treatment with glucosamine/chondroitin for three years did not appear to bring about relief in symptoms or retardation of disease progression.
Our data relating to symptomatic effects are consistent with recent systematic reviews (7,8) on single treatment with glucosamine or chondroitin and with independent long-term clinical trials on combination treatment with both supplements (41,42). The recently updated Cochrane review concluded that clinical trials with adequate allocation concealment did not demonstrate a superiority of glucosamine over placebo for pain or physical function (7). Likewise, a recent meta-analysis found that large-scale clinical trials using an intention-to-treat analysis reported minimal or nonexistent symptomatic benefits from chondroitin compared to placebo (8). Moreover, as far as we know, there are two published long-terms trials, i.e., the Glucosamine/Chondroitin Arthritis Intervention Trial (GAIT) (41) and the Long-term Evaluation of Glucosamine Sulfate (LEGS) study (42), which assessed the efficacy of combination treatment with both supplements in treating knee OA. Both studies found that combination treatment did not confer symptomatic benefits compared to treatment with either supplement or with placebo (41,42). Our data join a growing body of evidence suggesting that glucosamine/chondroitin has no impact on relieving OA symptoms.
With respect to the effect of glucosamine/chondroitin on structural progression, our findings are consistent with some (43), but not all of the literature (19,42). The GAIT study reported a difference of 0.028mm in joint space narrowing between the combination treatment group and the placebo group and concluded no benefits of modifying disease progression from combination treatment (43). On the contrary, the LEGS study found that the difference in joint space narrowing was 0.10mm after two-year follow-up, which was in favor of the combination treatment and was marginally statistically significant (p=0.046). This absolute reduction in joint space narrowing was comparable to that found in our study. Considering that the smallest detectable change in JSW measures was 0.2 mm (38), this reduction may be trivial.
Moreover, we are aware of another non-experimental study by Martel-Pelletier et al., which was based on OAI participants and assessed use of glucosamine/chondroitin on slowing OA progression (19). Using a different study design and analytic approaches, our study confirms their finding that combination use of glucosamine and chondroitin does not have an impact on slowing joint space narrowing. However, their study reported that glucosamine and chondroitin reduced loss of cartilage volume in some subregions of the tibiofemoral joint assessed with MRI (19). We interpret this conclusion cautiously. Over 60 comparisons were conducted to compare cartilage volume loss in different subregions of the knee joint between users and non-users of glucosamine/chondroitin, but no adjustments for multiple comparisons were made. Once Bonferroni corrections were applied (44), none of the comparisons would have been statistically significant.
Our study has some merits that are worth mentioning. First, we used a new-user design by excluding participants using glucosamine/chondroitin at baseline. A new-user design is considered a gold standard in pharmacoepidemiologic studies due to the well-recognized advantages of studying initiators of treatments (45). In particular, a new user design can avoid the selection of prevalent users who are responsive to the treatment and thus prevent overestimating the treatment benefits (20,45). In addition, a new-user design can avoid bias from adjusting for confounders that may be affected by previous treatments in prevalent users (20,45). Second, we used MSMs to estimate the treatment effects by adjusting for time-varying confounders which may also be intermediate variables and by controlling for bias from potential informative dropout (30,31). GEE models generally produce associative effects and may estimate treatment effects under very stringent assumptions, including the assumption that time-varying confounders are not influenced by prior treatments (30,31). GEE adjusts for time-varying confounders through conditioning analysis on these covariates and thus eliminates any indirect effect from prior treatments that are mediated by the time-varying confounders (30,31). Unlike GEE, MSM adjusts for time-varying confounders through assigning weights to participants and thus is capable of estimating the indirect effects from prior treatments, if they exist (30,31).
Notwithstanding, our findings must be considered with limitations in mind. First, there may be misclassification in use of glucosamine/chondroitin. Treatment use was assessed annually, and our analyses assumed that participants were on the treatment in the following year if they reported treatment use at an annual assessment. It is likely that participants were on and off the treatment during the intervals of assessments. If this misclassification was non-differential, we would have underestimated the treatment effects. Second, we do not have information on the glucosamine formulation (sulfate or hydrochloride) or treatment dosage or the extent of purity of the supplements. The supplements evaluated in our study were likely over-the-counter products, which have been reported to be different from those tested in clinical trials in terms of quality, strength, and composition (46). Third, despite that OAI administered comprehensive measurement on the disease severity that might affect patients in seeking treatment and that these indices were adjusted to deal with the potential confounding by indication, we could not rule out the possibility that our findings may still be biased by unmeasured confounding. Finally, the practice of adjusting for the concurrently measured disease characteristics as potential confounders may reduce the measurement error in confounders but may also induce bias due to the possible adjustment for intermediate variables.
In summary, the use of glucosamine/chondroitin as dietary supplements did not appear to relieve symptoms or modifying disease progression among radiographically confirmed OA patients. Our findings are consistent with the results from recent long-term clinical trials and support the latest guidelines for OA treatment which recommend against using the nutritional supplements of glucosamine and chondroitin (2).
Acknowledgments
The OAI is a public-private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Merck Research Laboratories; Novartis Pharmaceuticals Corporation, GlaxoSmithKline; and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. This manuscript was prepared using an OAI public use data set and does not necessarily reflect the opinions or views of the OAI investigators, the NIH, or the private funding partners.
References
- 1.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken) 2012;64:465–74. doi: 10.1002/acr.21596. [DOI] [PubMed] [Google Scholar]
- 3.Lapane KL, Sands MR, Yang S, McAlindon TE, Eaton CB. Use of complementary and alternative medicine among patients with radiographic-confirmed knee osteoarthritis. Osteoarthritis Cartilage. 2012;20:22–8. doi: 10.1016/j.joca.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCarty MF. The neglect of glucosamine as a treatment for osteoarthritis--a personal perspective. Med Hypotheses. 1994;42:323–7. doi: 10.1016/0306-9877(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 5.Sherman AL, Ojeda-Correal G, Mena J. Use of glucosamine and chondroitin in persons with osteoarthritis. PM R. 2012;4:S110–6. doi: 10.1016/j.pmrj.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 6.Huskisson EC. Glucosamine and chondroitin for osteoarthritis. J Int Med Res. 2008;36:1161–79. doi: 10.1177/147323000803600602. [DOI] [PubMed] [Google Scholar]
- 7.Towheed TE, Maxwell L, Anastassiades TP, Shea B, Houpt J, Robinson V, et al. Glucosamine therapy for treating osteoarthritis. Cochrane Database Syst Rev. 2005;2 doi: 10.1002/14651858.CD002946.pub2. CD002946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reichenbach S, Sterchi R, Scherer M, Trelle S, Bürgi E, Bürgi U, et al. Meta-analysis: chondroitin for osteoarthritis of the knee or hip. Ann Intern Med. 2007;146:580–90. doi: 10.7326/0003-4819-146-8-200704170-00009. [DOI] [PubMed] [Google Scholar]
- 9.McAlindon TE, LaValley MP, Gulin JP, Felson DT. Glucosamine and chondroitin for treatment of osteoarthritis: a systematic quality assessment and meta-analysis. JAMA. 2000;283:1469–75. doi: 10.1001/jama.283.11.1469. [DOI] [PubMed] [Google Scholar]
- 10.Vlad SC, LaValley MP, McAlindon TE, Felson DT. Glucosamine for pain in osteoarthritis: why do trial results differ? Arthritis Rheum. 2007;56:2267–77. doi: 10.1002/art.22728. [DOI] [PubMed] [Google Scholar]
- 11.Lee YH, Woo JH, Choi SJ, Ji JD, Song GG. Effect of glucosamine or chondroitin sulfate on the osteoarthritis progression: a meta-analysis. Rheumatol Int. 2010;30:357–63. doi: 10.1007/s00296-009-0969-5. [DOI] [PubMed] [Google Scholar]
- 12.Hochberg MC. Structure-modifying effects of chondroitin sulfate in knee osteoarthritis: an updated meta-analysis of randomized placebo-controlled trials of 2-year duration. Osteoarthritis Cartilage. 2010;18:S28–31. doi: 10.1016/j.joca.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 13.Wandel S, Jüni P, Tendal B, Nüesch E, Villiger P, Welton N, et al. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: network meta-analysis. BMJ. 2010;341:c4675. doi: 10.1136/bmj.c4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Felson DT. Chondroitin for pain in osteoarthritis. Ann Intern Med. 2007;146:611–2. doi: 10.7326/0003-4819-146-8-200704170-00014. [DOI] [PubMed] [Google Scholar]
- 15.Reginster JY, Deroisy R, Rovati LC, Lee RL, Lejeune E, Bruyere O, et al. Long-term effects of glucosamine sulphate on osteoarthritis progression: a randomised, placebo-controlled clinical trial. Lancet. 2001;357:251–6. doi: 10.1016/S0140-6736(00)03610-2. [DOI] [PubMed] [Google Scholar]
- 16.Pavelká K, Gatterová J, Olejarová M, Machacek S, Giacovelli G, Rovati LC. Glucosamine sulfate use and delay of progression of knee osteoarthritis: a 3-year, randomized, placebo-controlled, double-blind study. Arch Intern Med. 2002;162:2113–23. doi: 10.1001/archinte.162.18.2113. [DOI] [PubMed] [Google Scholar]
- 17.Nevitt MC, Felson DT, Lester G. The Osteoarthritis Initiative: protocol for the cohort study. 2006 Jun; URL: http://oai.epi-ucsf.org/datarelease/docs/StudyDesignProtocol.pdf.
- 18.Nallamothu BK, Hayward RA, Bates ER. Beyond the randomized clinical trial: the role of effectiveness studies in evaluating cardiovascular therapies. Circulation. 2008;118:1294–303. doi: 10.1161/CIRCULATIONAHA.107.703579. [DOI] [PubMed] [Google Scholar]
- 19.Martel-Pelletier J, Roubille C, Abram F, Hochberg MC, Dorais M, Delorme P, et al. First-line analysis of the effects of treatment on progression of structural changes in knee osteoarthritis over 24 months: data from the osteoarthritis initiative progression cohort. Ann Rheum Dis. 2013 doi: 10.1136/annrheumdis-2013-203906. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 20.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158:915–20. doi: 10.1093/aje/kwg231. [DOI] [PubMed] [Google Scholar]
- 21.Roos EM, Klässbo M, Lohmander LS. WOMAC osteoarthritis index. Reliability, validity, and responsiveness in patients with arthroscopically assessed osteoarthritis Western Ontario and MacMaster Universities. Scand J Rheumatol. 1999;28:210–5. doi: 10.1080/03009749950155562. [DOI] [PubMed] [Google Scholar]
- 22.Duryea J, Li J, Peterfy CG, Gordon C, Genant HK. Trainable rule-based algorithm for the measurement of joint space width in digital radiographic images of the knee. Med Phys. 2000;27:580–91. doi: 10.1118/1.598897. [DOI] [PubMed] [Google Scholar]
- 23.Duryea J, Neumann G, Niu J, Totterman S, Tamez J, Dabrowski C, et al. Comparison of radiographic joint space width with magnetic resonance imaging cartilage morphometry: analysis of longitudinal data from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken) 2010;62:932–7. doi: 10.1002/acr.20148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.University of California San Francisco OAI Coordinating Center. Central assessment of longitudinal knee x-rays for quantitative JSW. 2013 Jun; URL: https://oai.epi-ucsf.org/datarelease/SASDocs/kXR_QJSW_Duryea_descrip.pdf.
- 25.Okma-Keulen P, Hopman-Rock M. The onset of generalized osteoarthritis in older women: a qualitative approach. Arthritis Rheum. 2001;45:183–90. doi: 10.1002/1529-0131(200104)45:2<183::AID-ANR172>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 26.Ware J, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Reijman M, Pols HA, Bergink AP, Hazes JM, Belo JN, Lievense AM, et al. Body mass index associated with onset and progression of osteoarthritis of the knee but not of the hip: the Rotterdam Study. Ann Rheum Dis. 2007;66:158–62. doi: 10.1136/ard.2006.053538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008;12:1–23. [PubMed] [Google Scholar]
- 29.Greenland S, Finkle WD. A critical look at methods for handling missing covariates in epidemiologic regression analyses. Am J Epidemiol. 1995;142:1255–64. doi: 10.1093/oxfordjournals.aje.a117592. [DOI] [PubMed] [Google Scholar]
- 30.Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–60. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 31.Hernán MA, Hernández-Díaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15:615–25. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- 32.Platt RW, Brookhart M, Cole SR, Westreich D, Schisterman EF. An information criterion for marginal structural models. Stat Med. 2012;32:1383–93. doi: 10.1002/sim.5599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168:656–64. doi: 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hernán MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology. 2000;11:561–70. doi: 10.1097/00001648-200009000-00012. [DOI] [PubMed] [Google Scholar]
- 35.Greco NJ, Anderson AF, Mann BJ, Cole BJ, Farr J, Nissen CW, et al. Responsiveness of the International Knee Documentation Committee Subjective Knee Form in comparison to the Western Ontario and McMaster Universities Osteoarthritis Index, modified Cincinnati Knee Rating System, and Short Form 36 in patients with focal articular cartilage defects. Am J Sports Med. 2010;38:891–902. doi: 10.1177/0363546509354163. [DOI] [PubMed] [Google Scholar]
- 36.Angst F, Aeschlimann A, Stucki G. Smallest detectable and minimal clinically important differences of rehabilitation intervention with their implications for required sample sizes using WOMAC and SF-36 quality of life measurement instruments in patients with osteoarthritis of the lower extremities. Arthritis Rheum. 2001;45:384–91. doi: 10.1002/1529-0131(200108)45:4<384::AID-ART352>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 37.Escobar A, Quintana JM, Bilbao A, Aróstegui I, Lafuente I, Vidaurreta I. Responsiveness and clinically important differences for the WOMAC and SF-36 after total knee replacement. Osteoarthritis Cartilage. 2007;15:273–80. doi: 10.1016/j.joca.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 38.Hunter DJ. Risk stratification for knee osteoarthritis progression: a narrative review. Osteoarthritis Cartilage. 2009;17:1402–7. doi: 10.1016/j.joca.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 39.Vignon E, Piperno M, Le Graverand MP, Mazzuca SA, Brandt KD, Mathieu P, et al. Measurement of radiographic joint space width in the tibiofemoral compartment of the osteoarthritic knee: comparison of standing anteroposterior and Lyon schuss views. Arthritis Rheum. 2003;48:378–84. doi: 10.1002/art.10773. [DOI] [PubMed] [Google Scholar]
- 40.Pan W. Akaike’s information criterion in generalized estimating equations. Biometrics. 2001;57:120–5. doi: 10.1111/j.0006-341x.2001.00120.x. [DOI] [PubMed] [Google Scholar]
- 41.Sawitzke AD, Shi H, Finco MF, Dunlop DD, Harris CL, Singer NG, et al. Clinical efficacy and safety of glucosamine, chondroitin sulphate, their combination, celecoxib or placebo taken to treat osteoarthritis of the knee: 2-year results from GAIT. Ann Rheum Dis. 2010;69:1459–64. doi: 10.1136/ard.2009.120469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fransen M, Agaliotis M, Nairn L, Votrubec M, Bridgett L, Su S, et al. Glucosamine and chondroitin for knee osteoarthritis: a double-blind randomised placebo-controlled clinical trial evaluating single and combination regimens. Ann Rheum Dis. 2014 doi: 10.1136/annrheumdis-2013-203954. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 43.Sawitzke AD, Shi H, Finco MF, Dunlop DD, Bingham CO, Harris CL, et al. The effect of glucosamine and/or chondroitin sulfate on the progression of knee osteoarthritis: a report from the glucosamine/chondroitin arthritis intervention trial. Arthritis Rheum. 2008;58:3183–91. doi: 10.1002/art.23973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bland JM, Altman DG. Multiple significance tests: the Bonferroni method. BMJ. 1995;310:170. doi: 10.1136/bmj.310.6973.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Setoguchi S, Gerhard T. Comparator selection. In: Velentgas P, Dreyer NA, Nourjah P, editors. Developing a protocol for observational comparative effectiveness research: a User’s Guide. Rockville (MD): Agency for Healthcare Research and Quality; 2013. pp. 59–70. [PubMed] [Google Scholar]
- 46.Russell AS, Aghazadeh-Habashi A, Jamali F. Active ingredient consistency of commercially available glucosamine sulfate products. J Rheumatol. 2002;29:2407–9. [PubMed] [Google Scholar]


