Abstract
Background and Purpose
Minor stroke and rapidly improving stroke symptoms (RISS) are frequent exclusions for intravenous tissue-type plasminogen activator (t-PA). We explored factors influencing t-PA treatment decision for minor stroke/RISS.
Methods
A pilot survey including 110 case scenarios completed by 17 clinicians from two academic medical centers. Respondents were asked whether they would treat each case with t-PA at 60 min after Emergency Department admission. Cases varied by: (a) NIHSS score at treatment decision time; (b) symptom pattern over time [“improvement (IMP)” or “worsening and then improving (WI)”]; (c) type of neurological deficit [three main domains, “Motor (M)”, “Visual/Sensory/Ataxia (VSA)”, “Language/Neglect (LN)”]; and, (d) age/occupation (four profiles). Logistic regression was used to predict probability of omission (po). A binomial regression model was used to predict probability of treatment decision [p(t-PA)].
Results
P(t-PA) was affected by NIHSS score (p<0.001), age/occupation profiles (p<0.001), but not by symptom patterns (p=0.334). There were significant, albeit modest main effects on p(t-PA) for neurological domains. Responses were most likely omitted (p=0.027) for cases with “IMP” pattern and “LN” domain (po)=0.74; 95% [confidence interval (CI) 0.52-0.89] and with “VSA” domain (po=0.74; CI 0.37-0.93), as compared to “IMP” pattern and “M” domain (po =0.17; CI 0.06-0.42) and to any “WI” patterns (0.37<po<0.56).
Conclusions
This pilot survey provides the first quantitative evidence that NIHSS score is not the only determinant of treatment decision. An NIHSS score of 2 is the potential equipoise point, with the least consensus on treatment decision. These preliminary findings require validation in larger population surveys.
Keywords: acute stroke, thrombolysis, tissue plasminogen activator, rapidly improving stroke symptoms, minor stroke
Introduction
Minor stroke and rapidly improving stroke symptoms (RISS) are the most frequently cited exclusion criteria for intravenous tissue-type plasminogen activator (t-PA)1. Approximately half of all ischemic stroke cases have mild symptom severity at presentation (i.e. median NIHSS≤3, 25-75 IQR 1-7)2 with not always favorable outcomes when left untreated1,3-7.
These exclusion criteria are left to clinical judgments without standard or accepted guidelines8. The most appropriate diagnostic and management approach to minor strokes and/or RISS remain debatable5. A post-hoc analysis of outcomes of minor strokes included in the NINDS trials revealed too small a sample for definitive conclusions9. A currently ongoing randomized clinical trial, PRISMS10, is evaluating the safety and efficacy of t-PA for minor strokes.
Factors influencing treatment of minor stroke and/or RISS have not been systematically studied.
Our pilot study explored factors influencing the t-PA treatment decision for minor stroke and RISS among neurologists treating acute stroke. We hypothesized that different ages and occupations, symptom patterns over time, types of neurological deficits, and stroke severity at treatment decision time affect a clinician’s decision to use t-PA.
Methods
This study was approved by the IRB. A pilot survey including 110 case scenarios was provided to 40 physicians, 36 attendings (vascular and general neurologists) and 4 stroke fellows from two urban academic medical centers (The State University of New York, Downstate Medical Center, Brooklyn, NY and Tufts University Medical Center, Boston, MA). Answers were collected anonymously.
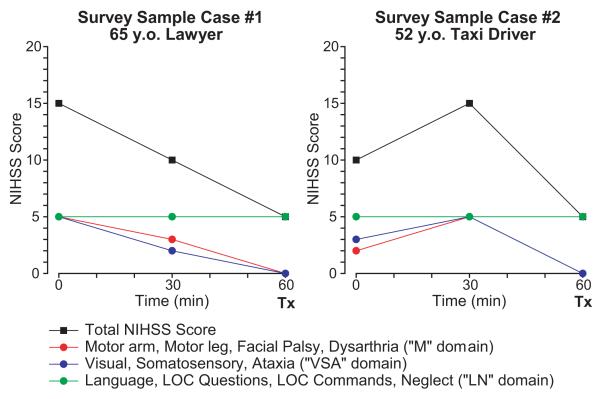
Each scenario provided an individual acute stroke patient with graphic representation of the patient’s stroke symptoms defined by NIHSS score at presentation to the Emergency Department (ED), and again at 30 minutes and 60 minutes after ED admission (Figure 1). For each scenario, respondents were asked whether they would treat with t-PA at 60 min after ED admission provided that there were no other contraindications for t-PA. Cases varied on the following factors: (a) NIHSS score (range 1-5), at treatment decision time; (b) symptom pattern measured by NIHSS over three time points [“continuous improvement (IMP) ” or “worsening and then improving (WI)”]; (c) type of neurological deficit grouped into three domains [“Motor (M)”, “Visual/Sensory/Ataxia (VSA)”, “Language/Neglect (LN)”]; and (d) age/occupation (4 profiles: “35/violinist”, “65/lawyer”, “52/taxi driver”, and “80/retired”).
Figure 1.
Survey Cases. Tx = treatment decision time.
The “M” domain included the NIHSS items #4. Facial Palsy, #5. Motor Arm, #6. Motor Leg and #10. Dysarthria; the “VSA” domain included NIHSS items #3.Visual, #7. Limb Ataxia, #8. Sensory; the “LN” domain included the NIHSS items #9. Best Language, #1b. Level of Consciousness (LOC) Questions, #1c. LOC Commands and #11. Extinction and Inattention (formerly Neglect). Survey samples are provided in figure 1.
Statistical analysis
The unit of analysis was the individual case scenario (N=110). Each scenario was dichotomized into those having at least one missing response or no missing response from any respondent. A logistic regression model was fitted to predict the probability of omitted responses to scenarios. After excluding omissions a binomial regression model was fitted predicting the probability of t-PA treatment recommendation. Predictors for the model were: NHSS score (linear, varying from 1-5), the specific neurological domain (“M”, “VSA”, or “LN”), symptom pattern over time (“IMP” or “WI”), and any potential interactions. Subsequent analyses included factor age/occupation included in 80 scenarios.
Results
Seventeen/40 (43%) physicians completed the survey.
Predicted probability of treatment recommendation [p(t-PA)]
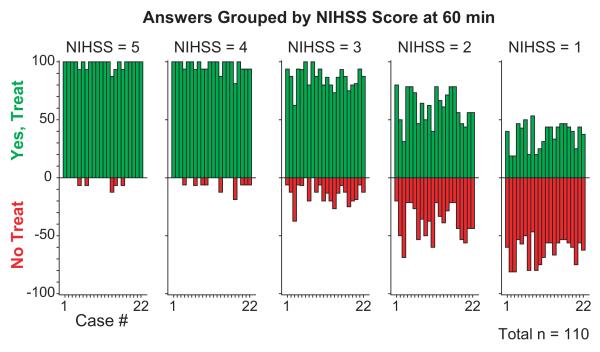
p(t-PA) was significantly affected by NIHSS score (p<0.001), decreasing with decreasing score, [p(t-PA)=0.98, 0.97, 0.83, 0.57, and 0.36 for NIHSS scores of 5, 4, 3, 2, and 1, respectively].
The closest p(t-PA) to equipoise was at an NIHSS of 2 (0.57, 95% CI 0.51-0.62) (Figure 2).
Figure 2.
Answers grouped by NIHSS score at 60 min after ED Admission. NIHSS = NIH Stroke Scale; ED = Emergency Department.
Treatment decision was also affected by age/occupation profiles (p<0.001). Treatment was less likely recommended for the “80/retired” cases. Model-estimated probabilities of treatment recommendation by age/occupation were as “35/violinist” 0.888 (95%CI 0.836, 0.925), “65/lawyer” 0.865 (95%CI 0.807, 0.908), “52/taxi driver” 0.885 (95% CI 0.836, 0.921), and “80/retired” 0.744 (95%CI 0.660, 0.812). There were no significant interactions between NIHSS and age/occupation.
There were significant, albeit modest main effects on p(t-PA) for neurological domains [“LN” 0.873 (95%CI 0.841, 0.900), “M” 0.825 (95% CI 0.782, 0.860), and “VSA” 0.850 (95% CI 0.800, 0.889)] (p=0.032) but not for symptom patterns (“IMP” or “WI”) (p=0.334).
Predicted Probability of Omissions (po)
Frequency of omitted responses was 2.8% of the total of 1,870 responses analyzed. Fifty-two of the 110 scenarios analyzed had exactly one missing response each. Model-estimated probabilities of omission by any respondent for any individual factor ranged from the “IMP” pattern associated with “LN” 0.74 (95% CI 0.523, 0.885) and “VSD” domain 0.74(95% CI 0.367, 0.934) to “IMP” pattern combined with the “M” domain 0.17 (95% CI 0.059, 0.416) and to any “WI” patterns (0.37<po<0.56). There was a significant 2-way interaction between symptom pattern over time and domains of neurological deficits (p=0.027). There was no significant effect involving NIHSS score (p=0.785).
Discussion
This pilot survey provides, to our knowledge, the first quantitative evidence that the NIHSS score at treatment time is not the only determinant of a clinician’s t-PA treatment decision. Type of neurological deficits at treatment decision time influenced the clinician’s recommendation: the “LN” domain deficits were more likely to be recommended for treatment than “M” domain deficits. We recognize that the “LN” domain included both language and neglect and therefore we cannot ascertain their individual importance. This is also true for the other domains where NIHSS items were combined. Our findings suggest the need to further explore, in a larger survey, specific, individual NIHSS items and to analyze their role in the clinician’s treatment decision.
An NIHSS score of 2 was identified as a potential “equipoise point”, with the least consensus on t-PA decision. To our knowledge, a specific NIHSS score reflecting the greatest level of uncertainty for a t-PA treatment decision has not been previously reported. We found that the lower the NIHSS score within the range of 1-5, the less likely the clinician would treat with t-PA. Even though the symptom pattern over time did not significantly affect the probability of treatment, in conjunction with the domains of neurological deficits, it was the strongest predictor of the clinician’s response omission, suggesting a greater level of uncertainty for giving t-PA when symptoms are fluctuating and not stable. It is also possible that answers were inadvertently skipped rather than omitted because of uncertainty.
Our study has several limitations. Response rate was 43%. We could not test the effect of each NIHSS item, age/occupation, and symptom pattern over time on p(t-PA) because the number of clinical scenarios requiring testing would be burdensome in this preliminary study. As we only piloted two urban academic medical centers, we may not be able to generalize these results to all academic stroke centers and to non-academic institutions. Further, we did not explore how training level may influence physicians’ treatment decision.
Conclusions
Our study provides objective evidence that there are various factors affecting clinician’s treatment decision and that an NIHSS score of 2 seems the equipoise point with the least consensus on treatment decision. These findings require to be validated in a larger population survey.
Acknowledgments
Sources of Funding: SRL was supported by the NIH grants 1RO1HL96944, 1U0NS1080377, and 1U01NS077378.
CB was supported by the American Heart Association/American Stroke Association/Founders Affiliate and the American Brain Foundation Lawrence M. Brass, M.D. Stroke Research Postdoctoral Fellowship Award.
Footnotes
Disclosures: CB, RB, EF, JW and DK report no disclosures.
SRL: Scientific Advisory Committee for PRISMS (An Ongoing Genentech, Inc. funded study – modest honorarium and travel expenses).
References
- 1.Smith EE, Fonarow GC, Reeves MJ, Cox M, Olson DM, Hernandez AF, et al. Outcomes in mild or rapidly improving stroke not treated with intravenous recombinant tissue-type plasminogen activator: findings from Get With The Guidelines-Stroke. Stroke. 2011;42:3110–3115. doi: 10.1161/STROKEAHA.111.613208. [DOI] [PubMed] [Google Scholar]
- 2.Reeves M, Khoury J, Alwell K, Moomaw C, Flaherty M, Woo D, et al. Distribution of National Institutes of Health stroke scale in the Cincinnati/Northern Kentucky Stroke Study. Stroke. 2013;44:3211–3213. doi: 10.1161/STROKEAHA.113.002881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nedeltchev K, Schwegler B, Haefeli T, Brekenfeld C, Gralla J, Fischer U, et al. Outcome of stroke with mild or rapidly improving symptoms. Stroke. 2007;38:2531–2535. doi: 10.1161/STROKEAHA.107.482554. [DOI] [PubMed] [Google Scholar]
- 4.Rajajee V, Kidwell C, Starkman S, Ovbiagele B, Alger JR, Villablanca P, et al. Early MRI and outcomes of untreated patients with mild or improving ischemic stroke. Neurology. 2006;67:980–984. doi: 10.1212/01.wnl.0000237520.88777.71. [DOI] [PubMed] [Google Scholar]
- 5.Balucani C, Levine SR. Mild stroke and rapidly improving symptoms: it’s not always a happy ending. Stroke. 2011;42:3005–3007. doi: 10.1161/STROKEAHA.111.628701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duncan PW, Samsa GP, Weinberger M, Goldstein LB, Bonito A, Witter DM, et al. Health status of individuals with mild stroke. Stroke. 1997;28:740–745. doi: 10.1161/01.str.28.4.740. [DOI] [PubMed] [Google Scholar]
- 7.Dhamoon MS, Moon YP, Paik MC, Boden-Albala B, Rundek T, Sacco RL, et al. Long-term functional recovery after first ischemic stroke: the Northern Manhattan Study. Stroke. 2009;40:2805–2811. doi: 10.1161/STROKEAHA.109.549576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jauch EC, Saver JL, Adams HP, Jr, Bruno A, Connors JJ, Demaerschalk BM, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870–947. doi: 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- 9.Khatri P, Kleindorfer DO, Yeatts SD, Saver JL, Levine SR, Lyden PD, et al. Strokes with minor symptoms: an exploratory analysis of the National Institute of Neurological Disorders and Stroke recombinant tissue plasminogen activator trials. Stroke. 2010;41:2581–2586. doi: 10.1161/STROKEAHA.110.593632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ClinicalTrials.gov [Accessed November 26, 2014];A service of the U.S. National Institutes of Health. A Study of the Efficacy and Safety of Activase (Alteplase) in Patients With Mild Stroke (PRISMS) http://clinicaltrials.gov/show/NCT02072226.