Abstract
Objective
IRF1 both mediates responses to type I interferons and the induction of interferons. It has been implicated in murine lupus models as a critical mediator of inflammation. A previous study of chromatin modifications in SLE patient monocytes implicated IRF1 as associated with increased histone acetylation in SLE patients. This study directly investigated IRF1 binding sites on chromatin using ChIP-seq.
Methods
Nine female SLE patients and seven female controls were examined. Monocytes were purified from peripheral blood and subjected to library preparation using a validated antibody to IRF1. The effect of IRF1 on target gene expression was confirmed using an overexpression system in cell lines and co-immunoprecipitation was used to define protein interactions.
Results
IRF1 binding around transcribed regions was increased in SLE patient monocytes but histone modifications at potential IRF1 binding sites without detectable IRF1 binding were also increased. IRF1 overexpression was sufficient to drive transcription of target genes. IRF1 overexpression was also able to alter histone modifications at a focus set of target genes and the use of an IRF1 inhibitor decreased both expression and histone modifications at target genes. IRF1 was found to interact with a select set of histone modifying enzymes and other transcription factors.
Conclusions
IRF1 is an important signaling protein in the interferon pathway. IRF1 not only activates gene expression as a transcription factor but may perpetuate disease by leading to a dysregulated epigenome.
Introduction
Systemic lupus erythematosus (SLE) is the quintessential systemic autoimmune disease. The current model for the development of SLE involves an imbalance between the production of apoptotic cells and their clearance with the residual nucleic acid-protein complexes driving a type I interferon response, maturation of dendritic cells and B cells with an associated loss of tolerance (1, 2). A central role for type I interferons was recognized in early studies from the 1970s identifying elevated interferon levels in the serum of patients with lupus (3).
The interferon regulatory factors (IRFs) are a family of transcription factors that regulate host defense. In monocytes, IRF1 enhances TLR-dependent gene expression (4). Low levels of type I interferons activate JAK and STAT pathways and maintain monocytes and macrophages in a primed state to respond rapidly to infectious challenges. This priming alters the epigenetic landscape and has been demonstrated to enhance the expression of interferon-β, IL-6, and TNF (5, 6). This altered epigenetic landscape translates into increased promoter occupancy by activating transcription factors (7–9). The combination of type I interferons and TNF leads to sustained activation of IRF1 and STAT1, driving a self-reinforcing circuit that could alter the pattern of gene regulation through chromatin alterations (10).
In a previous study, we found that 63% of the genes with H4 hyperacetylation in SLE had potential binding sites for IRF1 within the upstream region (11). IRF1 is known to associate with p300/CBP and PCAF potentially providing a mechanism for the hyperacetylation (12). IRF1 is notable from the perspective of the female preponderance of SLE because it is one of the major downstream mediators of prolactin signaling. Prolactin is immune stimulatory (13) and can break B cell tolerance (14). Hyperprolactinemia has been reported in 15–33 % of patients with SLE (15) and bromocriptine, which inhibits secretion of prolactin, has been shown to reduce SLE clinical activity (16, 17). Thus, IRF1 provides a potential nexus of female hormones, inflammation and type I interferon signals.
Studies from murine lupus models also support a role for IRF1. The IRF1KO bred onto the MRL/lpr background ameliorated the classic MRL/lpr skin disease (18). The mice also had decreased autoantibodies, less glomerular immune complex deposition, diminished glomerulonephritis, less proteinuria and improved survival (18). In a separate model, IRF1 KO mice had markedly ameliorated autoantibody production and renal disease (19). Therefore, IRF1, central to interferon-mediated induction of gene expression, appears to be pivotal in the lupus disease process.
In this study, we evaluated the role of IRF1 in modulating the epigenome and characterized its binding pattern in SLE. We found that both defined and potential gene targets of IRF1 had a significantly altered chromatin pattern and we found that we could replicate much of the effect by overexpressing IRF1 in cell lines.
Methods
Patients and cell purification
Primary human monocytes were purified from seven healthy controls and nine SLE patients by elutriation and adherence (6). Monocytes were more than 90% positive for CD14 staining. All controls and patients were female and an average of approximately 40 years of age. SLE patients had mild-moderate disease activity and no one was on high-level immune suppression (Supplemental Table 1). This study was IRB-approved.
Cell lines
D54MG is a glioma cell line that responds to TLR stimulation with the production of inflammatory mediators, similar to that seen in monocytes. It was used for both over-expression and IRF1 decoy experiments. D54MG cells tolerated IRF1 overexpression. The IRF1 cDNA was subcloned into pCMV6-A-puro (Origene, Rockville, MD) and the empty vector was used as a control. Both were transfected into D54MG, selected with puromycin and used as polyclonal populations. The cells were maintained in DMEM with 10% cosmic calf serum.
The IRF1 decoy was generated from a locked nucleic acid oligonucleotide of the sequence +G+G+AAGCGAAAATGAAATTGA+C+T where + indicates the use of the locked chemistry (20). The control oligonucleotide was +G+A+TTCAAATTGAAA+C. The oligonucleotides were purchased from Exiqon (Woburn, MA). Duplex oligonucleotides were transfected using Amaxa and samples were harvested 24 hours after transfection for either ChIP assay or RNA as above.
Protein analysis
The co-immunprecipitations utilized the IRF1 antibody from Santa Cruz Biotechnology (Dallas, RX) (catalog: sc-74530). Protein A beads were used for collection (Invitrogen Life Technologies, Carlsbad, CA). Protein was quantitated using a Bradford assay and were equally loaded on 4–12% NuPAGE gel (Invitrogen) and blotted with the following antibodies used at the manufacturer’s suggested dilutions: P300 (sc-584), HDAC3 (sc-11417), CBP (sc-365387), ATF2 (sc-187), HDAC11 (sc-130776), PCAF (sc-13124), SIRT1 (sc-15404), HAT1 (sc-376200), TIP60 (sc-5727), MOF (sc-271691), GCN5 (sc-6303), STAT2 (sc-839), NFκB p65 (sc-372) all from Santa Cruz Biotechnologies, and STAT1 (catalog #9172) from Cell Signaling Technology (Danvers, MA). Immunofluorescence was detected on a Zeiss Axio Observer B. The IRF1 antibody (above) and a secondary Goat anti-mouse AF546 (catalog A11003 Life Technologies) was used at a dilution of 1:50 on triton permeabilized cells with 4% paraformaldehyde fixation. Quantitation was performed using ImageJ.
Chromatin immunoprecipitation and qRT-PCR
ChIP experiments were carried out as previously described (6, 21–23) and utilized the IRF1 antibody from Santa Cruz Biotechnology (H205, Santa Cruz, CA.). The library preparation utilized the SOLiD ChIP-seq kit and was performed according to the manufacturer’s instructions. For confirmatory ChIP assays, the following antibodies were used: H4AC (Upstate Biology, Lake Placid, NJ), H3K4me3 (Active Motif, Carlsbad, CA), and GST (Invitrogen, Camarillo, CA).
qRT-PCR was used to define quantitative differences in RNA abundance. The Clontech Advantage RT for PCR kit (Clontech, Mountain View, CA) was used to generate cDNA. Gene expression was detected by real-time PCR using the TaqMan 7900. Transcript levels were normalized to the 18S or β-actin signal.
Bioinformatics
We used the CHOP-seq pipeline we developed and further refinements (24).
Results
IRF1 binding sites in primary monocytes
Our ultimate goal was to identify altered IRF1 binding in SLE patients, but initially we examined the landscape of IRF1 binding overall. We first identified monocyte IRF1 binding sites using all sixteen IRF1 ChIP-seq libraries (nine SLE patients and seven controls) and comparing to four control libraries (one IgG ChIP and three input DNA controls). We identified 1853 IRF1 peaks with high confidence. There was a 75× enrichment of sequencing reads around all genomic sites matching the 18bp motif identified in our previous study (25). Given such a strong association of IRF1 binding with the 18bp motif, we selected another 1,265 IRF1 binding sites, having ≥80% match to the 18bp motif, that had read enrichment with slightly less significance. The combined set of 3,118 binding sites was used for the rest of this study. The average sequencing depth at these sites was 78.7 and the average read enrichment of IRF1 ChIP libraries over controls was 5.1. The high baseline binding in resting monocytes is consistent with the concept of poised genes with pre-bound transcription factors (26).
To examine the genes likely to be impacted by IRF1, we defined the locations of IRF1 for this set of 3118 binding sites. 13.2% of IRF1 binding sites were located within 10kb promoters, 5’-UTR, or the first exon of known genes, while gene bodies, CpG islands, etc. were also enriched with binding sites (Supplemental Figure 1A). These genes included thirty-nine immune response genes and nine genes with variants that have been associated with SLE in previous studies (CYP1A1, DUSP1, HLADMB, MBP, MICA, TAP1, TAP2, TNFSF13B, TRIM21). Our previous RNA-seq study of this cohort identified 2,271 novel transcribed loci, many of which had SLE-specific transcription (27). Ninety of these novel transcripts or their 10kb upstream regions had IRF1 binding sites. The enrichment of IRF1 binding sites within the 10kb promoter of immune response genes and these novel transcripts is illustrated in Supplemental Figure 1B. A combined set of 548 IRF1 target genes (458 annotated and 90 novel loci) will be referred to as the defined IRF1 target gene set. DAVID analysis (28, 29) of this gene set revealed enrichment of antiviral responses and antigen processing genes (Supplemental Table 2).
We further examined 2,057 potential IRF1 binding sites defined as having ≥90% similarity to the 18bp IRF1 motif, but no detectable IRF1 binding in primary monocytes. These sites were found within 10kb promoter, 5’-UTR, or the first exon of 87 known genes, including immune response genes such as CCL8, IL18BP, CD37, and TRAF3IP2. These could represent targets in other cell types. Additionally, these sites were mapped to six novel transcripts. This combined set of 93 genes (87 known and 6 novel) will be referred to as potential IRF1 targets in the rest of this study.
To determine the chromatin characteristics of the IRF1 binding sites, we cross-referenced the sites with cell-specific DNase I hypersensitive (DHS) sites identified by the ENCODE project (30). The 3118 IRF1 binding sites were highly enriched within DHS regions in CD14+ monocytes (Supplemental Figure 2). To better understand the context of these sites, we examined which transcription factor motifs were most likely to be found adjacent to the IRF1 sites. Some of most enriched motifs belonged to NFκB complexes such as REL and RELA, STAT1, and chromatin remodeling factors such as RSC30 and TRIM28 (Supplemental Figure 3). IRF1 has been previously found to colocalize with STAT1, REL and RELA (30).
Histone modifications around IRF1 binding sites
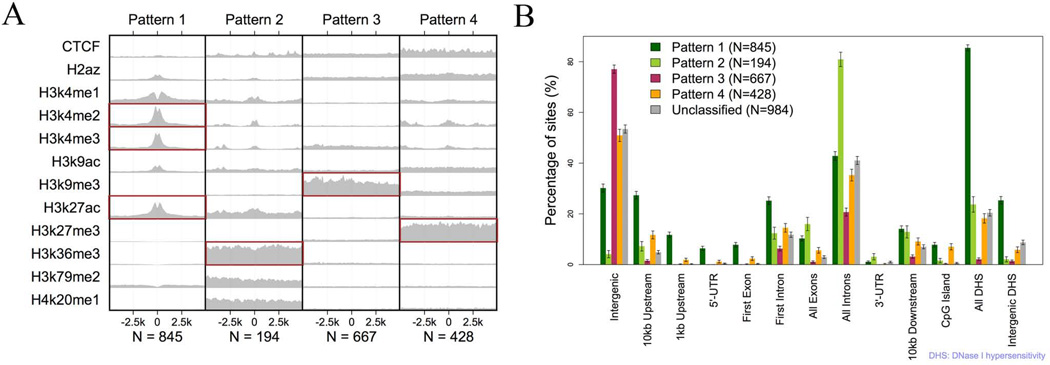
Transcriptional effects of regulators can be distinct in different chromatin contexts. Analysis of the ENCODE ChIP-seq data from CD14+ monocytes (30) identified four patterns of histone modifications around the 3118 IRF1 binding sites (Figure 1A). About 70% of the defined IRF1 sites fit one of these four patterns. Pattern I is typical for promoters and enhancers. It is exemplified by high levels of H3K4me2, H3K4me3, and H3K27ac. IRF1 binding sites fitting this pattern were enriched in DHS regions (85.4%) and highly enriched within the 1kb promoter (23.0 fold) and 5'-UTR (63.1 fold) regions. 75.6% sites mapped to novel transcripts also fit to this pattern. Pattern II is typical for gene bodies. It is exemplified by high levels of H3K36me3. Indeed, 185 of 194 sites fitting this pattern were in known exons (83.7 fold enrichment) or introns (25.7 fold enrichment). Pattern III is typical for constitutively repressed chromatin. It is exemplified by high levels of H3K9me3. 77.1% of binding sites fitting this pattern were intergenic and did not overlap with any known genes. Pattern IV is typical for cell-specific repression. It is exemplified by high levels of H3K27me3. Binding sites having this pattern were modestly enriched within the 1kb promoter (2.2 fold), 5'-UTR (6.8 fold), CpG island (9.9 fold) and DHS regions (4.7 fold). Lastly, unclassified binding sites were more likely to be found in intergenic regions. Figure 1B demonstrates the distribution of the four patterns according to annotated transcribed regions. These distinct patterns of chromatin around IRF1 binding sites reflect different states of gene expression but may also reflect distinct roles of IRF1 at those sites.
Figure 1.
Four distinctive patterns of histone modifications around IRF1 binding sites. A) Each block represents the average sequencing depth of a histone mark within 5kb from IRF1 binding sites after the depth was normalized by the total number of reads and then the average depth of input control. Each column has the same y-axis scale. The histone data was created by the ENCODE project from CD14+ monocytes. B) The location of IRF1 binding sites around transcribed regions according to their histone context is displayed. Error bars represent the standard deviation.
Expression of IRF1 targets in primary monocytes
These data supported a transcriptional effect of IRF1 binding but to further examine the relationship of IRF1 binding to transcription, we mined our RNA-seq data on the same patients and controls. Our RNA-seq data showed that the average transcription level of IRF1 defined targets was 75.8 % higher than that of non-targets with detectable transcription in primary monocytes. We examined the relationship of the transcript abundance to IRF1 binding and the surrounding histone modifications. Targets with pattern I sites (promoter/enhancer) were expressed 151.2% more than non-targets, while targets with pattern IV sites cell-specific repression) were expressed 75.1% less (Supplemental Figure 4). There was a statistically significant correlation between the transcript abundance of targets and IRF1 binding (r = 0.19, p = 6.9E-6). The binding sites with histone pattern IV had the strongest correlation of IRF1 peak height and transcript abundance (r = 0.36, p = 0.015), suggesting IRF1 maybe able to overcome a repressive environment. The six genes having both the top 5% IRF1 binding and transcription level were IFI6, GBP2, TAPBP, GRINA, PLEC, and PRELID1. The first three are known interferon response genes (31–33). These data demonstrate that the transcriptional effect of IRF1 is largely activation, even within repressed chromatin environments.
We additionally examined the relationship of IRF1 targets and gene expression with our published data on cytokine induction of gene expression in primary monocyte (34). Stimulation with α-interferon and γ-interferon increased the average expression of defined IRF1 targets by 62% (p = 2.7E-15) and 61% (p = 5.3E-19) respectively, but stimulation with interleukin 4 reduced their average by 7% (p = 0.0003). While this result is consistent with the canonical role of IRF1 as a mediator of interferon-induced activation, it also suggests that IRF1 binding is constitutive at some induced genes where other signals are required to activate transcription.
Differential IRF1 binding in SLE
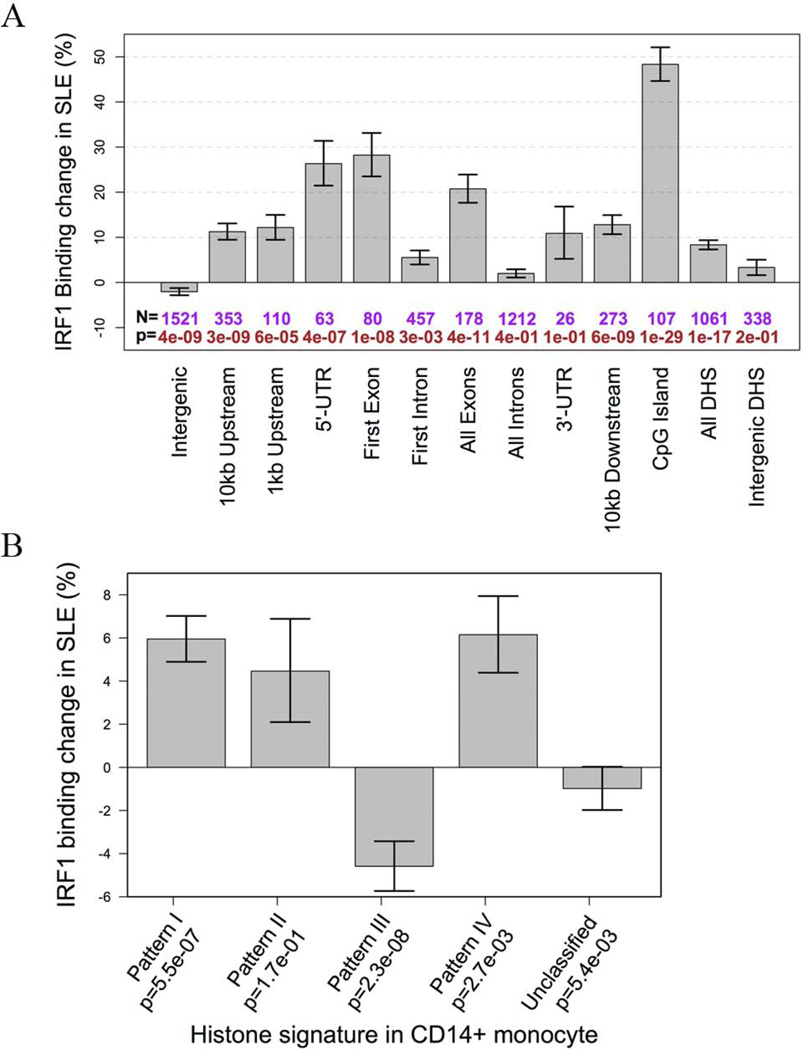
Our main goal was to examine whether IRF1 mediated altered gene expression in SLE patients. Differential binding (DB) of IRF1 between seven healthy controls and nine SLE patients was dependent on genomic location and nearby histone status of the binding sites. Binding sites at CpG islands were increased on average by ~50% in SLE. IRF1 binding was increased by 25% at sites within 5’-UTRs or the first exons (Figure 2A). In terms of chromatin context, sites with histone patterns I and IV had significantly increased binding in SLE while pattern III sites had a reduction of ~5% on average (Figure 2B). Therefore, IRF1 binding was increased in SLE but location and surrounding chromatin structure dictated some of the binding changes seen in SLE.
Figure 2.
Differential IRF1 binding in SLE. A) The average changes of IRF1 binding in SLE. Binding sites were grouped according to their location in annotated regions. The p value is given below each bar. B) The average changes of IRF1 binding in SLE. Binding sites were grouped according to their baseline histone status in CD14+ monocytes.
A permutation procedure identified 294 IRF1 DB sites with an estimated FDR equal to 0.2. These sites were grouped into three sets based on baseline and differential binding (Supplemental Figure 5). Set 1 DB sites had high baseline IRF1 binding, which was additionally increased by 66.3% in SLE on average. These sites were more likely to be found around the transcription start site (TSS). Set 1 DB sites were enriched for histone patterns I (promoter/enhancer) and IV (cell-specific repression). IRF1 target genes with such a binding site around their TSS included TAP1, TAP2, TRIM21, IFI35, TNFRSF14, and several proteasome subunits. Set 2 sites, on the other hand, had low or no detectable IRF1 binding in controls and had an average of 88.3% increase in binding in SLE, making them SLE-specific binding sites. These sites were significantly enriched within CpG islands and known exons. Set 2 DB sites were also enriched for histone patterns I and IV. STAT1 has such a site within a CpG island about 6.2kb upstream of the TSS. Set 3 DB sites had intermediate baseline binding, which was reduced in SLE. These sites were mostly intergenic. These data are the first to demonstrate differential binding of a pivotal transcription factor in SLE on a whole genome basis.
SLE-related H3K4me3 and H3K27me3 changes at IRF1 binding sites
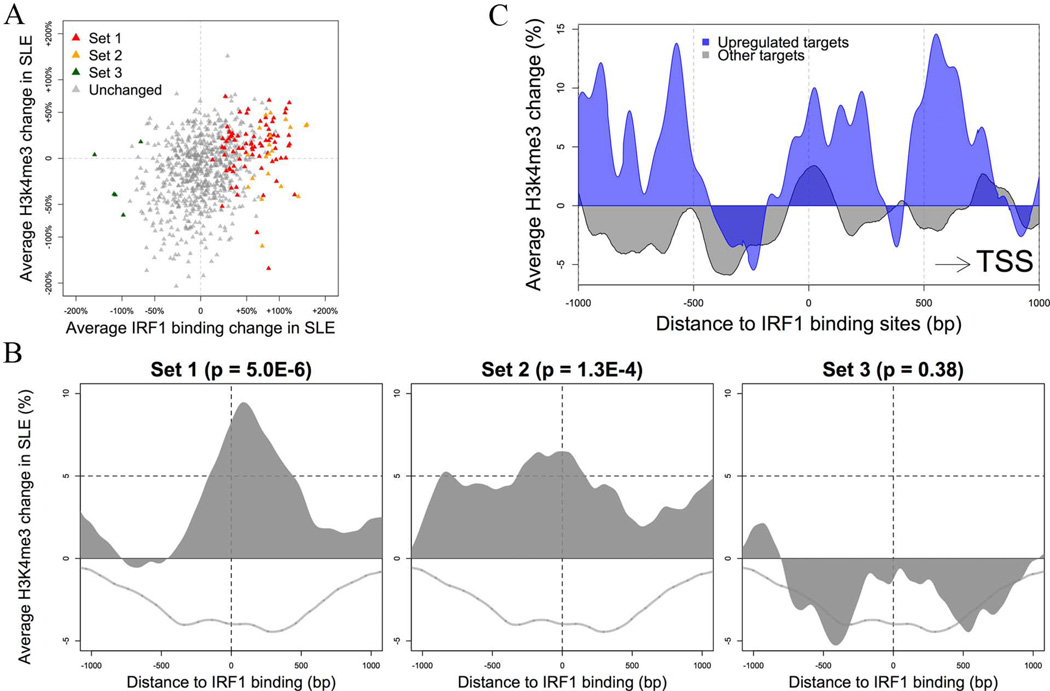
The histone modifications H3K4me3 and H3K27me3 were measured by ChIP-seq in five controls and five patients matched to the same samples as this study (manuscript in preparation). The controls exhibited patterns around IRF1 binding sites consistent with those seen in the published CD14+ monocyte data (30). Overall, H3K4me3 increased around sites with increased IRF1 binding in SLE patients with r = 0.132, p = 1.1E-13 (Figure3A and 3B).
Figure 3.
IRF1 and H3K4me3 peaks in SLE. A) There is a highly concordant change of IRF1 binding and H3K4me3 histone modification at IRF1 binding sites in SLE. Only sites with high baseline IRF1 binding (top 25%) were plotted. B) The average change of H3K4me3 in SLE according to the differential binding set assignment (see text). The p values indicate the difference between each set and sites with unchanged binding activity (grey line). C) The average change of H3K4me3 around IRF1 binding sites of target genes. Sites were split into two groups according to the differential expression of target genes. Targets significantly upregulated in SLE have a more distinctive, asymmetric pattern of H3K4me3 change. The direction was adjusted so that the closest TSS is always at the right side of the plot.
The H3K4me3 was largely restricted to sites with high IRF1 binding (Figure 3B). A clustering analysis of the H3K4me3 pattern around IRF1 binding sites identified a subset of 555 sites having an asymmetric pattern of H3K4me3 change in SLE (Supplemental Figure 6). Set 1 (high baseline with increase in SLE) DB sites were enriched in this subset (OR 2.71, p = 0.0004). H3K4me3 was increased by one-third at one side of these binding sites and decreased by one-third at the other side. The side with increased H3K4me3 is more likely to be proximal to the TSS (p = 0.0006). (Figure 3C). Target genes having IRF1 binding sites that fit this pattern included DUSP1, MICA, TAP2, TNFSF13B, IRF5, STAT1, and STAT2, the first four of which are known SLE-associated genes.
Differential expression (DE) of IRF1 targets in SLE
The average transcription level of the 548 defined IRF1 targets (458 known genes and 90 novel transcripts) was increased by 7.8% in SLE, while potential targets and non-targets with matching baseline transcription level had, respectively, a 14.0% and 2.5% decrease. 28 IRF1 targets, including HIVEP2, CCRL2, CD82, and at least seven interferon-inducible genes, were significantly upregulated in SLE (Supplemental Table 3). These analyses link IRF1 binding and increased expression of biologically relevant genes in SLE.
Although IRF1 target genes were more likely to be upregulated in SLE, the DE of these targets did not have a strong association with the DB of IRF1 binding (r = 0.01). There was, however, a connection between target DE and H3K4m3 change in SLE, as the upregulated targets had a stronger asymmetric pattern of H3K4me3 change around corresponding IRF1 binding sites (Figure 3C).
IRF1 overexpression
A number of analyses supported the concept that IRF1 had increased activity in SLE monocytes. The expression level of IRF1 itself was raised by 42.8% (p = 0.047), from the top 3.6% to the top 2.7% of all expressed genes. Correspondingly, IRF1 binding sites were more likely to have increased than decreased binding in SLE (250 vs. 44) and there were more sites having SLE-specific IRF1 binding than sites having control-specific binding (102 vs. 11). The average H3K4me3 near promoter IRF1 binding sites was increased (p = 0.003), and correspondingly, target genes were upregulated on average.
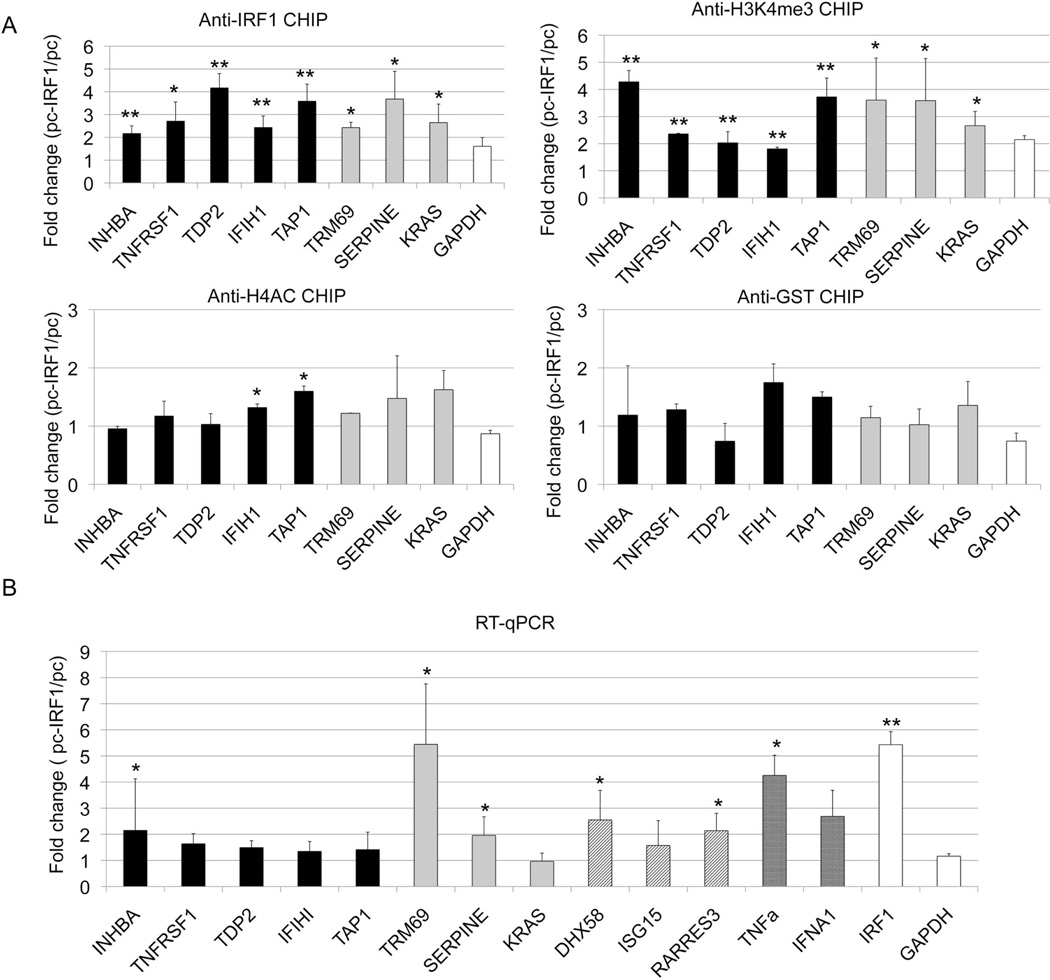
We used D54MG cells to determine if IRF1 could induce histone modifications at target genes. This cell line responds to inflammatory stimuli in a manner similar to that of monocytes. This cell line tolerated IRF1 over-expression. D54MG cells were transfected with an IRF1 overexpression vector or empty vector and selected in puromycin. For this study, we examined a set of five genes with increased IRF1 and mRNA in SLE and three genes with potential IRF1 binding sites. GAPDH was included as a negative control. We first ensured that overexpression of IRF1 increased genomic binding of IRF1 (Figure 4A). Our original observation that IRF1 binding motifs were highly enriched at sites with increased H4ac in SLE was the foundation for this study. We therefore examined H4ac at the defined and potential targets. Overexpression of IRF1 led to increased H4ac at two defined IRF1 targets. We also examined H3K4me3. We found that it was increased at both defined and potential IRF1 targets. In contrast, anti-GST recovery was no different between IRF1 overexpressing cells and the controls.
Figure 4.
The effect of IRF1 overexpression on histone modifications. D54MG cells were stably transfected by IRF1 cDNA over-expression vector (pc-IRF1) or control empty vector (pc). A) The cells were immunoprecipitated with antibody to IRF1, H3K4me3, H4ac or GST (a non-binding control antibody). The effect of overexpression was defined. The black bars represent the target genes with both increased IRF1 binding and mRNA expression in SLE patients. The grey bars represent potential IRF1 targets. GAPDH is a control gene, unaltered in SLE. B) RNA was harvested from transfected cells and cDNA was quantitated using qRT-PCR. The black bars represent targets with both increased IRF1 binding and mRNA in SLE (INHBA, TNFRSF14, TAP1, TDP2). The grey bars signify genes with potential IRF1 binding sites (TRIM69, SERPINE1, KRAS). The diagonal lines signify genes with very high IRF1 peaks (DHX58, ISG15, RARRES3, IFIH1). The cross hatched bars represent cytokine genes known to be overexpressed in SLE. IRF1 and GAPDH are shown as controls. Error bars indicate standard error. N=3. Student's t-test was used to determine statistical significance (* indicates p<0.05, ** indicates p<0.005).
The link between IRF1 and chromatin was established but we needed to understand why differential expression failed to directly correlate with differential IRF1 binding in SLE. We first examined published data on IRF1 overexpression in Huh-7 hepatoma cells and STAT1 −/− skin fibroblasts [GEO GSE26817]. The IRF1 target genes identified from our monocyte data were increased by 33.6% and 35.1% respectively while the targets upregulated in SLE were respectively increased by 133.3% and 147.4% (Supplemental Figure 7A). Although different cell types were used for these studies, these results demonstrated that IRF1 over-expression can replicate a portion of SLE-induced over-expression of genes, supporting an important role for IRF1 in SLE (Supplemental Figure 7B). Therefore, increased IRF1 activity appears to be at least partially responsible for the transcriptome changes in SLE.
We selected 15 genes for qRT-PCR analysis (Figure 4B). Five genes had increased expression in SLE and increased IRF1 binding in SLE. Three genes had a very high IRF1 peak that was unchanged in the SLE patients and three genes had potential IRF1 binding sites but no binding above background by ChIP-seq. We also included TNF, interferon A1, IRF1 itself, and GAPDH.. GAPDH expression was not changed by IRF1 overexpression. Of the genes with defined or potential IRF1 binding sites, 5/11 had significantly increased expression in the IRF1 overexpressing cells compared to vector-transfected cells and 5/11 of the remaining genes had increased expression that did not achieve statistical significance. We hypothesized that IRF1 was prebound to some targets as a results of environmental stimuli but additional stimuli or overexpression of IRF1 could drive transcription. IRF1, by interacting with chromatin modifying enzymes, could serve to target these complexes to specific loci.
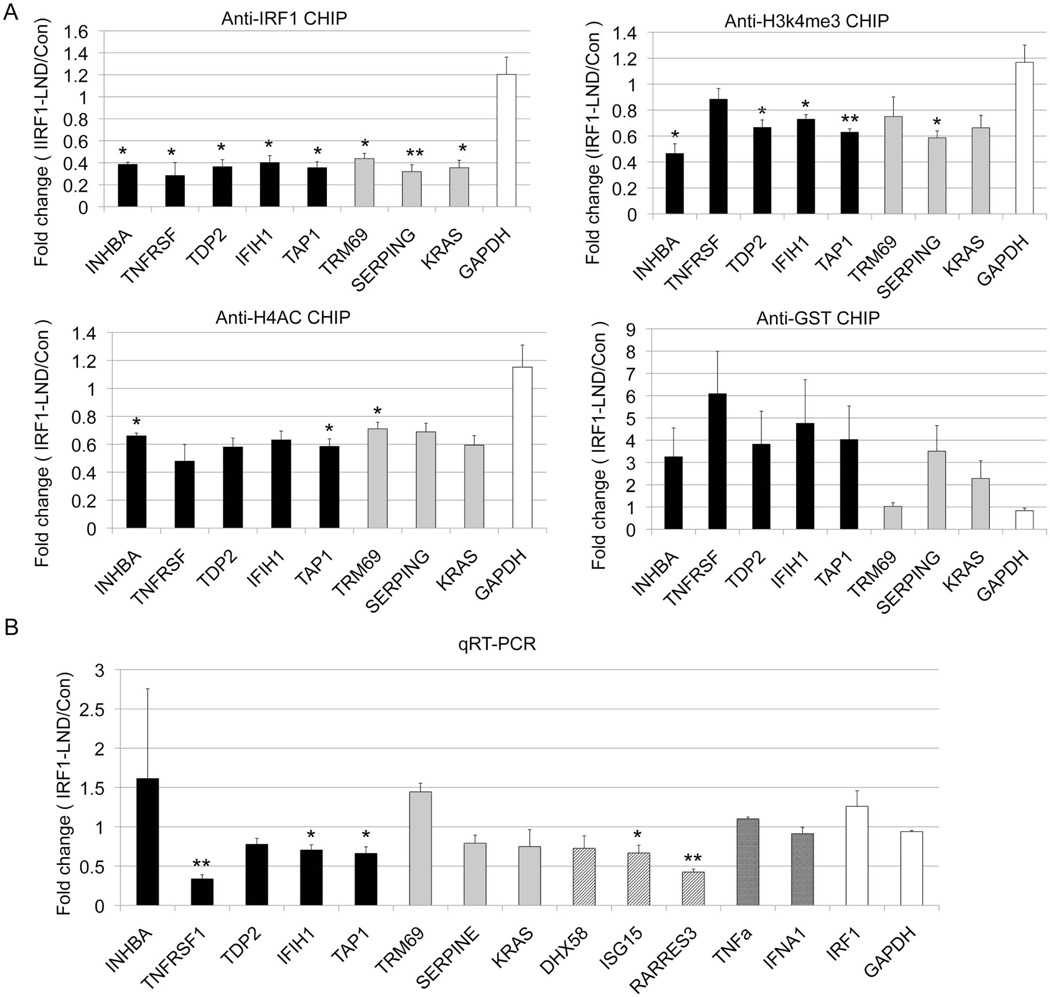
To further confirm the role of IRF1, we utilized a novel transcription factor decoy system. Locked nucleic acid:DNA oligonucleotides matched to the IRF1 binding site were transfected into D54MG cells. These decoys acted as “sponges” to prevent translocation of cytoplasmic IRF1 into the nucleus. We used qRT-PCR to define target gene expression and ChIP assays to analyze histone modifications. Transfection of the decoys successfully decreased IRF1 binding at target genes (Figure 5A). Expression of all but two target gene was decreased after decoy treatment (Figure 5B) and H3K4me3 and H4ac were also diminished after treatment, albeit at a subset of target genes. These data support a model wherein activated IRF1 binds to target genes and participates in chromatin modifications through recruitment of specific enzymes, thereby providing sequence specificity.
Figure 5.
The effect of IRF1 decoy treatment. D54MG cells were transfected with the IRF1 decoy oligonucleotide (IRF1-LND) or the control oligonucleotide (Con). A) The cells were immunoprecipitated with the indicated antibody, GST is a control non-binding antibody. The effect of the IRF1 decoy was defined by qPCR. B) RNA was harvested and cDNA was quantitated using qRT-PCR. .INHBA, TNFRSF14, TAP1, TDP2 represent the target genes with both increased IRF1 binding and mRNA expression in SLE patients (black bar). TRIM69, SERPINE1 and KRAS represent potential IRF1 targets(grey bar). The diagonal lines signify genes with very high IRF1 peaks (DHX58, ISG15, RARRES3, IFIH1). The cross hatched bars signify genes with potential IRF1 binding sites (TRIM69, SERPINE1, KRAS). IRF1 and GAPDH are shown as controls. GAPDH is unaltered in SLE. Error bars indicate standard error. N=3. Student's t-test was used to determine statistical significance (* indicates p<0.05, ** indicates p<0.005).
IRF1 protein analysis
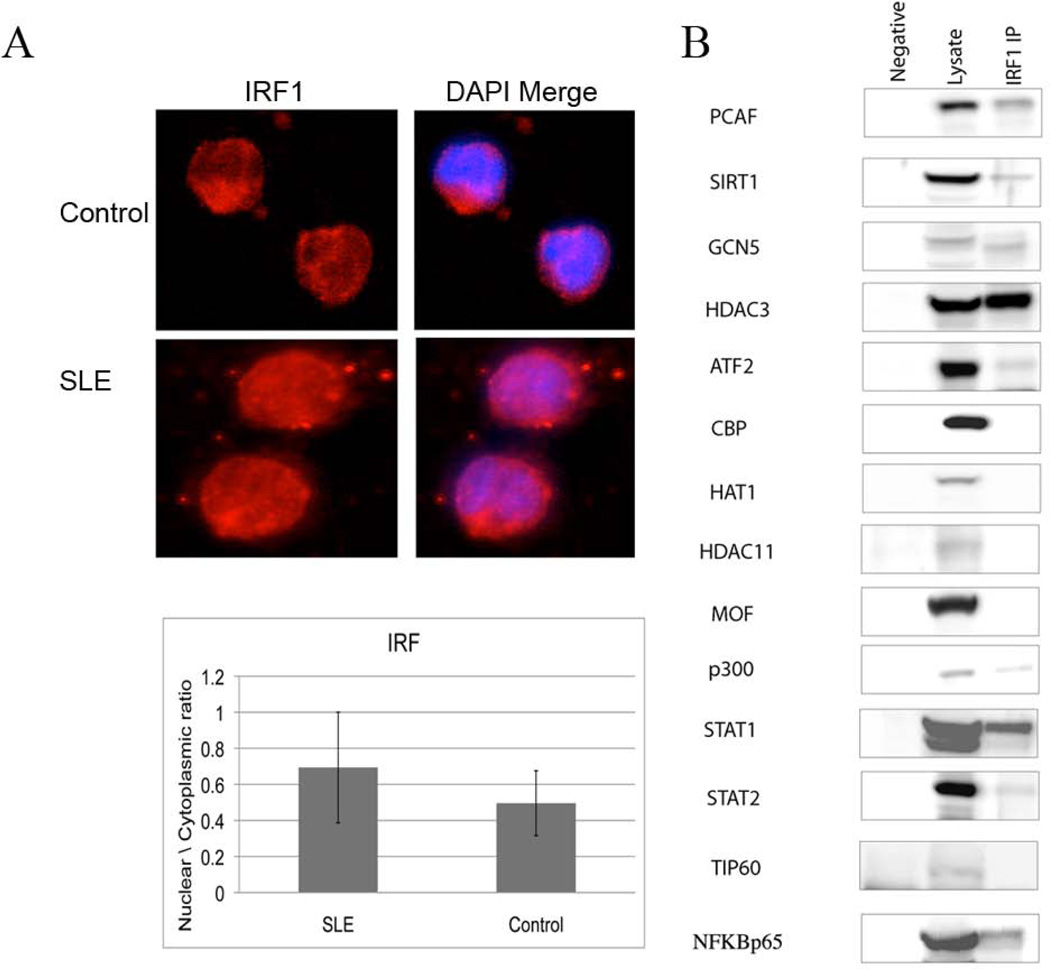
Phosphorylation, dimerization and translocation to the nucleus accompany IRF1 activation. To examine the state of IRF1 in SLE patients, monocytes from four controls and four SLE patients were stained for IRF1. Thirteen cells from each sample were analyzed and averaged. Monocytes from SLE patients exhibited a higher nuclear/cytoplasmic ratio of IRF1 than controls (Figure 6A).
Figure 6.
IRF1 protein. A) IRF1 nuclear translocation is increased in SLE. IRF1 was detected by quantitative immunofluorescence and the ratio of nuclear to cytoplasmic fluorescence analyzed. A representative cell from a control and a patient is shown and the average of four patients (n=13 cells × 4) and four controls (n=13 cells × 4) is displayed in the graph. The difference is significant with p=0.002. B) IRF1 immunoprecipitation was performed on D54MG cells overexpressing IRF1 and then blotted for the indicated proteins. The first lane is a negative control immunoprecipitated with beads only. The second lane is whole cell lysate and the third lane is the IRF1 immunoprecipitated material.
To examine whether IRF1 could directly interact with chromatin modifying enzymes, we performed an IRF1 immunoprecipitation in over-expressing D54MG cells. We then blotted for various chromatin modifying proteins. PCAF, p300, STAT1, NFκB, and HDAC3 were found to associate with IRF1 in resting cells (Figure 6B). This provides a potential mechanism for the association of IRF1 binding sites and the elevated H4ac in SLE that was previously identified (11).
Discussion
These data are the first to demonstrate altered transcription factor binding in SLE patients on a genome-wide basis. Our hypothesis that IRF1 binding was increased in SLE was based on our prior ChIP-chip study where increased H4 acetylation in SLE was found predominantly at IRF1 DNA motifs (11). IRF1 sites were found in different chromatin contexts, however, the increased IRF1 binding in SLE was primarily associated with promoter/enhancers and sites with chromatin characteristics of cell-specific repression. When we assessed the magnitude of the change in IRF1 binding in SLE, the strongest correlation was with the magnitude of the change in H3K4me3 in SLE at the same loci. IRF1 targets globally had increased expression in SLE, but the correlation between site-specific increased IRF1 binding and increased expression was weak. We hypothesized that IRF1 may be important for chromatin, but another transcription factor may be the critical driver of transcription. Indeed, IRF1 sites were associated with STAT1, NFκB and both proteins associated with IRF1.
We overexpressed IRF1 in D54MG cells to determine whether IRF1 binding sites could be activated in this setting. We examined a mix of targets with defined IRF1 sites and potential IRF1 sites as well as genes impacted by IRF1 but not thought to be directly bound by IRF1 but all exhibited increased expression compared to vector-transfected cells. These data suggest that many IRF1 sites in the genome exhibit inducible binding and that IRF1 binding may be transient in nature, responding to episodic environmental cues that drive interferon production. Furthermore, decoy data supported the critical role of IRF1 in the induction of histone modifications.
We examined whether a select set of histone modifying proteins and transcription factors could interact with IRF1. We found that IRF1 could be co-immunoprecipitated with PCAF and p300, two histone acetyltransferases. The focus group of enzymes was not exhaustive and did not assess the methyltransferases because the specificity of those enzymes is less well understood than the specificity of the histone acetyltransferases. Nevertheless, it is clear that IRF1 has the potential to interact with histone modifying enzymes and other transcription factors. These data support a model where environmental triggers such as infection or prolactin drive IRF1 onto binding sites transiently. With increased stimulation, additional relevant transcription factors are recruited that drive transcription. IRF1 nucleates a chromatin modification complex at target genes and even after resolution of the stimulus and departure of IRF1, some targets retain chromatin modifications that facilitate subsequent expression. We were able to link many of our findings through the RIG-I- MAVS pathway (Supplemental Figure 8). While critically important in innate anti-viral responses and interferon production, it has received less attention than the toll-like receptor pathway in SLE (35–43).
There were some weaknesses in this study. The patient sample size was relatively small for a study such as this. This precluded analysis of clinical subgroups. We felt that by working with a purified cell type, we could increase the signal and by examining multiple marks from the same cells, we would increase our statistical power. The ChIP-seq IRF1 signal was strong in this cell type, which led to confidence in our analyses. The focus set of target genes also represented a small group but the data did not suggest that IRF1 binding was significantly different between defined and potential sites in the overexpression system. Nevertheless, additional patient samples and the examination of additional targets will be required for confirmation.
IRF1 not only activates gene expression as a transcription factor but may contribute to disease chronicity by driving an altered epigenome that alters responses to subsequent stimuli. The fact that it has been previously identified as a pivotal transcription factor in murine lupus models further supports its relevance in SLE (18, 19). This pathway represents a novel therapeutic target and efforts at epigenetic therapeutics are already a major effort by the pharmaceutical industry.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge the contributions of patients, nurses and physicians and we thank Eric Rappaport, Stephen Mahoney and Kristen Hunter from the nucleic acid core facility at CHOP for SOLiD sample preparation as well as Juan Perin for alignments. This study was supported in part by a generous donation from the Irvin Siegel Lupus Research Fund, the Wallace Chair of Pediatrics, and NIH grants R01 ES017627 and AR43727.
References
- 1.Teichmann LL, Ols ML, Kashgarian M, Reizis B, Kaplan DH, Shlomchik MJ. Dendritic cells in lupus are not required for activation of T and B cells but promote their expansion, resulting in tissue damage. Immunity. 2010;33(6):967–978. doi: 10.1016/j.immuni.2010.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banchereau J, Pascual V. Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity. 2006;25(3):383–392. doi: 10.1016/j.immuni.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 3.Hooks JJ, Moutsopoulos HM, Geis SA, Stahl NI, Decker JL, Notkins AL. Immune interferon in the circulation of patients with autoimmune disease. N Engl J Med. 1979;301(1):5–8. doi: 10.1056/NEJM197907053010102. [DOI] [PubMed] [Google Scholar]
- 4.Pollara G, Handley ME, Kwan A, Chain BM, Katz DR. Autocrine type I interferon amplifies dendritic cell responses to lipopolysaccharide via the nuclear factor-kappaB/p38 pathways. Scandinavian Journal of Immunology. 2006;63(3):151–154. doi: 10.1111/j.1365-3083.2006.01727.x. [DOI] [PubMed] [Google Scholar]
- 5.Testoni B, Vollenkle C, Guerrieri F, Gerbal-Chaloin S, Blandino G, Levrero M. Chromatin dynamics of gene activation and repression in response to interferon alpha (IFN(alpha)) reveal new roles for phosphorylated and unphosphorylated forms of the transcription factor STAT2. J Biol Chem. 2011;286(23):20217–20227. doi: 10.1074/jbc.M111.231068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garrett S, Dietzmann-Maurer K, Song L, Sullivan KE. Polarization of primary human monocytes by IFN-gamma induces chromatin changes and recruits RNA Pol II to the TNF-alpha promoter. J Immunol. 2008;180(8):5257–5266. doi: 10.4049/jimmunol.180.8.5257. [DOI] [PubMed] [Google Scholar]
- 7.Chen J, Ivashkiv LB. IFN-gamma abrogates endotoxin tolerance by facilitating Toll-like receptor-induced chromatin remodeling. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(45):19438–19443. doi: 10.1073/pnas.1007816107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foster SL, Hargreaves DC, Medzhitov R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature. 2007;447(7147):972–978. doi: 10.1038/nature05836. [DOI] [PubMed] [Google Scholar]
- 9.Fang TC, Schaefer U, Mecklenbrauker I, Stienen A, Dewell S, Chen MS, et al. Histone H3 lysine 9 di-methylation as an epigenetic signature of the interferon response. J Exp Med. 2012;209(4):661–669. doi: 10.1084/jem.20112343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yarilina A, Park-Min KH, Antoniv T, Hu X, Ivashkiv LB. TNF activates an IRF1-dependent autocrine loop leading to sustained expression of chemokines and STAT1-dependent type I interferon-response genes. Nat Immunol. 2008;9(4):378–387. doi: 10.1038/ni1576. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Z, Song L, Maurer K, Petri MA, Sullivan KE. Global H4 acetylation analysis by ChIP-chip in systemic lupus erythematosus monocytes. Genes Immun. 2010;11(2):124–133. doi: 10.1038/gene.2009.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dornan D, Eckert M, Wallace M, Shimizu H, Ramsay E, Hupp TR, et al. Interferon regulatory factor 1 binding to p300 stimulates DNA-dependent acetylation of p53. Molecular and Cellular Biology. 2004;24(22):10083–10098. doi: 10.1128/MCB.24.22.10083-10098.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karmali RA, Lauder I, Horrobin DF. Letter: Prolactin and the immune response. Lancet. 1974;2(7872):106–107. doi: 10.1016/s0140-6736(74)91670-5. [DOI] [PubMed] [Google Scholar]
- 14.Peeva E, Michael D, Cleary J, Rice J, Chen X, Diamond B. Prolactin modulates the naive B cell repertoire. The Journal of Clinical Investigation. 2003;111(2):275–283. doi: 10.1172/JCI16530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petri M. Sex hormones and systemic lupus erythematosus. Lupus. 2008;17(5):412–415. doi: 10.1177/0961203308090026. [DOI] [PubMed] [Google Scholar]
- 16.Walker SE. Bromocriptine treatment of systemic lupus erythematosus. Lupus. 2001;10(10):762–768. doi: 10.1191/096120301717165010. [DOI] [PubMed] [Google Scholar]
- 17.Alvarez-Nemegyei J, Cobarrubias-Cobos A, Escalante-Triay F, Sosa-Munoz J, Miranda JM, Jara LJ. Bromocriptine in systemic lupus erythematosus: a double-blind, randomized, placebo-controlled study. Lupus. 1998;7(6):414–419. doi: 10.1191/096120398678920334. [DOI] [PubMed] [Google Scholar]
- 18.Reilly CM, Olgun S, Goodwin D, Gogal RM, Jr, Santo A, Romesburg JW, et al. Interferon regulatory factor-1 gene deletion decreases glomerulonephritis in MRL/lpr mice. European Journal of Immunology. 2006;36(5):1296–1308. doi: 10.1002/eji.200535245. [DOI] [PubMed] [Google Scholar]
- 19.Pollard KM, Hultman P, Toomey CB, Cauvi DM, Hoffman HM, Hamel JC, et al. Definition of IFN-gamma-related pathways critical for chemically-induced systemic autoimmunity. Journal of Autoimmunity. 2012;39(4):323–331. doi: 10.1016/j.jaut.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crinelli R, Bianchi M, Gentilini L, Magnani M. Design and characterization of decoy oligonucleotides containing locked nucleic acids. Nucleic Acids Research. 2002;30(11):2435–2443. doi: 10.1093/nar/30.11.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garrett S, Fitzgerald MC, Sullivan KE. LPS and poly I:C induce chromatin modifications at a novel upstream region of the IL-3 p19 promoter. Inflammation. 2008;31(4):235–246. doi: 10.1007/s10753-008-9070-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee JY, Kim NA, Sanford A, Sullivan KE. Histone acetylation and chromatin conformation are regulated separately at the TNF alpha promoter in monocytes and macrophages. Journal of Leukocyte Biology. 2003;73:862–871. doi: 10.1189/jlb.1202618. [DOI] [PubMed] [Google Scholar]
- 23.Sullivan KE, Reddy AB, Dietzmann K, Suriano AR, Kocieda VP, Stewart M, et al. Epigenetic regulation of tumor necrosis factor alpha. Mol Cell Biol. 2007;27(14):5147–5160. doi: 10.1128/MCB.02429-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc. 2012;7(3):562–578. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi L, Perin JC, Leipzig J, Zhang Z, Sullivan KE. Genome-wide analysis of interferon regulatory factor I binding in primary human monocytes. Gene. 2011;487(1):21–28. doi: 10.1016/j.gene.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muse GW, Gilchrist DA, Nechaev S, Shah R, Parker JS, Grissom SF, et al. RNA polymerase is poised for activation across the genome. Nat Genet. 2007;39(12):1507–1511. doi: 10.1038/ng.2007.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi L, Zhang Z, Yu A, Wang W, Wei Z, Akhter E, et al. The SLE transcriptome exhibits evidence of chronic endotoxin exposure and has widespread dysregulation of non-coding and coding RNAs. PLOS One. 2014 doi: 10.1371/journal.pone.0093846. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dennis G, Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, et al. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003;4(5):P3. [PubMed] [Google Scholar]
- 29.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 30.Bernstein BE, Birney E, Dunham I, Green ED, Gunter C, Snyder M. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489(7414):57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Porter AC, Chernajovsky Y, Dale TC, Gilbert CS, Stark GR, Kerr IM. Interferon response element of the human gene 6–16. EMBO J. 1988;7(1):85–92. doi: 10.1002/j.1460-2075.1988.tb02786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramsauer K, Farlik M, Zupkovitz G, Seiser C, Kroger A, Hauser H, et al. Distinct modes of action applied by transcription factors STAT1 and IRF1 to initiate transcription of the IFN-gamma-inducible gbp2 gene. Proc Natl Acad Sci U S A. 2007;104(8):2849–2854. doi: 10.1073/pnas.0610944104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zimmerer JM, Lesinski GB, Radmacher MD, Ruppert A, Carson WE., 3rd STAT1-dependent and STAT1-independent gene expression in murine immune cells following stimulation with interferon-alpha. Cancer Immunol Immunother. 2007;56(11):1845–1852. doi: 10.1007/s00262-007-0329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Z, Song L, Maurer K, Bagashev A, Sullivan KE. Monocyte polarization: the relationship of genome-wide changes in H4 acetylation with polarization. Genes Immun. 2011 doi: 10.1038/gene.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seth RB, Sun L, Ea CK, Chen ZJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell. 2005;122(5):669–682. doi: 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 36.Sun Q, Sun L, Liu HH, Chen X, Seth RB, Forman J, et al. The specific and essential role of MAVS in antiviral innate immune responses. Immunity. 2006;24(5):633–642. doi: 10.1016/j.immuni.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 37.Kato H, Sato S, Yoneyama M, Yamamoto M, Uematsu S, Matsui K, et al. Cell type-specific involvement of RIG-I in antiviral response. Immunity. 2005;23(1):19–28. doi: 10.1016/j.immuni.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 38.Pichlmair A, Schulz O, Tan CP, Naslund TI, Liljestrom P, Weber F, et al. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5'-phosphates. Science. 2006;314(5801):997–1001. doi: 10.1126/science.1132998. [DOI] [PubMed] [Google Scholar]
- 39.Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol. 2004;5(7):730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- 40.Boule MW, Broughton C, Mackay F, Akira S, Marshak-Rothstein A, Rifkin IR. Toll-like receptor 9-dependent and -independent dendritic cell activation by chromatin-immunoglobulin G complexes. J Exp Med. 2004;199(12):1631–1640. doi: 10.1084/jem.20031942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lau CM, Broughton C, Tabor AS, Akira S, Flavell RA, Mamula MJ, et al. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J Exp Med. 2005;202(9):1171–1177. doi: 10.1084/jem.20050630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pawar RD, Patole PS, Zecher D, Segerer S, Kretzler M, Schlondorff D, et al. Toll-like receptor-7 modulates immune complex glomerulonephritis. J Am Soc Nephrol. 2006;17(1):141–149. doi: 10.1681/ASN.2005070714. [DOI] [PubMed] [Google Scholar]
- 43.Tian J, Avalos AM, Mao SY, Chen B, Senthil K, Wu H, et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol. 2007;8(5):487–496. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.