Abstract
Psilocybin-occasioned mystical experiences have been linked to persisting effects in healthy volunteers including positive changes in behavior, attitudes, and values, and increases in the personality domain of openness. In an open-label pilot-study of psilocybin-facilitated smoking addiction treatment, 15 smokers received 2 or 3 doses of psilocybin in the context of cognitive behavioral therapy (CBT) for smoking cessation. Twelve of 15 participants (80%) demonstrated biologically verified smoking abstinence at 6-month follow-up. Participants who were abstinent at 6 months (n=12) were compared to participants still smoking at 6 months (n=3) on measures of subjective effects of psilocybin. Abstainers scored significantly higher on a measure of psilocybin-occasioned mystical experience. No significant differences in general intensity of drug effects were found between groups, suggesting that mystical-type subjective effects, rather than overall intensity of drug effects, were responsible for smoking cessation. Nine of 15 participants (60%) met criteria for “complete” mystical experience. Smoking cessation outcomes were significantly correlated with measures of mystical experience on session days, as well as retrospective ratings of personal meaning and spiritual significance of psilocybin sessions. These results suggest a mediating role of mystical experience in psychedelic-facilitated addiction treatment.
Keywords: Mystical experience, hallucinogen, tobacco, smoking cessation, nicotine, addiction, psilocybin, psychedelic
Introduction
From the late 1950s through early 1970s, serotonin 2A receptor (5-HT2AR) agonist psychedelics (i.e. classic hallucinogens) were examined as potential medications for the treatment of addiction. This research primarily focused on LSD for treatment of alcoholism, producing mixed results, likely due to methodological inconsistencies [1-5]. However, a meta-analysis of six randomized controlled studies (total N=536) found that a single dose of LSD in the context of alcoholism treatment significantly decreased alcohol misuse at the initial follow-up assessment compared with non-psychedelic control treatments, with a large effect size (odds ratio: 1.96) [6]. Some of the pioneering clinical work in this field was conducted in Saskatchewan, Canada by Humphry Osmond and Abram Hoffer, who treated more than 700 individuals suffering from alcoholism using a combination of LSD and psychotherapy [1,7-9]. Their approach emphasized the subjective nature of the psychedelic experience as an important factor affecting treatment outcomes. Similarly, early researchers such as Walter Pahnke, William Richards, and Stanislav Grof focused on psychedelics’ unique ability to facilitate powerful, and sometimes spiritual or mystical-type experiences as a means of producing therapeutic benefit in addiction treatment as well as end-of-life anxiety [10-13].
In a controlled study (N=74) examining LSD-facilitated treatment for opioid-dependent parolees, researchers found a statistically significant difference in biologically verified opioid abstinence at 6 months in the inpatient LSD treatment group, compared to a treatment-as-usual outpatient control group (32% vs. 8%, respectively) [14]. Although, a potential cofound was that the LSD group, but not control group, received residential treatment, the authors noted that the increased efficacy observed in the LSD condition may also have been due to the qualitative nature of the drug’s effects, described as, “cosmic, mystical, oceanic, peak, transpersonal, transcendental, etc. To the recipient they are experiences which seem to have ultimate metaphysical relevance, conversion-like experiences which, by definition, imply change” [14, p. 809].
Since the 1970s human research with psychedelics has been limited due to legal barriers and a lack of funding [15]. However, a recent resurgence in human psychedelic research has demonstrated that in healthy individuals, the naturally occurring 5-HT2AR agonist psilocybin is capable of eliciting highly meaningful and spiritually significant experiences with lasting positive effects [16-19]. Furthermore, a growing body of evidence suggests that when administered to well-prepared clinical populations in controlled settings, psilocybin and similar drugs may hold considerable therapeutic potential in the treatment of a variety of conditions including end-of-life anxiety, and smoking addiction [20-22].
In an open-label pilot study conducted by the authors that was the first to examine the feasibility and potential efficacy of psilocybin as an adjunct in the treatment of smoking addiction, 12 of 15 participants (80%) demonstrated biologically verified 7-day point-prevalence smoking abstinence1 at 6-month follow-up [22]. These results should be interpreted with caution, as researchers and participants were not blind to drug treatment conditions, and no control group was employed. However, it is noteworthy that the most successful current treatments for smoking cessation generally show far less efficacy, with typical abstinence rates below 35% at 6 months [23-24].
Mechanisms of Psychedelic-facilitated Addiction Treatment
Investigators have speculated about the potential mechanisms of psychedelics in addiction treatment. At the biological level, such explanations have focused on the molecular and neurological actions of these drugs involving serotonergic, glutamatergic, and dopaminergic signaling, as well as local brain metabolic activity and functional connectivity among brain regions including the amygdala, thalamus, and anterior and posterior cingulate cortex [25-28]. Effects of psychedelic-occasioned experiences on higher-order psychological constructs have also been implicated. For instance, reductions in craving and anxiety, increases in motivation and self-efficacy, and acute alterations in autobiographical recall and cognitive bias have been hypothesized to mediate potential efficacy of psychedelics in clinical treatment contexts [29-30]. Furthermore, the ability of psychedelics to elicit mystical, transcendent, or peak experiences has also been proposed as a potential psychological mechanism in precipitating insight and behavior change [1, 7, 10, 14, 17, 29, 31-32].
The present article provides a secondary analysis of data from our psilocybin-facilitated smoking cessation treatment study [22]. These analyses better characterize the mystical-type experiences occasioned by psilocybin and present additional subjective effects questionnaire data as well as excerpts from participants’ first-person accounts of these experiences. The purpose of this secondary analysis is to probe potential psychological mechanisms mediating treatment outcomes in psilocybin-facilitated addiction treatment.
Methods
Participants
Participants were recruited locally in the Baltimore area with advertisements offering a novel treatment involving psilocybin for smoking cessation. Fifteen participants (10 males) were enrolled and completed the study. Participants were physically and psychiatrically healthy adult smokers who were not regularly taking any psychotropic medications, who met minimum daily smoking criteria (≥10 cigarettes/day), who had multiple past unsuccessful quit attempts, and still desired to quit smoking. Participants had a mean (SD) age of 51 (10.5) years, and reported smoking a mean (SD) of 19 (2.9) cigarettes/day for a mean of 31 (9.9) years, with an average (SD) of 6 (3.6) previous lifetime quit attempts.
Participants were relatively well educated, with all individuals reporting some college, and 11 (73.3%) having received at least a Bachelor’s degree. The sample was racially homogeneous, including 14 (93%) White participants and 1 (7%) Asian participant. This study was approved by the Johns Hopkins University School of Medicine Institutional Review Board, and all participants provided informed consent. Participants received no financial compensation. For additional details on the study sample and procedures, refer to our previously published report [22].
Procedures
Participants underwent a 15-week smoking cessation treatment intervention, with moderate (20mg/70kg) and high (30mg/70kg) dose psilocybin sessions occurring in weeks 5, and 7 respectively, and an optional third high dose session in week 13. The manualized treatment intervention consisted of cognitive-behavioral therapy (CBT) based largely on the Quit for Life program [33-34], and additional elements of mindfulness training [35-36], and guided imagery exercises [37] shown to be effective in smoking cessation research.
The intervention consisted of weekly meetings for 15 weeks, and was delivered by a team of 2 to 3 facilitators. The Target-Quit Date (TQD) was set for week 5 of treatment, concurrent with the first psilocybin session. Afterwards, study treatment facilitators met weekly with participants to discuss psilocybin session experiences, encourage and socially reinforce the use of CBT techniques, and to provide support for smoking abstinence. Biological samples were collected at each weekly meeting to assess participants’ smoking status, and psychological measures were administered at multiple points throughout the intervention and at 6-month follow-up.
Measures
Smoking Biomarkers
Two biomarkers of recent smoking were used to assess participants’ smoking status weekly from study intake (week 1) through end of treatment (week 15), and again at 6-month follow-up. Breath carbon monoxide (CO) was measured using a Bedfont Micro III Smokerlyzer (Haddonfield, NJ), which is able to detect smoking within approximately the past 24 hours. Urine samples were also collected and sent to an independent laboratory for analysis (Friends Medical Laboratory, Baltimore, MD) to assess levels of cotinine, a metabolite of nicotine, which can identify smoking within approximately the past six days. For this study, cutoff values of ≤6ppm for breath CO, and <200 ng/mL for urine cotinine were considered biological verification of non-smoking status [38-40].
Timeline Follow-back (TLFB)
At study intake participants provided self-reported estimates of their daily cigarette consumption over the past 30 days with the TLFB, a widely used retrospective measure of daily substance use [41]. During each following study visit participants provided TLFB self-report data regarding the number of cigarettes they smoked daily since their last visit.
Questionnaire on Smoking Urges (QSU)
The QSU is a 32-item measure of smoking craving with subscales assessing intention to smoke, desire to smoke, anticipation of positive outcome, and relief of withdrawal [42]. QSU data were collected at study intake, weekly post-TQD, and at 6-month follow-up.
Smoking Abstinence Self-Efficacy Scale (SASE)
The SASE is a two-dimensional measure assessing confidence to abstain from smoking, and temptation to smoke in 20 hypothetical situations (e.g. ‘With friends at a party’) [43-44]. SASE data were collected at study intake, weekly post-TQD, and at 6-month follow-up.
Hallucinogen Rating Scale (HRS)
The HRS is a 99-item questionnaire assessing subjective effects of hallucinogens in six domains: intensity, somaesthesia, affect, perception, cognition, and volition [45]. This scale was originally designed to study the 5-HT2AR agonist N, N-dimethyltryptamine (DMT), though it has since been used to research other hallucinogens including psilocybin [18-19], Ayahuasca [46], and Salvia divinorum [47-48]. The HRS was administered at the end of each psilocybin session (approximately 7 hours after capsule ingestion), and participants were instructed to respond with reference to their experience during that particular session day.
Mysticism Scale
Originally designed to assess the occurrence of mystical experience across an individual’s lifetime [49], this 32-item questionnaire has previously shown sensitivity to the effects of psilocybin [16, 18, 22]. The mysticism scale includes a three-factor structure assessing dimensions referred to as introvertive, extrovertive, and interpretation [50]. For this study, the Mysticism scale was adapted to ask about participants’ experiences during a specific psilocybin session, and was administered at the conclusion of each session.
States of Consciousness Questionnaire (SOCQ)
This 100-item measure has previously been used to characterize the subjective effects of psilocybin [16, 18], including 43 items dedicated to evaluating mystical experience as quantified by Pahnke and Richards [13, 51-52], and 57 distracter items. Based on the descriptive work of Stace [53], Pahnke and Richards [13] described mystical experience as exhibiting these key features: a sense of unity (i.e. loss of [internal] boundaries within the self, or [external] boundaries between self and environment); transcendence of time and/or space; ineffability and paradoxicality (i.e. that the experience is difficult to describe or conceptualize); a sense of sacredness or awe; a noetic quality (i.e. sense of direct knowledge of ultimate or higher reality); and deeply felt positive mood (e.g. joy, peace, love).
Scores on the SOCQ and its component subscales are calculated using the 43 items assessing mystical experience, and are expressed here as a proportion of maximum possible score. Consistent with prior research [16, 18, 51], criteria for a complete mystical experience consisted of a score of ≥60% on each of the following subscales: unity (either internal or external unity subscales, whichever had a higher score), transcendence of time and space, ineffability, sacredness, noetic quality, and positive mood. As described previously [16], a mean total SOCQ score was calculated as a mean of all items from the preceding six scales. The SOCQ was administered at the end of each psilocybin session, and specifically asked that participants respond regarding their experience during that session day.
Ratings of Personal Meaning, Spiritual Significance, and Well-being
One week after each psilocybin session participants were asked to rate the personal meaning, spiritual significance, and impact on well-being or life satisfaction attributed to their most recent session. Participants were also asked to rate these retrospectively with reference to their cumulative psilocybin experiences during the final treatment meeting (in week 15), as well as providing open-ended comments on their session experiences in their own words at that time. One participant unexpectedly had to leave the country on business prior to finishing treatment, and was unable to provide these ratings at end of treatment. For this participant, ratings at 12-month follow-up were used to calculate mean scores and provide open-ended comments.
Data Analysis
Analyses were performed to assess whether differences in observed smoking cessation outcomes at 6 months might be attributed to pre-existing differences between groups. Specifically, two-tailed unpaired t-tests compared all smoking-related measures at study intake between participants who showed biologically confirmed 7-day point-prevalence smoking abstinence at 6-month follow-up (n=12), and participants who were still smoking at 6 months (n=3).
Analyses were performed to assess whether smoking cessation outcomes at 6 months were associated with subjective effects questionnaire data about psilocybin session experiences. Specifically, two-tailed unpaired t-tests were also used to evaluate differences between participants who were abstinent at 6 months (n=12) and participants who were still smoking at 6 months (n=3) on SOCQ, Mysticism scale, and HRS Intensity subscale scores, as well as ratings of psilocybin sessions’ personal meaning, spiritual significance, and impact on well-being. For data showing unequal between-group variance, Welch’s correction was used [54-55].
Analyses were performed to ascertain the frequency of psilocybin-occasioned mystical experience in an addiction treatment context. Occurrence of “complete” mystical experiences during the study treatment was assessed with SOCQ data according to a priori criteria used in prior studies as previously described [16, 18, 51].
Change scores from study intake to 6-month follow-up were calculated for the following smoking-related measures: breath carbon monoxide and urine cotinine, Timeline-follow back (TLFB), Questionnaire on Smoking Urges (QSU), and Smoking Abstinence Self-Efficacy (SASE) confidence and temptation scales. For these measures, change scores consisted of each participant’s score at study intake subtracted from their score at 6-month follow-up. For the TLFB this was calculated as mean cigarettes per day in the 30 days preceding study intake, subtracted from mean cigarettes per day from Target-Quit Date (TQD) to 6-month follow-up.
In order to determine correlates of smoking cessation success, Pearson’s correlations were calculated among participants’ change scores on smoking-related measures (i.e. smoking biomarkers, TLFB, QSU, and SASE), mean ratings of personal meaning, spiritual significance, and impact on well-being, and mean scores on the States of Consciousness Questionnaire (SOCQ), Mysticism scale, and HRS intensity subscale. The HRS intensity subscale was included in these analyses to test the hypothesis that smoking cessation would be more strongly related to mystical-type effects of psilocybin rather than overall intensity of drug effects.
Results
Differences in Smoking-related Measures at Study Intake
Two-tailed unpaired t-tests found significantly higher urine cotinine levels at study intake (t(13)=2.49, p=0.027) among participants who were still smoking at 6-month follow-up (n=3) with a mean (SD) of 2329 (771) ng/mL, compared with those who were abstinent at 6 months (n=12), who exhibited a mean (SD) of 1513 (443) ng/mL. The remaining smoking-related measures (i.e. breath CO, TLFB, QSU, SASE) showed no significant between-group differences at study intake.
Differences in Measures of Psilocybin Session Experiences
Two-tailed unpaired t-tests found significantly higher SOCQ scores among smoking-abstinent participants, compared with those who continued smoking at 6 months (Table 1). Scores on the Mysticism Scale and HRS Intensity subscale showed no significant differences between groups. Ratings of personal meaning, spiritual significance and impact on well-being were also significantly higher in participants no longer smoking at 6 months compared with those who were still smoking at 6 months.
Table 1.
Psilocybin session measures and unpaired t-test results for differences between groups.
| Measure | Quitters (n=12) a | Still Smokers (n=3) a | p Value* | (t, df) |
|---|---|---|---|---|
| SOCQ b | 65.8 (2.8) | 52.1 (7.9) | 0.049* | (2.02, 40) |
| MScale c | 217.6 (6.8) | 203.9 (18.2) | 0.398 | (0.85, 40) |
| HRS Intensity d | 2.51 (0.07) | 2.50 (0.13) | 0.962 | (0.05, 40) |
| Personal Meaning e | 6.49 (0.12) | 5.08 (0.62) | 0.047* | (2.22, 12) |
| Spiritual Significance f | 4.44 (0.18) | 3.50 (0.53) | 0.039* | (2.11, 55) |
| Impact on Wellbeing g | 2.49 (0.17) | 1.67 (0.45) | 0.043* | (2.07, 55) |
p<0.05
Data are mean scores with 1 SEM shown in parentheses.
SOCQ=States of Consciousness Questionnaire; Data expressed as percentage of maximum possible score.
MScale=Mysticism Scale; Possible score range: 0-288
HRS=Hallucinogen Rating Scale; Possible score range: 0-4
Possible score range: 1-8; 1=no more than routine, everyday experiences. 2=similar to meaningful experiences that occur on average once or more a week. 3=similar to meaningful experiences that occur on average once a month. 4=similar to meaningful experiences that occur on average once a year. 5=similar to meaningful experiences that occur on average once every 5 years. 6=among the 10 most meaningful experiences of my life. 7=among the five most meaningful experiences of my life. 8=the single most meaningful experience of my life.
Possible score range: 1-6; 1=not at all. 2=slightly. 3=moderately. 4=very much. 5=among the 5 most spiritually significant experiences of my life. 6=the single most spiritually significant experience of my life.
Possible score range: −3 to +3; −3=decreased very much. −2=decreased moderately. −1=decreased slightly. 0=no change. 1=increased slightly. 2=increased moderately. 3=increased very much.
Occurrence of “Complete” Mystical Experience
During the study there were a total of 42 psilocybin sessions across the 15 participants [22]. As previously reported, six participants (40%) reported at least one challenging psilocybin experience characterized by feelings of fear, fear of insanity, or feeling trapped. However these acute effects were readily managed by study staff, and had resolved by the end of the sessions. Aside from these short-lived dysphoric reactions, no clinically significant adverse events occurred during the study treatment [22].
Thirteen of the 42 psilocybin sessions (31%) were categorized as “complete” mystical experiences according to a priori criteria used in previous research [16, 18, 51]. Ten of 13 (77%) “complete” mystical experiences occurred during high dose (30mg/70kg) sessions, and the remaining three (23%) occurred during moderate dose (20mg/70kg) sessions. Using these same SOCQ score criteria, 9 of 15 participants (60%) had a “complete” mystical experience during at least one psilocybin session.
One of the three participants who continued smoking through 6-month follow-up had no “complete” mystical experience during study treatment, another had one “complete” mystical experience, and the third had two “complete” mystical experiences. Of the 12 participants who were abstinent at 6-month follow-up, five had no “complete” mystical experiences, four had one “complete” mystical experience, and three had two “complete” mystical experiences.
Table 2 presents verbatim written comments about the nature of the psilocybin session experiences for the 12 participants who were abstinent at 6 months.
Table 2.
Verbatim written comments for all volunteers who demonstrated biologically verified smoking abstinence at 6-month follow-up (n=12). These comments were excerpted from the States of Consciousness Retrospective Questionnaire (administered at end of treatment, in week 15) that asked open-ended questions about what was most memorable and what was most spiritually significant about the psilocybin session experiences.
| Participant ID | Verbatim Comments |
|---|---|
| 402 | Feelings of gratefulness, a great (powerful) remembrance of humility… of my experience of being, the experience of my being in and within the infinite. |
| 403 | Not at all religious but significant in motivating me to nurture my spiritual life. |
| 405 | It changes what I believe… We are all one and divine. |
| 406 1 | The awareness that all is one and then the realization that I am an integral piece of the one’s puzzle. |
| 410 | Oneness with universe; being forgiven. |
| 413 | Rich joy and awe. My body melting and becoming one with the universe felt both painless and profound… Feeling complete as a person and physically a part of all things. |
| 416 | There is a meaningful presence that humbles any human heart. |
| 417 | Simultaneously being aware and saturated in the majesty of existence. |
| 421 | Seeing God speaks for itself; seeing and feeling forever was like traveling through space-time. |
| 422 | The sessions permitted me to go inside and see and feel the nature of the mind. |
| 423 | Recognizing the source and manifestation of visions… source of unconscious content. |
| 427 | I believe I channeled the power of the Goddess and that I hold that power in me. I believe she exists everywhere and I look for her to add spark, life, and joy to everyday ordinary situations. |
This participant was out of the country at end of treatment, and provided these retrospective comments at 12-month follow-up.
Correlations between Measures of Psilocybin Session Experience and Smoking Cessation
As expected, significant correlations were found between change scores of all smoking-related measures (Table 3). Likewise, mean scores on SOCQ were significantly correlated with mean Mysticism scale, and HRS Intensity subscale scores, as well as mean ratings of psilocybin sessions’ spiritual significance, and impact on well-being. However, SOCQ mean scores were not significantly correlated with average ratings of personal meaning of psilocybin sessions.
Table 3.
Pearson’s r correlation matrix of change scores of smoking-related measures, mean scores of mystical experience, and mean ratings of personal meaning, spiritual significance, and well-being (N=15).
| CO a | Cotinine | TLFB b | QSU c | Conf d | Tempt e | SOCQ f | M Scale g | Intensity | Meaning | SpiritSig h | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cotinine 1 | 0.78*** | ||||||||||
| TLFB 1 | 0.83*** | 0.78*** | |||||||||
| QSU 1 | 0.63* | 0.67** | 0.71** | ||||||||
| Conf 1 | −0.83*** | −0.72** | −0.81*** | −0.68** | |||||||
| Tempt 1 | 0 79*** | 0.74** | 0.83*** | 0.70** | −0.98*** | ||||||
| SOCQ 2 | −0.32 | −0.56* | −0.38 | −0.65** | 0.39 | −0.43 | |||||
| M Scale 2 | −0.05 | −0.35 | −0.22 | −0.37 | 0.20 | −0.26 | 0.81*** | ||||
| Intensity 2 | −0.06 | −0.06 | −0.003 | −0.34 | 0.09 | −0.15 | 0.56* | 0.35 | |||
| Meaning 3 | −0.55* | −0.61* | −0.55* | −0.45 | 0.68** | −0.70** | 0.49 | 0.37 | 0.01 | ||
| SpiritSig 3 | −0.40 | −0.54* | −0.51 | −0.43 | 0.60* | −0.63* | 0.62* | 0.46 | 0.08 | 0.93*** | |
| Wellbeing 3 | −0.42 | −0.54* | −0.59* | −0.31 | 0.56* | −0.61* | 0.53* | 0.41 | 0.02 | 0.85*** | 0.92*** |
Note:
=p<.05
=p<.01
=p<.001
CO = Breath Carbon Monoxide
TLFB = Timeline follow-back (self-reported cigarettes per day)
QSU=Questionnaire on Smoking Urges
Conf = Smoking Abstinence Self-Efficacy (SASE) Confidence to abstain from smoking
Tempt = SASE Temptation to smoke
SOCQ = States of Consciousness Questionnaire.
M Scale = Mysticism Scale
SpiritSig = Ratings of Spiritual Significance.
Correlations were calculated using participants’ change scores from study intake to 6-month follow-up.
Correlations were calculated using participants’ mean scores across all psilocybin sessions (taken same day).
Correlations were calculated using participants’ mean ratings across all psilocybin sessions (taken 7 days after each session) and retrospective ratings at end of treatment (week 15).
Significant correlations between mean SOCQ scores and change scores of QSU craving, and urine cotinine were found. Furthermore, average ratings of psilocybin sessions’ personal meaning exhibited significant correlations with change scores of breath CO, urine cotinine, TLFB daily smoking, SASE confidence to abstain, and SASE temptation to smoke.
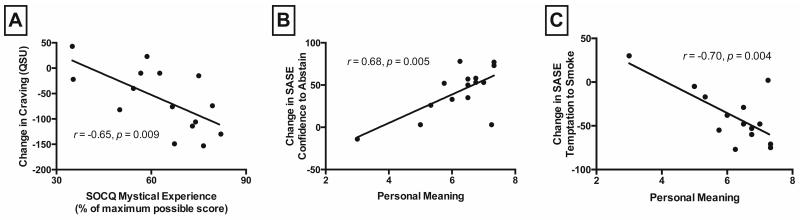
Mean ratings of spiritual significance were significantly correlated with change scores of urine cotinine, SASE confidence to abstain, and SASE temptation to smoke. Finally, mean ratings of psilocybin sessions’ impact on well-being and life satisfaction were significantly correlated with change scores of urine cotinine, TLFB daily smoking, SASE confidence to abstain, and SASE temptation to smoke. Three correlations between measures of psilocybin session experience and smoking cessation reached significance at p<.01 (Figure 1).
Figure 1. Relationships between psilocybin session experiences and changes in smoking-related measures.
Change in Questionnaire on Smoking Urges (QSU) smoking craving from study intake to 6-month follow-up as a function of mean States of Consciousness Questionnaire (SOCQ) mystical experience score (A). Change in Smoking Abstinence Self-Efficacy (SASE) Confidence to abstain (B), and SASE Temptation to smoke (C) from study intake to 6-month follow-up as a function of mean rating of how personally meaningful psilocybin session experiences were. Data points show data from each of the 15 individual participants with best-fit linear regression.
Discussion
The present results suggest that psilocybin, administered to drug-dependent individuals in the context of an addiction treatment program, is capable of occasioning “complete” mystical experiences at rates comparable to those previously exhibited in healthy volunteers [16, 18]. Participants’ open-ended comments about their psilocybin sessions (Table 2) were generally consistent with features of mystical-type experience (e.g. unity, sacredness). Although definitive conclusions about the role of psilocybin in this study’s smoking cessation outcomes cannot be drawn due to the open-label design and lack of control group, the mystical-type qualities of psilocybin sessions (measured the same day), as well as their personal meaning, spiritual significance, and impact on well-being (measured 7 days after these experiences) are significantly correlated with measures of smoking cessation treatment outcomes at 6-month follow-up. Furthermore, intensity of psilocybin session experiences was not significantly associated with smoking cessation treatment outcomes, suggesting that mystical-type effects specifically, rather than general intensity of subjective drug effects, are associated with long-term abstinence.
Exactly how psychedelic-occasioned mystical experiences may elicit profound changes in addictive behavior is still not well-understood, though research and anecdotal reports have corroborated the occurrence of dramatic improvements in substance abuse after psychedelic-occasioned and spontaneously occurring mystical experiences [1, 7, 14, 56-58]. Other models, such as the “conversion experience” [58], and more recently “quantum change” [56], are related and perhaps overlapping frameworks describing similarly sudden, positive transformations in personality and/or behavior. However, these remain as poorly elucidated as mystical experience in terms of ultimate causes and mechanisms.
Two specific hypotheses that have been proposed in the literature, and in part borne out by these results, are that the high degree of personal meaning attributed to participants’ psilocybin session experiences are associated with subsequent decrease in temptation to use tobacco, and increase in self-efficacy to abstain from smoking [29]. Furthermore, the magnitude of the mystical qualities of the psilocybin experiences as measured with the SOCQ seem to be predictive of subsequent decrease in tobacco craving and use, as observed from reductions in QSU scores and urinary cotinine levels. These findings are consistent with a recent study showing that mystical-type effects occasioned by the non-classic hallucinogen ketamine mediated reductions in cocaine craving among 8 non-treatment-seeking cocaine-dependent individuals [59]. It should be noted that while SOCQ data were collected the day of psilocybin sessions, participant ratings of meaning, spiritual significance, and impact on well-being were collected one week after each psilocybin session, and may have therefore been confounded by smoking cessation outcome.
That mystical experiences can be safely and reliably occasioned in both healthy and clinical populations through use of 5-HT2AR agonists provides the unprecedented ability to study them rigorously using all the tools available to modern scientific inquiry. Research on the acute and long-term effects of psychedelic-occasioned mystical experiences is still in its nascence, although the results of this, and previous studies offer a compelling glimpse into a class of experience that may hold a variety of important therapeutic applications, as well as continuing to further our understanding of basic neuroscience and behavioral biology.
The results of the present study are limited by the relatively small and homogeneous sample. Therefore, these findings must be considered preliminary. Although mystical-type effects were associated with positive treatment outcome in this small sample, other aspects of the experience that were not examined (perhaps correlated with mystical-type experience) may have played a causal role in abstinence, consistent with the fact that not all long-term abstainers had “complete” mystical experiences, and not all participants who had “complete” mystical experiences went on to quit smoking. Future research will benefit from larger and more diverse samples, as well as consideration of other (i.e. non-mystical) drug effects that may be mediating treatment outcomes. Additionally, these pilot findings should be interpreted with caution due to the post-hoc nature of the data analyses and multiple comparisons performed.
The association between psilocybin-occasioned mystical experience and higher order psychological constructs relevant across addictions (e.g. craving, temptation, self-efficacy), is consistent with prior research showing efficacy of psychedelics for treatment of alcoholism and opioid dependence [6, 14]. Perhaps the most exciting implication is that this drug class could be used to treat a wide variety of drug addictions, including smoking, alcoholism, and opioid dependence, as well as non-drug addictions (e.g. gambling addiction). Given the relatively low success rates of current addiction treatments, and the global morbidity and mortality associated with addictive disorders, further research into psychedelic-facilitated treatment of addiction is both timely and important.
The idea that a single discrete experience can result in lasting beneficial effects in an individual’s attitudes or behavior is highly unusual if not unprecedented within the modern biomedical paradigm, wherein curative or therapeutic processes are often conceptualized as occurring gradually (e.g. a course of medication to treat an illness). Yet there does exist a parallel phenomenon that seems to function similarly, though in the opposite direction; that is, a salient adverse event (i.e. trauma) may result in lasting negative effects in a person’s physical and mental life [60-67]. The acquired nature of post-traumatic stress disorder (PTSD) and the observation that acute adverse events are capable of producing enduring detrimental brain changes are well-documented. It is our contention that in a similar fashion, the psychedelic-occasioned peak experience may function as a salient, discrete event producing inverse PTSD-like effects-that is, persisting changes in behavior (and presumably the brain) associated with lasting benefit. By “PTSD-like” we are not presuming that these experiences necessarily share common biological mechanisms with PTSD. Rather, we are proposing that these experiences are “PTSD-like” in the sense that a single discrete event can cause lasting behavioral (and likely biological) changes, and “inverse” in the sense that these lasting changes are beneficial in nature, as opposed to deleterious. We hope that this conceptualization may prompt further research into this potentially important class of experiences.
Acknowledgements
Amanda Feilding of the Beckley Foundation provided initial encouragement and funding for this research, with continued funding provided by Heffter Research Institute. Mary Cosimano, MSW, Margaret Klinedinst, BS, Patrick Johnson, PhD, Matthew Bradstreet, PhD, Rosemary Scavullo Flickinger, BA, Fred Reinholdt, MA, Samantha Gebhart, BS, Grant Glatfelter, BS, and Toni White, BA assisted in data collection. Annie Umbricht, MD, and Leticia Nanda, CRNP provided medical screening and coverage. William A. Richards, STM, PhD provided valuable clinical consultation. August Holtyn, PhD, and Frederick S. Barrett, PhD provided comments on the manuscript.
Funding/Support: The Beckley Foundation provided initial funding for this research, with continued funding provided by Heffter Research Institute. Support for Dr. Garcia-Romeu was provided by NIDA Grant T32DA07209. Support for Dr. Griffiths was provided in part by NIDA Grant R01DA003889.
Footnotes
7-day point-prevalence smoking abstinence is defined as self-report of not smoking even a puff of a cigarette in the past 7 days, with biological verification from breath and urine samples.
Financial Disclosure: Dr. Griffiths is on the board of directors of the Heffter Research Institute.
References
- [1].Chwelos N, Blewett DB, Smith CM, et al. Use of d-lysergic acid diethylamide in the treatment of alcoholism. Quart J Stud Alcohol. 1959;20:577–90. [PubMed] [Google Scholar]
- [2].Hollister LE, Shelton J, Krieger G. A controlled comparison of lysergic acid diethylamide (LSD) and dextroamphetamine in alcoholics. Am J Psychiatry. 1969;125(10):1352–7. doi: 10.1176/ajp.125.10.1352. [DOI] [PubMed] [Google Scholar]
- [3].Ludwig A, Levine J, Stark L, et al. A clinical study of LSD treatment in alcoholism. Am J Psychiatry. 1969;126(1):59–69. doi: 10.1176/ajp.126.1.59. [DOI] [PubMed] [Google Scholar]
- [4].Mangini M. Treatment of alcoholism using psychedelic drugs: a review of the program of research. J Psychoactive Drugs. 1998;30(4):381–418. doi: 10.1080/02791072.1998.10399714. [DOI] [PubMed] [Google Scholar]
- [5].Smart RG, Storm T, Baker EFW, et al. A controlled study of lysergide in the treatment of alcoholism: The effects on drinking behavior. Quart J Stud Alcohol. 1966;27(3):469–482. [PubMed] [Google Scholar]
- [6].Krebs TS, Johansen PØ. Lysergic acid diethylamide (LSD) for alcoholism: meta-analysis of randomized controlled trials. J Psychopharmacol. 2012;26(7):994–1002. doi: 10.1177/0269881112439253. [DOI] [PubMed] [Google Scholar]
- [7].Osmond H. A review of the clinical effects of psychotomimetic agents. Ann N Y Acad Sci. 1957;66(3):418–434. doi: 10.1111/j.1749-6632.1957.tb40738.x. [DOI] [PubMed] [Google Scholar]
- [8].Sarett M, Cheek F, Osmond H. Reports of wives of alcoholics of effects of LSD-25 treatment of their husbands. Arch Gen Psychiatry. 1966;14(2):171–178. doi: 10.1001/archpsyc.1966.01730080059009. [DOI] [PubMed] [Google Scholar]
- [9].Dyck E. “Hitting highs at rock bottom”: LSD treatment for alcoholism, 1950–1970. Soc Hist Med. 2006;19(2):313–329. [Google Scholar]
- [10].Pahnke WN, Kurland AA, Unger S, Savage C, Grof S. The experimental use of psychedelic (LSD) psychotherapy. JAMA. 1970;212(11):1856–1863. [PubMed] [Google Scholar]
- [11].Kurland AA, Savage C, Pahnke WN, Grof S, Olsson JE. LSD in the Treatment of Alcoholics*. Pharmacopsychiatry. 1971;4(2):83–94. [Google Scholar]
- [12].Grof S, Goodman LE, Richards WA, Kurland AA. LSD-assisted psychotherapy in patients with terminal cancer. Int Pharmacopsychiatry. 1973;8:129–144. doi: 10.1159/000467984. [DOI] [PubMed] [Google Scholar]
- [13].Pahnke WN, Richards WA. Implications of LSD and experimental mysticism. J Relig Health. 1966;5(3):175–208. doi: 10.1007/BF01532646. [DOI] [PubMed] [Google Scholar]
- [14].Savage C, McCabe OL. Residential psychedelic (LSD) therapy for the narcotic addict: a controlled study. Arch Gen Psychiatry. 1973;28(6):808–814. doi: 10.1001/archpsyc.1973.01750360040005. [DOI] [PubMed] [Google Scholar]
- [15].Nutt DJ, King LA, Nichols DE. Effects of Schedule I drug laws on neuroscience research and treatment innovation. Nat Rev Neurosci. 2013;14(8):577–585. doi: 10.1038/nrn3530. [DOI] [PubMed] [Google Scholar]
- [16].Griffiths RR, Johnson MW, Richards WA, et al. Psilocybin occasioned mystical-type experiences: immediate and persisting dose-related effects. Psychopharmacology (Berl) 2011;218(4):649–665. doi: 10.1007/s00213-011-2358-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Griffiths RR, Richards WA, Johnson MW, et al. Mystical-type experiences occasioned by psilocybin mediate the attribution of personal meaning and spiritual significance 14 months later. J Psychopharmacol. 2008;22(6):621–632. doi: 10.1177/0269881108094300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Griffiths RR, Richards WA, McCann U, et al. Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacology (Berl) 2006;187(3):268–283. doi: 10.1007/s00213-006-0457-5. 2006. [DOI] [PubMed] [Google Scholar]
- [19].MacLean KA, Johnson MW, Griffiths RR. Mystical experiences occasioned by the hallucinogen psilocybin lead to increases in the personality domain of openness. J Psychopharmacol. 2011;25(11):1453–1461. doi: 10.1177/0269881111420188. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gasser P, Holstein D, Michel Y, et al. Safety and efficacy of lysergic acid diethylamide-assisted psychotherapy for anxiety associated with life-threatening diseases. J Nerv Ment Dis. 2014;202(7):513–520. doi: 10.1097/NMD.0000000000000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Grob CS, Danforth AL, Chopra GS, et al. Pilot study of psilocybin treatment for anxiety in patients with advanced-stage cancer. Arch Gen Psychiatry. 2011;68(1):71–78. doi: 10.1001/archgenpsychiatry.2010.116. [DOI] [PubMed] [Google Scholar]
- [22].Johnson MW, Garcia-Romeu A, Cosimano MP, et al. Pilot study of 5-HT2AR agonist psilocybin in the treatment of tobacco addiction. J Psychopharmacol. doi: 10.1177/0269881114548296. e-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Cahill K, Stevens S, Lancaster T. Pharmacological Treatments for Smoking Cessation. JAMA. 2014;311(2):193–194. doi: 10.1001/jama.2013.283787. [DOI] [PubMed] [Google Scholar]
- [24].Mottillo S, Filion KB, Bélisle P, et al. Behavioural interventions for smoking cessation: a meta-analysis of randomized controlled trials. Eur Heart J. 2009;30(6):718–730. doi: 10.1093/eurheartj/ehn552. [DOI] [PubMed] [Google Scholar]
- [25].Carhart-Harris RL, Erritzoe D, Williams T, et al. Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proc Natl Acad Sci U S A. 2012;109(6):2138–2143. doi: 10.1073/pnas.1119598109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kraehenmann R, Preller KH, Scheidegger M, et al. Psilocybin-induced decrease in amygdala reactivity correlates with enhanced positive mood in healthy volunteers. Biol Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.04.010. Forthcoming. [DOI] [PubMed] [Google Scholar]
- [27].Ross S. Serotonergic hallucinogens and emerging targets for addiction pharmacotherapies. Psychiatr Clin North Am. 2012;35(2):357–374. doi: 10.1016/j.psc.2012.04.002. [DOI] [PubMed] [Google Scholar]
- [28].Vollenweider FX, Kometer M. The neurobiology of psychedelic drugs: Implications for the treatment of mood disorders. Nat Rev Neurosci. 2010;11(9):642–651. doi: 10.1038/nrn2884. [DOI] [PubMed] [Google Scholar]
- [29].Bogenschutz MP, Pommy JM. Therapeutic mechanisms of classic hallucinogens in the treatment of addictions: From indirect evidence to testable hypotheses. Drug Test Anal. 2012;4(7-8):543–555. doi: 10.1002/dta.1376. [DOI] [PubMed] [Google Scholar]
- [30].Carhart-Harris RL, Leech R, Williams TM, et al. Implications for psychedelic-assisted psychotherapy: functional magnetic resonance imaging study with psilocybin. Br J Psychiatry. 2012;200(3):238–244. doi: 10.1192/bjp.bp.111.103309. [DOI] [PubMed] [Google Scholar]
- [31].Richards WA, Rhead JC, DiLeo FB, et al. The peak experience variable in DPT-assisted psychotherapy with cancer patients. J Psychoactive Drugs. 1977;9(1):1–10. [Google Scholar]
- [32].Maslow AH. Religions, values, and peak-experiences. Viking; New York: 1964. [Google Scholar]
- [33].Marks DF, Sykes CM. Randomized controlled trial of cognitive behavioural therapy for smokers living in a deprived area of London: outcome at one-year follow-up. Psychol Health Med. 2002;7(1):17–24. [Google Scholar]
- [34].Sykes CM, Marks DF. Effectiveness of a cognitive behaviour therapy self-help programme for smokers in London, UK. Health Promot Int. 2001;16(3):255–260. doi: 10.1093/heapro/16.3.255. [DOI] [PubMed] [Google Scholar]
- [35].Kabat-Zinn J. Full catastrophe living: Using the wisdom of your body and mind to face stress, pain, and illness. Delacorte; New York: 1990. [Google Scholar]
- [36].Brewer JA, Mallik S, Babuscio TA, et al. Mindfulness training for smoking cessation: results from a randomized controlled trial. Drug Alcohol Depend. 2011;119(1):72–80. doi: 10.1016/j.drugalcdep.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zernig G, Wallner R, Grohs U, et al. A randomized trial of short psychotherapy versus sustained- release bupropion for smoking cessation. Addiction. 2008;103(12):2024–2031. doi: 10.1111/j.1360-0443.2008.02348.x. [DOI] [PubMed] [Google Scholar]
- [38].Bramer SL, Kallungal BA. Clinical considerations in study designs that use cotinine as a biomarker. Biomarkers. 2003;8(3-4):187–203. doi: 10.1080/13547500310012545. [DOI] [PubMed] [Google Scholar]
- [39].Javors MA, Hatch JP, Lamb RJ. Cut-off levels for breath carbon monoxide as a marker for cigarette smoking. Addiction. 2005;100(2):159–167. doi: 10.1111/j.1360-0443.2004.00957.x. [DOI] [PubMed] [Google Scholar]
- [40].Middleton ET, Morice AH. Breath carbon monoxide as an indication of smoking habit. Chest. 2000;117(3):758–763. doi: 10.1378/chest.117.3.758. [DOI] [PubMed] [Google Scholar]
- [41].Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Litten R, Allen J, editors. Measuring Alcohol Consumption. Humana Press; Rockville, MD: 1992. pp. 207–224. [Google Scholar]
- [42].Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urges. Br J Addict. 1991;86(11):1467–1476. doi: 10.1111/j.1360-0443.1991.tb01732.x. [DOI] [PubMed] [Google Scholar]
- [43].DiClemente CC, Prochaska JO. Processes and stages of change: Coping and competence in smoking behavior change. In: Shiffman S, Wills TA, editors. Coping and Substance Abuse. Academic Press; New York, NY: 1985. pp. 319–343. [Google Scholar]
- [44].DiClemente CC, Prochaska JO, Gibertini M. Self-efficacy and the stages of self-change of smoking. Cognit Ther Res. 1985;9(2):181–200. [Google Scholar]
- [45].Strassman RJ, Qualls CR, Uhlenhuth EH, et al. Dose-response study of N, N-dimethyltryptamine in humans. II. Subjective effects and preliminary results of a new rating scale. Arch Gen Psychiatry. 1994;51:98–108. doi: 10.1001/archpsyc.1994.03950020022002. [DOI] [PubMed] [Google Scholar]
- [46].Riba J, Rodriguez-Fornells A, Strassman RJ, et al. Psychometric assessment of the hallucinogen rating scale. Drug Alcohol Depend. 2001;62(3):215–223. doi: 10.1016/s0376-8716(00)00175-7. [DOI] [PubMed] [Google Scholar]
- [47].Addy PH. Acute and post-acute behavioral and psychological effects of salvinorin A in humans. Psychopharmacology (Berl) 2012;220(1):195–204. doi: 10.1007/s00213-011-2470-6. [DOI] [PubMed] [Google Scholar]
- [48].Johnson MW, MacLean KA, Reissig CJ, et al. Human psychopharmacology and dose-effects of salvinorin A, a kappa opioid agonist hallucinogen present in the plant Salvia divinorum. Drug Alcohol Depend. 2011;115(1):150–155. doi: 10.1016/j.drugalcdep.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Hood RW. The construction and preliminary validation of a measure of reported mystical experience. J Sci Study Relig. 1975;14(1):29–41. [Google Scholar]
- [50].Hood RW, Ghorbani N, Watson PJ, et al. Dimensions of the Mysticism Scale: Confirming the Three-Factor Structure in the United States and Iran. J Sci Study Relig. 2001;40(4):691–705. [Google Scholar]
- [51].Pahnke WN. Psychedelic drugs and mystical experience. Int Psychiatry Clin. 1969;5:149–162. [PubMed] [Google Scholar]
- [52].Richards WA. Counseling, peak experiences and the human encounter with death: an empirical study of the efficacy of DPT-assisted counseling in enhancing the quality of life of persons with terminal cancer and their closest family members [dissertation] Catholic University of America; Washington, DC: 1975. [Google Scholar]
- [53].Stace WT. Mysticism and philosophy. MacMillan; New York: 1960. 1960. [Google Scholar]
- [54].Welch BL. The generalization of Student’s’ problem when several different population variances are involved. Biometrika. 1947;34(1-2):28–35. doi: 10.1093/biomet/34.1-2.28. [DOI] [PubMed] [Google Scholar]
- [55].Ruxton GD. The unequal variance t-test is an underused alternative to Student’s t-test and the Mann–Whitney U test. Beh Ecology. 2006;17(4):688–690. [Google Scholar]
- [56].Miller WR. The phenomenon of quantum change. J Clin Psychol. 2004;60(5):453–460. doi: 10.1002/jclp.20000. [DOI] [PubMed] [Google Scholar]
- [57].White LW. Recovery from alcoholism: Transpersonal dimensions. J Transpersonal Psychol. 1979;11(2):117–128. [Google Scholar]
- [58].James W. The varieties of religious experience. Harvard University Press; Cambridge, MA: 1902. [Google Scholar]
- [59].Dakwar E, Anerella C, Hart CL, et al. Therapeutic infusions of ketamine: Do the psychoactive effects matter? Drug Alcohol Depend. 2014;136:153–157. doi: 10.1016/j.drugalcdep.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Admon R, Milad MR, Hendler T. A causal model of post-traumatic stress disorder: disentangling predisposed from acquired neural abnormalities. Trends Cogn Sci. 2013;17(7):337–347. doi: 10.1016/j.tics.2013.05.005. [DOI] [PubMed] [Google Scholar]
- [61].Andero R, Dias BG, Ressler KJ. A Role for Tac2, NkB, and Nk3 Receptor in Normal and Dysregulated Fear Memory Consolidation. Neuron. 2014;83:1–11. doi: 10.1016/j.neuron.2014.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Elzinga BM, Bremner JD. Are the neural substrates of memory the final common pathway in posttraumatic stress disorder (PTSD)? J Affect Disord. 2002;70(1):1–17. doi: 10.1016/s0165-0327(01)00351-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Kühn S, Gallinat J. Gray matter correlates of posttraumatic stress disorder: a quantitative meta-analysis. Biol Psychiatry. 2013;73(1):70–74. doi: 10.1016/j.biopsych.2012.06.029. [DOI] [PubMed] [Google Scholar]
- [64].Hopper JW, Frewen PA, van der Kolk BA, et al. Neural correlates of reexperiencing, avoidance, and dissociation in PTSD: Symptom dimensions and emotion dysregulation in responses to script-driven trauma imagery. J Trauma Stress. 2007;20(5):713–725. doi: 10.1002/jts.20284. [DOI] [PubMed] [Google Scholar]
- [66].Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann N Y Acad Sci. 2006;1071(1):67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- [67].Tan L, Zhang L, Qi R, et al. Brain structure in post-traumatic stress disorder: A voxel-based morphometry analysis. Neural Regen Res. 2013;8(26):2405–2414. doi: 10.3969/j.issn.1673-5374.2013.26.001. [DOI] [PMC free article] [PubMed] [Google Scholar]