Abstract
Background and Purpose
The ATP-binding cassette transporter A-1 (ABCA1) gene is a key target of the transcription factors liver-X-receptors (LXRs). LXR activation has anti-inflammatory and neuroprotective effects in animal ischemic stroke models. Here, we tested the hypothesis that brain ABCA1 reduces blood-brain barrier (BBB) and white matter (WM) impairment in the ischemic brain after stroke.
Methods
Adult brain-specific ABCA1 deficient (ABCA1−B/−B) and floxed-control (ABCA1fl/fl) mice were subjected to permanent distal middle cerebral artery occlusion (dMCAo) and were sacrificed 7 days after dMCAo. Functional outcome, infarct volume, BBB leakage, and WM-damage were analyzed.
Results
Compared to ABCA1fl/fl mice, ABCA1−B/−B mice showed marginally (P=0.052) increased lesion volume, but significantly increased BBB leakage and WM-damage in the ischemic brain, and more severe neurological deficits. Brain-ABCA1 deficient mice exhibited increased the level of matrix-metalloproteinase-9 (MMP9) and reduced the level of insulin-like growth factor-1 (IGF1) in the ischemic brain. BBB leakage was inversely correlated (r=−0.073, P<0.05) with aquaporin-4 (AQP4) expression. Reduction of IGF1 and AQP4, but upregulation of MMP9 expression were also found in the primary astrocyte-cultures derived from ABCA1−B/−B mice. Cultured primary-cortical-neurons (PCNs) derived from C57BL/6 wild-type mice with ABCA1−B/−B astrocyte-conditioned-medium exhibited decreased neurite-outgrowth compared to culture with ABCA1fl/fl astrocyte-conditioned-medium. ABCA1−B/−B PCNs show significantly decreased neurite-outgrowth, which was attenuated by IGF1 treatment.
Conclusions
We demonstrate that brain ABCA1-deficiency increases BBB leakage, WM/axonal damage and functional deficits after stroke. Concomitant reduction of IGF1 and upregulation of MMP9 may contribute to brain ABCA1-deficiency induced BBB and WM/axonal damage in the ischemic brain.
Keywords: ATP-binding cassette transporter A-1 (ABCA1), blood-brain barrier (BBB), aquaporin-4 (AQP4), insulin-like growth factor 1 (IGF1), white matter (WM), stroke
The ATP binding cassette transporter subfamily member A 1 (ABCA1) regulates efflux of cellular cholesterol and phospholipids to form Apolipoprotein E (ApoE)-containing lipoprotein particles and plays a critical role in high-density lipoprotein (HDL) cholesterol homeostasis and metabolism in the central nervous system (CNS).1-3 ABCA1 gene expression is regulated by liver X receptors (LXRs), transcriptional factors involved in lipid metabolisms and inflammation4, 5. Activation of LXRs using synthetic LXRs agonist, T0901317 and GW3965, decrease neuroinflammation and promotes synaptic plasticity and axonal regeneration and improves neurological functional outcome following cerebral ischemia.4, 6, 7 ABCA1 has anti-inflammatory effects in both brain and the peripheral blood system, which implies its neuroprotective potential.5, 8 Compared with wild-type (WT) mice, mice lacking brain ABCA1 exhibit fewer synapses and synaptic vesicles changes in neurons and increased neuroinflammation in the brain.3, 5
The blood-brain barrier (BBB) is composed by brain capillary endothelial cells (BCECs) connected by tight junctions and pericytes that share common basement membrane extracellular matrix proteins that form the perivascular space. Vascular cells including smooth muscle cells, BCECs and pericytes, neural cells, i.e. neurons, astrocytes and oligodendrocytes, and microglia form the neurovascular-unit (NVU), which is the functional unit of the BBB.9, 10 Cells forming the NVU express ABCA1 which plays an important role in maintenance of BBB and NVU stability.2, 11 However, the effects of ABCA1 on stroke-induced BBB and white matter (WM) damage have not been investigated.
Insulin-like growth factor 1 (IGF1) is not only involved in brain growth, development and myelination, but also in neurogenesis and oligodendrogenesis.12 IGF1 has neuroprotective properties, and attenuates stroke induced BBB damage concomitantly with rapid immunosuppression and sustained antiinflammation in brain.13 IGF1 decreases cholesterol efflux via ABCA1 and scavenger receptor class B type I expression.14 MMP9 is a major contributing factor to BBB leakage after stroke15, 16. Whether MMP9 and IGF1 mediate brain ABCA1 deficiency induced BBB and WM-damages after stroke have not been investigated. In this study, we tested the effects and underlying mechanisms of brain ABCA1 deficiency in the regulation of BBB, WM and axonal damage and functional outcome after stroke.
Methods
Animal model and experimental group
Adult (2 to 3 months) male brain ABCA1 knockout (ABCA1-floxed x nestin-Cre positive, ABCA1−B/−B) and control (ABCA1-floxed, ABCA1fl/fl) mice were generated using the Cre/loxP recombination system.3 Mice were subjected to permanent distal middle cerebral artery occlusion (dMCAo),17 and were sacrificed 7 days after dMCAo for neurological functional evaluation and immunostaining (n=9 in ABCA1fl/fl mice, n=11 in ABCA1−B/−B mice), Western blot and real time-quantitative polymerase chain reaction (RT-qPCR) assays (n=4/group). Sham operated mice (n=6/group) underwent the same surgical procedure without ligation of the dMCAo.
Functional test
The adhesive removal test, a sensitive test of somatosensory function in mice for dMCAo model,17 was performed prior to dMCAo and at 1, 3 and 7 days after dMCAo by an investigator, who was blinded to the experimental groups, as previously described.17
Histological and immunohistochemical assessment and lesion volume measurement
Animal brains were fixed and embedded in paraffin. The cerebral tissues were cut into seven equally spaced (1 mm) coronal blocks. A series of adjacent 6 μm thick sections were cut from each block and stained with hematoxylin and eosin (HE) for the calculation of lesion volumes. Every 10th coronal section was cut from the center of the lesion (bregma -1 mm to +1 mm), and a total 5 of sections were used for immunostaining. Histochemical double staining for Bielschowsky silver (BS, an axon marker) and Luxol Fast Blue (LFB, a myelin marker),6 immunostaining for albumin (infiltration into the brain is a marker of BBB leakage,15 1:500, Abcam), Aquaporin-4 (AQP4, a marker of astrocytic endfeet, an important functional component of the BBB and NVU,9, 18 1:500, Abcam), adenomatous polyposis coli (APC, a marker of mature oligodendrocytes,19 1:100, GenWay) and platelet-derived growth factor receptor alpha (PDGFRα, a marker of oligodendrocyte progenitor cell-OPCs,20 1:100, Chemicon) were employed.
Immunostaining quantization
Images were acquired from five slides from each brain, with each slide containing 4 field views from the ischemic core, ischemic boundary zone (IBZ) of the cortex or the corpus callosum, respectively (Figure 1A). The area that stained positive for albumin in the ischemic core area of cortex and for AQP4 in the IBZ of cortex, the number of PDGFRα+− and APC+− cells in the IBZ of cortex or corpus callosum, and the percentage of BS+/LFB+−area in the IBZ of the corpus callosum were analyzed at 40× magnification with a Micro Computer Imaging Device (MCID) imaging analysis system (Imaging Research).
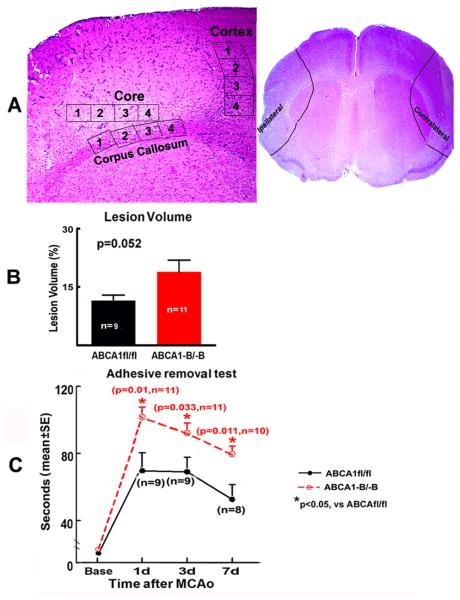
Figure 1.
ABCA1−B/−B marginally increases lesion volume and decreases functional outcome after dMCAo. A. Schematic diagram shows the IBZ in the cortex and corpus callosum and the ischemic core in the cortex for immunostaining, and isolated brain tissue area from the ipsilateral brain or sham brain for Western blot and RT-qPCR; B: Lesion volume; C: Functional outcome.
Primary brain astrocyte cultures
The pregnant ABCA1fl/fl mice were sacrificed and E15 embryos were harvested. Genotyping was performed to identify ABCA1−B/−B (n=7) or ABCA1fl/fl (n=6) embryos. Primary astrocytes were isolated and cultured, as previously described.21 Passage 2 astrocytes were used and cultured with DMEM medium with L-glutamine without D-glucose and sodium-pyruvate (Gibco) followed by 3 hours of oxygen-glucose deprivation (OGD).7 The astrocyte-conditioned-medium was collected and stored in −80°C for primary cortical neuron (PCN) culture use. The astrocytes were used for Western blotting and RT-qPCR assay.
PCN culture and neurite-ougrowth measurements
First, we tested whether ABCA1 knockdown in astrocyte regulates PCN neurite-ougrowth; PCN culture with astrocyte-conditioned-medium derived from ABCA1−B/−B and ABCA1fl/fl mice were employed. PCNs were isolated from E15 embryos from a C57BL/6 WT mouse (Jackson Laboratory, n=8). On day in vitro 3 (DIV 3), the PCNs were subjected to 3 hour OGD for hypoxic ischemia, then treated with conditioned-medium derived from ABCA1−B/−B or ABCA1fl/fl astrocytes for 24 hours (n=6 well/group).
Second, we tested whether reduced ABCA1 in neurons regulates neurite-ougrowth, and whether IGF1 mediates the neurite-ougrowth, PCN cultures were isolated from E15 embryos of ABCA1−B/−B (n=7) and ABCA1fl/fl (n=6). After 3 hour OGD, the experimental groups include: 1) ABCA1fl/fl-PCNs; 2) ABCA1−B/−B-PCNs; 3) ABCA1−B/−B-PCNs treated with IGF1 (recombinant mouse IGF1 protein, 100 ng/ml, Abcam, Cat# ab9861, n=6 well/group) for 24 hours. PCN cultures were immunostained for TUJ1- (a phenotypic marker of neural cells, 1:1000, Covance) with Cy3. TUJ1-positive cells and neurites were photographed at 10× magnification using a fluorescent microscope. The average neurite length of the 20 longest neurites in each well (6 wells /group) was measured using the MCID analysis system.
RT-qPCR
The ipsilateral brain and the homologous areas from the sham brain (Figure 1A) and the harvested astrocyte-cultures were used for RT-qPCR. The following primers were designed using Primer Express software (ABI). GAPDH: FWD: AGAACATCATCCCTGCATCC; REV: CACATTGGGGGTAGGAACAC; IGF1: FWD: TGGATGCTCTTCAGTTCGTG, REV: TGGTAGATGGGGGCTGATAC; MMP9: FWD: ATCTCTTCTAGAGA-CTGGGAAGGAG; REV: AGCTGATTGACTAAAGTAGCTGGA; AQP4: FWD: CGGTTCATGGAAACCTCACT; REV: CATGCTGGCTCCGGTATAAT.
Western blotting
Specific proteins were visualized using Luminal Reagent (Santa Cruz). Anti-AQP4 (1:1000, Abcam), anti-IGF1 (1:1000, Abcam), anti-MMP9 (1:500, Santa Cruz), and anti-β-actin (1:10,000, Abcam) were used, as previously described.20
Statistical analysis
Differences in the functional outcome and lesion volume were analyzed using Student's t-test. The percentage of albumin+−, AQP4+−, BS+/LFB+− area and APC+−, PDGFRα+− cell numbers, protein and mRNA expression were analyzed using two-factor ANOVA followed by post-hoc Bonferroni test. One-way ANOVA and Least Significant Difference (LSD) test were performed for neurite-ougrowth. Correlation between the percentage of AQP4+− and albumin+− area was tested by Pearson's correlation coefficients.
Results
Brain ABCA1 deficiency worsens functional outcome after stroke
There was a marginal increase in the lesion volume (P=0.052, Figure 1B) and a significant increase in neurological deficits at 1, 3 and 7 days after dMCAo in ABCA1−B/−B mice, compared to ABCA1fl/fl mice (P<0.05, Figure 1C).
Brain ABCA1 deficiency increases BBB dysfunction after stroke
To test whether brain ABCA1 deficiency regulates BBB leakage after stroke, albumin and AQP4 expression in the ischemic brain were measured. There was no albumin infiltration, evident in the non-stroke brains in either the ABCA1−B/−B or in ABCA1fl/fl mice receiving sham surgery. However, albumin infiltration was observed near the ischemic core in both ABCA1−B/−B and ABCA1fl/fl mice. Albumin density was significantly increased in ABCA1−B/−B mice (P<0.05, n=11) compared with ABCA1fl/fl mice (n=9) 7 days after stroke (Figure 2A).
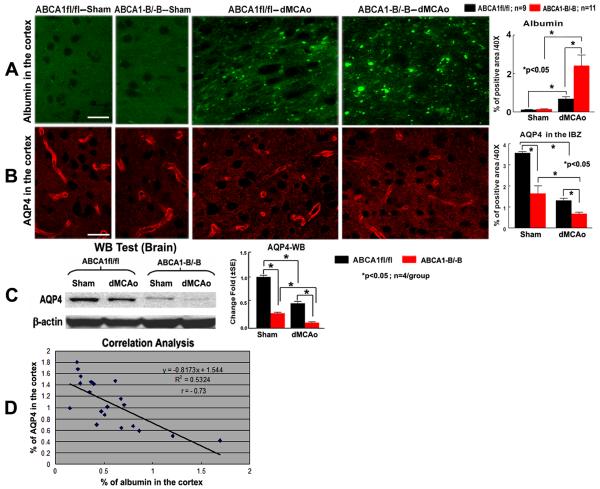
Figure 2.
ABCA1−B/−B increases BBB leakage in the ischemic brain and decreases AQP4 expression in both the sham and the ischemic brain after dMCAo. A, B: Albumin and AQP4 immunostaining and quantitative data; C: AQP4 Western blot and quantitative data; D: Correlation of AQP4 and albumin immunostaining. Scare bar in A and B = 40 μm.
AQP4 protein expression was significantly decreased in both the sham brains and the IBZ of the cortex in the ABCA1−B/−B mice, compared with ABCA1fl/fl mice after stroke measured by immunostaining (Figure 2B) and Western blot (Figure 2C, P<0.05) 7 days after dMCAo. In addition, the density of AQP4 was inversely correlated with the amount of albumin accumulation in the IBZ (Figure 2D, r = − 0.73, P<0.05).
Brain ABCA1 deficiency increases axonal and WM-damage in the ischemic brain after stroke
WM is composed of bundles of myelinated axons, and oligodendrocytes are the only myelin-forming cells in the CNS and maintain long-term axonal integrity.19, 22 To test whether brain ABCA1 deficiency regulates axon and WM-damage after stroke, the density of BS+/LFB+ and the number of APC+−cells in the IBZ of corpus callosum were measured. There was no significant difference in BS+/LFB+−density and APC+−cell numbers in the sham brains between ABCA1−B/−B and ABCA1fl/fl mice. However, the BS+/LBF+−density and APC+−cell numbers were significantly decreased in the IBZ of the corpus callosum in ABCA1−B/−B mice (n=11) compared with the ABCA1fl/fl mice (n=9) after stroke (Figure 3A and B, P<0.05).
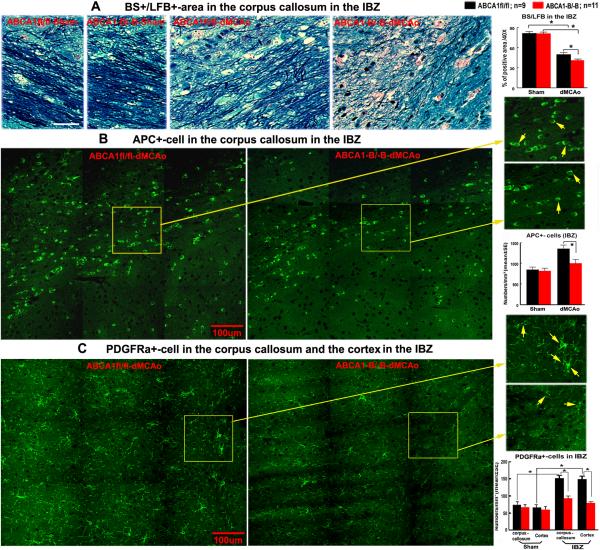
Figure 3.
ABCA1−B/−B increases WM-damage and decreases oligodendrocytes and OPCs in the ischemic brain after dMCAo. A: BS-axon (black) and LFB-myelin (blue) double-staining and quantitative data. B, C: APC and PDGFRα immunostaining and quantitative data. Scare bar in A = 40 μm, in B, C = 100 μm.
In adult animals, OPCs are present in the brain parenchyma after stroke and are generated in the subventricular zone of the lateral ventricle, where they proliferate, migrate and differentiate into new oligodendrocytes.19, 22 To test whether brain ABCA1 deletion decreases OPC numbers, the number of PDGFRα+−OPCs was also measured. We found that the number of PDGFRα+−OPCs in the IBZ of the corpus callosum and cortex significantly increased in ABCA1fl/fl mice after stroke compared with sham brain. In addition, compared with the ABCA1fl/fl mice, ABCA1−B/−B stroke mice exhibited significant decreases in PDGFRα+−OPC numbers in the IBZ of the corpus callosum and cortex, which indicate that ABCA1 may regulate OPC generation after stroke (Figure 3C, P<0.05).
Brain ABCA1 deficiency increases MMP9 and decreases IGF1 in the ischemic brain
MMP9 is a marker of neuroinflammation.16 IGF1 has anti-inflammatory properties. 12, 13 To elucidate the mechanisms responsible for the increased BBB leakage and WM-damage and worse functional outcome in ABCA1−B/−B mice, MMP9 and IGF1 expression were measured by Western blot (Figure 4A) and RT-qPCR (Figure 4B), respectively. The data show that MMP9 expression in the ischemic brains was significantly increased, whereas IGF1 expression was significantly decreased in both sham and ischemic brain in ABCA1−B/−B mice compared with ABCA1fl/fl mice (P<0.05, n=4/group).
Figure 4.
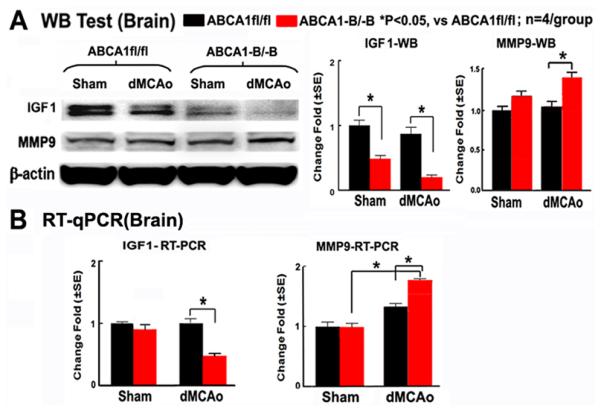
ABCA1−B/−B increases MMP9 in the ischemic brain and decreases IGF1 expression both in the sham and ischemic brain. A: Western blot and quantification data. B: RT-qPCR data.
ABCA1−B/−B decreases AQP4 and IGF1 and increases MMP9 expression in primary cultured astrocytes
To investigate whether ABCA1 knockdown altered AQP4, IGF1 and MMP9 expression in astrocytes, primary brain astrocyte cultures were employed. Both the protein and mRNA level of MMP9 was increased, while AQP4 and IGF1 were decreased (Figure 5A and B) in the primary cultured ABCA1−B/−B astrocytes (n=7) compared with ABCA1fl/fl astrocytes (P<0.05, n=6) after OGD.
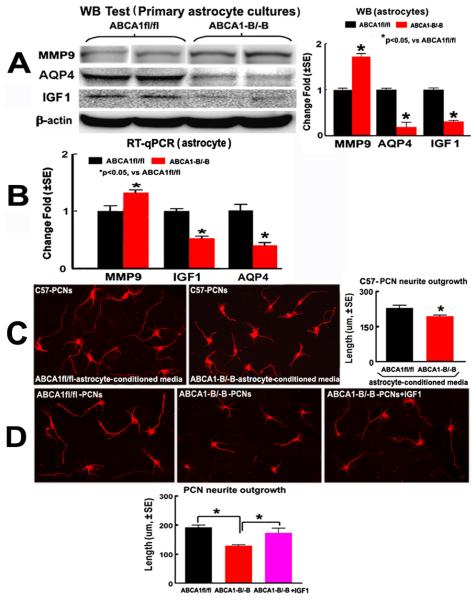
Figure 5.
ABCA1−B/−B decreases AQP4 and IGF1 but increases MMP9 expression in the primary cultured astrocytes, decreases WT-PCN neurite-ougrowth cultured with the ABCA1−B/−B astrocyte-conditioned-medium compared with the ABCA1fl/fl astrocyte-conditioned-medium, which attenuated by IGF1-treatment after OGD. A, B: Western blot (A) and RT-qPCR data (B) in astroctyes. C, D: Neurite-outgrowth data in WT-PCNs (C), ABCA1−B/−B and ABCA1fl/fl PCNs (D).
Brain ABCA1 deficiency decreases neurite-ougrowth, while IGF attenuates this reduction
To investigate whether ABCA1 knockdown in both neurons and astrocytes regulates neurite-ougrowth, the PCN and astrocyte cultures derived from E15 ABCA1−B/−B and ABCA1fl/fl embryos were employed. Neurite-ougrowth was significantly decreased in WT-PCNs cultured with ABCA1−B/−B astrocyte conditioned-medium, as compared with the ABCA1fl/fl astrocyte-conditioned-medium treatment group (Figure 5C, P<0.05, n=6/group).
To further elucidate the pathway responsible for the reduced neurite-outgrowth in ABCA1 deficiency, IGF1 treatment was employed. Neurite-ougrowth was significantly decreased in ABCA1−B/−B PCNs compared to ABCA1fl/fl PCNs after OGD, while IGF1 (100 ng/ml) treatment significantly attenuated this reduction (Figure 5D, P<0.05, n=6/group).
Discussion
Stroke leads to BBB disruption, WM and axonal damage which are associated with long-term disability after stroke. Astrocytic endfeet are tightly attached to neurons on one side and to BCECs and pericytes of the BBB on the other side, thereby connecting neurons and vascular cells, and play a decisive role in BBB maintenance.9 In rodent brain, AQP4 is primarily expressed in astrocytic endfeet in contact with BBB and mediates water homeostasis.23 AQP4 may have biphasic effects in stroke injury and neuroprotection, for example, AQP4 is related to the early stages of cerebral edema and neuroinflammation within 2 to 4 days after ischemia.23,24 However, at a later phase post brain injury (7 days to 1 month), AQP4 may play a beneficial role, and may inhibit activation of microglia and promot resolution of edema.25 The upregulation of AQP4 causes increased water clearance from the tissue, and decreased BBB disruption and neutrophil infiltration, as well as decreased pro-inflammatory cytokines.25 Using nestin-Cre system, ABCA1 was primarily knocked out from neural cells, but not from BCECs in ABCA1−B/−B mice.3 In this study, we found that brain ABCA1 deficiency significantly increased BBB leakage in the ischemic brain, but decreased AQP4 expression in both the sham and the ischemic brain after stroke, and BBB leakage was inversely correlated with the AQP4 expression in the ischemic brain after stroke. In addition, a decrease of AQP4 expression was found in primary astrocytes cultures after OGD. These data indicated that brain ABCA1 deficiency decrease astrocytic endfeet protein expression, which may contribute to BBB dysfunction in the sham brain, and increased BBB leakage after stroke. Pericytes also play a pivotal role in the formation and maintenance of the adult BBB.10 Pericytes project elongated, stellate-shaped finger-like processes that unsheathe >70% of the abluminal side of the capillary wall10, 26 and astrocytes directly communicate with pericytes in addition to endothelial cells.27 Pericyte deficiency in the CNS leads to BBB breakdown and brain hypoperfusion resulting in secondary neurodegenerative changes.10 Because pericytes also express nestin,28 therefore, we can not exclude the possibility that ABCA1 is also deleted from pericytes, in addition to neural cells. Further studies on this issue are warranted.
BBB leakage postischemic stroke might in-part explained by the increase vascular associated MMP9 activity because tight junction proteins and basement membrane extracellular matrix proteins are substrates for MMP9.16,9 MMP9 correlates to early stage vascular dysfunction and BBB disruption as well as WM-damages;29 inhibition of MMP9 effectively reduces cerebral-vascular permeability and reduces brain infarction in mice after MCAo.30 Knockout of MMP9 significantly decreases BBB disruption and myelin basic protein reduction and prevents macrophage induced axonal retraction after ischemia;31 MMP9 activity was significantly increased in the ischemic brain of type 2 diabetic mice which correlated with the increased WM-damage after stroke.20 Neuroinflammation exacerbates BBB leakage in the ischemic brain, resulting in increased lesion volume and neurological deficits.29 ABCA1 is a potent anti-inflammatory factor. The systemic anti-inflammatory role of ABCA1 in the periphery has been studied using global ABCA1 knockout mice and macrophage-specific ABCA1 knockout mice.8 ABCA1−B/−B mice also exhibit neuroinflammatory response including increased nuclear factor kappa B (NFκB) and tumor necrosis factor alpha (TNFα) gene expression in the brain.5 BBB dysfunction also promotes WM and axonal damage.15 Ischemia induced WM injury is generated by selectively up-regulating neuroinflammation and BBB damage, which may be associated with the increased infarction volume and neurological deficits.29 In this study, we found that brain ABCA1 deficiency increases MMP9 expression in both the ischemic brain and cultured astrocytes under OGD condition, which may contribute to the induction of BBB leakage and WM-damage in the ischemic brain. However, MMPs may be beneficial during delayed phases after stroke because MMPs modulate the brain extracellular matrix and may mediate and facilitate beneficial plasticity and remodeling during stroke recovery.32 MMP9 was upregulated in peri-infarct cortex at 7-14 days after stroke and is colocalized with markers of neurovascular remodeling. Treatment with MMP inhibitors at 7 days after stroke suppresses neurovascular remodeling, increases ischemic brain injury and impairs functional recovery at 14 days.32 Therefore, the longterm effects of MMP9 post of stroke using this model warrants investigation.
IGF1 is not thought to easily cross the BBB. Local production from astrocytes and microglia are the main source of IGF1 in the brain.33 Astrocyte-specific overexpression of IGF1 protects hippocampal neurons and reduces behavioral deficits following traumatic brain injury.34 Exogenous administration of IGF1 into the cerebral lateral ventricle significantly reduces the BBB breakdown, brain edema formation, and cellular/tissue injuries.13 Administration of IGF1 within a few hours after brain injury is protective in both gray and white matter and leads to improved somatic function.35 For example, intra-cerebraventricularly infusion of IGF1 prevented loss of oligodendrocytes and myelin basic protein density 4 days after ischemia reperfusion.35 In the present study, brain ABCA1 deficiency decreased IGF1 expression in the sham and the ischemic brain as well as the hypoxic ischemic astrocyte cultures, which coexist with BBB dysfunction and axonal impairment in the sham brain and increased BBB leakage and WM-damage in the ischemic brain. These data indicate that brain ABCA1 deficiency induced decrease in IGF1 may also contribute to the increased BBB leakage and WM-damage after stroke. However, the mechanism underlying how ABCA1 regulates IGF1 expression is not clear and warrants investigation.
Conclusions
In this study, to our knowledge, we are the first to demonstrate that brain ABCA1 deficiency increases BBB leakage, WM/axonal damage and functional deficits after stroke. Concomitant reduction of IGF1 and elevation of MMP9 may contribute to brain ABCA1 deficiency induced BBB and WM/axonal damage (Figure 6).
Figure 6.
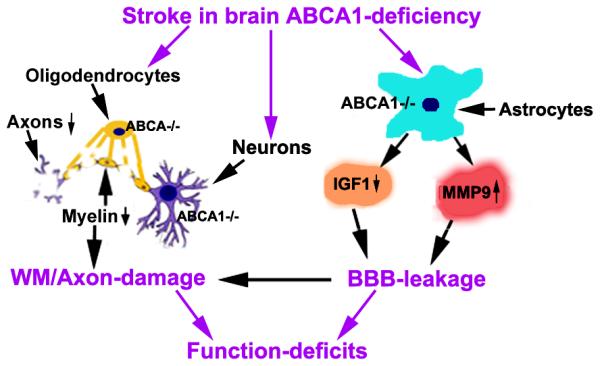
Diagram shows the mechanism of brain ABCA1-deficiency induced BBB and WM/axon damage after stroke. ABCA1-deficiency in neurons and oligodendrocytes increases WM/axon-damage; ABCA1-deficiency in astrocytes increases MMP9 and decreases IGF1 expression and enhances BBB-leakage after stroke; In turn, BBB-leakage stimulates MMP9 expression in astrocytes and further exacerbates WM/axon-damage; BBB-leakage and WM/axon-damage contribute to the functional deficits.
Acknowledgements
The authors wish to thank Cynthia Roberts, Qinge Lu and Sutapa Santra for technical assistance.
Sources of funding
This work was supported by American Heart Association grant 12SDG9300009 (X.C.) and 14GRNT20460026 (J.C.); National Institute on Aging RO1 AG037506 (M.C.), National Institute of Neurological Disorders and Stroke RO1 NS083078 (J.C.) and 1R41NS080329-01A1 (J C).
Footnotes
Disclosure
None.
References
- 1.Hirsch-Reinshagen V, Zhou S, Burgess BL, Bernier L, McIsaac SA, Chan JY, et al. Deficiency of abca1 impairs apolipoprotein e metabolism in brain. J Biol Chem. 2004;279:41197–41207. doi: 10.1074/jbc.M407962200. [DOI] [PubMed] [Google Scholar]
- 2.Wahrle SE, Jiang H, Parsadanian M, Legleiter J, Han X, Fryer JD, et al. Abca1 is required for normal central nervous system apoe levels and for lipidation of astrocyte-secreted apoe. J Biol Chem. 2004;279:40987–40993. doi: 10.1074/jbc.M407963200. [DOI] [PubMed] [Google Scholar]
- 3.Karasinska JM, Rinninger F, Lutjohann D, Ruddle P, Franciosi S, Kruit JK, et al. Specific loss of brain abca1 increases brain cholesterol uptake and influences neuronal structure and function. J Neurosci. 2009;29:3579–3589. doi: 10.1523/JNEUROSCI.4741-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng O, Ostrowski RP, Liu W, Zhang JH. Activation of liver x receptor reduces global ischemic brain injury by reduction of nuclear factor-kappab. Neuroscience. 2010;166:1101–1109. doi: 10.1016/j.neuroscience.2010.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karasinska JM, de Haan W, Franciosi S, Ruddle P, Fan J, Kruit JK, et al. Abca1 influences neuroinflammation and neuronal death. Neurobiology of disease. 2013;54:445–455. doi: 10.1016/j.nbd.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Zacharek A, Cui X, Shehadah A, Jiang H, Roberts C, et al. Treatment of stroke with a synthetic liver x receptor agonist, to901317, promotes synaptic plasticity and axonal regeneration in mice. J Cereb Blood Flow Metab. 2010;30:102–109. doi: 10.1038/jcbfm.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cui X, Chopp M, Zacharek A, Cui Y, Roberts C, Chen J. The neurorestorative benefit of gw3965 treatment of stroke in mice. Stroke. 2013;44:153–161. doi: 10.1161/STROKEAHA.112.677682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang C, Liu Y, Kessler PS, Vaughan AM, Oram JF. The macrophage cholesterol exporter abca1 functions as an anti-inflammatory receptor. J Biol Chem. 2009;284:32336–32343. doi: 10.1074/jbc.M109.047472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zlokovic BV. Neurovascular pathways to neurodegeneration in alzheimer's disease and other disorders. Nature reviews. Neuroscience. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nature neuroscience. 2011;14:1398–1405. doi: 10.1038/nn.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim WS, Guillemin GJ, Glaros EN, Lim CK, Garner B. Quantitation of atp-binding cassette subfamily-a transporter gene expression in primary human brain cells. Neuroreport. 2006;17:891–896. doi: 10.1097/01.wnr.0000221833.41340.cd. [DOI] [PubMed] [Google Scholar]
- 12.Lin S, Fan LW, Pang Y, Rhodes PG, Mitchell HJ, Cai Z. Igf-1 protects oligodendrocyte progenitor cells and improves neurological functions following cerebral hypoxia-ischemia in the neonatal rat. Brain research. 2005;1063:15–26. doi: 10.1016/j.brainres.2005.09.042. [DOI] [PubMed] [Google Scholar]
- 13.Bake S, Selvamani A, Cherry J, Sohrabji F. Blood brain barrier and neuroinflammation are critical targets of igf-1-mediated neuroprotection in stroke for middle-aged female rats. PLoS ONE. 2014;9:e91427. doi: 10.1371/journal.pone.0091427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang SL, Chen WJ, Yin K, Zhao GJ, Mo ZC, Lv YC, et al. Papp-a negatively regulates abca1, abcg1 and sr-b1 expression by inhibiting lxralpha through the igf-i-mediated signaling pathway. Atherosclerosis. 2012;222:344–354. doi: 10.1016/j.atherosclerosis.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Wallin A, Sjogren M, Edman A, Blennow K, Regland B. Symptoms, vascular risk factors and blood-brain barrier function in relation to ct white-matter changes in dementia. European neurology. 2000;44:229–235. doi: 10.1159/000008242. [DOI] [PubMed] [Google Scholar]
- 16.Wu Y, Wang YP, Guo P, Ye XH, Wang J, Yuan SY, et al. A lipoxin a4 analog ameliorates blood-brain barrier dysfunction and reduces mmp-9 expression in a rat model of focal cerebral ischemia-reperfusion injury. Journal of molecular neuroscience : MN. 2012;46:483–491. doi: 10.1007/s12031-011-9620-5. [DOI] [PubMed] [Google Scholar]
- 17.Freret T, Bouet V, Leconte C, Roussel S, Chazalviel L, Divoux D, et al. Behavioral deficits after distal focal cerebral ischemia in mice: Usefulness of adhesive removal test. Behavioral neuroscience. 2009;123:224–230. doi: 10.1037/a0014157. [DOI] [PubMed] [Google Scholar]
- 18.Nicchia GP, Nico B, Camassa LM, Mola MG, Loh N, Dermietzel R, et al. The role of aquaporin-4 in the blood-brain barrier development and integrity: Studies in animal and cell culture models. Neuroscience. 2004;129:935–945. doi: 10.1016/j.neuroscience.2004.07.055. [DOI] [PubMed] [Google Scholar]
- 19.Zhang RL, Chopp M, Roberts C, Wei M, Wang X, Liu X, et al. Sildenafil enhances neurogenesis and oligodendrogenesis in ischemic brain of middle-aged mouse. PLoS ONE. 2012;7:e48141. doi: 10.1371/journal.pone.0048141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen J, Cui X, Zacharek A, Cui Y, Roberts C, Chopp M. White matter damage and the effect of matrix metalloproteinases in type 2 diabetic mice after stroke. Stroke. 2011;42:445–452. doi: 10.1161/STROKEAHA.110.596486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crocker SJ, Frausto RF, Whitton JL, Milner R. A novel method to establish microglia-free astrocyte cultures: Comparison of matrix metalloproteinase expression profiles in pure cultures of astrocytes and microglia. Glia. 2008;56:1187–1198. doi: 10.1002/glia.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang R, Chopp M, Zhang ZG. Oligodendrogenesis after cerebral ischemia. Frontiers in cellular neuroscience. 2013;7:201. doi: 10.3389/fncel.2013.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Badaut J, Lasbennes F, Magistretti PJ, Regli L. Aquaporins in brain: Distribution, physiology, and pathophysiology. J Cereb Blood Flow Metab. 2002;22:367–378. doi: 10.1097/00004647-200204000-00001. [DOI] [PubMed] [Google Scholar]
- 24.He L, Zhang X, Wei X, Li Y. Progesterone attenuates aquaporin-4 expression in an astrocyte model of ischemia/reperfusion. Neurochemical research. 2014;39:2251–2261. doi: 10.1007/s11064-014-1427-7. [DOI] [PubMed] [Google Scholar]
- 25.Fukuda AM, Badaut J. Aquaporin 4: A player in cerebral edema and neuroinflammation. Journal of neuroinflammation. 2012;9:279. doi: 10.1186/1742-2094-9-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68:409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bell RD, Winkler EA, Singh I, Sagare AP, Deane R, Wu Z, et al. Apolipoprotein e controls cerebrovascular integrity via cyclophilin a. Nature. 2012;485:512–516. doi: 10.1038/nature11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dore-Duffy P, Katychev A, Wang X, Van Buren E. Cns microvascular pericytes exhibit multipotential stem cell activity. J Cereb Blood Flow Metab. 2006;26:613–624. doi: 10.1038/sj.jcbfm.9600272. [DOI] [PubMed] [Google Scholar]
- 29.Jalal FY, Yang Y, Thompson J, Lopez AC, Rosenberg GA. Myelin loss associated with neuroinflammation in hypertensive rats. Stroke. 2012;43:1115–1122. doi: 10.1161/STROKEAHA.111.643080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yin KJ, Deng Z, Hamblin M, Zhang J, Chen YE. Vascular ppardelta protects against stroke-induced brain injury. Arterioscler Thromb Vasc Biol. 2011;31:574–581. doi: 10.1161/ATVBAHA.110.221267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Asahi M, Wang X, Mori T, Sumii T, Jung JC, Moskowitz MA, et al. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J Neurosci. 2001;21:7724–7732. doi: 10.1523/JNEUROSCI.21-19-07724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao BQ, Wang S, Kim HY, Storrie H, Rosen BR, Mooney DJ, et al. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nature medicine. 2006;12:441–445. doi: 10.1038/nm1387. [DOI] [PubMed] [Google Scholar]
- 33.Russo VC, Gluckman PD, Feldman EL, Werther GA. The insulin-like growth factor system and its pleiotropic functions in brain. Endocrine reviews. 2005;26:916–943. doi: 10.1210/er.2004-0024. [DOI] [PubMed] [Google Scholar]
- 34.Madathil SK, Carlson SW, Brelsfoard JM, Ye P, D'Ercole AJ, Saatman KE. Astrocyte-specific overexpression of insulin-like growth factor-1 protects hippocampal neurons and reduces behavioral deficits following traumatic brain injury in mice. PLoS ONE. 2013;8:e67204. doi: 10.1371/journal.pone.0067204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guan J, Bennet L, Gluckman PD, Gunn AJ. Insulin-like growth factor-1 and post-ischemic brain injury. Progress in neurobiology. 2003;70:443–462. doi: 10.1016/j.pneurobio.2003.08.002. [DOI] [PubMed] [Google Scholar]