Abstract
In vitro and ex-vivo studies assessing the impact of stress hormones on immune competence commonly replace the natural milieu of leukocytes with an artificial medium, excluding plasma factors, hormones, and cytokines. Given prevalent inconsistencies between in vitro, ex-vivo, and in vivo findings, we studied whether such procedures could yield misleading outcomes regarding the impact of stress hormones on NK cell cytotoxicity (NKCC), using fresh human whole blood samples. We found that in the presence of plasma 10-30-fold higher concentrations of cortisol, epinephrine, and prostaglandin-E2 (PGE2) were required to reach suppression levels evident in the context of artificial medium. Importantly, whereas the NK suppressive effects of PGE2 occurred immediately and remained stable upon prolonged exposure, the suppressive effects of cortisol slowly increased over time. Last, to simulate the exclusion of stress factors in the ex-vivo approach, we subjected whole blood to stress hormones (as occurs in vivo), and abruptly removed them. We found that the effects of epinephrine and PGE2 quickly disappeared, while the effects of cortisol persisted. Overall, these findings demonstrate the potential misleading nature of in vitro and ex-vivo procedures, and specifically suggest that (i) the common in vitro findings of profound suppression of NKCC by stress hormones are overestimation of their direct effects expected in vivo; and (ii) the common ex-vivo approach cannot reflect the direct in vivo suppressive effects of epinephrine and PGE2 on NKCC, while inflating the effects of glucocorticoids. Some of these fallacies may be circumvented by using non-delayed whole blood NKCC assays in humans.
Keywords: NK cytotoxicity, cortisol, prostaglandin, epinephrine, in vitro, ex-vivo, in vivo, stress, human, whole blood
1. Introduction
Stress and stress hormones are known to alter the function of Natural Killer (NK) cells. However, significant inconsistencies are prevalent between in vitro, ex-vivo, and in vivo findings regarding the nature and direction of the effects of specific stress hormones or stress paradigms on NK cell cytotoxicity (NKCC) [1]. For example, epinephrine was reported to suppress NKCC in vitro, both in human and animal blood [2–5], through activating NK cell adrenergic receptors and the consequent increase in intracellular cAMP levels [3]. However, ex-vivo human and animal studies reported contradictory results; many have demonstrated that administration of epinephrine, acute stress exposure, or exercise enhances NKCC [6–11], whereas some have reported suppression of NKCC [12–14]. Animal studies employing in vivo procedures generally inferred a suppressive effect of epinephrine on NK activity [12, 15–17].
Glucocorticoids in physiological concentrations were repeatedly shown to markedly suppress human and animal NKCC in vitro [18–20]. However, several ex-vivo studies in humans and animals have suggested that no such suppression occurs in vivo [21, 22], and a recent in vivo animal study has supported this suggestion, indicating specific conditions under which corticosterone may exerts some effects [16].
Unlike catecholamines and glucocorticoids, the release of prostaglandins (PGs) is not controlled centrally. Rather, PGs are released locally by a variety of cells [23, 24], including malignant cells [25], and as a response to tissue damage [26]. Under some conditions (e.g., surgery) local release can markedly increase systemic PGs levels [27, 28]. In vitro studies showed that prostaglandin-E2 (PGE2) can markedly suppresses NK activity [19, 29, 30], and in vivo studies reported deleterious impacts of PGs on resistance to cancer metastases [22], which is allegedly mediated through in vivo suppression of NK cells [31]. However, in a recent study in rats, we have provided evidence indicating that a direct in vivo suppressive effect of PGE2 on NKCC cannot be evident in an ex-vivo assessment of NKCC [5]
We hypothesize that most inconsistencies regarding the impact of stress hormones on NKCC originate from methodological obstacles and specific procedures that yield misleading outcomes. These procedures include: (1) exclusion or distortion of the natural milieu when conducting ex-vivo or in vitro testing, such as replacement of plasma with a hormone- and cytokine-free artificial medium, or testing cytotoxicity in purified NK cells; (2) overlooking the kinetics of the effects of a hormone in its presence and following its exclusion; (3) disregarding the in vivo effects of a stress hormone on NK cell trafficking, which may manifest itself as a change in function; and (4) disregarding the existence of different NK cell subpopulations with different cytotoxicity capacity, in conjunction with stress-induced redistribution of NK cells that is subpopulation-specific (specifically in ex-vivo studies). Some alleged inconsistencies result from differences in stress paradigms or hormone levels/concentrations, which we do not consider as inconsistent findings, but rather as reflecting the complexity of the effects of stress.
Although the impact of stress on immune competence should ideally be studied in vivo, in humans this approach is commonly impractical. Thus, it is important to elucidate the degree to which the standard in vitro and ex-vivo approaches, used in human studies of NKCC, would reflect in vivo outcomes. It would be instrumental to point at specific distortions caused by these approaches, if exist. To start addressing these issues in humans, we herein simulated several critical procedural aspects of the standard in vitro and ex-vivo approaches, employing fresh whole human blood. Admittedly, such an in vitro study may seem limited and paradoxical in examining potential limitations of in vitro and ex-vivo approaches. Thus, we restricted the scope of the study to the assessment of aspects that can be simulated or examined by this methodology, aiming at identifying inherent impediments in standard in vitro and ex-vivo approaches. Specifically, we address the potential effects of (i) studying human NKCC in the absence of plasma factors, and (ii) disregarding the kinetics of the impact of stress hormones and of their removal from the assay medium. We address these issues employing three different putative immunemodulating stress hormones acting through different cellular or intracellular mechanisms, namely epinephrine, PGE2, and cortisol.
2. Materials and Methods
2.1. Subjects and their baseline stress levels
In all experiments, all manipulations were conducted within subject. In each experiment, 6–12 healthy volunteers (Mean age 33.07, SD 7.60) provided morning blood samples. Healthy subjects were recruited by an advertisement posted at the Tel Aviv University campus, offering financial compensation for their time. Exclusion criteria were acute sickness during the last week, alcoholism, and drug abuse. Females constituted 25% of all subjects. The study was approved by the Institutional Review Board (Helsinki committee) of Sheba Medical Center, and all participants gave written informed consent before donating their blood.
All subjects have donated blood several times in different circumstances, and were made familiar with the experimental setting of blood withdrawal. Most contributed blood for several of the 8 experiments reported herein. Before blood withdrawal, subjects were allowed to rest, and filled a questionnaire regarding their health status, medication usage, caffeine intake, exercise habits, and existing stress levels. Specifically, subjects rated their current stress levels between 1 and 10 – 1 being the lowest possible stress level ("not at all") and 10 the highest possible stress level ("extremely stressed").
2.2. Blood withdrawal and use
Venous blood (between 40–100 ml, depending on specific experiment) was collected from each subject between 8:00 and 10:00 A.M. into heparinized syringes (30 U of preservative-free heparin per ml blood). Blood samples were immediately subjected to the procedures described below.
2.3. Serum cortisol levels
Serum cortisol levels were assessed employing an ELISA kit (MP Biomedicals, Orangeburg, NY) according to manufacture instruction.
2.4. Drugs
The concentrations of the hormones used in the study cover their physiological systemic levels. For epinephrine and PGE2 higher concentrations were also used, as these hormones, but not cortisol, also interact locally with NK cells at higher concentrations – catecholamines at immunological sympathetic synapses [32, 33] and PGE2 at areas of tissue damage or malignant tissue that release PGs [26, 34].
Cortisol (hydrocortisone-Water Soluble), and epinephrine (both from Sigma–Aldrich, Israel) were dissolved in complete medium. Prostaglandin-E2 (ENCO, Israel) was dissolved in ethanol and diluted in PBS, then further diluted in complete medium, to reach a final concentration of 0.003% or less ethanol in the assay medium. In previous studies we found that a 30-fold higher concentration of ethanol is needed to start impacting NK cytotoxicity in vitro [35].
2.5. Complete Medium (CM)
CM is RPMI-1640 supplemented with 10% heat-inactivated fetal calf serum (FCS), 50 µg/mL of gentamicin, 2 mM of l-glutamine, 0.1 mM of nonessential amino-acids, and 1 mM of sodium pyruvate. All compounds were purchased from Biological Industries, Kibbutz Beit Haemek, Israel.
2.6. Maintenance and radiolabeling of K562 target cells
The standard K562 tumor cell line (NK-sensitive erythromyeloid) [36] was used as target cells in the NK cytotoxicity assay. Cells were grown in CM at 37 °C in 5% CO2. For radiolabeling, 25 × 106 cells were incubated for 1 hr with 200 µCi of 51Cr (Rotem Tassiot, Dimona, Israel) in 200 µl FCS, and 100 µl CM. After incubation, cells were washed three times with CM (335g, for 10 min).
2.7. NK cytotoxicity assay
A whole blood 4h 51Cr release assay was used. This procedure assesses anti-tumor NKCC without excluding any cell subtype, and potentially without excluding plasma factors. Earlier studies have indicated that cytotoxicity measured using this procedure is attributable to NK cells, rather than other cell types or soluble factors, as the selective depletion of NK cells abolishes all target-cell killing [37–39]. The advantages of this procedure include shorter preparation time, less interference with the effector cells, and better representation of the original in vivo milieu and cell composition.
Aliquots of 220µl blood from each subject were placed in a 96-well microtiter plate. In all studies, each plate contained blood from one subject, subjected to drug treatments (in up to seven columns) and triplicates of control (vehicle) conditions. The outcomes from the three controls conditions were averaged, and drug effects were expressed as percent of averaged control levels within plate.
To minimize dilution of plasma, 40µl of tumor cells and 20µl of drug were added to the 220µl of blood. To determine spontaneous and maximal 51Cr release, blood was substituted with CM or Triton 100 (Sigma, Jerusalem, Israel), respectively. To form six E:T (Effector:Target) ratios, 2 × 105 K562 cells were used for the lowest E:T ratio, and half the number of tumor cells was used for each of the consecutive 5 E:T ratios. The plates were centrifuged at 596g for 10 min and were then incubated for 4 h in 5% CO2 at 37 °C. Following incubation, plates were centrifuged again at 4 °C, and 80µl of the supernatant were recovered from each well for assessment of radioactivity in a γ-counter.
Each experiment was conducted in several replications, employing different subjects. In each replication, blood samples were subjected to all treatments. However, in some experiments/replications the highest or lowest non-physiological doses were tested only in some subjects, and thus in these doses the sample sizes (“n”) are smaller than in theft physiological doses.
2.8. Data analysis
2.8.1. NK cytotoxicity
To evaluate percent-specific lysis, the following formula was used: (experimental release × correction - spontaneous release)/(maximal release - spontaneous release) × 100, where correction is the percentage of the supernatant within the volume of the well (i.e., excluding the hematocrit volume). This correction is necessary because chromium released by labeled target cells is found only in the supernatant above the red blood cells [38].
2.8.2. Lytic units 45 (LU45)
The LU approach transforms the cytotoxicity levels evident in the different E:T ratios (6 in our study), to a single index, indicating the number of aliquots/units contained in the sample tested for cytotoxicity that can each exert a designated level of cytotoxicity (e.g., 39% specific lysis in Fig 1A). In this approach, the comparison of different conditions is based on the horizontal shift between the different cytotoxicity killing curves, rather than the vertical difference [40]. In the current study, we defined and quantified LU45 within each microtiter plate as the number of LU at a 45% increase between the minimal and maximal cytotoxicity in the control condition (i.e., in Fig. 1A – 45% increase from 15% to 69% specific lysis designate “39% specific lysis” as the levels for calculating LU for all cytotoxicity curves in this plate). The regression exponential fit method was used to calculate LU for each condition, based on the six E:T ratios [40].
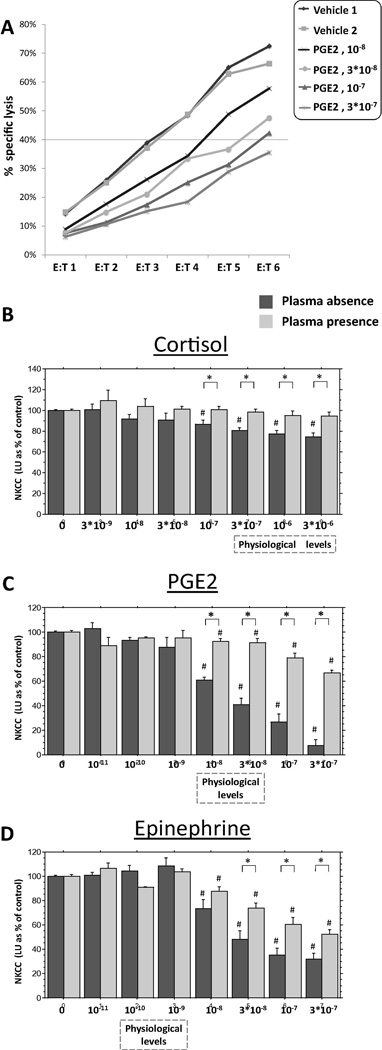
Fig. 1.
The effects of different hormones on NKCC against K562 target cells, in the presence of plasma and in its absence. (A) A representative E:T cytotoxicity curves in a single plate, including two replicates of control condition and 4 concentrations of PGE2, in the absence of plasma. Each curve is expressed as a single value (LU45, as explained in details in the Method section), and all results are presented as % of control levels of this index (1B–D). (B) Cortisol dose-dependently suppressed NKCC, but only in the absence of plasma. (C) PGE2 and (D) epinephrine dose-dependently suppressed NKCC, but this suppression was markedly more profound in the absence of plasma. ⍰ Indicates a significant difference between the presence and absence of plasma. # Indicates a significant reduction in NKCC compared to the respective control (Vehicle) levels. The concentrations of epinephrine and PGE2 spanned beyond systemic physiological levels, as these hormones, but not cortisol, also interact locally with NK cells at higher concentrations. Data presented as mean + SEM.
2.8.3. Statistical analysis
Throughout the study, drug effects are expressed as percent of control levels within each microtiter plate, and thus the control condition is always at 100%, with deviations stemming from duplicates or triplicates of the control condition. Each plate always contained blood from one type of blood preparation (e.g., presence or absence of plasma) and from a single subject. This approach overcomes individual differences in baseline levels of NKCC, and enables focusing on the effects of the drugs in each of the different blood preparations. Two- or three-way analyses of variance (ANOVA) with a predetermined significance level of 0.05 were conducted to test for main effects and interactions. Provided significant group differences were found, Fisher’s protected least significant differences (Fisher’s PLSD) contrasts were performed to compare specific pairs of groups, based on a priori hypotheses. We chose not to conduct repeated measure ANOVAs, as the use of the “% of control” index within each plate/subject already addresses individual differences, and the qualitatively different preparations of the blood, used within a subject blood sample, questions the appropriateness of using a within subject analysis. The between-subject analyses that we have conducted are more conservative.
3. Procedures and results
3.1. Subjects exhibit low stress levels at blood withdrawal
3.1.1. Self-report
All subjects reported low levels of stress at the time of blood withdrawal. The average score reported was 1.86 (SD 0.56) on a scale of 1 to 10, with a median of 2.
3.1.2. Serum cortisol levels
The average serum cortisol level in the blood withdrawn from subjects was 3.7*10−7 M (SD 1.7*10−7), with a median of 3.1*10−7 M.
3.2. Experiment 1: Markedly higher suppression of NKCC is induced in the absence of plasma compared to its presence by cortisol, PGE2, and epinephrine
This study was designed to compare whole blood (containing subjects’ original plasma) to blood without plasma (replaced with CM, as in the case of the standard in vitro approach) with respect to the acute effects of different concentrations of stress hormones on NKCC. Thus, in this experiment, the stress hormones were applied simultaneously with the initiation of the cytotoxicity assay, while in experiments 2 and 3 the stress hormones were also applied at different time intervals prior to the cytotoxicity assay.
3.2.1. Procedure
Blood was collected into heparinized syringes. Half of the blood was washed and plasma was replaced with CM (plasma absence), and the remaining blood was kept at room temperature (plasma presence). For plasma removal, aliquots of 3 ml of blood were washed once with 12 ml of PBS and twice with 12 ml of CM (centrifuged at 596g for 10 min, and reconstituted to their original volume each wash). Following blood wash, aliquots from each subject were pooled together, and blood samples were immediately co-incubated with different concentrations of cortisol, PGE2, epinephrine, or with vehicle, as seen in Fig. 1 B–D, which cover the physiological ranges of plasma levels of these hormones. Immediately after hormones were introduced into the blood, NKCC was assessed in their presence. The study was conducted in four replicates, employing different subjects, and each replicate included all experimental conditions.
3.2.2. Results
A representative E:T cytotoxicity curve is presented for a single plate, including two replicates of control condition and 4 doses of PGE2, in the absence of plasma. (Fig. 1 A). Each curve was expressed as a single LU45 value, which served as the dependent value in all statistical analyses.
The four replicates of the study yielded similar patterns of results and were combined based on percent of control levels in each plate (see Materials and Methods, 2.8.3. Statistical analysis). For each drug, an 8×2 ANOVA was conducted (8 drug concentrations including vehicle, by plasma presence/absence) with percent of averaged control levels as the dependent variable. By and large, in the presence of plasma markedly higher doses of each of the drugs were needed to cause NKCC suppression, compared to similar effects in the absence of plasma, as detailed below.
3.2 .2.1. Cortisol
A two-way ANOVA revealed main effects for cortisol concentrations (F(7,156) = 12.404, p < 0.0001) and for plasma presence (presence vs. absence of plasma) (F(1,156) = 34.325, p < 0.0001), and a significant interaction (F(7,156) = 5.887, p < 0.0001). Specifically, cortisol dose-dependently suppressed NKCC, but this suppression was evident only in the absence of plasma. Fisher’s PLSD indicated that in the absence of plasma cortisol concentrations of 3 × 10−6, 10−6, 3 × 10−7 and 10−7 M significantly reduced NKCC compared to the control (p < 0.0001), and compared to the presence of plasma (p < 0.03 or smaller). No significant effects were evident in any of the concentrations used in the presence of plasma (Fig. 1 B).
3.2.2.2. PGE2
A two-way ANOVA revealed a main effect for PGE2 concentration (F(7,164) = 120.37, p < 0.0001) and for plasma presence (F(1,164) = 101.207, p < 0.0001), and a significant interaction (F(7,164) = 40.473, p < 0.0001). Specifically, PGE2 dose-dependently suppressed NKCC, but this suppression was markedly more profound in the absence of plasma. In fact, more than 30-fold higher concentrations of PGE2 were needed in the presence of plasma to reach similar levels of suppression evident in its absence. Fisher’s PLSD indicated that PGE2 concentrations of 3 × 10−7, 10−7, 3 × 10−8 and 10−8 M significantly reduced NKCC in the absence of plasma more than in its presence (p < 0.0001). Additionally, concentrations of 3 × 10−7, 10−7, 3 × 10−8 and 10−8 M significantly reduced NKCC both in the absence and in the presence of plasma compared to the relevant control condition (p < 0.0001, and p < 0.02 or smaller, respectively) (Fig. 1 C).
3.2.2.3. Epinephrine
A two-way ANOVA revealed a main effect for concentration (F(7,160) = 110.338, p < 0.0001) and for plasma presence (F(1,160) = 11.459, p < 0.001), and a significant interaction (F(7,160) = 6.821, p < 0.0001). Specifically, epinephrine dose-dependently suppressed NKCC, but this suppression was markedly more profound in the absence of plasma, as more than 10-fold higher concentrations of epinephrine were needed in the presence of plasma to reach similar levels of suppression evident in its absence. Fisher’s PLSD indicated that epinephrine concentrations of 3 × 10−7, 10−7, and 3 × 10−8 M significantly reduced NKCC in the absence of plasma more than in its presence (p < 0.01). Additionally, concentrations of 3 × 10−7, 10−7, 3 × 10−8 and 10−8 M significantly reduced NKCC both in the absence and in the presence of plasma compared to the relevant control condition (p < 0.0001, and p < 0.01 or smaller, respectively) (Fig. 1 D).
3.3. Experiment 2: Suppression of NKCC by cortisol increases with time, whereas suppression induced by PGE2 occurs immediately and remains stable
This study was conducted to examine potential time-dependent effects of different stress hormones on NKCC, in the presence of plasma and in its absence. In this experiment, the stress hormones were introduced (i) simultaneously with the initiation of the cytotoxicity assay (0 h), or (ii) 2 h, or (iii) 6 h prior to the cytotoxicity assay. In all three conditions stress hormones remained present throughout the cytotoxicity assay.
Whereas in Exp. 1 NKCC assessment was performed shortly after blood was withdrawn, in Exp. 2 all blood samples were maintained for 6 hours prior to NKCC assessment (to enable the 6 pre-incubation period).
In this experiment, different mechanisms of action are represented through cortisol and PGE2. Cortisol was chosen due to its activation of a nuclear transcriptional mechanism [41], whereas PGE2 activates an immediate cytoplasmatic cAMP-dependent mechanism [42]. Epinephrine and PGs act through the same intracellular mechanism to suppress NKCC [3], but the effects of PGE2 were more potent at physiological levels than of epinephrine. Thus, and given limited amount of blood available per participant (100 ml), PGE2 was favored over epinephrine for this experiment.
3.3.1. Procedure
Blood was collected into heparinized syringes at a single time point. Half of the blood was washed and plasma was replaced while the remaining whole blood was kept at room temperature. For plasma removal, aliquots of 3 ml of blood were washed and centrifuged (room temperature, 596g for 10 min), once with 12 ml of PBS and twice with 12 ml of complete medium, and reconstituted to its original volume. Following blood wash, aliquots from each subject were pooled together.
Blood samples were co-incubated with different concentrations of cortisol, PGE2, or vehicle, as seen in Fig. 2, which cover the physiological ranges of plasma levels of these hormones. Hormones were first introduced to the 6 h co-incubation condition. Thereafter, all plates were kept in the incubator (5% CO2 at 37 °C). 4 hours later hormones were introduced to the 2 h co-incubation condition and placed back in the incubator. 2 hours later hormones were introduced to the 0 h condition (i.e., 6 hours following the introduction of hormones to the 6 h condition), and NKCC was assessed for all conditions/time intervals in the presence of the hormones. The study was conducted in two replicates, employing different subjects.
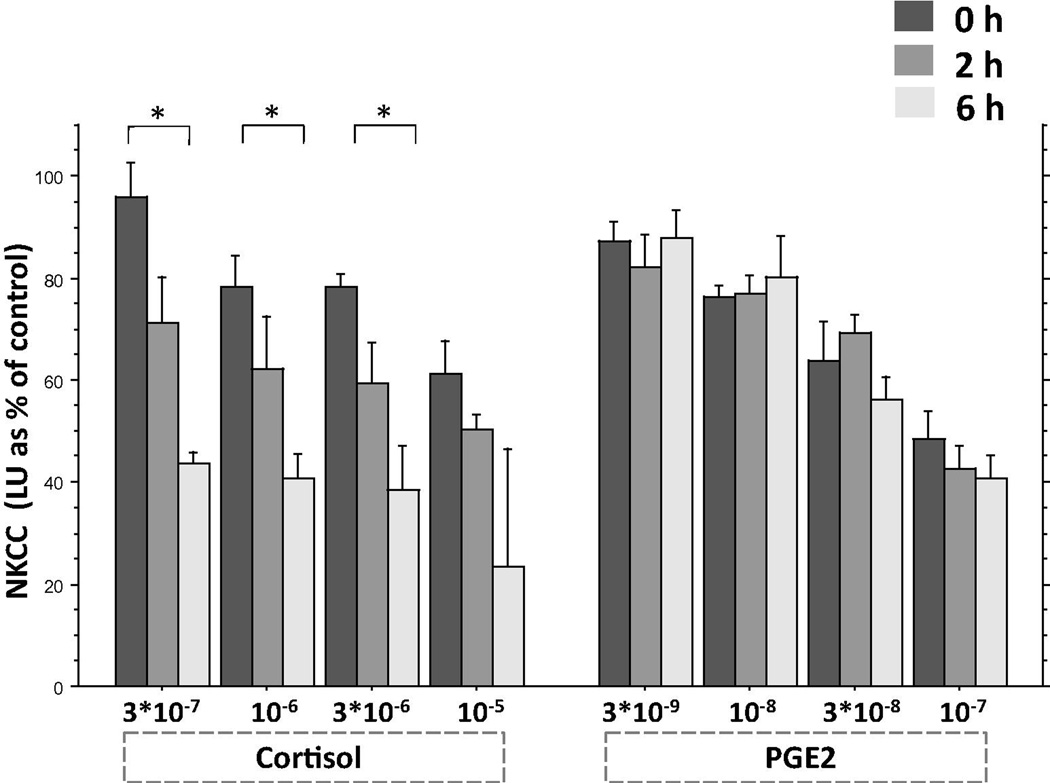
Fig. 2.
Exposure-time-dependent effects of cortisol and PGE2 on NKCC. Cortisol significantly reduced NKCC in 6 h more than in 0 h (indicated by *), whereas PGE2 exerts the same levels of suppression at all time-intervals. The figure presents the findings in the absence of plasma. Results are presented (mean + SEM) as % of vehicle (control condition) levels (not shown).
3.3.2 Results
The two runs of the study yielded similar patterns of results and were combined based on percent of control levels in each plate (see Materials and Methods, 2.8.3. Statistical analysis). As the main focus of the study was to compare the time-dependent effects between the different drugs, a 2×4×3 ANOVA was conducted separately for the replacement of plasma and its presence (2 drugs, 4 concentrations, 3 time intervals), with percent of averaged control levels as dependent variable.
3.3.2.1. Plasma absence
A three-way ANOVA revealed main effects for drug (F(1,84) = 8.886, p < 0.005), concentration (F(3,84) = 18.839, p < 0.0001), and time (F(2,84) = 18.051, p < 0.0001), and a significant drug by time interaction (F(2,84) = 13.797, p < 0.0001), indicating that PGE2 and cortisol exert significantly different time-dependent NK suppressive effects. Specifically, Fisher’s PLSD indicated that cortisol significantly reduced NKCC in 6 h more than in 0 h (in concentration of 3 × 10−6, 10−6, and 3 × 10−7 M, p < 0.003 or smaller), whereas PGE2 exerted the same levels of suppression at all time-intervals tested (Fig. 2).
3.3.2.2. Plasma presence
Similar effects were evident in the presence of plasma as described above in the absence of plasma, except that the suppression of NKCC by cortisol was markedly lower in the presence of plasma, as expected. A three-way ANOVA revealed main effects for drug (F(1,84) = 55.187, p < 0.0001), concentration (F(3,84) = 25.021, p < 0.0001), and time (F(2,84) = 6.638, p < 0.003), and significant interactions between drug by concentration (F(3,84) = 12.750, p < 0.0001) and drug by time (F(2,84) = 4.409, p < 0.03), the latter indicating that PGE2 and cortisol exert significantly different time-dependent NK suppressive effects. Specifically, Fisher’s PLSD indicated that cortisol significantly reduced NKCC in 6 h more than in 0 h (in concentration of 3 × 10−6, 10−6, and 3 × 10−7 M, p < 0.05 or smaller), whereas PGE2 exerted the same levels of suppression at all time-intervals tested (data not shown).
3.4. Experiment 3: The effects of PGE2 and epinephrine on NKCC quickly disappear following their removal, while the effects of cortisol persist
As the standard ex-vivo approach incorporates the replacement of plasma by an artificial medium, and thus also the exclusion of stress hormones, in the current experiment we studied the kinetics of the effects of stress hormones on NKCC following their removal. Specifically, to simulate the in vivo impact of the hormones, whole blood (containing plasma) was maintained in the incubator for 2 h while subjected to different stress hormones. Thereafter, the hormones were either (i) maintained in the assay milieu while testing NKCC (hormone presence), (ii) washed away and blood immediately tested for NKCC (hormone exclusion), or (iii) washed away and blood maintained at room temperature for 4 h before testing for NKCC (hormone exclusions + 4 h). The last two conditions aimed to simulate the standard ex-vivo exclusion of hormones and plasma before testing for NKCC.
3.4.1. Procedure
Blood was collected into heparinized syringes, and aliquots of whole blood (containing subjects’ plasma) were co-incubated for 2 hours with different concentrations of cortisol, PGE2, epinephrine, or vehicle, as seen in Fig. 3 A (covering the physiological ranges of plasma levels of these hormones). Additionally, a combination of cortisol and epinephrine, each in an effective concentration, was studied, as seen in Fig 3 B. NKCC was assessed either in the presence of these hormones, immediately after they were washed away, or 4 h after they were washed away. Except for the initial 2 hours of co-incubation with hormones, blood was processed and maintained at room temperature until assessment of NKCC. For hormone exclusion, aliquots of 1.6 ml of blood were washed twice with 3.5 ml of PBS while reestablishing volume of 200µl above hematocrit levels, and twice with 3 ml of complete medium while reconstituting to original volume of 1.6 ml (centrifuged at 596g for 10 min each time). This procedure diluted the original hormone concentrations by 7,600-fold. The study was conducted in two replicates, employing different subjects, each replicate including all experimental conditions.
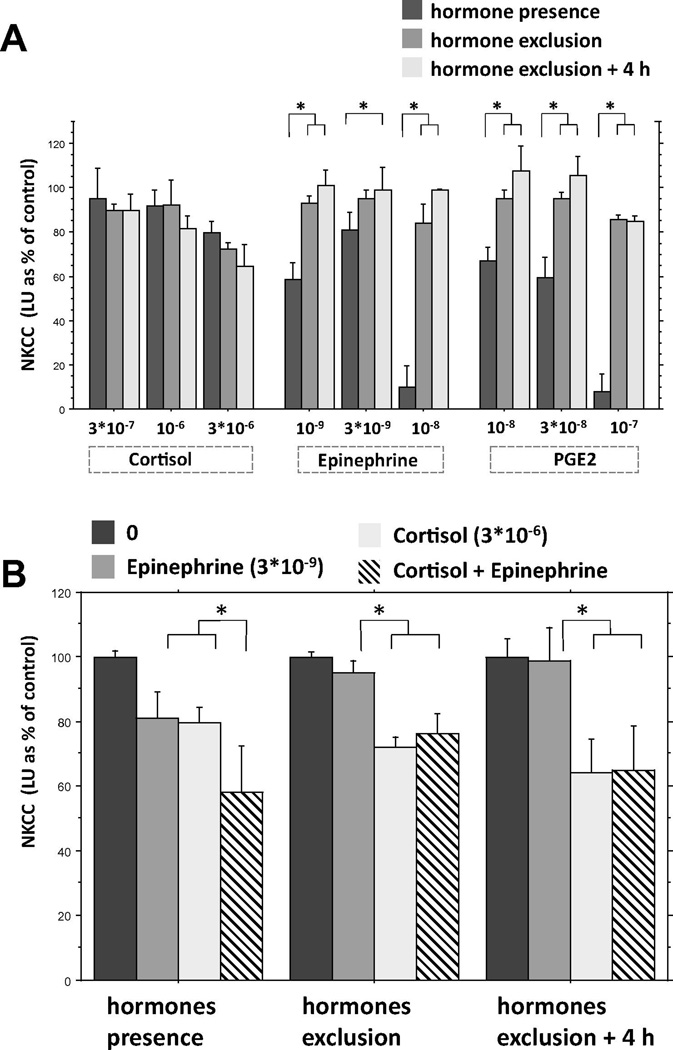
Fig.3.
The effects of different hormones on NKCC against K562 target cells. Hormones were either (i) maintained in the assay milieu while testing for NKCC (hormone presence), (ii) washed Jaway and blood immediately tested for of NKCC (hormone exclusion), or (iii) washed away and blood maintained at room temperature for 4 h before testing for NKCC (hormone exclusions + 4 h). (A) Epinephrine and PGE2 suppressed NKCC only when present in the in vitro setting, and their effects disappeared immediately following their removal, whereas suppression levels induced by cortisol did not change following its removal. (B) In the presence of these hormones, the combination of cortisol and epinephrine yielded significantly greater suppression of NKCC than each of these hormones individually. Following the exclusion of the hormones and 4 hours later, the combination of cortisol and epinephrine suppressed NKCC similarly to cortisol alone, and significantly greater than epinephrine alone, for which the effects vanished. * Indicates a significant difference in NKCC levels between the different hormone impact approaches (A) or different drug combinations (B). Results are presented (mean + SEM) as % of vehicle (control condition) levels (not shown).
3.4.2 Results
The two replicates of the study yielded similar patterns of results and were combined based on percent of control levels in each replicate (see Materials and Methods, 2.8.3. Statistical analysis). A 3×3×3 ANOVA was conducted (3 drugs, 3 concentrations, 3 hormone impact approaches), with percent of averaged control levels as dependent variable (Fig. 3 A). In addition, a 3×3 ANOVA was conducted (3 drugs, 3 hormone impact approaches) in order to compare the individual effects of cortisol and epinephrine to their combined impact on NKCC employing a single concentration of each drug (Fig. 3 B).
3.4.2.1. Effects of cortisol, epinephrine, and PGE2
A three-way ANOVA revealed main effects for concentration (F(2, 117) = 14.466, p < 0.0001), and hormone impact approaches (F(2,117) = 27.745, p < 0.0001), and significant interactions for drug by hormone impact approach (F(4,117) = 11.297, p < 0.0001) and concentration by hormone impact approaches (F(4,117) = 2.730, p < 0.05). Importantly, Fisher’s PLSD indicated that epinephrine and PGE2 suppressed NKCC only when they were present in the in vitro setting, and their effects disappeared immediately following their removal ( p < 0.05 or smaller), whereas suppression levels of cortisol did not change following its removal as seen in Fig 3 A.
3.4.2.2. Effects of cortisol, epinephrine, and their combination
A two-way ANOVA revealed a main effect for drug (F(3,120) = 21.354, p < 0.0001), with no significant interaction. Fisher’s PLSD indicated that in the presence of these hormones, the combination of cortisol and epinephrine yielded significantly greater suppression of NKCC than each of these hormones individually (p < 0.03 or smaller), exhibiting an additive effect of these hormones. However, following the exclusion of the hormones and 4 hours later, the combination of cortisol and epinephrine suppressed NKCC similarly to cortisol alone, and significantly more profoundly than epinephrine alone, where the effect disappeared (p < 0.05 or smaller) (Fig. 3 B).
4. Discussion
The present study suggests that the common findings of in vitro suppression of NKCC by stress hormones are an overestimation of the effects expected in vivo. Specifically, in the presence of plasma, 10–30 fold higher concentrations of stress hormones were needed to reach the same levels of NKCC suppression evident in artificial medium (absence of plasma), which is the standard in vitro milieu (e.g., RPMI-1640 + various additives + 10% FCS). Moreover, at acute exposure to systemic physiological levels, minute or no effects of stress hormones were evident in the presence of plasma, while marked suppression was evident in artificial medium. It should be noted that when blood was drawn, plasma cortisol levels were at low physiological levels (~3*10−7 M), and thus subjecting these blood samples to high physiological concentrations (10−6 to 3*10−6 M) constitutes a remarkable increase in cortisol levels, which, nevertheless, had no immediate effect on NKCC in the presence of plasma. These findings question the notion that acute exposure to physiological levels of stress hormones (e.g., up to 60 min of a stress paradigm), especially cortisol, directly suppress circulating NKCC in vivo on a per NK cell basis. Thus, we suggest that when ex-vivo studies report suppression of NKCC by acute stress exposure, such findings may primarily reflect redistribution effects induced by stress hormones [12], such as an influx of NK cells of an a priori lower cytotoxicity levels into the circulation.
On the other hand, under different conditions stress hormones may in fact impact NKCC on a per NK cell basis. Specifically, this may occur through a more prolonged exposure, which was evident herein to exert a direct in vitro NK suppressive effects in the presence of plasma, and was reported by in vivo studies [16]. Additionally, suppression of NKCC may occur through (i) indirect effects of stress hormones on NKCC, such as through reduction in Th1 cytokine levels [43], (ii) through direct suppression of NKCC by catecholamines and PGs when locally released at supra-systemic levels [26, 32–34], and/or (iii) through suppression of non-circulating unique NK cell subpopulations [44–46] of different susceptibility to stress hormones.
The current study also indicates that the duration of exposure of NK cells to stress hormones is a critical factor with potential confounding or misleading impact. The NK suppressive effects of cortisol increased over time (from 0 to 2 to 6 h of pre-incubation before being challenged with tumor cells), whereas the effects of PGE2 occurred immediately (0 h) and remained stable at different durations of pre-incubation with this hormone (i.e., at 2 and 6 h). These findings correspond well with the known nuclear transcriptional mechanisms activated by cortisol (and thus its delayed outcome) [41], and the immediate cytoplasmatic cAMP-dependent mechanisms activated by PGE2 [42] and epinephrine [3]. Therefore, when employing stress paradigms or in vitro exposure to stress hormones, the duration of the paradigm/exposure period may determine the impact of specific stress hormones, and such studies should thus include a complete time course for each hormone.
Clearly supporting the above conclusions are animal and human studies employing physiological levels of corticosterone or cortisol. In vitro studies, in which plasma was replaced by an artificial medium, demonstrated marked suppression of NKCC at physiological levels [18, 20]. However, a recent animal in vivo study demonstrated that no such suppression occurs within the animals at short exposure to corticosterone (less than 3 hr), and only a moderate suppression of NKCC appears following prolonged exposure (3–8 hr) to high physiological levels – an effect that was mainly ascribed to corticosterone-induced potentiation of an in vivo NK-suppressive effects of epinephrine [16]. A pivotal study in healthy humans also concluded that in vivo physiological changes in plasma cortisol levels along a one-hr period do not affect NKCC. Specifically, the administration of ACTH or metyrapone, which markedly increased or decreased plasma cortisol levels in these subjects, respectively, did not affect NK cytotoxicity levels. Additionally, no significant correlations were evident between levels of cortisol and levels of NK cytotoxicity, at baseline or following these manipulations, despite marked variations in both indices [21]. One factor that might explain the protective effects of plasma and of the in vivo milieu against suppression of NKCC by glucocorticoids is the presence of corticosteroid-binding-globulins, which bind up to 90% of glucocorticoid molecules [47], reducing the effective concentrations of glucocorticoids, and potentially buffering their effects [16].
Most importantly, our findings suggest that direct in vivo suppressive effects of epinephrine and PGE2 on NKCC would not be reflected in ex-vivo studies, while effects of glucocorticoids may be exaggerated. We demonstrated that when hormones are removed from the assay milieu, simulating their exclusion in the ex-vivo approach, the effects of epinephrine and PGE2 immediately vanish, while the effects of cortisol remain stable or increase with time. Thus, the interpretation of findings from numerous ex-vivo studies of NKCC, including our own, should be carefully reexamined, as is further elaborated below.
The current findings are aligned with recent animal studies we have conducted, directly indicating that an in vivo suppression of NKCC by PGE2 was not evident in an ex-vivo assessment, while a weaker in vivo effect of corticosterone was robustly evident ex-vivo [5], as would indeed be expected from the current study. Additionally, in rats’ blood, in vitro suppression of NKCC by PGE2 and by epinephrine vanished following their removal from the in vitro milieu, and even reversed (rebounded), while the impact of corticosterone persisted or increased after its removal [5], as similarly indicated herein in human blood.
To overcome some of the above limitations of the ex-vivo approach in humans, we have used a whole blood cytotoxicity assay, where NKCC is tested immediately upon blood withdrawal and without removing the plasma, its many factors, and the various cellular blood components [48–53]. We believe that compared to prolonged and manipulative procedures that enrich NK cells and eliminate the natural plasma milieu, this immediate whole blood approach better reflects the in vivo capacity of circulating NK cells to lyse target cells, as well as the modulation of this capacity by various stress responses.
As are most in vitro studies, the present study is clearly limited in simulating the complex in vivo milieu. Although hormonal concentrations in this study cover physiological levels, it is difficult to compare in vitro to in vivo concentrations, and local in vivo hormonal concentrations might be higher than their systemic levels, especially regarding catecholamines and PGs. Additionally, although plasma was maintained at some of the experimental conditions, one cannot assume the maintenance of all its factors and the simulation of their in vivo dynamics. Nevertheless, the current findings clearly suggest specific methodological impediments and distortions that are inherent to standard in vitro and ex-vivo approaches, and these same impediments have also been indicated in in vivo animal studies that do not suffer from most of the above limitations [5, 16].
It is worthy to note that the in vitro and ex-vivo approaches are valuable tools when their limitations are understood and circumvented. Specifically, the study of cellular and intracellular mechanisms is greatly advanced through in vitro approaches, albeit in an artificial context and commonly irrespective of significant modulating interactions with serum factors and other leukocytes [54–59]. The ex-vivo approach is unique in reflecting organ-specific cell redistribution (NK and others), and some, but not other, characteristics of these cell populations.
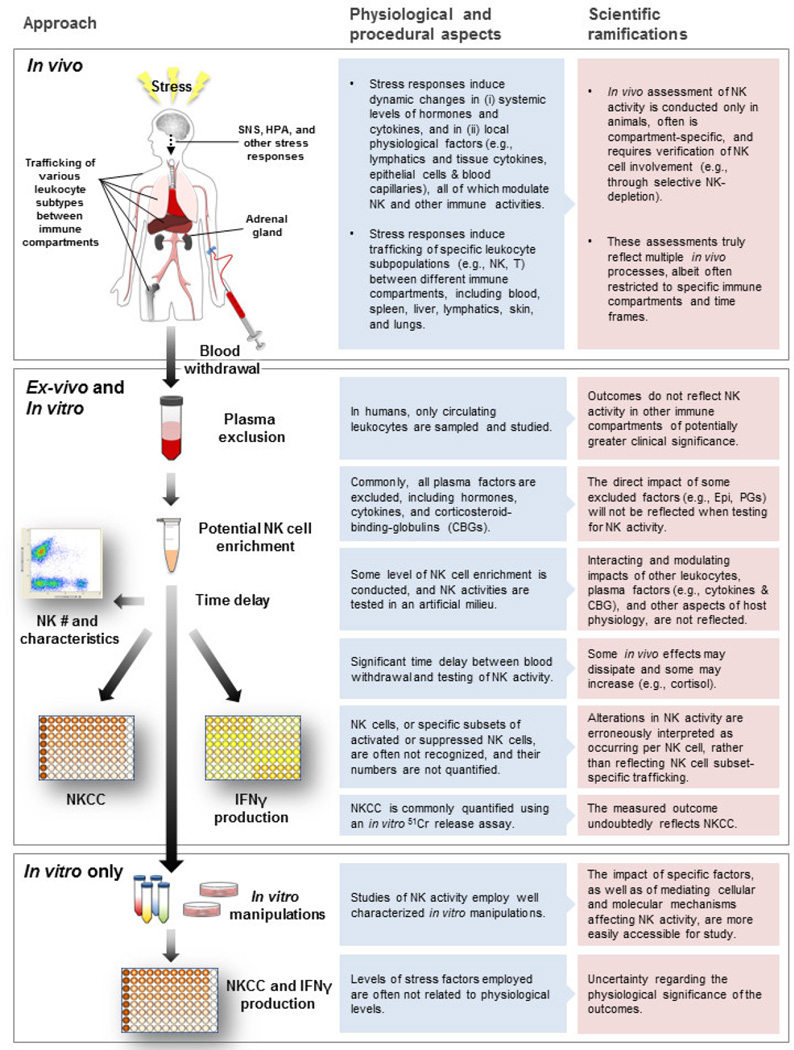
Additional prevalent obstacles to understanding the in vivo status of the NK cell system by ex-vivo and in vivo studies, which are not addressed herein, are (i) unrecognized stress-induced alterations in the number of NK cells in a cell population (ex-vivo) or in an immune compartment (in vivo) tested for NKCC, and (ii) the existence of NK cell subpopulations of different cytotoxic capacity, but of similar NK cell identifying markers [22, 44], that are differentially redistributed by stress hormones (also see Scheme 1). Such processes may lead to false interpretations, allegedly suggesting alterations in cytotoxicity per NK cell, while in fact the outcomes reflect NK cell trafficking or NK-subtype specific redistribution patterns. For example, unrecognized reduced proportion of NK cells (or of activated NK cells) within a sample of mononuclear cells tested for NKCC will result in lowered NKCC in such a sample, and will erroneously be interpreted as indicating suppression of NKCC per NK cell, while it might actually only reflect the reduction in the total number of NK cells (or activated NK cells) in the tested sample. Similarly, an in vivo reduction in NK cell number within the lungs’ capillaries, where circulating tumors cells are being trapped and destroyed by marginating pulmonary NK cells [22, 44], may impact an in vivo index of NK activity (e.g., lung tumor cell retention), not necessarily due to suppression of NKCC per NK cell. Thus, redistribution of NK cells, leading to reduction or enhancement in their numbers at specific strategic locations (e.g., lungs’ capillaries or the skin) should be considered a regional immune suppression/enhancement. Such regional immune modulations are biologically significant [44, 60–62], and may bear clinical implications in cancer patients.
Scheme 1.
Different approaches to study the impact of stress on NK activity: Physiological and procedural aspects of the approaches and their scientific ramifications
Although the direct effects of PGs and epinephrine on NKCC are not expected to be evident in ex-vivo assessments, some studies have reported ex-vivo enhancement of NKCC through these factors and following stress exposure [8, 9], and others reported suppression of NKCC [12–14, 17, 45, 63]. Therefore, we suggest that all these findings should be reinterpreted in light of insights from the present and other studies [5, 16]. Ex-vivo studies that reported enhanced NKCC by catecholamines may have reflected (i) a rebound effect induced by the removal of these hormones from the sample tested for cytotoxicity [5], (ii) an indirect effect of such hormones, and/or (iii) alterations in NK cell numbers or influx of more active NK cell subpopulation into the immune compartment studied. On the other hand, we reported ex-vivo suppression of NKCC by surgery, which was blocked by antagonizing catecholamines and PGs [22, 45, 64]. However, the blockade of catecholamines and PGs also reduced corticosterone levels [43, 64], which we now believe was the actual factor causing the evident ex-vivo suppression of NKCC and its attenuation by the antagonists to catecholamines and PGs. Irrespective of this ex-vivo suppression by corticosterone, we have found that the in vivo release of catecholamines and PGs following stress or surgery causes marked in vivo suppression of NKCC [12, 22, 31], without exerting these effects in ex-vivo assessments, and not through elevating corticosterone levels [5].
The finding presented herein should stimulate researchers to assess whether similar processes and fallacies exist with respect to the impact of stress on other immune indices. For example, cytokine levels may differentially be affected in vivo and in vitro, as is reported by a recent study regarding IL-12 regulation by stress hormones [43]. Antibody production seems to be a systemic prolonged process that may be less affected by transient stress responses. However, it may involve critical sub-processes, such as initial interactions with pathogens, which may be restricted to a specific immune compartment that is affected by leukocyte trafficking and specific regional humoral milieu. Clearly, each immune index should be studied in accordance with its unique characteristics.
In summary, the limitations of the in vitro and ex-vivo approaches studied herein stem from the inadequate representation and preservation of the natural in vivo milieu. Given the complex and dynamic nature of NK cell modulation by stress hormones and cytokines, also evident in the current study, these inadequacies are doomed to yield misleading outcomes, as specified above. In human studies, some distortions might be minimized by using a non-delayed whole blood NK cytotoxicity assay [53]. Understanding the specific limitations of ex-vivo and in vitro approaches in the context of NKCC, combining them with in vivo studies when feasible, and considering the potential impact of redistribution of subpopulations of NK cells by stress hormones, may help reinterpret previous literature and devise more advance future studies. Such considerations may also be applicable to other immune indices, especially those of a highly dynamic nature.
The following are authors’ definitions.
In vitro: “in glass” (Latin). Studies in which the experimental manipulations and the assessment of the outcomes are both conducted in test-tubes, petri dishes, and alike. For example, studying the impact of epinephrine on cytotoxicity of purified NK cells - all conducted in testing tubes.
Ex-vivo: “out of the living” (Latin). Studies in which the experimental manipulations are conducted in the living organism, followed by in vitro assessments of the measured outcomes. For example, subjecting animals to swim-stress, followed by blood withdrawal and in vitro assessment of NK cytotoxicity.
In vivo: “within the living” (Latin). Studies in which the experimental manipulations are conducted in the living organism, followed by either (i) assessments of outcome indices in the living organism (e.g., imaging of a developing malignant mass or freezing behavior), or (ii) assessment of an index in an approach that undoubtedly preserves and reflects its status in the living organism (e.g., number of lung metastases counted following animal euthanasia, or serum antibody levels).
Highlights.
Exclusion of plasma renders NK cells 10–30 fold more susceptible to stress hormones
In vitro (no plasma), physiological levels of CORT, PGE, and Epi suppress NKCC
Acute physiological CORT levels are unlikely to affect NKCC in vivo (plasma milieu)
Prolonged CORT exposure may suppress NKCC in vivo; effects may be inflated ex-vivo
In vivo profound suppression of NKCC by PGE or Epi would not be detected ex-vivo
Acknowledgements
This work was supported by NIH/NCI grant # R01CA172138-01 (SBE), and by the Israeli Science Foundation (SBE).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ben-Eliyahu S, Page GG, Schleifer SJ. Stress, NK cells, and cancer: Still a promissory note. Brain Behav Immun. 2007 doi: 10.1016/j.bbi.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 2.Takamoto TY, et al. Norepinephrin inhibits human natural killer cell activity in vitro. International Journal of Neuroscience. 1991;58:127. doi: 10.3109/00207459108987189. [DOI] [PubMed] [Google Scholar]
- 3.Whalen MM, Bankhurst AD. Effects of beta-adrenergic receptor activation, cholera toxin and forskolin on human natural killer cell function. Biochemical Journal. 1990;272(2):327–331. doi: 10.1042/bj2720327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hellstrand K, Hermodsson S. An immunopharmacological analysis of adrenaline-induced suppression of human natural killer cell cytotoxicity. International Archives of Allergy and Applied Immunology. 1989;89(4):334–341. doi: 10.1159/000234972. [DOI] [PubMed] [Google Scholar]
- 5.Meron G, et al. PGE (2) suppresses NK activity in vivo directly and through adrenal hormones: Effects that cannot be reflected by ex vivo assessment of NK cytotoxicity. Brain, behavior, and immunity. 2013;28:128–138. doi: 10.1016/j.bbi.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greisen J, et al. Acute pain induces an instant increase in natural killer cell cytotoxicity in humans and this response is abolished by local anaesthesia. Br J Anaesth. 1999;83(2):235–240. doi: 10.1093/bja/83.2.235. [DOI] [PubMed] [Google Scholar]
- 7.Pedersen BK, et al. Modulation of natural killer cell activity in peripheral blood by physical exercise. Scandinavian Journal of Immunology. 1988;27(6):673–678. doi: 10.1111/j.1365-3083.1988.tb02400.x. [DOI] [PubMed] [Google Scholar]
- 8.Schedlowski M, et al. Changes of natural killer cells during acute psychological stress. Journal of Clinical Immunology. 1993;13(2):119–126. doi: 10.1007/BF00919268. [DOI] [PubMed] [Google Scholar]
- 9.Tarr AJ, et al. beta-Adrenergic receptor mediated increases in activation and function of natural killer cells following repeated social disruption. Brain Behav Immun. 2012;26(8):1226–1238. doi: 10.1016/j.bbi.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tonnesen E, Christensen NJ, Brinklov MM. Natural killer cell activity during cortisol and adrenaline infusion in healthy volunteers. European Journal of Clinical Investigation. 1987;17(6):497–503. doi: 10.1111/j.1365-2362.1987.tb01148.x. [DOI] [PubMed] [Google Scholar]
- 11.Tonnesen E, Tonnesen J, Christensen NJ. Augmentation of cytotoxicity by natural killer (NK) cells after adrenaline administration in man. Acta Pathologica, Microbiologica, et Immunologica Scandandinavica. Section C. 1984;92(1):81–83. [PubMed] [Google Scholar]
- 12.Shakhar G, Ben-Eliyahu S. In vivo beta-adrenergic stimulation suppresses natural killer activity and compromises resistance to tumor metastasis in rats. Journal of Immunology. 1998;160(7):3251–3258. [PubMed] [Google Scholar]
- 13.Ben-Eliyahu S, et al. Evidence that stress and surgical interventions promote tumor development by suppressing natural killer cell activity. Int J Cancer. 1999;80(6):880–888. doi: 10.1002/(sici)1097-0215(19990315)80:6<880::aid-ijc14>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 14.Andersen BL, et al. Stress and immune responses after surgical treatment for regional breast cancer. Journal of the National Cancer Institute. 1998;90(1):30–36. doi: 10.1093/jnci/90.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ben-Eliyahu S, et al. Suppression of NK cell activity and of resistance to metastasis by stress: a role for adrenal catecholamines and beta-adrenoceptors. Neuroimmunomodulation. 2000;8(3):154–164. doi: 10.1159/000054276. [DOI] [PubMed] [Google Scholar]
- 16.Rosenne E, et al. In vivo suppression of NK cell cytotoxicity by stress and surgery: glucocorticoids have a minor role compared to catecholamines and prostaglandins. Brain Behav Immun. 2014;37:207–219. doi: 10.1016/j.bbi.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inbar S, et al. Do stress responses promote leukemia progression? An animal study suggesting a role for epinephrine and prostaglandin-E2 through reduced NK activity. PLoS One. 2011;6(4):e19246. doi: 10.1371/journal.pone.0019246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cox WI, Holbrook NJ, Friedman H. Mechanism of glucocorticoid action on murine natural killer cell activity. Journal of the National Cancer Institute. 1983;71(5):973–981. [PubMed] [Google Scholar]
- 19.Gatti G, et al. Cortisol at physiological concentrations and prostaglandin E2 are additive inhibitors of human natural killer cell activity. Immunopharmacology. 1986;11(2):119–128. doi: 10.1016/0162-3109(86)90032-9. [DOI] [PubMed] [Google Scholar]
- 20.Shakhar G, et al. Amelioration of operation-induced suppression of marginating pulmonary NK activity using poly IC: a potential approach to reduce postoperative metastasis. Ann Surg Oncol. 2007;14(2):841–852. doi: 10.1245/s10434-006-9078-9. [DOI] [PubMed] [Google Scholar]
- 21.Bodner G, Ho A, Kreek MJ. Effect of endogenous cortisol levels on natural killer cell activity in healthy humans. Brain Behav Immun. 1998;12(4):285–296. doi: 10.1006/brbi.1998.0533. [DOI] [PubMed] [Google Scholar]
- 22.Melamed R, et al. Marginating pulmonary-NK activity and resistance to experimental tumor metastasis: suppression by surgery and the prophylactic use of a beta-adrenergic antagonist and a prostaglandin synthesis inhibitor. Brain Behav Immun. 2005;19(2):114–126. doi: 10.1016/j.bbi.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Liedtke CM. Differentiated properties of rabbit tracheal epithelial cells in primary culture. Am J Physiol. 1988;255(6 Pt 1):C760–C770. doi: 10.1152/ajpcell.1988.255.6.C760. [DOI] [PubMed] [Google Scholar]
- 24.Ueda F, et al. Adrenergic regulation of prostaglandin biosynthesis in cultured rabbit gastric epithelial cells. Jpn J Pharmacol. 1994;65(2):113–120. doi: 10.1254/jjp.65.113. [DOI] [PubMed] [Google Scholar]
- 25.Wojtowicz-Praga S. Reversal of tumor-induced immunosuppression: a new approach to cancer therapy. J Immunother. 1997;20(3):165–177. [PubMed] [Google Scholar]
- 26.Buvanendran A, et al. Upregulation of prostaglandin E2 and interleukins in the central nervous system and peripheral tissue during and after surgery in humans. Anesthesiology. 2006;104(3):403–410. doi: 10.1097/00000542-200603000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Baxevanis CN, et al. Abnormal cytokine serum levels correlate with impaired cellular immune responses after surgery. Clin Immunol Immunopathol. 1994;71(1):82–88. doi: 10.1006/clin.1994.1055. [DOI] [PubMed] [Google Scholar]
- 28.Vitoratos N, et al. Prostaglandin F1a and prostaglandin E2 plasma levels after transvaginal cervical cerclage. Clin Exp Obstet Gynecol. 1996;23(1):21–25. [PubMed] [Google Scholar]
- 29.Baxevanis CN, et al. Elevated prostaglandin E2 production by monocytes is responsible for the depressed levels of natural killer and lymphokine-activated killer cell function in patients with breast cancer. Cancer. 1993;72(2):491–501. doi: 10.1002/1097-0142(19930715)72:2<491::aid-cncr2820720227>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 30.Skibinski G, et al. Relative immunosuppressive activity of human seminal prostaglandins. J Reprod Immunol. 1992;22(2):185–195. doi: 10.1016/0165-0378(92)90015-v. [DOI] [PubMed] [Google Scholar]
- 31.Yakar I, et al. Prostagland in e(2) suppresses NK activity in vivo and promotes postoperative tumor metastasis in rats. Ann Surg Oncol. 2003;10(4):469–479. doi: 10.1245/aso.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 32.Felten DL, et al. Noradrenergic and peptidergic innervation of lymphoid tissue. Journal of Immunology. 1985;135(2 Suppl):755s–765s. [PubMed] [Google Scholar]
- 33.Felten SY, Olschowka J. Noradrenergic sympathetic innervation of the spleen: II. Tyrosine hydroxylase (TH)-positive nerve terminals form synapticlike contacts on lymphocytes in the splenic white pulp. J Neurosci Res. 1987;18(1):37–48. doi: 10.1002/jnr.490180108. [DOI] [PubMed] [Google Scholar]
- 34.Kim R, et al. Tumor-driven evolution of immunosuppressive networks during malignant progression. Cancer Res. 2006;66(11):5527–5536. doi: 10.1158/0008-5472.CAN-05-4128. [DOI] [PubMed] [Google Scholar]
- 35.Yirmiya R, et al. Ethanol increases tumor progression in rats: possible involvement of natural killer cells. Brain, Behavior, and Immunity. 1992;6(1):74–86. doi: 10.1016/0889-1591(92)90061-r. [DOI] [PubMed] [Google Scholar]
- 36.Lozzio BB, Lozzio CB. Properties and usefulness of the original K-562 human myelogenous leukemia cell line. Leuk Res. 1979;3(6):363–370. doi: 10.1016/0145-2126(79)90033-x. [DOI] [PubMed] [Google Scholar]
- 37.Ben-Eliyahu S, et al. Increased susceptibility to metastasis during pro-oestrus/oestrus in rats: possible role of oestradiol and natural killer cells. British Journal of Cancer. 1996;74(12):1900–1907. doi: 10.1038/bjc.1996.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ottenhof PC, Morales A, Baines MG. Quantitation of a whole blood assay for human natural killer cell activity. Journal of Immunological Methods. 1981;42(3):305–318. doi: 10.1016/0022-1759(81)90159-9. [DOI] [PubMed] [Google Scholar]
- 39.Ree RC, Platts AA. A modified short-term cytotoxicity test: assessment of natural cell mediated cytotoxicity in whole blood. J Immunol methods. 1983;62:79–83. doi: 10.1016/0022-1759(83)90113-8. [DOI] [PubMed] [Google Scholar]
- 40.Pollock RE, et al. Lytic units reconsidered: pitfalls in calculation and usage. Journal of clinical laboratory analysis. 1990;4:274–282. doi: 10.1002/jcla.1860040408. [DOI] [PubMed] [Google Scholar]
- 41.Zhou J, et al. Glucocorticoid regulation of natural cytotoxicity: effects of cortisol on the phenotype and function of a cloned human natural killer cell line. Cell Immunol. 1997;178(2):108–116. doi: 10.1006/cimm.1997.1138. [DOI] [PubMed] [Google Scholar]
- 42.Torgersen KM, et al. Selective activation of cAMP-dependent protein kinase type I inhibits rat natural killer cell cytotoxicity. J Biol Chem. 1997;272(9):5495–5500. doi: 10.1074/jbc.272.9.5495. [DOI] [PubMed] [Google Scholar]
- 43.Shaashua L, et al. Plasma IL-12 levels are suppressed in vivo by stress and surgery through endogenous release of glucocorticoids and prostaglandins but not catecholamines or opioids. Psychoneuroendocrinology. 2014;42:11–23. doi: 10.1016/j.psyneuen.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Melamed R, et al. The marginating-pulmonary immune compartment in rats: characteristics of continuous inflammation and activated NK cells. J Immunother. 2010;33(1):16–29. doi: 10.1097/CJI.0b013e3181b0b146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Benish M, et al. Perioperative use of beta-blockers and COX-2 inhibitors may improve immune competence and reduce the risk of tumor metastasis. Ann Surg Oncol. 2008;15(7):2042–2052. doi: 10.1245/s10434-008-9890-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benish M, et al. The marginating-pulmonary immune compartment in mice exhibits increased NK cytotoxicity and unique cellular characteristics. Immunol Res. 2014;58(1):28–39. doi: 10.1007/s12026-013-8435-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lewis JG, Elder PA. Intact or “active” corticosteroid-binding globulin (CBG) and total CBG in plasma: Determination by parallel ELISAs using monoclonal antibodies. Clin Chim Acta. 2012 doi: 10.1016/j.cca.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 48.Shakhar K, et al. Differences in number and activity of peripheral natural killer cells in primary versus secondary recurrent miscarriage. Fertil Steril. 2003;80(2):368–375. doi: 10.1016/s0015-0282(03)00611-3. [DOI] [PubMed] [Google Scholar]
- 49.Shakhar K, et al. High NK cell activity in recurrent miscarriage: what are we really measuring? Hum Reprod. 2006;21(9):2421–2425. doi: 10.1093/humrep/del131. [DOI] [PubMed] [Google Scholar]
- 50.Shakhar K, et al. Timing within the menstrual cycle, sex, and the use of oral contraceptives determine adrenergic suppression of NK cell activity. Br J Cancer. 2000;83(12):1630–1636. doi: 10.1054/bjoc.2000.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yovel G, Shakhar K, Ben-Eliyahu S. The effects of sex, menstrual cycle, and oral contraceptives on the number and activity of natural killer cells. Gynecol Oncol. 2001;81(2):254–262. doi: 10.1006/gyno.2001.6153. [DOI] [PubMed] [Google Scholar]
- 52.Yovel G, et al. Higher natural killer cell activity in schizophrenic patients: the impact of serum factors, medication, and smoking. Brain Behav Immun. 2000;14(3):153–169. doi: 10.1006/brbi.1999.0574. [DOI] [PubMed] [Google Scholar]
- 53.Greenfeld K, et al. Immune suppression while awaiting surgery and following it: dissociations between plasma cytokine levels, their induced production, and NK cell cytotoxicity. Brain Behav Immun. 2007;21(4):503–513. doi: 10.1016/j.bbi.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 54.Marcenaro E, et al. IL-12 or IL-4 prime human NK cells to mediate functionally divergent interactions with dendritic cells or tumors. J Immunol. 2005;174(7):3992–3998. doi: 10.4049/jimmunol.174.7.3992. [DOI] [PubMed] [Google Scholar]
- 55.Nguyen KB, et al. Interferon alpha/beta-mediated inhibition and promotion of interferon gamma: STAT1 resolves a paradox. Nat Immunol. 2000;1(1):70–76. doi: 10.1038/76940. [DOI] [PubMed] [Google Scholar]
- 56.Nguyen KB, et al. Coordinated and distinct roles for IFN-alpha beta, IL-12, and IL-15 regulation of NK cell responses to viral infection. J Immunol. 2002;169(8):4279–4287. doi: 10.4049/jimmunol.169.8.4279. [DOI] [PubMed] [Google Scholar]
- 57.Sareneva T, Julkunen I, Matikainen S. IFN-alpha and IL-12 induce IL-18 receptor gene expression in human NK and T cells. J Immunol. 2000;165(4):1933–1938. doi: 10.4049/jimmunol.165.4.1933. [DOI] [PubMed] [Google Scholar]
- 58.Fernandez NC, et al. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nat Med. 1999;5(4):405–411. doi: 10.1038/7403. [DOI] [PubMed] [Google Scholar]
- 59.Della Chiesa M, et al. Multidirectional interactions are bridging human NK cells with plasmacytoid and monocyte-derived dendritic cells during innate immune responses. Blood. 2006;108(12):3851–3858. doi: 10.1182/blood-2006-02-004028. [DOI] [PubMed] [Google Scholar]
- 60.Dhabhar FS. Stress, leukocyte trafficking, and the augmentation of skin immune function. Ann N Y Acad Sci. 2003;992:205–217. doi: 10.1111/j.1749-6632.2003.tb03151.x. [DOI] [PubMed] [Google Scholar]
- 61.Dhabhar FS, et al. Short-term stress enhances cellular immunity and increases early resistance to squamous cell carcinoma. Brain Behav Immun. 2010;24(1):127–137. doi: 10.1016/j.bbi.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Benish M, et al. The marginating-pulmonary immune compartment in mice exhibits increased NK cytotoxicity and unique cellular characteristics. Immunol Res. 2013 doi: 10.1007/s12026-013-8435-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Levi B, et al. Continuous stress disrupts immunostimulatory effects of IL-12. Brain, Behavior, and Immunity. 2011;25(4):727–735. doi: 10.1016/j.bbi.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Glasner A, et al. Improving survival rates in two models of spontaneous postoperative metastasis in mice by combined administration of a beta-adrenergic antagonist and a cyclooxygenase-2 inhibitor. J Immunol. 2010;184(5):2449–2457. doi: 10.4049/jimmunol.0903301. [DOI] [PubMed] [Google Scholar]