Introduction
Stroke is an important acute neurological condition in children with an annual incidence ranging from 2.3 to 13 per 100,000 children.1-3 While most children who suffer stroke do not die of the acute disorder, the consequences of the brain injury are amortized over the lengthy lifespan that follows.4-8 Potential for reduction in lifelong morbidity by timely and effective intervention with a thrombolytic agent such as tissue plasminogen activator (tPA) in children with acute arterial ischemic stroke (AIS) constituted the core rationale for study of tPA treatment of acute AIS in children. The perceived high potential for benefit following treatment justified assumption of risk for intracranial hemorrhage (ICH) following its use.9 Since in adults, the risk of hemorrhage following tPA use was thought to be related to infarct volume, this principle was assumed for children. The known developmental trajectory of the fibrinolytic system includes lower levels of endogenous tPA and higher levels of plasminogen activator inhibitor 1 (PAI-1) in young children than are found in adults, and warranted a dose-finding study beginning at doses lower than that used in adults with incremental increase through the currently used adult dose of 0.9 mg/kg, and careful assessment of tPA pharmacokinetics.10
Currently, information regarding children treated with tPA consists of case reports, small case series, and hospital database documentation. Best practice for treatment of children with acute stroke has received little rigorous study. Clinical approach varies widely among centers, and reflects a dearth of research on which to base treatment protocols. Although tPA is not approved for use in childhood stroke, up to 2% of children with acute stroke are reported to have been treated with tPA in the United States despite lack of safety and efficacy data.11-14
In 2010 the NINDS funded the first prospective treatment trial in acute pediatric stroke, the Thrombolysis in Pediatric Stroke (TIPS) trial (NIH grant R01NS065848). TIPS reflected a multi-institutional, multidisciplinary design to determine safety, best dose and feasibility of treatment with intravenous (IV) tPA of children who present with AIS. Secondary aims comprised the determination of the pharmacokinetics of tPA in children and assessment of the 90 day clinical outcome among treated patients. TIPS was closed by the NIH in December, 2013 for lack of accrual. Herein, we summarize the lessons learned during the development and initial execution of the TIPS trial, the protocol synopsis, and the results of the challenges in implementation of the study.
The occurrence of symptomatic intracranial hemorrhage (SICH) following use of tPA in children with acute ischemic stroke constitutes a principal concern. When administered to adults according to NINDS guidelines, intravenous (IV) tPA therapy for AIS is associated with SICH in 6.4%.15 This risk may be lower in younger patients as none of 48 young adults (16 to 49 years of age) treated with IV tPA for AIS developed SICH.16 In adults with acute stroke, increasing hemorrhagic volume following IV tPA treatment correlated with increasingly poor neurologic outcome.17
During childhood, the fibrinolytic system is not yet mature.10,18-20 Baseline free tPA concentration is decreased and PAI-1 concentration, an inhibitor of tPA, is increased, compared to adults.20,21 In addition, children have an increased volume of distribution22,23 and more rapid hepatic clearance suggesting they will clear tPA more quickly.24 This raises the possibility that a higher dose of tPA than is used in adults may be needed to promote thrombolysis in children who present with acute AIS.
Most children with acute AIS present for care within 0-6 hours.25,26 However, there can be significant in-hospital delay to diagnosis.15,25-29 All TIPS sites were expected to establish and maintain acute stroke protocols for rapid diagnosis of acute stroke in children and ensure swift treatment with tPA in qualifying patients. Importantly, procedures for evaluation of children who present with symptoms of acute stroke were assessed at each site at the beginning of, during preparation for, and at site activation for TIPS.30
The challenges in protocol development for TIPS therefore encompassed: (1) the ethics of treatment children with potentially risky therapy for acute ischemic stroke; (2) the risk of SICH following treatment with tPA; (3) the effects of developmental hemostatic differences between children and adults that might affect the dosing, safety, and efficacy of tPA in acute pediatric arterial ischemic stroke; (4) tPA dose selection for children; (5) frequency and type of neuroimaging to be used in both initial assessment for tPA use and for follow-up after tPA administration; (6) inclusion and exclusion criteria; and (7) overcoming diagnostic delays to achieve eligibility for thrombolysis.
Methods
The TIPS study was an international multi-center, dose-adaptive, phase 1 cohort study. Study design spanned several years with input from members of the International Pediatric Stroke Study (IPSS), adult stroke specialists, and TIPS site investigators. Work in preparation for TIPS spanned 200 meetings over 3 years, including NINDS and FDA oversight in study design. As minimal safety and efficacy data on tPA use in childhood AIS exists, the most ethical way to proceed was through design of a consensus protocol for prospective enrollment and careful, complete follow-up, thereafter.
Children age 2 through 17 years of age who presented with acute AIS were eligible for enrollment to receive IV tPA if initiated within 4.5 hours of stroke onset (see Table 1 for inclusion and exclusion criteria). Importantly, at most centers, TIPS required confirmation of acute stroke by magnetic resonance imaging (MRI), using diffusion weighted imaging (DWI) to prove acute ischemic injury and magnetic resonance angiography (MRA) to identify some amount of arterial obstruction. In several centers, head computerized tomography (CT) showing a normal brain parenchyma or minimal early ischemic change and CT angiogram showing partial or complete arterial occlusion of the corresponding intracranial artery were used, consistent with pre-existing care pathways. Three dosing tiers were planned (0.75, 0.9, 1.0 mg/kg of IV tPA), with a maximum dose reached at 90 kg body weight. The IV tPA dose was to be given over one hour; ten percent of the total dose as a bolus over 5 minutes with the remaining 90% over the subsequent 55 minutes. The dosing tier for a given patient was determined using a Bayesian dose finding method, as below.31 All patients underwent follow-up neuroimaging 24 hours after tPA infusion. Standard protocols were made available to all centers for the investigation and management of pediatric stroke. Guidelines for management of potential complications (ICH, systemic bleeding, angioedema, hypotension) of tPA treatment were provided to all centers. [Please see supplementary material on-line http://stroke.ahajournals.org]
Table 1.
TIPS Inclusion and Exclusion Criteria
| Inclusion Criteria |
| Age 2 to 17 years |
| Acute ischemic stroke defined as acute onset neurological deficit with a pattern consistent with arterial ischemia |
| PedNIHSS ≥ 4 and ≤ 24 |
| Treatment can be adminsitered within 4.5 of stroke onset |
| Radiological confirmation of an acute ischemic stroke by: |
| (a) MR showing acute stroke on diffusion imaging plus MRA showing arterial partial or complete arterial occlusion of the corresponding intracranial artery |
| (b) CT and CT angiogram confirmation showing a normal brain parenchyma or minimal early ischemic change plus partial or complete arterial occlusion of the corresponding intracranial artery |
| No evidence of any intracranial hemorrhage. |
| Children with seizure at onset may be included as long as they fulfill the criteria above |
| Exclusion Criteria |
| Unknown time of symptoms onset |
| Pregnancy |
| Clinical presentation suggestive of SAH even if brain imaging is negative for blood. |
| Patient who would decline blood transfusion if indicated |
| History of prior intracranial hemorrhage |
| Known cerebral arterial venous malformation, aneurysm, or neoplasm |
| Persistent Systolic Blood Pressure > 15% above the 95th percentile for age while sitting or supine |
| Glucose <50 mg/dl (2.78 mmol/l) or > 400 mg/dl (22.22 mmol/l) |
| Bleeding diathesis including platelets < 100,000, PT > 15 sec (INR > 1.4) or elevated PTT > upper limits of the normal range. |
| Clinical presentation consistent with acute myocardial infarction (MI) or post-MI pericarditis that requires evaluation by cardiology prior to treatment |
| Prior stroke, major head trauma, or intracranial surgery within the past 3 months |
| Major surgery or parenchymal biopsy within 10 days (relative contraindication) |
| Gastrointestinal or urinary bleeding within 21 days (relative contraindication) |
| Arterial puncture at noncompressible site or lumbar puncture within 7 days (relative contraindication). Patients who have had a cardiac catheterization via a compressible artery are not excluded. |
| Patient with malignancy or within 1 month of completion of treatment for cancer |
| Patients with an underlying significant bleeding disorder. Patients with a mild platelet dysfunction, mild von Willebrand Disease or other mild bleeding disorders are not excluded. |
| Stroke related exclusion criteria |
| Mild deficit (PedNIHSS < 4) at start of tPA infusion or at time of sedation for neuroimaging, if applicable. |
| Severe deficit suggesting very large territory stroke, with pre-tPA PedNIHSS > 24, regardless of the infarct volume seen on neuroimaging. |
| Stroke suspected to be due to subacute bacterial endocarditis, moyamoya, sickle cell disease, meningitis, bone marrow, air or fat embolism. |
| Previously diagnosed primary angiitis of the central nervous system (PACNS) or secondary CNS vasculitis. Focal cerebral arteriopathy (FCA) of childhood is not a contraindication. |
| Neuro-imaging related exclusions: |
| Intracranial hemorrhage (HI-1, HI-2, PH-1 or PH-2) on pretreatment head MRI or head CT |
| Intracranial dissection (defined as at or distal to the opthalmic artery) |
| Large infarct volume, defined by the finding of acute infarct on MRI involving 1/3 or or more of the complete MCA territory involvement |
| Drug Related exclusions: |
| Known allergy to recombinant tissue plasminogen activator |
| Patient on anticoagulation therapy must have INR ≤ 1.4 |
| Patient who received heparin within 4 hours must have aPTT in normal range |
| LMWH within past 24 hours (aPTT and INR will not reflect LMWH effect) |
Assays of free tPA activity, tPA antigen and plasminogen activator inhibitor-1 level were planned to determine tPA pharmacokinetics in children. Neuroimaging consisting of diagnostic and follow-up imaging obtained 24 hours following completion of the tPA dose was to be sent to the Imaging Core for review.
The clinical course following tPA treatment was to have been followed carefully. Patients were to be scored on the pediatric version of the NIH Stroke Scale (PedNIHSS)32 at 2 hours, 12 hours, 24 hours, 36 hours, 48 hours, 7 days (or day of discharge if earlier) and at 3 months post-tPA administration. In addition, the Pediatric Stroke Outcome Measure (PSOM)33,34 was to have been used at 7 days, and 3 months post-intervention. Finally, the King's Outcome Scale for Childhood Head Injury (KOSCHI)35 and the Pediatric Evaluation of Disability Inventory (PEDI)36 were planned at 3 months post-intervention.
Statistical methods
Dose limiting toxicity (DLT) was defined as presence within 36 hours of tPA administration of any of the following: (1) type 2 parenchymal hemorrhage (involving > 30% of the infarcted area; PH2)37,38 or worse, regardless of whether or not it was associated with clinical deterioration; (2) any intracranial hemorrhage accompanied by neurological deterioration; or (3) any hemorrhage resulting in the need for transfusion, discontinuation of study drug, surgical evacuation of hemorrhage, or death. The target acceptable probability of dose-limiting toxicity was set at 10%. The maximal tolerated dose (MTD) was defined as the dose tier for which the estimated probability of dose-limiting toxicity is closest to 10% among the evaluated tiers for which the likelihood of having unacceptably high toxicity does not exceed 90%.
The study design was based on the Bayesian method of toxicity probability intervals31 to guide the dose-modification process and the selection of the MTD among the three candidate dose tiers, with the entire process performed separately in two age group strata (2-10 years and 11-17 years). The final design is described below in short, with additional details of the parameter settings, dose-modification and final MTD estimation plans described in the on-line supplement. [Please see supplementary material on line http://stroke.ahajournals.org]
Up to 18 patients per stratum were expected to be recruited in cohorts of three starting at the lowest dose tier. The dose for each subsequent cohort chosen was based on the number of DLTs observed in all patients treated at the current dose using the cutoffs shown in Table 2 (Cutoffs for dose modification decisions). For example, if 6 patients (i.e. two cohorts) have already been treated at a given dose, then the next three patients would be enrolled at the next higher dose if no patients had a DLT, at the same dose if 1 patient had a DLT, and at the next lower dose if 2 or more patients had DLTs. In the latter case the dose will also be declared to have unacceptably high toxicity and the tier was to have been closed for any future enrollment. If the table called for escalation beyond the highest candidate tier or to a tier that had been previously closed due to excessive toxicity, then the dose would have been maintained at the current tier. If the lowest candidate tier was closed due to excessive toxicity, the study arm would have been stopped and no MTD selected. Otherwise, after 18 treated patients the MTD was to have been estimated. The design had a provision of enrolling up to 6 additional patients to ensure that the dose tier selected as the MTD had been evaluated in at least 6 patients.
Table 2.
Cutoffs for dose modification decisions
| Cumulative number of patients at the dose | Cumulative number of DLTs for each decision | ||
|---|---|---|---|
| Escalate to next tier | Stay at current tier | De-escalate and close tier | |
| 3 | 0 | 1 | 2+ |
| 6 | 0 | 1 | 2+ |
| 9 | 0 | 1-2 | 3+ |
| 12 | 0-1 | 2 | 3+ |
| 15 | 0-1 | 2-3 | 4+ |
| 18 | n/a | n/a | 4+ |
Results
At the study's official start date, April 20, 2012, 22 primary sites were working towards study start-up. Of the 22 sites, 14 (63%) were activated at the time of study closure (12/21/2013). On average, these sites required 10 months to prepare for patient enrollment. While several obstacles were encountered during the site start-up period, site preparation to diagnose and treat patients immediately constituted a formidable challenge the response to which has been reported previously.30 Human subjects approval was often slow. The cost of study start-up at individual sites was an ongoing challenge, the most frequent of which was cost for tPA storage and immediately available preparation at study site pharmacies. At several institutions, the pK sample processing could only be done by a research support lab during weekdays, requiring study site teams to make provision to process samples outside of these times which included site-mandated training and certification to work in a study lab. Other challenges faced by sites included resolution of feasibility issues, dearth of dedicated research coordinator support, site requirements to establish data transfer agreements with Data Cores, and change in site principal investigator.
Of the twenty-two initially identified primary sites, 6 (27%) were unable to complete the start-up process for various reasons including feasibility, indemnity issues (international sites), or lack of dedicated coordinator time. In response to dropped sites, the TIPS Steering Committee instituted rolling site recruitment for participation in the TIPS study. Twelve additional sites were selected and at the time of study closure three had been activated, bringing the total number of activated sites to 17.
The 17 activated patient enrollment sites demonstrated the ability to triage and diagnose stroke promptly, obtain urgent diagnostic neuroimaging, and treat potential complications of stroke and thrombolysis, including coagulopathy, intensive care, neurosurgical emergencies. In addition, each had institutional-based guidelines for the evaluation and treatment of children who presented with acute AIS. Sites were active for a mean of 9 months (median 9.5 months, range 2-15 months). One child was enrolled but not treated due to a complication following extubation prior to tPA administration. Another 7 sites had completed preparation and were to be activated in January, 2014. The study was closed in December 2013 by the sponsor (NINDS) for lack of recruitment.
A total of 93 children aged 2-17 years were screened for TIPS, defined as a patient considered a possible candidate for the study based on the information provided to the study PI at initial contact (Figure 1). Almost half (43/93 or 46%) of children screened for possible acute stroke had a confirmed AIS. The remainder (50/93 or 44%) had a stroke mimic. Among children with confirmed stroke, 21 had a medical contraindication to receiving tPA including moyamoya arteriopathy (5 patients), anticoagulation treatment (5 patients), malignancy (3 patients), recent stroke (2 patients, one of whom was anticoagulated). Two patients had sickle cell disease, one of whom had a hemorrhagic infarct and one of whom had a PedNIHSS of 25. Two patients were excluded due to lack of occlusion on arterial imaging. Ten were outside the treatment window but presented within 12 hours, and at least 7 of these presented within approximately 5 hours. One arrived 3.5 hours after documented onset of AIS symptoms, but failed anesthesia, and one missed the window by 15 minutes due to a delay at the scanner. Six children were excluded based on pediatric NIHSS below the study cut-off of 6, ranging from 1-5, prior to revision downward of the PedsNIHSS to 4 or higher for patient inclusion in TIPS. Among children without confirmed stroke on MRI, three-quarters (31/41 or 76%) had a stroke mimic, defined as a child presenting with features compatible with ischemic stroke but subsequently proven by imaging to be caused by something other than arterial ischemic stroke. Patients with moyamoya with presumed TIA but not stroke were included in this category. The remaining 10 had a non-benign mimic, defined as a patient not expected to return to baseline. (Table 3. Characteristics of 93 patients age 2 -17 years screened for TIPS)
Figure 1.
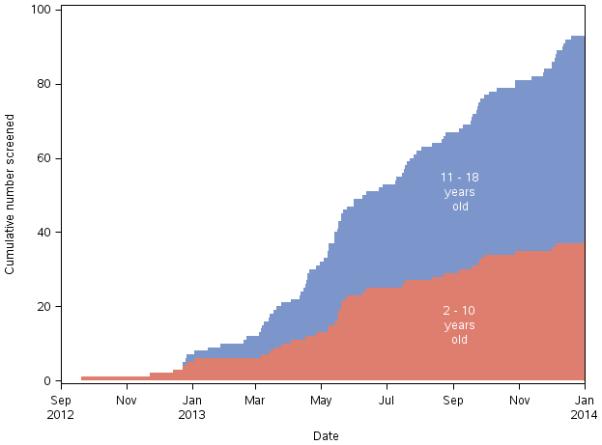
Children screened for TIPS
Table 3.
Characteristics of 93 patients age 2 -17 years screened for TIPS
| Screens | No (%) | Reason patient not enrolled | |
|---|---|---|---|
| 43 (46%) confirmed stroke | 21(22%) | Medical contraindications ± time to presentation > 12 hours | |
| 5(5%) | PedNIHSS < 6 (2,2,3,4, < 6) | ||
| 10(11%) | Diagnosed at 4.5 to 12 hours | ||
| 4(5%) | PedNHSS < 6 and outside time window | ||
| 2(2%) | Without clot visualized | ||
| 1(1%) | Not applicable, child was enrolled | ||
| 50 (54%) stroke mimics | 38(41%) | Benign mimic | |
| 12(13%) | Non-benign mimic | 2-hemorrhagic stroke | |
| 3-mass lesion | |||
| 2-infection | |||
| 2-methotrexate toxicity | |||
| 2-posterior reversible encephalopathy syndrome | |||
Conclusion
The TIPS study arose from two major concerns in the pediatric stroke community. The first comprised safety of tPA administration to children. The second related to the absence of accurate outcome data for use of tPA in childhood stroke. These two key concerns persist. The TIPS study has begun to address the first issue by defining, through expert consensus and collective experience, safety criteria to guide the use of tPA in childhood. These criteria are available (refer to eSupplementary material) and could serve as a guideline for future studies in this area. These criteria still need to be tested, perhaps now in a funded international registry akin to the SITS-MOST39 adult stroke registry in Europe. In the 10 months following study closure by the NINDS, former TIPS sites have continued to identify patients who would have been eligible for TIPS and have treated children with tPA using the TIPS protocol.
The TIPS study did not recruit pediatric ischemic stroke patients rapidly enough for continuation of funding by the NIH. Despite an enthusiastic and increasingly well established pediatric stroke community, the number of children with acute AIS who qualify for thrombolytic treatment within the therapeutic time window of 4.5 hours appears to be small. Even among the screened patients, nearly half had an alternate diagnosis and most of those with ischemic stroke presented a contraindication to treatment. Ultimately, only 1 of 93 screened children was enrolled into the study. It may be that pediatric regional centers for acute stroke evaluation and care would offer the best opportunity for efficient identification, transfer, and diagnosis leading to thrombolytic and other treatments for acute stroke in children. Such a regional center would work with other hospitals in the region to establish channels and protocols for rapid identification and transport of children believed to have acute arterial stroke to the regional primary pediatric stroke center. In such a plan some diagnostic evaluation may best be conducted at the referring hospital. Telemedicine methods may prove helpful. Thrombolytic treatment could in some cases begin during transport to the pediatric primary stroke center.
The difficulties of enrollment in TIPS suggest that establishing an acute stroke program must precede a prospective acute interventional clinical trial in acute childhood stroke. Routine stroke recognition, rapid diagnosis and management are not widespread in the community at large. The insitution of TIPS led to the development and refinement of Primary Pediatric Stroke centers able to provide acute care to children with acute stroke and it is hoped that these guidelines will allow children to be represented in future trials of acute stroke intervention.30 The pediatric stroke community must now continue to work together to ensure that if tPA is given in childhood ischemic stroke, that standard safety protocols are not only established but followed and outcomes are collected and used to guide further recommendations.
Supplementary Material
Acknowledgments
Funding: National Institutes of Health (NIH) grant R01NS065848 “Thrombolysis in Pediatric Stroke (TIPS)”
Footnotes
Disclosures: MDH received study drug (TNK-tPA) from Hoffman La Roche Canada LTD for a stroke clinical trial in 2012-2014.
REFERENCES
- 1.Fullerton HJ, Wu YW, Zhao S, Johnston SC. Risk of stroke in children: ethnic and gender disparities. Neurology. 2003;61:189–94. doi: 10.1212/01.wnl.0000078894.79866.95. [DOI] [PubMed] [Google Scholar]
- 2.Lynch JK, Hirtz DG, DeVeber G, Nelson KB. Report of the National Institute of Neurological Disorders and Stroke workshop on perinatal and childhood stroke. Pediatrics. 2002;109:116–23. doi: 10.1542/peds.109.1.116. [DOI] [PubMed] [Google Scholar]
- 3.Schoenberg BS, Mellinger JF, Schoenberg DG. Moyamoya disease in children. South Med J. 1978;71:237–41. doi: 10.1097/00007611-197803000-00005. [DOI] [PubMed] [Google Scholar]
- 4.De Schryver EL, Kappelle LJ, Jennekens-Schinkel A, Boudewyn Peters AC. Prognosis of ischemic stroke in childhood: a long-term follow-up study. Developmental Medicine and Child Neurology. 2000;42:313–8. doi: 10.1017/s0012162200000554. [DOI] [PubMed] [Google Scholar]
- 5.deVeber GA, MacGregor D, Curtis R, Mayank S. Neurologic outcome in survivors of childhood arterial ischemic stroke and sinovenous thrombosis. Journal of Child Neurology. 2000;15:316–24. doi: 10.1177/088307380001500508. [DOI] [PubMed] [Google Scholar]
- 6.Fullerton HJ, Chetkovich DM, Wu YW, Smith WS, Johnston SC. Deaths from stroke in US children, 1979 to 1998. Neurology. 2002;59:34–9. doi: 10.1212/wnl.59.1.34. [DOI] [PubMed] [Google Scholar]
- 7.Ganesan V, Hogan A, Shack N, Gordon A, Isaacs E, Kirkham FJ. Outcome after ischaemic stroke in childhood. Developmental Medicine and Child Neurology. 2000;42:455–61. doi: 10.1017/s0012162200000852. [DOI] [PubMed] [Google Scholar]
- 8.Lo W, Zamel K, Ponnappa K, Allen A, Chisolm D, Tang M, Kerlin B, et al. The cost of pediatric stroke care and rehabilitation. Stroke. 2008;39:161–5. doi: 10.1161/STROKEAHA.107.497420. [DOI] [PubMed] [Google Scholar]
- 9.Jauch EC, Saver JL, Adams HP, Jr., Bruno A, Connors JJ, Demaerschalk BM, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870–947. doi: 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- 10.Parmar N, Albisetti M, Berry LR, Chan AK. The fibrinolytic system in newborns and children. Clinical Laboratory. 2006;52:115–24. [PubMed] [Google Scholar]
- 11.Amlie-Lefond C, deVeber G, Chan AK, Benedict S, Bernard T, Carpernter J, et al. Use of alteplase in childhood arterial ischaemic stroke: a multicentre, observational, cohort study. Lancet Neurol. 2009;8:530–6. doi: 10.1016/S1474-4422(09)70106-1. [DOI] [PubMed] [Google Scholar]
- 12.Janjua N, Nasar A, Lynch JK, Qureshi AI. Thrombolysis for ischemic stroke in children: data from the nationwide inpatient sample. Stroke. 2007;38:1850–4. doi: 10.1161/STROKEAHA.106.473983. [DOI] [PubMed] [Google Scholar]
- 13.Monagle P, Chan AK, Goldenberg NA, Ichord RN, Journeycake JM, Nowak-Göttl U, et al. Antithrombotic therapy in neonates and children: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e737S–801S. doi: 10.1378/chest.11-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roach ES, Golomb MR, Adams R, Biller J, Daniels S, deveber G, et al. Management of stroke in infants and children: a scientific statement from a Special Writing Group of the American Heart Association Stroke Council and the Council on Cardiovascular Disease in the Young. Stroke. 2008;39:2644–91. doi: 10.1161/STROKEAHA.108.189696. [DOI] [PubMed] [Google Scholar]
- 15.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group Tissue plasminogen activator for acute ischemic stroke. The New England Journal of Medicine. 1995;333:1581–7. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 16.Putaala J, Metso TM, Metso AJ, Mäkelä E, Haapaniemi E, Salonen O, et al. Thrombolysis in young adults with ischemic stroke. Stroke. 2009;40:2085–91. doi: 10.1161/STROKEAHA.108.541185. [DOI] [PubMed] [Google Scholar]
- 17.Dzialowski I, Pexman JH, Barber PA, Demchuk AM, Buchan AM, Hill MD, et al. Asymptomatic hemorrhage after thrombolysis may not be benign: prognosis by hemorrhage type in the Canadian alteplase for stroke effectiveness study registry. Stroke. 2007;38:75–9. doi: 10.1161/01.STR.0000251644.76546.62. [DOI] [PubMed] [Google Scholar]
- 18.Andrew M, Vegh P, Johnston M, Bowker J, Ofosu F, Mitchell L. Maturation of the hemostatic system during childhood. Blood. 1992;80:1998–2005. [PubMed] [Google Scholar]
- 19.Monagle P, Barnes C, Ignjatovic V, Furmedge J, Newall F, Chan A, et al. Developmental haemostasis. Impact for clinical haemostasis laboratories. Thromb Haemost. 2006;95:362–72. doi: 10.1160/TH05-01-0047. [DOI] [PubMed] [Google Scholar]
- 20.Siegbahn A, Ruusuvaara L. Age dependence of blood fibrinolytic components and the effects of low-dose oral contraceptives on coagulation and fibrinolysis in teenagers. Thrombosis and haemostasis. 1988;60:361–4. [PubMed] [Google Scholar]
- 21.Monagle P, Chan AK, Albisetti M, Vegh P, Andrew M, Mitchell L. Fibrinolytic system in adolescents: response to venous occlusion stress tests. Pediatric Research. 2003;53:333–7. doi: 10.1203/01.PDR.0000047644.97422.F2. [DOI] [PubMed] [Google Scholar]
- 22.Evans WE, Relling MV, de Graaf S, Rodman JH, Pieper JA, Christensen ML, et al. Hepatic drug clearance in children: studies with indocyanine green as a model substrate. J Pharm Sci. 1989;78:452–6. doi: 10.1002/jps.2600780605. [DOI] [PubMed] [Google Scholar]
- 23.Strolin BBE. Drug metabolism and disposition in children. Fundam Clin Pharmacol. 2003;17:281–99. doi: 10.1046/j.1472-8206.2003.00140.x. [DOI] [PubMed] [Google Scholar]
- 24.Murry DJ, Crom WR, Reddick WE, Bhargava R, Evans WE. Liver volume as a determinant of drug clearance in children and adolescents. Drug Metabolism and Disposition: the Biological Fate of Chemicals. 1995;23:1110–6. [PubMed] [Google Scholar]
- 25.McGlennan C, Ganesan V. Delays in investigation and management of acute arterial ischaemic stroke in children. Developmental Medicine and Child Neurology. 2008;50:537–40. doi: 10.1111/j.1469-8749.2008.03012.x. [DOI] [PubMed] [Google Scholar]
- 26.Rafay MF, Pontigon AM, Chiang J, Adams M, Jarvis DA, Silver F, et al. Delay to diagnosis in acute pediatric arterial ischemic stroke. Stroke. 2009;40:58–64. doi: 10.1161/STROKEAHA.108.519066. [DOI] [PubMed] [Google Scholar]
- 27.Gabis LV, Yangala R, Lenn NJ. Time lag to diagnosis of stroke in children. Pediatrics. 2002;110:924–8. doi: 10.1542/peds.110.5.924. [DOI] [PubMed] [Google Scholar]
- 28.Braun KP, Kappelle LJ, Kirkham FJ, Deveber G. Diagnostic pitfalls in paediatric ischaemic stroke. Developmental Medicine and Child Neurology. 2006;48:985–90. doi: 10.1017/S0012162206002167. [DOI] [PubMed] [Google Scholar]
- 29.Srinivasan J, Miller SP, Phan TG, Mackay MT. Delayed recognition of initial stroke in children: need for increased awareness. Pediatrics. 2009;124:e227–34. doi: 10.1542/peds.2008-3544. [DOI] [PubMed] [Google Scholar]
- 30.Bernard TJ, Rivkin MJ, Scholz K, deVeber G, Kirton A, Gill JC, et al. Emergence of the Primary Pediatric Stroke Center: Impact of the Thrombolysis in Pediatric Stroke Trial. Stroke. 2014;45:2018–2023. doi: 10.1161/STROKEAHA.114.004919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ji Y, Li Y, Nebiyou Bekele B. Dose-finding in phase I clinical trials based on toxicity probability intervals. Clin Trials. 2007;4:235–44. doi: 10.1177/1740774507079442. [DOI] [PubMed] [Google Scholar]
- 32.Ichord RN, Bastian R, Abraham L, Askalan R, Benedict S, Bernard TJ, et al. Interrater reliability of the Pediatric National Institutes of Health Stroke Scale (PedNIHSS) in a multicenter study. Stroke. 2011;42:613–7. doi: 10.1161/STROKEAHA.110.607192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lo W, Gordon AL, Hajek C, Gomes A, Greenham M, Anderson V, et al. Pediatric Stroke Outcome Measure: Predictor of Multiple Impairments in Childhood Stroke. Journal of Child Neurology. 2013;29:1524–1530. doi: 10.1177/0883073813503186. [DOI] [PubMed] [Google Scholar]
- 34.Kitchen L, Westmacott R, Friefeld S, MacGregor D, Curtis R, Allen A, et al. The pediatric stroke outcome measure: a validation and reliability study. Stroke. 2012;43:1602–8. doi: 10.1161/STROKEAHA.111.639583. [DOI] [PubMed] [Google Scholar]
- 35.Calvert S, Miller HE, Curran A, Hameed B, McCarter R, Edwards RJ, et al. The King's Outcome Scale for Childhood Head Injury and injury severity and outcome measures in children with traumatic brain injury. Developmental Medicine and Child Neurology. 2008;50:426–31. doi: 10.1111/j.1469-8749.2008.02061.x. [DOI] [PubMed] [Google Scholar]
- 36.Feldman AB, Haley SM, Coryell J. Concurrent and construct validity of the Pediatric Evaluation of Disability Inventory. Physical Therapy. 1990;70:602–10. doi: 10.1093/ptj/70.10.602. [DOI] [PubMed] [Google Scholar]
- 37.Berger C, Fiorelli M, Steiner T, Schäbitz W, Bozzao L, Bluhmki E, et al. Hemorrhagic transformation of ischemic brain tissue: asymptomatic or symptomatic? Stroke. 2001;32:1330–5. doi: 10.1161/01.str.32.6.1330. [DOI] [PubMed] [Google Scholar]
- 38.Hacke W, Kaste M, Fieschi C, Toni D, Lesaffre E, von Kummer R, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA. 1995;274:1017–25. [PubMed] [Google Scholar]
- 39.Toni D, Lorenzano S, Puca E, Prencipe M. The SITS-MOST registry. Neurological Sciences. 2006;27(Suppl 3):S260–2. doi: 10.1007/s10072-006-0632-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


