Supplemental digital content is available in the text.
Key Words: BENIGN PROSTATIC HYPERPLASIA, LOWER URINARY TRACT SYMPTOMS, NOCTURIA, PHYSICAL ACTIVITY
ABSTRACT
Introduction
Benign prostatic hyperplasia (BPH) and its associated lower urinary tract symptoms (LUTS), including nocturia, are extremely common among middle- and older-age American men. Although studies on physical activity (PA) and prevalent BPH-related outcomes suggest that PA may protect against the development of this common condition, only a few studies have examined the relation between PA and incident BPH-related outcomes and LUTS with mixed findings. In addition, although nocturia is the most commonly reported and most bothersome LUTS in men with or without evidence of BPH, few studies have examined the association of PA and nocturia independent of BPH. The purpose of this analysis was to examine the association of PA with BPH-related outcomes and nocturia in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening trial.
Methods
We examined this association with both prevalent (n = 28,404) and incident (n = 4710) BPH-related outcomes (measured by self-report of physician diagnosis, BPH surgery, finasteride use, and clinical indicators) and nocturia. Poisson regression with robust variance was used to calculate prevalence ratios and relative risks.
Results
PA was weakly positively associated with several prevalent BPH-related outcomes and was strongly inversely associated with prevalent nocturia. In incident analyses, PA was only associated with nocturia. Men who were active ≥1 h·wk−1 were 13% less likely (95% confidence interval, 2%–22%) to report nocturia and 34% less likely (95% confidence interval, 15%–49%) to report severe nocturia as compared with men who reported no PA. The associations were similar for men with and without additional BPH-related outcomes, except for prevalent nocturia, where the association was stronger for men without other BPH-related outcomes.
Conclusions
Combined with other management strategies, PA may provide a strategy for the management of BPH-related outcomes, particularly nocturia.
Benign prostatic hyperplasia (BPH) is a benign condition of the prostate gland characterized histologically by hyperplastic nodules in the periurethral region and transition zone of the prostate and clinically by a palpably enlarged prostate and lower urinary tract symptoms (LUTS) (29,41). These LUTS, which are typically measured by the International Prostate Symptom Score (IPSS, previously known as the American Urological Association Symptom Index (4)), include three irritative or storage symptoms (increased frequency, increased urgency, and nocturia or waking during the night to urinate) and four obstructive or voiding symptoms (poor stream, hesitancy, intermittency, and feeling of incomplete bladder emptying) (29). Irritative and obstructive LUTS may have differing pathologies, with irritative symptoms thought to be related to heightened prostate smooth muscle tone and obstructive symptoms to prostate enlargement (29). On the basis of autopsy studies, the underlying pathology of BPH and associated LUTS is believed to begin in men as young as their 20s or 30s (5,17), such that by the time men reach their 60s, approximately half are believed to have prevalent BPH (29).
In addition to BPH, LUTS may also be related to other underlying conditions. For instance, nocturia, the most common and bothersome LUTS in men, can relate to a broader overproduction of urine, increase in nighttime urine production, bladder storage or capacity problems, sleep disturbance, or a combination (13,14). The causes of nocturia are multifactorial for most and may remain unclear in a significant number of patients (14). Nocturia increases with age and is estimated to occur in over 50% of men over the age of 45 (regardless of BPH status) (21,37). It is also associated with numerous adverse health outcomes including depression and poor sleep (6,8,21).
Physical activity (PA) has myriad health benefits, which may also extend to protection against BPH and LUTS development and progression. PA has been proposed to protect against BPH/LUTS by several possible mechanisms, including reducing body size, decreasing sympathetic nervous system activity (27,36), and lowering levels of systemic inflammation (3,39). Consistent with this hypothesis, several studies have observed protective findings between PA and BPH-related outcomes (e.g., physician diagnosis of BPH, BPH surgery, BPH medication use, and clinical indicators) and LUTS (23,39). However, most of these studies included men who had either a diagnosis of BPH or LUTS at the time of PA assessment (9,10,12,15,19,20,22,25,27,30,32,33,40), raising concerns about the influence of recall bias (i.e., men may have reported their PA differently after diagnosis of BPH or LUTS onset) and reverse causation (i.e., men may have reduced their PA because of LUTS) to their findings. Considerably fewer studies have examined the possible relation between PA and incident disease without concerns of recall bias and reverse causation. Findings from these few incident analyses have been equivocal, with no association observed in one (18) and inverse or suggestively inverse association in the other two (24,42), although only crude results were presented in the study that observed suggestive results (42). With respect to earlier-life PA, which may potentially protect against initiation of BPH or LUTS in men during their 20s and 30s, only a few studies, to our knowledge, have addressed this question. These studies generally observed variable findings (9,11,19), and all were limited to prevalent disease.
Nocturia has not been well studied in relation to lifestyle interventions separate from other LUTS. Only one study, to our knowledge (38), has explicitly studied this in men. In addition, studies on nocturia among men tend to not distinguish nocturia concurrent with BPH-related outcomes from nocturia that occurs in the absence of other BPH-related outcomes (21). Understanding whether BPH-related nocturia (or other LUTS) shares common risk factors with non-BPH-related nocturia may also help target interventions.
Therefore, studies focusing on PA in early and late adulthood in relation to incident BPH-related outcomes and LUTS, including nocturia, are necessary for the targeting of appropriate and evidence-based intervention strategies.
MATERIALS AND METHODS
The Prostate, Lung, Colorectal, and Ovarian Cancer (PLCO) Screening Trial is a large ongoing clinical trial designed to investigate the effects of prostate, lung, colorectal, and ovarian cancer screening on cancer-specific mortality. Its methods are well described (28). Human Subjects’ approval was obtained at each screening center, and each participant provided an informed consent. Briefly, from 1993 to 2001, 76,705 men were recruited into PLCO at 10 screening centers across the United States. Men 55–74 yr of age with no reported history of prostate cancer or prostatectomy were eligible for the trial. Men who had used finasteride in the previous 6 months were not eligible. At baseline, participants in both arms of the trial completed a questionnaire, which included questions on lifestyle and BPH-related outcomes and nocturia, the most common LUTS among men (21). Participants in the intervention arm also completed a food frequency and PA questionnaire and underwent a baseline prostate-specific antigen (PSA) test and digital rectal examination (DRE). During follow-up, participants completed brief annual study update questionnaires to update their cancer information and provide information on finasteride use. Participants in the intervention arm also underwent additional five annual PSA tests and three annual DRE. Finally, in 2006–2008, participants completed a supplemental questionnaire that included additional BPH-related outcomes, nocturia, and PA questions. Only men in the intervention arm were included in this analysis because only these men provided complete baseline information on PA.
PA
On the baseline questionnaire, intervention participants were asked to report their current levels of “vigorous activities, such as swimming, brisk walking, etc.” and their levels at 40 yr of age in categories of hours per week (none, <1, 1, 2, 3, and ≥4 h·wk−1). The specific question used in PLCO is associated with many expected factors (e.g., lower risk of postprostatectomy incontinence (41), higher fruit, vegetable (17), and energy intake (37), and vitamin D (6)), indicating that it has strong criterion validity. In our analysis, we considered the effects of each level of PA from none through ≥4 h·wk−1 and the effects of PA ≥1 h·wk−1 to more closely align with current PA recommendations.
The supplemental questionnaire included more detailed PA assessment. Participants were asked to report their typical frequency in the past year of PA “strenuous enough to work up a sweat or to increase [their] breathing and HR to very high levels” (none or <1, 2–3, 4–5, or 6–7 d·wk−1) and the duration of each session (none or <15, 16–19, 20–29, 30–39, or ≥40 min). The same questions were asked for moderate PA (“worked up a light sweat or increased [their] breathing and HR to moderately higher levels”). We converted responses to these four questions into the same categories of PA per week (moderate and strenuous combined) as on the baseline questionnaire by 1) assigning the midpoint of each category of frequency and duration for both strenuous and moderate PA to each participant, 2) calculating the total amount of time spent engaged in strenuous and moderate PA per week as the sum of the products of weekly frequency and duration of both strenuous and moderate PA, and 3) dividing this sum into the categories of weekly moderate and vigorous PA used on the baseline questionnaire.
Prevalent BPH-related outcomes
BPH is difficult to measure in epidemiologic studies because no universally accepted definition suitable for use in large population-based studies exists. Therefore, most studies collect several BPH- and LUTS-related measures and use these measures to examine individual aspects of BPH and LUTS, and/or overall BPH/LUTS, on the basis of a composite definition. Because of its large sample size and broad disease focus, PLCO did not collect as many BPH/LUTS-related measures as those in other studies, particularly those that are smaller or urology or treatment focused. However, PLCO did still collect a wide range of BPH/LUTS-related measures that together capture many facets of BPH/LUTS, including prostate enlargement, nocturia, and severity of symptoms (e.g., finasteride use).
The presence of BPH-related outcomes at baseline was determined among men without prostate cancer on the baseline screen using information obtained from the baseline PSA test, DRE, and questionnaire (Table 1). PSA concentration was recorded for each PSA test, and PSA values >1.4 ng·mL−1 were considered indicative of BPH-related PSA elevation in men without history of BPH surgery (36). Although PSA is not specific for BPH, it has been found to be significantly correlated with other non–cancer-related BPH/LUTS measures, such as ultrasound-determined prostate volume (r = 0.54 (39)), maximum flow rate (r = −0.29 (39)), and, more importantly, long-term outcomes of BPH/LUTS, including future prostate growth, (30) acute urinary retention, and BPH surgery in men without prostate cancer (31).
TABLE 1.
Prevalent and incident BPH-related outcomes and nocturia definitions.
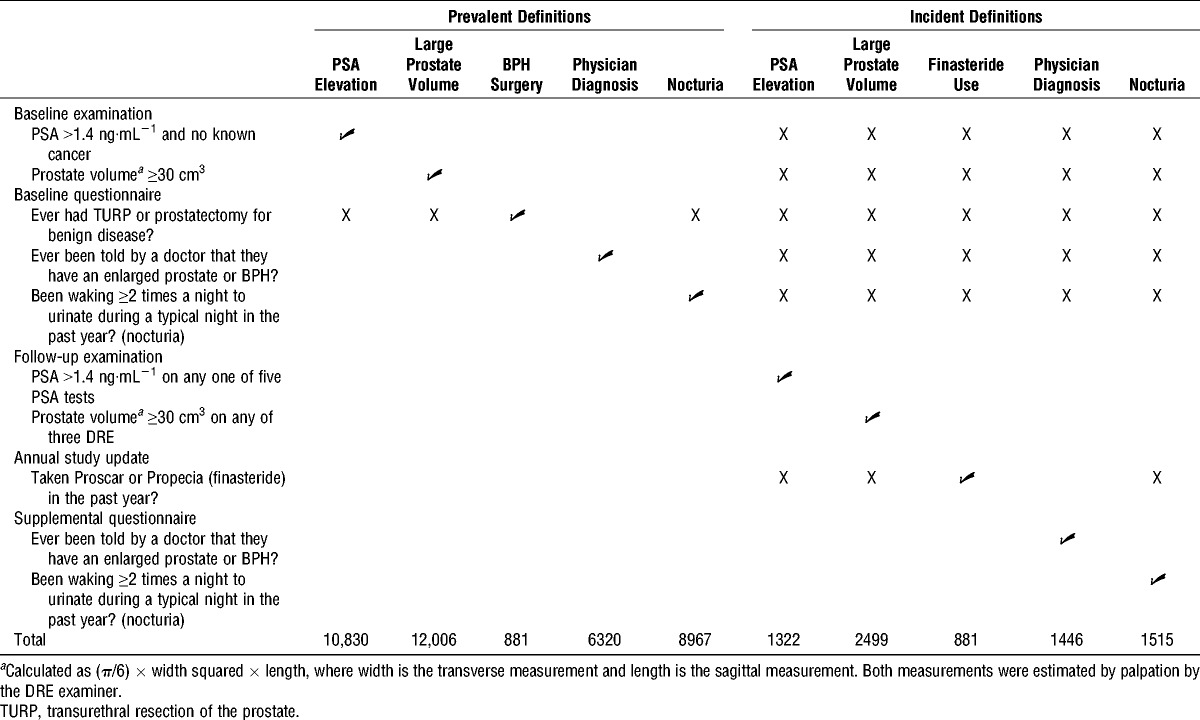
Prostate volume was derived from transverse (width) and sagittal (length) prostate measurements estimated by trained DRE examiners and was calculated using the following formula: volume = (π/6) × width squared × length. To reduce measurement error, only values from examiners who had performed at least 100 DRE were included and measures were corrected for examiner bias (i.e., some examiners routinely estimate higher or lower than other examiners) (26). Although prostate volume is typically estimated by transrectal ultrasound in clinical settings where prostate imaging is available, this type of invasive imaging could not be performed on all approximately 38,000 in the invention arm of PLCO. Therefore, we chose instead to use corrected DRE-estimated volume because it has been found to be associated with all expected variables in PLCO, as follows: age, PSA, transrectal ultrasound-determined prostate volume (only available for 653 men who underwent prostate biopsy) (26), race (unpublished data), and obesity (in preparation). Volumes ≥30 cm3 were considered indicative of BPH-related outcomes in men without history of BPH-related surgery.
Finally, on the baseline questionnaire, three BPH-related outcome items were included, as follows: history of surgical procedures of the prostate, including transurethral resection of the prostate and prostatectomy for benign disease, history of a physician diagnosis of “an enlarged prostate or benign prostatic hypertrophy,” and nocturia in the past year. Nocturia, the most common and bothersome LUTS (1), is highly predictive of moderate and severe LUTS as assessed by the full IPSS minus nocturia (r = 0.63–0.67) (4). Nocturia was defined as waking ≥2 times during the night to urinate (2), and severe nocturia was defined as waking ≥3 times during the night in men without history of BPH-related surgery.
Incident BPH-related outcomes
Similar to prevalent BPH-related outcomes at baseline, we combined follow-up examination and supplemental questionnaire data to indicate incident BPH-related outcomes and nocturia (Table 1). PSA levels were obtained and DRE were performed as on the baseline examination. Incident PSA elevation was defined as a PSA value >1.4 ng·mL−1 on any PSA test after baseline, and incident large prostate volume was defined as an estimated prostate volume ≥30 cm3 on any follow-up DRE. Both definitions were limited to men with no reported finasteride (Proscar or Propecia) use during follow-up, which was queried on each annual study update questionnaire. Incident finasteride use was defined as a report of finasteride use on any of these annual study update questionnaires. Finally, on the supplemental questionnaire, two sets of BPH-related outcome questions were included. Participants were asked to report a physician diagnosis of an enlarged prostate or BPH and nocturia in the past year. We used this information to define incident physician diagnosis of an enlarged prostate/BPH, incident nocturia (waking ≥2 times during the night to urinate (2)), and incident severe nocturia (waking ≥ 3 times during the night). Nocturia definitions were limited to men with no reported finasteride use.
Analysis of prevalent BPH-related outcomes
After limiting the analysis of prevalent BPH-related outcomes to men in the intervention arm (n = 38,340), we further excluded participants who 1) reported history of cancer (except basal or squamous-cell skin cancer) at baseline (n = 827), 2) were diagnosed with prostate cancer on the baseline prostate cancer screen to avoid including men who may have reported BPH-related outcomes because of prevalent, possibly advanced stage prostate cancer (n = 609), 3) did not complete the baseline questionnaire (n = 887), 4) provided incomplete information on BPH-related outcomes or nocturia (n = 64), 5) missed or had an invalid baseline PSA test or DRE result among those who did not report BPH surgery (n = 3023), 6) did not complete the baseline food frequency and PA questionnaire (n = 3997), and 7) did not provide complete information on PA and body mass index (BMI) (n = 539). After these exclusions, 28,404 men remained in the prevalent analysis.
Analysis of incident BPH-related outcomes
For our investigation of incident BPH-related outcomes and nocturia, we further excluded men who 1) had any evidence of BPH-related outcomes or nocturia at baseline (n = 21,927), 2) were diagnosed with prostate cancer before completion of the supplemental questionnaire because prostate cancer or its treatment may alter the risk of BPH-related outcomes and LUTS (e.g., PSA elevation, prostate enlargement, and nocturia, n = 101), 3) did not complete the supplemental questionnaire (n = 1556), 4) provided incomplete information on BPH-related outcomes or nocturia on the supplemental questionnaire (n = 84), and 5) did not have either a valid baseline PSA test result and at least one valid follow-up PSA test result or a valid baseline DRE result and at least one valid follow-up DRE result among those who did not report finasteride use (n = 26). These exclusion criteria resulted in an incident cohort of 4710 men.
Statistical analysis
We investigated associations for PA with prevalent and incident BPH-related outcomes and nocturia by calculating prevalence ratios (PR) and relative risks (RR), respectively, using Poisson regression with robust variance estimation. PR and RR were calculated rather than odds ratios because BPH-related outcomes and nocturia are common outcomes. All regression models included terms for age. Regression models for incident PSA elevation included additional terms for number of PSA tests and time between participants’ first and last PSA test; those for incident large prostate volume included number of DRE and time between their first and last DRE, and those for incident self-reported BPH-related outcomes and nocturia included follow-up time to account for differing time of enrollment. We investigated as potential confounders variables associated with access to and use of medical care (race, education, marital status, and prevalence of chronic conditions including hypertension, CHD, stroke, emphysema, bronchitis, diabetes, arthritis, osteoporosis, hepatitis, cirrhosis, diverticulitis, colon polyps, and gallbladder disease), variables found to be associated with prevalent or incident BPH-related outcomes/nocturia in previous PLCO analyses (smoking history, alcohol intake, and nonsteroidal anti-inflammatory drug use (16)), BMI across the life course (baseline BMI, BMI at age 20, and BMI at age 50), and dietary variables found to be associated with prevalent or incident disease in previous studies on BPH-related outcomes and LUTS (intakes of total energy, CHO, protein, fruit, vegetables, red meat, alcohol, fats, polyunsaturated fatty acids, and antioxidant nutrients, including beta-carotene, selenium, vitamins A, C, E, and zinc from the diet and supplements, and dietary alpha-carotene, beta-cryptoxanthin, lutein and zeaxanthin, and lycopene). We also considered mutual adjustment for current PA and PA at age 40.
To investigate possible misclassification of BPH-related outcomes, we performed sensitivity analyses 1) limiting the self-reported analyses to men without other medical conditions with similar symptoms as BPH and 2) limiting the analyses of PSA elevation to men without history of clinical prostatitis. For finasteride, we performed sensitivity analyses limiting the finasteride definition to men who also reported a diagnosis of an enlarged prostate/BPH because finasteride can be used for both BPH (Proscar) and hair loss (Propecia). We also explored alternate definitions for elevated PSA and large prostate volume. For elevated PSA, these definitions were baseline PSA >1.5 ng·mL−1 (prevalent analysis), at least two follow-up or two consecutive follow-up values >1.4 ng·mL−1 (incident analysis), at least two follow-up or two consecutive follow-up values >1.5 ng·mL−1 (incident analysis), and a large change in PSA over follow-up (PSA slope >80th percentile (35)). Alternate ways of defining prevalent large prostate volume were baseline prostate volume ≥40 cm3, uncorrected baseline prostate volume ≥30 cm3, and examiner-noted prostate enlargement on the baseline DRE. Alternate incident definitions were 1) prostate volume ≥30 cm3 on at least two follow-up or two consecutive follow-up DRE, 2) prostate volume ≥40 cm3 on at least two follow-up or two consecutive follow-up DRE, 3) uncorrected prostate volume ≥30 cm3 on at least one or two follow-up DRE, and 4) examiner-noted prostate enlargement on at least one or two follow-up DRE. Results from all sensitivity analyses were similar to those from the main analyses, except where indicated in the Results section.
Finally, because nocturia can be a symptom of other underlying conditions besides BPH (such as bladder dysfunction), we investigated the association of nocturia with PA in men with nocturia but no other evidence of BPH or LUTS separately from men who experienced nocturia and either a physician diagnosis of BPH, elevated PSA, or large prostate volume.
RESULTS
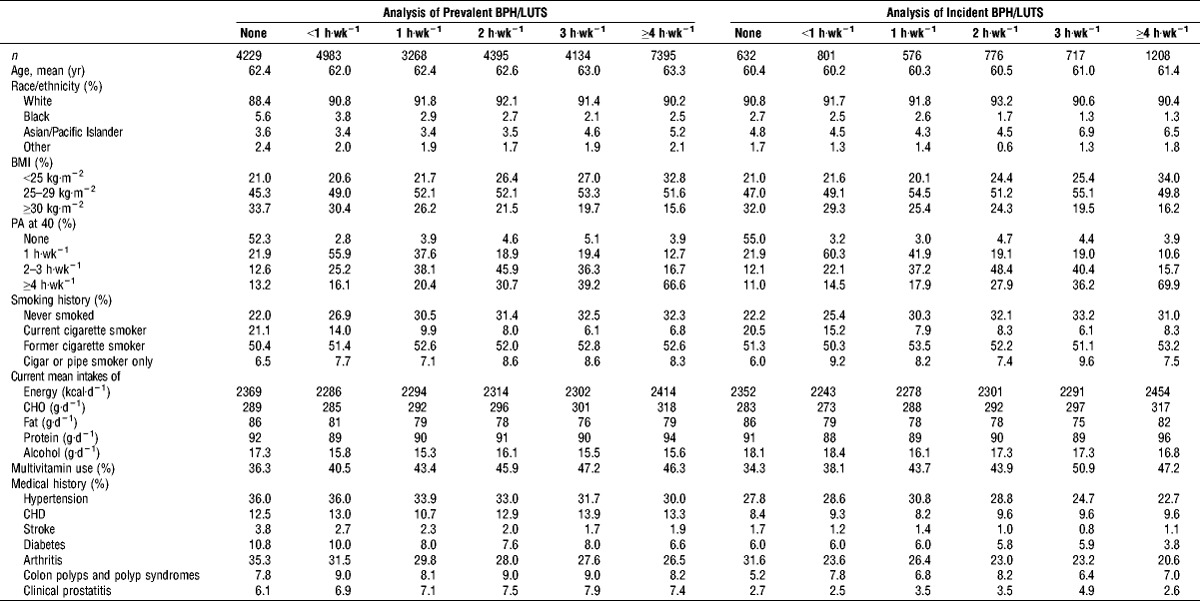
Of 28,404 eligible participants in the prevalent analysis, 4229 (14.9%) reported no current PA, with 7395 (26.0%) men reporting ≥4 h·wk−1. The majority of PLCO participants were White (90.7%), with a mean sample age of 62.7 yr. Most participants were overweight (50.6%) or obese (23.6%) and were former smokers (52.0%) (Table 1). Of the 4710 men in the incident analysis, 632 (13.4%) reported no PA, whereas 1208 (25.6%) reported ≥4 h·wk−1. The characteristics of the sample for the incident analyses were similar to those in the prevalent sample. PA levels did not vary by race. Men who were not active were more likely to be obese, to have been inactive at age 40, and to be current smokers. Inactive men reported higher fat and alcohol intakes and were less likely to take a multivitamin (Table 2). Inactive men were also more likely to report history of hypertension, stroke, diabetes, and arthritis.
TABLE 2.
Age-adjusted baseline characteristics of male participants eligible for the analysis of PA and BPH-related outcomes and nocturia in the intervention arm of the PLCO screening trial by baseline PA, 1993–2001.
Current PA
Prevalent BPH-related outcomes and nocturia
The prevalence of the BPH-related outcomes and nocturia varied, with 881 men reporting BPH surgery, 6320 reporting a physician diagnosis of BPH, 10,830 men having elevated PSA, 12,006 having large prostate volume, 8967 reporting nocturia, and 2411 reporting severe nocturia (Table 1). Of the men with nocturia, 2315 (26%) had nocturia only, with the remaining men reporting nocturia and other BPH-related outcomes. Similarly, 22% of men with severe nocturia had no other BPH-related outcomes. In bivariate analysis, baseline PA was associated with prevalent BPH-related outcomes using several outcome definitions, but the direction of the association varied by outcome definition. PA was positively associated with prevalent BPH-related outcomes when defined as having had BPH surgery; men exercising ≥1 h·wk−1 were 28% more likely (PR, 1.28; 95% confidence interval (CI), 1.05–1.56) to report BPH surgery than men who did not report any PA. There was a significant trend (P = 0.01); men exercising ≥4 h·wk−1 were 30% more likely (PR, 1.30; 95% CI, 1.04–1.61) to report BPH surgery than men who did not report any PA. PA was also associated with weak but statistically significant increase in the prevalence of BPH-related outcomes when defined as having an elevated PSA or a physician diagnosis of BPH (Table 3).
TABLE 3.
PR and 95% CI of prevalent BPH-related outcomes and nocturia by current PA; PLCO screening trial.
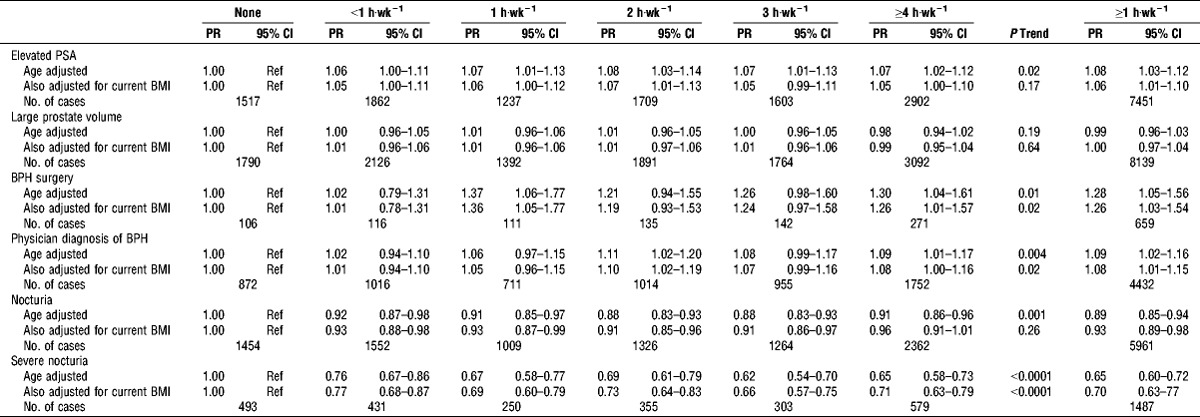
In contrast, PA was inversely associated with prevalent nocturia. Men who reported PA ≥1 h·wk−1 were 35% less likely to report severe nocturia (PR, 0.65; 95% CI, 0.60–0.72). When separating men with nocturia alone from those with other BPH-related outcomes, we found the association was stronger in men with nocturia alone (See Table, Supplemental Digital Content 1, Prevalent nocturia with and without BPH-related outcomes, http://links.lww.com/MSS/A426). Men who reported PA ≥1 h·wk−1 were 18% less likely to report nocturia only (PR, 0.82; 95% CI, 0.74–0.91) and 39% less likely to report severe nocturia only (PR, 0.61; 95% CI, 0.49–0.76; P trend < 0.001) than men who did not report any PA. Among men who reported nocturia and either 1) physician diagnosis of BPH, 2) elevated PSA, or 3) large prostate volume, the association between PA and nocturia was weaker and had a nonsignificant trend when all levels of activity were considered (P trend = 0.17). The association between PA and severe nocturia was similar but to that of men with nocturia only (PR ≥1 h·wk−1, 0.67; 95% CI, 0.60–0.74; P trend < 0.001).
When we adjusted for current BMI, all associations for prevalent outcomes attenuated, although the trends for BPH surgery, a physician diagnosis of an enlarged prostate/BPH, and severe nocturia remained statistically significant. Because none of the other potential confounders meaningfully affected the effect estimates, we present only those adjusted for age and current BMI.
Incident BPH-related outcomes and nocturia
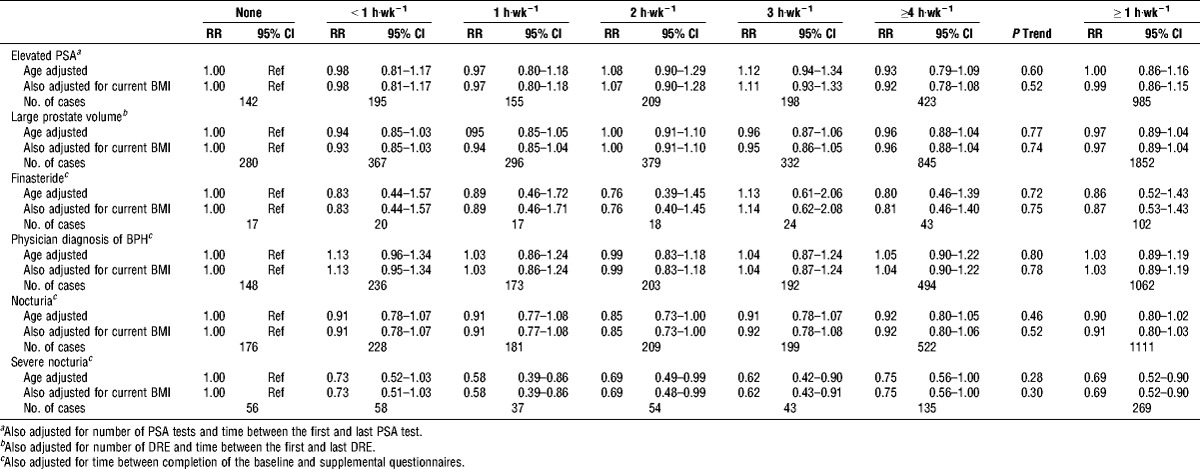
The incidence of the BPH-related outcomes and nocturia varied, with 881 men reporting finasteride use, 1446 reporting a physician diagnosis of BPH, 1322 men having elevated PSA, 2499 having large prostate volume, 1515 reporting nocturia, and 383 reporting severe nocturia. Of the men with nocturia, 24% reported experiencing nocturia only and 76% reported nocturia and at least one other BPH-related outcome. Of the men with severe nocturia, 23% reported experiencing nocturia only and no other BPH-related outcome. Baseline PA was not associated with incident elevated PSA, large prostate volume, finasteride use, or physician diagnosis of BPH (Table 4). It was, however, associated with nocturia. Men who reported PA ≥1 h·wk−1 were 13% (RR, 0.87; 95% CI, 0.78–0.98) less likely to report nocturia and 34% (RR, 0.66; 95% CI, 0.51–0.85) less likely to report severe nocturia than men who were not active, with significant trends across activity level.
TABLE 4.
Risk ratios (RR) and 95% CI of incident BPH-related outcomes and nocturia by current PA; PLCO screening trial.
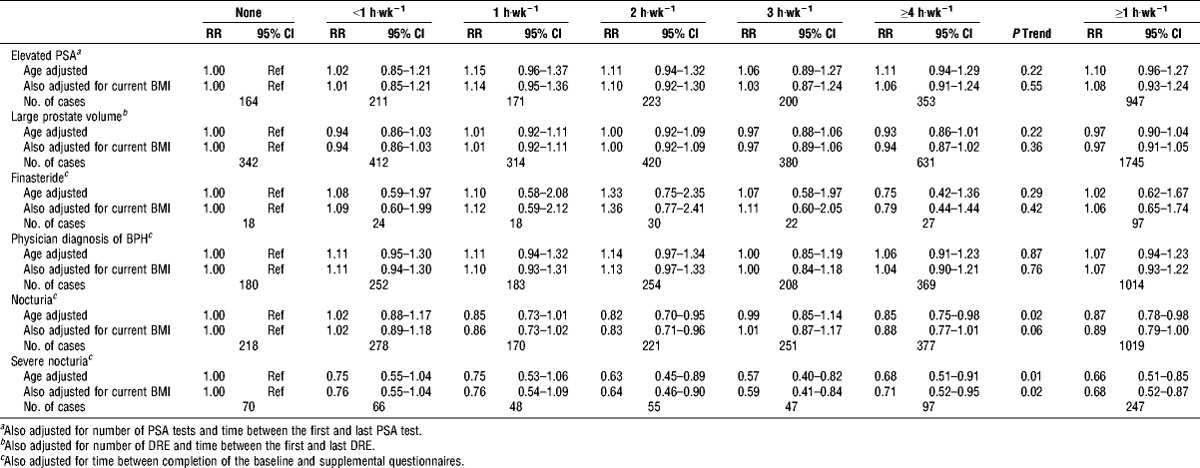
Recognizing the small number of subjects in some analyses, we explored the associations of PA and incident nocturia for men with and without other BPH-related outcomes (Table 5). We found no significant association of PA with nocturia in either group, although the effect estimates were similar in magnitude to each other and to the inverse estimate for nocturia among all men combined. The association of PA with severe nocturia in men with nocturia only was also not significant, whereas the association with severe nocturia in men who reported both incident nocturia and incidence of either 1) physician diagnosis of BPH, 2) elevated PSA, or 3) large prostate volume was significantly inverse; men who reported PA ≥1 h·wk−1 were 32% less likely to experience incident severe nocturia (RR, 0.68; 95% CI, 0.51–0.91; P trend = 0.02).
TABLE 5.
RR and 95% CI of incident nocturia for men with and without other BPH-related outcomes by current PA; PLCO screening trial.
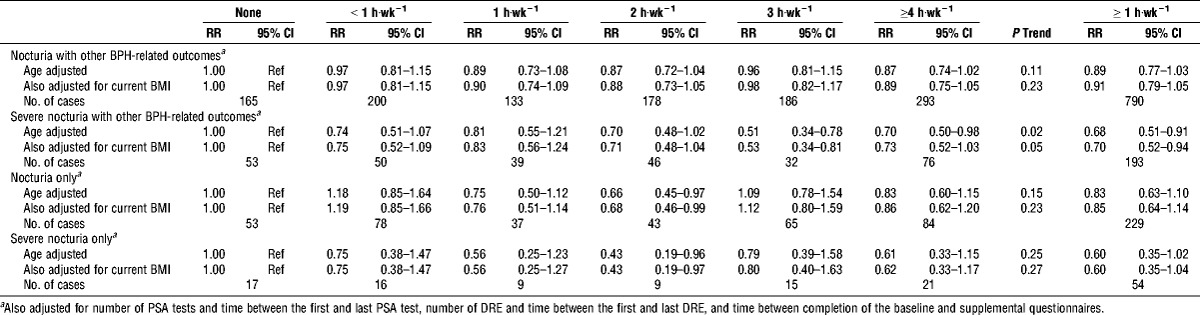
None of the potential confounders, except current BMI, altered the results. Adjusting for current BMI did not significantly change the effect estimate but did result in borderline significant trends for nocturia among all men and severe nocturia among men who reported other BPH-related outcomes.
PA across the Life-span
It has been suggested that consistent engagement in PA may be necessary to alter risk of several health outcomes. In prevalent analyses, PA level at age 40 was not significantly associated with any BPH-related outcome definition (data not shown), with the exception of severe nocturia; men reporting ≥1 h·wk−1 of PA had 15% (95% CI, 5%–25%) lower prevalence of severe nocturia.
In incident analyses, a similar pattern of findings was observed for PA at age 40 as for baseline PA, but none of the findings were statistically significant, except for severe nocturia (Table 6). Men who were active ≥1 h·wk−1 were 31% less likely to experience severe nocturia (RR, 0.69; 95% CI, 0.52–0.90). We also evaluated whether the risk of incident BPH-related outcomes and nocturia differed for men who were active at age 40 and baseline (participating in ≥1 h·wk−1 of PA at each) versus men who were inactive at both times (participating in <1 h·wk−1 of PA at each) (data not shown). The results were consistent with the data for baseline PA; PA change was only associated with the nocturia outcome. Men who were active at both times had lower risk of nocturia (RR, 0.88; 95% CI, 0.78–0.98), those who were active only at baseline had nonsignificantly lower risk of nocturia (RR, 0.89; 95% CI, 0.76–1.06), and those who became inactive had no protection (RR, 1.06; 95% CI, 0.92–1.22) compared with men who were inactive at both times.
TABLE 6.
RR and 95% CI of incident BPH-related outcomes and nocturia by PA at age 40; PLCO screening trial.
We also explored whether men with prevalent nocturia who increased their PA from the baseline questionnaire to the supplemental questionnaire saw a corresponding change in their nocturia over the same period and found no association (data not shown).
DISCUSSION
Although PA has been examined in association with prevalent BPH-related outcomes and LUTS in several studies, few incident analyses exist. Furthermore, few studies have examined the relation between PA and nocturia, the most common and bothersome LUTS among men, independently of BPH. Therefore, our study is one of the first to examine PA in relation to both prevalent and incident BPH-related outcomes as well as in relation to nocturia alone. We found that PA was significantly inversely associated with prevalent nocturia among all men and when separating nocturia concurrent with BPH-related outcomes from independent nocturia. Similar findings were observed for incident nocturia, although our stratified findings were not statistically significant likely because of lower power. PA was not associated with other incident BPH-related outcomes definitions. These data suggest that PA may be protective for the development of nocturia regardless of its underlying pathology. Future studies may also wish to consider exploring nocturia independently of other BPH-related outcomes to investigate the mechanisms underlying nocturia associations.
As noted before, understanding the association with incident BPH-related outcomes, including nocturia, is important for informing potential interventions and clinical care. We investigated incident disease because of typical methodological concerns of recall bias and reverse causation in case–control studies. However, where outcome definitions overlapped, we did not see any differences in the results for prevalent versus incident disease (except for differences in statistical significance due to the very large sample size of the prevalent analysis), suggesting that recall bias and reverse causation are of less concern for prevalent findings of PA and BPH-related outcomes.
In contrast to previous research on prevalent BPH-related outcomes, we found a positive association of PA with prevalent BPH surgery. Although this may be a true association, BPH surgery as an outcome definition has important limitations that should be considered; it is only observed in men with BPH or LUTS who have access to or who physically qualify for surgery. We found only weak associations of PA with the other prevalent BPH-related outcome definitions, except for nocturia, which held in the incident analyses. We did not have data on incident BPH surgery with which to evaluate whether this unexpected finding is a function of it being a prevalent outcome.
Our finding of an inverse association with nocturia is novel yet consistent with the very limited literature in this area. Two previous studies have examined the potential of PA intervention to reduce nocturia. Sugaya et al. (38) enroled 47 elderly men in a walking intervention. Among the 30 men who completed assessment, episodes of nocturia and daytime urinary frequency significantly decreased and nighttime sleep was deeper for 67% of subjects. In a trial of nocturia, which included men and women, Soda et al. (34) found that an intervention of fluid restriction, reduced hours in bed (to improve sleep quality), PA, and keeping warm in bed significantly reduced nocturnal voids and volume. We did not find an improvement in nocturia among men with prevalent nocturia who increased their PA levels over time. However, the association may be more proximal than could be captured by our instruments, which were designed to capture variation over months or years and not days. These findings suggest that PA may be a valuable strategy in the management of nocturia in older men. Our findings are also consistent with the existing literature that has shown variability in the association of PA and BPH-related outcomes with the outcome definition used.
PA has been proposed to protect against BPH-related outcomes and LUTS development by several possible mechanisms, including reducing body size and decreasing sympathetic nervous system activity (27). Individuals who are more physically active have also been found to have lower levels of systemic inflammation (3), which may potentially reduce their risk of BPH-related outcomes. Sugaya et al. (38) hypothesized that the association between PA and nocturia could be due to improved (deeper) sleep (21). If this is indeed the mechanism behind the association, PA may not have a significant effect on BPH/LUTS more broadly because it fails to address the other LUTS associated with the condition but does address nocturia associated with sleep disturbance. This mechanism is consistent with a broader literature indicating that PA improves sleep quality (21). However, as nocturia can be attributed to multiple causes, including those unrelated to BPH, it is possible that the association found is related to those conditions. Sleep disturbance is one such condition. Furthermore, as we did not collect information on other irritative LUTS, it is also possible that our observed association is attributable to other irritative LUTS mechanisms, such as heightened prostate smooth muscle tone, and is not specific to nocturia and sleep disturbance. Therefore, future studies should explore nocturia, and other irritative LUTS, to determine the specificity of our association and to inform underlying mechanisms.
Our results indicate that only recent PA is associated with incident BPH-related outcomes. When examining consistency of activity over time, we found that only men who maintained their PA or who became active by the time of entry into the PLCO trial had lower risk of nocturia; men active at age 40 who became inactive over time had no protection. This suggests that recent PA may be most important for decreasing risk of nocturia. It may also suggest that consistency of PA matters because men who are recently active are often consistently active. Mechanistically, these findings suggest that PA does not prevent the initiation of BPH-related outcomes and LUTS but does lead to reduced symptom experiences that typically drive the diagnosis of BPH/LUTS. Together, these findings suggest that men who have been consistently active may have delayed diagnosis and easier symptom management. The findings also suggest that men who have not been physically active can still benefit from PA interventions, which may help with symptom management and delayed diagnosis.
Our findings should be viewed in light of the limitations of the PA and BPH-related measures used as well as the study design. With respect to PA, although only one question was asked to participants, this question has been found to be associated with many expected factors (e.g., lower risk of postprostatectomy incontinence (41) and higher fruit, vegetable (17), and energy intake (37), and vitamin D (6)), indicating that it captures PA to the degree necessary to identify associations. However, although this instrument has criterion validity, a higher than expected percentage of the men in the study reported being regularly physically active likely because they responded to the “brisk walking” versus “vigorous” aspect of the question. This contrasts with data from nationally representative studies that indicate that the US male population is largely sedentary (7). Overreporting of PA in our sample may result in underestimation of the true effect. As noted previously, the large size and broad scope of the PLCO trial also limited the urology-focused measures included in the study questionnaires and examinations. As a result, we did not have access to prostate imaging, uroflowmetry, or postvoid residual volume measurement and did not collect information on the full IPSS, incident BPH surgery, or use of BPH/ LUTS drugs beyond finasteride. However, the items PLCO did collect are consistently correlated with other more time-consuming, invasive, and/or non–cancer-related measures not collected in PLCO. In addition, although information on incident BPH surgery or alpha-blocker use was not collected in PLCO, these men should still be classified correctly as having developed BPH-related outcomes by our definition on the basis of a physician diagnosis of an enlarged prostate/BPH. In addition, we considered each BPH-related outcome separately so that the limitations of any single outcome variable did not influence the other variables. Similar to many previous studies, our prevalence analyses are limited by the cross-sectional nature of the data; however, the prospective nature of the PLCO trial allowed us to also examine incident outcomes. The limitations in the measures imposed by the parent trial were offset by the novel access to incident data and a large study sample size in this underexplored area.
Our findings are among a small, but growing, literature that distinguishes prevalent from incident BPH-related outcomes and LUTS, such as nocturia. Our study found no significant association for PA with most definitions of BPH-related outcomes, although we did find strong and significant association between physically active lifestyle and nocturia. Future studies in men with BPH-related outcomes and LUTS, and nocturia in particular, should explore this as a potential symptom management strategy, with particular attention to the dose of PA necessary and the mechanisms that might underlie the association.
Supplementary Material
Acknowledgments
This work was supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases, R21DK090595.
The findings presented here were also presented as a poster at the American College of Sports Medicine 2013 Annual Meeting.
The authors have no conflicts to declare.
The results of the present study do not constitute endorsement by the American College of Sports Medicine.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.acsm-msse.org).
REFERENCES
- 1. Abrams P. Nocturia: the major problem in patients with lower urinary tract symptoms suggestive of benign prostatic obstruction (LUTS/BPO). Eur Urol. 2005; 3: 8– 16. [Google Scholar]
- 2. Abrams P, Swift S. Solifenacin is effective for the treatment of OAB dry patients: a pooled analysis. Eur Urol. 2005; 48 (3): 483– 7. [DOI] [PubMed] [Google Scholar]
- 3. Abramson JL, Vaccarino V. Relationship between physical activity and inflammation among apparently healthy middle-aged and older US adults. Arch Intern Med. 2002; 162 (11): 1286– 92. [DOI] [PubMed] [Google Scholar]
- 4. Barry MJ, Fowler FJJ, O apos Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992; 148 (5): 1549– 57, discussion 1564. [DOI] [PubMed] [Google Scholar]
- 5. Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. J Urol. 1984; 132 (3): 474– 9. [DOI] [PubMed] [Google Scholar]
- 6. Brock K, Huang W-Y, Fraser DR, et al. Low vitamin D status is associated with physical inactivity, obesity and low vitamin D intake in a large US sample of healthy middle-aged men and women. J Steroid Biochem Mol Biol. 2010; 121 (1–2): 462– 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention (CDC). Adult participation in aerobic and muscle-strengthening physical activities—United States, 2011. MMWR Morb Mortal Wkly Rep. 2013; 62 (17): 326– 30. [PMC free article] [PubMed] [Google Scholar]
- 8. Coyne KS, Zhou Z, Thompson C, et al. The impact on health-related quality of life of stress, urge and mixed urinary incontinence. BJU Int. 2003; 92 (7): 731– 5. [DOI] [PubMed] [Google Scholar]
- 9. Dal Maso L, Zucchetto A, Tavani A, et al. Lifetime occupational and recreational physical activity and risk of benign prostatic hyperplasia. Int J Cancer. 2006; 118 (10): 2632– 5. [DOI] [PubMed] [Google Scholar]
- 10. Fowke JH, Phillips S, Koyama T, et al. Association between physical activity, lower urinary tract symptoms (LUTS) and prostate volume. BJU Int. 2012; 111 (1): 122– 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fritschi L, Tabrizi J, Leavy J, Ambrosini G, Timperio A. Risk factors for surgically treated benign prostatic hyperplasia in Western Australia. Public Health. 2007; 121 (10): 781– 9. [DOI] [PubMed] [Google Scholar]
- 12. Gann PH, Hennekens CH, Longcope C, Verhoek-Oftedahl W, Grodstein F, Stampfer MJ. A prospective study of plasma hormone levels, nonhormonal factors, and development of benign prostatic hyperplasia. Prostate. 1995; 26 (1): 40– 9. [DOI] [PubMed] [Google Scholar]
- 13. Jin MH, Moon DG. Practical management of nocturia in urology. Indian J Urol. 2008; 24 (3): 289– 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Johnson TM. Nocturia: Clinical presentation, diagnosis, and treatment. Topic 6877 Version 17.0 In: UpToDate. O’Leary MP, Sokol N, editors. Waltham (MA): UpToDate; Available from: http://www.uptodate.com/contents/nocturia-clinical-presentation-diagnosis-and-treatment?source=search_result&search=Nocturia%3A+Clinical+presentation%2C+diagnosis%2C+and+treatment.&selectedTitle=1~150. Accessed 1 May 2014.
- 15. Joseph MA, Harlow SD, Wei JT, et al. Risk factors for lower urinary tract symptoms in a population-based sample of African-American men. Am J Epidemiol. 2003; 157 (10): 906– 14. [DOI] [PubMed] [Google Scholar]
- 16. Kang D, Andriole GL, Van De Vooren RC. Risk behaviours and benign prostatic hyperplasia. BJU Int. 2004; 93 (9): 1241– 5. [DOI] [PubMed] [Google Scholar]
- 17. Kirsh VA, Peters U, Mayne ST, et al. Prospective study of fruit and vegetable intake and risk of prostate cancer. J Natl Cancer Inst. 2007; 99 (15): 1200– 9. [DOI] [PubMed] [Google Scholar]
- 18. Kristal AR, Arnold KB, Schenk JM, et al. Race/ethnicity, obesity, health related behaviors and the risk of symptomatic benign prostatic hyperplasia: results from the prostate cancer prevention trial. J Urol. 2007; 177 (4): 1395– 400, quiz, 1591. [DOI] [PubMed] [Google Scholar]
- 19. Lacey JV, Deng J, Dosemeci M, et al. Prostate cancer, benign prostatic hyperplasia and physical activity in Shanghai, China. Int J Epidemiol. 2001; 30 (2): 341– 9. [DOI] [PubMed] [Google Scholar]
- 20. Lagiou A, Samoli E, Georgila C, et al. Occupational physical activity in relation with prostate cancer and benign prostatic hyperplasia. Eur J Cancer Prev. 2008; 17 (4): 336– 9. [DOI] [PubMed] [Google Scholar]
- 21. Markland AD, Vaughan CP, Johnson TM, Goode PS, Redden DT, Burgio KL. Prevalence of nocturia in United States men: results from the National Health and Nutrition Examination Survey. J Urol. 2011; 185 (3): 998– 1002. [DOI] [PubMed] [Google Scholar]
- 22. Meigs JB, Mohr B, Barry MJ, Collins MM, McKinlay JB. Risk factors for clinical benign prostatic hyperplasia in a community-based population of healthy aging men. J Clin Epidemiol. 2001; 54 (9): 935– 44. [DOI] [PubMed] [Google Scholar]
- 23. Parsons JK, Kashefi C. Physical activity, benign prostatic hyperplasia, and lower urinary tract symptoms. Eur Urol. 2008; 53 (6): 1228– 35. [DOI] [PubMed] [Google Scholar]
- 24. Parsons JK, Messer K, White M, et al. Obesity increases and physical activity decreases lower urinary tract symptom risk in older men: the Osteoporotic Fractures in Men study. Eur Urol. 2011; 60 (6): 1173– 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Penson DF, Munro HM, Signorello LB, Blot WJ, Fowke JH, Urologic Diseases in America Project. Obesity, physical activity and lower urinary tract symptoms: results from the Southern Community Cohort Study. J Urol. 2011; 186 (6): 2316– 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pinsky PF, Kramer BS, Crawford ED, et al. Prostate volume and prostate-specific antigen levels in men enrolled in a large screening trial. Urology. 2006; 68 (2): 352– 6. [DOI] [PubMed] [Google Scholar]
- 27. Platz EA, Kawachi I, Rimm EB, et al. Physical activity and benign prostatic hyperplasia. Arch Intern Med. 1998; 158 (21): 2349– 56. [DOI] [PubMed] [Google Scholar]
- 28. Prorok PC, Andriole GL, Bresalier RS, et al. Design of the prostate, lung, colorectal and ovarian (PLCO) cancer screening trial. [Internet]. Controlled Clinical Trials. 2000; 21 (0197–2456 (Print)): 273S– 309S Available from: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=11189684&retmode=ref&cmd=prlinks. [DOI] [PubMed] [Google Scholar]
- 29. Roehrborn CG, McConnell JD. Benign prostatic hyperplasia: Etiology, pathophysiology, epidemiology, and natural history. In: Wein AJ, Kavoussi LR, editors. Campbell-Walsh Urology. Philadelphia (PA): Saunders Elsevier; 2007. p. 2649– 73. [Google Scholar]
- 30. Roehrborn CG, McConnell JD, Bonilla J, et al. Serum prostate specific antigen is a strong predictor of future prostate growth in men with benign prostatic hyperplasia. PROSCAR long-term efficacy and safety study. J Urol. 2000; 163 (1): 13– 20. [PubMed] [Google Scholar]
- 31. Roehrborn CG, McConnell JD, Lieber M, et al. Serum prostate-specific antigen concentration is a powerful predictor of acute urinary retention and need for surgery in men with clinical benign prostatic hyperplasia. PLESS Study Group. Urology. 1999; 53 (3): 473– 80. [DOI] [PubMed] [Google Scholar]
- 32. Rohrmann S, Crespo CJ, Weber JR, SMIT E, Giovannucci E, Platz EA. Association of cigarette smoking, alcohol consumption and physical activity with lower urinary tract symptoms in older American men: findings from the third National Health And Nutrition Examination Survey. BJU Int. 2005; 96 (1): 77– 82. [DOI] [PubMed] [Google Scholar]
- 33. Safarinejad MR. Prevalence of benign prostatic hyperplasia in a population-based study in Iranian men 40 years old or older. Int Urol Nephrol. 2008; 40 (4): 921– 31. [DOI] [PubMed] [Google Scholar]
- 34. Soda T, Masui K, Okuno H, Terai A, Ogawa O, Yoshimura K. Efficacy of nondrug lifestyle measures for the treatment of nocturia. J Urol. 2010; 184 (3): 1000– 4. [DOI] [PubMed] [Google Scholar]
- 35. St. Sauver JL, Jacobson DJ, Girman CJ, Lieber MM, McGree ME, Jacobsen SJ. Tracking of longitudinal changes in measures of benign prostatic hyperplasia in a population based cohort. J Urol. 2006; 175 (3 Pt 1): 1018–22–discussion 1022. [DOI] [PubMed] [Google Scholar]
- 36. St. Sauver JL, Jacobson DJ, McGree ME, Lieber MM, Jacobsen SJ. Protective association between nonsteroidal antiinflammatory drug use and measures of benign prostatic hyperplasia. Am J Epidemiol. 2006; 164 (8): 760– 8. [DOI] [PubMed] [Google Scholar]
- 37. Sue LY, Schairer C, Ma X, et al. Energy intake and risk of postmenopausal breast cancer: an expanded analysis in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO) cohort. Cancer Epidemiol Biomarkers Prev. 2009; 18 (11): 2842– 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sugaya K, Nishijima S, Owan T, Oda M, Miyazato M, Ogawa Y. Effects of walking exercise on nocturia in the elderly [Internet]. Biomed. Res. 2007; 28 (2): 101– 5 Available from: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=17510495&retmode=ref&cmd=prlinks. [DOI] [PubMed] [Google Scholar]
- 39. Vesely S, Knutson T, Damber JE, Dicuio M, Dahlstrand C. Relationship between age, prostate volume, prostate-specific antigen, symptom score and uroflowmetry in men with lower urinary tract symptoms. Scand J Urol Nephrol. 2003; 37 (4): 322– 8. [DOI] [PubMed] [Google Scholar]
- 40. Williams PT. Effects of running distance and performance on incident benign prostatic hyperplasia. Med Sci Sports Exerc. 2008; 40 (10): 1733– 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wolin KY, Luly J, Sutcliffe S, Andriole GL, Kibel AS. Risk of urinary incontinence following prostatectomy: the role of physical activity and obesity. J Urol. 2011; 183 (2): 629– 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wong SY, Woo J, Leung JC, Leung PC. Depressive symptoms and lifestyle factors as risk factors of lower urinary tract symptoms in Southern Chinese men: a prospective study. Aging Male. 2010; 13 (2): 113– 9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


