Abstract
Introduction:
The efficacy of epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors in EGFR mutation-positive non–small-cell lung cancer (NSCLC) patients necessitates accurate, timely testing. Although EGFR mutation testing has been adopted by many laboratories in Asia, data are lacking on the proportion of NSCLC patients tested in each country, and the most commonly used testing methods.
Methods:
A retrospective survey of records from NSCLC patients tested for EGFR mutations during 2011 was conducted in 11 Asian Pacific countries at 40 sites that routinely performed EGFR mutation testing during that period. Patient records were used to complete an online questionnaire at each site.
Results:
Of the 22,193 NSCLC patient records surveyed, 31.8% (95% confidence interval: 31.2%–32.5%) were tested for EGFR mutations. The rate of EGFR mutation positivity was 39.6% among the 10,687 cases tested. The majority of samples were biopsy and/or cytology samples (71.4%). DNA sequencing was the most commonly used testing method accounting for 40% and 32.5% of tissue and cytology samples, respectively. A pathology report was available only to 60.0% of the sites, and 47.5% were not members of a Quality Assurance Scheme.
Conclusions:
In 2011, EGFR mutation testing practices varied widely across Asia. These data provide a reference platform from which to improve the molecular diagnosis of NSCLC, and EGFR mutation testing in particular, in Asia.
Keywords: EGFR mutation, Non–small-cell lung cancer, Testing, Survey, Multicenter
Non–small-cell lung cancer (NSCLC) accounts for approximately 85% of lung cancers,1 with an estimated 1.8 million new lung cancer cases worldwide in 2012, including 1 million cases in the Southeast Asian and Western Pacific regions combined.2
Recent advances in lung cancer research have resulted in the identification of several mutations that contribute to carcinogenesis of NSCLC.1,3 The epidermal growth factor receptor (EGFR) is encoded by one of the most commonly mutated genes in NSCLC, with activating mutations detected in up to 15% of adenocarcinoma (ADC) and less than 5% of squamous cell carcinomas (SCC) within the Caucasian patient population.1,4 Mutation rates are higher in Asian populations, with EGFR mutations reported in up to 59% of ADC and 5.4% of SCC cases.5 Mutations are also more common in never-smokers and women patients.6 The landmark Iressa Pan-Asia Study was the first Phase III study to demonstrate improved progression-free survival, response rate, symptom control, and quality of life in EGFR mutation-positive patients treated with an EGFR-tyrosine kinase inhibitor compared with doublet chemotherapy.5 Subsequent Phase III trials confirmed this finding.5,7–11 EGFR mutations are now well recognized as the most potent predictive biomarkers of treatment outcome to first-line EGFR-tyrosine kinase inhibitors, confirming the need for accurate, timely EGFR mutation testing worldwide.5,12,13
Although EGFR mutation testing has been adopted by many laboratories in Asia, accurate data are lacking on the proportion of NSCLC patients tested in each country, and the most commonly used testing methods. For example, a previous study showed that in 2010, the EGFR mutation testing rate was less than 10% in China because of the limited prevalence of testing technology.14 To increase the understanding of real-world testing practices in this region, a formal internet-based survey of EGFR mutation testing practices was conducted in centers throughout Asia Pacific. The aim of the survey was to gather information about the NSCLC population in Asia Pacific that is tested for EGFR mutations, along with the proportion tested, the outcomes of EGFR mutation testing, and the most commonly used testing methods and sample types.
MATERIALS AND METHODS
Participating Sites and Study Design
A retrospective database survey of records from NSCLC patients tested for EGFR mutations from January 1, 2011 to January 1, 2012 was conducted at participating sites in 11 countries across the Asia Pacific region. Site eligibility was assessed using feedback obtained by Astra Zeneca affiliate offices in each country. Of 71 sites initially shortlisted, 40 sites were selected as being both eligible and willing to participate. Eligible sites had to perform EGFR mutation testing for clinical purposes, and should have tested at least 100 samples from NSCLC cases during this period. If, in a given country, no site could meet these criteria, the site that performed the highest number of EGFR mutation tests during this period was surveyed. On-site training was given to ensure that the survey was completed according to the study protocol.
The survey took the form of an online questionnaire using SurveyMonkey Inc. (Palo Alto, CA) that was available in English (Supplementary data, Supplementary Digital Content 1, http://links.lww.com/JTO/A741), Japanese, and Chinese. The survey was completed between April 1 and November 1, 2013 by a qualified individual at each site (pathologist, oncologist, or laboratory head) who had access to data sources containing information about the tested NSCLC population, i.e., patient and laboratory records. Survey response data were stored in a central database that was not accessible to the investigators until the survey closing date, and the survey was programmed so that responders were only able to complete the questionnaire once.
On the survey closing date, the final dataset was downloaded from the central online database into a Microsoft Excel spreadsheet. Data quality was assured via an independent check of the data. Any data discrepancies were queried with the respective sites, and the data were corrected if appropriate. Where discrepancies could not be resolved or explained, the data were treated as missing and only the validated, cleaned data were used in the analyses.
This noninterventional retrospective study was performed in accordance with ethical principles that are consistent with the Declaration of Helsinki, International Conference on Harmonisation Good Clinical Practice guidelines, and the applicable legislation on Non-Interventional Studies. Patient-level data were not reported, hence patient consent was not required. The protocol was approved by the Institutional Review Board for each site.
Study Objectives
The primary objective of the study was to determine the number of NSCLC patients who were tested for EGFR mutations at the surveyed sites, to estimate the rate of EGFR mutation positivity in the tested samples: overall, and by NSCLC histological subtype, sex, and smoking status.
Secondary objectives were: (1) to estimate the proportion of NSCLC patients who were tested in the participating countries: overall, and by NSCLC histological subtype, sex and smoking status (this was calculated based on responses regarding all NSCLC patients who were newly diagnosed at the hospitals from which participating sites received samples from January to December 2011); (2) to determine which EGFR mutation testing methods are most commonly used in the participating countries and the characteristics of samples tested; and (3) to evaluate the source of the captured data and utilize this information to interpret the outcomes. To this end, questions were included in the survey to determine the type of laboratories (commercial or hospital, and whether they test samples from only their own or from several hospitals); QA scheme participation; accreditation by a local or international accrediting body; and the turnaround time for testing, defined as the period from which the sample is received by the laboratory to when the report is sent out. The survey also included questions about the nature of the source records (paper or electronic). Respondents were asked whether the source records were centrally located, contained data from all available patients, were stored consecutively so that no patients could have been missed, and whether or not there was a possibility of duplicate records among the source records.
Statistical Methods
Where provided, the number of NSCLC patients who were tested for EGFR mutations during 2011 was reported. The proportions of EGFR mutation-positive patients were calculated along with the 95% confidence interval based on the Wilson score method in the overall study population, as well as for subgroups based on histological subtypes and demographic factors. Other variables were summarized descriptively. If the answer to a question about the number of cases in a subgroup was “Not known”, then the data from that site were not used to calculate the proportions stated above.
Role of Funding
This investigator-initiated study was funded by AstraZeneca. Employees of the sponsor (AstraZeneca) played a role in the study design, data analysis, data interpretation, and writing the report. The corresponding author had full access to all data and was responsible for the decision to submit for publication.
RESULTS
Assurance of the Data Reliability
Asked about the type of data source records the participating sites consulted to provide answers to the survey questions, 67.5% used hospital electronic records, 55.0% used laboratory electronic records, 35.0% used laboratory paper-based records, and 15.0% used hospital paper-based records, with several sites using multiple data sources. In the subset of sites that used paper-based records, 15 sites responded that they were stored in a single central location, and one site responded that each laboratory staff member stored their own records. At 95.0% of sites, the records used contained all available patient data and at 82.5% of sites, records were confirmed as being collected consecutively. Data were not collected consecutively at 17.5% sites, meaning that some patients could have been missed; three sites (7.5%) indicated a possibility of duplicates among the source records.
Proportion, Demographics and Histology of NSCLC Patients Tested for EGFR Mutation
Respondents from 40 sites in 11 Asian Pacific countries successfully completed the survey: China (nine sites); Hong Kong (two sites); Indonesia (one site); Japan (eight sites); Korea (eight sites); Malaysia (one site); Philippines (one site); Singapore (one site); Taiwan (six sites); Thailand (two sites); and Vietnam (one site). Of the 22,193 patients who were reported as diagnosed with NSCLC at hospitals that sent samples to the participating sites during 2011, 31.8% overall were tested for EGFR mutations (Table 1, Supplementary Table S1.1, Supplementary Digital Content 2, http://links.lww.com/JTO/A742). The highest EGFR mutation testing rate was observed in Japan (64.8%), and the lowest in China (18.3%). Data on total NSCLC cases diagnosed were not provided by the participating sites in Indonesia, Malaysia, Singapore, the Philippines or Vietnam, so the EGFR mutation testing rate could not be calculated for those countries. Not all sites in China, Thailand and Korea were able to provide these data; hence, the testing rates for those countries were based only on data from the sites that provided it.
Table 1.
Proportion of NSCLC Patients who were Tested for EGFR mutations during 2011
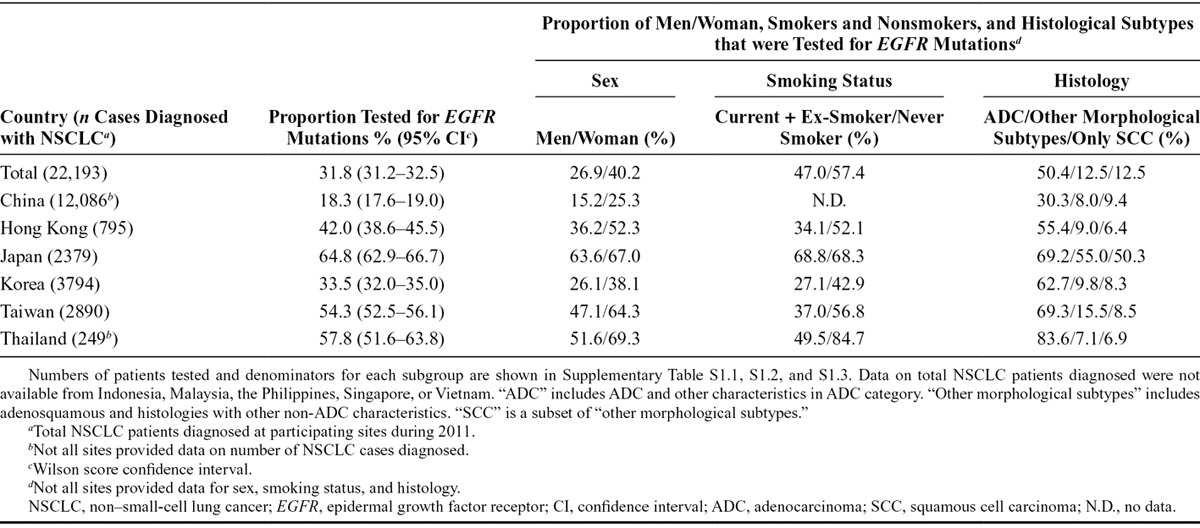
In all countries, a larger proportion of women than men patients with NSCLC were tested for EGFR mutations (Table 1, Supplementary Table S1.1, S1.2, Supplementary Digital Content 2, http://links.lww.com/JTO/A742). Smoking status amongst tested patients varied widely between countries; proportions with a history of smoking who were tested ranged from 27.1% in Korea to 68.8% in Japan.
In the survey, histological groupings were defined as ADC or “other morphological subtypes,” with SCC cases included as a subset of “other.” Overall, 50.4% of patients with ADC-subtype NSCLC were tested for EGFR mutations, compared with 12.5% of those with other morphology and 12.5% of those with SCC (Table 1, Supplementary Table S1.3, Supplementary Digital Content 2, http://links.lww.com/JTO/A742). The proportion of histology subtypes tested was generally similar in all countries except for Japan, where 55% of non-ADC morphology and 50.3% of SCC cases were tested.
EGFR Mutation Positivity in Relation to Demography and Histology at Participating Sites
Data on EGFR mutation positivity rates were available from all sites included in the analysis. Of the 10,687 cases tested for EGFR mutation that were surveyed (Table 2, Supplementary Table S2.1, Supplementary Digital Content 2, http://links.lww.com/JTO/A742), 39.6% (95% confidence interval [CI]: 38.7%–40.5%) were EGFR mutation-positive, with mutation positivity rates varying between countries. EGFR mutation frequency was higher in samples from woman (56.5% overall, 95% CI: 55.0%–58%) than from men (27.8%, 95% CI: 26.6%–29.0%) in all countries except Indonesia, and from never-smokers (60.8%, 95% CI: 58.6%–63.0%) than from current or ex-smokers (27.4%, 95% CI: 25.6%–29.4%; Table 2, Supplementary Table S2.1, S2.2, Supplementary Digital Content 2, http://links.lww.com/JTO/A742).
Table 2.
EGFR Mutation Positivity at Participating Sites, Overall and by Demography and Histology

The frequency of EGFR mutation positivity among the different histological subgroups was 46.5% (95% CI: 45.4%–47.6%) overall in ADC samples, 13.3% (95% CI: 11.7%–15.0%) in tumor samples with other morphologies, and 7.6% (95% CI: 6.1%–9.6%) in SCC samples (Table 2, Supplementary Table S2.3, Supplementary Digital Content 2, http://links.lww.com/JTO/A742). The proportion of samples where the EGFR mutation test did not yield a result was low—1.1% overall.
Sample Types, EGFR Mutation Testing Methods and Pathology Support
The majority of samples tested for EGFR mutations were biopsy and/or cytology samples (71.4% overall; Fig. 1). High proportions of EGFR mutation tests on cytology samples were carried out in Indonesia (98.0%), the Philippines (73.3%), and Vietnam (66.7%). Overall, 75.6% of the tested samples came from the primary tumor, 21.1% were from a metastatic site (including pleural effusion), and 3.3% came from an unknown site.
FIGURE 1.
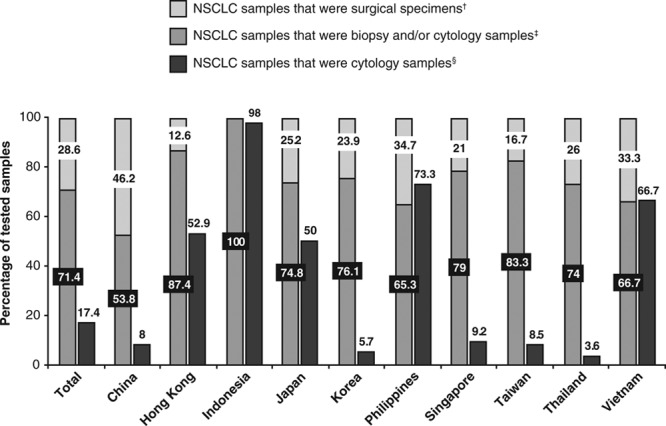
Nature of samples tested for EGFR mutations at participating sites. No data were available for Malaysia. †Data from responses to question: “(Amongst NSCLC cases tested for EGFR mutations in your laboratory, what is the) number of surgical specimen samples tested?” ‡Data from responses to question: “(Amongst NSCLC cases tested for EGFR mutations in your laboratory, what is the) number of biopsy and/or cytology samples tested?” §Data from responses to question: “How many samples tested for EGFR mutation during 2011 were cytology samples?” Proportions of biopsy and/or cytology samples and of surgical specimens were calculated using data from only those sites that provided answers to both questions on “NSCLC samples that were biopsy and/or cytology samples” and “NSCLC samples that were surgical specimens.” The proportions of cytology samples were calculated using data from the sites that provided answers to all three questions, i.e., on “NSCLC samples that were biopsy and/or cytology samples,” “NSCLC samples that were surgical specimens” and “NSCLC samples that were cytology samples.” EGFR, epidermal growth factor receptor; NSCLC, non–small-cell lung cancer.
Seventy-five per cent of sites reported routine use of immunohistochemistry (IHC) to predict the subtypes of samples that had poorly differentiated morphology. A pathology report was available to 60.0% of the participating testing sites, with a further 17.5% of sites indicating that a pathology report was sometimes available.
DNA sequencing was cited as the most commonly used EGFR mutation testing method for both tissue and cytology samples by the largest proportion of participating sites overall (Fig. 2), but the most commonly used methods varied among countries. PCR-INVADER was the most commonly used test in 50.0% of Japanese sites for both tissue and cytology samples, followed by PNA-LNA PCR clamp (37.5% of sites). PNAClamp Mutation Detection was the most commonly used method for testing cytology samples at 50% of Korean sites and 33.3% of sites in China selected “other commercial in vitro diagnostic kit” as their most common testing method for tissue samples. Most laboratories (70.0%) had a turnaround time of 5–10 days, and 20.0% had a turnaround time of less than 5 days.
FIGURE 2.
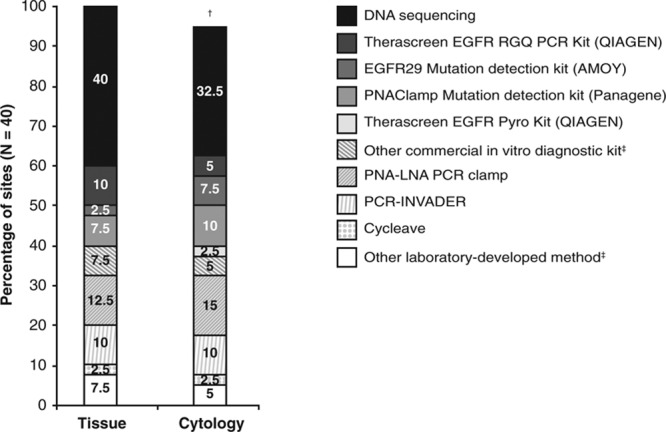
Most commonly used EGFR mutation testing methods for tissue and cytology samples across Asia, determined as a percentage of participating sites. Cobas EGFR Mutation Test (Roche) was included in the survey as an option, but was not selected by any site as the most commonly used test. †Two sites (5.0%) had missing data or did not test cytology samples. ‡Nature of methods not ascertained. EGFR, epidermal growth factor receptor.
Mutation Testing Laboratories
Participating sites included commercial and hospital laboratories; the largest proportion (57.5%) comprised hospital laboratories that tested samples from their own and other hospitals (Fig. 3A). Local accreditation was more common than accreditation by international accrediting bodies (Fig. 3B), with 20.0% of participating sites (all in Japan) being neither accredited nor participating in a Quality Assurance scheme. Only 52.5% of the surveyed sites were members of a Quality Assurance scheme (Fig. 3C).
FIGURE 3.

Information about EGFR mutation testing sites that participated in the survey. A, Type of laboratory; B, accreditation; and C, participation in QA schemes amongst participating laboratories. EGFR, epidermal growth factor receptor; QA, quality assurance.
DISCUSSION
This large retrospective database study of more than 22,000 newly diagnosed NSCLC patients from 40 sites provides insight into the “real-world” EGFR mutation testing practices in Asia in 2011. The frequency of EGFR mutation positivity in the EGFR mutation-tested NSCLC population was 39.6% overall, in line with the EGFR mutation frequency reported in Asia.15,16 The variations in EGFR mutation positivity between countries are likely the result of differences in clinical/case selection of the tested populations (e.g., the presence or absence of reimbursement for testing within healthcare systems, proportion of smokers, or larger or smaller proportions of ADC samples being tested [Supplementary Figure, Supplementary Digital Content 3, http://links.lww.com/JTO/A743]), and also possibly due to differences in EGFR mutation testing methods.17 For example, Japan had the lowest rate of EGFR mutation positivity of all countries surveyed, but tested a wider spectrum of patients, including 63.6% of men and 68.8% of smokers, which was substantially higher than the average testing rates in these patient groups elsewhere in the region (Supplementary Figure, Supplementary Digital Content 3, http://links.lww.com/JTO/A743). In addition, Japan reported higher testing rates in non-ADC NSCLC samples (55.0%) than any other country, most likely because EGFR mutation testing is reimbursed for all patients with lung cancer in this country.
The EGFR mutation frequency observed in the different histological subtypes was also generally in line with published reports.15,16 However, EGFR mutation in SCC samples appeared to be higher than expected in some countries. This finding might be associated with poor cooperation between pathology and molecular testing laboratories: 40% of the laboratories did not always refer to the pathology report and 25% did not use IHC to subtype poorly differentiated tumors. Therefore, EGFR testing can be considered even in patients with SCC if the clinicopathological features suggest the possibility of EGFR mutations. This is in agreement with molecular testing guidelines published by experts representing the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology (CAP/IASLC/AMP).18
Data on total NSCLC cases diagnosed were not available in Indonesia, Malaysia, the Philippines, Singapore, or Vietnam; hence, the proportion of cases tested for EGFR mutations could not be estimated for those countries. Japan, where mutation testing for cancer is well established, had a relatively high EGFR mutation testing rate (64.8%), mainly because (1) sample logistics and testing are well established and are largely carried out in a centralized manner at three major commercial laboratories, and (2) cytology samples are routinely tested due to the practice of using methods optimal for these samples (i.e., sensitive allele-specific PCR-based methods in all sites),19 which increases the ability to test for EGFR mutations in patients from whom tumor tissue samples are not available.
In all countries, the majority of samples tested for EGFR mutations were biopsy or cytology samples. The use of tissue resections or biopsies is generally preferable for EGFR mutation testing because of the greater amount of sample that is available. However, in many countries, cytology samples are commonly used for EGFR testing despite the common use of direct sequencing. As recommended in the CAP/IASLC/AMP molecular testing guidelines,18 more sensitive assays should be applied for patients in whom only cytology samples are available. Increasingly, cytology cell blocks are being used to provide adequate material for additional molecular tests, including anaplastic lymphoma kinase IHC and fluorescence in situ hybridization (FISH). Cell block preparation of cytology specimens is recommended in recently published molecular testing guidelines for treatment selection among lung cancer patients.18 It will be interesting to see how the uptake of new methodologies, such as next-generation sequencing and multiplex panels, impacts testing in the future.
Only 60% of the survey’s participating sites routinely received pathology information such as percentage tumor content along with the samples they tested, and this is an area that could be targeted for improvement. Particularly, EGFR testing is conducted frequently with small biopsy/cytology samples, in which false negative results can occur more frequently due to insufficient cancer cell contents and poor DNA quantity. Therefore, the CAP/IASLC/AMP guideline recommends active engagement of pathologists to molecular testing, i.e., checking the adequacy of specimens.18 The turnaround time was generally acceptable, with all sites generating EGFR mutation test results within 15 days (and 20% within 5 days). Further improvements may help ensure that patients receive results in a more timely manner to facilitate appropriate first-line treatment decisions, particularly for urgent cases.
As a retrospective database survey, this study had limitations. Not all the testing sites had access to data on numbers of patients diagnosed with NSCLC at the hospitals from which they received samples; hence, proportions of NSCLC patients tested could not be calculated for some countries. Not all sites were able to provide answers to all the questions, and thus some calculations, for example the proportion of EGFR mutation positivity in men versus woman, and in smokers versus nonsmokers, were performed on the subset of data generated from responses that were received. At a minority of sites (4 of 40), the number of samples used in diagnosis or tested for EGFR mutations exceeded the number of patients tested. This was explained, in most cases, by the testing of more than one sample from a single patient, for example following disease progression or via a secondary biopsy. EGFR mutation status in Table 2 was reported on a per-patient basis, and so was not impacted by the issue of multiple samples. The lack of exact matching of samples to patients meant that sample data (Fig. 1) could not be directly linked back to patients in all cases, although it should be noted that the discrepancy between the number of samples diagnosed and number of patients was less than 0.5%. Another potential source of concern was the nature and quality of the data. However, given that 38 of 40 (95.0%) sites used source records containing all available patient data; at 33 of 40 (82.5%) sites, records were collected consecutively; and only three sites (7.5%) noted a possibility of duplicates among the source records, the data captured are likely to reflect accurately the testing practices across Asia in 2011.
The survey showed that 20% of laboratories surveyed in 2011 were not accredited by international or national bodies and 47.5% did not participate in a Quality Assurance scheme, an aspect of testing practice that clearly requires immediate improvement.
The data collected in this survey indicate that, despite the high incidence of the EGFR mutation in Asian populations, EGFR mutation testing practices varied widely across the region in 2011. The survey revealed several areas where improvements are required, and may provoke changes in the health measures and/or policy in the individual countries by clarifying the differences on EGFR mutation testing among the countries. The data also provides a baseline against which the impact of the 2013 CAP/IASLC/AMP molecular testing guidelines,18 evolving histological classifications (e.g., the upcoming 2015 WHO classification), and development of multiplex mutation analyses can be assessed.
Acknowledgements
This investigator-initiated retrospective study was funded by AstraZeneca. The authors thank the staff who completed the online survey at the participating testing sites; the CRO, WorldWide Clinical Trials; and AstraZeneca’s Observational Research Centre.
Uploading and management of the online survey, and editorial assistance with drafting the manuscript were provided by Samantha Santangelo, PhD of MediTech Media Asia Pacific, funded by AstraZeneca.
Footnotes
Disclosure: The survey was funded by AstraZeneca. Yasushi Yatabe, Keith Kerr, Teh-Ying Chou, Jabed Iqbal, Jin-Haeng Chung, Koichi Hagiwara, Zhiyong Liang, Nicola Normanno, Keunchil Park, Shinichi Toyooka, Chun-Ming Tsai, Paul Waring, Li Zhang and Tony Mok previously received consulting fees or honoraria from AstraZeneca as well as support for travel to the 2012 expert opinion meeting described in the manuscript. Yasushi Yatabe has previously received consultancy fees from AstraZeneca, Novartis, Roche and Pfizer and payment for lectures from AstraZeneca, Novartis, Roche, Pfizer, Eli Lilly and Abbott Molecular. Keith Kerr has current consultancy agreements with and currently receives payment for lectures from AstraZeneca, Roche, Pfizer, Eli Lilly, Novartis, Merck Serono, Boehringer Ingelheim, and GSK. Ahmad Utomo, Xiang Du and Geon Kook Lee have no conflicts of interest to declare. Ma. Luisa Enriquez’s institution was the recipient of a previous research grant from AstraZeneca and in the past she received support for travel from AstraZeneca to attend an oncology meeting. Jabed Iqbal’s institution currently receives funding from Astra Zeneca for this study. Pathmanathan Rajadurai has an ongoing consultancy agreement with AstraZeneca and also receives payment for local and international CME activities from AstraZeneca. Li Zhang’s institution receives an ongoing grant from AstraZeneca and Roche related to this study, and previously received honoraria from AstraZeneca, Boehringer Ingelheim and Roche; he has also previously received payment for board membership from Eli Lilly and Pfizer, and honoraria from Novartis and Eli Lilly. Van Khanh Tran’s institution is the recipient of an ongoing grant from AstraZeneca and Roche. Shanop Shuangshoti’s institution is the recipient of a previous grant from AstraZeneca. Shinichi Toyooka previously received lecture fees from Taiho Pharmaceutical Co, Sanofi K.K. and Chugai Pharmaceutical Co., Ltd. Keunchil Park receives ongoing consultancy fees from Eli Lilly, Roche, KyowaHakkoKirin, Novartis, Astellas, Clovis and previously received payment for lectures from Eli Lilly. Nicola Normanno’s institution previously received a grant from AstraZeneca to support research activity on EGFR mutations in NSCLC. Teh-Ying Chou has an ongoing consultancy agreement with Roche and currently receives lecture fees from AstraZeneca, Roche, Pfizer, Merck and Eli Lilly. Paul Waring has previously received consultancy fees from Amgen, Roche, Merck, AstraZeneca, was employed by Genentech and was a Board Member of Ventana Medical Systems; he currently has stock options in Roche and a patent with Ventana Medical Systems. Tony Mok has current consultancy agreements with AstraZeneca, Roche, Eli Lilly, Merck Serono, Eisai, BMS, BeiGene, AVEO, Pfizer, Taiho, Boehringer Ingelheim, GSK Biologicals, Clovis Oncology, Amgen, Janssen and Biomarin and is President of IASLC. Rose McCormack, Marianne Ratcliffe, and Yohji Itoh are employees of AstraZeneca and hold shares in AstraZeneca. Masatoshi Sugeno is an employee of AstraZeneca and has previously received funding from Applied Biomathematics Inc. and The Research Foundation for The State University of New York.
REFERENCES
- 1.Dearden S, Stevens J, Wu YL, Blowers D. Mutation incidence and coincidence in non small-cell lung cancer: Meta-analyses by ethnicity and histology (mutMap). Ann Oncol. 2013;24:2371–2376. doi: 10.1093/annonc/mdt205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GLOBOCAN (IARC) 2012. Lung cancer fact sheet. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx. Accessed March 17, 2014. [Google Scholar]
- 3.Ding L, Getz G, Wheeler DA, et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature. 2008;455:1069–1075. doi: 10.1038/nature07423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pao W, Girard N. New driver mutations in non-small-cell lung cancer. Lancet Oncol. 2011;12:175–180. doi: 10.1016/S1470-2045(10)70087-5. [DOI] [PubMed] [Google Scholar]
- 5.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 6.Mitsudomi T, Yatabe Y. Mutations of the epidermal growth factor receptor gene and related genes as determinants of epidermal growth factor receptor tyrosine kinase inhibitors sensitivity in lung cancer. Cancer Sci. 2007;98:1817–1824. doi: 10.1111/j.1349-7006.2007.00607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee JS, Park K, Kim SW, et al. A randomized phase III study of gefitinib (IRESSA) versus standard chemotherapy (gemcitabine plus cisplatin) as a first-line treatment for never smokers with advanced or metastatic adenocarcinoma of the lung. J Thorac Oncol. 2009;4:S283–S284. [Google Scholar]
- 8.Maemondo M, Inoue A, Kobayashi K, et al. North-East Japan Study Group. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 9.Mitsudomi T, Morita S, Yatabe Y, et al. West Japan Oncology Group. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 10.Rosell R, Carcereny E, Gervais R, et al. Spanish Lung Cancer Group in collaboration with Groupe Français de Pneumo-Cancérologie and Associazione Italiana Oncologia Toracica. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 11.Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): A multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 12.Marchetti A, Normanno N, Pinto C, et al. AIOM - SIAPEC-IAP; Italian Association of Medical Oncology; Italian Society of Anatomic Pathology and Diagnostic Cytopathology. Recommendations for mutational analysis of EGFR in lung carcinoma. Pathologica. 2010;102:119–126. [PubMed] [Google Scholar]
- 13.Pirker R, Herth FJ, Kerr KM, et al. European EGFR Workshop Group. Consensus for EGFR mutation testing in non-small cell lung cancer: Results from a European workshop. J Thorac Oncol. 2010;5:1706–1713. doi: 10.1097/JTO.0b013e3181f1c8de. [DOI] [PubMed] [Google Scholar]
- 14.Xue C, Hu Z, Jiang W, et al. National survey of the medical treatment status for non-small cell lung cancer (NSCLC) in China. Lung Cancer. 2012;77:371–375. doi: 10.1016/j.lungcan.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 15.Tokumo M, Toyooka S, Kiura K, et al. The relationship between epidermal growth factor receptor mutations and clinicopathologic features in non-small cell lung cancers. Clin Cancer Res. 2005;11:1167–1173. [PubMed] [Google Scholar]
- 16.Yoshida K, Yatabe Y, Park JY, et al. Prospective validation for prediction of gefitinib sensitivity by epidermal growth factor receptor gene mutation in patients with non-small cell lung cancer. J Thorac Oncol. 2007;2:22–28. [PubMed] [Google Scholar]
- 17.Ellison G, Zhu G, Moulis A, et al. EGFR mutation testing in lung cancer: A review of available methods and their use for analysis of tumour tissue and cytology samples. J Clin Pathol. 2013;66:79–89. doi: 10.1136/jclinpath-2012-201194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindeman NI, Cagle PT, Beasley MB, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: Guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. Arch Pathol Lab Med. 2013;137:828–860. doi: 10.5858/arpa.2012-0720-OA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goto K, Satouchi M, Ishii G, et al. An evaluation study of EGFR mutation tests utilized for non-small-cell lung cancer in the diagnostic setting. Ann Oncol. 2012;23:2914–2919. doi: 10.1093/annonc/mds121. [DOI] [PubMed] [Google Scholar]


