Abstract
Acute heart failure is a common condition associated with considerable morbidity, mortality, and cost. However, evidence-based data on treating heart failure in the acute setting are limited, and current individual treatment options have variable efficacy. The healthcare team must often individualize patient care in ways that may extend beyond available clinical guidelines. In this review, we address the question, “How do you do the best you can clinically with incomplete evidence and imperfect drugs?” Expert opinion is provided to supplement guideline-based recommendations and help address the typical challenges that are involved in the management of patients with acute heart failure. Specifically, we discuss 4 key areas that are important in the continuum of patient care: differential diagnosis and risk stratification; choice and implementation of initial therapy; assessment of the adequacy of therapy during hospitalization or observation; and considerations for discharge/transition of care. A case study is presented to highlight the decision-making process throughout each of these areas. Evidence is accumulating that should help guide patients and healthcare providers on a path to better quality of care.
Keywords: acute heart failure, treatment, management, dyspnea
Clinical guidelines for heart failure focus primarily on chronic rather than acute disease management,1,2 despite the substantial morbidity, mortality, and cost associated with acute heart failure (AHF).3,4 This is a reflection of sparse evidence-based data in the acute setting and begs the question, “How do you do the best you can clinically with incomplete evidence and imperfect drugs?” In this article, we review the most recent American College of Cardiology Foundation/American Heart Association guidelines1 and, beyond these recommendations, provide expert opinion to help providers address the common challenges that are involved in the management of patients with AHF. Only summary statements are made with respect to the American College of Cardiology Foundation/American Heart Association guidelines as it was not our intention to fully reproduce the guidelines in detail. We discuss 4 key areas: differential diagnosis and risk stratification; choice and implementation of initial therapy; assessment of the adequacy of therapy during hospitalization or observation; and considerations for discharge/transition of care. A case study representing a typical patient is interwoven throughout each of these sections.
DIFFERENTIAL DIAGNOSIS AND RISK STRATIFICATION
Case Study
Josephine was a 70-year-old white woman measuring 5 feet 4 inches tall who was a former heavy smoker. She presented to the emergency department (ED) complaining of progressively worsening breathlessness over 7 days, now present at rest, with the onset of orthopnea, mildly productive cough, and wheezing for the past 2 days. Josephine had a history of hypertension, chronic heart failure, and chronic obstructive pulmonary disease. She had been admitted for AHF 1 year ago and pneumonia 4 months ago. Her left ventricular ejection fraction measured 3 months ago was 47%. On physical examination, her blood pressure was 160/100 mm Hg, heart rate was 95 beats/min and regular, and temperature was 38.0°C. Body weight was 165 pounds and had increased by 6 pounds since it was last measured 3 months ago. The patient had wet rales and some wheezes, more prominent on the left side, 10 cm of jugular venous distension, an audible fourth heart sound, and mild pitting edema in both legs. A chest radiograph showed pulmonary venous congestion and no infiltrates. A 12-lead electrocardiogram showed left ventricular hypertrophy and normal sinus rhythm, but no indication of ischemia or infarction. Noteworthy laboratory findings included a positive high-sensitivity cardiac troponin I of 100 ng/L (upper reference limit of 24–30 ng/L5), blood urea nitrogen level of 47 mg/dL, serum creatinine level of 1.1 mg/dL, estimated glomerular filtration rate of 52 mL/min/1.73 m2, a white blood cell count of 12,500 cells/mm3, and mildly increased liver enzymes. Josephine was already being treated with enalapril 10 mg twice daily, bisoprolol 10 mg once daily, and triamterene 100 mg twice daily for her chronic heart failure and hypertension. A brain natriuretic peptide level was not sampled upon presentation.
Differential Diagnosis
Dyspnea is the most common symptom of patients presenting with AHF in the emergency setting. However, this symptom is nonspecific because many other medical conditions are commonly associated with dyspnea, including pneumonia, exacerbations of chronic obstructive pulmonary disease, pulmonary embolism, acute coronary syndrome, and asthma. This can make the diagnosis or exclusion of AHF a challenge.
American College of Cardiology Foundation/American Heart Association Guidelines
A thorough medical history, physical examination, laboratory evaluation, chest radiograph, 12-lead electrocardiogram, and echocardiogram are recommended for all patients.1 The foundation of assessment remains a medical history and physical examination. Some find it helpful to classify patients based on the adequacy of peripheral perfusion (“warm” or “cold”) and degree of congestion (“dry” or “wet”). Recommended laboratory tests include complete blood count, urinalysis, serum electrolytes (including calcium and magnesium), blood urea nitrogen, serum creatinine, glucose, fasting lipid profile, liver function tests, and thyroid-stimulating hormone. A chest radiograph can identify cardiomegaly and pulmonary congestion, and may reveal alternate causes of the patient’s symptoms. Of note, a normal chest radiograph does not exclude the presence of AHF. A 2-dimensional echocardiogram with Doppler is the most useful diagnostic test and can help detect abnormalities of the myocardium, heart valves, and pericardium. Each of the aforementioned guideline-recommended tests carries a class of recommendation I and level of evidence C. If there is uncertainty with respect to the diagnosis of AHF, measurement of brain natriuretic peptide or N-terminal probrain natriuretic peptide levels can be useful to support clinical judgment (class of recommendation I, level of evidence A), although cutoff points are not specified in the guidelines.
Expert Opinion
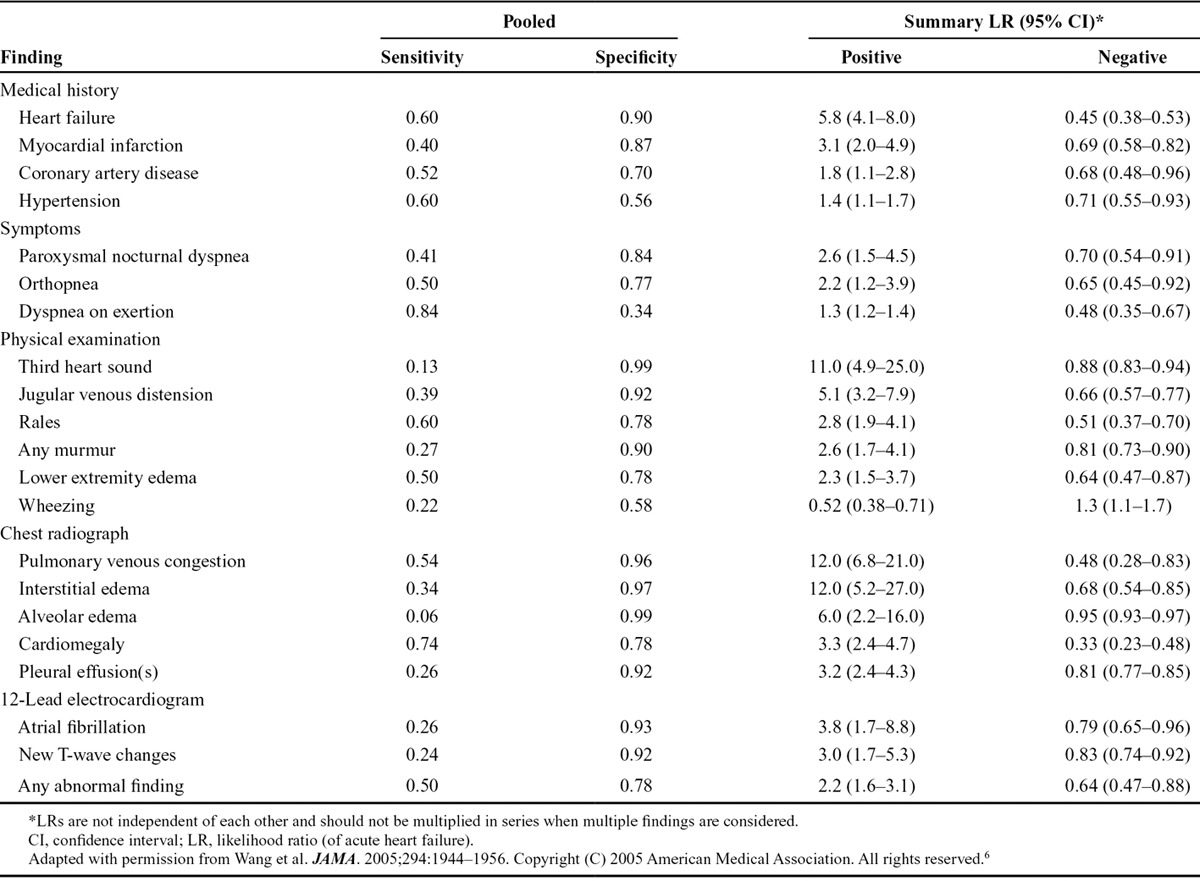
Based on results of the above assessments, the presence (or absence) of certain features increases (or decreases) the likelihood of AHF, although, unfortunately, none achieve 70% sensitivity and specificity (Table 1).6 Those characteristics associated with the highest positive likelihood ratios for AHF include history of heart failure, paroxysmal nocturnal dyspnea, third heart sound (S3 gallop), pulmonary venous congestion on chest radiograph, and atrial fibrillation on electrocardiogram. In contrast, absence of history of heart failure, dyspnea on exertion, rales, peripheral edema, cardiomegaly on chest radiograph, or any abnormality on electrocardiogram are the characteristics that most reduce the likelihood of AHF. Awareness of these relationships can help physicians in making a correct diagnosis. Classical signs and symptoms of AHF may be absent despite this diagnosis. Evidence of congestion, either “right” or “left” sided, is present in more than 90% of patients diagnosed with AHF in general practice.7
TABLE 1.
Presence/Absence of Findings Significantly Increasing/Decreasing the Likelihood of Acute Heart Failure in Adult Patients Presenting With Dyspnea to the Emergency Department
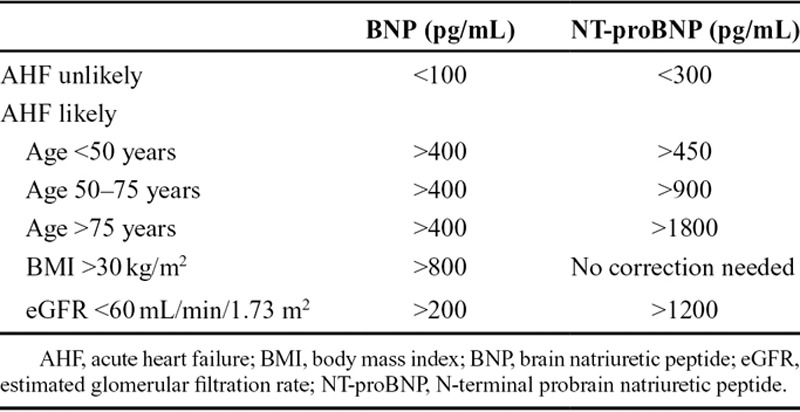
Brain natriuretic peptide and N-terminal probrain natriuretic peptide levels also play an important role in the diagnosis of AHF (Table 2),8 although no values are 100% diagnostic. Patients presenting with dyspnea are highly unlikely to have AHF at brain natriuretic peptide levels <100 pg/mL, whereas a diagnosis of AHF is likely at brain natriuretic peptide levels >400 pg/mL. For N-terminal probrain natriuretic peptide, the optimal cutoff level for ruling out a diagnosis of AHF is <300 pg/mL, whereas values >450 pg/mL (younger than 50 years), >900 pg/mL (50–75 years), and >1800 pg/mL (older than 75 years) all substantially increase the probability of diagnosis. Different thresholds should be used for obese patients and those with renal dysfunction. Given the linear decrease in brain natriuretic peptide levels with increasing body mass index, obese patients (>30 kg/m2) should have their brain natriuretic peptide values doubled to use the standard cutoff levels; no corrections are necessary for N-terminal probrain natriuretic peptide levels. Increases in natriuretic peptide levels in patients with renal dysfunction have been attributed more to the presence and extent of cardiac pathology than to impaired renal clearance.8 The cutoff levels suggested for patients with an estimated glomerular filtration rate of <60 mL/min/1.73 m2 are 200 pg/mL (brain natriuretic peptide) and 1200 pg/mL (N-terminal probrain natriuretic peptide).9 Although the American College of Cardiology Foundation/American Heart Association guidelines state that both brain natriuretic peptide and N-terminal probrain natriuretic peptide values are useful in diagnosis (but cannot be used interchangeably), they do not indicate a preference for the use of one over the other.1 Clinician choice of which natriuretic peptide to assess may be influenced by fluctuations with age, central laboratory preference, half-life, and other factors, as described above. Many experts believe that even if the diagnosis of AHF is clear, a natriuretic peptide level will give important prognostic information to help triage patients to the appropriate level of care (eg, intensive care unit, telemetry, floor, or home). In addition, compared with physician estimation, brain natriuretic peptide levels have been suggested to be more predictive of near-term events.10
TABLE 2.
Optimal Cutoff Points of Natriuretic Peptide Levels for Diagnosis of Acute Heart Failure8,9
Approximately 25% of patients have natriuretic peptide levels in the “gray zone” [brain natriuretic peptide: 100–400 pg/mL; N-terminal probrain natriuretic peptide: 300–450 pg/mL (younger than 50 years), 300–900 pg/mL (50–75 years), and 300–1800 pg/mL [older than 75 years)] and therefore additional clinical information is required for differential diagnosis.8 Other conditions that cause a rise in brain natriuretic peptide/N-terminal probrain natriuretic peptide levels, such as those causing myocardial stretch (eg, acute pulmonary embolus, acute coronary syndrome, and primary pulmonary hypertension), should be considered. A biomarker of emerging interest for individuals within the gray zone is midregional pro-atrial natriuretic peptide. The literature suggests that measurement of a midregional pro-atrial natriuretic peptide level, although not routinely performed outside of academic centers, adds to the diagnostic capability of brain natriuretic peptide or N-terminal probrain natriuretic peptide levels.11 A midregional pro-atrial natriuretic peptide cutoff level of 120 pmol/L can be used to rule out a diagnosis of AHF in most patients, with a lower cutoff value recommended for patients younger than 50 years and obese patients.12
Delays in diagnosis, including those due to the measurement of natriuretic peptide levels, are strongly associated with delays in the treatment of AHF, which in turn, are independently associated with worse outcomes such as increased mortality. In an analysis of patients in the Acute Decompensated Heart Failure National Registry who required ED admission and treatment with intravenous diuretics, time to diuretic treatment and time to brain natriuretic peptide level measurement were divided into quartiles for 58,465 episodes from 209 hospitals. Patients who experienced the longest time between admission and measurement of brain natriuretic peptide levels also experienced the longest time to diuretic treatment. In addition, the longer the wait for diuretic treatment, the smaller the percentage of patients who were asymptomatic at discharge, and treatment delay was associated with increased in-hospital mortality (Fig. 1).13 We therefore recommend assessment of biomarker levels as quickly as possible in the setting of an uncertain diagnosis, ideally within the first 60 minutes after presentation in patients with high acuity.
FIGURE 1.

Relationship of hospital mortality (%) to time of initial treatment (quartiles) and initial BNP levels being drawn (quartiles). BNP, brain natriuretic peptide; IV, intravenous. Reprinted with permission from Maisel et al. J Am Coll Cardiol. 2008;52:534–540.13
Risk Stratification
Nearly 1 million annual ED visits between 2006 and 2010 were for AHF.14 Likely due to the lack of good risk-stratification tools,15,16 and unacceptable rates of relapse and mortality after an ED discharge,17,18 more than 80% resulted in a hospital admission.14 This accounts for a substantial portion of the projected $70 billion to be spent on heart failure care by 2030.3,4 Unfortunately, up to 20% of patients will be readmitted within 30 days as a result of both heart failure–related issues and other concomitant comorbidities. Heart failure is the primary reason for Medicare hospital readmissions.19,20 Recent policy changes have driven pressure on hospitals and medical systems to change this cycle of admission-readmission or face financial consequences.21
Once admitted, in-hospital mortality rates among patients with AHF generally range from 4% to 7%, but may be substantially greater in high-risk subsets.22 Mortality is high after an episode of AHF with 60-day and 1-year mortality rates of approximately 10% and 20%, respectively, but again higher in high-risk subsets.23,24 It has been estimated that up to 50% of admitted patients are at low risk for short-term morbidity and mortality and may be candidates for aggressive outpatient therapy and careful follow-up.23 In contrast, high-risk patients should receive aggressive in-hospital treatment.1 The importance of accurate risk stratification cannot be underestimated.
American College of Cardiology Foundation/American Heart Association Guidelines
Several multivariable risk scores are recommended (class of recommendation IIa, level of evidence B) to predict outcomes for patients with AHF.1 Guidelines specifically mention the Acute Decompensated Heart Failure National Registry Classification and Regression Tree Model, American Heart Association Get With The Guidelines Score, Enhanced Feedback for Effective Cardiac Treatment Risk Score, Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness Risk Model and Discharge Score, and Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure Risk Prediction Nomogram. These risk scores objectively estimate the risk of mortality, but have not performed as well in predicting which patients will worsen in hospital or in estimating the risk of readmission. Moreover, they have not been useful in identifying a patient at low enough risk to avoid an inpatient admission. Measurement of biomarkers of myocardial injury (troponin T or I) or fibrosis (soluble ST2 and galectin-3) may be considered for additive risk stratification (class of recommendation IIa, level of evidence A). Increased levels of these biomarkers are associated with worse clinical outcomes, but no specific cutoffs are provided in the guidelines and such tests are not always available in routine practice.
Expert Opinion
The lack of evidence-based guidelines for interventions that improve morbidity and mortality in AHF reflects the lack of progress in the development of such therapies despite extensive efforts. Clinical experience and judgment in this setting is key to identifying those patients at higher risk and then tailoring available therapies as best as possible.
A variety of factors have been identified that place patients at high risk of morbidity and mortality, including prior hospitalizations for AHF, low serum sodium (<135 mEq/L), increased blood urea nitrogen (>40 mg/dL) or creatinine (>3 mg/dL), increased liver function tests (eg, typically a substantial, rapid increase in aminotransferase and lactic dehydrogenase levels 10–20 times normal in 1–3 days after hemodynamic insult), low systolic blood pressure (<100 mm Hg), ischemic changes on electrocardiogram, and increased troponin T or I.25–27 For example, a positive troponin test is an independent predictor of adverse outcomes,26,28 with higher levels indicative of greater risk for both short-term and long-term mortality.29 Patients with any of the above high-risk factors should be evaluated and considered for treatment in an inpatient setting. The same approach should be considered for patients with an intermediate level of risk, including those with a substantial increase in brain natriuretic peptide level (>1000 pg/mL) or N-terminal probrain natriuretic peptide level (>5000 pg/mL), or borderline low-normal systolic blood pressure (100–120 mm Hg).25 If a patient is determined to be at high risk upon initial assessment, it should inform decision making throughout hospitalization and follow-up.
Identifying low-risk patients is more difficult but equally important because it will help to reduce unnecessary hospitalizations. Determining AHF clinical profiles safe for either ED or acute care clinic discharge, or discharge after a brief period of treatment and observation, would be of great utility. Unfortunately, risk-prediction instruments in AHF have been largely unsuccessful when attempting to define a cohort of patients safe for early discharge and at low-risk of 30-day mortality and readmission.23,30 More importantly, risk tools have not been implemented in these settings to determine how they add to, or detract from, clinician gestalt. As a result, published tools have had little impact on ED or acute care clinic disposition decision making.
These limitations notwithstanding, the absence of any high-risk factors or normal troponin levels each has been associated with lower risk for subsequent morbidity and mortality,25,31 although further research is needed. Results of the ongoing Identifying High- and Low-Risk Heart Failure Patients in the Emergency Department study, expected in December 2014, should help in this regard.32 In the Identifying High- and Low-Risk Heart Failure Patients in the Emergency Department study, clinical and laboratory information obtained prospectively from ED patients with suspected AHF will be used to develop a decision tool to help predict risk for inpatient and outpatient complications from AHF. A fertile area of ongoing research is the evaluation of novel biomarkers for risk stratification (eg, copeptin, midregional adrenomedullin), including in combination with clinical variables or natriuretic peptides and how they might complement one another in the assessment of risk.33,34 For example, in the Multinational Observational Cohort on Acute Heart Failure study, the addition of biomarkers to a clinical prediction model incrementally improved risk stratification for predicting 30-day and 1-year mortality.34
Because the rate of admission for patients with AHF is high (~80% nationally), and has remained largely unchanged over the past few years,14,15 we recommend a more comprehensive approach for risk assessment to possibly modify this clinical inertia. We suspect the complicated and heterogeneous AHF patient may need both an objective evaluation of physiologic risk and an assessment of barriers to ideal self-care (eg, medication access, transportation, health literacy, diet, exercise facilities, caregiver support), along with strategies to address these challenges.35–39 Social, behavioral, and environmental factors strongly influence one’s ability to implement the healthy lifestyle required to optimally manage chronic illness.40,41 Efforts should be aimed at combining existing and future risk-prediction tools with clinical gestalt, identifying important self-care behaviors and existing patient barriers, and improving collaboration between providers, patients, and caregivers. Such collaboration from all stakeholders encourages interaction to establish a care path based on mutual agreement, or “shared decision making.”42,43 Most important is the active engagement of patients when healthcare decisions are made (eg, convergence of medical options) that have potentially serious consequences and long-term implications.44 Examples include decisions on diagnostic testing, treatments, and admission; all may result in compelling quality-of-life issues and further downstream interventions.15,45
Case Study—Revisited
In our case study, the medical history, current presentation, and diagnostic evaluation suggested that Josephine’s dyspnea was due to AHF, but a pulmonary process such as pneumonia was not ruled out in the ED and was considered the most likely cause of her dyspnea by the healthcare team. She was treated with antibiotics and bronchodilators and remained in the ED for 24 hours until a bed opened up in the intermediate care unit. Once there, she was noted to have ongoing dyspnea at rest and a blood pressure of 160/95 mm Hg. Because of persistent dyspnea, her first brain natriuretic peptide level was obtained after transfer and returned as 650 pg/mL. This established that AHF was present, but the correct diagnosis had been delayed by nearly 36 hours.
Josephine has comorbidities that are typical of patients with AHF (ie, hypertension, moderate renal dysfunction), along with several laboratory findings that place her at significant risk for morbidity and mortality (eg, positive troponin, moderate renal insufficiency, increased liver function tests).46 Registry data from the United States (Acute Decompensated Heart Failure National Registry study) highlight the fact that the majority of patients admitted to hospital for AHF have increased systolic blood pressure (50% had a first recorded systolic blood pressure >140 mm Hg).47 Increased blood pressure has been found in nearly 80% with left ventricular ejection fraction 40% to 55%.48 In addition, about 43% have moderate renal dysfunction, although many cases are undiagnosed.49 Based on the Acute Decompensated Heart Failure National Registry Classification and Regression Tree Model, Josephine would be classified as intermediate risk level 2, which has an associated in-hospital mortality rate of 6.41%.22 Immediate treatment is warranted, and enough high-risk factors are present to justify hospitalization. An alternative to hospitalization for lower-risk patients is management in an observation unit.50 Of note, whereas penalties are instituted for excessive rehospitalizations, the Centers for Medicare and Medicaid Services provides reimbursement for AHF care in an observation unit.
AHF—A COMPLEX SYSTEMIC DISEASE
AHF is a complex systemic disease that causes organ damage and can cause death if not quickly treated (Fig. 2). Despite advancements in the clinical knowledge of AHF, there remains an incomplete understanding of all the processes involved in its development and progression. Initial treatment must be focused on relieving symptoms and hemodynamically stabilizing the patient to prevent further organ damage. Traditionally, AHF was considered a disorder of fluid overload caused by increased renal sodium and water retention. Increased fluid volume was felt to be due to noncompliance with medications and/or diet. Patients were treated with diuretics to normalize fluid volume levels and alleviate congestion. However, findings that AHF is not associated with increased weight gain due to fluid retention led to the belief that congestion can also be caused by fluid redistribution rather than just fluid accumulation.51 Thus, treatment of AHF with diuretics alone is not sufficient to prevent the damage that can occur to multiple organ systems, which may explain the lack of evidence base with these agents concerning improved long-term outcomes.
FIGURE 2.
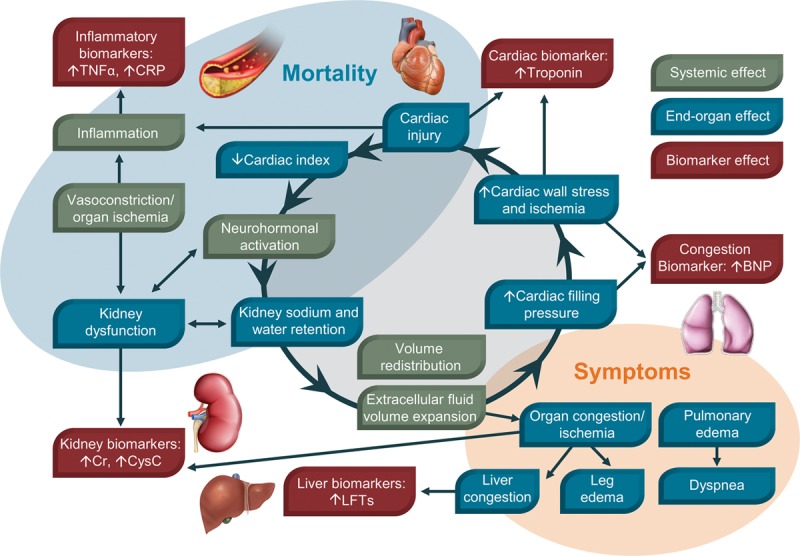
Acute heart failure is a complex systemic disease involving multiple organ systems. BNP, brain natriuretic peptide; Cr, creatinine; CRP, C-reactive protein; CysC, cystatin C; LFTs, liver function tests; TNF, tumor necrosis factor.
AHF is a clinical syndrome with heterogeneous underlying contributing factors and a somewhat variable clinical presentation, depending on the components responsible for its initiation. A history of ischemic heart disease is common in these patients, and subsequent myocyte necrosis can be a trigger for AHF.52 In addition, decreased coronary perfusion during AHF can cause myocardial ischemia and myocyte damage. Venous congestion has recently been reported to cause inflammation and neurohormonal and endothelial activation.53 Vasoconstriction can impair renal blood flow during AHF, resulting in renal dysfunction and activation of the renin-angiotensin-aldosterone system. Renin-angiotensin-aldosterone system activation promotes tubular absorption of sodium and water and thus plays a role in the progression of fluid imbalance. Renal insufficiency is frequently associated with AHF and can also result from decreased renal perfusion and, regardless of cause, is associated with poor patient outcome.54 An estimated two-third of patients with AHF have at least moderate renal insufficiency at presentation,49 and about 25% develop cardiorenal syndrome after hospitalization.55 AHF has been reported to cause hepatic dysfunction as a result of reduced blood flow and cases of acute liver failure have been documented in this population, especially with right-sided failure and hepatic congestion.56 Increased levels of inflammatory cytokines have been observed in AHF, but therapeutic interventions to treat inflammation have not proven successful.57 However, it is believed that inflammatory activation in AHF can lead to pulmonary fluid overload without increasing total body fluid.51 In addition, increased expression of regulatory neurohormonal molecules (eg, norepinephrine, angiotensin II, endothelin, and aldosterone) contributes to disease progression.58
Approximately half of patients with heart failure have a preserved ejection fraction, the incidence of which is increasing at a significant rate relative to heart failure with reduced ejection fraction.59 This is felt to be due to a combination of better interventions for the treatment of acute coronary syndromes and also greater awareness of heart failure with a preserved ejection fraction as a distinct entity. Heart failure with a preserved ejection fraction is characterized by normal or near-normal systolic function with some evidence of diastolic dysfunction. These individuals are more likely to be older, female, obese, diabetic, hyperlipidemic, hypertensive, and to have coronary artery disease, but less likely to have had a myocardial infarction.60,61 AHF with underlying heart failure with reduced ejection fraction is often associated with some degree of systolic pump failure; in cases of heart failure with a preserved ejection fraction, a stiff noncompliant ventricle along with noncardiac pathophysiologic factors predominate.
The detection of heart failure with a preserved ejection fraction is more challenging than heart failure with reduced ejection fraction because of the necessity of excluding other potential noncardiac causes of symptoms suggestive of heart failure. Like heart failure with reduced ejection fraction, heart failure with a preserved ejection fraction is associated with significant morbidity and mortality. Therapies targeting neurohormonal activation pathways such as the renin-angiotensin-aldosterone system have been ineffective in treating heart failure with a preserved ejection fraction, and novel treatment strategies are necessary for this population.62 The disparate ejection fraction values found between heart failure with a preserved ejection fraction and heart failure with reduced ejection fraction are likely due to differences in myocardial remodeling. In heart failure with a preserved ejection fraction, increased oxidative stress reduces myocardial nitric oxide bioavailability and cardiomyocytes become stiff and hypertrophied as a result of reduced protein kinase G activity.63 Myocardial remodeling in heart failure with reduced ejection fraction is driven by cardiomyocyte death caused by ischemia, infection, or toxicity resulting in increased collagen production.63 If ischemic conditions such as coronary artery disease and acute coronary syndrome can be better treated in AHF, the proportion of patients with heart failure with a preserved ejection fraction is likely to rise.
Initial Therapy Options for AHF
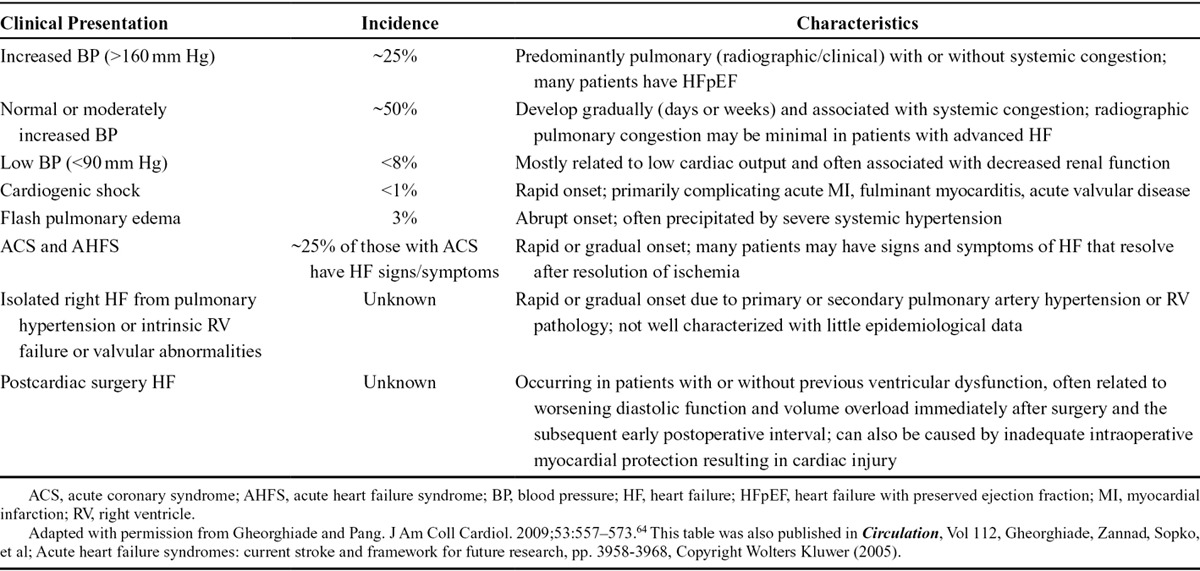
To facilitate treatment of AHF, patients can be classified into subgroups depending on their clinical presentation (Table 3).64 Blood pressure is particularly important in this regard. Many patients (~50%) present with normal or moderately increased blood pressure, and about 25% have high blood pressure (systolic >160 mm Hg). Few patients (<8%) present with low blood pressure (<90 mm Hg). Each subgroup is associated with unique characteristics that necessitate a personalized treatment approach.
TABLE 3.
Clinical Profiles of Patients Who Present With Acute Heart Failure
American College of Cardiology Foundation/American Heart Association Guidelines
Diuretic therapy is the primary method of reducing fluid imbalance in AHF. The most common and effective diuretics used in AHF are loop diuretics. These agents inhibit activity of the sodium-potassium-chloride cotransporter in the thick ascending limb of the loop of Henle and prevent reabsorption of sodium and water. The American College of Cardiology Foundation/American Heart Association guidelines recommend that hospitalized patients with AHF with significant fluid overload receive prompt treatment with intravenous loop diuretics (class of recommendation I, level of evidence B).1
Nitrodilators such as nitroglycerin have been used for decades to treat vasoconstriction in AHF. Upon administration, nitrates break down to produce nitric oxide, which directly activates the protein guanylate cyclase in vascular smooth muscle cells and induces systemic relaxation. Because they have a direct chemical action, nitrodilators can be considered “chemovasodilators.” Nesiritide, a recombinant formulation of brain natriuretic peptide, also stimulates vasodilation by activating guanylate cyclase in vascular smooth muscle cells. Because it works via a receptor, nesiritide can be considered a “biovasodilator.” The American College of Cardiology Foundation/American Heart Association guidelines recommend that if symptomatic hypotension is absent, use of intravenous nitroglycerin, nitroprusside, or nesiritide may be considered an adjuvant to diuretic therapy for relief of dyspnea in patients admitted with AHF (class of recommendation IIb, level of evidence A).1 The Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure trial demonstrated no long-term benefit of nesiritide over placebo, but put to rest claims of worsened outcomes and worsened renal function with nesiritide therapy.65
Therapy with parenteral inotropes increases cardiac output by improving myocardial contractility and reducing left and right ventricle filling pressure. These agents may be the best option in the subset of patients with severe systolic dysfunction who present with low blood pressure and significantly depressed cardiac output to maintain systemic perfusion and preserve end-organ performance (class of recommendation IIb, level of evidence B).1 However, in the absence of these findings, use of inotropes is potentially harmful (class of recommendation III, level of evidence B).
Expert Opinion
Diuretics remain the mainstay of treatment for AHF despite concern about potential adverse effects. Sustained use of diuretics can lead to the development of diuretic resistance such that higher doses will be required to obtain a response. When given at high doses, diuretics can cause renin-angiotensin-aldosterone system and sympathetic nervous system activation, electrolyte disturbances, nephrotoxicity, and ototoxicity.66 Some pharmacokinetic and pharmacodynamic data suggested potential benefits with continuous infusion over intermittent bolus dosing, but results from the Diuretic Optimization Strategies Evaluation trial found no significant difference in patients’ global assessment of symptoms or change in renal function when comparing these 2 treatment strategies.67 Moreover, there were no significant differences in the aforementioned parameters when comparing high- and low-dose administration. Because patients in the Diuretic Optimization Strategies Evaluation trial had a median time from presentation to randomization of 14.6 hours, these findings may not reflect early diuretic effects.67 Previous findings of an association between poor patient outcome and high doses of diuretics may have been due to the severity of disease in patients receiving high doses rather than the diuretic dose. However, in an analysis of Acute Decompensated Heart Failure National Registry, the nearly 20,000 patients who received furosemide ≥160 mg were found to have a higher risk of in-hospital mortality, intensive care unit stay, prolonged hospitalization, or adverse renal effects compared with the nearly 63,000 patients who received furosemide <160 mg.68 Clinical experience suggests that this association may have been due to the need for higher diuretic doses in patients who already had more severe disease and a poorer prognosis. In the Renal Optimization Strategies Evaluation trial, which evaluated whether addition of low-dose dopamine (2 μg/kg/min) to diuretic therapy or addition of low-dose nesiritide (0.005 μg/kg/min without bolus) to diuretic therapy could enhance decongestion and preserve renal function compared with the addition of placebo to diuretic therapy in patients with AHF and renal dysfunction, neither strategy was able to provide these benefits.69
There is a sense among some experts that vasodilator therapy is underutilized in the management of AHF, although compelling supportive data are lacking. As reviewed elsewhere, low doses of nitrodilators have been shown to improve some aspects of dyspnea shortly after administration, and early treatment with high-dose nitrodilators was found to improve arterial oxygenation and possibly lower the risk of myocardial infarction.70 However, tolerance to the hemodynamic effects of these agents may develop after a few hours of treatment.71 Blood pressure must be closely monitored during administration of nitrodilators to prevent the development of hypotension. Care must also be taken when stopping treatment as abrupt discontinuation can induce neurohormonal activation and cause rebound vasoconstriction, resulting in an increase in blood pressure.70 The lack of adequately powered prospective studies with nitrodilators and findings that these agents may lead to worse patient outcome, especially in individuals with myocardial ischemia, necessitate that these drugs be judiciously used.70
Nesiritide was initially found to reduce pulmonary capillary wedge pressure and improve dyspnea shortly after administration.72,73 Questions about the effect of nesiritide on renal function74 and survival75 gave rise to the Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure trial to assess short-term (30 days) and long-term (6 months) clinical outcomes in a broad population of patients with acute decompensated heart failure.65 In the Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure trial, nesiritide had a neutral effect on mortality at 30 and 180 days and had no significant effect on dyspnea in this large-scale trial. In addition, renal function was not impaired by nesiritide. The rate of hypotension was increased in patients on nesiritide, but this effect was not related to the occurrence of severe adverse events. Although these findings were reassuring about the safety of vasodilator therapy, we agree with the authors’ conclusion that, based on the results from this study, “nesiritide cannot be recommended for routine use in the broad population of patients with acute heart failure.”
Inotropic agents should only be used as rescue therapy for patients with “low cardiac output” syndrome, which is characterized by peripheral hypoperfusion that is refractory to volume replacement, diuretics, and nitrodilators. Use in patients at risk for myocardial ischemia should be carefully considered because of increased rates of myocardial oxygen consumption after inotropic therapy. Potentially harmful results have been documented by findings of an increased risk of in-hospital mortality after use of these agents in a retrospective analysis of several AHF registries.76,77 However, the increased mortality could be due to confounding factors such as the most severely affected patients receiving inotropic agents and the use of such treatment for recovery of vasodilator-induced hypotension.77 In the Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure trial, treatment with milrinone did not decrease the length of hospitalization for cardiac causes and resulted in a greater risk of sustained hypotension and new atrial arrhythmias.78
Case Study—Initial Treatment
Josephine was ordered to bed rest and given 6 L of oxygen via a nasal cannula. Oxygen saturation levels were monitored for a target saturation of 94% to 98%. The diuretic furosemide was given intravenously at 40 mg to treat fluid accumulation. For relief of breathlessness and because this patient was hypertensive, intravenous nitroglycerin was initiated at 10 μg/min and up-titrated until response. Treatment with inotropic agents was not warranted.
The primary goals of initial treatment in AHF are hemodynamic stabilization of the patient, alleviation of symptoms, reduction of congestion, and prevention of further organ damage.79 An important consideration in the initial management of AHF is whether or not the patient has increased blood pressure, a common trigger of AHF. Hypertension is prevalent at the time of presentation in the majority of patients with AHF,47,64,80 including Josephine, and timely treatment is critical for preventing organ damage caused by impaired perfusion. Patients with heart failure with reduced ejection fraction should be treated in the long term with inhibitors of neurohormonal activation such as angiotensin-converting enzyme inhibitors, beta blockers, and aldosterone antagonists to enhance diuresis and reduce vasoconstriction. Josephine’s left ventricular function is more on the preserved side, but these agents may be considered for her associated cardiovascular disease. She is already on 2 of these agents, but could be considered for aldosterone antagonists as well. Continuing and optimizing these life-saving medications as tolerated during hospitalization is important, and their use should be a major consideration at discharge and during chronic follow-up.
Morphine can be used to alleviate breathlessness in patients with AHF, but caution must be taken because its use may be associated with increased length of hospitalization and higher mortality.81 In the case of AHF with hyponatremia, the V2-selective vasopressin antagonist tolvaptan should be used to improve neurocognition.1 Hydralazine and isosorbide dinitrate therapy is indicated in African American patients with heart failure with reduced ejection fraction who remain symptomatic despite concomitant use of angiotensin-converting enzyme inhibitors, beta blockers, and aldosterone antagonists.1
Assessing Adequacy of AHF Therapy
Residual congestion at discharge is associated with an increased risk of morbidity and mortality,82 underscoring the need for assessing the adequacy of therapy throughout hospitalization.
American College of Cardiology Foundation/American Heart Association Guidelines
These guidelines recommend that the effect of treatment should be monitored with careful measurement of fluid intake and output, vital signs, body weight that is determined at the same time each day, and clinical signs and symptoms of systemic perfusion and congestion. Daily serum electrolytes, blood urea nitrogen, and creatinine concentrations should be measured during the use of intravenous diuretics or active titration of medications (class of recommendation I, level of evidence C).1
Patients with AHF often have impaired renal function, which can reduce the effectiveness of loop diuretics. The American College of Cardiology Foundation/American Heart Association guidelines recommend that low-dose dopamine infusion may be considered, in addition to loop diuretic therapy, to improve diuresis and better preserve renal function and renal blood flow (class of recommendation IIb, level of evidence B).1
An alternative approach to diuretics for volume removal in AHF is ultrafiltration. Ultrafiltration facilitates the extracorporeal removal of plasma water from whole blood across a semipermeable membrane with a transmembrane pressure gradient.83 Ultrafiltration has several potential advantages over traditional therapy for diuresis, including closer regulation of the hourly rate of fluid removal, avoidance of diuretic-associated electrolyte abnormalities, and a greater level of sodium removal for a given amount of volume.84 Although initial studies provided promising safety and efficacy data,85,86 a subsequent study in patients with cardiorenal syndrome and persistent congestion did not demonstrate a significant advantage of ultrafiltration over bolus diuretic therapy.87 Larger studies in broader patient populations are needed to determine the risk:benefit profile and cost-effectiveness of ultrafiltration as a method of fluid removal in AHF.84 The American College of Cardiology Foundation/American Heart Association guidelines recommend that, if all diuretic strategies are unsuccessful, ultrafiltration may be considered for patients with (a) obvious volume overload to alleviate congestive symptoms and fluid weight (class of recommendation IIb, level of evidence B) and (b) refractory congestion not responding to medical therapy (class of recommendation IIb, level of evidence C).1 However, limited clinical experience, accessibility, and financial limitations may prevent its use in some care settings.88
The validation of brain natriuretic peptide or N-terminal probrain natriuretic peptide as biomarkers for the assessment of AHF led to investigation of their ability to guide patient-care decisions. The American College of Cardiology Foundation/American Heart Association guidelines recommend monitoring levels of either of these natriuretic peptides to provide guidance for AHF therapy (class of recommendation IIb, level of evidence C).1
Expert Opinion
Clinical trials have found inpatient improvements in levels of decongestion to be variable, with significant numbers of patients still experiencing symptoms of congestion upon discharge.84 Dyspnea can aid in the guidance of therapy because lack of dyspnea relief has been associated with slower rates of decongestion and worse patient outcome.89 Hemoconcentration is associated with aggressive diuretic use and worsening of renal function, but improved survival.90 Therefore, we recommend the aggressive use of diuretics, even in the presence of an initial worsening of renal function, to continue the process of decongestion.
The initial relief of dyspnea caused by pulmonary congestion is a welcome initial improvement in patients hospitalized with AHF. However, the clinical assessment should include orthopnea and dyspnea on exertion because these can often reveal residual congestion requiring further diuresis. Even in patients reporting symptomatic improvement, the presence of residual congestion at the time of discharge has been associated with a poor prognosis.91,92
In addition to persistent and residual congestion, episodes of in-hospital worsening heart failure have emerged as an important early prognostic sign because they have been associated with slower improvement of dyspnea, longer hospitalizations, higher readmission rates, and increased mortality.93,94 Patients with worsening heart failure should be considered treatment failures because worsening heart failure is defined as a failure in improvement of dyspnea on initial therapies or a subsequent need during hospitalization for increased or rescue intervention with additional parenteral pharmacological or mechanical support. Worsening heart failure occurs in up to 40% of patients hospitalized with AHF and identifies those who may benefit from intensive management.94,95 If risk factors for worsening heart failure can be identified, earlier interventions may prevent such episodes and lead to improved outcomes.96
Observational studies have shown that improved clinical outcomes are associated with lowering of brain natriuretic peptide/N-terminal probrain natriuretic peptide levels,97,98 but randomized controlled trials have yielded mixed results on the benefit of using natriuretic peptide levels to guide therapy.99 Criticism of some of the negative studies was due to the fact that natriuretic peptide levels were not drawn with any frequency and, if they were, results were not acted upon. Interestingly, a comprehensive meta-analyses showed that brain natriuretic peptide-guided therapy reduced all-cause mortality compared with standard clinical care, especially in patients younger than 75 years.100,101 Taken with the American College of Cardiology Foundation/American Heart Association guidelines, these findings, and those reviewed by others,102–104 suggest that guidance of patient care by brain natriuretic peptide/N-terminal probrain natriuretic peptide levels may be an effective method of managing AHF.
Case Study—Reassessment
At 48 hours after hospital admission, Josephine was resting in the intermediate care unit with improved symptoms. Her blood pressure had decreased to 136/92 mm Hg but was still increased due to background hypertension. She had lost 3 pounds since admission. Her symptoms of dyspnea, cough, wheezing, and leg edema had improved. However, her orthopnea and dyspnea on exertion persisted. Brain natriuretic peptide and troponin I levels were reduced to 275 pg/mL and 50 ng/L, respectively. Blood urea nitrogen levels had decreased to 25 mg/dL, serum creatinine levels had increased to 1.3 mg/dL, and estimated glomerular filtration rate had decreased to 45 mL/min/1.73 m2. Additional intravenous doses of furosemide were administered and resulted in further decongestion. Her white blood cell count decreased to 9000 cells/mm3 and liver enzyme levels returned to normal. Electrolyte levels were normal. Chronic therapeutic administration of enalapril, bisoprolol, and triamterene was continued during hospitalization.
Significant progress had been made in treating Josephine since her presentation at the ED. Going forward, it is important to educate the patient about further steps to help with blood pressure control such as losing weight and regular physical activity. Her symptoms of congestion were alleviated by reducing fluid volume, leading to reduced stress and anxiety. However, the presence of residual orthopnea and dyspnea on exertion indicated that substantial congestion remained. Furosemide therapy should be continued, even though her serum creatinine level had increased, to complete the removal of excess fluid and decongestion. Biomarker levels taken after initial treatment can provide valuable information about patient outcome because reductions in admission brain natriuretic peptide levels are associated with improved clinical outcome. The >46% reduction from Josephine’s admission brain natriuretic peptide levels, coupled with brain natriuretic peptide levels <300 pg/mL, are suggestive of a positive patient outcome.105
A common oversight that can negatively impact regulation of hypertension and fluid retention is the discontinuation of chronic oral medications after admission to the hospital and thus it is important to ensure continuation of these therapies as tolerated. Patients experiencing large degrees of volume loss may experience hypotension and should have their antihypertensive agents adjusted as needed.
Preparing for Discharge and Beyond
In preparation for discharge, patient education concerning management of AHF and any comorbidities is beneficial in reducing future hospitalizations, mortality, and healthcare costs.106 A social support system is also valuable in helping the patient comply with medication and follow-up recommendations, maintain a healthy lifestyle, and reduce stress. Lack of a social support system has been associated with an increased risk of rehospitalization and mortality.107
American College of Cardiology Foundation/American Heart Association Guidelines
These guidelines have several recommendations for management of AHF after discharge.1 Patients should receive specific education to facilitate self-care (class of recommendation I, level of evidence B). In addition, patients with chronic heart failure should have a clear, detailed, and evidence-based plan of care that ensures the achievement of guideline-directed medical therapy goals, effective management of comorbid conditions, timely follow-up with the healthcare team, appropriate dietary and physical activities, and compliance with Secondary Prevention Guidelines for cardiovascular disease (class of recommendation I, level of evidence C). This plan of care should be updated regularly and made readily available to all members of each patient’s healthcare team.
Expert Opinion
Identification of high-risk patients, as previously described, is important for determining when patients should be discharged. Early discharge, especially when residual congestion is present, can increase the likelihood of further morbidity, rehospitalization, and result in increased healthcare costs. For low-risk patients, discharge may be appropriate after a short observation period, but randomized clinical trials are necessary to determine the effectiveness of such an approach.50 Patient outcome is associated with levels of congestion at time of discharge and typically decongestion should be optimal before the patient’s discharge. Methods of outpatient decongestion are not as effective and, in patients with persistent congestion, rehospitalization could be required. It is also clear that utilization of biomarkers near the time of discharge provides an important element in risk stratification. Biomarkers of wall stress (natriuretic peptides), necrosis (troponins), inflammation (ST2), fibrosis (galectin-3), and kidney injury (neutrophil gelatinase-associated lipocalin, cystatin C) may all prove helpful in this regard, albeit availability of such tests may be limited in routine practice.
A personalized treatment plan should be developed before discharge that takes into account the physiological characteristics of the patient’s disease in addition to their social support system, resources, motivation, and ability to understand their disease. Using a teach-back method can aid in confirming that the patient understands the reasons for pharmacologic therapy and lifestyle adjustments. Medications should be reviewed for those that can cause fluid retention or that have negative inotropic effects. Dietary sodium requires careful restriction and water intake should be decreased as needed; dietary adjustments are an important part of the discharge education program to promote compliance with low sodium intake. The patient should be encouraged to increase levels of physical activity because exercise training (or regular physical activity) has been shown to be safe and may be beneficial in this population.15 For patients with sleep apnea, continuous positive airway pressure may be appropriate to help prevent congestion and to improve left ventricular ejection fraction and cardiac function.108
Another component of patient education that can help reduce rehospitalization rates is addressing known triggers of AHF. As mentioned previously, hypertension is a common precipitant of AHF hospitalization and thus pharmacologic regulation of blood pressure is important for patient management. Patient education about the rationale for prescribed drugs can help improve compliance. Atherosclerosis is another common condition in patients with AHF; hyperlipidemia can be treated with statins to reduce the risk of AHF. Glucose levels should also be regulated in diabetic patients with AHF. Chronic kidney disease is also common in patients with heart failure, so it is important to evaluate the patient’s renal function and plan heart failure treatment accordingly; agents that exacerbate kidney dysfunction should be avoided.109 Obesity has been found to be associated with an increased risk for AHF and thus patients should be encouraged to lose weight through exercise and diet modification. Education about the detrimental effects of tobacco, alcohol, cocaine, and amphetamine use should be conducted, as appropriate. Drugs for comorbid conditions such as cardiotoxic chemotherapy regimens and trastuzumab can trigger AHF. In these patients, heart function should be closely monitored for left ventricular dysfunction, and an angiotensin-converting-enzyme inhibitor, such as enalapril, may be given early on to prevent cardiotoxicity.110
Patient education should begin early after admission and continue through all levels of care in the hospital. This requires effort from multiple members of the patient care team, including physicians and nurses. Education should not be limited to the patient alone; family members or caregivers should be included when possible. Care of the patients does not end when they are discharged from the hospital because patients will likely require additional assistance from pharmacists, social workers, and their own family.
Case Study—Discharge
Josephine was prepared for discharge Friday afternoon, approximately 5.5 days after presentation to the ED. On Friday morning, Josephine had an hour-long education session with a nurse educator. The nurse educator detailed the causes of AHF and the rationale for pharmacologic therapies using the teach-back method. Josephine was instructed to limit her sodium intake to <3000 mg/d and fluid intake to ≤2000 mL/d. She was also instructed to make daily recordings of her weight to detect fluid retention. Finally, Josephine was encouraged to begin a daily exercise program and warned about the risks of tobacco and heavy alcohol consumption. Josephine’s daughter, Catherine, will make regular visits to help Josephine make healthy food choices, participate in physical activity, and be compliant with her drug prescriptions.
Rehospitalization of patients with AHF is common and results in substantial healthcare costs. Patient education concerning approaches to prevent the recurrence of AHF is critical. Identifying a strong support network before discharge can decrease the chance of readmission. Congestion can quickly develop after discharge and require readmission. A follow-up visit should be conducted soon after discharge to monitor for increased fluid retention and adjust medications as needed. Many disease management programs are targeting follow-up to occur within 1 week of discharge. Patient management plans must include strategies to optimize patient compliance, because noncompliance with follow-up visits, treatment regimens, and dietary restrictions is common and leads to poor outcomes.111 In a recently published analysis of 30-day readmission rates of Medicare beneficiaries hospitalized for heart failure, pneumonia, or acute myocardial infarction, the 30-day readmission rate for patients with heart failure was 24.8%, and 35.2% of those readmissions were for recurrent heart failure. The majority of readmissions (61%) for patients initially hospitalized with heart failure occurred within the first 15 days of the 30-day period, demonstrating the need for early, effective transitional care strategies.19
THE FUTURE—AHF THERAPIES IN DEVELOPMENT
A number of new treatment options are being studied that may become available for the future treatment of AHF. Several of these agents are well into clinical development, including levosimendan, serelaxin, omecamtiv mecarbil, and ularitide. Levosimendan is an inotropic agent with additional vasodilatory properties.112 These effects are mediated by enhanced sensitivity of troponin C to intracellular calcium and opening of adenosine triphosphate-dependent potassium channels in smooth muscle cells, respectively. Clinical trials with levosimendan have had mixed results. In the randomized, double-blind, active-controlled phase 3 Survival of Patients With Acute Heart Failure in Need of Intravenous Inotropic Support trial, a short-term intravenous infusion of levosimendan did not improve all-cause mortality at 31 or 180 days compared with dobutamine.113 The Randomized Evaluation of Intravenous Levosimendan Efficacy I and II double-blind, placebo-controlled trials showed that intravenous infusion of levosimendan over 24 hours, when added to standard treatment, improved the short-term clinical course of patients with acute decompensated heart failure. However, treatment with levosimendan was associated with an increased incidence of hypotension and cardiac arrhythmias during the infusion period, and deaths were numerically higher in levosimendan-treated patients.114 Levosimendan is currently approved for use in approximately 40 countries, but it is not approved for use in the United States.
Serelaxin is a recombinant form of human relaxin-2, an endogenous hormone that mediates maternal adaptations to pregnancy through binding to the relaxin receptor. Serelaxin induces receptor-mediated initiation of vasodilation (biovasodilation) resulting in increased renal blood flow, arterial compliance, and cardiac output.115 This mechanism of action clearly distinguishes it from nitrodilators that do not act in a receptor-dependent manner. Animal studies have identified other activities that may contribute to the beneficial effects of serelaxin, including antifibrotic and antiinflammatory effects.115 In the Relaxin in Acute Heart Failure trial, intravenous serelaxin relieved dyspnea, reduced evidence of organ damage, reduced the incidence of in-hospital worsening of heart failure (as measured by the need for intravenous therapies and mechanical or ventilator support), lowered long-term mortality across multiple subgroups of patients with AHF, and had a benign safety profile.46,116,117 The response to serelaxin was consistent, regardless of left ventricular ejection fraction.117,118 For enrollment into the Relaxin in Acute Heart Failure trial, patients were required to have systolic blood pressure >125 mm Hg and increased brain natriuretic peptide levels, but could have reduced or preserved ejection fraction. Therefore, although the trial population contains a large fraction of all patients with AHF, it does not encompass all subgroups of AHF patients such as those with hypotension. In addition, rehospitalization rates were unchanged, a finding that could be due in part to reductions in mortality. The randomized, double-blind, placebo-controlled, phase 3 Relaxin in Acute Heart Failure-2 trial is ongoing.119 More than 6000 patients with AHF are expected to be enrolled with patients receiving study medication in addition to standard treatment. The primary efficacy endpoint is time to confirmed cardiovascular death during the follow-up period of 180 days. The Relaxin in Acute Heart Failure-2 trial is expected to be completed in June 2016.
Omecamtiv mecarbil is an investigational inotropic agent that selectively activates cardiac myosin by a unique mechanism. The rate of myosin cross-bridge formation is increased by activation of myocardial adenosine triphosphatase and reduced phosphate binding to myosin, resulting in greater myocardial contractility. Intracellular levels of calcium are not altered by omecamtiv. In contrast to existing inotropic agents, omecamtiv increases the duration of systole without changing the rate of left ventricular pressure development.120 In healthy volunteers, intravenous infusion of omecamtiv increased ejection fraction and fractional shortening and prolonged systolic ejection time.121 In a small clinical trial, omecamtiv was found to improve cardiac function in stable heart failure patients with left ventricular dysfunction.122 To date, the efficacy of omecamtiv for the treatment of AHF has not been reported in full, but a randomized, double-blind, placebo-controlled, dose-finding phase 2 trial in patients with AHF and left ventricular systolic dysfunction was recently completed. In this study, the Acute Treatment with Omecamtiv Mecarbil to Increase Contractility in Acute Heart Failure trial, the primary endpoint of dyspnea relief was not met.123 However, the cohort of patients treated with the highest dose of the drug, which was to achieve plasma concentrations of 310 ng/mL, showed greater dyspnea relief compared with placebo (51% versus 37%, P = 0.03). Other favorable trends were observed, including reductions in worsening heart failure and supraventricular arrhythmias, and no increase in ventricular arrhythmias. Postrandomization myocardial infarctions (nonfatal, all-index hospitalization) were numerically increased in the omecamtiv groups (5 of 303 patients) than in the placebo groups (2 of 303 patients) but this was not of concern because 2 of the 5 events in the omecamtiv groups occurred long after the infusion had taken place; 1 of the 5 events occurred after the patient had received an intervention for angina that was present before randomization; and the 2 other events occurred in patients with troponin elevations typical of AHF.124 This study will help identify the dosing regimen to be used in future phase 3 trials.
Ularitide is a synthetic form of the renal natriuretic peptide urodilatin. The effects of ularitide are mediated by cyclic guanosine monophosphate and include vasodilation and inhibition of renal sodium reabsorption and the renin-angiotensin-aldosterone system. In 2 randomized, double-blind, placebo-controlled trials, intravenous infusion of ularitide had significant beneficial effects on hemodynamics and symptoms in patients with decompensated AHF.125,126 Specifically, pulmonary capillary wedge pressure, systemic vascular resistance, cardiac index, and dyspnea were improved, without deleterious effects on renal function. The randomized, double-blind, placebo-controlled, phase 3 Efficacy and Safety of Ularitide for the Treatment of Acute Decompensated Heart Failure trial is ongoing.127 More than 2000 patients are expected to be enrolled, with patients receiving study medication in addition to standard treatment. The primary efficacy endpoint is improvement in a hierarchical clinical composite that includes the following: patient global assessment of symptoms, persistent or worsening heart failure requiring an intervention, and all-cause mortality. This trial is expected to be completed in March 2015.
It is the hope that 1 or more of these agents will help to satisfy the unmet need for therapies that enhance symptom relief and reduce the substantial morbidity and mortality associated with AHF.
CONCLUSIONS
The management of AHF currently remains clinically challenging. However, even with incomplete evidence and imperfect drugs, we can pay careful attention to diagnosis, choice of initial therapy, in-hospital monitoring of response, and discharge planning to optimize outcomes. These approaches can ensure that the best care is provided to patients until new information and new therapies become available.
DISCLOSURES
Technical assistance with editing, figure preparation, and styling of the article for submission was provided by Oxford PharmaGenesis Inc., Newtown, Pennsylvania, and was funded by Novartis Pharmaceuticals Corporation, East Hanover, New Jersey. The authors were fully responsible for all content and editorial decisions and received no financial support or other form of compensation related to the development of this article. The opinions expressed in the article are those of the authors and Novartis Pharmaceuticals Corporation had no influence on the contents. S.L.T. is a consultant for Novartis, and BEAT Biotherapeutics. A.S.M. is a consultant for BG Medicine and Alere; research support was received from BG Medicine, Alere, Abbott, Nexus, and Novartis. A.B.S. received current grant support from Abbott Diagnostics, Centers for Medicaid and Medicare Services (CMS), NIH/NHLBI (K12HL1090), UL1TR000445 National Center for Advancing Translational Sciences, Centers for Disease Control, and Roche Diagnostics; current consultant for Roche Diagnostics, Novartis, Alere Diagnostics, and Trevena.
REFERENCES
- 1.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–e319. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 2.Lindenfeld J, Albert NM, Boehmer JP, et al. Heart Failure Society of America. HFSA 2010 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2010;16:e1–e194. [Google Scholar]
- 3.Go AS, Mozaffarian D, Roger VL, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heidenreich PA, Albert NM, Allen LA, et al. American Heart Association Advocacy Coordinating Committee; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Stroke Council. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–619. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thygesen K, Mair J, Giannitsis E, et al. Study Group on Biomarkers in Cardiology of ESC Working Group on Acute Cardiac Care. How to use high-sensitivity cardiac troponins in acute cardiac care. Eur Heart J. 2012;33:2252–2257. doi: 10.1093/eurheartj/ehs154. [DOI] [PubMed] [Google Scholar]
- 6.Wang CS, FitzGerald JM, Schulzer M, et al. Does this dyspneic patient in the emergency department have congestive heart failure? JAMA. 2005;294:1944–1956. doi: 10.1001/jama.294.15.1944. [DOI] [PubMed] [Google Scholar]
- 7.Joseph SM, Cedars AM, Ewald GA, et al. Acute decompensated heart failure: contemporary medical management. Tex Heart Inst J. 2009;36:510–520. [PMC free article] [PubMed] [Google Scholar]
- 8.Maisel A, Mueller C, Adams K, Jr, et al. State of the art: using natriuretic peptide levels in clinical practice. Eur J Heart Fail. 2008;10:824–839. doi: 10.1016/j.ejheart.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 9.Kim HN, Januzzi JL., Jr Natriuretic peptide testing in heart failure. Circulation. 2011;123:2015–2019. doi: 10.1161/CIRCULATIONAHA.110.979500. [DOI] [PubMed] [Google Scholar]
- 10.Maisel A, Hollander JE, Guss D, et al. Rapid Emergency Department Heart Failure Outpatient Trial investigators. Primary results of the Rapid Emergency Department Heart Failure Outpatient Trial (REDHOT). A multicenter study of B-type natriuretic peptide levels, emergency department decision making, and outcomes in patients presenting with shortness of breath. J Am Coll Cardiol. 2004;44:1328–1333. doi: 10.1016/j.jacc.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 11.Maisel A, Mueller C, Nowak R, et al. Mid-region pro-hormone markers for diagnosis and prognosis in acute dyspnea: results from the BACH (Biomarkers in Acute Heart Failure) trial. J Am Coll Cardiol. 2010;55:2062–2076. doi: 10.1016/j.jacc.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 12.Daniels LB, Clopton P, Potocki M, et al. Influence of age, race, sex, and body mass index on interpretation of midregional pro atrial natriuretic peptide for the diagnosis of acute heart failure: results from the BACH multinational study. Eur J Heart Fail. 2012;14:22–31. doi: 10.1093/eurjhf/hfr157. [DOI] [PubMed] [Google Scholar]
- 13.Maisel AS, Peacock WF, McMullin N, et al. Timing of immunoreactive B-type natriuretic peptide levels and treatment delay in acute decompensated heart failure: an ADHERE (Acute Decompensated Heart Failure National Registry) analysis. J Am Coll Cardiol. 2008;52:534–540. doi: 10.1016/j.jacc.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Storrow AB, Jenkins C, Self W, et al. The burden of acute heart failure on US emergency departments. JACC Heart Fail. 2014;2:269–277. doi: 10.1016/j.jchf.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collins SP, Storrow AB. Moving toward comprehensive acute heart failure risk assessment in the emergency department: the importance of self-care and shared decision making. JACC Heart Fail. 2013;1:273–280. doi: 10.1016/j.jchf.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collins S, Storrow AB, Kirk JD, et al. Beyond pulmonary edema: diagnostic, risk stratification, and treatment challenges of acute heart failure management in the emergency department. Ann Emerg Med. 2008;51:45–57. doi: 10.1016/j.annemergmed.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Lee DS, Schull MJ, Alter DA, et al. Early deaths in patients with heart failure discharged from the emergency department: a population-based analysis. Circ Heart Fail. 2010;3:228–235. doi: 10.1161/CIRCHEARTFAILURE.109.885285. [DOI] [PubMed] [Google Scholar]
- 18.Ezekowitz JA, Bakal JA, Kaul P, et al. Acute heart failure in the emergency department: short and long-term outcomes of elderly patients with heart failure. Eur J Heart Fail. 2008;10:308–314. doi: 10.1016/j.ejheart.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 19.Dharmarajan K, Hsieh AF, Lin Z, et al. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309:355–363. doi: 10.1001/jama.2012.216476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 21.Vaduganathan M, Bonow RO, Gheorghiade M. Thirty-day readmissions: the clock is ticking. JAMA. 2013;309:345–346. doi: 10.1001/jama.2012.205110. [DOI] [PubMed] [Google Scholar]
- 22.Fonarow GC, Adams KF, Jr, Abraham WT, et al. ADHERE Scientific Advisory Committee, Study Group, and Investigators. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA. 2005;293:572–580. doi: 10.1001/jama.293.5.572. [DOI] [PubMed] [Google Scholar]
- 23.Collins SP, Storrow AB. Acute heart failure risk stratification: can we define low risk? Heart Fail Clin. 2009;5:75–83, vii. doi: 10.1016/j.hfc.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 24.Marik PE, Flemmer M. Narrative review: the management of acute decompensated heart failure. J Intensive Care Med. 2012;27:343–353. doi: 10.1177/0885066611403260. [DOI] [PubMed] [Google Scholar]
- 25.Collins SP, Gheorghiade M, Weintraub NL, et al. Society of Chest Pain Centers Acute Heart Failure Committee. Risk stratification. Crit Pathw Cardiol. 2008;7:96–102. [Google Scholar]
- 26.Peacock WF, 4th, De Marco T, Fonarow GC, et al. ADHERE Investigators. Cardiac troponin and outcome in acute heart failure. N Engl J Med. 2008;358:2117–2126. doi: 10.1056/NEJMoa0706824. [DOI] [PubMed] [Google Scholar]
- 27.Samsky MD, Patel CB, DeWald TA, et al. Cardiohepatic interactions in heart failure: an overview and clinical implications. J Am Coll Cardiol. 2013;61:2397–2405. doi: 10.1016/j.jacc.2013.03.042. [DOI] [PubMed] [Google Scholar]
- 28.Felker GM, Hasselblad V, Tang WH, et al. Troponin I in acute decompensated heart failure: insights from the ASCEND-HF study. Eur J Heart Fail. 2012;14:1257–1264. doi: 10.1093/eurjhf/hfs110. [DOI] [PubMed] [Google Scholar]
- 29.Arenja N, Reichlin T, Drexler B, et al. Sensitive cardiac troponin in the diagnosis and risk stratification of acute heart failure. J Intern Med. 2012;271:598–607. doi: 10.1111/j.1365-2796.2011.02469.x. [DOI] [PubMed] [Google Scholar]
- 30.Collins SP, Lindsell CJ, Jenkins CA, et al. Risk stratification in acute heart failure: rationale and design of the STRATIFY and DECIDE studies. Am Heart J. 2012;164:825–834. doi: 10.1016/j.ahj.2012.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Collins SP, Lindsell CJ, Naftilan AJ, et al. Low-risk acute heart failure patients: external validation of the Society of Chest Pain Center’s recommendations. Crit Pathw Cardiol. 2009;8:99–103. doi: 10.1097/HPC.0b013e3181b5a534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. ClinicalTrials.gov. Identifying high-and low-risk heart failure patients in the emergency department (the STRATIFY study). Available at: http://clinicaltrials.gov/show/NCT00508638. Accessed April 25, 2014.
- 33.Maisel AS, Choudhary R. Biomarkers in acute heart failure—state of the art. Nat Rev Cardiol. 2012;9:478–490. doi: 10.1038/nrcardio.2012.60. [DOI] [PubMed] [Google Scholar]
- 34.Lassus J, Gayat E, Mueller C, et al. GREAT-Network. Incremental value of biomarkers to clinical variables for mortality prediction in acutely decompensated heart failure: the Multinational Observational Cohort on Acute Heart Failure (MOCA) study. Int J Cardiol. 2013;168:2186–2194. doi: 10.1016/j.ijcard.2013.01.228. [DOI] [PubMed] [Google Scholar]
- 35.Estabrooks PA, Boyle M, Emmons KM, et al. Harmonized patient-reported data elements in the electronic health record: supporting meaningful use by primary care action on health behaviors and key psychosocial factors. J Am Med Inform Assoc. 2012;19:575–582. doi: 10.1136/amiajnl-2011-000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Connor CM, Whellan DJ, Lee KL, et al. HF-ACTION Investigators. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1439–1450. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riegel B, Moser DK, Anker SD, et al. American Heart Association Council on Cardiovascular Nursing; American Heart Association Council on Cardiovascular Nursing; American Heart Association Council on Clinical Cardiology; American Heart Association Council on Nutrition, Physical Activity, and Metabolism; American Heart Association Interdisciplinary Council on Quality of Care and Outcomes Research. State of the science: promoting self-care in persons with heart failure: a scientific statement from the American Heart Association. Circulation. 2009;120:1141–1163. doi: 10.1161/CIRCULATIONAHA.109.192628. [DOI] [PubMed] [Google Scholar]
- 38.DeWalt DA, Malone RM, Bryant ME, et al. A heart failure self-management program for patients of all literacy levels: a randomized, controlled trial [ISRCTN11535170]. BMC Health Serv Res. 2006;6:30. doi: 10.1186/1472-6963-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jovicic A, Holroyd-Leduc JM, Straus SE. Effects of self-management intervention on health outcomes of patients with heart failure: a systematic review of randomized controlled trials. BMC Cardiovasc Disord. 2006;6:43. doi: 10.1186/1471-2261-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu JR, Moser DK, Lennie TA, et al. Medication adherence in patients who have heart failure: a review of the literature. Nurs Clin North Am. 2008;43:133–153; vii. doi: 10.1016/j.cnur.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 41.van der Wal MH, Jaarsma T. Adherence in heart failure in the elderly: problem and possible solutions. Int J Cardiol. 2008;125:203–208. doi: 10.1016/j.ijcard.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 42.Légaré F, Witteman HO. Shared decision making: examining key elements and barriers to adoption into routine clinical practice. Health Aff (Millwood) 2013;32:276–284. doi: 10.1377/hlthaff.2012.1078. [DOI] [PubMed] [Google Scholar]
- 43.Makoul G, Clayman ML. An integrative model of shared decision making in medical encounters. Patient Educ Couns. 2006;60:301–312. doi: 10.1016/j.pec.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 44.Barry MJ, Edgman-Levitan S. Shared decision making—pinnacle of patient-centered care. N Engl J Med. 2012;366:780–781. doi: 10.1056/NEJMp1109283. [DOI] [PubMed] [Google Scholar]
- 45.Flynn D, Knoedler MA, Hess EP, et al. Engaging patients in health care decisions in the emergency department through shared decision-making: a systematic review. Acad Emerg Med. 2012;19:959–967. doi: 10.1111/j.1553-2712.2012.01414.x. [DOI] [PubMed] [Google Scholar]
- 46.Metra M, Cotter G, Davison BA, et al. RELAX-AHF Investigators. Effect of serelaxin on cardiac, renal, and hepatic biomarkers in the Relaxin in Acute Heart Failure (RELAX-AHF) development program: correlation with outcomes. J Am Coll Cardiol. 2013;61:196–206. doi: 10.1016/j.jacc.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 47.Adams KF, Jr, Fonarow GC, Emerman CL, et al. ADHERE Scientific Advisory Committee and Investigators. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J. 2005;149:209–216. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 48.Sweitzer NK, Lopatin M, Yancy CW, et al. Comparison of clinical features and outcomes of patients hospitalized with heart failure and normal ejection fraction (> or =55%) versus those with mildly reduced (40% to 55%) and moderately to severely reduced (<40%) fractions. Am J Cardiol. 2008;101:1151–1156. doi: 10.1016/j.amjcard.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heywood JT, Fonarow GC, Costanzo MR, et al. ADHERE Scientific Advisory Committee and Investigators. High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database. J Card Fail. 2007;13:422–430. doi: 10.1016/j.cardfail.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 50.Collins SP, Pang PS, Fonarow GC, et al. Is hospital admission for heart failure really necessary? The role of the emergency department and observation unit in preventing hospitalization and rehospitalization. J Am Coll Cardiol. 2013;61:121–126. doi: 10.1016/j.jacc.2012.08.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cotter G, Metra M, Milo-Cotter O, et al. Fluid overload in acute heart failure–re-distribution and other mechanisms beyond fluid accumulation. Eur J Heart Fail. 2008;10:165–169. doi: 10.1016/j.ejheart.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 52.Metra M, Felker GM, Zacà V, et al. Acute heart failure: multiple clinical profiles and mechanisms require tailored therapy. Int J Cardiol. 2010;144:175–179. doi: 10.1016/j.ijcard.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 53.Colombo PC, Onat D, Harxhi A, et al. Peripheral venous congestion causes inflammation, neurohormonal, and endothelial cell activation. Eur Heart J. 2014;35:448–454. doi: 10.1093/eurheartj/eht456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith GL, Lichtman JH, Bracken MB, et al. Renal impairment and outcomes in heart failure: systematic review and meta-analysis. J Am Coll Cardiol. 2006;47:1987–1996. doi: 10.1016/j.jacc.2005.11.084. [DOI] [PubMed] [Google Scholar]
- 55.Ronco C, Cicoira M, McCullough PA. Cardiorenal syndrome type 1: pathophysiological crosstalk leading to combined heart and kidney dysfunction in the setting of acutely decompensated heart failure. J Am Coll Cardiol. 2012;60:1031–1042. doi: 10.1016/j.jacc.2012.01.077. [DOI] [PubMed] [Google Scholar]
- 56.Saner FH, Heuer M, Meyer M, et al. When the heart kills the liver: acute liver failure in congestive heart failure. Eur J Med Res. 2009;14:541–546. doi: 10.1186/2047-783X-14-12-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heymans S, Hirsch E, Anker SD, et al. Inflammation as a therapeutic target in heart failure? A scientific statement from the Translational Research Committee of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2009;11:119–129. doi: 10.1093/eurjhf/hfn043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mann DL, Bristow MR. Mechanisms and models in heart failure: the biomechanical model and beyond. Circulation. 2005;111:2837–2849. doi: 10.1161/CIRCULATIONAHA.104.500546. [DOI] [PubMed] [Google Scholar]
- 59.Owan TE, Hodge DO, Herges RM, et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 60.Yancy CW, Lopatin M, Stevenson LW, et al. ADHERE Scientific Advisory Committee and Investigators. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am Coll Cardiol. 2006;47:76–84. doi: 10.1016/j.jacc.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 61.Lam CS, Donal E, Kraigher-Krainer E, et al. Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13:18–28. doi: 10.1093/eurjhf/hfq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J. 2011;32:670–679. doi: 10.1093/eurheartj/ehq426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 64.Gheorghiade M, Pang PS. Acute heart failure syndromes. J Am Coll Cardiol. 2009;53:557–573. doi: 10.1016/j.jacc.2008.10.041. [DOI] [PubMed] [Google Scholar]
- 65.O’Connor CM, Starling RC, Hernandez AF, et al. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011;365:32–43. doi: 10.1056/NEJMoa1100171. [DOI] [PubMed] [Google Scholar]
- 66.Felker GM, O’Connor CM, Braunwald E Heart Failure Clinical Research Network Investigators. Loop diuretics in acute decompensated heart failure: necessary? Evil? A necessary evil? Circ Heart Fail. 2009;2:56–62. doi: 10.1161/CIRCHEARTFAILURE.108.821785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Felker GM, Lee KL, Bull DA, et al. NHLBI Heart Failure Clinical Research Network. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011;364:797–805. doi: 10.1056/NEJMoa1005419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Peacock WF, Costanzo MR, De Marco T, et al. ADHERE Scientific Advisory Committee and Investigators. Impact of intravenous loop diuretics on outcomes of patients hospitalized with acute decompensated heart failure: insights from the ADHERE registry. Cardiology. 2009;113:12–19. doi: 10.1159/000164149. [DOI] [PubMed] [Google Scholar]
- 69.Chen HH, Anstrom KJ, Givertz MM, et al. NHLBI Heart Failure Clinical Research Network. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: the ROSE acute heart failure randomized trial. JAMA. 2013;310:2533–2543. doi: 10.1001/jama.2013.282190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Metra M, Teerlink JR, Voors AA, et al. Vasodilators in the treatment of acute heart failure: what we know, what we don’t. Heart Fail Rev. 2009;14:299–307. doi: 10.1007/s10741-008-9127-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Elkayam U, Bitar F, Akhter MW, et al. Intravenous nitroglycerin in the treatment of decompensated heart failure: potential benefits and limitations. J Cardiovasc Pharmacol Ther. 2004;9:227–241. doi: 10.1177/107424840400900403. [DOI] [PubMed] [Google Scholar]
- 72.Colucci WS, Elkayam U, Horton DP, et al. Intravenous nesiritide, a natriuretic peptide, in the treatment of decompensated congestive heart failure. Nesiritide Study Group. N Engl J Med. 2000;343:246–253. doi: 10.1056/NEJM200007273430403. [DOI] [PubMed] [Google Scholar]
- 73.Publication Committee for the VMAC Investigators. Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial. JAMA. 2002;287:1531–1540. doi: 10.1001/jama.287.12.1531. [DOI] [PubMed] [Google Scholar]
- 74.Sackner-Bernstein JD, Skopicki HA, Aaronson KD. Risk of worsening renal function with nesiritide in patients with acutely decompensated heart failure. Circulation. 2005;111:1487–1491. doi: 10.1161/01.CIR.0000159340.93220.E4. [DOI] [PubMed] [Google Scholar]
- 75.Sackner-Bernstein JD, Kowalski M, Fox M, et al. Short-term risk of death after treatment with nesiritide for decompensated heart failure: a pooled analysis of randomized controlled trials. JAMA. 2005;293:1900–1905. doi: 10.1001/jama.293.15.1900. [DOI] [PubMed] [Google Scholar]
- 76.Abraham WT, Adams KF, Fonarow GC, et al. ADHERE Scientific Advisory Committee and Investigators; ADHERE Study Group. In-hospital mortality in patients with acute decompensated heart failure requiring intravenous vasoactive medications: an analysis from the Acute Decompensated Heart Failure National Registry (ADHERE). J Am Coll Cardiol. 2005;46:57–64. doi: 10.1016/j.jacc.2005.03.051. [DOI] [PubMed] [Google Scholar]
- 77.Teerlink JR, Metra M, Zacà V, et al. Agents with inotropic properties for the management of acute heart failure syndromes. Traditional agents and beyond. Heart Fail Rev. 2009;14:243–253. doi: 10.1007/s10741-009-9153-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cuffe MS, Califf RM, Adams KF, Jr, et al. Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF) Investigators. Short-term intravenous milrinone for acute exacerbation of chronic heart failure: a randomized controlled trial. JAMA. 2002;287:1541–1547. doi: 10.1001/jama.287.12.1541. [DOI] [PubMed] [Google Scholar]
- 79.Weintraub NL, Collins SP, Pang PS, et al. American Heart Association Council on Clinical Cardiology and Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation. Acute heart failure syndromes: emergency department presentation, treatment, and disposition: current approaches and future aims: a scientific statement from the American Heart Association. Circulation. 2010;122:1975–1996. doi: 10.1161/CIR.0b013e3181f9a223. [DOI] [PubMed] [Google Scholar]
- 80.Gheorghiade M, Abraham WT, Albert NM, et al. OPTIMIZE-HF Investigators and Coordinators. Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure. JAMA. 2006;296:2217–2226. doi: 10.1001/jama.296.18.2217. [DOI] [PubMed] [Google Scholar]
- 81.Peacock WF, Hollander JE, Diercks DB, et al. Morphine and outcomes in acute decompensated heart failure: an ADHERE analysis. Emerg Med J. 2008;25:205–209. doi: 10.1136/emj.2007.050419. [DOI] [PubMed] [Google Scholar]
- 82.Metra M, Davison B, Bettari L, et al. Is worsening renal function an ominous prognostic sign in patients with acute heart failure? The role of congestion and its interaction with renal function. Circ Heart Fail. 2012;5:54–62. doi: 10.1161/CIRCHEARTFAILURE.111.963413. [DOI] [PubMed] [Google Scholar]
- 83.Felker GM, Mentz RJ. Diuretics and ultrafiltration in acute decompensated heart failure. J Am Coll Cardiol. 2012;59:2145–2153. doi: 10.1016/j.jacc.2011.10.910. [DOI] [PubMed] [Google Scholar]
- 84.Muñoz D, Felker GM. Approaches to decongestion in patients with acute decompensated heart failure. Curr Cardiol Rep. 2013;15:335. doi: 10.1007/s11886-012-0335-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bart BA, Boyle A, Bank AJ, et al. Ultrafiltration versus usual care for hospitalized patients with heart failure: the Relief for Acutely Fluid-Overloaded Patients With Decompensated Congestive Heart Failure (RAPID-CHF) trial. J Am Coll Cardiol. 2005;46:2043–2046. doi: 10.1016/j.jacc.2005.05.098. [DOI] [PubMed] [Google Scholar]
- 86.Costanzo MR, Guglin ME, Saltzberg MT, et al. UNLOAD Trial Investigators. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol. 2007;49:675–683. doi: 10.1016/j.jacc.2006.07.073. [DOI] [PubMed] [Google Scholar]
- 87.Bart BA, Goldsmith SR, Lee KL, et al. Heart Failure Clinical Research Network. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med. 2012;367:2296–2304. doi: 10.1056/NEJMoa1210357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kazory A, Bellamy FB, Ross EA. Ultrafiltration for acute decompensated heart failure: financial implications. Int J Cardiol. 2012;154:246–249. doi: 10.1016/j.ijcard.2011.05.073. [DOI] [PubMed] [Google Scholar]
- 89.Metra M, Teerlink JR, Felker GM, et al. Dyspnoea and worsening heart failure in patients with acute heart failure: results from the Pre-RELAX-AHF study. Eur J Heart Fail. 2010;12:1130–1139. doi: 10.1093/eurjhf/hfq132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Testani JM, Chen J, McCauley BD, et al. Potential effects of aggressive decongestion during the treatment of decompensated heart failure on renal function and survival. Circulation. 2010;122:265–272. doi: 10.1161/CIRCULATIONAHA.109.933275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kociol RD, McNulty SE, Hernandez AF, et al. NHLBI Heart Failure Network Steering Committee and Investigators. Markers of decongestion, dyspnea relief, and clinical outcomes among patients hospitalized with acute heart failure. Circ Heart Fail. 2013;6:240–245. doi: 10.1161/CIRCHEARTFAILURE.112.969246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ambrosy AP, Pang PS, Khan S, et al. EVEREST Trial Investigators. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: findings from the EVEREST trial. Eur Heart J. 2013;34:835–843. doi: 10.1093/eurheartj/ehs444. [DOI] [PubMed] [Google Scholar]
- 93.Weatherley BD, Milo-Cotter O, Felker GM, et al. Early worsening heart failure in patients admitted with acute heart failure—a new outcome measure associated with long-term prognosis? Fundam Clin Pharmacol. 2009;23:633–639. doi: 10.1111/j.1472-8206.2009.00697.x. [DOI] [PubMed] [Google Scholar]
- 94.Cotter G, Metra M, Weatherley BD, et al. Physician-determined worsening heart failure: a novel definition for early worsening heart failure in patients hospitalized for acute heart failure—association with signs and symptoms, hospitalization duration, and 60-day outcomes. Cardiology. 2010;115:29–36. doi: 10.1159/000249280. [DOI] [PubMed] [Google Scholar]
- 95.Torre-Amione G, Milo-Cotter O, Kaluski E, et al. Early worsening heart failure in patients admitted for acute heart failure: time course, hemodynamic predictors, and outcome. J Card Fail. 2009;15:639–644. doi: 10.1016/j.cardfail.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 96.O’Connor CM, Mentz RJ, Cotter G, et al. The PROTECT in-hospital risk model: 7-day outcome in patients hospitalized with acute heart failure and renal dysfunction. Eur J Heart Fail. 2012;14:605–612. doi: 10.1093/eurjhf/hfs029. [DOI] [PubMed] [Google Scholar]
- 97.Bettencourt P, Azevedo A, Pimenta J, et al. N-terminal-pro-brain natriuretic peptide predicts outcome after hospital discharge in heart failure patients. Circulation. 2004;110:2168–2174. doi: 10.1161/01.CIR.0000144310.04433.BE. [DOI] [PubMed] [Google Scholar]
- 98.Dhaliwal AS, Deswal A, Pritchett A, et al. Reduction in BNP levels with treatment of decompensated heart failure and future clinical events. J Card Fail. 2009;15:293–299. doi: 10.1016/j.cardfail.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 99.O’Donoghue M, Braunwald E. Natriuretic peptides in heart failure: should therapy be guided by BNP levels? Nat Rev Cardiol. 2010;7:13–20. doi: 10.1038/nrcardio.2009.197. [DOI] [PubMed] [Google Scholar]
- 100.Porapakkham P, Porapakkham P, Zimmet H, et al. B-type natriuretic peptide-guided heart failure therapy: A meta-analysis. Arch Intern Med. 2010;170:507–514. doi: 10.1001/archinternmed.2010.35. [DOI] [PubMed] [Google Scholar]
- 101.Troughton RW, Frampton CM, Brunner-La Rocca HP, et al. Effect of B-type natriuretic peptide-guided treatment of chronic heart failure on total mortality and hospitalization: an individual patient meta-analysis. Eur Heart J. 2014;35:1559–1567. doi: 10.1093/eurheartj/ehu090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Troughton R, Michael Felker G, Januzzi JL., Jr Natriuretic peptide-guided heart failure management. Eur Heart J. 2014;35:16–24. doi: 10.1093/eurheartj/eht463. [DOI] [PubMed] [Google Scholar]
- 103.Fiuzat M, O’Connor CM, Gueyffier F, et al. Biomarker-guided therapies in heart failure: a forum for unified strategies. J Card Fail. 2013;19:592–599. doi: 10.1016/j.cardfail.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 104.Pruett AE, Lee AK, Patterson JH, et al. Biomarker guided therapy for heart failure: focus on natriuretic peptides. Curr Cardiol Rev. doi: 10.2174/1573403X09666131117123525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Di Somma S, Magrini L, Pittoni V, et al. In-hospital percentage BNP reduction is highly predictive for adverse events in patients admitted for acute heart failure: the Italian RED Study. Crit Care. 2010;14:R116. doi: 10.1186/cc9067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Koelling TM, Johnson ML, Cody RJ, et al. Discharge education improves clinical outcomes in patients with chronic heart failure. Circulation. 2005;111:179–185. doi: 10.1161/01.CIR.0000151811.53450.B8. [DOI] [PubMed] [Google Scholar]
- 107.Luttik ML, Jaarsma T, Moser D, et al. The importance and impact of social support on outcomes in patients with heart failure: an overview of the literature. J Cardiovasc Nurs. 2005;20:162–169. doi: 10.1097/00005082-200505000-00007. [DOI] [PubMed] [Google Scholar]
- 108.Kasai T. Sleep apnea and heart failure. J Cardiol. 2012;60:78–85. doi: 10.1016/j.jjcc.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 109.Metra M, Cotter G, Gheorghiade M, et al. The role of the kidney in heart failure. Eur Heart J. 2012;33:2135–2142. doi: 10.1093/eurheartj/ehs205. [DOI] [PubMed] [Google Scholar]
- 110.Cardinale D, Colombo A, Sandri MT, et al. Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation. 2006;114:2474–2481. doi: 10.1161/CIRCULATIONAHA.106.635144. [DOI] [PubMed] [Google Scholar]
- 111.Grady KL, Dracup K, Kennedy G, et al. Team management of patients with heart failure: A statement for healthcare professionals from The Cardiovascular Nursing Council of the American Heart Association. Circulation. 2000;102:2443–2456. doi: 10.1161/01.cir.102.19.2443. [DOI] [PubMed] [Google Scholar]
- 112.Nieminen MS, Fruhwald S, Heunks LM, et al. Levosimendan: current data, clinical use and future development. Heart Lung Vessel. 2013;5:227–245. [PMC free article] [PubMed] [Google Scholar]
- 113.Mebazaa A, Nieminen MS, Packer M, et al. SURVIVE Investigators. Levosimendan vs dobutamine for patients with acute decompensated heart failure: the SURVIVE Randomized Trial. JAMA. 2007;297:1883–1891. doi: 10.1001/jama.297.17.1883. [DOI] [PubMed] [Google Scholar]
- 114.Packer M, Colucci W, Fisher L, et al. REVIVE Heart Failure Study Group. Effect of levosimendan on the short-term clinical course of patients with acutely decompensated heart failure. JACC Heart Fail. 2013;1:103–111. doi: 10.1016/j.jchf.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 115.Teichman SL, Unemori E, Teerlink JR, et al. Relaxin: review of biology and potential role in treating heart failure. Curr Heart Fail Rep. 2010;7:75–82. doi: 10.1007/s11897-010-0010-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Teerlink JR, Cotter G, Davison BA, et al. RELAXin in Acute Heart Failure (RELAX-AHF) Investigators. Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): a randomised, placebo-controlled trial. Lancet. 2013;381:29–39. doi: 10.1016/S0140-6736(12)61855-8. [DOI] [PubMed] [Google Scholar]
- 117.Metra M, Ponikowski P, Cotter G, et al. Effects of serelaxin in subgroups of patients with acute heart failure: results from RELAX-AHF. Eur Heart J. 2013;34:3128–3136. doi: 10.1093/eurheartj/eht371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Filippatos G, Teerlink JR, Farmakis D, et al. Serelaxin in acute heart failure patients with preserved left ventricular ejection fraction: results from the RELAX-AHF trial. Eur Heart J. 2014;35:1041–1050. doi: 10.1093/eurheartj/eht497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. ClinicalTrials.gov. Efficacy, safety and tolerability of serelaxin when added to standard therapy in AHF (RELAX-AHF-2). Available at: http://clinicaltrials.gov/ct2/show/NCT01870778. Accessed April 25, 2014.
- 120.Malik FI, Hartman JJ, Elias KA, et al. Cardiac myosin activation: a potential therapeutic approach for systolic heart failure. Science. 2011;331:1439–1443. doi: 10.1126/science.1200113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Teerlink JR, Clarke CP, Saikali KG, et al. Dose-dependent augmentation of cardiac systolic function with the selective cardiac myosin activator, omecamtiv mecarbil: a first-in-man study. Lancet. 2011;378:667–675. doi: 10.1016/S0140-6736(11)61219-1. [DOI] [PubMed] [Google Scholar]
- 122.Cleland JG, Teerlink JR, Senior R, et al. The effects of the cardiac myosin activator, omecamtiv mecarbil, on cardiac function in systolic heart failure: a double-blind, placebo-controlled, crossover, dose-ranging phase 2 trial. Lancet. 2011;378:676–683. doi: 10.1016/S0140-6736(11)61126-4. [DOI] [PubMed] [Google Scholar]
- 123.Teerlink JR, Felker GM, McMurray JJ, et al. ATOMIC-AHF Investigators and Patients. Amsterdam, The Netherlands: Presented at: European Socity of Cardiology (ESC) Congress; 2013. A phase 2 study of intravenous omecamtiv mecarbil, a novel cardiac myosin activator, in patients with acute heart failure. Available at: www.clinicaltrialresults.org/Slides/ESC%202013/Teerlink_ATOMIC.ppt. Accessed April 22, 2014. [Google Scholar]
- 124.Kaiser C. MIs in ATOMIC-HF trial pose no problems. Available at: http://www.medpagetoday.com/MeetingCoverage/HFSA/41817. Accessed April 25, 2014.
- 125.Mitrovic V, Lüss H, Nitsche K, et al. Effects of the renal natriuretic peptide urodilatin (ularitide) in patients with decompensated chronic heart failure: a double-blind, placebo-controlled, ascending-dose trial. Am Heart J. 2005;150:1239. doi: 10.1016/j.ahj.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 126.Mitrovic V, Seferovic PM, Simeunovic D, et al. Haemodynamic and clinical effects of ularitide in decompensated heart failure. Eur Heart J. 2006;27:2823–2832. doi: 10.1093/eurheartj/ehl337. [DOI] [PubMed] [Google Scholar]
- 127. ClinicalTrials.gov. Efficacy and safety of ularitide for the treatment of acute decompensated heart failure (TRUE-AHF). Available at: http://clinicaltrials.gov/ct2/show/nct01661634. Accessed April 25, 2014.


