Article first published online 24 December 2014.
Supplemental Digital Content is Available in the Text.
Key Words: iron deficiency anemia, inflammatory bowel disease, ulcerative colitis, Crohn's disease, ferric maltol
Abstract
Background:
Iron deficiency anemia (IDA) is frequently seen in inflammatory bowel disease. Traditionally, oral iron supplementation is linked to extensive gastrointestinal side effects and possible disease exacerbation. This multicenter phase-3 study tested the efficacy and safety of ferric maltol, a complex of ferric (Fe3+) iron with maltol (3-hydroxy-2-methyl-4-pyrone), as a novel oral iron therapy for IDA.
Methods:
Adult patients with quiescent or mild-to-moderate ulcerative colitis or Crohn's disease, mild-to-moderate IDA (9.5–12.0 g/dL and 9.5–13.0 g/dL in females and males, respectively), and documented failure on previous oral ferrous products received oral ferric maltol capsules (30 mg twice a day) or identical placebo for 12 weeks according to a randomized, double-blind, placebo-controlled study design. The primary efficacy endpoint was change in hemoglobin (Hb) from baseline to week 12. Safety and tolerability were assessed.
Results:
Of 329 patients screened, 128 received randomized therapy (64 ferric maltol-treated and 64 placebo-treated patients) and comprised the intent-to-treat efficacy analysis: 55 ferric maltol patients (86%) and 53 placebo patients (83%) completed the trial. Significant improvements in Hb were observed with ferric maltol versus placebo at weeks 4, 8, and 12: mean (SE) 1.04 (0.11) g/dL, 1.76 (0.15) g/dL, and 2.25 (0.19) g/dL, respectively (P < 0.0001 at all time-points; analysis of covariance). Hb was normalized in two-thirds of patients by week 12. The safety profile of ferric maltol was comparable with placebo, with no impact on inflammatory bowel disease severity.
Conclusions:
Ferric maltol provided rapid clinically meaningful improvements in Hb and showed a favorable safety profile, suggesting its possible use as an alternative to intravenous iron in IDA inflammatory bowel disease.
Anemia is a frequent complication of inflammatory bowel disease (IBD) estimated to occur in more than 70% of patients with Crohn's disease (CD) or ulcerative colitis (UC).1–3 Iron deficiency is the most common cause of anemia in IBD and arises due to insufficient dietary intake, malabsorption, chronic inflammation, and/or intestinal bleeding.4–6 Iron deficiency anemia (IDA) in IBD has been shown to have important detrimental effects on patient's day-to-day functioning, with a wide range of clinical symptoms and psychosocial impairments including fatigue, headache, dizziness, shortness of breath, tachycardia, reduced cognitive function, depression, and decreased ability to work.4,5,7 Alone or in combination, these symptoms substantially impair quality of life (QoL) and, in severe cases, can lead to hospitalization and serious comorbidities.1,8–10
Oral ferrous (Fe2+) iron preparations such as ferrous sulfate, ferrous gluconate, and ferrous fumarate have traditionally been used to treat IDA-associated with IBD and a range of other conditions including nutritional deficiency, chronic kidney disease, and obstetric and gynecological conditions.6,11 However, a major difficulty with oral ferrous iron salts is that a large proportion of the administered iron is not absorbed and subsequently undergoes oxidation in the gut lumen and/or mucosa. This leads to the generation of reactive oxygen species, which can damage the intestine and cause a range of adverse gastrointestinal effects such as abdominal pain, nausea, diarrhea, and constipation.7,12–14 Poor gastrointestinal tolerance and the potential risk of an IBD flare after oral iron supplementation can reduce patient compliance with oral ferrous iron-based therapy.2,15,16
The intestinal mucosa in patients with IBD is particularly sensitive to iron ingestion.2,17–19 Experimental and clinical data suggest that reactive oxygen species derived from oral ferrous iron might lead to re-activation or worsening of IBD, although clinical trial findings are not consistent in this respect.17,20–24 Nevertheless, based on accumulated knowledge, the labeling for a number of branded ferrous sulfate- and ferrous fumarate-based formulations in a number of countries now carries contraindications or special warnings regarding their use in patients with IBD.
For all patients with IDA, and for patients with IBD in particular, there is an ongoing need for well-tolerated oral iron therapies that rapidly normalize hemoglobin (Hb) without adversely affecting IBD activity.4,5 Current guidelines for treating IDA in patients with clinically active IBD address well-known drawbacks of oral therapy with ferrous iron salts, including poor gastrointestinal tolerability, slow treatment effects, and a potential for disease worsening.6 In Europe, intravenous iron is recommended for the treatment of patients with IBD with previous intolerance to oral iron and those with severe anemia (Hb <10 g/dL).6,25 Although intravenous iron is undoubtedly effective in correcting anemia, it is associated with higher health care costs and the inconvenience of intravenous infusion,1,6,26 with a small risk of anaphylactic reactions.27–29
Ferric maltol is a novel oral iron therapy based on a stable complex of ferric (Fe3+) iron with maltol (3-hydroxy-2-methyl-4-pyrone), a naturally occurring sugar derivative that has been used for many years as a food additive.30 After oral ingestion of ferric maltol, ferric iron reaches the intestinal mucosa in complex form, which is believed to allow more efficient uptake of elemental ferric iron into enterocytes compared with ferrous iron salts.31–33 In addition, iron uptake can be achieved at a relatively low daily oral iron dose.34–36 Early, experimental data indicate a clear potential for ferric maltol to provide effective oral iron treatment with a substantially reduced risk of gastrointestinal side effects compared with ferrous salts.31–33,37,38 A 12-week open-label, uncontrolled proof-of-concept study assessed the efficacy and tolerability of ferric maltol in a mixed cohort of patients with and without IBD with IDA and documented intolerance to ferrous sulfate.39 Ferric maltol increased mean Hb from 10.6 ± 1.5 g/dL at baseline to 12.6 ± 1.6 g/dL after 12 weeks, and safety monitoring indicated a favorable tolerability profile.39
Here, we report data from a phase-3 clinical trial program assessing the efficacy and tolerability of ferric maltol during the treatment of IDA over 12 weeks in patients with IBD who were known to be intolerant of, or unresponsive to, available oral ferrous iron preparations.
METHODS
Study Design
This phase-3 clinical trial program comprised 2 identical clinical trials that evaluated patients with UC and CD according to a 12-week, randomized, double-blind, placebo-controlled, multicenter study design. As UC and CD represent the major subgroups of IBD, data from both trials were prospectively collected and analyzed as a single data set based on a predefined statistical analysis plan, which was devised after consultation with regulatory authorities.
The program was conducted across centers in Austria, Germany, Hungary, and the United Kingdom between August 2011 and December 2013. After a 7- to 14-day screening period, all patients underwent 12 weeks of randomized, double-blind treatment. Randomization to either ferric maltol or placebo was conducted through an interactive voice response system according to a centralized randomization list (SAS v9.2). Patients and all sponsor, clinical research and clinical staff were blinded to the randomization code until all randomized trial processes were complete.
Patients
Patients were males or females aged ≥18 years with a confirmed diagnosis of UC or CD. All patients were required to be in remission or to have a mild-to-moderate disease activity of either UC (as defined by a Simple Clinical Colitis Activity Index [SCCAI] score <4 at screening and randomization) or CD (as defined by a Crohn's Disease Activity Index [CDAI] score of <220 at randomization). All patients were required to have mild-to-moderate IDA, as defined by an Hb concentration ≥9.5 g/dL and <12.0 g/dL for females and ≥9.5 g/dL and <13.0 g/dL for males40 and serum ferritin levels <30 μg/L at screening. Patients were also required to have previously failed on treatment with oral ferrous products (OFP) for 1 or more of the following reasons: (1) adverse drug effects that led to withdrawal from OFP (at least one of nausea, diarrhea, constipation, abdominal pain, flatulence); (2) deterioration of the primary disease caused by OFP; (3) lack of efficacy; and (4) other signs of failure of OFP (or documented reasons why OFP could not be used).
Patients receiving protocol-allowed immunosuppressive and immunomodulatory agents (i.e., thiopurines and antitumor necrosis factor) at screening were required to have been on a stable dose for ≥4 weeks before randomization. Patients with anemia unrelated to iron deficiency or who had received depot iron preparations, erythropoietin, or blood transfusions within 12 weeks of screening were excluded. Other reasons for exclusion were oral iron treatment within 4 weeks of randomization; treatment with immunosuppressants known to induce anemia (e.g., methotrexate, cyclosporin A, tacrolimus); folate deficiency, uncorrected vitamin B12 deficiency; serum creatinine >2.0 mg/dL (176 μmol/L); abnormal liver function tests; and pregnancy.
Treatment
After screening, enrolled patients were randomized in a 1:1 ratio to receive either oral ferric maltol or placebo for 12 weeks. Previously published data suggested that in a compliant study population, 60 mg per day of ferric maltol, administered in 2 divided doses (30 mg twice a day), would result in correction of baseline anemia in the majority of subjects.39 Ferric maltol was therefore supplied in hard orange gelatin capsules, each containing 231.5 mg of ferric maltol (equivalent to 30 mg of elemental iron), to be taken orally on an empty stomach with water first thing in the morning before breakfast and last thing at night (twice a day dosing). Placebo capsules were identical in appearance to ferric maltol and were administered similarly. All study medication was packaged in white polypropylene secure containers and was supplied to patients in study drug kits that were identified by unique numbers from the randomization list. Treatment compliance was evaluated by capsule count at the end of each month (weeks 4, 8, and 12); Patients were considered non-compliant with the study medication if they were <80% or >100% compliant with the dosage schedule.
Assessments
Both primary and secondary efficacy assessments focused on changes in Hb concentration, as evaluated during routine laboratory measurements at randomization (baseline) and weeks 4, 8, and 12. All laboratory measurements were performed at a central laboratory. The primary efficacy endpoint was change in Hb concentration from baseline to week 12. Secondary efficacy endpoints included changes in Hb concentration from baseline to weeks 4 and 8, serum ferritin concentration, and percentage transferrin saturation (TSAT). A responder analysis was also conducted, where responders to treatment were defined as patients who achieved increases in Hb of ≥1 g/dL or ≥2 g/dL, or Hb normalization by week 12. Normalization of Hb was defined based on Hb values ≥12 g/dL for females or ≥13 g/dL for males.40
Clinical symptoms were evaluated at randomization and weeks 4, 8, and 12 using the SCCAI41 in patients with UC and the CDAI42 in patients with CD. Disease-specific QoL was assessed at randomization and week 12 using country-specific, validated versions of the Inflammatory Bowel Disease Questionnaire (IBDQ).43,44 General QoL was also assessed based on the 36-item Short-Form (SF-36) questionnaire.45
Safety and tolerability were assessed based on adverse events (AEs) (recorded according to MedDRA preferred terms46), vital signs measurements, and routine hematological and blood chemistry indices. Patients who developed Hb concentrations ≤8.5 g/dL and/or flare-up of UC (as defined by an SCCAI score ≥5) or CD (as defined by a CDAI score ≥320) were to be withdrawn from the study to receive standard medical treatment. Urine pregnancy tests were conducted in women, and concomitant medications were evaluated in all patients at each clinic visit. Other safety-related stopping rules for premature discontinuation included pregnancy and serious adverse events (SAEs) considered related to the study medication (based on investigator opinion that there was a reasonable possibility that the event may have been caused by the study drug).
Data Analysis
Allowing for dropouts, a sample size of 60 patients per treatment group was calculated as providing sufficient power to detect a minimum of 1.3 g/dL difference (considered the smallest clinically significant benefit) with ferric maltol over placebo based on the Wilcoxon (Mann–Whitney) rank-sum test at the 0.025 one-sided significance level and with an estimated SD of 0.165.
The primary efficacy endpoint was evaluated based on analysis of covariance (ANCOVA), with treatment, gender, and disease as factors and baseline Hb as a covariate. Multiple imputations for missing values were conducted using SAS procedures PROC MI and PROC MIANALYZE. Absolute mean and SD Hb values as well as adjusted mean treatment differences, associated standard errors (SE), and one-sided 97.5% confidence limit (CL) are presented. Changes in Hb concentration from baseline to weeks 4 and 8 were also analyzed using ANCOVA.
For the responder analysis, the proportions of patients who achieved ≥1 or ≥2 g/dL increases or normalization of Hb from baseline were analyzed by logistic regression with treatment as a fixed effect and baseline Hb as a covariate. Ferric maltol–placebo treatment effects are presented as odds ratios (ORs) with respective 95% confidence intervals (CI). Time to normalization of Hb was assessed using Kaplan–Meier methods, and median time to normalization is presented. The other secondary efficacy endpoints—iron indices, patient symptoms, and QoL—are summarized using descriptive statistics (n, mean, SD, median, and/or range).
A post hoc analysis of Hb response to ferric maltol was conducted based on subgroups of patients categorized according to their baseline disease severity (i.e., quiescent and mild/moderate). Patients were categorized separately based on IBD etiology: SCCAI scores for patients with UC and CDAI scores for patients with CD. Statistical analyses were conducted using methodology identical to that used for the primary efficacy analysis. The second post hoc analysis was also conducted to identify any effect that changes in disease activity on the primary endpoint, where patients were analyzed in categories for time since last disease flare (<6 or ≥6 mo).
Primary and secondary efficacy evaluations were based on an intention-to-treat (ITT) analysis of all randomized patients who received at least 1 dose of study medication (i.e., the ITT full analysis set [FAS]). The prespecified statistical analysis plan also included ITT efficacy analysis of the first 120 subjects randomized, as per the planned study size. A sensitivity analysis was conducted that compared findings in the FAS with the per-protocol (PP) population (i.e., all ITT patients who did not have any major protocol violations); patients who discontinued treatment early due to AEs were still counted as part of the PP population if they attended end-of-study assessments after week 12. Data from the “all-randomized” population (i.e., all patients who were randomized) also underwent statistical analysis identical to those for the FAS and PP population and constituted a further sensitivity analysis. At the time of data cutoff, patient symptom, QoL, and safety data (including AEs) were available from the first 120 randomized subjects (the preplanned population). SAEs were recorded among all randomized patients (i.e., based on the ITT FAS).
ETHICAL CONSIDERATIONS
The trial protocol and all associated materials and amendments were approved by relevant ethics committees. All study procedures were conducted in accordance with Good Clinical Practice Guidelines and the principles and recommendations outlined in the Declaration of Helsinki (1964 and subsequent revisions). Written informed consent was obtained from all patients before their participation. Trial registration was at ClinicalTrials.gov: identifier no. NCT01340872 or NCT01352221.
RESULTS
Patients
From a total of 329 patients screened, 128 were randomized to treatment (FAS); reasons for screening failure are listed in Figure 1. The PP population comprised 104 patients; reasons for exclusion from the PP population are summarized in Figure 1. A total of 55 ferric maltol-treated patients (86%) and 53 placebo patients (83%) completed 12 weeks of double-blind therapy.
FIGURE 1.
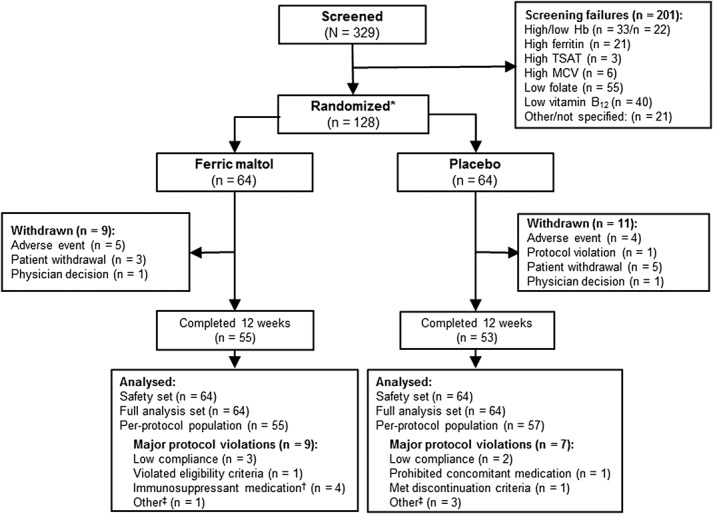
Patient disposition. *One hundred twenty-eight randomized patients were included in the main ITT FAS efficacy analysis, and the first 120 patients randomized were included in the safety analysis as predefined by statistical protocol; †Due to dose changes (n = 2) or duration of treatment (n = 1); ‡Due to timings of assessments. MCV, mean corpuscular volume.
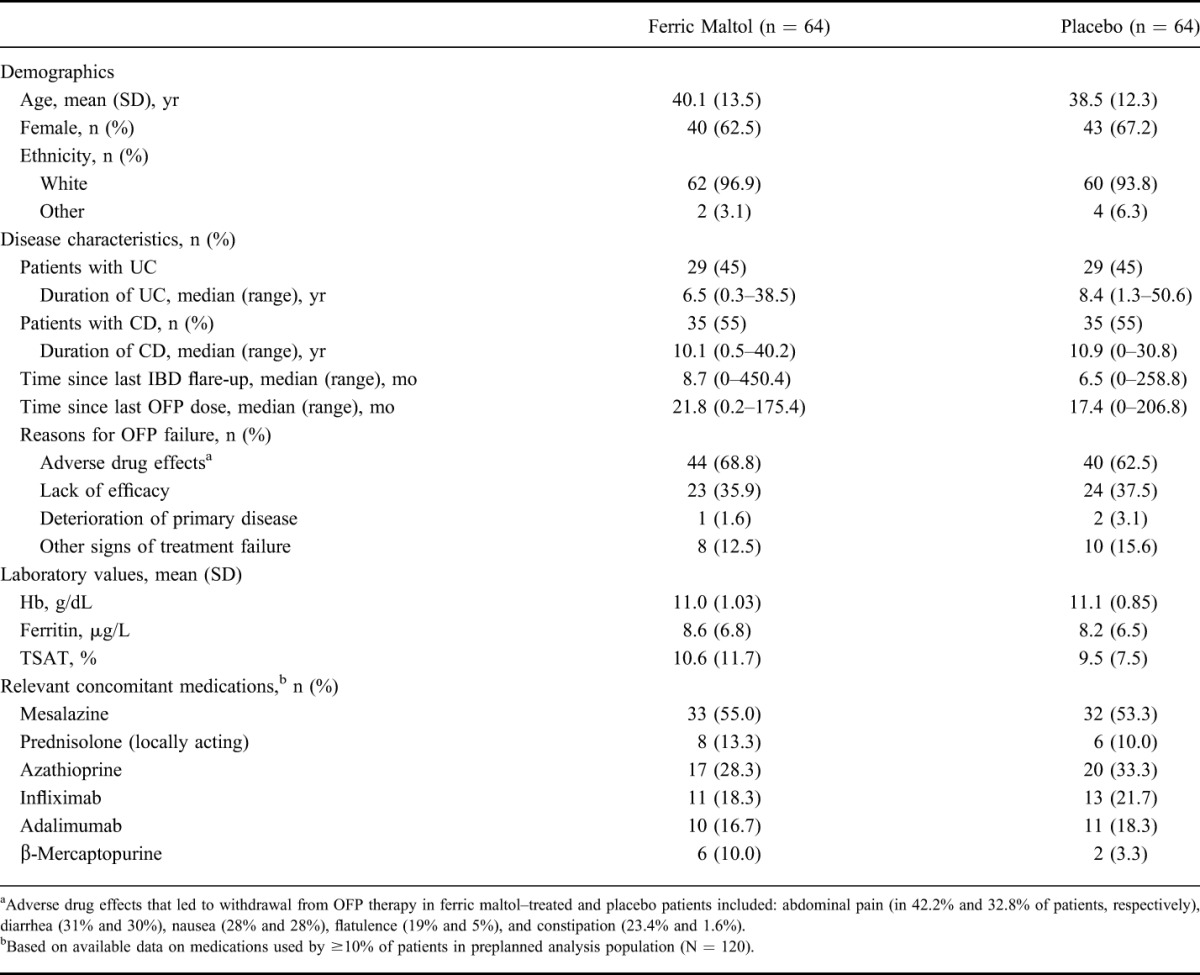
Baseline demographics and disease characteristics were generally comparable between the 2 treatment groups (Table 1). The proportions of patients with UC and CD were identical in the ferric maltol and placebo groups (45% and 55%, respectively). Adverse drug effects and lack of efficacy were the most common reasons for withdrawal from previous OFP therapy. Use of concomitant medications was also comparable between the 2 treatment groups.
TABLE 1.
Demographics and Disease Characteristics at Baseline (ITT FAS)
Treatment
Overall, patients received study medication for a median of 85 days in both treatment groups, during which time ferric maltol–treated patients received a median (range) total elemental iron dose of 4830 (330–5220) mg. The median level of treatment compliance was 98% in both groups.
Efficacy
Hemoglobin
In the primary efficacy analysis, a highly statistically significant increase in Hb concentration was observed in the ferric maltol group compared with placebo at week 12 (Fig. 2). The mean (SE) improvement in Hb in the ferric maltol group versus placebo was 2.25 (0.12) g/dL (one-sided 97.5% CL, 1.81; P < 0.0001 based on ANCOVA) (see Fig., Supplemental Digital Content 1, http://links.lww.com/IBD/A710). Absolute mean (SD) Hb concentrations improved from 11.00 (1.03) g/dL at baseline to 13.20 (1.04) g/dL at week 12 in the ferric maltol group. In the placebo group, mean (SD) Hb values were similar at baseline and week 12: 11.10 g/dL (0.85) and 11.20 g/dL (0.98), respectively. The statistical significance of these primary efficacy findings was supported by sensitivity analyses in the PP and all-randomized populations (P < 0.0001 in both populations). The preplanned analysis of the first 120 randomized patients produced very similar results to the total randomized population (mean [SE] improvement in Hb in the ferric maltol group versus placebo was 2.25 [0.19] g/dL; one-sided 97.5% CL, 1.88; P < 0.0001 based on ANCOVA; see Fig., Supplemental Digital Content 2, http://links.lww.com/IBD/A711).
FIGURE 2.
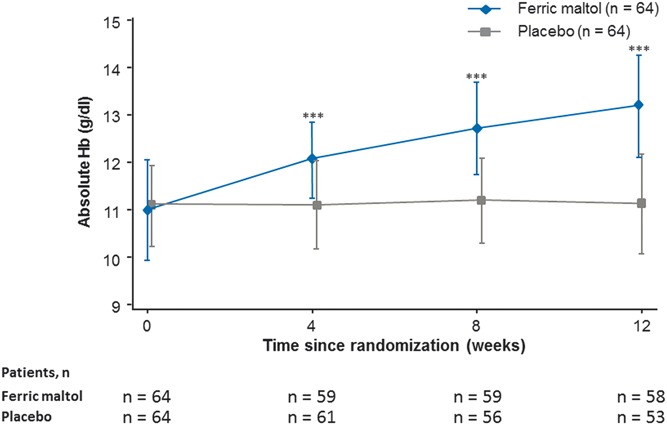
Hb concentration from baseline to week 12 (ITT FAS). Data are mean ± SD; ***P < 0.0001 (ferric maltol versus placebo based on ANCOVA).
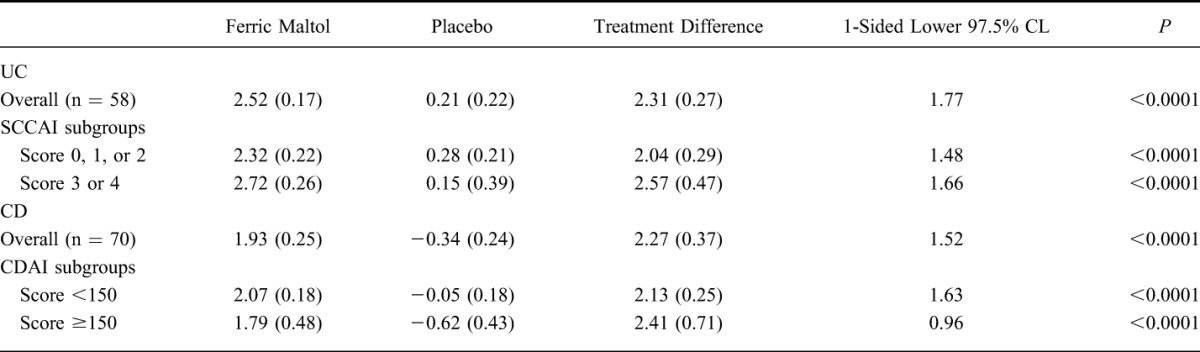
Post hoc analysis of Hb responses according to baseline disease activity scores (i.e., SCCAI scores in patients with UC and CDAI scores in patients with CD), separating patients into quiescent and mild/moderate subgroups, showed significant increases in Hb in each disease severity subgroup (Table 2). Changes were similar in magnitude to those described above for the whole patient population with IBD; despite the reduced number of patients per disease severity subgroup compared with the overall population, the lower end of the 97.5% CLs remained well above zero in patients with both UC and CD. In addition, further post hoc analysis of change in Hb according to time since last flare (<6 or ≥6 mo) did not reveal any effect of disease activity on Hb response in either patient subgroups with UC or CD (see Table, Supplemental Digital Content 3, http://links.lww.com/IBD/A712).
TABLE 2.
Change in Mean (SE) Hb (in grams per deciliters) from Baseline to Week 12 by Severity Subgroup
Secondary efficacy evaluations of changes from baseline to weeks 4 and 8 in the FAS-indicated improved Hb concentrations at both time points with ferric maltol. Absolute mean (SD) Hb concentrations improved from baseline to 12.05 (0.80) g/L at week 4 and 12.8 (0.97) g/L at week 8 in the ferric maltol group. In the placebo group, mean (SD) Hb values at these time points were 11.10 (0.97) g/L and 11.20 (0.98) g/L, respectively. Adjusted mean (SE) treatment differences (ferric maltol versus placebo) were 1.04 (0.11) g/dL at week 4 and 1.73 (0.15) g/dL at week 8 (P < 0.0001 in both cases based on ANCOVA). Again, sensitivity analyses in the PP and preplanned analysis patient populations supported these findings.
Findings from the responder analysis are summarized in Figure 3. The majority of ferric maltol–treated patients achieved ≥1 and ≥2 g/dL increases in Hb concentration or normalization of Hb by week 12. Logistic regression analysis showed that IBD etiology (UC or CD) had no effect on the achievement of these predefined treatment goals for Hb. In addition, a higher baseline Hb was associated with reduced likelihood of achieving either a ≥1 g/dL improvement from baseline (OR, 0.487; 95% CI, 0.273–0.870) or a ≥2 g/dL improvement (OR, 0.341; 95% CI, 0.176–0.660). Overall, the median time to normalization of Hb among evaluable ferric maltol–treated patients (n = 64) was 57 days. Time to normalization could not be calculated for the placebo group due to the low number of evaluable patients (n = 13).
FIGURE 3.
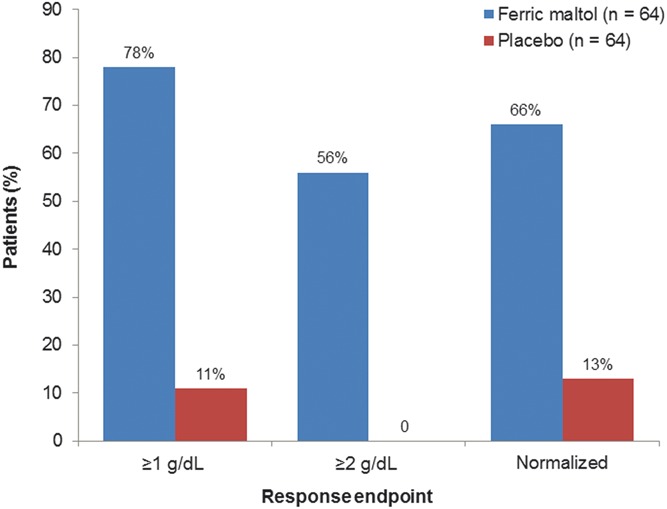
Responder analysis: patients achieving 1 and 2 g/dL increases, and normalization of Hb concentration between baseline and week 12 (ITT FAS). Ferric maltol versus placebo ORs were 41.8 (95% CI, 13.5–129.9) for ≥1 g/dL increases, not applicable for ≥2 g/dL increases, and 15.3 (95% CI, 5.9–39.3) for normalization.
Iron Indices
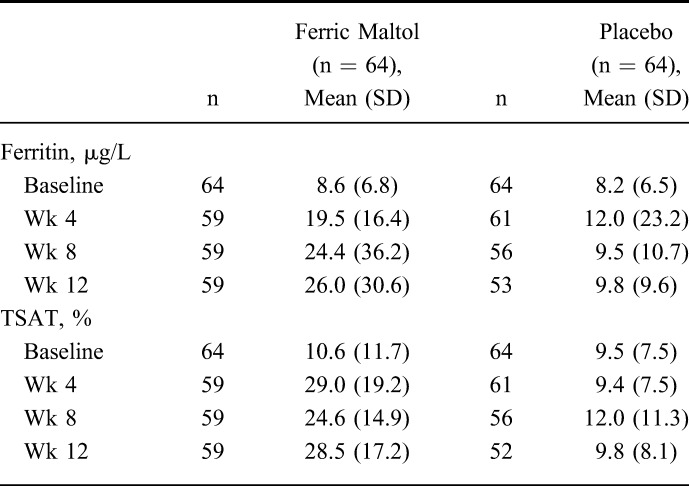
Mean absolute levels of serum ferritin and TSAT are summarized in Table 3 (see Fig., Supplemental Digital Content 2, http://links.lww.com/IBD/A711). Iron indices improved between baseline and week 12 in ferric maltol–treated patients but remained largely unchanged in the placebo group. The mean (SD) overall increase in serum ferritin was 17.3 (28.30) μg/L in ferric maltol–treated patients compared with 1.2 (7.85) μg/L in the placebo group: high variability among the placebo patients was indicated by the high SD. A mean (SD) increase of 18.0% (20.2) in TSAT was observed in the ferric maltol group compared with a small reduction (−0.4% [7.8]) in patients who received placebo.
TABLE 3.
Iron Indices Between Baseline and Week 12 (ITT FAS)
Patient Symptoms and QoL Scores
Among patients with UC, median (range) absolute SCCAI scores were 2.0 (0–3) at baseline and 2.5 (0–6) at week 12 in the ferric maltol group, and 2.0 (0–3) at baseline and 1.5 (0–7) at week 12 in the placebo group.41 Among patients with CD, median (range) absolute scores on the CDAI decreased from 75.0 (14–199) at baseline to 51.7 (2–231) at week 12 in the ferric maltol group but showed almost no change between baseline and week 12 in the placebo group, 108.0 (10–220) and 109.0 (21–285), respectively.
There were no meaningful changes in IBDQ scores in either treatment group between baseline and week 12. In ferric maltol–treated patients, mean (SD) IBDQ scores were 175.6 (31.4) at baseline and 179.7 (32.6) at week 12. Mean (SD) IBDQ scores in placebo patients at these time points were 171.0 (33.8) and 176.0 (32.2), respectively. Scores on all 10 of the SF-36 subscales either improved or remained stable between baseline and week 12 in both the ferric maltol and placebo groups. Percentage improvements in SF-36 subscores ranged from 0.3% to 18% and from −3.4% to 6.8%, respectively. The greatest increase was observed in the vitality domain: 8.2 (18%) in the ferric maltol group and 3.42 (7%) in the placebo group.
Safety and Tolerability
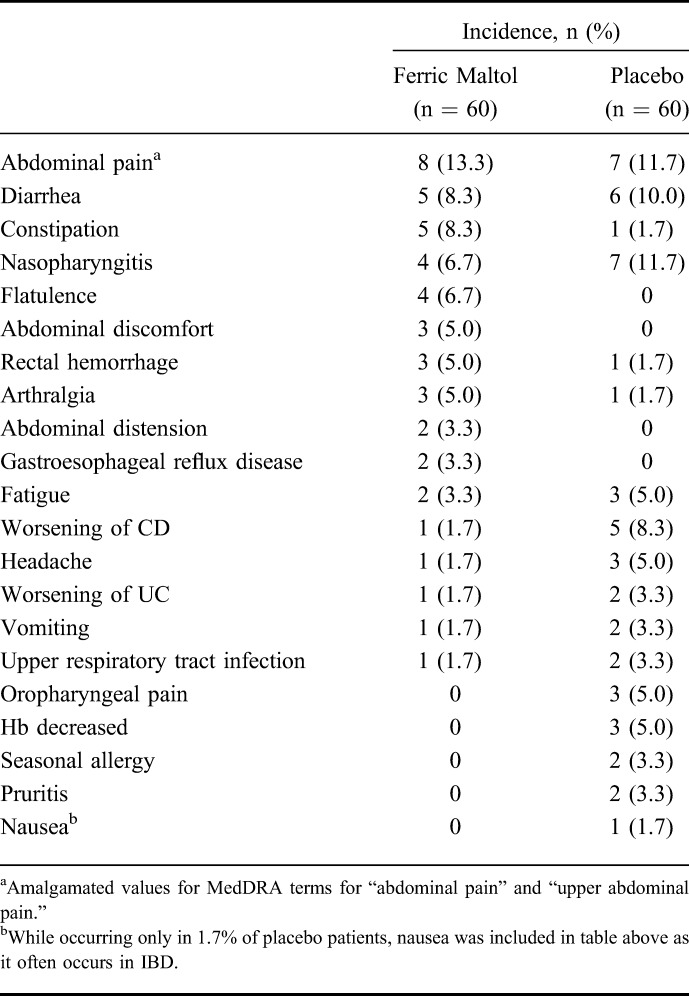
Treatment-emergent AEs were recorded in 35 patients (58%) in the ferric maltol group and in 43 patients (72%) in the placebo group. AEs were mainly gastrointestinal in nature, and most were of mild or moderate severity (Table 4). The overall incidence of gastrointestinal AEs was 23 of ferric maltol–treated patients (38.3%) and 24 placebo patients (40.0%). The most frequent individual gastrointestinal AEs in ferric maltol and placebo groups were abdominal pain (13.3% and 11.7%, respectively), diarrhea (8.3% and 10.0%, respectively), and constipation (8.3% and 1.7%, respectively). Nausea was not recorded in ferric maltol–treated patients but did occur in 1 patient (1.7%) in the placebo group. The only other treatment-emergent AE with an incidence >10% was nasopharyngitis, which was recorded in 6.7% of ferric maltol–treated patients and 11.7% of placebo patients.
TABLE 4.
Incidence of Treatment-Emergent AEs Occurring in >2% of Patients in Either Treatment Group (Preplanned Safety Set)
AEs were considered by investigators to be related to study medication in a total of 15 ferric maltol–treated patients (25.0%) and 7 placebo patients (11.7%), respectively. The most common treatment-related AEs in ferric maltol–treated patients were abdominal pain, constipation, and flatulence, each occurring in 4 patients (6.7%). The respective frequencies of these AEs in the placebo group were abdominal pain (5.0%), constipation (1.7%), and flatulence (0%). All other AEs considered treatment-related, including diarrhea and nausea, were only recorded in one patient in either group.
Study medication was discontinued prematurely due to AEs in a total of 8 patients (13%) in the ferric maltol group and 5 patients (8%) in the placebo group. All treatment-related AEs leading to discontinuation of study medication were gastrointestinal in nature: abdominal pain in 1 ferric maltol–treated patient and 2 placebo patients; diarrhea in 1 patient in each treatment group; and constipation and flatulence in 1 ferric maltol–treated patient each. Discontinuation AEs that were not related to study medication included flare-up of existing IBD in 2 patients in each treatment group (1 case each of CD and UC among ferric maltol–treated patients and 2 cases of CD with placebo), and rectal hemorrhage in 2 ferric maltol–treated patients.
SAEs were recorded in 2 patients in the ferric maltol group and 2 patients in the placebo group; all were gastrointestinal and were considered unrelated to study medication. There were no clinically meaningful safety-relevant trends among changes in routine clinical laboratory parameters, vital signs, or physical examination findings in either the ferric maltol or placebo groups. No deaths occurred during the study.
DISCUSSION
In this randomized phase-3 clinical trial program, we investigated the efficacy and tolerability of ferric maltol (ST-10) in the treatment of IDA in patients with IBD who had previously failed to respond to, or had been intolerant to, previous OFP therapy. A significant improvement in Hb concentration (2.25 g/dL) was observed in ferric maltol–treated patients (versus placebo) over 12 weeks. This is considered a clinically meaningful change based on current guidelines for the management of IDA in IBD.6 In addition, a rapid and clinically relevant increase in Hb was observed (1 g/dL by week 4), and this treatment response was not affected by baseline IBD severity (i.e., quiescent versus mild/moderate disease). Hb was normalized in two-third of patients by week 12, with a median time to normalization of less than 2 months. Substantial mean increases in iron indices (ferritin and TSAT) were also observed although data values were highly variable. Overall, oral ferric maltol was equally efficacious in patients with UC and CD, and crucially, ferric maltol demonstrated a safety profile that was comparable with placebo; only constipation occurred more frequently with ferric maltol. No detrimental impact on disease-specific QoL scores, and no adverse effect on disease severity were observed. In particular, these safety- and tolerability-related factors are worth highlighting in view of the fact that this patient cohort had a documented history of intolerance or “lack of efficacy” to OFPs.
These data demonstrate clear advantages for the use of oral ferric maltol in patients with poor tolerability on oral ferrous iron treatment and suggest that ferric maltol might be a useful alternative to intravenous iron. The favorable gastrointestinal tolerability of ferric maltol is likely due to the novel chemical and pharmacodynamic properties of the ferric iron–maltol complex. After oral ingestion, ferric iron is delivered to the intestinal mucosa in a biologically labile complex form that is believed to allow efficient uptake of elemental ferric iron into enterocytes.31–33 As a result, effective gastrointestinal iron uptake is achieved at a relatively low daily dose of elemental iron, which meets current recommendations for the use of oral iron in IBD.6,34–36 In addition, unlike unabsorbed ferrous iron, the ferric iron in iron–maltol complexes remains in a chelated form if it is not absorbed.32
The current efficacy findings seem in line with previous data reported by Harvey et al39 in the earlier proof-of-concept trial with a similar ferric–maltol complex formulation, despite some key methodological differences. Although the previous study was uncontrolled, this trial included a placebo comparator arm. The former study also included patients with and without IBD with IDA (approximately 25% with baseline Hb <9.5 g/dL), whereas only patients with IBD with mild-to-moderate anemia were included here. Nevertheless, the observed improvements in Hb were comparable, albeit slightly larger, in this study, and the proportions of patients with normalized Hb at 12 weeks were similar.
Comparisons of the current trial data with previous studies of oral ferrous salts and intravenous iron therapies are difficult due to differences in patient disease severity at baseline, elemental iron doses, treatment duration, primary study endpoints, and disease assessment scales. In a 4-week open-label study evaluating high-dose oral ferrous sulfate (200 mg thrice a day; i.e., 600 mg/d total dose) in patients with and without IBD, mean Hb increases in mildly anemic patients with UC and CD with inactive disease were 0.4 and 1.7 g/dL, respectively17; a comparably lower therapeutic response compared with that seen here. In a 12-week randomized controlled trial comparing intravenous ferric carboxymaltose (FeCarb) with oral ferrous sulfate 100 mg twice a day (FeSulf; 200 mg/d total dose) in patients with IBD, mean improvements in Hb were 2.6 and 3.0 g/dL, respectively.47 However, this FeCarb–FeSulf trial included patients who were considerably more anemic at baseline: median (range) Hb values were 8.7 (5.0–11.5) g/dL and 9.1 (5.3–11.1) g/dL, respectively. The second large, 12-week, randomized, controlled trial with intravenous FeCarb, this time versus intravenous iron sucrose (FeSuc), included patients with slightly greater anemia at baseline compared with this study (mean baseline Hb values were 10.1 and 10.3 g/dL, respectively).26 Improvements in Hb over 12 weeks with these intravenous iron formulations seemed similar in terms of magnitude and time course.
To date, no clinical study has shown a similar magnitude or time course of improvements in Hb with oral iron therapy in patients with mild-to-moderate anemia. Evaluation of ORs in the responder analysis in this study illustrated that it is more difficult to achieve a clinically significant improvement in Hb in patients with mild-to-moderate anemia than it is in patients with more severe anemia.34,35 Although inclusion of only patients with mild-to-moderate anemia was a prerequisite for the incorporation of a placebo control arm in this trial, it is tempting to speculate whether greater treatment effects could be achieved in patients with more severe anemia at baseline, as have been included in previous published studies assessing ferrous sulfate in IBD.17,47
Ferric maltol demonstrated a good tolerability profile, with a similar overall incidence of AEs compared with placebo. As a result, the proportion of patients who discontinued treatment was similar in ferric maltol–treated and placebo patients; adherence to medication was also very high in both groups. As expected, the majority of AEs in both treatment groups were gastrointestinal in nature, with most being mild or moderate in severity. Notably, only 2 patients in the ferric maltol group and 2 in the placebo groups had a flare-up of their IBD, none of which were considered related to study medication. Few patients discontinued due to study medication-related gastrointestinal AEs. These safety data compare favorably with previous studies assessing OFPs in IBD.16,17,22,48
The interpretation of data from this study is subject to a number of limitations related to the clinical trial design. First, the use of a placebo control arm as opposed to an active control limits the comparability of these findings. The principal objective of this study was to assess the use of ferric maltol in an underserved population: patients with UC and CD who were known to be intolerant of, or unresponsive to, available oral iron preparations. In line with current guidelines for the treatment of IDA in IBD, the only alternative iron replacement treatment for this group of patients is intravenous iron,6 but it was not considered appropriate to randomize patients to intravenous placebo injections to establish an active comparator group. Consequently, only patients with mild-to-moderate anemia were included for ethical reasons. Second, the low number of recorded AEs precluded any valid statistical comparison of AEs between the ferric maltol–treated and placebo groups. As a result, we can only assess the safety profile in a descriptive manner. Nevertheless, it is considered unlikely that the difference in incidence of, or instance, constipation, would constitute a statistically significant finding. Finally, it is noteworthy that although the screening failure rate in the current trial was quite high in this study, it was only 10% above those reported in other therapeutic trials in IBD.26,47 As summarized in Figure 1, to a degree this reflects the multifactorial nature of IDA in IBD. Aside from Hb outside selected inclusion limits, folate deficiency contributed the largest proportion of failures at screening. However, an amendment to the study protocol regarding folate deficiency criteria midway through this trial resulted in improved patient recruitment: some excluded patients were not “deficient” per se but had values less than or equal to the lower limit of normal.
In conclusion, this randomized controlled study demonstrated that the novel oral iron therapy, ferric maltol, provided rapid and clinically meaningful improvements in Hb concentration, normalized Hb in the majority of patients, and showed a favorable safety and tolerability profile. The favorable gastrointestinal tolerability with ferric maltol compared with currently available oral ferrous iron products, which is at least in part due to the lower doses of elemental iron allowed by this novel iron–maltol complex formulation, suggests that it may serve as an alternative to intravenous iron therapy in patients with IBD. Patients in this randomized controlled trial have continued ferric maltol treatment in a 12-month open-label extension study, and the long-term effects of ferric maltol on hemoglobin and patient QoL will be reported when data analyses are complete. Data available now from an interim safety analysis with a total treatment duration of 15 months have shown that no patients have had to withdraw due to lack of efficacy or anemia. Considering the possible implications for health care costs, convenience and safety together, these data suggest that ferric maltol could become the first choice treatment in IBD-associated IDA.
Supplementary Material
ACKNOWLEDGMENTS
AEGIS Study Group principal site investigators: Austria: Dr. Harry Fuchssteiner, Linz; Prof. Dr. C. Gasche, Wien; Dr. Michael Gschwantler, Wien; Prof. Dr. Peter Knoflach, Wels; Dr. Wolfgang Tillinger, Wien; Germany: Prof. Dr. D. C. Baumgart, Berlin; Dr. B. Bokemeyer, Minden; PD Dr. C. Büning, Berlin; PD Dr. Ulf Helwig, Oldenburg; Dr. S. Howaldt, Hamburg; Dr. Dietrich Hüppe, Herne; Dr. Annette Krummenerl, Halle; Dr. Thomas Krummenerl, Münster; Prof. Dr. Tanja Kühbacher, Hamburg; Dr. Wilfried Landry, Dachau; PD Dr. A. Lügering, Münster; Prof. Dr. Christian Maaser, Lüneburg; Dr. Michael Mroβ, Berlin; Prof. Dr. Ursula Seidler, Hannover; Prof. Dr. A. Stallmach, Jena; Prof. Dr. Jürgen Stein, Frankfurt; PD Dr. Niels Teich, Leipzig; Hungary: Dr. Gabor Horvath, Budapest; Dr. Tünde Kristóf, Miskolc; Dr. András László, Budapest; Dr. Tamás Molnár, Szeged; Dr. Ágnes Salamon, Szekszard; Prof. Dr. Z. Tulassay, Budapest; Prof. Dr. Áron Vincze, Pecs; Switzerland: PD Dr. Pierre Krayenbuehl, Uznach; UK: Dr. T. Ahmad, Exeter; Dr. Ian Beales, Norwich; Dr. Matthew Brookes, Wolverhampton; Dr. Simon Campbell, Manchester; Dr. Fraser Cummings, Southampton; Dr. Ronald Ede, Dartford; Dr. David Elphick, Chesterfield; Dr. Alan Ireland, Brighton; Dr. Deepak Kejariwal, Durham; Dr. Andy Li, Worthing; Dr. John Mansfield, Newcastle upon Tyne.
Matthew Reilly, PhD at InTouch Medical Ltd. provided editorial support in the preparation of this article, paid for by Iron Therapeutics Ltd.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.ibdjournal.org).
Supported by Iron Therapeutics Ltd.
A. Stallmach has served as a board member for Abbvie and MSD and has received consultancy fees from Takeda, speaker honoraria from Abbvie and Takeda, and travel expenses from Iron Therapeutics Ltd. B. Bokemeyer has received travel expenses from Iron Therapeutics Ltd., consultancy fees from Abbvie, MSD, Shire, Ferring, UCB, Hospira, Takeda, and Movetis, speaker honoraria from Abbvie, MSD, Ferring, Merck, FALK HLR, UCB, and Vifor and research grants from Abbvie, Ferring, and UCB. C. Gasche has received research grant and consultancy honoraria from Vifor International, speaker honoraria from Vifor Österreich, Vifor International, Fresenius Medical Care, Renapharma, and Biogena and research grants from Vifor International and AOP Pharmaceuticals. S. Howaldt has received consulting fees and travel expenses. T. Ahmad has received research funding from Iron Therapeutics Ltd., consultancy fees and research grants from Abbvie and Merck, and speaker honoraria from Merck. The remaining authors have no conflicts of interest to disclose.
REFERENCES
- 1.Stein J, Hartmann F, Dignass AU. Diagnosis and management of iron deficiency anemia in patients with IBD. Nat Rev Gastroenterol Hepatol. 2010;7:599–610. [DOI] [PubMed] [Google Scholar]
- 2.Kulnigg S, Gasche C. Systematic review: managing anaemia in Crohn's disease. Aliment Pharmacol Therap. 2006;24:1507–1523. [DOI] [PubMed] [Google Scholar]
- 3.Gasche C, Beguin Y, de Silva AD, et al. IBD and anemia. In: Gasche C, ed. Anemia in Inflammatory Bowel Diseases. Bremen: UNI-MED Verlag AG; 2008. [Google Scholar]
- 4.Weiss G, Goodnough LT. Anemia of chronic disease. New Engl J Med. 2005;352:1011–1023. [DOI] [PubMed] [Google Scholar]
- 5.Zimmermann MB, Hurrell RF. Nutritional iron deficiency. Lancet. 2007;370:511–520. [DOI] [PubMed] [Google Scholar]
- 6.Gasche C, Berstad A, Befrits R, et al. Guidelines on the diagnosis and management of iron deficiency and anemia in inflammatory bowel diseases. Inflamm Bowel Dis. 2007;13:1545–1553. [DOI] [PubMed] [Google Scholar]
- 7.Gasche C, Lomer MC, Cavill I, et al. Iron, anaemia, and inflammatory bowel diseases. Gut. 2004;53:1190–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cucino C, Sonnenberg A. Cause of death in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2001;7:250–255. [DOI] [PubMed] [Google Scholar]
- 9.Wells CW, Lewis S, Barton JR, et al. Effects of changes in hemoglobin level on quality of life and cognitive function in inflammatory bowel disease patients. Inflamm Bowel Dis. 2006;12:123–130. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell A, Guyatt G, Singer J, et al. Quality of life in patients with inflammatory bowel disease. J Clin Gastroenterol. 1988;10:306–310. [DOI] [PubMed] [Google Scholar]
- 11.Clark SF. Iron deficiency anemia: diagnosis and management. Curr Opin Gastroenterol. 2009;25:122–128. [DOI] [PubMed] [Google Scholar]
- 12.Van Assche G, Dignass A, Reinisch W, et al. The second European evidence-based Consensus on the diagnosis and management of Crohn's disease: special situations. J Crohns Colitis. 2010;4:63–101. [DOI] [PubMed] [Google Scholar]
- 13.Van Assche G, Dignass A, Bokemeyer B, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 3: special situations. J Crohns Colitis. 2013;7:1–33. [DOI] [PubMed] [Google Scholar]
- 14.Lund EK, Wharf SG, Fairweather-Tait SJ, et al. Oral ferrous sulfate supplements increase the free radical-generating capacity of feces from healthy volunteers. Am J Clin Nutr. 1999;69:250–255. [DOI] [PubMed] [Google Scholar]
- 15.Lugg S, Beal F, Nightingale P, et al. Iron treatment and inflammatory bowel disease: what happens in real practice? J Crohns Colitis. 2014;8:876–880. [DOI] [PubMed] [Google Scholar]
- 16.Schroder O, Mickisch O, Seidler U, et al. Intravenous iron sucrose versus oral iron supplementation for the treatment of iron deficiency anemia in patients with inflammatory bowel disease–a randomized, controlled, open-label, multicenter study. Am J Gastroenterol. 2005;100:2503–2509. [DOI] [PubMed] [Google Scholar]
- 17.de Silva AD, Tsironi E, Feakins RM, et al. Efficacy and tolerability of oral iron therapy in inflammatory bowel disease: a prospective, comparative trial. Aliment Pharmacol Ther. 2005;22:1097–1105. [DOI] [PubMed] [Google Scholar]
- 18.Erichsen K, Hausken T, Ulvik RJ, et al. Ferrous fumarate deteriorated plasma antioxidant status in patients with Crohn disease. Scand J Gastroenterol. 2003;38:543–548. [DOI] [PubMed] [Google Scholar]
- 19.Kawai M, Sumimoto S, Kasajima Y, et al. A case of ulcerative colitis induced by oral ferrous sulfate. Acta Paediatr Jpn. 1992;34:476–478. [DOI] [PubMed] [Google Scholar]
- 20.Carrier J, Aghdassi E, Platt I, et al. Effect of oral iron supplementation on oxidative stress and colonic inflammation in rats with induced colitis. Aliment Pharmacol Ther. 2001;15:1989–1999. [DOI] [PubMed] [Google Scholar]
- 21.Rizvi S, Schoen RE. Supplementation with oral vs. intravenous iron for anemia with IBD or gastrointestinal bleeding: is oral iron getting a bad rap? Am J Gastroenterol. 2011;106:1872–1879. [DOI] [PubMed] [Google Scholar]
- 22.Gisbert JP, Bermejo F, Pajares R, et al. Oral and intravenous iron treatment in inflammatory bowel disease: hematological response and quality of life improvement. Inflamm Bowel Dis. 2009;15:1485–1491. [DOI] [PubMed] [Google Scholar]
- 23.Erichsen K, Ulvik RJ, Grimstad T, et al. Effects of ferrous sulphate and non-ionic iron-polymaltose complex on markers of oxidative tissue damage in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2005;22:831–838. [DOI] [PubMed] [Google Scholar]
- 24.Millar AD, Rampton DS, Blake DR. Effects of iron and iron chelation in vitro on mucosal oxidant activity in ulcerative colitis. Aliment Pharmacol Ther. 2000;14:1163–1168. [DOI] [PubMed] [Google Scholar]
- 25.WGO. World Gastroenterology Organisation global guideline. Inflammatory bowel disease: a global perspective. 2009. Available at: http://www.guideline.gov/content.aspx?id=15231&search=iron+deficiency+anemia. Accessed May 2, 2014.
- 26.Evstatiev R, Marteau P, Iqbal T, et al. FERGIcor, a randomized controlled trial on ferric carboxymaltose for iron deficiency anemia in inflammatory bowel disease. Gastroenterology. 2011;141:846.e1–2–853.e1–2. [DOI] [PubMed] [Google Scholar]
- 27.EMA (European Medicines Agency). New recommendations to manage risk of allergic reactions with intravenous iron-containing medicines. 2013. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/IV_iron_31/WC500151308.pdf. Accessed May 1, 2014.
- 28.Auerbach M, Rodgers GM. Intravenous iron. N Engl J Med. 2007;357:93–94. [DOI] [PubMed] [Google Scholar]
- 29.Gomollon F, Chowers Y, Danese S, et al. Letter: European Medicines Agency recommendations for allergic reactions to intravenous iron-containing medicines. Aliment Pharmacol Ther. 2014;39:743–744. [DOI] [PubMed] [Google Scholar]
- 30.WHO. Toxicological evaluation of certain food additives—International Programme on Chemical Safety IPCS. FAO Nutrition Meetings Report Series. 1980;16:123–129. [Google Scholar]
- 31.Levey JA, Barrand MA, Callingham BA, et al. Characteristics of iron (III) uptake by isolated fragments of rat small intestine in the presence of the hydroxypyrones, maltol and ethyl maltol. Biochem Pharmacol. 1988;37:2051–2057. [DOI] [PubMed] [Google Scholar]
- 32.Barrand MA, Callingham BA, Dobbin P, et al. Dissociation of a ferric maltol complex and its subsequent metabolism during absorption across the small intestine of the rat. Br J Pharmacol. 1991;102:723–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barrand MA, Callingham BA. Evidence for regulatory control of iron uptake from ferric maltol across the small intestine of the rat. Br J Pharmacol. 1991;102:408–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kelsey SM, Blake DR, Hider RC, et al. Absorption of ferric maltol, a novel ferric iron compound, in iron-deficient subjects. Clin Lab Haematol. 1989;11:287–288. [DOI] [PubMed] [Google Scholar]
- 35.Kelsey SM, Hider RC, Bloor JR, et al. Absorption of low and therapeutic doses of ferric maltol, a novel ferric iron compound, in iron deficient subjects using a single dose iron absorption test. J Clin Pharm Ther. 1991;16:117–122. [DOI] [PubMed] [Google Scholar]
- 36.Barrand MA, Callingham BA, Hider RC. Effects of the pyrones, maltol and ethyl maltol, on iron absorption from the rat small intestine. J Pharm Pharmacol. 1987;39:203–211. [DOI] [PubMed] [Google Scholar]
- 37.Schneider W. The fate of iron compounds in the gastrointestinal tract. Coordination-chemical facts. Arzneimittelforschung. 1987;37:92–95. [PubMed] [Google Scholar]
- 38.Singh RK, Barrand MA. Lipid peroxidation effects of a novel iron compound, ferric maltol. A comparison with ferrous sulphate. J Pharm Pharmacol. 1990;42:276–279. [DOI] [PubMed] [Google Scholar]
- 39.Harvey RS, Reffitt DM, Doig LA, et al. Ferric trimaltol corrects iron deficiency anaemia in patients intolerant of iron. Aliment Pharmacol Ther. 1998;12:845–848. [DOI] [PubMed] [Google Scholar]
- 40.WHO. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Vitamin and Mineral Nutrition Information system (WHO/NMH/NHD/MNM/111). Geneva, Switzerland: World Health Organization; 2011:1–4. [Google Scholar]
- 41.Walmsley RS, Ayres RC, Pounder RE, et al. A simple clinical colitis activity index. Gut. 1998;43:29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Best WR, Becktel JM, Singleton JW, et al. Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology. 1976;70:439–444. [PubMed] [Google Scholar]
- 43.Hauser W, Dietz N, Grandt D, et al. Validation of the inflammatory bowel disease questionnaire IBDQ-D, German version, for patients with ileal pouch anal anastomosis for ulcerative colitis. Z Gastroenterol. 2004;42:131–139. [DOI] [PubMed] [Google Scholar]
- 44.de Boer AG, Wijker W, Bartelsman JF, et al. Inflammatory Bowel Disease Questionnaire: cross-cultural adaptation and further validation. Eur J Gastroenterol Hepatol. 1995;7:1043–1050. [DOI] [PubMed] [Google Scholar]
- 45.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 46.Brown EG, Wood L, Wood S. The medical dictionary for regulatory activities (MedDRA). Drug Saf. 1999;20:109–117. [DOI] [PubMed] [Google Scholar]
- 47.Kulnigg S, Stoinov S, Simanenkov V, et al. A novel intravenous iron formulation for treatment of anemia in inflammatory bowel disease: the ferric carboxymaltose (FERINJECT) randomized controlled trial. Am J Gastroenterol. 2008;103:1182–1192. [DOI] [PubMed] [Google Scholar]
- 48.Lindgren S, Wikman O, Befrits R, et al. Intravenous iron sucrose is superior to oral iron sulphate for correcting anaemia and restoring iron stores in IBD patients: a randomized, controlled, evaluator-blind, multicentre study. Scand J Gastroenterol. 2009;44:838–845. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


