Abstract
Background
Population pharmacokinetic (PK) studies of busulfan in children have shown that individualized model-based algorithms provide improved targeted busulfan therapy when compared to conventional dosing. The adoption of population PK models into routine clinical practice has been hampered by the tendency of pharmacologists to develop complex models too impractical for clinicians to use. The authors aimed to develop a population PK model for busulfan in children that can reliably achieve therapeutic exposure (concentration-at-steady-state, Css) and implement a simple, model-based tool for the initial dosing of busulfan in children undergoing HCT.
Patients and Methods
Model development was conducted using retrospective data available in 90 pediatric and young adult patients who had undergone HCT with busulfan conditioning. Busulfan drug levels and potential covariates influencing drug exposure were analyzed using the non-linear mixed effects modeling software, NONMEM. The final population PK model was implemented into a clinician-friendly, Microsoft Excel-based tool and used to recommend initial doses of busulfan in a group of 21 pediatric patients prospectively dosed based on the population PK model.
Results
Modeling of busulfan time-concentration data indicates busulfan CL displays non-linearity in children, decreasing up to approximately 20% between the concentrations of 250–2000 ng/mL. Important patient-specific covariates found to significantly impact busulfan CL were actual body weight and age. The percentage of individuals achieving a therapeutic Css was significantly higher in subjects receiving initial doses based on the population PK model (81%) versus historical controls dosed on conventional guidelines (52%) (p = 0.02).
Conclusion
When compared to the conventional dosing guidelines, the model-based algorithm demonstrates significant improvement for providing targeted busulfan therapy in children and young adults.
Keywords: usulfan, pharmacokinetics, pediatric, therapeutic drug monitoring, hematopoietic cell transplantation
INTRODUCTION
Busulfan (Busulfex® for injection) is a bifunctional alkylating agent routinely used in conditioning regimens prior to hematopoietic cell transplantation (HCT) for the treatment of a variety of childhood diseases including both malignant and nonmalignant disorders.[1] Because busulfan pharmacokinetics (PK) displays large inter-patient variability and exposure-response relationships have been described, the therapeutic drug monitoring (TDM) of busulfan is routinely performed as standard clinical care in children undergoing HCT.[2–7] Busulfan exposure can be estimated by several different strategies including area-under-the-curve (AUC) monitoring or estimation of a steady-state plasma concentration (Css = AUC / dosing interval)(Table 1).[8] Improved rates of engraftment and lower drug-related toxicity (e.g. mucositis, sinusoidal obstruction syndrome) have been demonstrated with a Css0–6hour of 600 – 900 ng/mL (approximately equivalent to a AUC0–6hour of 900 – 1350 uM min).[6, 9, 10] Most commonly in North America, initial doses of busulfan are determined based on actual body weight as defined by the US FDA-approved drug label for busulfan use in children and aims to achieve a Css of approximately 600 – 900ng/mL.[1] This “conventional dosing” nomogram recommends for patients weighing ≤12kg an initial dose of 1.1 mg/kg, and for patients weighing >12kg initiating therapy at 0.8 mg/kg/dose regardless of age.
Table 1.
Different PK parameters used in the clinical TDM of busulfan in children.
| PK parameter (units) | Equivalent value of the therapeutic range1 |
|---|---|
| Css (ng/mL) | 600 – 900 |
| AUC (uM min) | 900 – 1350 |
| AUC (mg hr/L) | 3.6 – 5.4 |
Equivalent values reflect the therapeutic range for a 6-hour dosing interval
The current suggested dosing regimen for busulfan use in children was determined based on the results of a single clinical trial of only 24 children undergoing HCT receiving busulfan in combination with cyclophosphamide.[1, 11] Based on simulation studies using a population PK model in pediatrics it is estimated that, at most, only about 60% of patients will fall within the desired therapeutic range for the first dose using the conventional busulfan dosing nomogram.[11] These percentages can vary widely for children of different ages and weights.[11] Additionally, optimal busulfan exposure may differ for each individual child depending on several factors including disease and donor source.[6, 12, 13] Unfortunately, the current conventional dosing guidelines do not allow for different targeted exposures. Achievement of individualized targeted busulfan therapy early on in the course of treatment is critical and failure to do so may lead to suboptimal therapy or toxicity.[14]
More recently, several population PK studies in children have shown that individualized (e.g. personalized) model-based algorithms for busulfan clearance (CL) which incorporate body size and/or age provide improved targeted therapy when compared to stratified weight or age-based regimens alone[15–19]. Unfortunately, the adoption of population PK models into mainstream clinical practice has been hampered by complicated software and the tendency by pharmacologists to develop complex models that are often impractical for clinicians to use. Furthermore, model validation, including prospective evaluation, is critical to ensure the predictability of any model. A user-friendly tool that is easily accessible to clinicians is required to ensure translation and adoption of useful yet complicated PK models into routine clinical practice. The objectives of this study were 2-fold: (1) to develop a dosing algorithm for personalized dosing of busulfan in children that can more reliably achieve therapeutic Css (Css 600 – 900 ng/mL) targeted exposures and (2) implement a clinician-friendly, easy-to-use tool for determining initial dosing in a prospective validation group of children undergoing HCT. The latter aims to bridge the gap from the laboratory to the patient, ensuring the information we gained from our research is adopted in routine clinical practice.
PATIENTS AND METHODS
Study Population
All patients and/or guardians provided written informed consent to participate in the routine therapeutic monitoring of busulfan as part of their specific transplant protocol. Consent for participation in the PK analysis was waived as part of the University of California San Francisco Committee on Human Subjects’ Research approval process. Eligibility criteria for busulfan PK analysis in this study included (1) autologous or allogeneic (related or unrelated) HCT that included intravenous busulfan therapy and (2) busulfan plasma time-concentration data available for analysis. Patients underwent HCT for a wide variety of malignant and nonmalignant pediatric disorders. Briefly, conditioning chemotherapy consisted of busulfan in combination with (1) fludarabine + serotherapy (anti-thymocyte globulin or alemtuzumab) (2) fludarabine + thiotepa + serotherapy; (3) fludarabine + clofarabine + serotherapy; or (4) melphalan + serotherapy. In all patients, busulfan therapy was administered by a 2-hour infusion, every 6 hours for a total of 16 doses. Seizure prophylaxis consisted of either lorazepam or levetiracetam.
Model Development Dataset
Model development was conducted using retrospective PK data available from the routine TDM of busulfan levels in 90 pediatric and young adult patients who had undergone HCT at UCSF Benioff Children’s Hospital between January 2007 and April 2013. Demographics of the 90 subjects are shown in Table 2. For the majority of patients (n=79), initial busulfan doses were determined based on the patient’s actual body weight according to the conventional dosing nomogram.[1] For patients weighing ≤12 kg, busulfan was initiated at 1.1 mg/kg/dose. An initial dose of 0.8 mg/kg/dose was used in children weighing greater than 12 kg. In eleven subjects, enrolled in a previously completed pilot study, PK data from a test dose (0.5mg/kg) given 3–4 days prior to the start of chemotherapy was used to estimate an individual’s CL and to determine the first dose of busulfan in mg.[12] Included in all transplant protocols, busulfan plasma concentrations were therapeutically targeted to achieve a Css within the range of 600 – 900ng/mL as part of routine clinical care. Blood collections for PK sampling were performed with dose 1 and used to determine subsequent doses, if needed. Following the first dose (and test dose if administered) samples were obtained at 2, 2.25, 2.5, 3, 4, 5 and 6 hours post start of the infusion. If dose modifications were performed, repeat drug levels were obtained following a steady-state dose (dose 9, 13) and blood samples collected at 0 (just prior to the start of infusion), 2.25, 2.5, 4, and 6 hours post start of infusion. Conditions of steady-state were assumed after the administration of 3 consecutive doses of busulfan and based on prior knowledge of estimated busulfan half-life in children. Plasma samples used for model development were analyzed by the University of Pennsylvania Medical Center, Department of Pathology and Laboratory Medicine Toxicology Laboratory using a validated reverse-phase high-performance liquid chromatography assay with tandem mass spectrometry (HPLC-MS) as previously described.[20] The assay was linear in the range of 40 to 2000ng/ml. Intra-day and inter-day assay variability was <6% and 9%, respectively.
Table 2.
Patient demographics and baseline characteristics.1
| Median (range) / N (%) | ||
|---|---|---|
| Model Development Data Set |
Validation Data Set |
|
| Number of Subjects | 90 | 21 |
| Age (years) | 7 (0.1–24) | 2.4 (0.4–13.3) |
| Weight (kg) | 22 (3–101) | 13 (6.6–55.5) |
| Male/Female | 53(59%) / 37(41%) | 17(81%) / 4(19%) |
| Serum creatinine (mg/dL) | 0.3 (0.3–0.95) | 0.3 (0.3–0.55) |
| Creatinine clearance (mL/min/m2)2 | 169 (70–286) | 155 (96–222) |
| Alkaline phosphatase (IU/L) | 159 (46–1760) | 160 (101–289) |
| Aspartate aminotransferase (IU/L) | 30 (10–265) | 26 (17–39) |
| Alanine aminotransferase (IU/L) | 28 (5–525) | 19 (10–44) |
| Total bilirubin (mg/dL) | 0.6 (0.1–3.7) | 0.3 (0.1–0.5) |
Laboratory data was collected was just prior to the first dose of busulfan.
Creatinine clearance was estimated in children using the Schwartz method and in young adults by the Cockcroft-Gault equation using ideal body weight.
Prospective Validation Cohort
External validation of the model was performed using PK data available from the routine TDM of busulfan levels in 21 pediatric patients prospectively dosed based on the population PK model. Demographics of the 21 subjects are shown in Table 1. Busulfan plasma concentrations were therapeutically monitored to achieve a Css within the range of 600 – 900ng/mL as part of routine care. Blood collections for PK sampling were performed with dose 3 and used to determine subsequent doses, if needed. Following the 3rd dose, samples were obtained at T=0 (just prior to start of infusion), and 2.25, 2.5, 4, and 6 hours post start of infusion. If dose modifications were performed, drug levels were repeated at dose 7 and 11, as needed. Plasma samples collected in the validation group were analyzed by the University of California-San Francisco, Department of Pathology and Laboratory Medicine laboratory using a validated LC-MS/MS assay described elsewhere in detail.[21] The assay was linear in the range of 6 to 2000 ng/ml. Assay intra-day, and inter-day precision were <3% and <6%, respectively.
Population PK Modeling
Model development using busulfan plasma concentration-time data was performed with the non-linear mixed effects modeling program NONMEM (version 7, ICON Development Solutions, Ellicott City, MD). Diagnostic graphics and post-processing of NONMEM output and simulations were performed using the statistical software R and Xpose.[22, 23]The first order conditional estimation method with interaction (FOCE-I) was used throughout the model building process to estimate PK parameters and variability. Model development was guided by exploratory analysis of the data, changes in the NONMEM objective function value (OFV), diagnostic plots, and the potential biological plausibility of a relationship between clinical covariates and drug exposure. Because many subjects had intensive sampling on more than one occasion, inter-occasion variability was investigated. Because inter-occasion variability showed the trend that CL may be changing with time, a mechanism-based model incorporating Michaelis-Menten elimination was investigated. Residual unexplained variability was characterized by an additive and proportional error model. Using standard principles of allometric scaling, weight was built into the base model a priori and scaled to a reference patient having a median weight of 22 kg.[24]
Patient specific factors considered for covariate testing included age, height, body surface area (BSA), sex, serum creatinine, creatinine clearance (CrCL), blood urea nitrogen, alkaline phosphatase, aspartate aminotransferase, alanine aminotransferase, and total bilirubin. Clinical data was collected on each day of PK sampling. Creatinine clearance was estimated in pediatric patients (≤17 years of age) by the Schwartz method and in young adults by the Cockcroft-Gault equation using ideal body weight.[25, 26] Difference covariate relationships on PK parameters were investigated and included power, linear, and exponential functions. The final PK model was built through the process of forward selection and backward elimination of clinical covariates. The likelihood ratio test was used to assess the significance of all covariates in the final model. During forward selection, covariates were univariately tested and deemed significant if the OFV decreased by at least 3.84 (χ2, P ≤ 0.05, df = 1) with its inclusion in the model. During backward elimination, significance of the covariates were confirmed by removing one at a time from the full model and required an increase in the OFV of at least 6.33 (X2, P ≤ 0.05, df=1) to remain in the model.
To evaluate the precision of the final model parameter estimates, a nonparametric bootstrap was performed. A total of 1000 bootstrap datasets were generated by repeated sampling with replacement from the original data and the final PK model fitted to each of the bootstrap datasets. The median, 5th and 95th percentiles were then obtained for each PK parameter and compared with the final model PK estimates.
Development of an Initial Dose Calculator Tool
The final population PK model was incorporated into a Microsoft Excel-based calculator tool (Microsoft® Excel® version 14.4.1, Microsoft Corporation, Redmond, CA), which could be used in the clinical setting to determine initial doses for individualized busulfan therapy. Based on our final model, the equation for determining individual doses of busulfan in units of mg in the prospective validation cohort was:
Parameters were fixed to the values estimated in the final model (Table 3), and along with the patient’s age (years) and weight (actual body weight, kg), used to calculate individual CL (CLi) and the first dose of busulfan. Model-based doses were calculated to achieve the midpoint AUC corresponding to a targeted Css range for exposure. For example, expressed in terms of mg, conventional exposure as proposed by the FDA-approved drug labeling for busulfan use in children was defined as an AUCtarget of 4.5 mg hr/L (range, 3.6–5.4 mg hr/L) over a 6-hour dosing interval. This target is equivalent to a Css of 750 ng/mL (range, 600–900 ng/mL) and AUC of 1098 uM min (range, 900–1350 uM min). The equation AUC / dose interval was used to calculate Css.
Table 3.
Final population PK model parameter estimates and bootstrap results.
| Final Model Results |
Bootstrap Results |
||||
|---|---|---|---|---|---|
| Population PK Parameters | Units | Typical Value Estimates1 |
RSE(%)2 | Mean Value |
95% Confidence Interval |
| CLin, intrinsic clearance3 | L/hr | 4.32 | 8 | 4.36 | 3.66 – 5.14 |
| Exponent for effect of weight on CLin | 0.75 (fixed) | ||||
| Vc, volume of central compartment | L | 15.7 | 3 | 15.6 | 14.8 – 16.6 |
| Exponent for effect of weight on Vc | 1 (fixed) | ||||
| SL1<BP, slope < breakpoint | 0.032 | 32 | 0.031 | 0.013 – 0.058 | |
| SL2≥BP, slope ≥ breakpoint | −0.0138 | 46 | −0.0128 | −0.002 – 0.033 | |
| BP, breakpoint for age effect on CLin | years | 12 (fixed) | |||
| Km, Michaelis-Menten constant | ng/mL | 6704 | 43 | 6340 | 3462 – 29042 |
| Inter-individual variability (IIV)4 | |||||
| IIV for CLin (%CV) | 22 | 19 | 22 | 17 – 26 | |
| IIV for Vc (%CV) | 29 | 45 | 28 | 18 – 42 | |
| Correlation CLin-V | 0.42 | 35 | 0.43 | 0.33 – 0.44 | |
| Residual unexplained variability | |||||
| Proportional error (%CV) | 14.8 | 14.6 | 14.6 | 9.2 – 18.3 | |
| Additive error (%CV) | ng/mL | 47 | 25 | 46.8 | 24 – 73 |
Mean typical value of the PK parameter in the final model for a child weight 22kg and 7 years of age.
Presented as Relative standard error (%)
Intrinsic clearance in the model was defined as Clin=Vmax/KM
Presented as CV % (the ratio of the standard deviation to the mean)
Model-Based Algorithm versus Conventional Dosing
Based on our final population PK model, busulfan doses were simulated using the model-based algorithm and compared to conventional dosing for achieving a targeted Css 750 ng/mL. Clinical covariates (age, weight) for a typical patient were based on 50th percentile estimates of weight per age as provided by the World Health Organization (WHO) growth standards for infants and children. Doses were aimed to achieve the desired therapeutic Css over a 6-hour dosing interval.
Prospective Validation
The validation dataset was used to both evaluate the performance of the model and the model-based dosing algorithm. A prediction-corrected visual predictive check using parameter estimates from the final model and comparing the concentration-time profiles predicted by the final model versus the observed concentrations in the validation dataset was performed and presented.[27] In addition, the percent of subjects achieving the therapeutic range Css of 600 – 900 ng/mL for conventional dosing (historical controls) and the model-based dosing (validation group) at the time of first PK sampling was calculated and compared using a Chi-square test for proportions. Historical controls consisted of 79 out of 90 subjects included in the model development dataset that had initial doses of busulfan determined by the conventional guidelines.
RESULTS
Population Modeling
A total of 1165 quantifiable concentrations were available for population PK model building and were best described with a 1-compartment base model with non-linear elimination. The range of observations was 34–3857 ng/mL. Irrespective of the assay, <1% of busulfan plasma concentrations were below the level of quantification and were included in the analysis.[28] A 1-compartment model with linear CL was compared to a 1-compartment model with inter-occasion variability (IOV) on CL. This resulted in a decrease in the OFV of −200, but shrinkage was observed (62% on occurrence 1 (test dose), 26% on occurrence 2 (1st dose) and 36.8% on occurrences 3 and 4 (steady-state doses). In addition, no improvement in the goodness of fit plots was observed. Therefore, the model without IOV was considered to be a more predictive model. When comparing plots obtained after a single dose (test dose or first dose) and multiple doses (in the model including IOV), a change in the CL of busulfan was noted. A trend in decreasing busulfan CL over time for individuals undergoing both first dose and steady-state PK sampling (n=58) was found (Figure 1, panel A). In order to address the question what might best explained the reduced CL with time, the model with linear elimination was compared with the following: (1) Michaelis-Menten kinetics (ΔOFV= −87, p<10−20); (2) time-dependent CL(ΔOFV= −144 p<10−20)[29]; and (3) reduced CL after 6, 12, or 24 hours estimated as a fraction of the CL at day 1 (ΔOFV=−175,−192 and −219, respectively p<10−20,). All three models showed a change in CL of approximately 15% at 24 hours after the start of therapy. From this dataset it is not distinguishable why this occurs (time-dependent or concentration-dependent). The model with Michaelis-Menten elimination demonstrates that CLin is a function of concentration, and we hypothesize this mechanistic approach to be more physiologically plausible compared to other time-dependent models tested. Therefore, we considered the model with Michaelis-Menten kinetics to be the final model and hypothesize that at steady-state concentrations busulfan CL shows non-linear PK. Michaelis-Menten kinetics was implemented into the model as follows:
for which CLin is the intrinsic CL of busulfan. The value of Km was high at 6704 ng/mL, but reasonably well estimated (RSE 43%). Representing the typical range of concentrations achieved with the therapeutic dosing, busulfan CL decreased up to approximately 20% between the concentrations of 250–2000 ng/mL (Figure 1, panel B).
Figure 1.
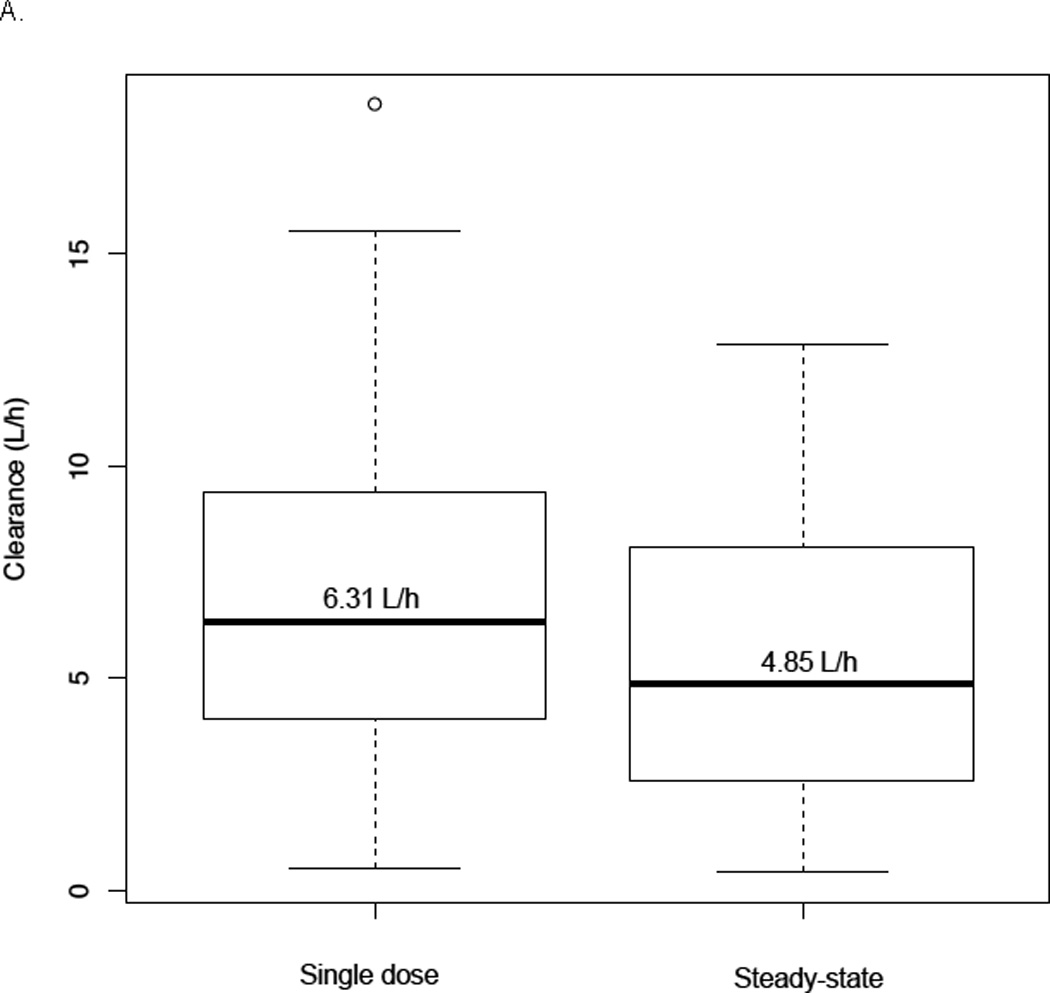
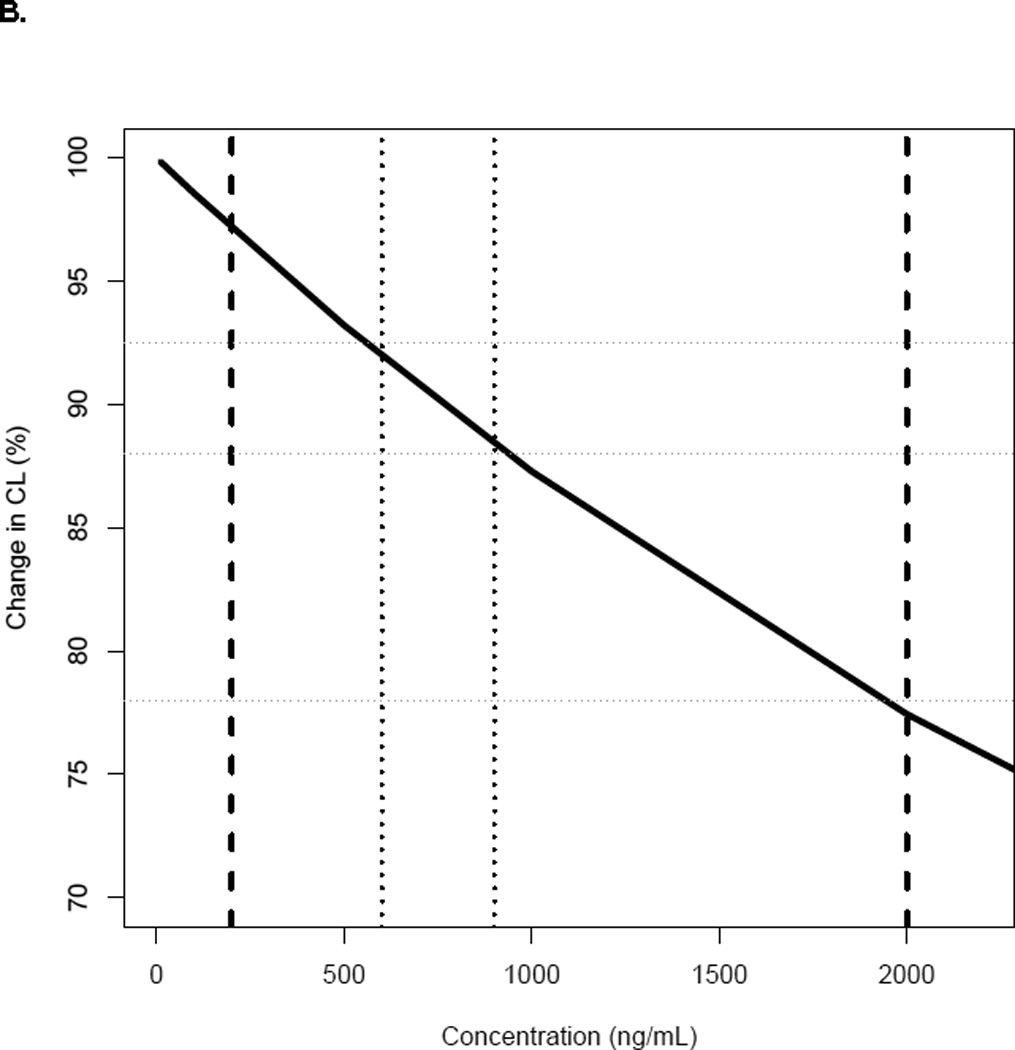
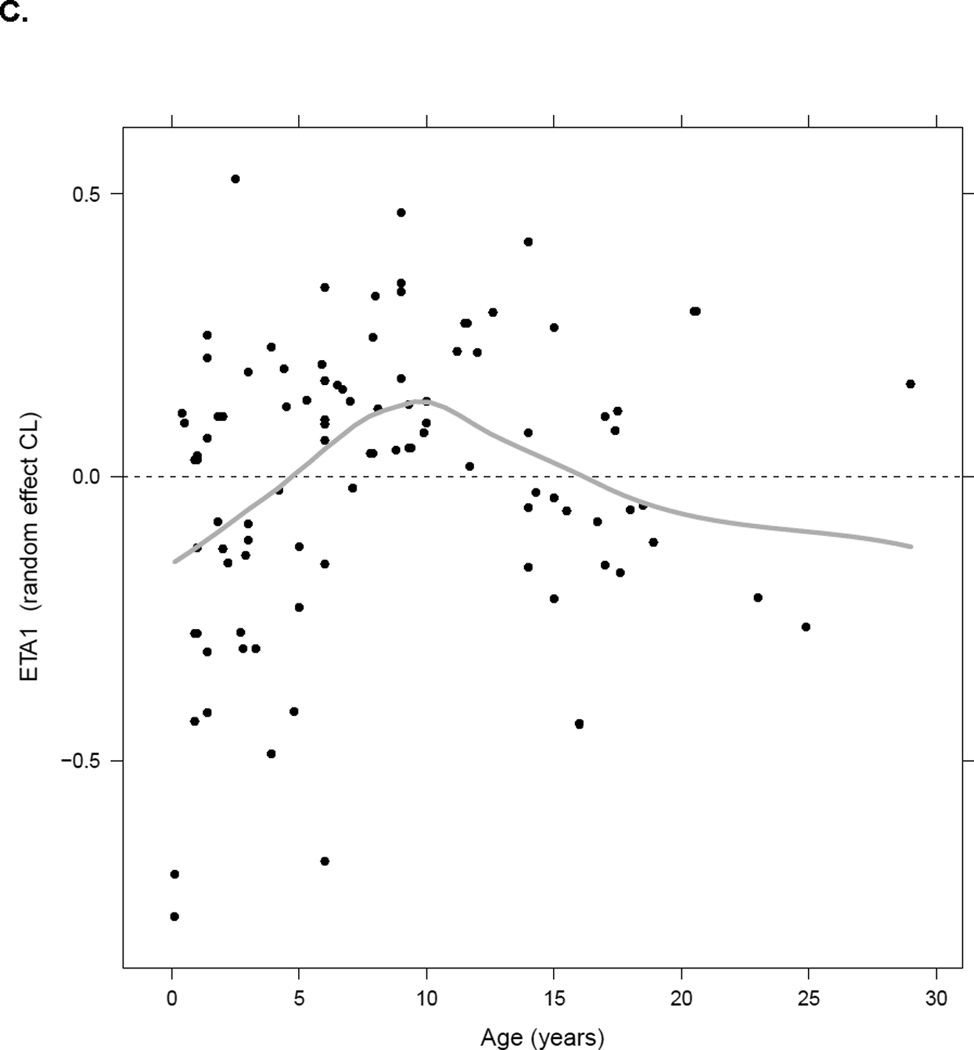
Panel A- Comparison between individual busulfan CL estimates with single dose (test dose or first dose) and steady-state doses (n=58); Panel B- Change in busulfan CLin for the typical patient with plasma concentrations1; Panel C- Plot of inter-individual variability of CLin versus age.2
1 Dark line represents the change in busulfan CL of a typical patient of 22 kg and 7 years of age with busulfan concentrations. Dashed lines represent the range of the observed busulfan concentrations in our study (250–2000 ng/mL); dotted lines are the target busulfan concentrations (600–900 ng/mL).
2 Black dots represent the individual values of ETA1 (random effect on CLin), the grey line represents the smooth line.
Important patient-specific covariates found to significantly impact busulfan CLin were actual body weight and age. All covariates identified were supported by individual Bayesian PK parameter estimates versus covariate plots. After actual body weight was implemented in the model using allometric scaling, plots of individual parameter estimates versus age suggested a bell-shape trend in the data with individual Bayes PK CLin estimates increasing with age up to 12 years of age and then slowly decreasing back to the baseline value (Figure 1, panel C). The simple “hockey-stick” function describing this maturation effect was implemented in the model and was significant (p<0.0001). This function consisted of two linear functions and a breakpoint. The first linear function described an increase of CLin with age, the breakpoint described the maximum of the function, e.g. age when CLin is at its maximum value, and the second linear function described the decrease of CLin with age past the breakpoint. During the modeling process, we attempted to estimate the breakpoint. The estimation process was associated with certain numerical difficulties due to data limitation; however its estimate was close to 12 years, which further supported the use of this value as a breakpoint. Different breakpoints ranging between 8–15 years were estimated around the visually observed value of 12 years (Figure 1, panel C). Sensitivity analysis was also performed with the breakpoint fixed to different values and this exercise also confirmed that 12 years was an optimal estimate. The breakpoint of 12 years was then fixed for further analyses. No significant impact of additional covariates including additional markers for body size (BSA, height) or clinical status (hepatic function) on busulfan CLin was identified.
The population PK parameters estimates and their relative standard errors (%) from the final model are presented in Table 3. The final models for busulfan CLin incorporating both a weight and maturation effect were as follows:
For children less than 12 years of age:
And for children equal to or greater than 12 years of age:
where 4.32 L/h is the typical value of busulfan CLin for a child weight 22kg and 7 years of age, Bp is the breakpoint for the age effect on CLin, Sl<bp is the slope of the age effect for children less than 12 years of age and Sl>bp is the slope of the age effect in children greater than 12 years of age. The goodness of fit plots for the base and final model showed clear improvement with good distribution of population-predicted concentration around the line of unity indicating the data were adequately described by the final model (data not shown). Ninety-five percent of conditional weighted residuals fell within 2 standard deviations demonstrating good predictability of the model. No trend in the residuals was observed.
The median PK parameter estimates and 95% confidence intervals from the bootstrap analysis are presented in Table 3. Median estimates of PK parameters, inter-patient variability, and residual unexplained variability derived from the bootstrap analysis were comparable with the typical values derived from the original population PK analysis. Predicted busulfan concentrations in the validation group using the population PK model and observed concentrations are plotted versus time in Figure 2, panel A. Concentrations were adequately predicted over the 6-hour time-concentration profile. The prediction-corrected visual predictive check showed the median and percentiles of the simulated data captures the median and percentiles of the observed PK data for the validation group well (Figure 2, panel B).
Figure 2.
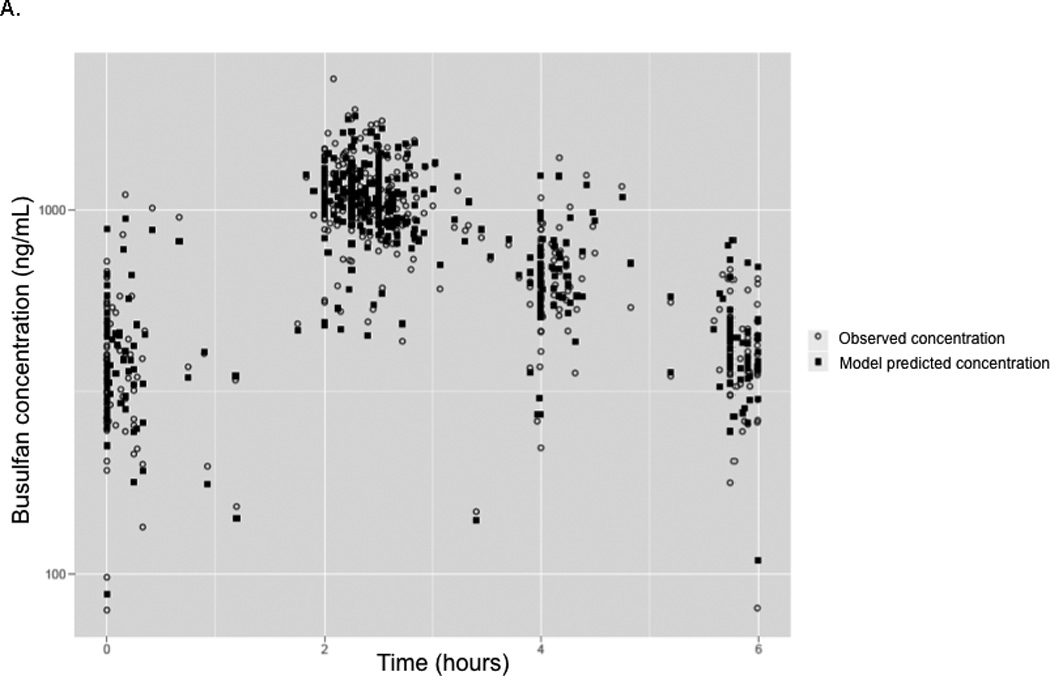
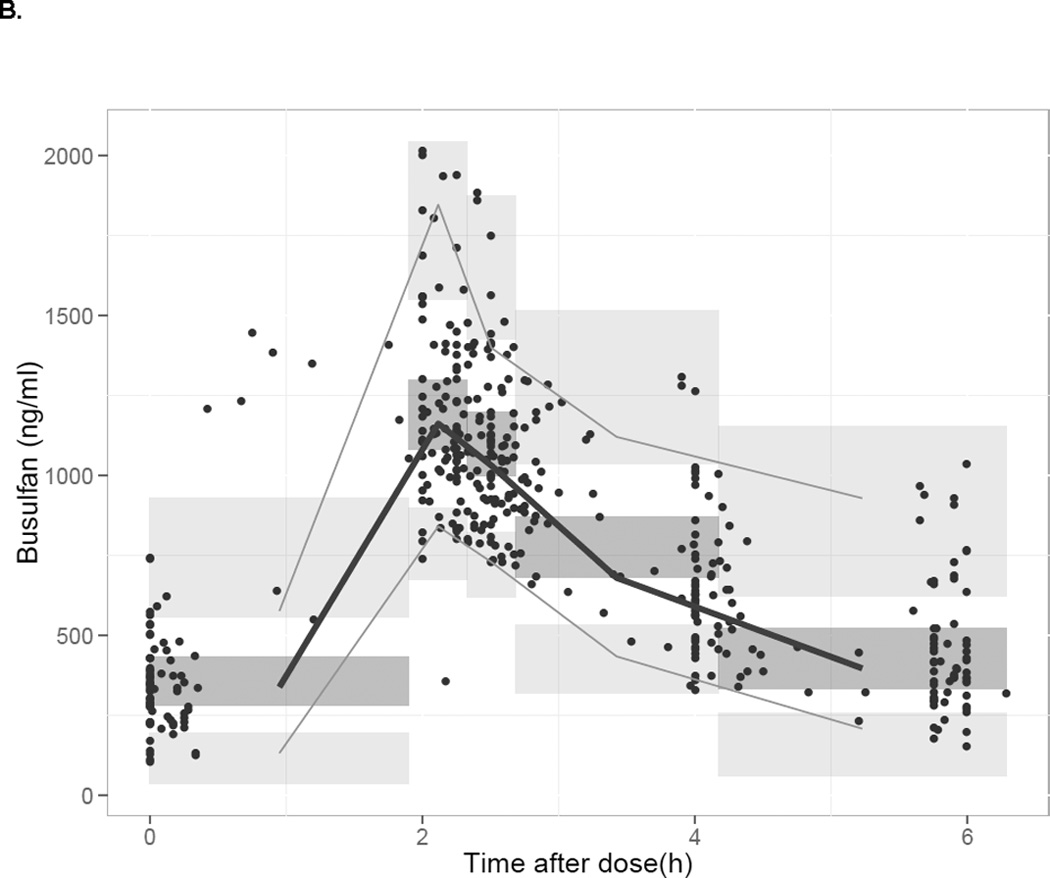
Panel A- Model predicted and measured (observed) plasma busulfan plasma concentrations obtained using the population model in the validation cohort plotted versus time. 1 Panel B- Prediction-corrected visual predictive check of the concentration-time profile predicted by the original busulfan PK model versus the concentrations observed in the validation dataset. 2
1 Panel A- Light grey circles represent the observed concentrations in the validation dataset. The filled dark squares represent the simulated concentration by the final population PK model.
2 Panel B- The solid grey lines represent the 2.5th percentile, median and 95th percentile of the prediction corrected observed plasma concentrations. The semitransparent dark grey field represents a simulation-based 95% confidence interval for the median and the semitransparent light grey fields show the 95% confidence intervals of the simulated data.
Development of the Dose Calculator Tool
The final population PK model was implemented as clinician-friendly, easy-to-use Microsoft Excel-based tool. Figure 3 shows the “front end” of the Excel worksheet of the busulfan dose calculator tool. Typical population estimates from the final model were fixed to the values listed in Table 2. The dosage tool can be used to recommend initial doses of busulfan based on patient-specific covariate data (age and weight). In addition to conventional exposure (referred to as “normal”, Css target of 750 ng/mL, range 600–900) the tool was designed to allow clinicians the option to select different therapeutic targets within the conventional therapeutic target range of 600 – 900 ng/mL, as these may different between patients or HCT protocols (low exposure Css of 650 ng/mL (range 600–700) or high exposure Css of 850ng/mL (range 800–900). Although not shown, the calculator tool can also determine initial doses for alternative dosing frequencies of busulfan therapy (every 12 hours, every 24 hours). In its current form, the dosing tool is only able to determine initial doses of busulfan given intravenously. It is available upon request from the authors via email.
Figure 3.

A computer screen shot of the Excel-based tool used to determine initial doses of busulfan based on the population PK model dosing algorithm.
Model-Based Dosing to Achieve Conventional Therapeutic Target
Initial estimated doses of busulfan by weight and age based on conventional dosing versus the model-based algorithm to achieve a targeted Css of 750 ng/mL over a 6-hour dosing interval are presented in Table 4. In general, dosing in mg/kg is higher when compared to conventional dosing over the span of age/weights evaluated (6 months to 14 years). For example, the model-predicted dose needed to achieve a Css of 750 ng/mL for a 6 year-old child weighing 20 kg would be 22.1 mg (1.07 mg/kg), representing an increase of approximately 25% compared to conventional dosing. A comparison of the percent of subjects achieving the therapeutic range Css of 600 – 900 ng/mL for historical controls versus the prospective validation group at the time of first PK sampling is presented in Table 5. The percent of individuals achieving a therapeutic level at the time of first PK collection (dose 1 or dose 3) was higher in subjects receiving initial doses based on the population PK model (81%) versus conventional guidelines (52%) (p = 0.02). For individuals with a Css outside the therapeutic range, exposure was more often sub-therapeutic with conventional dosing versus the model-based algorithm at 42% and 9.5%, respectively.
Table 4.
Estimated initial doses of busulfan by weight and age based on conventional dosing verses the model-based algorithm to achieve a targeted Css of 750ng/mL.1, 2
| 8kg (6 months) |
10kg (1 year) |
12kg (2 years) |
20kg (6 years) |
32kg (10 years) |
50kg (14 years) |
|
|---|---|---|---|---|---|---|
| Conventional dosing3 | ||||||
| Dose (mg) | 8.7 | 10.7 | 9.8 | 16.5 | 25.5 | 40.8 |
| Dose (mg/kg) | 1.1 | 1.1 | 0.8 | 0.8 | 0.8 | 0.8 |
| Model-based algorithm3 | ||||||
| Dose (mg) | 9.2 | 10.9 | 13.3 | 22.1 | 33.9 | 49.2 |
| Dose (mg/kg) | 1.16 | 1.12 | 1.09 | 1.07 | 1.06 | 0.96 |
The target Css of 750ng/mL (range 600–900ng/mL) is equivalent to an AUC of 1098µM*min (range 900–1350µM*min).
Based on 50th percentile estimates of weight per age as provided by the World Health Organization (WHO) growth standards for infants and children.
Doses are based on achievement of the desired therapeutic Css over a 6-hour dosing interval.
Table 5.
Comparison of the percent of subjects achieving the therapeutic range Css of 600–900ng/mL for conventional dosing versus the model-based dosing at the time of first PK sampling.
| Total No. of Subjects |
Number (%) of Subjects within Goal Css (600–900ng/mL |
Number (%) of Subjects with Css < 600ng/mL |
Number (%) of Subjects with Css > 900ng/mL |
|
|---|---|---|---|---|
| Conventional dosing1, 2 | 79 | 41 (52%) | 33 (42%) | 5 (6%) |
| Model-based algorithm3 | 21 | 17 (81%) | 2 (9.5%) | 2 (9.5%) |
Excludes eleven subjects from the model development dataset (n=90) for which PK data from a test dose (0.5mg/kg) was given 3–4 days prior to the start of chemotherapy and used to predict the initial dose of busulfan therapy.
For patients weighing ≤12 kg, busulfan was initiated at 1.1 mg/kg/dose. An initial dose of 0.8 mg/kg/dose was used in children weighing greater than 12 kg. PK sampling was collected with the first dose of busulfan.
Twenty-one subjects that were prospectively dosed on the model-based algorithm (validation data set). PK sampling was conducted with dose 3 of busulfan.
DISCUSSION
The role of body size and age on busulfan PK has been previously investigated in several pediatric studies with varying results.[7, 11, 15, 16, 18, 21, 30] Typically, PK studies conducted in children demonstrate a high correlation between body size and maturation.[24] The relationship between weight and age may be less consistent in the pediatric HCT population given the potential for children to be underweight for their age due to natural disease processes related to underlying diagnosis and/or prior chemotherapy. Thus, investigating age-related changes that occur as a result of biological and/or enzymatic processes can make the PK modeling of busulfan in children undergoing HCT particularly challenging.[31] Our covariate analysis found both weight and age to be independent significant patient-specific factors impacting busulfan CLin. Allometry allows PK parameters to be adapted according to body weight, thus taking growth into account. However, simple allometric scaling does not account for variations in the maturation of the metabolic pathways, which are reflected by age.[32] After inclusion of growth on CLin in the model using allometric scaling, we showed the predicted CLin of busulfan increases up through 12 years of age and then begins to decline to adult levels. These results are consistent with several other clinical studies of drugs metabolized by the liver, demonstrating an age-dependent increase in CL in children younger than 12–14 years of age.[33–35] For infants and toddlers (1 month to 23 months) and younger children (2–5 years of age) rapid changes in busulfan CL, may be due in part to the gradual maturation of metabolic pathways that occurs with age.[36, 37] In older children (6–11 years) and adolescents (12–17 years) variations in busulfan CL may more be related to changes in elimination capacity due to differences in the liver-mass-to-body-mass ratio.[33, 38]
Pharmacokinetic modeling of our busulfan time-concentration data indicates busulfan CL displays non-linearity over the therapeutic concentration ranges seen in children. For all models tested, a decrease in CL of approximately 15% at 24 hours after the start of treatment was captured. This change may be time-dependent or due to higher drug concentrations and less metabolism after multiple doses. The model with Michaelis-Menten elimination demonstrates that CLin is a function of concentration, and we hypothesize this mechanistic approach to be more physiologically plausible compared to other time-dependent models tested. While most other studies do not report a significant increase in busulfan exposure with time, our results are consistent with two recent reports in children showing a decrease in busulfan CL throughout the course of therapy. In a large, multicenter study of 245 children undergoing HCT for a variety of malignant and nonmalignant disorders, busulfan CL was 12% lower at days 2–4, as compared to day 1.[15] In a study of children with thalassemia, busulfan CL was 20% higher with first dose compared to subsequent doses.[39] Busulfan is metabolized extensively in the liver through conjugation with glutathione by glutathione s-transferase (GST) enzymes, predominantly GSTA1.[36, 40] It is plausible the concentration-dependent behavior of busulfan could arise if the saturation of elimination pathways occurs, especially with dose increases over the duration of therapy.
Given the short duration of busulfan therapy, achievement of individualized drug exposure early on in treatment is crucial and failure to do so may lead to suboptimal therapy or toxicity.[14] As Michaelis-Menten kinetics occurs with increasing drug concentrations, the non-linear model has minimal influence on the initial dose estimation provided in the Excel-based tool. The clinical implications of non-linear PK emerge as dose escalations or reductions of busulfan result in disproportionate increases or decreases in CL and exposure (AUC, Css). Currently, there are many logistical challenges with performing busulfan PK including the short duration of treatment and the need for rapid and accurate estimation of busulfan PK parameters.[8] At most institutions, PK sampling of busulfan occurs with dose 1 and dose-proportional kinetics is assumed for the adjustment of subsequent doses. With non-linear behavior, a decrease in busulfan CL from first-dose to steady-state implies PK sampling performed before the achievement of steady-state may not be optimal. Additionally, the ability for most centers to measure busulfan levels on site is not routinely available therefore limiting the ability to perform dose-modifications early on in therapy and the usefulness of repeat PK sampling.
Achievement of optimal busulfan exposure is necessary to promote engraftment and limit drug-related toxicity in children. Therapeutic targets can vary depending on several factors including donor source, diagnosis (malignant vs. nonmalignant), and other agents included in the preparative regimen.[6, 8, 12] However, the current conventional dosing guidelines cannot account for the use of different targeted exposures, thus these patients may not achieve optimal exposure. Offering a significant advantage over the conventional guidelines, our model-based dosing algorithm can be used to individualize therapy. The incorporation of age and weight relationships into our model for busulfan CL enables children of different ages and weights to have the same likelihood of reaching the desired therapeutic exposure, irrespective of the selected targeted goal. Although model-based dosing of busulfan offers several advantages to conventional dosing, it is important to point out that neither the model-based nor conventional regimens achieves 100% success with the first dose of busulfan. This is likely to occur because the therapeutic targets for busulfan are considerably narrow, taking into account the between-subject variability. Consequently, even with a model-based strategy there will be a proportion of patients who fail to achieve the therapeutic target with the first dose of busulfan, therefore reinforcing the need for repeat TDM. Additionally, we consider validation of the model to be an ongoing process until a larger number of patients spanning a wide range of ages and weights have been evaluated. Thus, we are currently working to improve on our dosing tool through the development of a web-based application for the Bayesian dose-individualization of busulfan using an individual’s time-concentration data. The framework will be able to collect and store data from clinicians and re-estimate the model parameters continually.
Although the PK parameters were well estimated with our final model, the residual unexplained variability remained approximately 15%. This suggests other clinical or patient-specific factors not tested in this analysis study may be important determinants of busulfan CL. Physiological changes induced by specific disease states including inborn errors of metabolism and thalassemia have been demonstrated to alter busulfan CL.[39, 41, 42] Unfortunately, given the limited number of subjects and heterogeneity of diseases included in our study population, covariate analysis of different disease groups was not feasible. Similarly, the impact of co-administered medications previously shown to alter busulfan PK through induction or inhibition of GSTs could not adequately be investigated. Drug-drug interactions between busulfan with azole anti-fungals and metronidazole have been shown to alter busulfan CL presumably through the inhibition of metabolic enzymes.[43, 44] Concomitant use of medications that deplete glutathione, such as the commonly prescribed anti-pyretic acetaminophen, may also contribute to changes in the metabolism of busulfan over the course of treatment.[45] Acetaminophen was permitted during busulfan conditioning, occurring most often with the administration of alemtuzumab. Unfortunately we are unable to test this specific covariate for the impact on busulfan PK, predominantly because an electronic medical record was not implemented at our institution until 2012. Other potential factors unexplored in this analysis include genetic variants of genes involved in busulfan metabolism and disposition. Specifically, in vitro studies have shown variants in GSTA1 result in functional alterations in activity leading to decreased enzymatic activity.[46] Clinically, the impact of several GST genetic variants on busulfan exposure has been investigated, reporting variable results.[47–50] Given the complexity of HCT recipients and likelihood of concomitant medications that may mask or confound variant effects, genetic studies of busulfan exposure may prove difficult and will require careful consideration.
CONCLUSION
In summary, we developed a model for busulfan CL that can be applied to determine initial doses for targeted-therapy in a pediatric HCT population based on age and weight. When compared to the conventional dosing guidelines, the individualized model-based calculation provided an improved dosing strategy for achieving targeted exposure in children and young adults. The final population PK model was implemented as a clinician-friendly, Microsoft Excel-based tool, which can easily be used in the clinical setting to recommend initial doses of intravenous busulfan in children. With improved dosing accuracy, clinicians will be able to more effectively reach their therapeutic target quickly without exceeding toxic thresholds and improve outcomes.
Acknowledgments
FINANCIAL DISCLOSURE STATEMENTS
This work was supported in part by NIH-NIAID grant U54 AI082973 (MJC and CCD) and NIH-NIAID grant R13 AI094943 (MJC).
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.IV Busulfex (Busulfan) Package Insert [package insert] Tokyo, Japan: Otsuka America Pharmaceutical, Inc; [Google Scholar]
- 2.McCune JS, Gooley T, Gibbs JP, et al. Busulfan concentration and graft rejection in pediatric patients undergoing hematopoietic stem cell transplantation. Bone Marrow Transplant. 2002;30:167–173. doi: 10.1038/sj.bmt.1703612. [DOI] [PubMed] [Google Scholar]
- 3.Vassal G, Gouyette A, Hartmann O, Pico JL, Lemerle J. Pharmacokinetics of high-dose busulfan in children. Cancer Chemother Pharmacol. 1989;24:386–390. doi: 10.1007/BF00257448. [DOI] [PubMed] [Google Scholar]
- 4.Vassal G, Deroussent A, Hartmann O, et al. Dose-dependent neurotoxicity of high-dose busulfan in children: a clinical and pharmacological study. Cancer Res. 1990;50:6203–6207. [PubMed] [Google Scholar]
- 5.Slattery JT, Sanders JE, Buckner CD, et al. Graft-rejection and toxicity following bone marrow transplantation in relation to busulfan pharmacokinetics. Bone Marrow Transplant. 1995;16:31–42. [PubMed] [Google Scholar]
- 6.Bolinger AM, Zangwill AB, Slattery JT, et al. Target dose adjustment of busulfan in pediatric patients undergoing bone marrow transplantation. Bone Marrow Transplant. 2001;28:1013–1018. doi: 10.1038/sj.bmt.1703264. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen L, Fuller D, Lennon S, Leger F, Puozzo CIV. busulfan in pediatrics: a novel dosing to improve safety/efficacy for hematopoietic progenitor cell transplantation recipients. Bone Marrow Transplant. 2004;33:979–987. doi: 10.1038/sj.bmt.1704446. [DOI] [PubMed] [Google Scholar]
- 8.McCune JS, Gibbs JP, Slattery JT. Plasma concentration monitoring of busulfan: does it improve clinical outcome? Clin Pharmacokinet. 2000;39:155–165. doi: 10.2165/00003088-200039020-00005. [DOI] [PubMed] [Google Scholar]
- 9.Lindley C, Shea T, McCune J, et al. Intraindividual variability in busulfan pharmacokinetics in patients undergoing a bone marrow transplant: assessment of a test dose and first dose strategy. Anticancer Drugs. 2004;15:453–459. doi: 10.1097/01.cad.0000127145.50172.51. [DOI] [PubMed] [Google Scholar]
- 10.Vassal G, Michel G, Esperou H, et al. Prospective validation of a novel IV busulfan fixed dosing for paediatric patients to improve therapeutic AUC targeting without drug monitoring. Cancer Chemother Pharmacol. 2008;61:113–123. doi: 10.1007/s00280-007-0455-2. [DOI] [PubMed] [Google Scholar]
- 11.Booth BP, Rahman A, Dagher R, et al. Population pharmacokinetic-based dosing of intravenous busulfan in pediatric patients. J Clin Pharmacol. 2007;47:101–111. doi: 10.1177/0091270006295789. [DOI] [PubMed] [Google Scholar]
- 12.Law J, Cowan MJ, Dvorak CC, et al. Busulfan, Fludarabine, and Alemtuzumab As a Reduced Toxicity Regimen for Children with Malignant and Nonmalignant Diseases Improves Engraftment and Graft-versus-Host Disease without Delaying Immune Reconstitution. Biol Blood Marrow Transplant. 2012;18:1656–1663. doi: 10.1016/j.bbmt.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Horn B, Baxter-Lowe LA, Englert L, et al. Reduced intensity conditioning using intravenous busulfan, fludarabine and rabbit ATG for children with nonmalignant disorders and CML. Bone Marrow Transplant. 2006;37:263–269. doi: 10.1038/sj.bmt.1705240. [DOI] [PubMed] [Google Scholar]
- 14.Dix SP, Wingard JR, Mullins RE, et al. Association of busulfan area under the curve with veno-occlusive disease following BMT. Bone Marrow Transplant. 1996;17:225–230. [PubMed] [Google Scholar]
- 15.Bartelink IH, van Kesteren C, Boelens JJ, et al. Predictive performance of a busulfan pharmacokinetic model in children and young adults. Therapeutic drug monitoring. 2012;34:574–583. doi: 10.1097/FTD.0b013e31826051bb. [DOI] [PubMed] [Google Scholar]
- 16.Trame MN, Bergstrand M, Karlsson MO, Boos J, Hempel G. Population pharmacokinetics of busulfan in children: increased evidence for body surface area and allometric body weight dosing of busulfan in children. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:6867–6877. doi: 10.1158/1078-0432.CCR-11-0074. [DOI] [PubMed] [Google Scholar]
- 17.Bleyzac N, Souillet G, Magron P, et al. Improved clinical outcome of paediatric bone marrow recipients using a test dose and Bayesian pharmacokinetic individualization of busulfan dosage regimens. Bone Marrow Transplant. 2001;28:743–751. doi: 10.1038/sj.bmt.1703207. [DOI] [PubMed] [Google Scholar]
- 18.Tse WT, Duerst R, Schneiderman J, et al. Age-dependent pharmacokinetic profile of single daily dose i.v. busulfan in children undergoing reduced-intensity conditioning stem cell transplant. Bone Marrow Transplant. 2009;44:145–156. doi: 10.1038/bmt.2008.437. [DOI] [PubMed] [Google Scholar]
- 19.Paci A, Vassal G, Moshous D, et al. Pharmacokinetic behavior and appraisal of intravenous busulfan dosing in infants and older children: the results of a population pharmacokinetic study from a large pediatric cohort undergoing hematopoietic stem-cell transplantation. Therapeutic drug monitoring. 2012;34:198–208. doi: 10.1097/FTD.0b013e31824c2f60. [DOI] [PubMed] [Google Scholar]
- 20.Lai WK, Pang CP, Law LK, et al. Routine analysis of plasma busulfan by gas chromatography-mass fragmentography. Clinical chemistry. 1998;44:2506–2510. [PubMed] [Google Scholar]
- 21.French D, Sujishi KK, Long-Boyle JR, Ritchie JC. Development and validation of a liquid chromatography-tandem mass spectrometry assay to quantify plasma busulfan. Therapeutic drug monitoring. 2014;36:169–174. doi: 10.1097/01.ftd.0000443060.22620.cd. [DOI] [PubMed] [Google Scholar]
- 22.R: A language and enviroment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2010. [computer program] [Google Scholar]
- 23.Jonsson EN, Karlsson MO. Xpose--an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Computer methods and programs in biomedicine. 1999;58:51–64. doi: 10.1016/s0169-2607(98)00067-4. [DOI] [PubMed] [Google Scholar]
- 24.Meibohm B, Laer S, Panetta JC, Barrett JS. Population pharmacokinetic studies in pediatrics: issues in design and analysis. AAPS J. 2005;7:E475–E487. doi: 10.1208/aapsj070248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwartz GJ, Gauthier B. A simple estimate of glomerular filtration rate in adolescent boys. J Pediatr. 1985;106:522–526. doi: 10.1016/s0022-3476(85)80697-1. [DOI] [PubMed] [Google Scholar]
- 26.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 27.Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011;13:143–151. doi: 10.1208/s12248-011-9255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Byon W, Fletcher CV, Brundage RC. Impact of censoring data below an arbitrary quantification limit on structural model misspecification. Journal of pharmacokinetics and pharmacodynamics. 2008;35:101–116. doi: 10.1007/s10928-007-9078-9. [DOI] [PubMed] [Google Scholar]
- 29.van Hest RM, van Gelder T, Bouw R, et al. Time-dependent clearance of mycophenolic acid in renal transplant recipients. Br J Clin Pharmacol. 2007;63:741–752. doi: 10.1111/j.1365-2125.2006.02841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nguyen L. Integration of modelling and simulation into the development of intravenous busulfan in paediatrics: an industrial experience. Fundam Clin Pharmacol. 2008;22:599–604. doi: 10.1111/j.1472-8206.2008.00651.x. [DOI] [PubMed] [Google Scholar]
- 31.Barrett JS, Della Casa Alberighi O, Laer S, Meibohm B. Physiologically based pharmacokinetic (PBPK) modeling in children. Clinical pharmacology and therapeutics. 2012;92:40–49. doi: 10.1038/clpt.2012.64. [DOI] [PubMed] [Google Scholar]
- 32.Bouillon-Pichault M, Jullien V, Bazzoli C, Pons G, Tod M. Pharmacokinetic design optimization in children and estimation of maturation parameters: example of cytochrome P450 3A4. Journal of pharmacokinetics and pharmacodynamics. 2011;38:25–40. doi: 10.1007/s10928-010-9173-1. [DOI] [PubMed] [Google Scholar]
- 33.Kearns GL, Abdel-Rahman SM, Alander SW, et al. Developmental pharmacology--drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349:1157–1167. doi: 10.1056/NEJMra035092. [DOI] [PubMed] [Google Scholar]
- 34.Karlsson MO, Lutsar I, Milligan PA. Population pharmacokinetic analysis of voriconazole plasma concentration data from pediatric studies. Antimicrobial agents and chemotherapy. 2009;53:935–944. doi: 10.1128/AAC.00751-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Friberg LE, Ravva P, Karlsson MO, Liu P. Integrated population pharmacokinetic analysis of voriconazole in children, adolescents, and adults. Antimicrobial agents and chemotherapy. 2012;56:3032–3042. doi: 10.1128/AAC.05761-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gibbs JP, Liacouras CA, Baldassano RN, Slattery JT. Up-regulation of glutathione S-transferase activity in enterocytes of young children. Drug metabolism and disposition: the biological fate of chemicals. 1999;27:1466–1469. [PubMed] [Google Scholar]
- 37.Gibbs JP, Murray G, Risler L, et al. Age-dependent tetrahydrothiophenium ion formation in young children and adults receiving high-dose busulfan. Cancer Res. 1997;57:5509–5516. [PubMed] [Google Scholar]
- 38.van den Anker JN, Schwab M, Kearns GL. Developmental pharmacokinetics. Handbook of experimental pharmacology. 2011;205:51–75. doi: 10.1007/978-3-642-20195-0_2. [DOI] [PubMed] [Google Scholar]
- 39.Gaziev J, Nguyen L, Puozzo C, et al. Novel pharmacokinetic behavior of intravenous busulfan in children with thalassemia undergoing hematopoietic stem cell transplantation: a prospective evaluation of pharmacokinetic and pharmacodynamic profile with therapeutic drug monitoring. Blood. 2010;115:4597–4604. doi: 10.1182/blood-2010-01-265405. [DOI] [PubMed] [Google Scholar]
- 40.Hassan M, Ljungman P, Bolme P, et al. Busulfan bioavailability. Blood. 1994;84:2144–2150. [PubMed] [Google Scholar]
- 41.Vassal G, Fischer A, Challine D, et al. Busulfan disposition below the age of three: alteration in children with lysosomal storage disease. Blood. 1993;82:1030–1034. [PubMed] [Google Scholar]
- 42.Bertholle-Bonnet V, Bleyzac N, Galambrun C, et al. Influence of underlying disease on busulfan disposition in pediatric bone marrow transplant recipients: a nonparametric population pharmacokinetic study. Therapeutic drug monitoring. 2007;29:177–184. doi: 10.1097/FTD.0b013e318039b478. [DOI] [PubMed] [Google Scholar]
- 43.Nilsson C, Aschan J, Hentschke P, et al. The effect of metronidazole on busulfan pharmacokinetics in patients undergoing hematopoietic stem cell transplantation. Bone Marrow Transplant. 2003;31:429–435. doi: 10.1038/sj.bmt.1703896. [DOI] [PubMed] [Google Scholar]
- 44.Buggia I, Zecca M, Alessandrino EP, et al. Itraconazole can increase systemic exposure to busulfan in patients given bone marrow transplantation. GITMO (Gruppo Italiano Trapianto di Midollo Osseo) Anticancer research. 1996;16:2083–2088. [PubMed] [Google Scholar]
- 45.Zhao L, Pickering G. Paracetamol metabolism and related genetic differences. Drug metabolism reviews. 2011;43:41–52. doi: 10.3109/03602532.2010.527984. [DOI] [PubMed] [Google Scholar]
- 46.Hu X, Pal A, Krzeminski J, et al. Specificities of human glutathione S-transferase isozymes toward anti-diol epoxides of methylchrysenes. Carcinogenesis. 1998;19:1685–1689. doi: 10.1093/carcin/19.9.1685. [DOI] [PubMed] [Google Scholar]
- 47.Johnson L, Orchard PJ, Baker KS, et al. Glutathione S-transferase A1 genetic variants reduce busulfan clearance in children undergoing hematopoietic cell transplantation. J Clin Pharmacol. 2008;48:1052–1062. doi: 10.1177/0091270008321940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abbasi N, Vadnais B, Knutson JA, et al. Pharmacogenetics of intravenous and oral busulfan in hematopoietic cell transplant recipients. J Clin Pharmacol. 2011;51:1429–1438. doi: 10.1177/0091270010382915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ansari M, Krajinovic M. Can the pharmacogenetics of GST gene polymorphisms predict the dose of busulfan in pediatric hematopoietic stem cell transplantation? Pharmacogenomics. 2009;10:1729–1732. doi: 10.2217/pgs.09.135. [DOI] [PubMed] [Google Scholar]
- 50.Zwaveling J, Press RR, Bredius RG, et al. Glutathione S-transferase polymorphisms are not associated with population pharmacokinetic parameters of busulfan in pediatric patients. Therapeutic drug monitoring. 2008;30:504–510. doi: 10.1097/FTD.0b013e3181817428. [DOI] [PubMed] [Google Scholar]


