Abstract
Accelerated pubertal development has been linked to adverse early environments and may heighten subsequent mental and physical health risks. Hypothalamic–pituitary–adrenal axis functioning has been posited as a mechanism whereby stress may affect pubertal development, but the literature lacks prospective tests of this mechanism. The current study assessed 277 youth (M = 10.84 years, SD = 1.14), 138 boys and 139 girls, who reported on their pubertal development and underwent the Trier Social Stress Test for Children at baseline and returned to the laboratory approximately 1 year later (M = 1.12 years, range = 0.59–1.98 years). For girls, lower cortisol area under the curve (with respect to ground) at Time 1 predicted more advanced pubertal development at Time 2, controlling for Time 1 pubertal development. This association persisted after additional covariates including age, body mass index, race, and maltreatment history were introduced, and was driven by adrenal rather than gonadal development. Cortisol was not linked to boys’ subsequent pubertal development, and no interaction by gender or by maltreatment appeared. These results suggest that attenuated cortisol, reported in other studies of children exposed to early adversity, may contribute to accelerated pubertal tempo in girls.
Pubertal development signals an important juncture in the transition from childhood to adulthood. The timing and tempo of puberty are variable, and individual differences have been linked with health outcomes (Marceau, Ram, Houts, Grimm, & Susman, 2011). For example, early puberty has been associated with heightened risk of psychological symptoms (Graber, Lewinsohn, Seeley, & Brooks-Gunn, 1997; Negriff & Susman, 2011) as well as physical health problems like obesity (Wang, 2002) and breast cancer (Bernstein, 2002; Sellers et al., 1992). Although genetic effects have been found to account for 50%–80% of the variability in pubertal timing across studies (Palmert & Hirschhorn, 2003), environmental factors, including psychosocial stressors, have also been associated with pubertal development (Belsky, Steinberg, & Draper, 1991; Graber, Brooks-Gunn, & Warren, 1995). The question of how stress influences pubertal development is complex, given evidence that stress both delays pubertal development in some cases (as has been found during extreme privation, e.g., famine and war) and accelerates it in others (e.g., in the context of father absence or family adversity; Ellis, 2004).
At the hormonal level, the hypothalamic–pituitary–adrenal (HPA) axis, which secretes glucocorticoids, the main one cortisol, is known to have a suppressive effect on the hypothalamic–pituitary–gonadal (HPG) axis (Chrousos & Gold, 1992). The HPG axis is responsible for reproduction, communicates with the gonads, and coordinates the release of sex hormones as well as multiple other metabolic processes. When threat is imminent, the body diverts resources toward the threat and away from longer range goals such as reproduction, growth, and immunity. When a stressful experience or anticipation activates the HPA axis, reproductive system activity is correspondingly dampened via the suppressive effects of HPA axis hormones on the luteinizing hormone-releasing hormone. HPA axis activation also inhibits pituitary gonadatrophs and the gonad itself, and renders target tissues for sex steroids (i.e., testes and ovaries) more resistant to these hormones (Chrousos & Gold, 1992). This inhibiting effect of physiological or emotional stress on the reproductive system, in the context of puberty, has been labeled “stress-suppression theory” (Ellis, 2004) and has been supported by evidence that puberty appears delayed among subordinate primates (French, 1997) and among youth exposed to extreme stress and trauma, such as during war (Prebeg & Bralic, 2000). However, a more recent study found changes in the coupling of the HPA and HPG axes over adolescence that appeared modulated by early life stress (Ruttle, Shirtcliff, Armstrong, Klein, & Essex, 2013). Specifically, HPA–HPG “cross-talk” appeared weakly positive at age 11 but shifted to more adult like negative coupling by age 15. Girls exposed to early stress showed an earlier shift to the latter pattern, with negative HPA–HPG coupling appearing by age 13.
In contrast to stress-suppression theory, other theories of environmental effects on puberty (e.g., psychosocial acceleration theory, paternal investment theory, and child development theory; reviewed by Ellis, 2004) posit an opposite direction of effects, with more stressful rearing environments predicting accelerated pubertal development. These theories have received empirical support, with factors like father absence, harsh or unsupportive parenting, parental unemployment, and family conflict associated with earlier pubertal development in girls (e.g., Arim et al., 2011; Belsky et al., 2007; Deardorff et al., 2011; Ellis & Garber, 2000), while positive family characteristics like approval and warmth have been linked with delayed pubertal development (Graber et al., 1995). These findings of psychosocial effects on puberty appear to be robust across a variety of assessment approaches, including physician assessment of Tanner stages (as used in papers such as Belsky et al., 2007, and Deardorff et al., 2011), and self-reported or parent-reported pubertal development using measures such as the Pubertal Development Scale (PDS; Petersen, Crockett, Richards, & Boxer, 1988; used in Arim et al., 2011; Ellis & Garber, 2000). A number of possible mechanisms have been proposed to explain these findings, such as genes, pheromones (related to the presence of familial or extrafamilial males), body fat (with stress-related weight gain triggering earlier puberty), and stress hormones. Despite the fact that stress hormones have been mentioned as a possible mechanism, few studies have actually measured stress hormones or used prospective designs, so the contributions of HPA axis functioning to psychosocial effects on puberty remain unstudied. In speculating about the HPA axis as a mechanism linking stress with accelerated pubertal development, researchers (e.g., Ellis & Garber, 2000; Ellis, 2004) have pointed to the interaction of the HPA and HPG axes, but they have noted that findings to date (linking higher family stress to earlier onset and faster tempo of puberty) present a paradox given that the HPA axis is known to suppress the HPG axis.
The “attenuation hypothesis” (Susman, 2006) helps to reconcile this apparently “paradoxical” relationship between psychosocial stress and accelerated puberty. According to this hypothesis, early, chronic stressful experiences may act to downregulate the stress system of some children, an adaptive strategy that protects the organism from the costs of continued adrenocortical overload. In support of this hypothesis, studies have linked early adversity with low baseline cortisol and attenuated reactivity to stress (e.g., Carpenter et al., 2007; Elzinga et al., 2008; Granger et al., 1998; Luecken, Kraft, & Hagan, 2009; Roisman et al., 2009). Previous investigations of the sample studied in this paper reported a history of maltreatment to be linked with low or attenuated patterns of cortisol responses to a stress task (Trickett, Gordis, Peckins, & Susman, in press). Youth whose maltreatment included physical or sexual abuse showed more pronounced attenuation, relative to youth who had experienced only neglect or emotional abuse, indicating that more severe maltreatment has a more suppressive effect on the HPA axis.
Evidence that early life stress may dampen, rather than heighten, HPA axis functioning in some contexts suggests the intriguing and untested possibility that youths with attenuated HPA axis functioning might be at risk for earlier puberty through the reduction of HPA axis-mediated suppression of the HPG axis, or the removal of a “hormonal brake” regulating puberty. Besides the current study, the only study, to our knowledge, to explore HPA axis functioning as predictive of pubertal tempo (Ellis, Shirtcliff, Boyce, Deardorff, & Essex, 2011) reported results consistent with the above possibility. The authors sampled cortisol when children (59% girls) were in first grade and took self-report and mother-report PDS measures of pubertal development (averaged together) up to four times when children were between third and ninth grades. Children whose cortisol levels increased over a 4-hr laboratory protocol showed a trend (significant in the case of pubic hair development) toward slower initial tempo of pubertal development (up to age 12.5) followed by faster subsequent pubertal tempo (after age 12.5). The association between higher cortisol and slower initial pubic hair development was stronger among children who received supportive parenting, a finding the authors interpret in terms of the “biological sensitivity to context” hypothesis (e.g., youth who are more physiologically sensitive to their environments have especially positive outcomes in high-resource environments and especially negative outcomes in aversive environments; Boyce & Ellis, 2005). However, the study was not designed primarily to examine cortisol and is limited by the lack of a standardized stress protocol, such as the Trier Social Stress Test, designed to elicit a cortisol response; instead, the authors used the slope of cortisol (increasing or decreasing) over the course of the entire laboratory visit to index “adrenocortical reactivity.”
The current report examines how HPA axis functioning in early to mid-puberty (ages 9–12) affects the tempo of pubertal development across approximately 1 year in a large, ethnically diverse sample of maltreated and nonmaltreated boys and girls. Because of its prospective design, this report can make a unique contribution to understanding the mechanisms of pubertal development. We hypothesize that HPA axis functioning at the first assessment (Time 1 [T1]) will be linked with tempo, modeled as pubertal stage at a second assessment (Time 2 [T2]), controlling for T1 pubertal stage. Given evidence that HPA activity suppresses HPG activity, we expect that attenuated cortisol, which has previously been associated with maltreatment in this sample (Trickett et al., in press), will predict accelerated puberty. In addition to looking at an overall measure of pubertal development, we will examine components of puberty that are assumed to reflect adrenal development (e.g., pubic hair development) and gonadal (breast/genital) development separately given that these are mediated by different hormones. Hypotheses will be tested separately for girls and for boys because the majority of past studies have focused exclusively on girls (Ellis, 2004), and then with boys and girls together to test the moderating role for gender. We also examined a possible moderating role for maltreatment, investigating whether an association between cortisol and puberty is stronger or weaker among youth with a history of maltreatment.
Methods
Participants
The present study used data from the first two assessments (~1 year apart) of an ongoing longitudinal study examining the effects of maltreatment on adolescent development. T1 data collection took place from 2002 to 2005 and involved 454 adolescents aged 9–13 years (241 males, 213 females). The final sample of youth who had available cortisol and puberty data at both time points, and were not dropped from these analyses for other reasons described below, consisted of 277 youth (138 boys, 139 girls). Maltreated youth comprised 64% of the sample (172 youth: 79 boys, 97 girls) and comparison youth constituted 36% of the sample (97 youth: 57 boys, 40 girls). Of maltreated youth, 61 had only experienced neglect or emotional abuse (35.5%), and 111 (64.5%) had experienced physical and/or sexual abuse (in addition to, or instead of, neglect and emotional abuse). In terms of ethnic background, 97 children identified as African American (35.5% of the sample), 31 as White (11.4%), 112 as Latino (41%), and 33 as mixed/biracial (12.1%). Other sample descriptives are shown in Table 1. Zero-order correlations between study variables are shown in Table 2.
Table 1.
Age, BMI, pubertal stage, and cortisol among participating children
| Variable | M | SD | Range |
|---|---|---|---|
| Males (n = 138) | |||
|
| |||
| Age (years) at T1 | 10.94 | 1.17 | 8.79–13.68 |
| Age (years) at T2 | 12.07 | 1.22 | 9.90–15.37 |
| BMI | 21.42 | 4.94 | 14.1–45.1 |
| Self-reported pubertal stage at T1 | 1.89 | 0.74 | 1–4 |
| Adrenal subscale | 1.80 | 0.95 | 1–4 |
| Gonadal subscale | 2.17 | 1.15 | 1–5 |
| Self-reported pubertal stage at T2 | 2.42 | 0.93 | 1–4.5 |
| Adrenal subscale | 2.18 | 1.10 | 1–4 |
| Gonadal subscale | 2.40 | 1.22 | 1–5 |
| Cortisol AUCg | −215.52 | 41.29 | −335.97 to −111.24 |
|
| |||
| Females (n = 139) | |||
|
| |||
| Age (years) at T1 | 10.74 | 1.12 | 8.50–13.47 |
| Age (years) at T2 | 11.84 | 1.13 | 9.51–14.56 |
| BMI | 21.91 | 4.90 | 14.1–37.7 |
| Self-reported pubertal stage at T1 | 2.15 | 0.88 | 1–4.5 |
| Adrenal subscale | 2.05 | 1.17 | 1–5 |
| Gonadal subscale | 2.32 | 0.90 | 1–4.5 |
| Self-reported pubertal stage at T2 | 2.73 | 0.92 | 1–4.5 |
| Adrenal subscale | 2.73 | 1.23 | 1–5 |
| Gonadal subscale | 2.54 | 0.82 | 1–4.5 |
| Cortisol AUCg | −229.86 | 46.71 | −345.40 to −78.83 |
Note: BMI, Body mass index; AUCg, area under the curve with respect to ground; T1, Time 1; T2, Time 2.
Table 2.
Zero-order correlations between study variables
| Measure | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| 1. Youth AUCg | — | ||||||
| 2. Age at T2 | .06 | — | |||||
| 3. Child’s gender | −.16** | −.10 | — | ||||
| 4. Race (Afr. Am./not Afr. Am.) | −.06* | .003 | −.05 | — | |||
| 5. Body mass index | −.10* | .27*** | .05 | .04 | — | ||
| 6. Maltreatment type | −.14* | −.09 | .14* | .12† | −.09 | — | |
| 7. Self-reported pubertal stage at T1 | −.02 | .54*** | .16* | .12† | .17** | .03 | — |
| Adrenal subscale | −.03 | .37*** | .12† | .17** | .14* | −.02 | .83*** |
| Gonadal subscale | −.003 | .20** | .05 | .08 | .14* | −.03 | .81*** |
| 8. Self-reported pubertal stage at T2 | −.08 | .50*** | .17*** | .05 | .16* | .06 | .69*** |
| Adrenal subscale | −.13* | .48*** | .23*** | .07 | .16** | .04 | .41*** |
| Gonadal subscale | .03 | .37*** | .06 | .01 | .07 | .01 | .43*** |
Note: AUCg, Area under the curve with respect to ground; T2, Time 2; T1, Time 1.
p < .1.
p < .05.
p < .01.
p < .001.
Recruitment
Participants in the maltreatment group were recruited from active cases in the Los Angeles Department of Children and Family Services (DCFS). The inclusion criteria were as follows: a new substantiated referral to DCFS in the preceding month for any type of maltreatment (e.g., neglect, physical abuse, sexual abuse); child age 9–12 years; child identified as Latino, African American, or Caucasian (non-Latino); and child residing in one of 10 zip codes in a designated county at the time of referral to DCFS. With the approval of DCFS and the institutional review board of the affiliated university, potential participants were contacted via postcard and then received a follow-up phone call.
According to information abstracted from the DCFS case records, most children in the maltreated group experienced multiple forms of maltreatment and had multiple referrals as well (for details, see Mennen, Kim, Sang, & Trickett, 2010). The majority of the maltreatment sample experienced neglect in some form, about half of the sample experienced physical abuse and/or emotional abuse, and approximately one fifth experienced sexual abuse. On average, the participants had experienced two types of maltreatment and had four referrals to DCFS.
The comparison group was recruited using names from school lists of children, obtained with institutional review board approval through a marketing firm, aged 9–12 years, and residing in the same 10 zip codes as the maltreated sample but without DCFS reports on file. Caretakers of potential participants were contacted by postcard and phone.
Upon enrollment in the study, the maltreatment and comparison groups were compared on a number of demographic variables. The two groups were similar on neighborhood characteristics (based on census block information) at enrollment. However, they differed in living arrangements: 93% of nonmaltreated and 52% of the maltreated children lived with a biological parent. The remainder of the maltreatment group was living in kinship or foster care, not unusual for adolescents involved with social services. Within the final sample, maltreated children were, on average, 3 months younger than comparison group children (mean age of maltreated children = 10.75, mean of comparison group children = 11.03), t (287) = −2.01, p = .05, and were more likely to be girls, t (287) = 2.42, p = 0.2. They did not differ in race/ethnicity, body mass index (BMI), or time elapsed between T1 and T2 (all ps > .17).
Attrition
The attrition rate between T1 and T2 was 13.4% (n = 61), and based on binary logistic regression analyses, this rate was nonrandom; participants in the maltreatment group were less likely to return at T2 (odds ratio = 4.38, p < .01; Nagelkerke R2 = .10).
We dropped youth from analyses if more than 2 years had elapsed between T1 and T2 study visits, resulting in the removal of 21 youth, or 7% of the sample (16 boys, 5 girls). Therefore, the average time elapsed between T1 and T2 assessments was 1.12 years (~1 year, 1.5 months; SD = 0.21 years, range = 7.2 months to 1.98 years).
Procedures
Assessments were conducted at an urban research university. After assent and consent were obtained from the adolescent and caretaker, respectively, the adolescent was administered questionnaires and tasks during a 4-hr protocol. The measures used in the following analyses represent a subset of the questionnaires administered during the protocol. Both the child and the caretaker were paid for their participation according to the guidelines of the National Institutes of Health Healthy Volunteer Program.
Stress paradigm and saliva collection
During the scheduling phone call, caregivers were told that children should not eat or drink anything (other than water) for 4 hr prior to their study visit. This information was also included in the confirmation letter they received and again in the reminder phone call. During their laboratory visit, children responded to a questionnaire that included items about medications currently being taken (including separate items asking whether they had taken any steroids, inhaled medications, or creams/lotions) and other variables that might affect cortisol concentrations. Youth who reported taking steroid medications or using inhalers at T1 (38 boys, 22 girls) were dropped from the sample, as was 1 girl who reported being a smoker in T1.
Over the course of 90 min, six saliva samples were obtained. The first sample occurred 45 min before the stressor. The second sample was 10 min before the stressor, immediately after a 5-min relaxation protocol (listening to soft music while viewing a still slide of a beach scene). Adolescents then engaged in a version of the Trier Social Stressor Test modified for children (Buske-Kirschbaum, et al., 1997). During this procedure, the interviewer and a panel of two judges entered the room. The interviewer told the participant that he or she would read the beginning of a story, and the participant would have 5 min to develop the next part of the story, after which the participant would present the next part of the story to the panel of judges for 4 min. The interviewer and judges then left the room for 5 min. When they reentered, the interviewer asked the adolescent to present the story. The interviewer and judges maintained neutral facial expressions throughout the task. If the youth did not fill the 4 min, the interviewer used a standard set of prompts to encourage him or her to continue. After the 4 min, the interviewer asked the youth to perform a 4-min serial subtraction task before the judges. This task was designed to be challenging for the youth (e.g., subtracting 7 serially beginning with 758). The third sample was obtained immediately after the stressor was complete, and the fourth, fifth, and sixth samples occurred 10, 20, and 30 min after the end of the stressor, respectively.
Saliva samples were collected via passive drool through a short straw into a vial. Data collection occurred primarily in the afternoon, with an average start time of 2:45 p.m. (SD = 73 min, range = 12:24–5:27 p.m.). Saliva samples were immediately frozen and subsequently transported on ice to Salimetrics LLC and stored frozen at −80 °C until assayed for cortisol. On the day of testing, all samples were centrifuged at 3000 rpm for 15 min to remove mucins.
Measures
PDS
Youth self-report of pubertal stage was used in this study because physician assessment was not feasible. The PDS is a measure of the exogenous physical changes associated with pubertal development. It was developed as an alternative to physician rating measures and has shown to have adequate reliability and validity (Petersen et al., 1988). On a 4-point scale, ranging from 1 (has not yet started) to 4 (has completed), each subject is asked to indicate the level of development on each of the physical changes. Five items were used for both males and females (height spurt, body hair, skin changes, breast growth/deepening of voice, and menarche/facial hair; α = 0.61 for males, α = 0.76 for females). A coding system developed by Shirtcliff, Dahl, and Pollak (2009) was used to convert the PDS scores to a 5-point scale to parallel the Tanner stages. This coding system was validated using comparison to hormonal measures and physician ratings. Adrenal and gonadal subscales were also computed (adrenal items included skin changes and pubic hair; gonadal items included growth spurt, breast development, and menarche for girls, and growth spurt, voice change, and facial hair for boys). Thus, we created an overall pubertal stage score composed of an adrenal sub-scale score and a gonadal subscale score.
Sixty-nine children, or approximately 20% of the sample, reported lower pubertal stage scores in T2 than T1. In most cases, this was a small decrease, less than 0.5, and occurred mostly for boys in the earliest stages of puberty. Our average ages at T1 (10.94 for boys, 10.74 for girls) and T2 (12.07 for boys, 11.84 for girls) correspond to a fairly early stage of visually apparent puberty for most children. A study of males and females in a similar age range that compared self-report with physical examination (Schlossberger, Turner, & Irwin, 1992) found that participants tended to overestimate their development at early stages and underestimate their development at later stages of puberty. Because true reversal of pubertal development is very rare, we adjusted for possible overestimation at T1 and underestimation at T2 by taking the mean of both ratings and imputed these at both time points for those whose score decreased.
BMI
Trained graduate student research assistants took weight measurements using a Healthometer scale and height measurements using a mounted stadiometer after asking the child to remove shoes and any bulky outerwear. Weight was measured three times (to the nearest 0.5 lb) and height measured twice (to the nearest 0.25 in.). BMI was calculated using the standard formula: BMI = weight (lb)× 703/height2 (in.).
Cortisol
Raw cortisol values (μg/dl) were examined closely for outliers and truncated if out of range (>3 SD above the mean value for each time point), a common approach with extreme cortisol values (e.g., Dettling, Gunnar, & Donzella, 1999). Between three and nine samples were dropped at each time point; altogether, 16 youth (~5% of the sample) had out-of-range values at one or more time point. Cortisol values were then log-transformed to adjust for skewness. Area under the curve with respect to ground (AUCg), a measure of total cortisol excretion across the laboratory task, was calculated using logged values and following the trapezoidal formula supplied by Pruessner, Kirschbaum, Meinlschmidt, and Hellhammer (2003) and included all six cortisol data points. Area under the curve with respect to increase (AUCi) was also calculated using logged values and using the same formula but minus the initial starting value of cortisol, or “ground.”
Data cleaning and all analyses were done using SPSS Statistics (IBM).
Results
Regression models predicting pubertal tempo from T1 cortisol
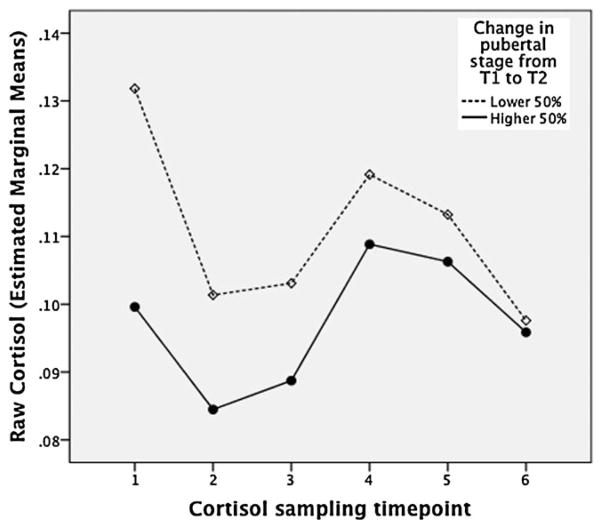
We hypothesized that HPA axis functioning at T1 would be linked to pubertal tempo, operationalized as T2 pubertal stage when controlling for prior pubertal stage. We tested this hypothesis using linear regression analyses. The first model predicting T2 pubertal stage included only cortisol AUCg, saliva sampling start time (an essential covariate due to the diurnal rhythm of cortisol), and T1 pubertal development. We split the sample by gender to be consistent with the literature and because boys’ and girls’ pubertal development unfolds differently (e.g., differences in timing of puberty and reproductive target tissues). As shown in Table 3, T1 cortisol AUCg emerged as a predictor of self-reported T2 pubertal stage in girls when controlling for T1 stage, with lower cortisol levels in T1 linked with more mature pubertal stage in T2. This association was not significant in boys. Cortisol AUCi was not associated with pubertal stage at T2 when controlling for T1 pubertal stage and saliva sampling start time (p > .30 for both boys and girls). Figure 1 illustrates these results by showing girls’ cortisol levels at all six sampling time points. This figure was created by a repeated-measures analysis with starting time of cortisol sampling entered as a covariate and a median split separating faster developing and slower developing girls (based on a change score calculated by subtracting T1 pubertal stage from T2 pubertal stage). As is evident from Figure 1, girls’ reactivity to the stress task is similar in both pubertal-tempo groups, but cortisol levels are lower at every time point in the group that showed faster pubertal development from T1 to T2.
Table 3.
Linear regression analyses showing effects of cortisol on pubertal tempo (T1 pubertal stage and cortisol AUCg predicting T2 pubertal stage)
| Beta | t | |
|---|---|---|
| Males: R (135, 3) = .65, F = 31.66*** | ||
|
| ||
| Constant | 1.09 | |
| Cortisol collection start time | 0.02 | 0.29 |
| T1 self-reported pubertal stage | 0.64 | 9.68*** |
| T1 cortisol AUCg | 0.04 | 0.52 |
|
| ||
| Females: R (136, 3) = .72, F = 48.84*** | ||
|
| ||
| Constant | 1.63 | |
| Cortisol collection start time | −0.07 | −1.08 |
| T1 self-reported pubertal stage | 0.70 | 11.72*** |
| T1 cortisol AUCg | −0.17 | −2.62** |
Note: T1, Time 1; AUCg, area under the curve with respect to ground; T2, Time 2.
p < .01.
p < .001.
Figure 1.
Change in cortisol levels over all sampling time points; separate lines depict faster developing girls (higher 50%ile for change in self-reported pubertal stage from Time 1 to Time 2) and slower developing girls (lower 50%ile for change from Time 1 to Time 2).
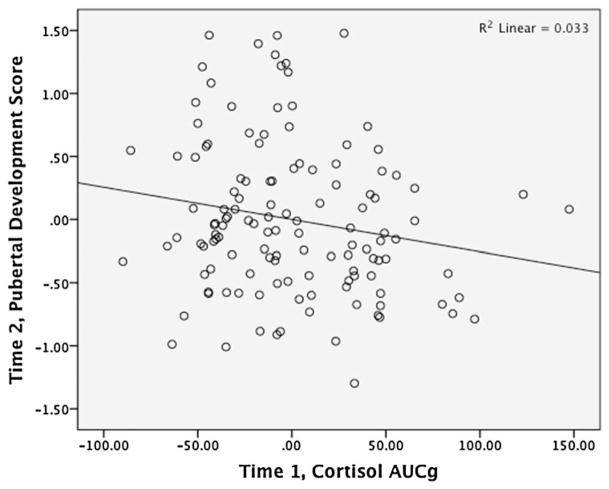
Next, we added covariates that have been shown elsewhere in the literature to be linked with puberty: age, BMI, race (coded as African American vs. not African American), and maltreatment type (coded as 0 = no maltreatment; 1 = emotional abuse and/or neglect; 2 = physical abuse and/or sexual abuse). We also included time elapsed between the T1 and T2 study visits. Once again, as shown in Table 4, cortisol AUCg was negatively associated with girls’ self-reported pubertal stage in the following year. Figure 2 shows a partial regression plot illustrating this result. The only control variable to remain significant in the full model was age, which was positively associated with T2 puberty for both boys and girls.
Table 4.
Full regression model showing effects of cortisol on pubertal tempo
| Beta | t | |
|---|---|---|
| Males: R (128, 8) = .69, F = 13.64*** | ||
|
| ||
| Constant | −1.29 | |
| Cortisol collection start time | .01 | −0.19 |
| Age at T2 | .27 | 3.41*** |
| Time elapsed between T1 and T2 | .03 | 0.43 |
| Race (Afr. Am./not Afr. Am.) | −.06 | −0.92 |
| BMI | −.02 | −0.33 |
| Maltreatment type | .07 | 1.01 |
| T1 self-reported pubertal stage | .57 | 7.88*** |
| T1 cortisol AUCg | −.04 | −0.52 |
|
| ||
| Females: R (127, 8) = .77, F = 21.82*** | ||
|
| ||
| Constant | −1.61 | |
| Cortisol collection start time | −.08 | −1.22 |
| Age at T2 | .29 | 3.93*** |
| Time elapsed between T1 and T2 | .09 | 1.46 |
| Race (Afr. Am./not Afr. Am.) | .04 | 0.71 |
| BMI | .002 | 0.03 |
| Maltreatment type | −.01 | −0.19 |
| T1 self-reported pubertal stage | .55 | 7.71*** |
| T1 cortisol AUCg | −.13 | −2.02* |
Note: T2, Time 2; T1, Time 1; BMI, body mass index; AUCg, area under the curve with respect to ground.
p < .05.
p < .001.
Figure 2.
Partial regression plot showing Time 1 cortisol area under the curve with respect to ground predicting Time 2 self-reported pubertal development in girls, controlling for Time 1 pubertal stage and other covariates (age, body mass index, race, maltreatment type, and cortisol sampling start time).
Moderation analyses
Next, we tested for possible moderators of cortisol effects on pubertal tempo by adding interaction terms. To examine the interaction of cortisol with maltreatment, we added a “cortisol by maltreatment” term to the regression model described above. The result was not significant (for boys, b = 0.11, t = 0.97, p = .33; for girls, b = −0.06, t = −0.68, p = .50). We also combined boys’ and girls’ data and tested for the interaction of sex by cortisol, sex by maltreatment group (maltreated vs. nonmaltreated), and the three-way interaction of sex by cortisol by maltreatment. Whether we added these interaction terms separately or together, no significant results emerged, suggesting that the results were not moderated by maltreatment or gender. In an additional test of moderating effects, we split the sample by gender and maltreatment group and tested the effect of T1 cortisol on T2 pubertal stage, controlling for T1 pubertal stage. Significant results did not emerge for boys. The effect of T1 cortisol on pubertal tempo remained significant for maltreated girls, b (95) = −0.21, t = 2.25, p = .03, but became marginally significant among non-maltreated girls, b (38) = −0.27, t = 1.97, p = .06.
Adrenal and gonadal development
Next, we ran separate models testing the adrenal and gonadal subscales of the PDS measure separately. Consistent with the results for overall PDS scores, T1 cortisol AUCg was negatively associated with T2 adrenal development for girls. This was the case whether the model included only cortisol sampling time and T1 adrenal development as covariates (b = −0.29, t = 3.73, p = .001) or additional control variables age, race, BMI, and maltreatment type (b = −0.24, t = −3.18, p = .002). Cortisol was not predictive of T2 adrenal development in boys or T2 gonadal development in girls or boys (p > .30).
When we split the sample by gender and maltreatment group, as above, T1 cortisol was predictive of T2 adrenal development in both maltreated (b = −0.29, t = −2.96, p = .004) and nonmaltreated (b = −0.34, t = −2.40, p = .02) girls.
Discussion
This study found girls’ attenuated cortisol to be predictive of a faster tempo of pubertal development, measured over two assessments spaced approximately 1 year apart. Girls with lower cortisol secretion at T1, based on cortisol samples collected during a laboratory stress paradigm, showed more advanced self-reported pubertal development (notably adrenal development) at T2 when controlling for prior-year pubertal stage. These results held when including covariates also associated with pubertal development, such as age, BMI, ethnic minority status, and maltreatment. Boys’ T1 cortisol did not appear to be predictive of T2 pubertal stage, and no significant interaction effects emerged for sex or maltreatment group status. The results were driven by the adrenal development scale (body hair and skin changes), which fits expectations given that adrenal development is linked more closely with the HPA axis and gonadal development with the HPG axis (Dorn & Biro, 2011). These results were specific to AUCg, a measure of total cortisol excretion over the laboratory visit, and did not extend to AUCi, a measure of reactivity that does not include the starting or basal level of cortisol.
This report constitutes one of the first prospective tests of HPA axis activity predicting the tempo of pubertal development. Although researchers have theorized that environmental stress might contribute to both the acceleration and the deceleration of puberty, our results help to reconcile prior ambiguities in the literature by suggesting that the attenuation in cortisol secretion found among some youth exposed to adversity might contribute to accelerated pubertal development among girls. This may occur due to the modulation of the HPA axis’s known suppressive effect on the HPG axis (Chrousos & Gold, 1992). In other words, the “turning down” of the HPA axis in conditions of early chronic stress, which has been seen in numerous studies of youth exposed to psychosocial adversity (Susman, 2006) may facilitate the HPG system’s “turning up” and thereby hasten the tempo of puberty. Another possibility is that early life stress may affect the coupling of the HPA and HPG axes in girls (Ruttle et al., 2013).
Much like the dampening of HPA axis activation in a chronic stress/threat context, accelerated pubertal tempo may be adaptive in the short-term but carry longer-term consequences. In the short term, earlier physical maturation may promote children’s independence from an aversive environment. This is consistent with Ellis’ (2004) child development theory, which argued that delayed puberty is adaptive when the childhood environment is nurturing but not when it is harsh or neglectful. For example, girls who become sexually mature sooner may be able to form romantic partnerships that enable escape from an abusive family of origin. However, in the long-term, such a strategy may bring costs, because these romantic partnerships may perpetuate a cycle of early pregnancy and family violence. Early pregnancy increases girls’ risk of single motherhood and poor economic and educational outcomes, and may also disadvantage offspring (e.g., Black & DeBlassie, 1985; Furstenberg, Brooks-Gunn, & Chase-Landsdale, 1989). An accelerated tempo of puberty has been associated with mental and physical health problems, including increased risk of substance use (Arim et al, 2011) and both internalizing and externalizing problems in girls (Marceau et al., 2011).
Our results dovetail with those of Ellis et al. (2011), who found first graders’ higher cortisol increases in response to stress and challenge tasks to be linked with a slower tempo of pubertal development up to age 12.5 (and a faster tempo after age 12.5). Our participants were mostly in the pre-12.5 age range, and we also found higher cortisol to be linked with a slower tempo of puberty. Their results were specific to pubic hair development, which is consistent with our significant findings for adrenal but not for gonadal development.
Ellis et al. (2011) also found an interaction between family history and cortisol, such that higher cortisol youth who received more supportive parenting had more delayed pubertal development. While we did not examine supportive parenting in this study, we did not find interaction effects for maltreatment in these analyses. Maltreated youth in this sample had lower cortisol responses to the child version of the Trier Social Stress Test (Trickett, in press), but we did not find maltreatment to be correlated with T1 or T2 pubertal stage or to moderate the effect of T1 cortisol on T2 pubertal stage. Instead, we found similar directions of effects among both maltreated and nonmaltreated girls, with lower cortisol associated with a faster subsequent tempo of pubertal development in both cases. Statistically, this result became marginally significant for nonmaltreated girls when we split the sample, but this was likely due to power because the nonmaltreated sample was smaller than the maltreated sample. Therefore, our results point to a main effect for dampened cortisol on girls’ pubertal tempo rather than the interaction of family history and cortisol that would be consistent with a “sensitivity to context” or “differential susceptibility” (Belsky & Pluess, 2009) framework. The majority of our participants were maltreated youth, and comparison youth were recruited from the same high-density and high-crime areas in south and east Los Angeles. It is likely that the level of community violence exposure was relatively high in both groups and that even our comparison-group girls might have experienced early adversity.
Our results were specific to total cortisol excretion over the laboratory visit (AUCg), a parameter that includes starting values, and did not extend to a measure of cortisol reactivity (AUCi) that only reflected change from the beginning to the end of the visit. As is apparent from the figure showing raw cortisol values over the course of the visit, girls in the faster maturing and slower maturing groups showed similar responses to the stress task, but average levels of cortisol were lower at all time points for the faster maturing girls. The AUCg maps onto “total hormonal output” and has been found to be more closely linked to raw measurements of cortisol than AUCi (Fekedulegn et al., 2007), suggesting that our AUCg measure might have been a better approximation of girls’ day-to-day basal cortisol than the AUCi measure. Daily, chronic HPA axis activity might be more likely to influence pubertal development, which unfolds slowly over months and years, than the less frequently deployed acute stress response. Further studies that include ambulatory sampling of cortisol over several days would elucidate this finding.
We also did not find effects for boys or a moderating effect of gender. Most of the literature on environmental effects on puberty has focused on girls, perhaps because they tend to show stronger associations between psychosocial stressors and pubertal development (Ellis, 2004). A study of neuroendocrine coupling across adolescence found that early life stress shifted cortisol–testosterone coupling for girls only (Ruttle et al, 2013), suggesting that the pattern of female-only findings in the extant literature may be linked to a gender difference in how life experiences modulate the interaction of hormonal systems implicated in puberty. Our lack of results for boys might also have been driven by measurement issues: for example, boys had low reliability (α = 0.61) on the pubertal measure we used. Future research can help to illuminate more of the environmental antecedents of pubertal development in boys, because boys represent an understudied group within this literature. We also did not find a link between participants’ cortisol and their gonadal development subscale. One likely explanation is that adrenal hormones, such as cortisol, influence adrenal development more strongly than gonadal development. Another possibility is that because adrenal development typically precedes gonadal development, and most of our participants were in early to mid-puberty, we did not capture the window of gonadal development. Our results were in the same direction for both adrenal and gonadal development, which tend to be tightly correlated in early puberty.
This study had a number of limitations. For example, we relied on a self-report measure of pubertal development rather than physical examination. Because self-report can add bias, particularly when adolescents are substantially more or less physically developed than their peers (Schlossberger et al., 1992), this method limits the validity of our results. However, the coding system that we used to convert PDS scores into Tanner stages has been found in other studies to correlate highly with both a picture-based interview about puberty and a physical exam, as well as with adrenal and gonadal hormones (Shirtcliff et al., 2009). Another limitation is that our first measurement of cortisol occurred when participants were, on average, 10.9 years of age, meaning that pubertal development had already begun for most of the sample. A truer prospective test would have been to sample cortisol several years earlier, before pubertal processes had started. However, our data arguably provide a more proximal test of the effects of stress hormones on pubertal development than Ellis et al. (2011), who measured cortisol when participants were in first grade. Finally, our measurement of maltreatment came from DCFS reports. While this offers a more objective and proximal measure of maltreatment than the retrospective self-report measures used in many studies, it does not allow us to conclusively report the duration, severity, or chronicity of maltreatment given that some episodes of maltreatment may not have been formally reported. While comparison-group children were not in the DCFS database, they could have experienced unreported maltreatment. Moreover, we did not measure other types of stressful life events in this sample, although it is likely that other significant stressors were present given the sample demographics.
In conclusion, our results identify a possible mechanism (the suppression of the HPA axis in a context of early chronic adversity) by which early life stress may hasten the tempo of girls’ puberty, and thereby contribute to cascading negative outcomes across the lifespan. The complex relationship between stress and pubertal maturation warrants further exploration. However, our findings provide new terrain in which to explore the adaptive and maladaptive trade-offs of a dampened stress response in youth exposed to risky environments. These findings may inform intervention by helping clinicians, parents, and teachers identify which youth are most likely to experience accelerated pubertal maturation and its accompanying risks.
Acknowledgments
This study was supported by NIH Grant R01 HD 39129 (P.K.T., Principal Investigator).
References
- Arim RG, Tramonte L, Shapka JD, Dahinten VS, Willms JD. The family antecedents and the subsequent outcomes of early puberty. Journal of Youth and Adolescence. 2011;40:1423–1435. doi: 10.1007/s10964-011-9638-6. [DOI] [PubMed] [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: Differential susceptibility to environmental influences. Psychological Bulletin. 2009;135:885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Belsky J, Steinberg L, Draper P. Childhood experience, interpersonal development, and reproductive strategy: An evolutionary theory of socialization. Child Development. 1991;62:647–670. doi: 10.1111/j.1467-8624.1991.tb01558.x. [DOI] [PubMed] [Google Scholar]
- Belsky J, Steinberg LD, Houts RM, Friedman SL, DeHart G, Cauffman E, et al. Family rearing antecedents of pubertal timing. Child Development. 2007;78:1302–1321. doi: 10.1111/j.1467-8624.2007.01067.x. [DOI] [PubMed] [Google Scholar]
- Bernstein L. Epidemiology of endocrine-related risk factors for breast cancer. Journal of Mammary Gland Biology and Neoplasia. 2002;7:3–15. doi: 10.1023/a:1015714305420. [DOI] [PubMed] [Google Scholar]
- Black C, DeBlassie RR. Adolescent pregnancy: Contributing factors, consequences, treatment, and plausible solutions. Adolescence. 1985;20:281–289. [PubMed] [Google Scholar]
- Boyce WT, Ellis BJ. Biological sensitivity to context: An evolutionary-developmental theory of the origins and functions of stress reactivity. Development and Psychopathology. 2005;17:271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- Buske-Kirschbaum A, Jobst S, Psych D, Wustmans A, Kirschbaum C, Rauh W, et al. Attenuated free cortisol response to psychosocial stress in children with atopic dermatitis. Psychosomatic Medicine. 1997;59:419–426. doi: 10.1097/00006842-199707000-00012. [DOI] [PubMed] [Google Scholar]
- Carpenter LL, Carvalho JP, Tyrka AR, Wier LM, Mello AF, Mello MF, et al. Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biological Psychiatry. 2007;62:1080–1087. doi: 10.1016/j.biopsych.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos G, Gold P. The concepts of stress and stress system disorders: Overview of physical and behavioral homeostasis. Journal of the American Medical Association. 1992;267:1244. [PubMed] [Google Scholar]
- Deardorff J, Ekwaru JP, Kushi LH, Ellis BJ, Greenspan LC, Mirabedi A, et al. Father absence, body mass index, and pubertal timing in girls: Differential effects by family income and ethnicity. Journal of Adolescent Health. 2011;48:441–447. doi: 10.1016/j.jadohealth.2010.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettling AC, Gunnar MR, Donzella B. Cortisol levels of young children in full-day childcare centers: Relations with age and temperament. Psychoneuroendocrinology. 1999;24:519–536. doi: 10.1016/s0306-4530(99)00009-8. [DOI] [PubMed] [Google Scholar]
- Dorn LD, Biro FM. Puberty and its measurement: A decade in review. Journal of Research on Adolescence. 2011;21:180–195. [Google Scholar]
- Ellis BJ. Timing of pubertal maturation in girls: An integrated life history approach. Psychological Bulletin. 2004;130:920–958. doi: 10.1037/0033-2909.130.6.920. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Garber J. Psychosocial antecedents of variation in girls’ pubertal timing: Maternal depression, stepfather presence, and marital and family stress. Child Development. 2000;71:485–501. doi: 10.1111/1467-8624.00159. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Shirtcliff EA, Boyce WT, Deardorff J, Essex MJ. Quality of early family relationships and the timing and tempo of puberty: Effects depend on biological sensitivity to context. Development and Psychopathology. 2011;23:85–99. doi: 10.1017/S0954579410000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzinga BM, Roelofs K, Tollenaar MS, Bakvis P, Van Pelt J, Spinhoven P. Diminished cortisol responses to psychosocial stress associated with lifetime adverse events a study among healthy young subjects. Psychoneuroendocrinology. 2008;33:227–237. doi: 10.1016/j.psyneuen.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Fekedulegn DB, Andrew ME, Burchfiel CM, Violanti JM, Hartley TA, Charles LE, et al. Area under the curve and other summary indicators of repeated waking cortisol measurements. Psychosomatic Medicine. 2007;69:651–659. doi: 10.1097/PSY.0b013e31814c405c. [DOI] [PubMed] [Google Scholar]
- French J. Proximate regulation of singular breeding in callitrichid primates. In: Solomon N, French J, editors. Cooperative breeding in mammals. New York: Cambridge University Press; 1997. [Google Scholar]
- Furstenberg FF, Brooks-Gunn J, Chase-Lansdale L. Teenaged pregnancy and childbearing. American Psychologist. 1989;44:313–320. [PubMed] [Google Scholar]
- Graber JA, Brooks-Gunn J, Warren MP. The antecedents of menarcheal age: Heredity, family environment, and stressful life events. Child Development. 1995;66:346–359. doi: 10.1111/j.1467-8624.1995.tb00875.x. [DOI] [PubMed] [Google Scholar]
- Graber JA, Lewinsohn PM, Seeley JR, Brooks-Gunn J. Is psychopathology associated with the timing of pubertal development? Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36:1768–1776. doi: 10.1097/00004583-199712000-00026. [DOI] [PubMed] [Google Scholar]
- Granger DA, Serbin LA, Schwartzman A, Lehoux P, Cooperman J, Ikeda S. Children’s salivary cortisol, internalising behaviour problems, and family environment: Results from the Concordia Longitudinal Risk Project. International Journal of Behavioral Development. 1998;22:707–728. [Google Scholar]
- Luecken LJ, Kraft A, Hagan MJ. Negative relationships in the family-of-origin predict attenuated cortisol in emerging adults. Hormones and Behavior. 2009;55:412–417. doi: 10.1016/j.yhbeh.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marceau K, Ram N, Houts R, Grimm K, Susman E. Individual differences in boys’ and girls’ timing and tempo of puberty: Modeling development with nonlinear growth models. Developmental Psychology. 2011;47:1389–1409. doi: 10.1037/a0023838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennen FE, Kim K, Sang J, Trickett PK. Child neglect: Definition and identification of youth’s experiences in official reports of maltreatment. Child Abuse and Neglect. 2010;34:647–658. doi: 10.1016/j.chiabu.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negriff S, Susman EJ. Pubertal timing, depression, and externalizing problems: A framework, review, and examination of gender differences. Journal of Research on Adolescence. 2011;21:717–746. [Google Scholar]
- Palmert MR, Hirschhorn JN. Genetic approaches to stature, pubertal timing, and other complex traits. Molecular Genetics and Metabolism. 2003;80:1–10. doi: 10.1016/s1096-7192(03)00107-0. [DOI] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Prebeg Z, Bralic I. Changes in menarcheal age in girls exposed to war conditions. American Journal of Human Biology. 2000;12:503–508. doi: 10.1002/1520-6300(200007/08)12:4<503::AID-AJHB10>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Pruessner J, Kirschbaum C, Meinlschmidt G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Roisman GI, Susman E, Barnett-Walker K, Booth-LaForce C, Owen MT, Belsky J, et al. Early family and child-care antecedents of awakening cortisol levels in adolescence. Child Development. 2009;80:907–920. doi: 10.1111/j.1467-8624.2009.01305.x. [DOI] [PubMed] [Google Scholar]
- Ruttle PL, Shirtcliff EA, Armstrong JM, Klein MH, Essex MJ. Neuroendocrine coupling across adolescence and the longitudinal influence of early life stress. Developmental Psychobiology. 2013 doi: 10.1002/dev.21138. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlossberger NM, Turner RA, Irwin CE., Jr Validity of self-report of pubertal maturation in early adolescents. Journal of Adolescent Health. 1992;13:109–113. doi: 10.1016/1054-139x(92)90075-m. [DOI] [PubMed] [Google Scholar]
- Sellers TA, Kushi LH, Potter JD, Kaye SA, Nelson CL, McGovern PG, et al. Effect of family history, body-fat distribution, and reproductive factors on the risk of postmenopausal breast cancer. New England Journal of Medicine. 1992;326:1323–1329. doi: 10.1056/NEJM199205143262004. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Dahl RE, Pollak SD. Pubertal development: Correspondence between hormonal and physical development. Child Development. 2009;80:327–337. doi: 10.1111/j.1467-8624.2009.01263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susman EJ. Psychobiology of persistent antisocial behavior: Stress, early vulnerabilities and the attenuation hypothesis. Neuroscience & Bio-behavioral Reviews. 2006;30:376–389. doi: 10.1016/j.neubiorev.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Trickett PK, Gordis E, Peckins MK, Susman EJ. Stress reactivity in maltreated and comparison male and female young adolescents. Child Maltreatment. doi: 10.1177/1077559513520466. in press. [DOI] [PubMed] [Google Scholar]
- Wang Y. Is obesity associated with early sexual maturation? A comparison of the association in American boys versus girls. Pediatrics. 2002;110:903–910. doi: 10.1542/peds.110.5.903. [DOI] [PubMed] [Google Scholar]