Abstract
Background and Purpose
There is controversy and little information concerning whether individual proton pump inhibitors (PPIs) differentially alter the effectiveness of clopidogrel in reducing ischemic stroke risk. We therefore aimed to elucidate the risk of ischemic stroke among concomitant users of clopidogrel and individual PPIs.
Methods
We conducted a propensity score-adjusted cohort study of adult new users of clopidogrel, using 1999–2009 Medicaid claims from 5 large states. Exposures were defined by prescriptions for esomeprazole, lansoprazole, omeprazole, rabeprazole and pantoprazole—with pantoprazole serving as the referent. The endpoint was hospitalization for acute ischemic stroke, defined by International Classification of Diseases 9th Revision Clinical Modification codes in the principal position on inpatient claims, within 180 days of concomitant therapy initiation.
Results
Among 325,559 concomitant users of clopidogrel and a PPI, we identified 1,667 ischemic strokes for an annual incidence of 2.4% (95% confidence interval: 2.3–2.5). Adjusted hazard ratios for ischemic stroke vs. pantoprazole were: 0.98 (0.82–1.17) for esomeprazole; 1.06 (0.92–1.21) for lansoprazole; 0.98 (0.85–1.15) for omeprazole; and 0.85 (0.63–1.13) for rabeprazole.
Conclusions
PPIs of interest did not increase the rate of ischemic stroke among clopidogrel users when compared to pantoprazole, a PPI thought to be devoid of the potential to interact with clopidogrel.
Keywords: Cohort studies, Drug interactions, Medicaid, Pharmacoepidemiology, Platelet aggregation inhibitors, Proton pump inhibitors, Stroke
INTRODUCTION
Clopidogrel reduces the rate of cerebrovascular and cardiovascular events in patients with acute coronary syndrome (ACS), recent acute myocardial infarction (AMI) or stroke, and those with peripheral artery disease. Clopidogrel itself is pharmacologically inactive and must be converted to an active metabolite through a multi-step process mediated by multiple cytochrome P-450 (CYP) isozymes.1 CYP2C19 is thought to contribute to 45% of the metabolism of clopidogrel to an inactive intermediate, and to 21% of the conversion of the intermediate to the active form.2 This has led to significant concern that drugs inhibiting CYP2C19 (including some commonly-used proton pump inhibitors [PPIs]) might reduce clopidogrel’s effectiveness. For example, a 2009 Food and Drug Administration (FDA) public health advisory warned that patients taking clopidogrel should avoid omeprazole and esomeprazole, both of which inhibit CYP2C19.3 Despite the warning, others have questioned the importance of CYP2C19 in clopidogrel activation4,5 in favor of CYP3A4 and paraoxonase-1 pathways.6,7
Many studies have examined effects of the potential drug interaction between clopidogrel and PPIs on major adverse cardiovascular events (MACE) as a combined endpoint and on AMI. Fewer studies have examined effects of the potential interaction on ischemic stroke.8–18 Among these, only one small study examined the comparative safety of individual PPIs in patients taking clopidogrel.15 This question is important, as the effects of PPIs on stroke might differ from the effects on the composite MACE endpoint. Therefore, we sought to examine the comparative safety of individual PPIs with respect to risk of acute ischemic stroke among users of clopidogrel.
METHODS
Overview and study population
We conducted a propensity score-adjusted retrospective cohort study of adult new users of clopidogrel. Our cohort consisted exclusively of person-time exposed to clopidogrel plus one of the following PPIs: esomeprazole, lansoprazole, omeprazole, pantoprazole, or rabeprazole. We compared risks among PPIs, rather than comparing PPI exposed to unexposed subjects, since PPIs have different strengths of CYP2C19 inhibition and to avoid potential confounding by the clinical indication for the PPI, which could occur if patients with gastrointestinal disorders were also at increased risk of stroke. Data for this study consisted of 1999–2009 Medicaid claims from California, Florida, New York, Ohio, and Pennsylvania.19 These states comprise about 38% of the US Medicaid population,20 with the 11-year dataset recording the experience of over 59 million cumulative enrollees and 179 million person-years (p-y) of observation. Because up to 27% of Medicaid beneficiaries (varying by state and year) were co-enrolled in Medicare (i.e., dually enrolled),21–23 we also obtained Medicare claims (including Part D prescription data from 2006 onward) to ascertain a more complete picture of enrollees’ healthcare.24
Defining the study cohort
We defined new users of clopidogrel as those with at least 12 months of Medicaid enrollment before their first clopidogrel prescription was dispensed. Among such persons, we then identified the day on which they were first concomitantly-exposed to clopidogrel and a PPI; this served as the cohort entry date. Persons were able to enter the cohort in one of three ways: 1) clopidogrel was added to ongoing PPI therapy; 2) clopidogrel and the PPI were initiated on the same day; or 3) a PPI was added to ongoing clopidogrel therapy. Given the aforementioned enrollment requirement, persons had at least one year of time prior to cohort entry, yet were permitted to have baseline periods of variable length (≥12 months) so long as this period was devoid of enrollment interruption. Use of a variable baseline period, such as this, results in less biased estimates and minimizes residual confounding vs. use of a fixed period.25
Persons were excluded from study if <18 years of age. Persons with a stroke during the baseline period were not excluded, as this could have been the indication for their clopidogrel therapy; rather, history of prior stroke was included in the propensity score.
Follow-up began upon cohort entry and continued until the first occurrence of the following: a) outcome of interest (defined below); b) death, as assessed by linkage to the Social Security Administration Death Master File; c) the 181st day of follow-up (see rationale in outcome subsection below); d) >15-day gap in either clopidogrel or PPI therapy; e) switch to another PPI; f) loss of Medicaid eligibility; or g) the end of the dataset. Follow-up time occurring during a period of hospitalization was excluded, although hospitalization did not serve as a censoring event.
Exposure and covariate ascertainment
Exposure was defined by the specific PPI agent (dispensed in capsule or tablet form) active on the day of cohort entry. Pantoprazole was selected as the reference PPI, as it: 1) is not a potent inhibitor of CYP2C19;26–28 2) is considered to have a low potential for drug-drug interactions with clopidogrel;29 3) may not be associated with an increased risk of MACE;1,30 and 4) has been recommended by FDA as a PPI to be considered in clopidogrel-treated patients.31 We considered using rabeprazole as the reference PPI since its metabolism is primarily non-enzymatic.29 However, rabeprazole was used very infrequently, which would have resulted in very wide 95% confidence intervals (CIs) for effect estimates of PPIs vs. rabeprazole.
We measured numerous baseline potential confounders in the following categories: 1) demographics—age, sex, race, state of residence, calendar year, and dual-eligibility status; 2) health system use factors, measured during baseline—such as numbers of emergency department visits, hospitalizations, ambulatory visits, nursing home residence, and prescription dispensings for unique drugs;32 3) diseases, measured during baseline—such as chronic illnesses (e.g., diabetes mellitus), potential risk factors for the outcome (e.g., hypertension), medical device utilization (e.g., stent placement), and labeled and off-labeled indications for clopidogrel (e.g., AMI); 4) drug markers of chronic diseases, measured during baseline (e.g., antidiabetic agents, as a marker of diabetes mellitus); 5) acutely-occurring diseases, measured 90 days prior to cohort entry (e.g., infection); and 6) recent drug exposures, measured 30 days prior to cohort entry (7 days for antimicrobials)—for agents posited to increase or decrease outcome risk (e.g., nonsteroidal anti-inflammatory drugs and warfarin, respectively), CYP inhibitors and inducers, and drug markers of acutely-occurring diseases (e.g., antimicrobial agents). Covariates within each of these groups were used in calculating the propensity scores; see Supplemental Table I for a complete listing of covariates used.
Outcome ascertainment
The outcome of interest was hospitalization for acute ischemic stroke occurring within 180 days of cohort entry. Operationally, ischemic stroke was defined by one of the following International Classification of Diseases 9th Revision Clinical Modification (ICD-9-CM) discharge diagnosis codes in the principal position on an inpatient claim: 433.X1 (occlusion and stenosis of precerebral arteries, with cerebral infarction); 434 (occlusion of cerebral arteries); 434.0 or 434.01 (cerebral thrombosis); 434.1 or 434.11 (cerebral embolism); 434.9 or 434.91 (cerebral artery occlusion, unspecified); or 436.X (acute, but ill-defined, cerebrovascular disease). Hospitalizations meeting this definition, yet with a concomitant discharge diagnosis for an intracranial injury (with or without skull fracture, ICD-9-CM 800.X–804.X or 850.X–854.X) were excluded,33 as such ischemic stroke events were likely due to the injury itself rather than a drug interaction. This algorithm, which we have used previously,34,35 has a sensitivity, specificity and positive predictive value of 74%, 95% and 88%, respectively.33
The restriction of ischemic stroke events of interest to the first 180 days of follow-up was intended to minimize the impact of depletion of susceptible person-time,36 as loss of the protective effect of clopidogrel would be expected to manifest relatively early.37,38 This restriction was lifted in a sensitivity analysis that identified ischemic stroke outcomes in all available person-time.
Statistical analysis
We first generated descriptive statistics for baseline covariates and calculated incidence and unadjusted association measures, the latter via Cox proportional-hazards regression. We then generated a propensity score vector using multinomial logistic regression39—with one propensity score calculated for each PPI vs. referent in a single model. For each subject, each PPI-specific propensity score was included in the outcome model as a continuous covariate.40 We assessed the goodness-of-fit of the propensity score model using Austin’s weighted conditional standardized difference method.41 This approach compares conditional differences in baseline covariates between exposure groups. Propensity score-adjusted hazard ratios (HRs) and 95% CIs were calculated via Cox proportional-hazards regression. Proportional hazards assumptions were examined via inclusion of an interaction term of exposure by survival time.
To account for potential residual imbalance in baseline differences (i.e., those not accounted for via propensity score adjustment), we conducted a sensitivity analysis in which we added covariates to the outcome model if at least one of their weighted conditional standardized differences exceeded 0.1.41
A pre-specified secondary analysis included the examination of ischemic stroke risk excluding persons enrolled in managed care plans, as Centers for Medicare and Medicaid Services claims may be incomplete for such persons.42 Post hoc secondary analyses included the examination of ischemic stroke risk among persons with a hospitalization on the day of or within the 29 days prior to cohort entry for: a) ACS; b) carotid revascularization/stenting; c) coronary stenting; d) other vascular stenting; and e) AMI. These may represent high-risk periods during which clopidogrel activation would be critical.43
Statistical analyses were conducted using SAS v9.3 (SAS Institute Inc.: Cary, NC) and Stata MP v13.1 (StataCorp LP: College Station, TX).
RESULTS
We identified 325,559 concomitant users of clopidogrel and a PPI. Overall, such persons contributed 70,274 p-y of concomitant exposure, among which we identified 1,667 ischemic stroke events (unadjusted rate = 2.4 per 100 p-y [95% CI: 2.3–2.5]). Unadjusted rate ratios vs. pantoprazole were: 0.62 (0.53–0.73) for esomeprazole; 0.92 (0.81–1.05) for lansoprazole; 0.75 (0.65–0.86) for omeprazole; and 0.64 (0.48–0.85) for rabeprazole. Highly-prevalent characteristics of study participants (defined as cohort prevalence >30% for diseases and >20% for drugs) stratified by PPI exposure group are presented in Table 1; all measured characteristics, without regard to cohort prevalence, are presented in Supplemental Table I. Standardized mean differences and weighted conditional standardized differences are presented to facilitate the evaluation of potential imbalance in baseline covariates vs. pantoprazole, before and after conditioning on propensity score, respectively. For a given PPI vs. pantoprazole, the vast majority of baseline covariates were balanced.
Table 1.
Highly-prevalent* characteristics of clopidogrel users, by proton pump inhibitor exposure group
| Pantoprazole | Esomeprazole | Lansoprazole | Omeprazole | Rabeprazole | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | N | N | N | N | ||||||||||||||
| Users, concomitant with clopidogrel | 57,089 | 59,821 | 106,470 | 88,069 | 14,110 | |||||||||||||
| Person-years of follow-up | 12,057 | 13,359 | 23,185 | 18,791 | 2,882 | |||||||||||||
| Acute ischemic stroke events | 350 | 238 | 617 | 408 | 54 | |||||||||||||
| Characteristic | Group | % | % | SMD | WCSD | % | SMD | WCSD | % | SMD | WCSD | % | SMD | WCSD | ||||
| Demographic factors | ||||||||||||||||||
| Method of cohort entry | clopidogrel added to ongoing PPI therapy | 35.1 | 44.0 | 0.18 | 0.13 | 44.5 | 0.19 | 0.16 | 38.2 | 0.06 | 0.10 | 42.5 | 0.15 | 0.13 | ||||
| clopidogrel and PPI initiated on same day | 30.6 | 18.9 | 0.27 | 0.22 | 21.1 | 0.22 | 0.17 | 19.7 | 0.25 | 0.21 | 18.0 | 0.30 | 0.25 | |||||
| PPI added to ongoing clopidogrel therapy | 34.3 | 37.2 | 0.06 | 0.08 | 34.5 | 0.00 | 0.06 | 42.1 | 0.16 | 0.11 | 39.5 | 0.11 | 0.10 | |||||
| Age at cohort entry (continuous)† | Median (Q1–Q3) | 72.0 (62.4–80.2) | 69.7 (59.8–77.5) | 0.19 | 0.13 | 71.5 (62.1–79.5) | 0.04 | 0.09 | 72.0 (62.4–80.2) | 0.00 | 0.11 | 71.5 (62.1–79.5) | 0.04 | 0.11 | ||||
| Sex | female | 63.9 | 62.7 | 0.03 | 0.06 | 64.3 | 0.01 | 0.02 | 63.0 | 0.02 | 0.01 | 64.8 | 0.02 | 0.02 | ||||
| Race† | white | 49.6 | 44.8 | 0.10 | 0.16 | 46.8 | 0.06 | 0.03 | 48.7 | 0.02 | 0.13 | 44.1 | 0.11 | 0.09 | ||||
| black | 14.3 | 12.7 | 0.05 | 0.12 | 13.2 | 0.03 | 0.09 | 13.2 | 0.03 | 0.06 | 9.0 | 0.17 | 0.08 | |||||
| other / unknown | 36.0 | 42.5 | 0.13 | 0.16 | 40.0 | 0.08 | 0.08 | 38.2 | 0.04 | 0.14 | 47.0 | 0.22 | 0.08 | |||||
| State of residence† | CA | 39.1 | 32.5 | 0.14 | 0.22 | 34.4 | 0.10 | 0.06 | 32.4 | 0.14 | 0.16 | 45.4 | 0.13 | 0.14 | ||||
| FL | 15.9 | 12.3 | 0.10 | 0.13 | 26.1 | 0.25 | 0.12 | 12.4 | 0.10 | 0.17 | 10.0 | 0.17 | 0.20 | |||||
| NY | 27.6 | 36.8 | 0.20 | 0.26 | 23.1 | 0.10 | 0.25 | 32.4 | 0.10 | 0.17 | 33.5 | 0.13 | 0.20 | |||||
| OH | 6.7 | 14.3 | 0.25 | 0.13 | 11.2 | 0.16 | 0.17 | 8.3 | 0.06 | 0.14 | 6.6 | 0.00 | 0.11 | |||||
| PA | 10.7 | 4.1 | 0.25 | 0.09 | 5.1 | 0.21 | 0.07 | 14.5 | 0.12 | 0.10 | 4.5 | 0.24 | 0.07 | |||||
| Calendar year of cohort entry† | 2000/2001 | 5.0 | 1.2 | 0.22 | 0.01 | 14.6 | 0.33 | 0.01 | 12.5 | 0.27 | 0.01 | 17.8 | 0.41 | 0.01 | ||||
| 2002 | 12.9 | 5.0 | 0.28 | 0.11 | 11.2 | 0.05 | 0.11 | 4.0 | 0.32 | 0.19 | 17.7 | 0.14 | 0.15 | |||||
| 2003 | 14.9 | 7.5 | 0.24 | 0.10 | 15.6 | 0.02 | 0.10 | 3.4 | 0.41 | 0.07 | 13.6 | 0.04 | 0.05 | |||||
| 2004 | 15.8 | 9.8 | 0.18 | 0.16 | 10.4 | 0.16 | 0.07 | 3.9 | 0.41 | 0.13 | 14.9 | 0.02 | 0.09 | |||||
| 2005 | 7.1 | 16.1 | 0.28 | 0.29 | 16.2 | 0.28 | 0.08 | 5.8 | 0.05 | 0.23 | 7.1 | 0.00 | 0.03 | |||||
| 2006 | 13.8 | 17.1 | 0.09 | 0.20 | 12.8 | 0.03 | 0.12 | 16.9 | 0.09 | 0.19 | 9.4 | 0.14 | 0.11 | |||||
| 2007 | 12.0 | 15.5 | 0.10 | 0.13 | 9.5 | 0.08 | 0.08 | 16.1 | 0.12 | 0.20 | 8.4 | 0.12 | 0.11 | |||||
| 2008 | 9.0 | 13.3 | 0.14 | 0.05 | 4.8 | 0.16 | 0.03 | 16.3 | 0.22 | 0.09 | 6.4 | 0.10 | 0.06 | |||||
| 2009 | 9.5 | 14.6 | 0.16 | 0.08 | 4.9 | 0.18 | 0.07 | 21.1 | 0.33 | 0.08 | 4.8 | 0.18 | 0.06 | |||||
| CMS dual-eligible† | Yes | 84.5 | 72.6 | 0.29 | 0.24 | 76.6 | 0.20 | 0.22 | 80.3 | 0.11 | 0.20 | 80.9 | 0.10 | 0.08 | ||||
| Health system use factors | ||||||||||||||||||
| ACS hospitalization 29 days prior to cohort entry | Yes | 15.5 | 8.8 | 0.21 | 0.17 | 10.6 | 0.14 | 0.09 | 9.2 | 0.19 | 0.11 | 7.5 | 0.25 | 0.15 | ||||
| Nursing home residence, ever during baseline† | Yes | 31.5 | 15.2 | 0.39 | 0.08 | 25.4 | 0.14 | 0.15 | 27.2 | 0.10 | 0.11 | 13.1 | 0.45 | 0.11 | ||||
| # circulatory system hospitalizations in prior year† (continuous) | Median (Q1–Q3) | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) | 0.03 | 0.17 | 0.0 (0.0–1.0) | 0.08 | 0.15 | 0.0 (0.0–1.0) | 0.00 | 0.14 | 0.0 (0.0–1.0) | 0.08 | 0.19 | ||||
| # non-circulatory system hospitalizations in prior year (continuous) | Median (Q1–Q3) | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) | 0.06 | 0.15 | 0.0 (0.0–1.0) | 0.07 | 0.13 | 0.0 (0.0–1.0) | 0.00 | 0.14 | 0.0 (0.0–1.0) | 0.07 | 0.22 | ||||
| # circulatory system ED visits in prior year† (continuous) | Median (Q1–Q3) | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) | 0.00 | 0.01 | 0.0 (0.0–1.0) | 0.03 | 0.03 | 0.0 (0.0–1.0) | 0.00 | 0.03 | 0.0 (0.0–1.0) | 0.03 | 0.04 | ||||
| # non-circulatory system ED visits in prior year† (continuous) | Median (Q1–Q3) | 1.0 (0.0–2.0) | 1.0 (0.0–2.0) | 0.01 | 0.06 | 1.0 (0.0–2.0) | 0.05 | 0.07 | 1.0 (0.0–2.0) | 0.00 | 0.06 | 1.0 (0.0–2.0) | 0.05 | 0.08 | ||||
| # circulatory system outpatient visits in prior year† (continuous) | Median (Q1–Q3) | 6.0 (2.0–13.0) | 7.0 (3.0–13.0) | 0.07 | 0.04 | 8.0 (4.0–15.0) | 0.08 | 0.06 | 6.0 (2.0–13.0) | 0.00 | 0.05 | 8.0 (4.0–15.0) | 0.08 | 0.06 | ||||
| # non-circulatory system outpatient visits in prior year† (continuous) | Median (Q1–Q3) | 23.0 (10.0–45.0) | 26.0 (14.0–52.0) | 0.14 | 0.11 | 28.0 (15.0–52.0) | 0.10 | 0.12 | 23.0 (10.0–45.0) | 0.00 | 0.10 | 28.0 (15.0–52.0) | 0.10 | 0.14 | ||||
| # unique drugs prescribed in prior year (continuous) | Median (Q1–Q3) | 16.0 (11.0–23.0) | 18.0 (13.0–25.0) | 0.20 | 0.12 | 18.0 (13.0–24.0) | 0.17 | 0.08 | 16.0 (11.0–23.0) | 0.00 | 0.08 | 18.0 (13.0–24.0) | 0.17 | 0.09 | ||||
| Diseases and Procedures | ||||||||||||||||||
| Angina pectoris | Yes | 46.0 | 48.2 | 0.04 | 0.10 | 45.4 | 0.01 | 0.07 | 41.0 | 0.10 | 0.08 | 45.8 | 0.00 | 0.09 | ||||
| Artery, arteriole, capillary disease | Yes | 62.3 | 58.3 | 0.08 | 0.10 | 60.4 | 0.04 | 0.08 | 57.0 | 0.11 | 0.10 | 54.2 | 0.16 | 0.10 | ||||
| Asthma, COPD, or emphysema | Yes | 55.2 | 54.7 | 0.01 | 0.08 | 54.9 | 0.01 | 0.06 | 50.5 | 0.09 | 0.08 | 49.6 | 0.11 | 0.09 | ||||
| Cancer | Yes | 30.4 | 30.0 | 0.01 | 0.06 | 29.2 | 0.02 | 0.04 | 27.9 | 0.05 | 0.07 | 29.6 | 0.02 | 0.04 | ||||
| Cerebrovascular disease, CAO and stenosis | Yes | 31.9 | 30.9 | 0.02 | 0.08 | 31.7 | 0.00 | 0.08 | 27.8 | 0.09 | 0.08 | 29.1 | 0.06 | 0.06 | ||||
| Cerebrovascular disease, ischemic stroke | Yes | 40.2 | 29.5 | 0.23 | 0.04 | 36.8 | 0.07 | 0.07 | 34.6 | 0.12 | 0.04 | 31.6 | 0.18 | 0.09 | ||||
| Cerebrovascular disease, other | Yes | 52.3 | 46.5 | 0.11 | 0.07 | 50.6 | 0.03 | 0.04 | 47.0 | 0.10 | 0.07 | 45.8 | 0.13 | 0.08 | ||||
| Cerebrovascular disease, other (w/o CAO or stenosis) | Yes | 39.3 | 31.1 | 0.17 | 0.05 | 35.1 | 0.09 | 0.05 | 35.2 | 0.08 | 0.06 | 30.9 | 0.18 | 0.07 | ||||
| Cardiac dysrhythmias, all | Yes | 61.4 | 54.1 | 0.15 | 0.10 | 55.8 | 0.11 | 0.07 | 53.2 | 0.17 | 0.10 | 51.9 | 0.19 | 0.09 | ||||
| Cardiac dysrhythmias, other | Yes | 55.2 | 49.7 | 0.11 | 0.09 | 50.6 | 0.09 | 0.07 | 47.4 | 0.16 | 0.10 | 47.1 | 0.16 | 0.09 | ||||
| Cardiovascular system symptoms† | Yes | 53.9 | 55.7 | 0.04 | 0.10 | 53.7 | 0.01 | 0.10 | 48.7 | 0.11 | 0.11 | 50.5 | 0.07 | 0.11 | ||||
| Circulatory system disease, other | Yes | 96.9 | 97.2 | 0.02 | 0.04 | 97.7 | 0.05 | 0.06 | 95.3 | 0.08 | 0.06 | 96.8 | 0.00 | 0.08 | ||||
| Diabetes mellitus | Yes | 67.2 | 67.4 | 0.00 | 0.08 | 65.4 | 0.04 | 0.06 | 65.3 | 0.04 | 0.05 | 62.5 | 0.10 | 0.07 | ||||
| Esophageal disease** | Yes | 51.3 | 58.3 | 0.14 | 0.10 | 52.7 | 0.03 | 0.03 | 52.2 | 0.02 | 0.10 | 54.4 | 0.06 | 0.09 | ||||
| Gastritis/duodenitis** | Yes | 42.7 | 49.4 | 0.13 | 0.09 | 47.4 | 0.09 | 0.05 | 39.6 | 0.06 | 0.08 | 49.0 | 0.13 | 0.09 | ||||
| Heart failure or cardiomyopathy† | Yes | 63.9 | 52.9 | 0.22 | 0.12 | 56.7 | 0.15 | 0.10 | 53.5 | 0.21 | 0.12 | 49.7 | 0.29 | 0.11 | ||||
| Hypertension | Yes | 93.1 | 93.7 | 0.02 | 0.06 | 93.5 | 0.01 | 0.07 | 91.1 | 0.08 | 0.05 | 92.3 | 0.03 | 0.09 | ||||
| Hypothyroidism | Yes | 37.0 | 38.2 | 0.02 | 0.10 | 36.3 | 0.01 | 0.07 | 34.8 | 0.05 | 0.08 | 37.4 | 0.01 | 0.08 | ||||
| Ischemic heart disease† | Yes | 82.4 | 79.0 | 0.08 | 0.11 | 78.5 | 0.10 | 0.09 | 76.8 | 0.14 | 0.09 | 76.7 | 0.14 | 0.09 | ||||
| Lipoid metabolism disorder† | Yes | 80.5 | 86.4 | 0.16 | 0.11 | 79.6 | 0.02 | 0.11 | 79.9 | 0.01 | 0.08 | 81.9 | 0.04 | 0.09 | ||||
| Mental disorder, depression | Yes | 42.2 | 44.0 | 0.04 | 0.08 | 44.8 | 0.05 | 0.05 | 41.6 | 0.01 | 0.04 | 36.0 | 0.13 | 0.10 | ||||
| Mental disorder, other† | Yes | 61.4 | 60.3 | 0.02 | 0.07 | 60.8 | 0.01 | 0.04 | 58.9 | 0.05 | 0.05 | 51.8 | 0.19 | 0.12 | ||||
| MI, acute† | Yes | 37.0 | 26.6 | 0.23 | 0.14 | 27.8 | 0.20 | 0.07 | 28.2 | 0.19 | 0.10 | 23.5 | 0.30 | 0.10 | ||||
| MI, old | Yes | 25.0 | 18.8 | 0.15 | 0.10 | 19.5 | 0.13 | 0.05 | 20.7 | 0.10 | 0.06 | 16.7 | 0.20 | 0.09 | ||||
| Nervous system disease, disorders of the eye and adnexa | Yes | 81.1 | 84.9 | 0.10 | 0.05 | 83.7 | 0.07 | 0.05 | 78.9 | 0.05 | 0.06 | 83.5 | 0.06 | 0.06 | ||||
| Nervous system disease, other† | Yes | 33.7 | 29.1 | 0.10 | 0.06 | 31.7 | 0.04 | 0.05 | 29.6 | 0.09 | 0.05 | 26.2 | 0.16 | 0.11 | ||||
| Nervous system disease, peripheral | Yes | 37.3 | 41.0 | 0.08 | 0.07 | 37.2 | 0.00 | 0.07 | 35.8 | 0.03 | 0.08 | 35.1 | 0.05 | 0.08 | ||||
| Renal failure, other† | Yes | 47.1 | 41.5 | 0.11 | 0.09 | 41.3 | 0.12 | 0.07 | 39.6 | 0.15 | 0.10 | 35.9 | 0.23 | 0.12 | ||||
| Drug markers of chronic diseases | ||||||||||||||||||
| Antiadrenergic agent | Yes | 27.9 | 28.0 | 0.00 | 0.07 | 26.7 | 0.03 | 0.06 | 25.1 | 0.06 | 0.07 | 24.6 | 0.07 | 0.08 | ||||
| Antidepressant | Yes | 55.1 | 59.4 | 0.09 | 0.06 | 59.0 | 0.08 | 0.03 | 55.4 | 0.01 | 0.02 | 53.4 | 0.03 | 0.05 | ||||
| Antidiabetic agent, insulin | Yes | 23.5 | 18.8 | 0.12 | 0.06 | 20.7 | 0.07 | 0.07 | 21.8 | 0.04 | 0.04 | 14.5 | 0.23 | 0.09 | ||||
| Antidiabetic agent, non-insulin | Yes | 40.1 | 40.0 | 0.00 | 0.05 | 38.4 | 0.04 | 0.03 | 39.8 | 0.01 | 0.01 | 35.7 | 0.09 | 0.04 | ||||
| Antipsychotic | Yes | 23.4 | 24.2 | 0.02 | 0.06 | 25.7 | 0.05 | 0.03 | 22.5 | 0.02 | 0.03 | 19.9 | 0.08 | 0.09 | ||||
| Aspirin (low dose)† | Yes | 35.6 | 45.7 | 0.21 | 0.14 | 37.8 | 0.05 | 0.06 | 35.6 | 0.00 | 0.04 | 41.3 | 0.12 | 0.06 | ||||
| Beta-adrenergic or alpha/beta-adrenergic blocking agent | Yes | 66.5 | 67.9 | 0.03 | 0.08 | 61.9 | 0.09 | 0.04 | 66.8 | 0.01 | 0.04 | 61.2 | 0.11 | 0.03 | ||||
| Bronchodilator or inhaled corticosteroid | Yes | 45.6 | 49.5 | 0.08 | 0.05 | 46.8 | 0.02 | 0.03 | 44.7 | 0.02 | 0.04 | 43.2 | 0.05 | 0.06 | ||||
| Calcium channel blocker | Yes | 58.2 | 58.0 | 0.00 | 0.07 | 57.5 | 0.01 | 0.06 | 54.5 | 0.07 | 0.06 | 57.7 | 0.01 | 0.07 | ||||
| Diuretic, loop | Yes | 47.1 | 38.9 | 0.17 | 0.10 | 42.2 | 0.10 | 0.04 | 41.0 | 0.12 | 0.08 | 35.6 | 0.24 | 0.08 | ||||
| Diuretic, other | Yes | 47.9 | 53.4 | 0.11 | 0.05 | 47.6 | 0.01 | 0.04 | 47.9 | 0.00 | 0.04 | 47.4 | 0.01 | 0.04 | ||||
| Potassium supplement | Yes | 32.5 | 26.5 | 0.13 | 0.09 | 29.9 | 0.06 | 0.03 | 28.1 | 0.10 | 0.06 | 24.1 | 0.19 | 0.05 | ||||
| Renin angiotensin system antagonist | Yes | 75.5 | 77.3 | 0.04 | 0.07 | 73.5 | 0.04 | 0.04 | 74.2 | 0.03 | 0.03 | 72.9 | 0.06 | 0.04 | ||||
| Statin | Yes | 65.9 | 74.4 | 0.19 | 0.10 | 64.6 | 0.03 | 0.05 | 70.0 | 0.09 | 0.03 | 66.3 | 0.01 | 0.05 | ||||
| Vasodilator | Yes | 52.5 | 50.1 | 0.05 | 0.08 | 50.4 | 0.04 | 0.04 | 48.2 | 0.09 | 0.06 | 50.7 | 0.04 | 0.06 | ||||
| Recent drug exposures | ||||||||||||||||||
| Anti-infective agent | Yes | 39.7 | 40.7 | 0.02 | 0.06 | 41.7 | 0.04 | 0.03 | 37.9 | 0.04 | 0.05 | 39.8 | 0.00 | 0.04 | ||||
| Beta-adrenergic blocking agent | Yes | 26.9 | 28.3 | 0.03 | 0.04 | 26.5 | 0.01 | 0.03 | 29.9 | 0.07 | 0.06 | 26.2 | 0.02 | 0.06 | ||||
| Calcium channel blocking agent | Yes | 21.8 | 23.2 | 0.03 | 0.01 | 23.5 | 0.04 | 0.02 | 22.9 | 0.03 | 0.02 | 23.4 | 0.04 | 0.02 | ||||
| Renin angiotensin system antagonist | Yes | 36.6 | 41.3 | 0.10 | 0.05 | 38.7 | 0.04 | 0.04 | 39.6 | 0.06 | 0.05 | 39.4 | 0.06 | 0.06 | ||||
| Statin | Yes | 31.2 | 38.1 | 0.15 | 0.08 | 31.9 | 0.02 | 0.03 | 37.1 | 0.13 | 0.05 | 33.5 | 0.05 | 0.06 | ||||
SMD = standardized mean difference (vs. pantoprazole); WCSD = weighted conditional standardized difference (vs. pantoprazole); PPI = proton pump inhibitor; CMS = Centers for Medicare and Medicaid Services; COPD = chronic obstructive pulmonary disease; CAO = coronary artery occlusion; HIV = human immunodeficiency virus; AIDS = acquired immune deficiency syndrome; MI = myocardial infarction; ICD = implantable cardioverter-defibrillator; MAOI = monoamine oxidase inhibitor; SNRI = serotonin and norepinephrine reuptake inhibitor; SSRI = selective serotonin reuptake inhibitor; NSAID = nonsteroidal anti-inflammatory drug
cohort prevalence >30% for disease and >20% for drugs
not considered in the propensity score
baseline covariate, in addition to propensity score, included in the proportional-hazards model for the sensitivity analysis to control for potential residual imbalance in exposure groups; covariates capturing numbers of healthcare visits by site were included in this model as categorical, not continuous measures; the following additional covariates, not meeting the cohort prevalence threshold described above, were also included in this model: acute renal failure, chronic renal failure, substance abuse, recent infectious and parasitic diseases (all other), and recent serious infection (not already coded within infectious and parasitic diseases).
Propensity score-adjusted HRs for ischemic stroke are presented in Figure 1. Both unadjusted and adjusted HRs for the sensitivity analyses that did not impose a maximum follow-up time of 180 days and excluded persons with managed care coverage, respectively, yielded HRs similar to those presented in Figure 1 (data not shown).
Figure 1.
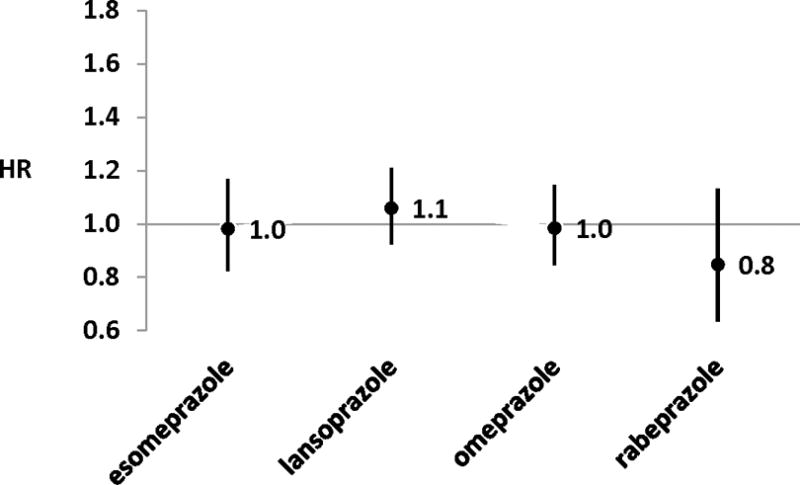
Propensity score-adjusted hazard ratios (HRs) for the rate of acute ischemic stroke within 180 days of cohort entry among clopidogrel users, by proton pump inhibitor of interest (vs. pantoprazole)
A sensitivity analysis to account for potential residual imbalance in baseline differences was conducted; this model adjusted for 29 covariates in addition to propensity scores, each of which had weighted conditional standardized differences >0.1. Adjusted HRs arising from this model were 0.99 (0.83–1.18) for esomeprazole, 1.05 (0.91–1.20) for lansoprazole, 0.98 (0.84–1.15) for omeprazole, and 0.85 (0.63–1.13) for rabeprazole, each vs. pantoprazole. The similarity of these results to those presented in Figure 1 suggests no effect of imbalance in measured covariates. Therefore, all other modeled results adjusted solely for the calculated propensity scores.
Results from post hoc sensitivity analyses examining potential high-risk subgroups of persons recently hospitalized (in which clopidogrel activation may be critical) as effect modifiers, are presented in Figure 2. None of the p-values for the interaction terms were statistically significant. Yet, among lansoprazole-treated persons with a recent hospitalization for ACS and recent hospitalization for AMI, adjusted HRs (vs. pantoprazole) were 1.39 (1.10–1.76) and 1.56 (1.10–2.23), respectively.
Figure 2.
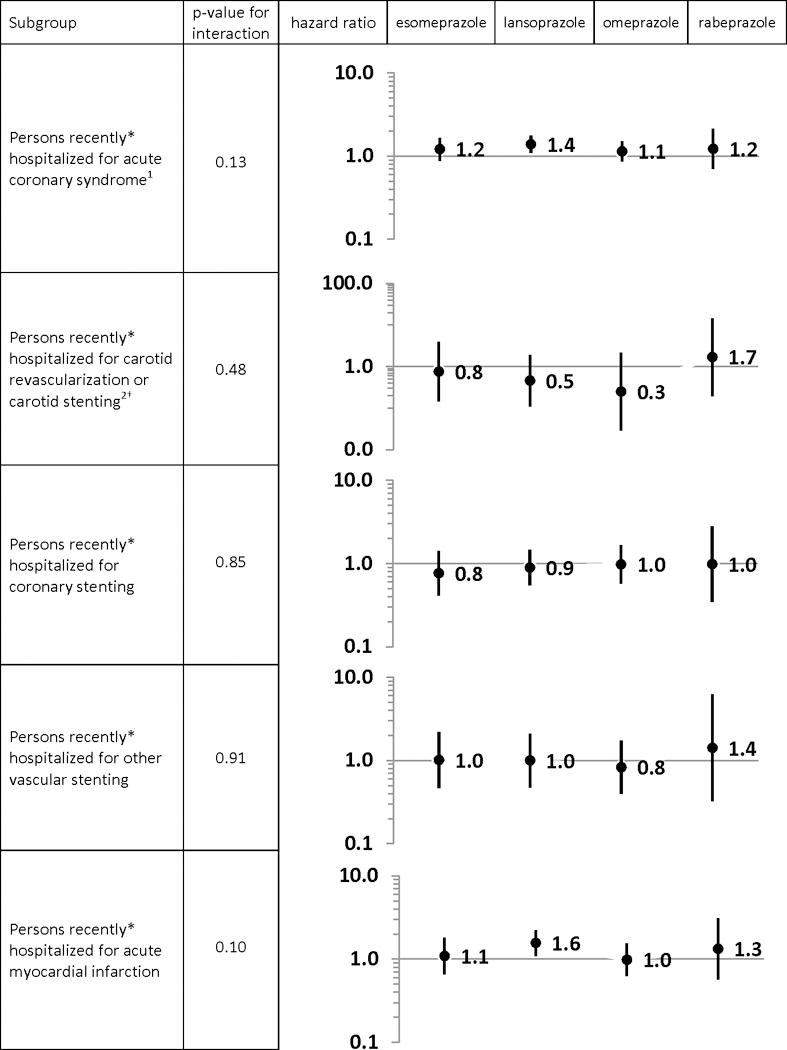
Propensity score-adjusted hazard ratios for the rate of acute ischemic stroke within 180 days of cohort entry among clopidogrel users, by proton pump inhibitor of interest (vs. pantoprazole), among subgroups of interest
*on the date of or within the 29 days prior to cohort entry
†please note the y-axis scale change
1defined as a hospitalization with a principal discharge diagnosis for stroke, transient cerebral ischemia, acute myocardial infarction or angina
2defined as carotid stenting, carotid endarterectomy, carotid angioplasty
DISCUSSION
Our study examined the risk of ischemic stroke among >325,000 persons receiving both clopidogrel and a PPI and found an annual event rate of 2.4%—consistent with major randomized trials (Atrial Fibrillation Clopidogrel Trial with Irbesartan for Prevention of Vascular Events [ACTIVE-A]44 and Secondary Prevention of Small Subcortical Strokes [SPS3]45) and with a recent cohort study.17 In propensity score-adjusted models, ischemic stroke rates for clopidogrel with individual PPIs of interest were no greater than that for clopidogrel+pantoprazole. While the HR for rabeprazole was consistent with a protective effect vs. pantoprazole, the 95% CI included the null value.
In the post hoc subgroup analysis of persons recently hospitalized for ACS, clopidogrel+lansoprazole was associated with a 40% increased rate of ischemic stroke vs. clopidogrel+pantoprazole. If causal, the magnitude of this association would suggest a complete nullification of clopidogrel’s effect, based on findings from the Clopidogrel in High-Risk Patients with Acute Nondisabling Cerebrovascular Events (CHANCE)46 and Fast Assessment of Stroke and Transient Ischaemic Attack to Prevent Early Recurrence (FASTER)47 trials in which clopidogrel+aspirin in patients with an acute transient ischemic attack or minor stroke was associated with HRs ~0.70 compared to aspirin alone (1/0.7 = 1.4). However, our subgroup finding for clopidogrel+lansoprazole should be interpreted with great caution for a number of reasons. First, we are unaware of a mechanism by which lansoprazole alone would exert this effect. Second, the complete nullification of clopidogrel’s pharmacodynamic effect by lansoprazole alone seems implausible, especially since PPIs have modest effects on adenosine diphosphate-induced platelet aggregation48,49 and aggregation inhibition is likely less for lansoprazole vs. other PPIs.50 Third, this subgroup analysis was not based on an a priori hypothesis. Finally, the overall p-values for the among-PPI difference in the rate of stroke among those with recent ACS hospitalization and among those with recent AMI were not statistically significant. Given this, we hesitate to interpret the potential importance of this subgroup finding and strongly suggest that it be independently confirmed in a study designed to answer this particular question.
Among the handful of studies that have examined the association of clopidogrel and PPIs on ischemic stroke as a stand-alone outcome (Supplemental Table II), only that by Simon et al15 reported risks by individual PPI. Yet, their study only examined stroke events (N = 7) occurring among ~1,500 inpatients admitted with an AMI. Interpretation of Simon et al’s results are limited by their comparison to a PPI-unexposed referent, examination of stroke only during hospitalization, very small sample size leading to wide CIs, and inability to calculate an effect estimate for lansoprazole. The prematurely-terminated Clopidogrel and the Optimization of Gastrointestinal Events Trial (COGENT)9—the only randomized study designed to test the hypothesis of a clopidogrel+PPI interaction—examined ischemic stroke risk only for omeprazole, finding no difference between fixed-dose combination clopidogrel+omeprazole vs. clopidogrel alone (p = 0.43). This is consistent with our findings.
Comparing our findings to expectations based on underlying biology is challenging given inconsistencies in measurements of the antiplatelet effect of clopidogrel (even in the absence of a PPI)1 and ongoing controversies regarding: 1) the metabolism of clopidogrel; 2) the concomitant effect of PPIs on inhibition of platelet reactivity; and 3) the role of genetics. If pharmacologic inhibition of CYP2C19 reduces the effect of clopidogrel, our finding of no difference in ischemic stroke risk among individual PPIs is inconsistent with in vitro findings suggesting that there are multi-fold differences in the strength of CYP2C19 inhibition by PPI.28 An alternative interpretation is that, despite a strong direct-acting inhibition of CYP2C19 by lansoprazole (the most potent inhibitor of this isozyme28), the interaction may rarely manifest clinically. This interpretation is consistent with findings that the inhibitory effect of lansoprazole on clopidogrel activation does not manifest in vivo51,52 Others though have argued that irreversible metabolism-dependent (rather than direct) inhibition of this isozyme by omeprazole and esomeprazole has more profound ramifications.26 Yet, our findings demonstrated no increase in ischemic stroke risk for omeprazole or esomeprazole.53,54
Our study has important strengths. It is the first to compare risk of ischemic stroke solely in community-dwelling users of clopidogrel plus individual PPIs. Our propensity score adjustment and subsequent sensitivity analysis served to minimize confounding. Further, our large sample size allowed for the examination of associations in subgroups of interest. Finally, our algorithm to identify stroke has an excellent positive predictive value and good sensitivity.
Our study also has limitations. First, we did not have access to biosamples and were therefore unable to examine genetic polymorphisms in CYP enzymes, p-glycoprotein transporters, or P2Y12 receptors. Second, we did not have data on adherence to prescribed clopidogrel and PPI therapies. Third, administrative databases may poorly capture some lifestyle behaviors and nonprescription therapies that may modify stroke risk. Regardless, such factors seem unlikely to differ substantially by PPI exposure. Fourth, as with all non-experimental studies, there may be residual confounding. Finally, our results may not be generalizable beyond a Medicaid population. Nevertheless, this population was specifically selected for study given its inherent vulnerability and inclusion of large numbers of women and minorities—groups typically understudied.
SUMMARY
This study provides evidence that the concomitant use of clopidogrel with esomeprazole, lansoprazole, omeprazole, or rabeprazole does not increase the risk of ischemic stroke when compared to pantoprazole, a PPI thought not to interact with clopidogrel.
Supplementary Material
Acknowledgments
We acknowledge Maximilian Herlim and Liu Qing from the University of Pennsylvania for their statistical programming support, Xu Han from the University of Pennsylvania for her pharmacology expertise, Hanieh Razzaghi formerly of the University of Pennsylvania for her project management support, and the University of Minnesota’s Research Data Assistance Center for their Centers for Medicare and Medicaid Services data support.
SOURCES OF FUNDING
This project was supported by R01AG025152 from the National Institute on Aging, R01DK102694 from the National Institute of Diabetes and Digestive and Kidney Diseases, and UL1TR000003 from the National Center for Advancing Translational Sciences.
Footnotes
POTENTIAL CONFLICTS OF INTEREST
None – Dr. Leonard, Ms. Brensinger, Dr. Flockhart
Ms. Freeman was supported by a study funded through a sponsored research agreement between the University of Pennsylvania and AstraZeneca and Bristol-Myers Squibb unrelated to the subject matter of this work. Dr. Kasner has received consulting fees from AstraZeneca, Merck, Novartis, and Pfizer, all unrelated to the subject matter of this work. Dr. Kimmel has consulted for Pfizer, unrelated to the subject matter of this work. Dr. Hennessy serves as an investigator for a study funded through a sponsored research agreement between the University of Pennsylvania and AstraZeneca and Bristol-Myers Squibb unrelated to the subject matter of this work. He has also served as a consultant to AbbVie Inc, AstraZeneca, Bayer Healthcare LLC, Clarus Therapeutics Inc, Hoffmann La-Roche, Bristol-Myers Squibb, Ferring Pharmaceuticals Inc, and Novartis unrelated to the subject matter of this work. The Center for Pharmacoepidemiology Research and Training receives support for pharmacoepidemiology training from Pfizer and Sanofi.
Subject Codes: Anticoagulants: platelet function inhibitors; Etiology: epidemiology; Stroke: acute cerebral infarction; Stroke: acute stroke syndromes; Stroke treatment – medical: antiplatelets; Treatment: cardiovascular pharmacology
References
- 1.Shah BS, Parmar SA, Mahajan S, Mehta AA. An insight into the interaction between clopidogrel and proton pump inhibitors. Curr Drug Metab. 2012;13:225–235. doi: 10.2174/138920012798918390. [DOI] [PubMed] [Google Scholar]
- 2.Kazui M, Nishiya Y, Ishizuka T, Hagihara K, Farid NA, Okazaki O, et al. Identification of the human cytochrome P450 enzymes involved in the two oxidative steps in the bioactivation of clopidogrel to its pharmacologically active metabolite. Drug Metab Dispos. 2010;38:92–99. doi: 10.1124/dmd.109.029132. [DOI] [PubMed] [Google Scholar]
- 3.Food and Drug Administration. Information for healthcare professionals: update to the labeling of clopidogrel bisulfate (marketed as Plavix) to alert healthcare professionals about a drug interaction with omeprazole (marketed as Prilosec and Prilosec OTC) 2013 Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm190787.htm; Last updated: 08/15/2013; Last accessed: 12/08/2014.
- 4.Ford NF, Taubert D. Clopidogrel, CYP2C19, and a Black Box. J Clin Pharmacol. 2013;53:241–248. doi: 10.1002/jcph.17. [DOI] [PubMed] [Google Scholar]
- 5.Taubert D, Bouman HJ, van Werkum JW. Cytochrome P-450 polymorphisms and response to clopidogrel. N Engl J Med. 2009;360:2249–50. doi: 10.1056/NEJMc090391. author reply 2251. [DOI] [PubMed] [Google Scholar]
- 6.Bouman HJ, Schömig E, van Werkum JW, Velder J, Hackeng CM, Hirschhäuser C, et al. Paraoxonase-1 is a major determinant of clopidogrel efficacy. Nat Med. 2011;17:110–116. doi: 10.1038/nm.2281. [DOI] [PubMed] [Google Scholar]
- 7.Zhu Y, Zhou J. Identification of the significant involvement and mechanistic role of CYP3A4/5 in clopidogrel bioactivation. ACS Med Chem Lett. 2012;3:844–849. doi: 10.1021/ml3002067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Boxel OS, van Oijen MG, Hagenaars MP, Smout AJ, Siersema PD. Cardiovascular and gastrointestinal outcomes in clopidogrel users on proton pump inhibitors: results of a large Dutch cohort study. Am J Gastroenterol. 2010;105:2430–6. doi: 10.1038/ajg.2010.334. [DOI] [PubMed] [Google Scholar]
- 9.Bhatt DL, Cryer BL, Contant CF, Cohen M, Lanas A, Schnitzer TJ, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med. 2010;363:1909–1917. doi: 10.1056/NEJMoa1007964. [DOI] [PubMed] [Google Scholar]
- 10.Muñoz-Torrero JF, Escudero D, Suárez C, Sanclemente C, Pascual MT, Zamorano J, et al. Concomitant use of proton pump inhibitors and clopidogrel in patients with coronary, cerebrovascular, or peripheral artery disease in the factores de Riesgo y ENfermedad Arterial (FRENA) registry. J Cardiovasc Pharmacol. 2011;57:13–19. doi: 10.1097/FJC.0b013e3181fc65e5. [DOI] [PubMed] [Google Scholar]
- 11.Mahabaleshwarkar RK, Yang Y, Datar MV, Bentley JP, Strum MW, Banahan BF, et al. Risk of adverse cardiovascular outcomes and all-cause mortality associated with concomitant use of clopidogrel and proton pump inhibitors in elderly patients. Curr Med Res Opin. 2013;29:315–323. doi: 10.1185/03007995.2013.772051. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka A, Sakakibara M, Okumura S, Okada K, Ishii H, Murohara T. Comparison of early outcomes after primary stenting in Japanese patients with acute myocardial infarction between clopidogrel and ticlopidine in concomitant use with proton-pump inhibitor. J Cardiol. 2012;60:7–11. doi: 10.1016/j.jjcc.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Chitose T, Hokimoto S, Oshima S, Nakao K, Fujimoto K, Miyao Y, et al. Clinical outcomes following coronary stenting in Japanese patients treated with and without proton pump inhibitor. Circ J. 2012;76:71–78. doi: 10.1253/circj.cj-11-0699. [DOI] [PubMed] [Google Scholar]
- 14.Charlot M, Ahlehoff O, Norgaard ML, Jorgensen CH, Sørensen R, Abildstrøm SZ, et al. Proton-pump inhibitors are associated with increased cardiovascular risk independent of clopidogrel use: a nationwide cohort study. Ann Intern Med. 2010;153:378–386. doi: 10.7326/0003-4819-153-6-201009210-00005. [DOI] [PubMed] [Google Scholar]
- 15.Simon T, Steg PG, Gilard M, Blanchard D, Bonello L, Hanssen M, et al. Clinical events as a function of proton pump inhibitor use, clopidogrel use, and cytochrome P450 2C19 genotype in a large nationwide cohort of acute myocardial infarction: results from the French Registry of Acute ST-Elevation and Non-ST-Elevation Myocardial Infarction (FAST-MI) registry. Circulation. 2011;123:474–482. doi: 10.1161/CIRCULATIONAHA.110.965640. [DOI] [PubMed] [Google Scholar]
- 16.Kreutz RP, Stanek EJ, Aubert R, Yao J, Breall JA, Desta Z, et al. Impact of proton pump inhibitors on the effectiveness of clopidogrel after coronary stent placement: the clopidogrel Medco outcomes study. Pharmacotherapy. 2010;30:787–796. doi: 10.1592/phco.30.8.787. [DOI] [PubMed] [Google Scholar]
- 17.Ray WA, Murray KT, Griffin MR, Chung CP, Smalley WE, Hall K, et al. Outcomes with concurrent use of clopidogrel and proton-pump inhibitors: a cohort study. Ann Intern Med. 2010;152:337–345. doi: 10.1059/0003-4819-152-6-201003160-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aihara H, Sato A, Takeyasu N, Nishina H, Hoshi T, Akiyama D, et al. Effect of individual proton pump inhibitors on cardiovascular events in patients treated with clopidogrel following coronary stenting: results from the Ibaraki Cardiac Assessment Study Registry. Catheter Cardiovasc Interv. 2012;80:556–563. doi: 10.1002/ccd.23327. [DOI] [PubMed] [Google Scholar]
- 19.Hennessy S, Carson JL, Ray WA, Strom BL. Medicaid Databases. In: Strom BL, editor. Pharmacoepidemiology. 4. Sussex: John Wiley; 2005. pp. 281–294. [Google Scholar]
- 20.Kaiser Family Foundation. Medicaid enrollment: June 2010 data snapshot. (Publication #8050-03).Kaiser Commission on Medicaid and the Uninsured: Medicaid Facts. kaiserfamilyfoundation.files.wordpress.com/2013/01/8050-03.pdf. 2011. Access date 01/05/15.
- 21.Holahan J, Ghosh A. Dual eligibles: Medicaid enrollment and spending for Medicare beneficiaries in 2003. (Publication #7346).Kaiser Commission on Medicaid and the Uninsured: Medicaid Facts. http://www.kff.org/medicaid/7346.cfm. 2005. Access date 01/05/15.
- 22.Holahan J, Miller DM, Rousseau D. Dual eligibles: Medicaid enrollment and spending for Medicare beneficiaries in 2005. (Publication #7846).Kaiser Commission on Medicaid and the Uninsured: Medicaid Facts. http://web.archive.org/web/20110608201215/http://www.kff.org/medicaid/upload/7846.pdf. 2009. Access date 01/05/15.
- 23.Rousseau D, Clemans-Cope L, Lawton E, Langston J, Connolly J, Howard J, et al. Dual eligibles: Medicaid enrollment and spending for Medicare beneficiaries in 2007. (Publication #7846-02).Kaiser Commission on Medicaid and the Uninsured: Medicaid Facts. http://www.ncbi.nlm.nih.gov/nlmcatalog/101560987. 2010. Access date 01/05/15.
- 24.Hennessy S, Leonard CE, Palumbo CM, Newcomb C, Bilker WB. Quality of Medicaid and Medicare data obtained through Centers for Medicare and Medicaid Services (CMS) Med Care. 2007;45:1216–1220. doi: 10.1097/MLR.0b013e318148435a. [DOI] [PubMed] [Google Scholar]
- 25.Brunelli SM, Gagne JJ, Huybrechts KF, Wang SV, Patrick AR, Rothman KJ, et al. Estimation using all available covariate information versus a fixed look-back window for dichotomous covariates. Pharmacoepidemiol Drug Saf. 2013;22:542–550. doi: 10.1002/pds.3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogilvie BW, Yerino P, Kazmi F, Buckley DB, Rostami-Hodjegan A, Paris BL, et al. The proton pump inhibitor, omeprazole, but not lansoprazole or pantoprazole, is a metabolism-dependent inhibitor of CYP2C19: implications for coadministration with clopidogrel. Drug Metab Dispos. 2011;39:2020–2033. doi: 10.1124/dmd.111.041293. [DOI] [PubMed] [Google Scholar]
- 27.Ohbuchi M, Noguchi K, Kawamura A, Usui T. Different effects of proton pump inhibitors and famotidine on the clopidogrel metabolic activation by recombinant CYP2B6, CYP2C19 and CYP3A4. Xenobiotica. 2012;42:633–640. doi: 10.3109/00498254.2011.653655. [DOI] [PubMed] [Google Scholar]
- 28.Li XQ, Andersson TB, Ahlström M, Weidolf L. Comparison of inhibitory effects of the proton pump-inhibiting drugs omeprazole, esomeprazole, lansoprazole, pantoprazole, and rabeprazole on human cytochrome P450 activities. Drug Metab Dispos. 2004;32:821–827. doi: 10.1124/dmd.32.8.821. [DOI] [PubMed] [Google Scholar]
- 29.Ogawa R, Echizen H. Drug-drug interaction profiles of proton pump inhibitors. Clin Pharmacokinet. 2010;49:509–533. doi: 10.2165/11531320-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 30.Rossini R, Capodanno D, Musumeci G, Lettieri C, Lortkipanidze N, Romano M, et al. Safety of clopidogrel and proton pump inhibitors in patients undergoing drug-eluting stent implantation. Coron Artery Dis. 2011;22:199–205. doi: 10.1097/MCA.0b013e328343b03a. [DOI] [PubMed] [Google Scholar]
- 31.Food and Drug Administration. Information on clopidogrel bisulfate (marketed as Plavix) Silver Spring, MD: Food and Drug Administration; 2010. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm190836.htm. Updated 08/19/13. Access date 01/05/15. [Google Scholar]
- 32.Toh S, García Rodríguez LA, Hernán MA. Confounding adjustment via a semi-automated high-dimensional propensity score algorithm: an application to electronic medical records. Pharmacoepidemiol Drug Saf. 2011;20:849–857. doi: 10.1002/pds.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tirschwell DL, Longstreth WT., Jr Validating administrative data in stroke research. Stroke. 2002;33:2465–2470. doi: 10.1161/01.str.0000032240.28636.bd. [DOI] [PubMed] [Google Scholar]
- 34.Schelleman H, Bilker WB, Kimmel SE, Daniel GW, Newcomb C, Guevara JP, et al. Amphetamines, atomoxetine and the risk of serious cardiovascular events in adults. PLoS One. 2013;8:e52991. doi: 10.1371/journal.pone.0052991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schelleman H, Bilker WB, Kimmel SE, Daniel GW, Newcomb C, Guevara JP, et al. Methylphenidate and risk of serious cardiovascular events in adults. Am J Psychiatry. 2012;169:178–185. doi: 10.1176/appi.ajp.2011.11010125. [DOI] [PubMed] [Google Scholar]
- 36.Moride Y, Abenhaim L. Evidence of the depletion of susceptibles effect in non-experimental pharmacoepidemiologic research. J Clin Epidemiol. 1994;47:731–737. doi: 10.1016/0895-4356(94)90170-8. [DOI] [PubMed] [Google Scholar]
- 37.Schelleman H, Brensinger CM, Bilker WB, Hennessy S. Antidepressant-warfarin interaction and associated gastrointestinal bleeding risk in a case-control study. PLoS One. 2011;6:e21447. doi: 10.1371/journal.pone.0021447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schelleman H, Bilker WB, Brensinger CM, Wan F, Yang YX, Hennessy S. Fibrate/Statin initiation in warfarin users and gastrointestinal bleeding risk. Am J Med. 2010;123:151–157. doi: 10.1016/j.amjmed.2009.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brookhart MA, Wyss R, Layton JB, Stürmer T. Propensity score methods for confounding control in nonexperimental research. Circ Cardiovasc Qual Outcomes. 2013;6:604–611. doi: 10.1161/CIRCOUTCOMES.113.000359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 41.Austin PC. Goodness-of-fit diagnostics for the propensity score model when estimating treatment effects using covariate adjustment with the propensity score. Pharmacoepidemiol Drug Saf. 2008;17:1202–1217. doi: 10.1002/pds.1673. [DOI] [PubMed] [Google Scholar]
- 42.Byrd VLH, Dodd AH. Assessing the usability of MAX 2008 encounter data for comprehensive managed care. Medicare Medicaid Res Rev. 2013;3:E1–E19. doi: 10.5600/mmrr.003.01.b01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK, et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345:494–502. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
- 44.ACTIVE Investigators. Connolly SJ, Pogue J, Hart RG, Hohnloser SH, Pfeffer M, et al. Effect of clopidogrel added to aspirin in patients with atrial fibrillation. N Engl J Med. 2009;360:2066–2078. doi: 10.1056/NEJMoa0901301. [DOI] [PubMed] [Google Scholar]
- 45.SPS3 Investigators. Benavente OR, Hart RG, McClure LA, Szychowski JM, Coffey CS, et al. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367:817–825. doi: 10.1056/NEJMoa1204133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Y, Wang Y, Zhao X, Liu L, Wang D, Wang C, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369:11–19. doi: 10.1056/NEJMoa1215340. [DOI] [PubMed] [Google Scholar]
- 47.Kennedy J, Hill MD, Ryckborst KJ, Eliasziw M, Demchuk AM, Buchan AM, et al. Fast assessment of stroke and transient ischaemic attack to prevent early recurrence (FASTER): a randomised controlled pilot trial. Lancet Neurol. 2007;6:961–969. doi: 10.1016/S1474-4422(07)70250-8. [DOI] [PubMed] [Google Scholar]
- 48.Andersson T, Nagy P, Niazi M, Nylander S, Galbraith H, Ranjan S, et al. Effect of esomeprazole with/without acetylsalicylic acid, omeprazole and lansoprazole on pharmacokinetics and pharmacodynamics of clopidogrel in healthy volunteers. Am J Cardiovasc Drugs. 2014;14:217–227. doi: 10.1007/s40256-014-0073-4. [DOI] [PubMed] [Google Scholar]
- 49.Small DS, Farid NA, Payne CD, Weerakkody GJ, Li YG, Brandt JT, et al. Effects of the proton pump inhibitor lansoprazole on the pharmacokinetics and pharmacodynamics of prasugrel and clopidogrel. J Clin Pharmacol. 2008;48:475–484. doi: 10.1177/0091270008315310. [DOI] [PubMed] [Google Scholar]
- 50.Frelinger AL, 3rd, Lee RD, Mulford DJ, Wu J, Nudurupati S, Nigam A, et al. A randomized, 2-period, crossover design study to assess the effects of dexlansoprazole, lansoprazole, esomeprazole, and omeprazole on the steady-state pharmacokinetics and pharmacodynamics of clopidogrel in healthy volunteers. J Am Coll Cardiol. 2012;59:1304–1311. doi: 10.1016/j.jacc.2011.12.024. [DOI] [PubMed] [Google Scholar]
- 51.Cavanaugh J, Winters E, Cohen A, Locke C, Braeckman R. Lack of effect of lansoprazole on steady state warfarin metabolism (abstract) Gastroenterology. 1991;100:A40. [Google Scholar]
- 52.Lefebvre RA, Flouvat B, Karolac-Tamisier S, Moerman E, Van Ganse E. Influence of lansoprazole treatment on diazepam plasma concentrations. Clin Pharmacol Ther. 1992;52:458–463. doi: 10.1038/clpt.1992.172. [DOI] [PubMed] [Google Scholar]
- 53.Food and Drug Administration. Guidance for industry: Drug interaction studies — study design, data analysis, implications for dosing, and labeling recommendations. Silver Spring, MD: Food and Drug Administration; 2012. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm292362.pdf. Access date 01/05/15. [Google Scholar]
- 54.Einolf HJ. Comparison of different approaches to predict metabolic drug-drug interactions. Xenobiotica. 2007;37:1257–1294. doi: 10.1080/00498250701620700. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


