Brief description of the problem
The “primary event” in the ischemic stroke is a rapid decline in the oxygen levels following the loss of blood flow in specific areas of the brain. Subsequent pathological processes results in a central core area of severely ischemic tissue surrounded by a region of moderate ischemic tissue (penumbra) with a preserved cellular metabolism. The outcome of an ischemic stroke depends on the size of the infarct core and the potential to salvage the cells in the penumbra, which is hypoperfused, and therefore at risk of infarction but still viable. Such viable penumbral tissue can be rescued by quick interventions that can increase oxygen levels and/or slow metabolism in the ischemic area and minimize oxidative injury on reperfusion.
Several strategies have been investigated to rescue ischemic tissue using experimental models, especially rodents, but largely failed in subsequent clinical trials. The rabbit model of ischemic stroke using embolic clot is a promising model for developing effective strategies. This rabbit model first led to the prediction of the clinical response of tissue plasminogen activator (tPA) to restore blood flow in patients1. The drug is currently recommended for administration within 3 h for best outcomes and has also shown modest benefit when administered within 4.5 – 6 h of clinical onset2. The rabbit model of embolic clot is now considered as a pertinent model for translational research by the Stroke Therapy Academic Industry Roundtable (STAIR) recommendations3.
In order to rationally develop effectual therapies, it is important to understand the effect of ischemic stroke on oxygen levels (pO2, partial pressure of oxygen) in the regions directly affected by the pathology as well as contralateral regions of the rabbit brain. The potential changes in tissue pO2 of contralateral regions may provide crucial information on adaptive response, if any, of the brain to counteract ischemic stroke. Such research will greatly benefit from the availability of oximetry techniques that can “directly” and “repeatedly” measure tissue pO2 in several regions of the brain.
Several methods are currently available for the assessment of brain pO2 including oxygen electrodes4, 5. However, their limited capability to directly and repeatedly measure brain pO2 in a minimally-invasive manner has restricted their effective application in ischemic stroke where monitoring oxygen levels are crucial for the development, and optimization of novel strategies for clinical translation. We report Electron Paramagnetic Resonance (EPR) oximetry using implantable resonators to monitor brain pO2 in rabbit with the goal to develop and test novel strategies that can significantly reduce brain loss in ischemic stroke. EPR oximetry using particulate probes, such as lithium phthalocyanine crystals or its derivatives, have been used to study tissue pO2 in a wide range of experimental systems, including muscle, heart, brain, kidney, and liver in rodents6-9 and is now being developed for clinical applications10, 11. Despite the benefits of EPR oximetry, the currently available hardware technology limits pO2 measurement to a depth of 1 cm at L-band frequencies (1.2 GHz). This is largely due to non-resonant losses of the microwave energy in the tissue of interest. The penetration of microwave energy can be increased up to approximately 7 cm by using lower frequencies but this decreases the signal-to-noise (S/N) ratio of the EPR signal, thus compromising the accuracy of measurements12. To resolve this problem, we have pioneered an innovative design of implantable resonators for pO2 measurement at depths greater than 1 cm, Figure 1. We have implemented this approach to simultaneously monitor tissue pO2 at two sites in each hemisphere of the rabbit brain. Our overall goal is to optimize the outcome of ischemic stroke for clinical translation. To the best of our knowledge, this is the first report of monitoring brain pO2 in multiple sites and at depths greater than 1 cm from the skull in rabbit by EPR oximetry.
Figure 1.
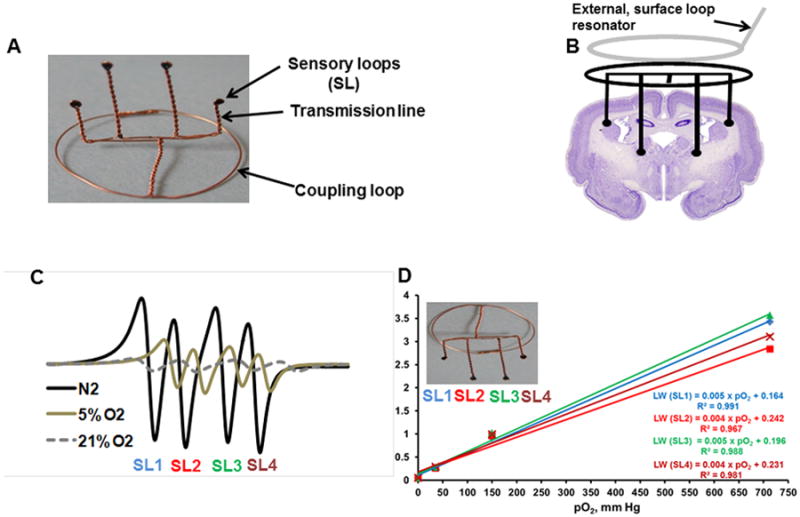
(A) Implantable resonator with 10 mm and 15 mm length of transmission line for pO2 measurement at different depths; (B) Schematic of a surface loop resonator coupled to the implantable resonator for pO2 measurement at four sites in a rabbit brain; (C) Typical EPR spectra acquired from the implantable resonator with sensory loops (SL1-SL4) perfused with N2, 5% O2 and 21 % O2 for the purpose of calibration; (D) Change in line-width with pO2 (calibration) of each sensor loop from left to right.
Description of the innovation
Implantable resonator
The implantable resonator is assembled with thin non-magnetic copper wire (0.3 mm wire gauge) and consists of a coupling loop (12-16 mm diameter) at one end and a transmission line with sensor loops (0.4 - 0.5 mm inner diameter) at the other end, Figure 1A. The sensor loops (or tips) are loaded with 30-50 μg of lithium phthalocyanine (LiPc, oximetry probe)13 crystals. The length of the transmission lines defines the depth and can be varied as needed for the experiment. The number of sensory loops and the distance between them can also be varied to measure pO2 at one or more sites in the brain of rabbits. The entire resonator is coated with a gas permeable and biocompatible Teflon AF240014. The mean area of the oximetry probe at the surface of each sensor loop is estimated to be 1.3 - 1.6 mm2, EPR oximetry therefore samples a region that includes many capillary segments and provides average pO2 at the site of sensor loop8, 15. Histological examination of the cerebral tissue in the rabbit euthanized 4 weeks after the placement of implantable resonator did not show any obvious accumulation of inflammatory cells or blood cells surrounding the sensor loops. Similar results were also evident as early as 7-days after the placement of implantable resonator with 6 mm length of transmission lines in the brain of rats 16.
Procedure for the placement of implantable resonator in the brain of rabbits
The surgical procedure for the placement of implantable resonator in the brain of rabbit was in strict accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee (IACUC) of Geisel School of Medicine at Dartmouth. The head of the anesthetized rabbit was antiseptically treated with Betadine, and 70% alcohol scrubs. A small incision (2-3 cm) was made on the skin and burr holes were gently created by using 18 gauge needle on the skull at predefined co-ordinates [AP (anterior-posterior from bregma): -2.0 mm; ML (medial-lateral from midline): 4 mm & 8 mm on each hemisphere; DV (dorsal-ventral from surface of skull): 15 mm & 10 mm in each hemisphere]. The sensor loops, SL1 and SL4, were located in the parietal cortex, while SL2 and SL3 were located in the sub-cortex (basal forebrain/internal capsule) region of the brain. The position of the sensor loops can be altered depending on the coordinates of the compromised tissue following ischemic stroke. The sensor loops were placed at the desired depth and the coupling loop was placed on the skull below the skin, to allow inductive coupling with the external surface loop resonator of the EPR spectrometer, Figure 1B. The incision on the skin was closed with non-absorbable 3-0 nylon suture and the rabbit was monitored for recovery as per the IACUC protocol. The repeated measurements of brain pO2 by EPR oximetry was started 72 h (day 3) after the placement of implantable resonator and the measurements were repeated for 4 weeks.
In vivo EPR oximetry
EPR oximetry requires one-time implantation of the oxygen probes (LiPc or implantable resonator), but rest of the procedure for pO2 measurement is entirely non-invasive and can be repeated as desired8, 17-19. The basis of oximetry is the paramagnetic nature of oxygen, which broadens the EPR signal of the probe in proportion to the amount of oxygen. EPR oximetry has unique capabilities and advantages compared to other techniques: (i) Direct measurement of absolute pO2 in the tissue of interest; (ii) pO2 is quantified through a physical interaction of oxygen with the probe (does not require oxygen consumption); (iii) pO2 measurements can be made continuously and repeatedly as desired, without a confounding influence of prior measurements; (iv) The oxygen sensors are metabolically inert and coated with Teflon, therefore do not perturb the tissue microenvironment including oxygen content, and (v) There is no other technique available at present to make repeated measurement of tissue pO2 without the need to reintroduce the probe for each measurement.
A 1.2 GHz EPR spectrometer equipped with a surface loop resonator and a set of gradient coil for multisite oximetry was used for monitoring brain pO2 in the rabbit. The anesthetized rabbit (2.5% isoflurane in 30% O2) was positioned in the EPR magnet and the external surface loop resonator was gently placed over the head region. A magnetic field gradient of 1.7 G/cm/amp was used to separate the EPR spectra from each sensory loop for simultaneous multisite oximetry20. The peak-to-peak line-widths of the EPR spectra were used to determine pO2 by using the calibration of implantable resonator, Figure 1C and 1D. The rabbit was maintained at 38 ± 1.0°C (monitored via a rectal probe) by keeping the animal under warm air during the EPR measurements. The EPR settings were: incident microwave power: 0.4-1.2 mW; modulation frequency 24 kHz; magnetic field center 410 G; scan time 10 sec, scan range 8-12 G, and modulation amplitude not exceeding one third of the line width. The implantable resonator appears as a signal void in T1-weighted MRI scans, which can be used to confirm their position in the brain of rabbits.
Pilot Results
Brain pO2 was measured for approximately 20-25 min on day 3 and the measurements were repeated on days 5, 7, 14, 21 and 28, Figure 2. The mean (SD) baseline pO2 at each site (SL1-SL4) in the brain on day 3 was 39.2 (2.2), 41.6 (1.4), 41.3 (1.7), 43.6 (2.0) mmHg, respectively and only a modest variation was observed in the measurements repeated subsequently for up to 4 weeks. In order to mimic the ischemic situation with low levels of oxygen and test the response of implantable resonator, the breathing gas was switched to 15% O2 for 15 min and then returned to 30% O2, Figure 3A. To test the potential effect of hyperoxia on brain pO2, the breathing gas was switched to carbogen (95% O2 + 5% CO2) for 20-25 min and then returned to 30% O2, Figure 3B. These experiments were performed on days 7, 14, 21 and 28. The brain pO2 measured at four-sites decreased by approximately 30% from baseline in rabbit breathing 15% O2. On the other hand, brain pO2 measured at four-sites increased significantly by at least 75% during carbogen breathing. An exponential quadratic function of time was used to determine minimum pO2 (pO2min) attained during 15% O2 on each day and the time to reach the pO2min (Tmin), Figure 4. Similar analysis was used to determine maximal pO2 (pO2max) attained on each day and the time to reach maximum pO2 (Tmax) during carbogen inhalation. These analyses suggest that it took approximately 10-14 min to reach a minimal or maximal pO2 when the breathing gas was switched from 30% O2 to 15% O2 or carbogen, respectively. A similar time-scale was noted for the brain pO2 to return to the baseline level when the breathing gas was switched from 15% O2 or carbogen to 30% O2. We anticipate that such temporal pO2 information will be extremely useful in designing hyperoxic therapies to modulate oxygen levels in ischemic stroke.
Figure 2.
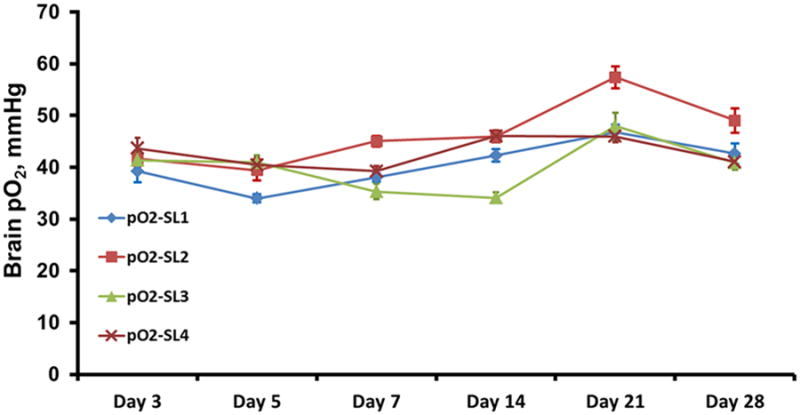
Tissue pO2 in the rabbit brain at two sites in each hemisphere measured simultaneously by multisite EPR oximetry. The SL1 and SL4 were at a depth of 10 mm, SL2 and SL3 were at a depth of 15 mm in the left and right-hemisphere, respectively. The brain pO2 measurements were repeated on days 3, 5, 7, 14, 21 and 28.
Figure 3.
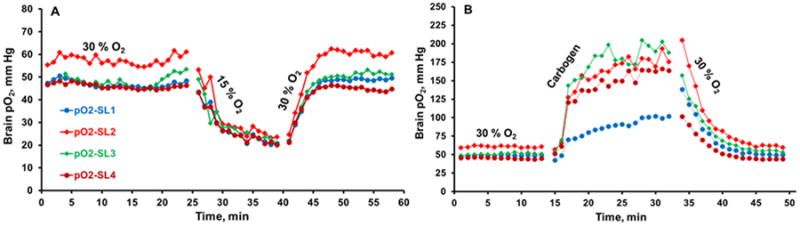
Typical changes in tissue pO2 of the rabbit brain at two sites in each hemisphere during (A) 30% O2 (baseline), 15% O2 and return to 30% O2 breathing, (B) 30% O2, carbogen and return to 30% O2 breathing. The experiment was repeated for four consecutive weeks.
Figure 4.
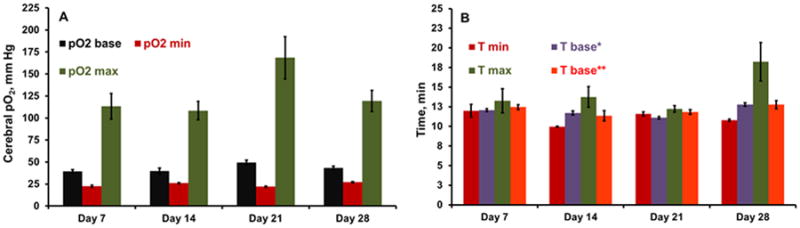
(A) Baseline (base), minimum (min) and maximum (max) brain pO2 determined using exponential quadratic function in rabbit breathing 30% O2, 15% O2 and carbogen respectively. (B) Time required to reach minimal pO2 on 15% O2 breathing (Tmin), maximal pO2 on carbogen breathing (T max), time required to return to baseline pO2 when the gas was switched from 15% O2 to 30% O2 (T base*), and time required to return to baseline pO2 when the gas was switched from carbogen to 30% O2 (T base**) in the experiments repeated on days 7, 14, 21 and 28. The pO2 obtained from all the sensor loops were pooled on each day to obtain average brain pO2 for these analyses.
Discussion
In order to fully comprehend the pathology of stroke and rationally develop strategies to rescue ischemic tissue, there is an unmet need to understand the complex temporal changes in tissue pO2 that occur during the course of ischemic stroke, a capability that previously has not been available. The results highlight the ability of EPR oximetry using implantable resonator for pO2 measurements at 4-sites simultaneously in the brain of a rabbit. The pO2 measurements can be repeated as desired. A rapid decline in tissue pO2 during 15% O2 breathing potentially highlights the immediate changes in the oxygen levels that may occur in ischemic stroke. The extent of increase in tissue pO2 during carbogen breathing is encouraging and can be potentially used as a therapeutic strategy to improve oxygen levels and thus save vital tissue loss in ischemic stroke.
For the multisite oximetry approach, the position of sensor loops should be carefully selected so that they are located in the region of interest i.e. infarct core and penumbra following ischemic stroke. The design of the implantable resonator including the length of transmission lines, number and distance between the sensory loops can be modified as needed for a particular experiment. A stable brain pO2 was observed from day 3, which suggest that the experiments to investigate ischemic stroke can be initiated as early as 3 days after the placement of implantable resonator in the brain of rabbits. The measurement of tissue pO2 in the non-ischemic contralateral brain can be used as internal control and investigate adaptive response of the brain to ischemic stroke. The brain pO2 data presented here was obtained in a rabbit to illustrate the capability of temporal monitoring by EPR oximetry. We are currently implementing this technique to investigate temporal changes in the brain pO2 in additional rabbits during hyperoxia and ischemic stroke.
Conclusions
We have demonstrated a direct and longitudinal measurement of absolute tissue pO2 at several sites simultaneously in the brain of rabbit by EPR oximetry using implantable resonators, a capability which was not available hitherto. Dynamic information of cerebral pO2 can be used to test and optimize strategies for improving treatment outcome of ischemic stroke. EPR oximetry with implantable resonators should also be useful to investigate the effect of other pathologies, such as traumatic brain injury and cold injury, on the oxygen levels in the brain of clinically pertinent animal models.
Acknowledgments
Funding Source: NIH grants R21NS082585 to NK, R01EB004031 to PK, and the Electron Paramagnetic Resonance (EPR) Center, Geisel School of Medicine at Dartmouth, Lebanon, NH
Footnotes
Disclosures: None
References
- 1.Lapchak PA. Translational stroke research using a rabbit embolic stroke model: A correlative analysis hypothesis for novel therapy development. Transl Stroke Res. 2010;1:96–107. doi: 10.1007/s12975-010-0018-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wardlaw JM, Murray V, Berge E, Del Zoppo G, Sandercock P, Lindley RL, et al. Recombinant tissue plasminogen activator for acute ischaemic stroke: An updated systematic review and meta-analysis. Lancet. 2012;379:2364–2372. doi: 10.1016/S0140-6736(12)60738-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albers GW, Goldstein LB, Hess DC, Wechsler LR, Furie KL, Gorelick PB, et al. Stroke treatment academic industry roundtable (stair) recommendations for maximizing the use of intravenous thrombolytics and expanding treatment options with intra-arterial and neuroprotective therapies. Stroke. 2011;42:2645–2650. doi: 10.1161/STROKEAHA.111.618850. [DOI] [PubMed] [Google Scholar]
- 4.Fisher M. The ischemic penumbra: A new opportunity for neuroprotection. Cerebrovasc Dis. 2006;21(Suppl 2):64–70. doi: 10.1159/000091705. [DOI] [PubMed] [Google Scholar]
- 5.Dengl M, Jaeger M, Renner C, Meixensberger J. Comparing brain tissue oxygen measurements and derived autoregulation parameters from different probes (licox vs. Raumedic) Acta neurochirurgica Supplement. 2012;114:165–168. doi: 10.1007/978-3-7091-0956-4_31. [DOI] [PubMed] [Google Scholar]
- 6.Helisch A, Wagner S, Khan N, Drinane M, Wolfram S, Heil M, et al. Impact of mouse strain differences in innate hindlimb collateral vasculature. Arteriosclerosis, thrombosis, and vascular biology. 2006;26:520–526. doi: 10.1161/01.ATV.0000202677.55012.a0. [DOI] [PubMed] [Google Scholar]
- 7.Jiang J, Nakashima T, Liu KJ, Goda F, Shima T, Swartz HM. Measurement of pO2 in liver using epr oximetry. J Appl Physiol. 1996;80:552–558. doi: 10.1152/jappl.1996.80.2.552. [DOI] [PubMed] [Google Scholar]
- 8.Khan N, Williams BB, Hou H, Li H, Swartz HM. Repetitive tissue pO2 measurements by electron paramagnetic resonance oximetry: Current status and future potential for experimental and clinical studies. Antioxidants & redox signaling. 2007;9:1169–1182. doi: 10.1089/ars.2007.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Towner RA, Sturgeon SA, Khan N, Hou H, Swartz HM. In vivo assessment of nodularin-induced hepatotoxicity in the rat using magnetic resonance techniques (mri, mrs and epr oximetry) Chemico-biological interactions. 2002;139:231–250. doi: 10.1016/s0009-2797(02)00002-9. [DOI] [PubMed] [Google Scholar]
- 10.Swartz HM, Williams BB, Zaki BI, Hartford AC, Jarvis LA, Chen EY, et al. Clinical epr: Unique opportunities and some challenges. Acad Radiol. 2014;21:197–206. doi: 10.1016/j.acra.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swartz HM, Hou H, Khan N, Jarvis LA, Chen EY, Williams BB, et al. Advances in probes and methods for clinical epr oximetry. Advances in experimental medicine and biology. 2014;812:73–79. doi: 10.1007/978-1-4939-0620-8_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halpern HJ, Yu C, Peric M, Barth E, Grdina DJ, Teicher BA. Oxymetry deep in tissues with low-frequency electron paramagnetic resonance. Proc Natl Acad Sci U S A. 1994;91:13047–13051. doi: 10.1073/pnas.91.26.13047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu KJ, Gast P, Moussavi M, Norby SW, Vahidi N, Walczak T, et al. Lithium phthalocyanine: A probe for electron paramagnetic resonance oximetry in viable biological systems. Proc Natl Acad Sci U S A. 1993;90:5438–5442. doi: 10.1073/pnas.90.12.5438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dinguizli M, Jeumont S, Beghein N, He J, Walczak T, Lesniewski PN, et al. Development and evaluation of biocompatible films of polytetrafluoroethylene polymers holding lithium phthalocyanine crystals for their use in epr oximetry. Biosens Bioelectron. 2006;21:1015–1022. doi: 10.1016/j.bios.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 15.O'Hara JA, Hou H, Demidenko E, Springett RJ, Khan N, Swartz HM. Simultaneous measurement of rat brain cortex ptO2 using epr oximetry and a fluorescence fiber-optic sensor during normoxia and hyperoxia. Physiological measurement. 2005;26:203–213. doi: 10.1088/0967-3334/26/3/006. [DOI] [PubMed] [Google Scholar]
- 16.Hou H, Dong R, Li H, Williams B, Lariviere JP, Hekmatyar SK, et al. Dynamic changes in oxygenation of intracranial tumor and contralateral brain during tumor growth and carbogen breathing: A multisite epr oximetry with implantable resonators. J Magn Reson. 2012;214:22–28. doi: 10.1016/j.jmr.2011.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmad R, Kuppusamy P. Theory, instrumentation, and applications of electron paramagnetic resonance oximetry. Chem Rev. 2010;110:3212–3236. doi: 10.1021/cr900396q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khan N, Mupparaju S, Hou H, Williams BB, Swartz H. Repeated assessment of orthotopic glioma pO2 by multi-site epr oximetry: A technique with the potential to guide therapeutic optimization by repeated measurements of oxygen. Journal of neuroscience methods. 2012;204:111–117. doi: 10.1016/j.jneumeth.2011.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dunn JF, Swartz HM. In vivo electron paramagnetic resonance oximetry with particulate materials. Methods (San Diego, Calif) 2003;30:159–166. doi: 10.1016/s1046-2023(03)00077-x. [DOI] [PubMed] [Google Scholar]
- 20.Smirnov AI, Norby SW, Clarkson RB, Walczak T, Swartz HM. Simultaneous multi-site epr spectroscopy in vivo. Magn Reson Med. 1993;30:213–220. doi: 10.1002/mrm.1910300210. [DOI] [PubMed] [Google Scholar]


