Summary
Strategies to target angiogenesis include inhibition of the vessel-stabilizing properties of vascular pericytes. Pericyte depletion in early-stage non-hypoxic tumors suppressed nascent angiogenesis, tumor growth and lung metastasis. In contrast, pericyte depletion in advanced-stage hypoxic tumors with pre-established vasculature resulted in enhanced intra-tumoral hypoxia, decreased tumor growth and increased lung metastasis. Further, depletion of pericytes in post-natal retinal blood vessels resulted in abnormal and leaky vasculature. Tumor transcriptome profiling and biological validation revealed that angiopoietin signaling is a key regulatory pathway associated with pericyte targeting. Indeed, pericyte targeting in established mouse tumors increased angiopoietin-2 (ANG2/Angpt2) expression. Depletion of pericytes, coupled with targeting of ANG2 signaling, restored vascular stability in multiple model systems and decreased tumor growth and metastasis. Importantly, ANGPT2 expression correlated with poor outcome in patients with breast cancer. These results emphasize the potential utility of therapeutic regimens that target pericytes and ANG2 signaling in metastatic breast cancer.
Introduction
Angiogenesis that accompanies tumor progression provides cancer cells with a means to meet their increased demand for oxygen and nutrients and may also provide a route for cancer cell spread to distal tissues (Folkman, 2002; Zetter, 1998). The complexity of blood vessel growth regulation in tumors may partake in offering adaptive mechanisms to promote rapid emergence of resistance mechanisms in response to anti-angiogenic therapies, thereby limiting their efficacy (Vasudev and Reynolds, 2014). Inhibition of angiogenesis has been shown to suppress metastasis in some experimental tumors (Folkman, 2002; Kirsch et al., 2000; Mazzieri et al., 2011; O’Reilly et al., 1997; O’Reilly et al., 1994; Weidner et al., 1991), whereas in other studies it has been associated with enhanced intratumoral hypoxia and increased local tumor invasion and frequency of metastasis (Cooke et al., 2012; Ebos et al., 2009; Paez-Ribes et al., 2009). Previously, we reported that the depletion of pericytes in established tumors impaired the neovascularization response and suppressed tumor growth, but enhanced tumor hypoxia and cancer cell spread to target organs of metastasis (Cooke et al., 2012). While pericyte coverage in established tumor blood vessels may function as a gatekeeper of metastasis, the molecular mechanisms mediating the increased frequency of metastasis after pericyte targeting remain poorly characterized.
Pericytes are important regulators of angiogenesis and vascular stability in both developmental and pathological contexts (Armulik et al., 2005; Armulik et al., 2011; Bergers and Song, 2005; Hirschi and D’Amore, 1996). These specialized perivascular mesenchymal cells are embedded in the basement membrane of blood vessels (Armulik et al., 2011; Strasser et al., 2010) and secrete pro-angiogenic factors at the onset of angiogenesis (Bergers and Song, 2005; Bergers et al., 2003; Lu et al., 2007; Sennino et al., 2007; Song et al., 2005), while also establishing quiescence of endothelial cells and stabilizing mature blood vessels (Benjamin et al., 1998; Greenberg et al., 2008; Hammes et al., 2002; Nasarre et al., 2009; Orlidge and D’Amore, 1987). Such apparently opposed functions of pericytes are controlled by the evolving pericyte-endothelial cell crosstalk that occurs during tumor angiogenesis. Pericyte-endothelial cell signaling involves multiple pathways, including angiopoietin signaling (Armulik et al., 2005; Armulik et al., 2011). At its core, Angiopoietin-1 (ANG1/Angpt1) and angiopoietin-2 (ANG2/Angpt2) are ligands for the endothelial receptor TIE2. ANG1 is mainly secreted by pericytes, fibroblasts and cancer cells (Buchanan et al., 2012) and plays a key role in regulating blood vessel permeability. ANG1 also signals to enhance blood vessel stability by increasing the number of vessel-associated pericytes, i.e., pericyte coverage (Augustin et al., 2009; Fuxe et al., 2011; Gaengel et al., 2009; Thurston et al., 2000; Thurston et al., 1999). ANG2, which is largely produced by activated endothelial cells, antagonizes ANG1 and destabilizes blood vessels by promoting pericyte detachment and initiating endothelial cell sprouting (Augustin et al., 2009; Scharpfenecker et al., 2005; Thomas and Augustin, 2009). Hypoxia increases ANG2 expression (Kelly et al., 2003; Oh et al., 1999; Rigamonti et al., 2014; Skuli et al., 2012) and ANG2 induces abnormal angiogenesis in the presence of VEGF-A (Augustin et al., 2009; Lobov et al., 2002; Thomas and Augustin, 2009). ANG2 blockade was reported recently to limit the onset of tumor resistance to VEGF-A signaling inhibition in RIP1-Tag2 pancreatic neuroendocrine tumors (Rigamonti et al., 2014).
To gain a better understanding of pericytes and pericyte-endothelial cell interactions and signaling in blood vessel formation, we performed experiments using genetic and pharmacologic targeting of pericytes at various stages of tumor and developmental (retinal) angiogenesis. In an effort to unravel the mechanisms of resistance to anti-vascular therapies, we focused on elucidating the regulatory cellular signaling pathways and functional contribution of pericytes to blood vessel formation during tumor progression.
Results
Coverage and functional role of pericytes evolve during tumor progression
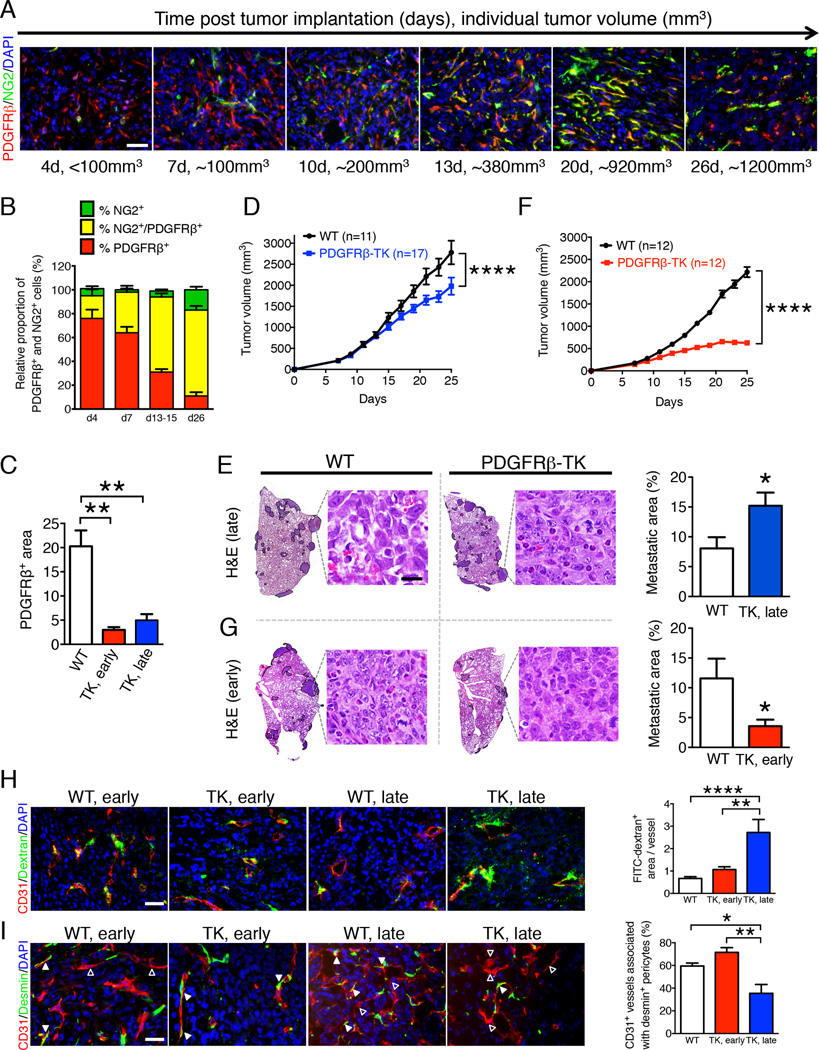
Anti-PDGFRβ and NG2 antibodies have been used to label pericytes (Song et al., 2005). Time-course analysis of pericyte labeling in orthotopically implanted 4T1 mouse mammary tumors showed PDGFRβ+ pericytes starting 4 days after injection of cancer cells (d4). PDGFRβ+ pericyte numbers increased as the tumor grew (d7, d13–15, d26) and their proportion relative to NG2+ pericytes was higher in early-stage non-hypoxic (smaller) tumors when compared to late-stage hypoxic tumors (Figure 1A–B, S1A). Here we refer to non-hypoxic tumors as early-stage and hypoxic tumors as late-stage. Fewer NG2+ pericytes, compared to PDGFRβ+ pericytes, were observed in the early-stage tumors, and the overlap of NG2 and PDGFRβ expression increased as tumors grew, resulting in a gradual increase in the PDGFRβ+/NG2+ double positive cells with tumor progression (Figure 1A–B, S1A). Thus, growing tumors present with dynamic pericyte coverage defined by sequential and partly overlapping PDGFRβ and NG2 expression.
Figure 1. Pericyte depletion at early and late stages of tumor progression differentially impacts tumor growth and metastasis.
A Double immunolabeling for PDGFRβ and NG2 (pericytes markers) in 4T1 tumors from wild-type (WT) mice at 4, 7, 10 13, 20 and 26 days after cancer cell orthotopic injection. Scale bar: 25µm. B Quantification of NG2+ and PDGFRβ+ cells in individual 4T1 orthotopic tumors over the course of cancer progression. The relative proportion of single and double positive cells are reported with 100% reflecting all of the NG2+ and PDGFRβ+ cells per field of view. d4, n=3; d7, n=3; d13–15, n=3; d26, n=5. C Quantification of the relative PDGFRβ+ area in tumors with early and late PDGFRβ+ pericyte depletion. WT, n=8; PDGFRβ-TK early (TK, early), n=5; PDGFRβ-TK late (TK, late), n=4. D Tumor volume measurements over time, late PDGFRβ+ pericyte depletion. GCV treatment started when total tumor burden reached 500 mm3. E H&E staining of lungs from WT and PDGFRβ-TK mice with late PDGFRβ+ cell depletion and respective quantification of the percent lung metastatic area. WT, n=11; PDGFRβ-TK, n=17. The metastatic nodules are encircled. High-magnification images of metastatic nodules are shown, scale bar: 20 µm. F Tumor volume measurements over time, early PDGFRβ+ pericyte depletion. GCV treatment started before total tumor burden reached 100 mm3. G H&E staining of lungs from WT and PDGFRβ-TK mice with early PDGFRβ+ cell depletion and respective quantification of the percent lung metastatic area. WT, n=12; PDGFRβ-TK, n=12. High-magnification images of metastatic nodules are shown, scale bar: 20 µm. H Representative images of tumors immunolabeled for CD31 and visualization of perfused 2,000 kDa FITC-dextran in the indicated experimental groups and quantitation of FITC-dextran+ area per vessel. WT early, n=12; PDGFRβ-TK early, n=7; PDGFRβ-TK late, n=6. Scale bar: 25µm. I Representative images of tumors immunolabeled for CD31 and desmin in the indicated experimental groups and quantification of the percent of CD31+ vessels associated with desmin labeling positive pericytes. Solid arrowheads: vessels with desmin+ pericyte coverage; empty arrowheads: vessels without desmin+ pericyte coverage. WT n=6; PDGFRβ-TK early, n=5; PDGFRβ-TK late, n=6. Scale bar: 25µm. Data are represented as the mean +/− SEM. In D & F: two-way ANOVA with Bonferroni’s multiple comparison test was used. For C, H-I: one-way ANOVA with Tukey post-hoc analysis was used. Unless otherwise noted, unpaired two-tailed t-test was used to determine statistical significance. * p<0.05, ** p<0.01, ****p < 0.0001. ns: not significant. See also Figure S1.
To functionally address the role of pericytes at different stages of tumor progression, 4T1 breast cancer cells were injected orthotopically into mammary fat pads of NG2-tymidine kinase (TK), PDGFRβ-TK or wild-type (WT) control mice. We previously showed that daily ganciclovir (GCV) administration specifically depletes proliferating NG2+ and PDGFRβ+ pericytes in NG2-TK and PDGFRβ-TK mice, respectively (Cooke et al., 2012). Depletion of 75.4% of PDGFRβ+ pericytes (Figure 1C, S1B) in advanced-stage tumors (initiated when the average tumor burden was greater than 500 mm3 and intratumoral hypoxia clearly detected (Figure S1C)) significantly slowed tumor growth (Figure 1D). We previously reported that depletion of NG2+ pericytes in advanced-stage tumors also decreased tumor growth (Cooke et al., 2012).
Depleting pericytes at an advanced tumor stage in NG2-TK and PDGFRβ-TK mice decreased the tumor volume by 60% (Cooke et al., 2012) and 25% (Figure 1D), respectively, and significantly increased the lung metastatic burden (Figure 1E and (Cooke et al., 2012)). In contrast, pericyte depletion early during tumor growth (Figure 1F, S1B) and prior to the onset of intratumoral hypoxia (Figure S1C) reduced the tumor mass by 36% and 72% in NG2-TK and PDGFRβ-TK mice, respectively (Figure 1F, S1D), but significantly decreased the incidence of lung metastasis only in PDGFRβ-TK mice (Figure 1G, S1E). The difference in the lung metastatic burden noted in the early pericyte depletion setting between NG2-TK and PDGFRβ-TK mice may be due to the important contribution of PDGFRβ+ pericytes to tumor angiogenesis at early stages of tumor growth, when NG2+ pericytes are still scarce (Figure 1A–B). PDGFRβ+ pericyte depletion did not affect metastasis in an experimental model of metastatic lung colonization after intravenous injection of 4T1 cancer cells (Figure S1F).
Together, the aforementioned data indicate that PDGFRβ+ pericytes broadly control metastasis of cancer cells at both early and late stages of tumor progression. We therefore focused subsequent studies on PDGFRβ-TK mice and pharmacological targeting of PDGFRβ signaling.
Pericyte depletion in advanced-stage tumors is associated with compromised blood vessel stability and enhanced intratumoral hypoxia
CD31 labeling of tumor blood vessels was significantly reduced in 4T1 tumors in both early and late pericyte depletion settings (Figure S1G). Intratumoral hypoxia, assayed by immunolabeling for pimonidazole adduct formation following injection of hypoxyprobe, was significantly reduced upon early depletion of pericytes (Figure S1H). In contrast, the smaller tumors in PDGFRβ-TK mice in the late pericyte depletion setting displayed significantly enhanced intratumoral hypoxia compared to WT control tumors (Figure S1H). The increased intratumoral hypoxia noted in the late pericyte depletion setting was associated with an increase in vascular leakage as determined by systemic FITC-dextran permeability studies (Figure 1H). A similar degree of permeability was noted in the tumor-associated blood vessels of mice subjected to early pericyte depletion (Figure 1H). The blood vessels in the late depletion setting were deficient in desmin+ cells, representing mature pericytes in structurally stable vessels (Chan-Ling et al., 2004; Kurz et al., 2008; Song et al., 2005; von Tell et al., 2006), suggesting that these vessels were less stable (Figure 1I). The increased intratumoral hypoxia in these tumors was accompanied by enhanced epithelial-to-mesenchymal transition (EMT) in cancer cells, as determined by increased numbers of CK8+ cancer cells acquiring mesenchymal gene expression (αSMA-positive), indicative of enhanced cancer cell invasiveness (Figure S1I).
Differential effects of pericyte depletion on early and late retinal development
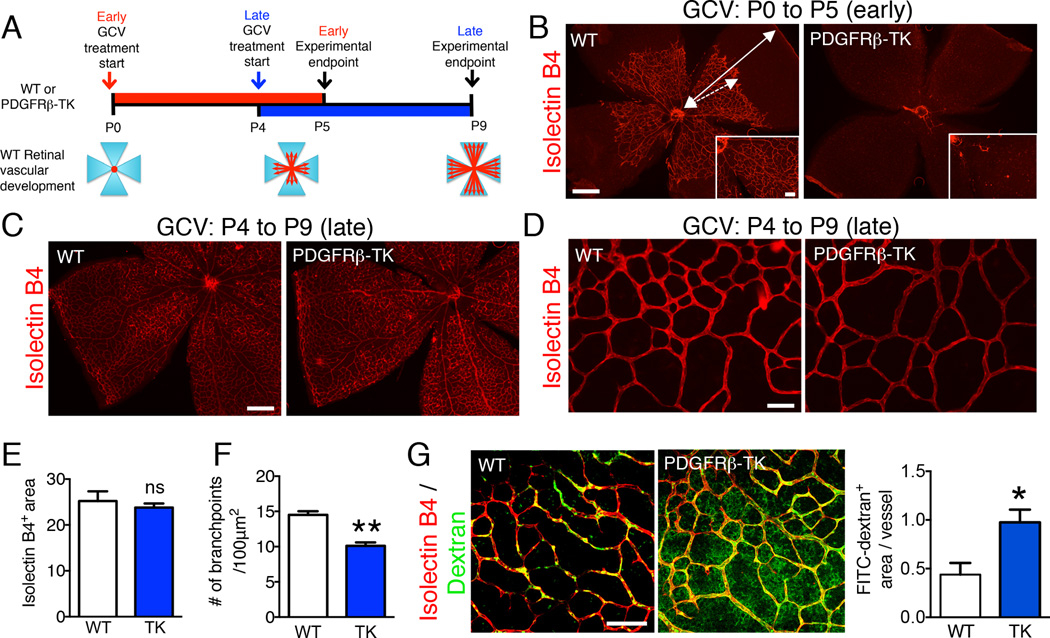
We utilized the retinal vasculature as a model system to further characterize the dynamic functional contributions of PDGFRβ+ pericytes to developmental angiogenesis (Figure 2A). In mouse neonatal pups, retinal blood vessels start forming after birth (P0) and extend from the center to the periphery in an organized network of branches and sprouts, providing an excellent model system to study developmental angiogenesis spatiotemporally (Stahl et al., 2010). To determine the effect of pericyte depletion at early stages of retinal angiogenesis, GCV treatment of PDGFRβ-TK or WT littermate pups was started on the day of birth (P0). Treatment was continued until P5, when the pups were sacrificed and the retinas harvested. To assess the effect of pericyte depletion at a later stage of development, when the retina vasculature was partially established, GCV treatment of PDGFRβ-TK or WT littermate pups was initiated at P4 and continued until P9. We noted that very few blood vessels developed in retinas when pericytes were depleted early in PDGFRβ-TK pups compared to WT littermates (Figure 2B, S2A). The lack of type IV collagen immunolabeling, which identifies the vascular basement membrane in the retinas, indicated that the lack of blood vessels after early PDGFRβ+ pericyte depletion was not due to vessel regression but rather impaired angiogenesis (Figure S2B).
Figure 2. Early (P0-P5) and late (P4-P9) pericyte depletion differentially alter developmental angiogenesis in the mouse retina.
A Schematic representation of stage-specific pericyte depletion in the development of the retina vasculature. B Isolectin B4 staining of retinas from P5 WT and PDGFRβ-TK pups treated with GCV from P0 to P5 (early depletion). Scale bar: 500 µm, insert scale bar: 100 µm. Dashed arrow shows the extent of vessel coverage in WT retina at P5 with respect to the edge of the retina (solid arrow). C–D Isolectin B4 staining of retinas from WT and PDGFRβ-TK mice treated with GCV from P4 to P9 (late depletion) Scale bars: 500 µm (C) and 100 µm (D). E Quantitation of the isolectin B4+ area/field (vessel area). WT, n=4; PDGFRβ-TK, n=3. F Quantitation of the number of branch points/100 µm2. WT, n=4; PDGFRβ-TK, n=3. G Isolectin B4 staining and visualization of 70 kDa FITC-dextran in WT and PDGFRβ-TK mice treated with GCV from P4 to P9 and quantitation of FITC-dextran+ area per vessel. WT, n=3; PDGFRβ-TK, n=5. Scale bar:
100 µm. Data are represented as the mean +/− SEM. Unpaired two-tailed t-test was used to determine statistical significance. * p<0.05, ** p<0.01. ns: not significant. See also Figure S2.
When pericyte depletion was delayed, we noted unperturbed blood vessel formation and sprouting angiogenesis, with blood vessels reaching the periphery of the retina (Figure 2C–D). In this setting, isolectin B4 staining showed that although the microvascular area was not detectably affected (Figure 2E), blood vessel branching was significantly reduced (Figure 2F). Severe vascular leakage in both the central remodeling zone and peripheral front of the expanding inner vascular plexus (shown by 70 kDa FITC-dextran perfusion) was apparent after late pericyte depletion (Figure 2G, S2C). The pericyte-depleted blood vessels also appeared dilated, with an increased vessel diameter allowing increased perfusion of 2,000 kDa FITC-dextran (Figure S2D). Together, these findings indicate differential effects of depleting pericytes during the early and late stages of retinal development.
Angiopoietin signaling is differentially modulated by temporal pericyte depletion during tumor progression and retinal angiogenesis
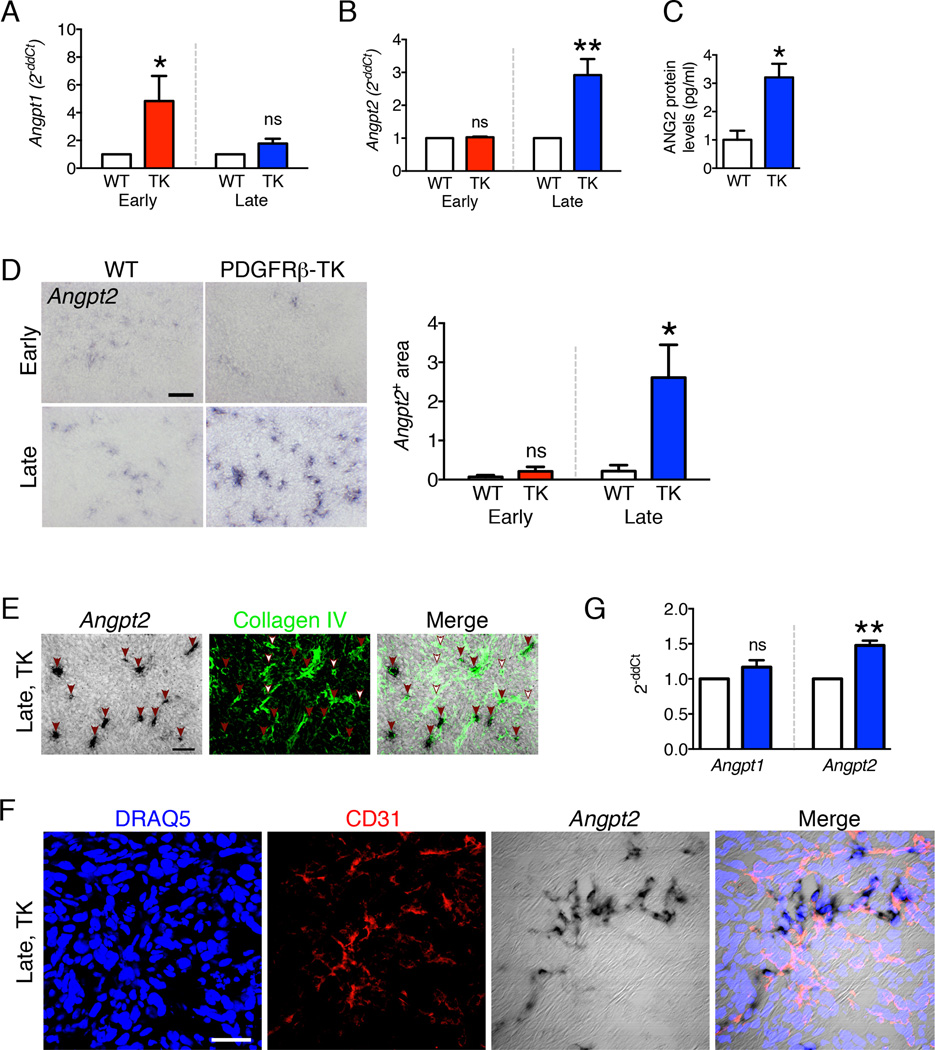
To identify the potential molecular effectors that may be responsible for enhanced metastasis following the late PDGFRβ+ cell depletion (in contrast to early PDGFRβ+ cell depletion), we compared the gene expression profiles of tumors subjected to either early or late PDGFRβ+ cell depletion. Growth factor signaling pathway analyses coupled to quantitative transcript level measurements revealed that the angiopoietin signaling pathway was deregulated both in the early and late pericyte depletion setting, along with the VEGF, PDGF and HGF signaling pathways (Figure S3A). While all growth factors and signaling targets evaluated by quantitative RT-PCR showed a similar up- or down-regulation in both the early and late pericyte-depleted experimental groups compared to their WT controls (Figure S3B–C), Anpgt1 and Anpgt2 were uniquely deregulated in the early vs. late experimental groups (Figure 3A–B). Specifically, in tumors with early pericyte depletion, Angpt1 transcript levels were elevated by 5-fold while Angpt2 transcript levels were unchanged (Figure 3A). In contrast, in tumors with late pericyte depletion, Angpt1 transcript levels were unchanged but Angpt2 transcript levels were elevated by 3-fold (Figure 3B) and ANG2 protein levels by 3-fold (Figure 3C). This significant deregulation in transcript and protein levels in early vs. late pericyte depletion was restricted to ANG1 and ANG2 (Figure 3A–B). These results indicate a switch in ANG1/ANG2 expression along with temporal targeting of PDGFRβ+ pericytes in tumors. In situ hybridization (ISH) supported the transcript data; indeed, we found no difference in Angpt2 signal in the early pericyte depletion setting (vs. controls), whereas there was a marked Angpt2 signal in the late pericyte depletion setting (Figure 3D). Angpt2 transcripts were detected primarily in foci co-localizing with collagen IV and CD31 immunolabeling, supporting a focal up-regulation of Angpt2 in endothelial cells (Figure 3E–F). While most blood vessels displayed high levels of Angpt2 (Figure 3E, red arrowheads), a few blood vessels lacked Angpt2 expression (Figure 3E, white arrowheads).
Figure 3. Angiopoietin-1 and Angiopoietin-2 expression is differentially modulated by pericyte depletion in a tumor stage-dependent manner.
A–B Transcript levels of Angpt1 (A) and Angpt2 (B) in 4T1 tumors from WT and PDGFRβ-TK mice with early and late pericyte depletion. Angpt1: WT early, n=6; PDGFRβ-TK early, n=6; WT late, n=5; PDGFRβ-TK late, n=7. Angpt2: WT early, n=6; PDGFRβ-TK early, n=4; WT late, n=5; PDGFRβ-TK late, n=7. The control group (WT) was arbitrarily set to 1, unpaired one-tailed t-test. C ANG2 protein levels in tumors from WT (n=4) and PDGFRβ-TK (n=4) mice with late pericyte depletion. D Angpt2 in situ hybridization on the frozen section of 4T1 tumors from WT and PDGFRβ-TK mice with either early or late pericyte depletion and quantification of Angpt2+ area/field of view. WT early, n=6; PDGFRβ-TK early, n=4; WT late, n=6; PDGFRβ-TK late, n=5. Scale bar: 50 µm. One-way ANOVA was used to determine statistical significance. E Angpt2 in situ hybridization followed by Collagen IV immunolabeling in tumors from PDGFRβ-TK mice with late pericyte depletion. Red arrowheads: Angpt2/Collagen IV double positive vessels. White arrowheads: Collagen IV positive vessels that lack Angpt2 expression. Scale bar: 50 µm. F. Angpt2 in situ hybridization followed by CD31 immunolabeling in tumors from PDGFRβ-TK mice (late pericyte depletion). Scale bar: 20 µm. G Transcript levels of Angpt1 and Angpt2 in retinas upon late pericyte depletion (P4-P7). Angpt1: WT, n=4; PDGFRβ-TK, n=7. Angpt2: WT, n=4; PDGFRβ-TK, n=7. The control group was arbitrarily set to 1, unpaired one-tailed t-test. Data are represented as the mean +/− SEM. Unless otherwise indicated, unpaired two-tailed t-test was used to determine statistical significance. * p<0.05, ** p<0.01. ns: not significant. See also Figure S3 and Table S1.
Differential expression of Angpt1 and Angpt2 associated with pericyte depletion was also analyzed in the retina angiogenesis model. Late depletion of retinal pericytes (P4-P7) showed unchanged Angpt1 transcript levels, whereas Angpt2 expression was increased (Figure 3G). Overall, these results indicate an inversed ANG1/ANG2 expression pattern in association with temporal targeting of PDGFRβ+ pericytes during both tumor growth and retinal angiogenesis.
Anti-ANG2 antibody treatment restores the integrity of pericyte-depleted leaky blood vessels and reduces metastasis
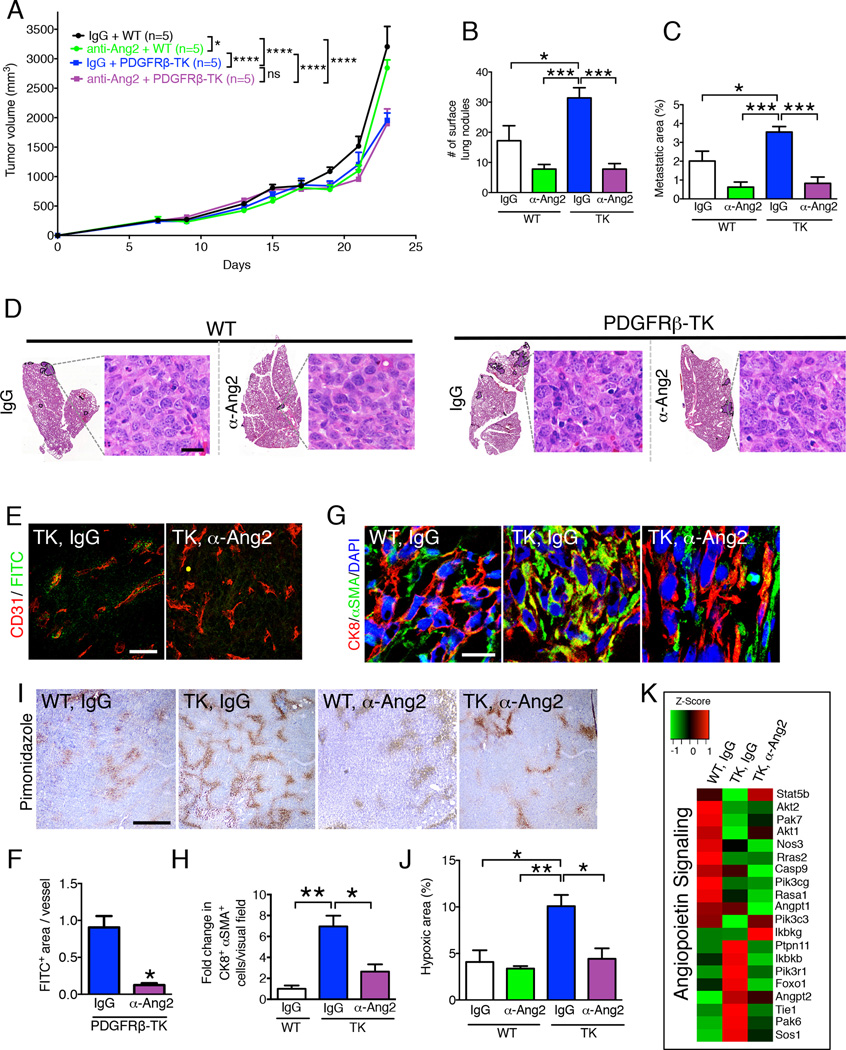
To determine whether the increase in lung metastasis observed in 4T1 mammary tumor-bearing PDGFRβ-TK mice was due to increased ANG2 expression in tumors with late pericyte depletion, we performed rescue experiments using a murinized anti-ANG2 neutralizing antibody (Srivastava et al., 2014). Control mice were treated with an isotype-matched IgG antibody. In WT mice without pericyte depletion, anti-ANG2 only moderately reduced tumor growth rate (Figure 4A). Pericyte depletion was initiated when tumor burden reached 500 mm3 of volume and this produced a significant reduction in tumor growth rate (Figure 4A). Although the anti-ANG2 antibody did not affect the tumor growth rate in pericyte-depleted tumors (Figure 4A), we observed a significant reduction in the frequency of lung metastasis (Figure 4B–D). Specifically, lung metastasis trended toward a decrease in WT mice treated with anti-ANG2, and the increased lung metastasis in PDGFRβ-TK mice was abated to levels observed in WT mice treated with the anti-ANG2 antibody (Figures 4B–D).
Figure 4. Anti-ANG2 antibody treatment in pericyte-depleted tumors restores vascular integrity and reduces lung metastasis.
A Tumor volume measurements over time in indicated experimental groups (IgG: control for anti-ANG2 antibody treatment). All mice were treated with ganciclovir (GCV) concurrently with the indicated treatment and treatment started when total tumor burden reached 500 mm3. Two-way ANOVA with Tukey’s multiple comparison test was used. B Quantification of the number of surface lung nodules. IgG + WT, n=5; anti-ANG2 + WT, n=5; IgG + PDGFRβ-TK, n=5; anti-ANG2 + PDGFRβ-TK, n=5. C Quantification of the percent lung metastatic area based on H&E staining of lung sections. IgG + WT, n=5; anti-ANG2 + WT, n=5; IgG + PDGFRβ-TK, n=5; anti-ANG2 + PDGFRβ-TK, n=5. D H&E staining of lungs from the indicated experimental groups. The metastatic nodules are encircled. High-magnification images of metastatic nodules are shown, scale bar: 20 µm. E–F Immunolabeling for CD31 and FITC for visualization of 2,000 kDa FITC-dextran in the indicated experimental groups and quantification of the FITC+ area per field of view. IgG + PDGFRβ-TK, n=3; anti-ANG2 + PDGFRβ-TK, n=3. Scale bar: 50 µm. Unpaired two-tailed t-test was used. G–H Representative images of tumors immunolabeled for CK8 and αSMA in the indicated experimental groups and quantification of the relative CK8+ αSMA+ cells per visual field. WT, n=3; PDGFRβ-TK, n=3; anti-ANG2+PDGFRβ-TK, n=3. Scale bar: 10µm. I–J Immunolabeling of hypoxyprobe (pimonidazole adduct formation) in the indicated experimental groups and quantitation of relative percent hypoxic area. IgG + WT, n=3; anti-ANG2 + WT, n=3; IgG + PDGFRβ-TK, n=4; anti-ANG2 + PDGFRβ-TK, n=3. Scale bar: 500 µm. K Heat map of the differentially regulated genes in the angiopoietin signaling pathways in the tumors of indicated experimental groups. Data are represented as the mean +/− SEM. Unless otherwise noted, one-way ANOVA with Tukey post-hoc analysis was used to determine statistical significance. * p<0.05, ** p<0.01, *** p< 0.001****, p < 0.0001. See also Figure S4.
Next, we assayed the impact of anti-ANG2 treatment on vascular leakage and intratumoral hypoxia. We found that anti-ANG2 treatment in the context of PDGFRβ+ pericyte depletion restored the structural stability of the blood vessels, as determined by reduced vascular leakage (Figure 4E–F), restored vessel diameter (Figure S4A–B), and diminished EMT program (Figure 4G–H, S4D–E) and intratumoral hypoxia (Figure 4I–J). Tumor lymphangiogenesis (mostly peri-tumoral in this model) assayed by LYVE-1 staining was unchanged in all experimental groups (Figure S4F). Pericyte depletion, with and without anti-ANG2 treatment, did not impact vascular TIE2 expression (Figure S4G), nor tumor infiltration by TIE2-expressing macrophages (Figure S4H), suggesting that ANG2 upregulation in pericyte-depleted tumors acts in an autocrine manner to destabilize endothelial cells, and that the effect of anti-ANG2 treatment is mediated by suppression of the imbalanced ANG2/TIE2 signaling without affecting TIE2 expression in endothelial cells or macrophages. Finally, global gene expression profiling of the WT, PDGFRβ-TK and PDGFRβ-TK tumors treated with the anti-ANG2 antibody revealed impaired angiopoietin signaling in pericyte-depleted tumors, and a gene expression pattern partially reverted to that of WT control mice after treatment with the anti-ANG2 antibody (Figure 4K).
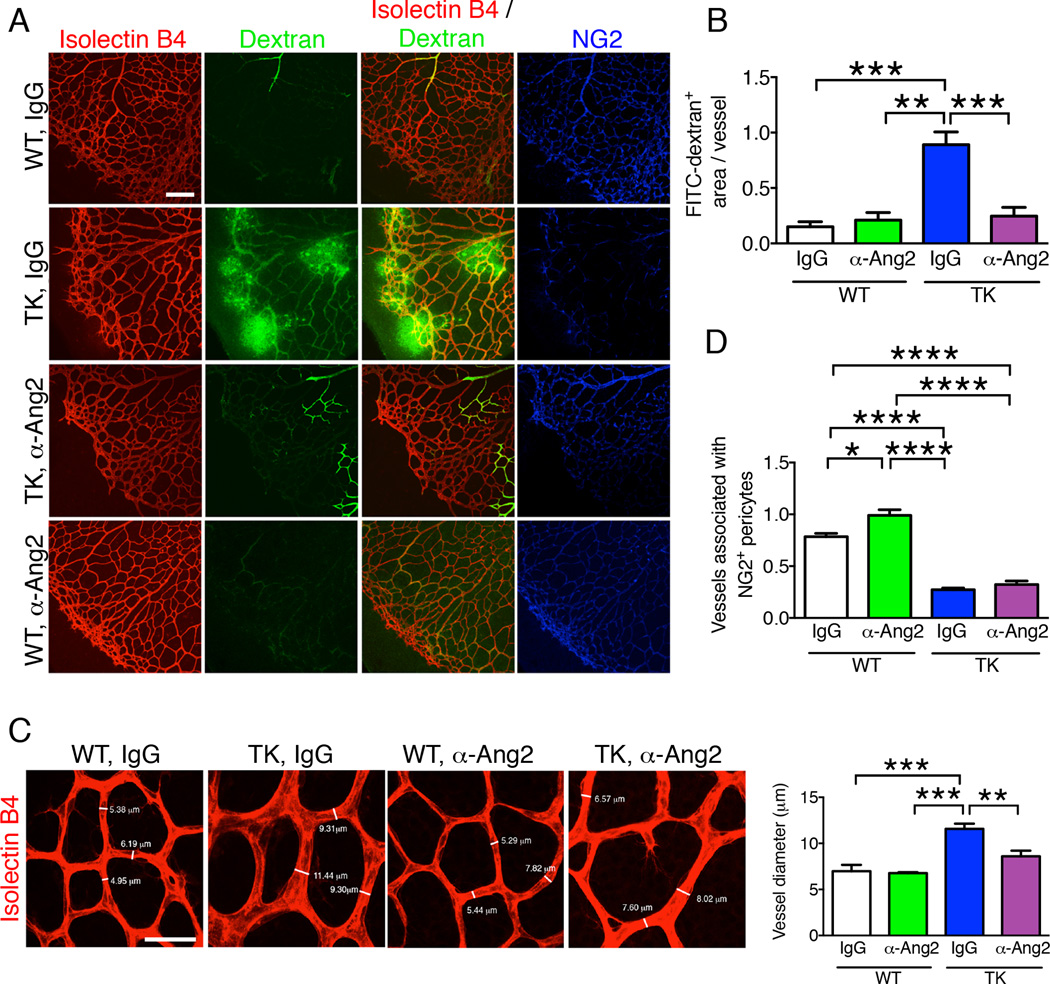
We then analyzed ANG2 signaling induced by late PDGFRβ+ cell depletion during retinal angiogenesis. Similar to the findings in tumors, anti-ANG2 rescued the vascular phenotype observed in the retina of PDGFRβ-TK pups. The enhanced vascular leakage at the central zone of the vascular plexus and at the sprouting edge of the primary plexus was observed specifically in PDGFRβ-TK mice in the late stage pericyte depletion setting; anti-ANG2 antibody treatment re-established the retinal vessel structural stability in PDGFRβ-TK mice (Figure 5A–B). Enhanced vessel diameter in PDGFRβ-TK mice (Figure S3D) was also restored by anti-ANG2 (Figure 5C). Anti-ANG2 treatment did not modify NG2+ pericyte coverage in retinal blood vessels (Figure 5A, 5D). Taken together, these results indicate that PDGFRβ+ pericyte depletion promotes an ANG2-mediated increase in vascular destabilization and permeability, both in tumors and the developing retinal vasculature.
Figure 5. Anti-ANG2 antibody treatment reduces vascular leakage of retinas with late pericyte depletion.
A Immunolabeling for NG2, isolectin B4 staining, and visualization of 2,000 kDa FITC-dextran of retinas from WT and PDGFRβ-TK pups treated with either control IgG or anti-ANG2 antibody from P4 to P9 (late pericyte depletion). All mice were treated with GCV. Scale bar: 200 µm. B Quantification of FITC-dextran+ area per vessel. IgG + WT, n=3; anti-ANG2 + WT, n=3; IgG + PDGFRβ-TK, n=3; anti-ANG2 + PDGFRβ-TK, n=5. C Isolectin B4 staining of the retina in the indicated experimental groups and quantification of the mean vessel diameter. IgG + WT, n=3; anti-ANG2 + WT, n=4; IgG + PDGFRβ-TK, n=5; anti-ANG2 + PDGFRβ-TK, n=5. Representative vessel diameters are listed. Scale bar: 40 µm. D Quantification of Isolectin B4+ vessels associated with NG2+ pericytes coverage. IgG + WT, n=3; anti-ANG2 + WT, n=4; IgG + PDGFRβ-TK, n=3; anti-ANG2 + PDGFRβ-TK, n=5. Data are represented as the mean +/− SEM. One-way ANOVA with Tukey post-hoc analysis was used to determine statistical significance. * p<0.05, ** p<0.01, *** p< 0.001, ****p < 0.0001.
Treatment of established breast tumors with imatinib and anti-ANG2 antibody restores blood vessel structural stability and limits metastasis
We previously reported that imatinib significantly reduced pericyte coverage in 4T1 tumors (Cooke et al., 2012). In larger tumors (tumor burden ~500 mm3), imatinib treatment mirrored the phenotype observed in PDGFRβ-TK and NG2-TK mice, which resulted in increased vascular leakage and intratumoral hypoxia associated with lung-enhanced metastasis (Cooke et al., 2012). To determine whether imatinib and ANG2 neutralization may synergistically decrease primary tumor burden and lung metastasis, we treated 4T1 tumor-bearing mice with a single agent (imatinib) or a combination therapy (imatinib and anti-ANG2). Control mice were treated with saline (PBS) and control IgG.
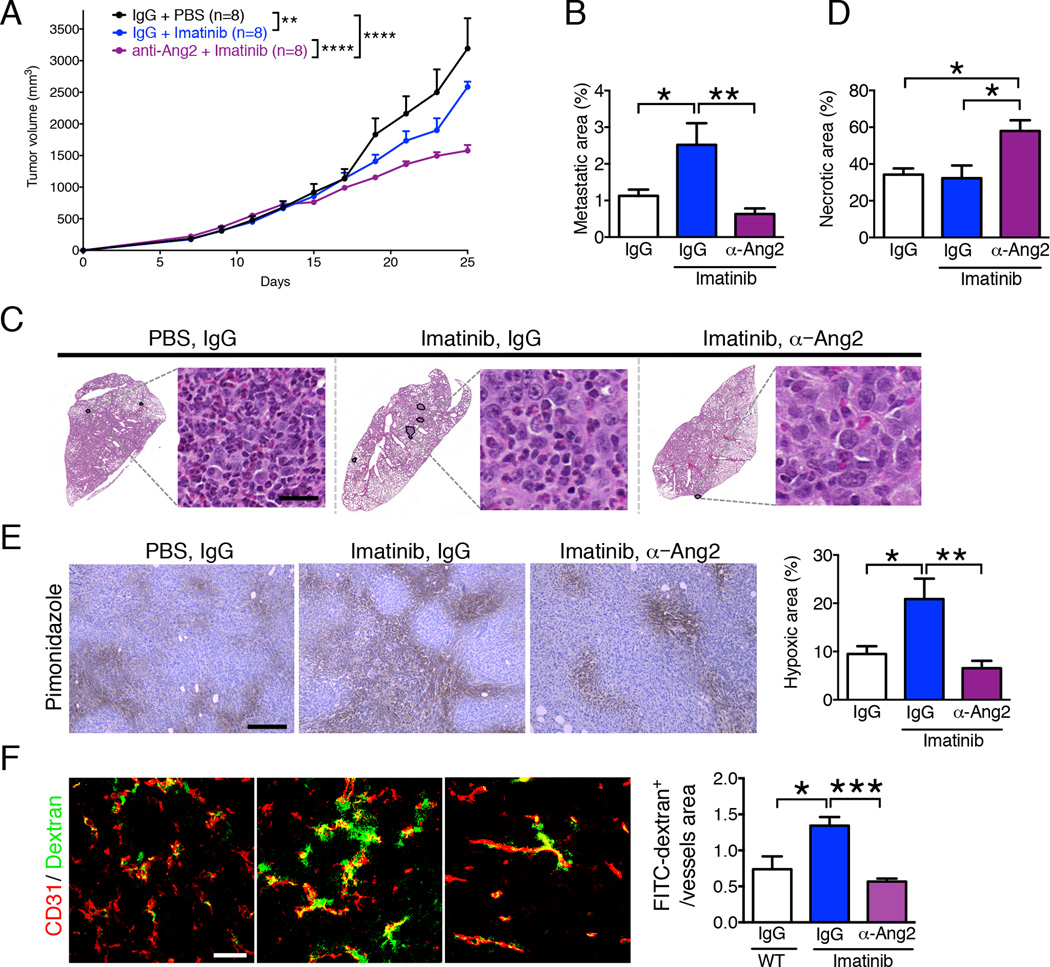
Single therapy with imatinib reduced primary tumor growth compared to control mice (Figure 6A), as previously reported (Cooke et al., 2012). Combination therapy with imatinib and anti-ANG2 antibody further increased inhibition of primary tumor growth when compared to imatinib monotherapy (Figure 6A). Imatinib treatment in established 4T1 tumors resulted in an increase in lung metastasis (Figure 6B–C; Cooke et al., 2012), however, the combination therapy resulted in a significant reduction of lung metastasis when compared to imatinib only (Figure 6B–C). Tumor necrosis was increased by anti-ANG2 (Figure 6D), as previously reported (Thomas et al., 2013), and may contribute to the stalled tumor growth (Figure 6A).
Figure 6. Imatinib and Anti-ANG2 antibody treatments synergistically control tumor growth and metastasis.
A Tumor volume measurements over time in indicated experimental groups (IgG: control for anti-ANG2 treatment, PBS: control for Imatinib). Treatment started when total tumor burden reached 500 mm3. Two-way ANOVA with Bonferroni’s multiple comparison test was used. B Quantification of the percent lung metastatic area. IgG + PBS, n=8; IgG + Imatinib, n=8; anti-ANG2 + Imatinib, n=8. C H&E staining of lungs from the indicated experimental groups. The metastatic nodules are encircled. High-magnification images of metastatic nodules are shown, scale bar: 20 µm. D Quantification of the percent tumor necrotic area. IgG + PBS, n=5; IgG + Imatinib, n=5; anti-ANG2 + Imatinib, n=5. E Immunolabeling of hypoxyprobe (pimonidazole adduct formation) in the indicated experimental groups and quantitation of percent hypoxic area. IgG + PBS, n=5; IgG + Imatinib, n=5; anti-ANG2 + Imatinib, n=5. Scale bar: 500 µm. F Immunolabeling for CD31 and visualization of 2,000 kDa FITC-dextran in the indicated experimental groups and quantification of the FITC-dextran+ area per vessel. IgG + PBS, n=3; IgG + Imatinib, n=5; anti-ANG2 + Imatinib, n=5. Scale bar: 50 µm. Data are represented as the mean +/− SEM. Unless otherwise noted, one-way ANOVA with Tukey post-hoc analysis was used to determine statistical significance. * p<0.05, ** p<0.01, *** p< 0.001. See also Figure S6.
Histological findings in the heart, bowel, kidney, and pancreas were unremarkable in control, imatinib alone, and imatinib/anti-ANG2 combination therapy (Figure S5A). A liver metastasis was noted in one of the mice treated with imatinib (in agreement with enhanced metastatic disease after imatinib monotherapy), and enlarged spleen with follicular expansion characteristic of tumor bearing mice was observed in all mice (Figure S5A).
Intratumoral hypoxia and vascular leakage were enhanced in tumors treated with imatinib (Figure 6E–F; Cooke et al., 2012). Concomitant anti-ANG2 and imatinib treatment reduced intratumoral hypoxia (Figure 6E), vascular leakage (Figure 6F), and vessel diameter (Figure S4A, S4C). Additional targets of imatinib, like c-Kit and Abl1, were unchanged in imatinib-treated mice (Figure S5B; (Cooke et al., 2012). Collectively, these results indicate that the anti-metastatic effects of ANG2 blockade may be due, at least in part, to improved vascular stabilization and decreased tumor hypoxia.
Normalization of angiopoietin signaling in PDGFRβ+ pericyte-depleted tumors promotes desmin+ pericyte coverage and increases endothelial cell-cell tight junctions
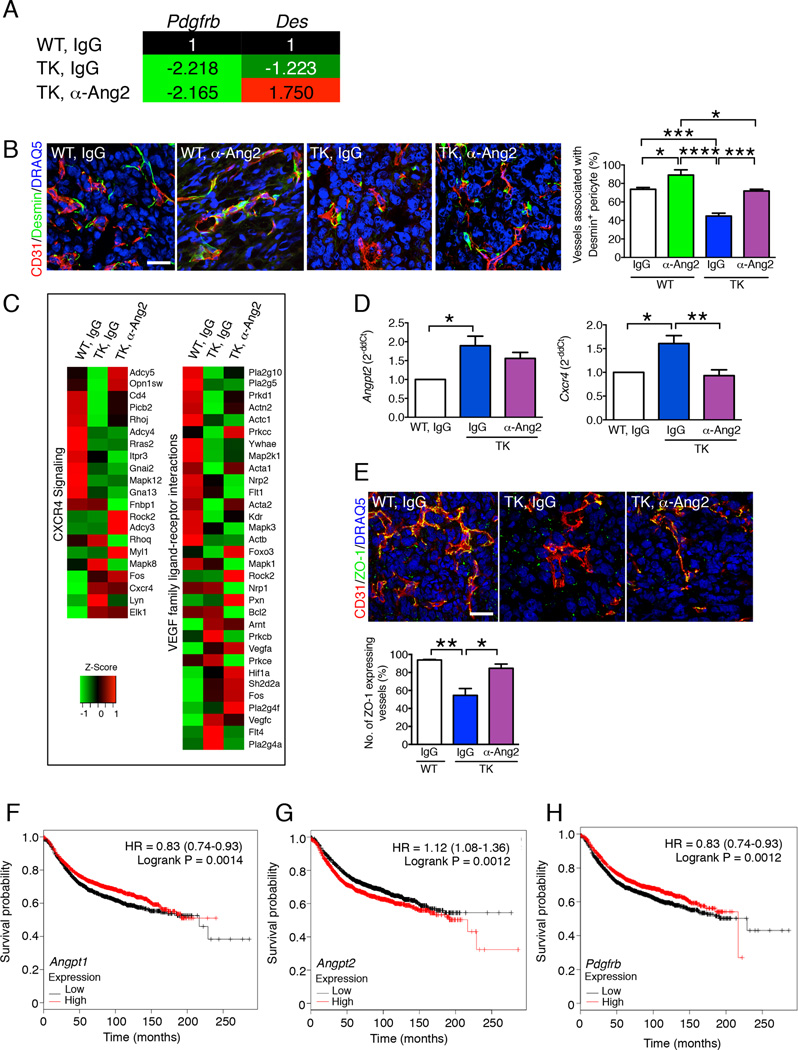
To determine the mechanistic underpinning of ANG2 neutralization in decreasing destabilized blood vessels in the context of genetic (PDGFRβ-TK mice, see Figure 4–5) or pharmacologic (imatinib, see Figure 6) targeting of PDGFRβ+ pericytes, we evaluated the differential expression of the mature pericyte marker desmin (Des). Gene expression profiling showed a sustained decrease in Pdgfrb transcript levels in tumors with PDGFRβ-TK mice despite anti-ANG2 antibody treatment (Figure 7A). ANG2 blockade limited the decrease in Des transcript levels observed in tumors of PDGFRβ-TK compared to control mice (Figure 7A). Desmin immunolabeling in proximity to CD31 vascular labeling indicated a significant increase in desmin+ mature pericyte coverage of tumor vessels in PDGFRβ+ pericyte-depleted tumors that were treated with the anti-ANG2 antibody (Figure 7B). ANG2 blockade also promoted desmin+ pericyte coverage of tumor vessels in WT mice without PDGFRβ+ pericyte depletion (Figure 7B). These results show that anti-ANG2 treatment up-regulated the expression of desmin and increased vascular coverage by mature pericytes, in spite of the loss of Pdgfrb expression caused by genetic targeting of PDGFRβ+ pericytes.
Figure 7. Neutralization of ANG2 in pericyte depleted tumors re-establish mature pericyte vessel coverage and endothelial cell-cell tight junction in association with restored CXCR4 and VEGF signaling pathways.
A Color-coded table listing the fold change in transcript levels of Pdgfrb and Des (desmin) based on global gene expression profiling of tumors from mice in the indicated experimental groups. B Representative images of tumors immunolabeled for CD31 and desmin in the indicated experimental groups and quantification of the percent CD31+ vessels associated with desmin+ pericytes. IgG + WT, n=3; anti-ANG2 + WT, n=3; IgG + PDGFRβ-TK, n=4; anti-ANG2 + PDGFRβ-TK, n=4. Scale bar: 25 µm. C Heat maps reflecting the differentially regulated genes in tumors of indicated experimental groups in each of the indicated pathways. D Quantitative RT-PCR for Angpt2 and Cxcr4 in tumors from the indicated experimental groups. Angpt2: IgG + WT, n=4; IgG + PDGFRβ-TK, n=5; anti-ANG2 + PDGFRβ-TK, n=5. Cxcr4: IgG + WT, n=4; IgG + PDGFRβ-TK, n=4; anti-ANG2 + PDGFRβ-TK, n=4. E Representative images of tumors immunolabeled for CD31 and ZO-1 in the indicated experimental groups and quantification of the percent of the number of ZO-1+ vessels. IgG + WT, n=3; IgG + PDGFRβ-TK, n=3; anti-ANG2 + PDGFRβ-TK, n=3. Scale bar: 25 µm. F–H Kaplan-Meier Plots depicting the recurrence free survival probability of patients with breast cancer (n= 3455) based on Angpt1 (F), Angpt2 (G), and Pdgfrb (H) tumor expression. HR: hazard ratio. LogRank test was used. Data are represented as the mean +/− SEM. Unless other wise noted, one-way ANOVA with Tukey post-hoc analysis was used to determine statistical significance. * p<0.05, ** p<0.01, *** p< 0.001, ****p < 0.0001.
Global gene expression signaling revealed partially restored VEGF family-ligand-receptor interactions and CXCR4 signaling pathways after anti-ANG2 treatment (Figure 7C). Transcriptome analyses showed that expression of Cxcr4 was increased in tumors from PDGFRβ-TK compared to WT control mice (both treated with control IgG); these results were validated by quantitative RT-PCR (Figure 7D). Cxcr4 expression in tumors from PDGFRβ-TK mice treated with anti-ANG2 antibody was restored to levels observed in WT tumors (Figure 7D). These results suggest a rescued vessel permeability defect following anti-ANG2 antibody treatment. Angpt2 transcript levels were elevated in PDGFRβ-TK tumors, corroborating our earlier observation (Figure 3A–C). Anti-ANG2 treatment did not suppress the transcription of Angpt2, consistent with previous findings (Mazzieri et al., 2011; Rigamonti et al., 2014), indicating that the therapeutic effect of antibody-mediated ANG2 blockade is mediated through downstream signaling rather than via transcriptional feedback down-regulation of Angpt2 (Figure 7D). These transcriptomic analyses support the notion that increased Angpt2 expression in tumors with late pericyte depletion may compromise vessel integrity via deregulation of CXCR4/CXCL12 signaling. Several studies have shown that CXCR4/CXCL12 plays a role in vascular permeability (Suri et al., 1998), endothelial cell patterning and morphology (Strasser et al., 2010), and transendothelial migration and metastasis of breast cancer cells (Lee et al., 2004; Mukherjee and Zhao, 2013; Muller et al., 2001). We also observed restored ZO-1 expression (a marker for endothelial tight junction) in endothelial cells of tumors from PDGFRβ-TK mice treated with the anti-ANG2 antibody, similar to levels of control tumors (WT mice treated with IgG control) (Figure 7E).
ANGPT2 and PDGFRB expression correlate with poor outcome in patients with breast cancer
We queried the Kaplan-Meier Plotter dataset (Gyorffy et al., 2012) and evaluated the survival probability of patients with breast cancer based on their tumor transcriptomes (Figure 7F–H). The median transcript level of ANGPT1, ANGPT2 and PDGFRB was used to stratify 3455 patient tumor transcriptomes into high and low expression for each gene. High ANGPT1 expression was associated with improved outcome (Figure 7F). In contrast, a significant association of high ANGPT2 expression and poor recurrence free survival was found (Figure 7G). In addition, low PDGFRB expression was associated with poor recurrence free survival (Figure 7H). These analyses are in agreement with results obtained in our mouse studies: whereas high ANGPT1 was associated with suppressed metastasis, high ANGPT2 was associated with enhanced metastasis in mice (Figure 1E, 3B–D). These results are also consistent with the higher metastatic tumor burden noted in PDGFRβ-TK mice (low PDGFRB), in which high ANGPT2 expression was noted (Figure 3B–D).
Discussion
Anti-angiogenic cancer therapies show efficacy but did not realize the full potential that preclinical studies had suggested, highlighting an unforeseen complexity of tumor neo-angiogenesis (Bisacchi et al., 2003; Folkman, 1972). Newly formed blood vessels, or lack thereof, affect the metabolic status of the primary tumor and may influence cancer cells’ metastatic potential in addition to providing routes to metastasis (Cooke et al., 2012; Folkman, 2002; Zetter, 1998). To shed light on the complex and dynamic nature of pericyte-endothelial cell interactions in various phases of tumor angiogenesis, we employed genetic targeting of pericytes in non-hypoxic (early stage) or hypoxic (advanced stage) tumors.
Our data indicated that anti-vascular therapy via depletion of PDGFRβ+ pericytes at early stages of tumor progression reduced metastasis, whereas targeting these cells at later stages enhanced metastasis. The stage-specific effects are a result of differential, dynamic PDGFRβ+ pericyte interactions with endothelial cells during the evolving angiogenic process. Early PDGFRβ+ pericyte depletion severely suppressed tumor angiogenesis, blunted primary tumor growth, and reduced intratumoral hypoxia. The desmin+ pericyte coverage in the small, PDGFRβ+ pericyte-depleted tumors suggests that impeded tumor angiogenesis at this early stage of tumor growth was associated with the development of a vascular network that adequately matched the tumor’s energetic needs with its growth rate.
In contrast with the early pericyte depletion experiments, the late depletion of pericytes in established primary tumors resulted in a moderate decrease of tumor growth but was associated with a significant increase of lung metastasis. The late pericyte-depleted tumors displayed an overall reduction of tumor blood vessels, with the remaining blood vessels characterized by hyperpermeability and poor desmin+ pericyte coverage, both likely contributing to the increased intratumoral hypoxia and metastatic dissemination. The stage-specific metastasis phenotypes may also underscore the relative contribution of specific pericyte subtypes to tumor angiogenesis. Our studies on early tumor development indeed showed a differential timing for PDGFRβ+ and NG2+ pericytes accumulation, with increasing overlap of expression, suggesting that PDGFRβ+ cells acquire NG2 expression. These findings may explain, at least in part, the differences observed in early ablation of PDGFRβ+ vs. NG2+ cells in 4T1 tumors, whereby early ablation of NG2+ cells has minimal effects on tumor growth and metastasis.
Our transcriptomic studies identified angiopoietin signaling as uniquely deregulated with respect to early vs. late pericyte targeting, and we further implicated ANG2 expression and signaling as a key mediator of the metastatic phenotype associated with tumor stage-specific PDGFRβ+ pericyte depletion. Using genetic depletion of PDGFRβ+ pericytes or pharmacological targeting of PDGFRβ signaling by imatinib, we showed that the leaky vascular phenotype in these tumors was associated with elevated ANG2 expression. Vessels with increased ANG2 stimulation in the Rip1Tag2 transgenic mouse model were previously characterized by reduced pericyte coverage and were found dilated, destabilized, and highly permeable (Fagiani et al., 2011), all of which are consistent with our observations. Furthermore, our analyses also highlighted a deregulation of CXCR4/CXCL12 signaling in association with late PDGFRβ+ pericytes depletion, also in concordance with its known implication in vessel permeability (Suri et al., 1998) and breast cancer cell transendothelial migration and metastasis (Lee et al., 2004; Mukherjee and Zhao, 2013; Muller et al., 2001). Our previous studies have shown that pericyte targeting in established tumors leads to increased EMT associated with increased c-MET expression in hypoxic cancer cells (Cooke et al., 2012). The current study suggests that such increase in c-MET expression in cancer cells could be facilitated by enhanced hypoxia resulting from ANG2-mediated vascular defects.
Our results indicate ANG2-mediated destabilization of sprouting blood vessels in the hypoxic environment of the late pericyte depletion setting, and high levels of Angpt2 in this setting may be restricted to endothelial cells in the more hypoxic regions of the tumor. ANG2 has been associated with metastasis in melanoma patients; indeed, increased circulating ANG2 levels were associated with tumor progression and metastasis (Helfrich et al., 2009). The association of ANG1 with metastasis has been uncertain in clinical contexts (Szarvas et al., 2008). Our results indicate that ANG1 expression is associated with decreased metastasis and increased desmin+ pericyte coverage, and high ANGPT1 is associated with increased survival probability in surveyed breast cancer transcriptomes.
While overexpression of ANG2 increases metastasis through enhanced vascular permeability (Holopainen et al., 2012), blocking of ANG2 signaling enhances pericyte coverage of blood vessels (Rigamonti et al., 2014) and strongly inhibits metastasis (Mazzieri et al., 2011), possibly by stabilizing the tumor-associated vasculature via enhanced endothelial cell-cell junctions (Holopainen et al., 2012) and by displacing macrophages that assist cancer cell intravasation from the tumor blood vessels (Mazzieri et al., 2011). Of note, anti-ANG2 suppresses both spontaneous metastasis of primary mammary tumors and the metastatic colonization of the lung after cancer cell seeding (Mazzieri et al., 2011). In this regards, anti-ANG2 antibody treatment was recently shown to suppress metastatic growth also in the adjuvant (post-surgical) setting in mice, an effect mediated via suppression of the endothelial cell proinflammatory phenotype at the metastatic site (Srivastava et al., 2014). While such anti-metastatic mechanism could also be at play in our studies, we also show that ANG2 blockade concomitant to PDGFRβ+ pericyte depletion during the late phases of tumor progression provides benefit by reducing both primary tumor growth and metastatic disease. Anti-ANG2 treatment rescued the vascular defects induced by pericyte targeting, and Imatinib and anti-ANG2 combination therapy suppressed primary tumor growth and controlled lung metastasis. The vascular defects associated with pericyte targeting in retinal angiogenesis were also restored upon anti-ANG2 therapy. ANG2 signaling inhibition enhanced endothelial cell-cell tight junctions, and these results altogether suggest that ANG2 signaling is central to the loss of vascular stability associated with PDGFRβ+ pericyte targeting in both tumor and developmental angiogenesis. Finally, the analysis of the tumor transcriptomes of patients with breast cancer revealed a robust correlation between recurrence-free survival and expression of ANGPT2 and PDGFRB.
Our results indicate that the enhanced cancer cell dissemination induced by targeting PDGFRβ+ pericytes in advanced stage tumors could be controlled by the administration of an anti-ANG2 antibody. Increased intratumoral hypoxia in late-stage tumors enhances ANG2 production by endothelial cells (Holash et al., 1999; Saharinen et al., 2011), further destabilizing the tumor vasculature and fueling a vicious cycle set forth by PDGFRβ+ pericyte targeting. While the anti-metastatic effects of the anti-ANG2 antibody may also be due to impaired angiogenesis and cancer cell growth at the metastatic site, our results indicate that its effects are also targeted to the primary tumors and their metastatic potential. Our findings further imply that vascular normalization and consequently improved vascular perfusion (Augustin et al., 2009; Carmeliet and Jain, 2011a; Carmeliet and Jain, 2011b; Jain, 2005; Li et al., 2007; Skuli et al., 2009) may be achieved by PGDFRβ+ pericyte targeting concomitant with anti-ANG2 antibody therapy. This strategy, if combined with chemotherapy, may potently suppress tumor growth by enhancing chemotherapeutic delivery while preserving the structural stability of the tumor blood vessels, thus minimizing hypoxia-induced metastatic dissemination.
Material and Methods
Cells and mice
4T1 Balb/c mammary tumor epithelial cells were obtained from ATCC and grown in DMEM media supplemented with 10% fetal bovine serum (FBS). PDGFRβ-TK and NG2-TK Balb/c mice were previously described (Cooke et al., 2012). Female mice, between the ages of 6 and 12 weeks, were used for orthotopic implantation of 4T1 mammary epithelial cancer cells, as described previously (Cooke et al., 2012). Mice received daily intraperitoneal (i.p.) GCV injections at a 50 mg/kg body weight (BW) dose at the indicated time points and, when indicated, also received i.p. injections of a neutralizing anti-ANG2 antibody (murinized LC06, Roche Diagnostics) (Srivastava et al., 2014) or control isotype-matched IgG antibody once a week (10 mg/kg BW in 0.2 ml PBS), or imatinib or PBS by oral gavage daily (50 mg/kg BW in 0.1 ml). All mouse experiments were reviewed and approved by the Institute of Animal Care and Use Committee at the Beth Israel Deaconess Medical Center (BIDMC), Boston, MA and the MD Anderson Cancer Center (MDACC), Houston, TX. Additional experimental details are listed in the Supplemental Experimental Procedures.
Tumor vessel leakage and metastatic burden
Mice were injected in the retro-orbital venous plexus with 100 µl of 10 mg/ml FITC-dextran (2,000,000 MW, Sigma) 5 minutes prior euthanasia. Tumor sections from O.C.T. embedded tumors were immunostained for CD31. FITC-dextran was visualized either directly by fluorescent microscopy under the green fluorescent filter or by immunostaining with anti-FITC antibody and a FITC-conjugated secondary antibody. FITC-dextran is quantified by ImageJ area fraction analysis. Multiple sections/tumor were analyzed at original magnification x40 with n ≥ 3 tumors/group. Histological assessment and quantitation of the metastatic and tumor necrosis areas are described in the Supplemental Experimental Procedures.
Microarray analysis and quantitative PCR analyses
Microarray analysis was performed using Mouse Ref8 or Ref 6 Gene Expression BeadChip (Illumina), and data were deposited in Gene Expression Omnibus (accession number: GSE55785). Details for microarray samples, quantitative RT PCR analyses and primer sequence information are listed in the Supplemental Experimental Procedures section and Table S1.
Statistical analysis
For comparison between two groups with one grouping variable, the unpaired two-tailed Student’s t-test was used. For RT-PCR analyses, the unpaired one-tailed Student’s t-test was used to compare dCt between experimental groups. To compare multiple groups, analysis of variance (ANOVA) was used with Prism™ software. For multiple groups with one grouping variable, one-way ANOVA with multiple comparisons with Tukey correction was performed and for multiple groups with two grouping variables, two-way ANOVA with Bonferroni’s multiple comparison test was used. P < 0.05 was considered statistically significant. All data are represented as the mean with error bars corresponding to Standard Error Mean (S.E.M.).
Supplementary Material
Acknowledgements
We wish to thank Markus Thomas and Joachim Muller (Roche, Penzberg, Germany) for providing the anti-ANG2 antibody, and Lauren Bizarro, Cristina Espinosa da Silva, and Laura Gibson for genotyping and animal husbandry support. We thank Erica Lawson, Komal Vadnagara, Donna Lundy, Sergio Dias and Oleg Tsinkalovsky for technical support. We also wish to thank Dr. Balázs Györffy for this help in mining the KM plotter datasets. This study was primarily supported by the NIH grant CA155370 (R.K.). R.K. is also supported by the Cancer Prevention and Research Institute of Texas and the Metastasis Research Center at MD Anderson Cancer Center, NIH grants CA125550, CA155370, CA151925 and CA163191. V.S.L. is supported by the NIH/NCI CCSG New Faculty Award P30CA016672 and UT MDACC Khalifa Bin Zayed Al Nahya Foundation. C.G. is supported by NIH RO1 NS064583.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
DK, JK, VG, C-CW, HS, and VSL performed experiments and analyzed data. MDP provided reagents and intellectual input, and edited the manuscript. CG provided reagents and intellectual input. DK and JK participated in the writing the manuscript and preparation of figures. RK and VSL wrote the manuscript, prepared figures and oversaw the study design, execution and analysis of the experiments.
References
- Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97:512–523. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Augustin HG, Koh GY, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol. 2009;10:165–177. doi: 10.1038/nrm2639. [DOI] [PubMed] [Google Scholar]
- Benjamin LE, Hemo I, Keshet E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development. 1998;125:1591–1598. doi: 10.1242/dev.125.9.1591. [DOI] [PubMed] [Google Scholar]
- Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol. 2005;7:452–464. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergers G, Song S, Meyer-Morse N, Bergsland E, Hanahan D. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J Clin Invest. 2003;111:1287–1295. doi: 10.1172/JCI17929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisacchi D, Benelli R, Vanzetto C, Ferrari N, Tosetti F, Albini A. Anti-angiogenesis and angioprevention: mechanisms, problems and perspectives. Cancer Detect Prev. 2003;27:229–238. doi: 10.1016/s0361-090x(03)00030-8. [DOI] [PubMed] [Google Scholar]
- Buchanan CF, Szot CS, Wilson TD, Akman S, Metheny-Barlow LJ, Robertson JL, Freeman JW, Rylander MN. Cross-talk between endothelial and breast cancer cells regulates reciprocal expression of angiogenic factors in vitro. J Cell Biochem. 2012;113:1142–1151. doi: 10.1002/jcb.23447. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011a;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov. 2011b;10:417–427. doi: 10.1038/nrd3455. [DOI] [PubMed] [Google Scholar]
- Chan-Ling T, Page MP, Gardiner T, Baxter L, Rosinova E, Hughes S. Desmin ensheathment ratio as an indicator of vessel stability: evidence in normal development and in retinopathy of prematurity. Am J Pathol. 2004;165:1301–1313. doi: 10.1016/S0002-9440(10)63389-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke VG, LeBleu VS, Keskin D, Khan Z, O’Connell JT, Teng Y, Duncan MB, Xie L, Maeda G, Vong S, et al. Pericyte depletion results in hypoxia-associated epithelial-to-mesenchymal transition and metastasis mediated by met signaling pathway. Cancer cell. 2012;21:66–81. doi: 10.1016/j.ccr.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer cell. 2009;15:232–239. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagiani E, Lorentz P, Kopfstein L, Christofori G. Angiopoietin-1 and −2 exert antagonistic functions in tumor angiogenesis, yet both induce lymphangiogenesis. Cancer research. 2011;71:5717–5727. doi: 10.1158/0008-5472.CAN-10-4635. [DOI] [PubMed] [Google Scholar]
- Folkman J. Anti-angiogenesis: new concept for therapy of solid tumors. Ann Surg. 1972;175:409–416. doi: 10.1097/00000658-197203000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29:15–18. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- Fuxe J, Tabruyn S, Colton K, Zaid H, Adams A, Baluk P, Lashnits E, Morisada T, Le T, O’Brien S, et al. Pericyte requirement for anti-leak action of angiopoietin-1 and vascular remodeling in sustained inflammation. Am J Pathol. 2011;178:2897–2909. doi: 10.1016/j.ajpath.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaengel K, Genove G, Armulik A, Betsholtz C. Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler Thromb Vasc Biol. 2009;29:630–638. doi: 10.1161/ATVBAHA.107.161521. [DOI] [PubMed] [Google Scholar]
- Greenberg JI, Shields DJ, Barillas SG, Acevedo LM, Murphy E, Huang J, Scheppke L, Stockmann C, Johnson RS, Angle N, Cheresh DA. A role for VEGF as a negative regulator of pericyte function and vessel maturation. Nature. 2008;456:809–813. doi: 10.1038/nature07424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyorffy B, Lanczky A, Szallasi Z. Implementing an online tool for genome-wide validation of survival-associated biomarkers in ovarian-cancer using microarray data from 1287 patients. Endocrine-related cancer. 2012;19:197–208. doi: 10.1530/ERC-11-0329. [DOI] [PubMed] [Google Scholar]
- Hammes HP, Lin J, Renner O, Shani M, Lundqvist A, Betsholtz C, Brownlee M, Deutsch U. Pericytes and the pathogenesis of diabetic retinopathy. Diabetes. 2002;51:3107–3112. doi: 10.2337/diabetes.51.10.3107. [DOI] [PubMed] [Google Scholar]
- Helfrich I, Edler L, Sucker A, Thomas M, Christian S, Schadendorf D, Augustin HG. Angiopoietin-2 levels are associated with disease progression in metastatic malignant melanoma. Clin Cancer Res. 2009;15:1384–1392. doi: 10.1158/1078-0432.CCR-08-1615. [DOI] [PubMed] [Google Scholar]
- Hirschi KK, D’Amore PA. Pericytes in the microvasculature. Cardiovasc Res. 1996;32:687–698. [PubMed] [Google Scholar]
- Holash J, Wiegand SJ, Yancopoulos GD. New model of tumor angiogenesis: dynamic balance between vessel regression and growth mediated by angiopoietins and VEGF. Oncogene. 1999;18:5356–5362. doi: 10.1038/sj.onc.1203035. [DOI] [PubMed] [Google Scholar]
- Holopainen T, Saharinen P, D’Amico G, Lampinen A, Eklund L, Sormunen R, Anisimov A, Zarkada G, Lohela M, Helotera H, et al. Effects of angiopoietin-2-blocking antibody on endothelial cell-cell junctions and lung metastasis. J Natl Cancer Inst. 2012;104:461–475. doi: 10.1093/jnci/djs009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- Kelly BD, Hackett SF, Hirota K, Oshima Y, Cai Z, Berg-Dixon S, Rowan A, Yan Z, Campochiaro PA, Semenza GL. Cell type-specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia-inducible factor 1. Circ Res. 2003;93:1074–1081. doi: 10.1161/01.RES.0000102937.50486.1B. [DOI] [PubMed] [Google Scholar]
- Kirsch M, Schackert G, Black PM. Angiogenesis, metastasis, and endogenous inhibition. J Neurooncol. 2000;50:173–180. doi: 10.1023/a:1006453428013. [DOI] [PubMed] [Google Scholar]
- Kurz H, Fehr J, Nitschke R, Burkhardt H. Pericytes in the mature chorioallantoic membrane capillary plexus contain desmin and alpha-smooth muscle actin: relevance for non-sprouting angiogenesis. Histochem Cell Biol. 2008;130:1027–1040. doi: 10.1007/s00418-008-0478-8. [DOI] [PubMed] [Google Scholar]
- Lee BC, Lee TH, Avraham S, Avraham HK. Involvement of the chemokine receptor CXCR4 and its ligand stromal cell-derived factor 1alpha in breast cancer cell migration through human brain microvascular endothelial cells. Molecular cancer research : MCR. 2004;2:327–338. [PubMed] [Google Scholar]
- Li JL, Sainson RC, Shi W, Leek R, Harrington LS, Preusser M, Biswas S, Turley H, Heikamp E, Hainfellner JA, Harris AL. Delta-like 4 Notch ligand regulates tumor angiogenesis, improves tumor vascular function, and promotes tumor growth in vivo. Cancer research. 2007;67:11244–11253. doi: 10.1158/0008-5472.CAN-07-0969. [DOI] [PubMed] [Google Scholar]
- Lobov IB, Brooks PC, Lang RA. Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. Proc Natl Acad Sci U S A. 2002;99:11205–11210. doi: 10.1073/pnas.172161899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Kamat AA, Lin YG, Merritt WM, Landen CN, Kim TJ, Spannuth W, Arumugam T, Han LY, Jennings NB, et al. Dual targeting of endothelial cells and pericytes in antivascular therapy for ovarian carcinoma. Clin Cancer Res. 2007;13:4209–4217. doi: 10.1158/1078-0432.CCR-07-0197. [DOI] [PubMed] [Google Scholar]
- Mazzieri R, Pucci F, Moi D, Zonari E, Ranghetti A, Berti A, Politi LS, Gentner B, Brown JL, Naldini L, De Palma M. Targeting the ANG2/TIE2 axis inhibits tumor growth and metastasis by impairing angiogenesis and disabling rebounds of proangiogenic myeloid cells. Cancer cell. 2011;19:512–526. doi: 10.1016/j.ccr.2011.02.005. [DOI] [PubMed] [Google Scholar]
- Mukherjee D, Zhao J. The Role of chemokine receptor CXCR4 in breast cancer metastasis. American journal of cancer research. 2013;3:46–57. [PMC free article] [PubMed] [Google Scholar]
- Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- Nasarre P, Thomas M, Kruse K, Helfrich I, Wolter V, Deppermann C, Schadendorf D, Thurston G, Fiedler U, Augustin HG. Host-derived angiopoietin-2 affects early stages of tumor development and vessel maturation but is dispensable for later stages of tumor growth. Cancer research. 2009;69:1324–1333. doi: 10.1158/0008-5472.CAN-08-3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- O’Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Moses M, Lane WS, Cao Y, Sage EH, Folkman J. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell. 1994;79:315–328. doi: 10.1016/0092-8674(94)90200-3. [DOI] [PubMed] [Google Scholar]
- Oh H, Takagi H, Suzuma K, Otani A, Matsumura M, Honda Y. Hypoxia and vascular endothelial growth factor selectively up-regulate angiopoietin-2 in bovine microvascular endothelial cells. J Biol Chem. 1999;274:15732–15739. doi: 10.1074/jbc.274.22.15732. [DOI] [PubMed] [Google Scholar]
- Orlidge A, D’Amore PA. Inhibition of capillary endothelial cell growth by pericytes and smooth muscle cells. J Cell Biol. 1987;105:1455–1462. doi: 10.1083/jcb.105.3.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Vinals F, Inoue M, Bergers G, Hanahan D, Casanovas O. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer cell. 2009;15:220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigamonti N, Kadioglu E, Keklikoglou I, Wyser Rmili C, Leow CC, De Palma M. Role of angiopoietin-2 in adaptive tumor resistance to VEGF signaling blockade. Cell reports. 2014;8:696–706. doi: 10.1016/j.celrep.2014.06.059. [DOI] [PubMed] [Google Scholar]
- Saharinen P, Eklund L, Pulkki K, Bono P, Alitalo K. VEGF and angiopoietin signaling in tumor angiogenesis and metastasis. Trends in molecular medicine. 2011;17:347–362. doi: 10.1016/j.molmed.2011.01.015. [DOI] [PubMed] [Google Scholar]
- Scharpfenecker M, Fiedler U, Reiss Y, Augustin HG. The Tie-2 ligand angiopoietin-2 destabilizes quiescent endothelium through an internal autocrine loop mechanism. J Cell Sci. 2005;118:771–780. doi: 10.1242/jcs.01653. [DOI] [PubMed] [Google Scholar]
- Sennino B, Falcon BL, McCauley D, Le T, McCauley T, Kurz JC, Haskell A, Epstein DM, McDonald DM. Sequential loss of tumor vessel pericytes and endothelial cells after inhibition of platelet-derived growth factor B by selective aptamer AX102. Cancer Res. 2007;67:7358–7367. doi: 10.1158/0008-5472.CAN-07-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skuli N, Liu L, Runge A, Wang T, Yuan L, Patel S, Iruela-Arispe L, Simon MC, Keith B. Endothelial deletion of hypoxia-inducible factor-2alpha (HIF-2alpha) alters vascular function and tumor angiogenesis. Blood. 2009;114:469–477. doi: 10.1182/blood-2008-12-193581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skuli N, Majmundar AJ, Krock BL, Mesquita RC, Mathew LK, Quinn ZL, Runge A, Liu L, Kim MN, Liang J, et al. Endothelial HIF-2alpha regulates murine pathological angiogenesis and revascularization processes. The Journal of clinical investigation. 2012;122:1427–1443. doi: 10.1172/JCI57322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Ewald AJ, Stallcup W, Werb Z, Bergers G. PDGFRbeta+ perivascular progenitor cells in tumours regulate pericyte differentiation and vascular survival. Nat Cell Biol. 2005;7:870–879. doi: 10.1038/ncb1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava K, Hu J, Korn C, Savant S, Teichert M, Kapel SS, Jugold M, Besemfelder E, Thomas M, Pasparakis M, Augustin HG. Postsurgical adjuvant tumor therapy by combining anti-angiopoietin-2 and metronomic chemotherapy limits metastatic growth. Cancer cell. 2014;26:880–895. doi: 10.1016/j.ccell.2014.11.005. [DOI] [PubMed] [Google Scholar]
- Stahl A, Connor KM, Sapieha P, Chen J, Dennison RJ, Krah NM, Seaward MR, Willett KL, Aderman CM, Guerin KI, et al. The mouse retina as an angiogenesis model. Invest Ophthalmol Vis Sci. 2010;51:2813–2826. doi: 10.1167/iovs.10-5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser GA, Kaminker JS, Tessier-Lavigne M. Microarray analysis of retinal endothelial tip cells identifies CXCR4 as a mediator of tip cell morphology and branching. Blood. 2010;115:5102–5110. doi: 10.1182/blood-2009-07-230284. [DOI] [PubMed] [Google Scholar]
- Suri C, McClain J, Thurston G, McDonald DM, Zhou H, Oldmixon EH, Sato TN, Yancopoulos GD. Increased vascularization in mice overexpressing angiopoietin-1. Science. 1998;282:468–471. doi: 10.1126/science.282.5388.468. [DOI] [PubMed] [Google Scholar]
- Szarvas T, Jager T, Totsch M, vom Dorp F, Kempkensteffen C, Kovalszky I, Romics I, Ergun S, Rubben H. Angiogenic switch of angiopietins-Tie2 system and its prognostic value in bladder cancer. Clin Cancer Res. 2008;14:8253–8262. doi: 10.1158/1078-0432.CCR-08-0677. [DOI] [PubMed] [Google Scholar]
- Thomas M, Augustin HG. The role of the Angiopoietins in vascular morphogenesis. Angiogenesis. 2009;12:125–137. doi: 10.1007/s10456-009-9147-3. [DOI] [PubMed] [Google Scholar]
- Thomas M, Kienast Y, Scheuer W, Bahner M, Kaluza K, Gassner C, Herting F, Brinkmann U, Seeber S, Kavlie A, et al. A novel angiopoietin-2 selective fully human antibody with potent anti-tumoral and anti-angiogenic efficacy and superior side effect profile compared to Pan-Angiopoietin-1/-2 inhibitors. PloS one. 2013;8:e54923. doi: 10.1371/journal.pone.0054923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurston G, Rudge JS, Ioffe E, Zhou H, Ross L, Croll SD, Glazer N, Holash J, McDonald DM, Yancopoulos GD. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med. 2000;6:460–463. doi: 10.1038/74725. [DOI] [PubMed] [Google Scholar]
- Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, McDonald DM. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999;286:2511–2514. doi: 10.1126/science.286.5449.2511. [DOI] [PubMed] [Google Scholar]
- Vasudev NS, Reynolds AR. Anti-angiogenic therapy for cancer: current progress, unresolved questions and future directions. Angiogenesis. 2014 doi: 10.1007/s10456-014-9420-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Tell D, Armulik A, Betsholtz C. Pericytes and vascular stability. Exp Cell Res. 2006;312:623–629. doi: 10.1016/j.yexcr.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- Zetter BR. Angiogenesis and tumor metastasis. Annu Rev Med. 1998;49:407–424. doi: 10.1146/annurev.med.49.1.407. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.