Abstract
Traumatic brain injury (TBI) represents a leading cause of morbidity and mortality among young individuals. Alcohol abuse is a risk factor associated with increased TBI incidence. In addition, up to 26% of TBI patients engage in alcohol consumption after TBI. Limited preclinical studies have examined the impact of post-injury alcohol exposure on TBI recovery. The aim of this study was to determine the isolated and combined effects of TBI and alcohol on cognitive, behavioral, and physical recovery, as well as on associated neuroinflammatory changes. Male Sprague-Dawley rats (~300 g) were subjected to a mild focal TBI by lateral fluid percussion (~30 PSI, ~25 ms) under isoflurane anesthesia. On day 4 after TBI, animals were exposed to either sub-chronic intermittent alcohol vapor (95% ethanol 14h on /10h off; BAL~200 mg/dL) or room air for 10 days. TBI induced neurological dysfunction reflected by an increased neurological severity score (NSS) showed progressive improvement in injured animals exposed to room air (TBI/air). In contrast, TBI animals exposed to alcohol vapor (TBI/alcohol) showed impaired NSS recovery throughout the 10-day period of alcohol exposure. Open-field exploration test revealed an increased anxiety-like behavior in TBI/alcohol group compared to TBI/air group. Additionally, alcohol-exposed animals showed decreased locomotion and impaired novel object recognition. Immunofluorescence showed enhanced reactive astrocytes, microglial activation, and HMGB1 expression localized to the injured cortex of TBI/alcohol as compared to TBI/air animals. The expression of neuroinflammatory markers showed significant positive correlation with NSS. These findings indicated a close relationship between accentuated neuroinflammation and impaired neurological recovery from post-TBI alcohol exposure. The clinical implications of long-term consequences in TBI patients exposed to alcohol during recovery warrant further investigation.
1. Introduction
Traumatic brain injury (TBI) represents a leading cause of morbidity and mortality among young individuals in the United States. The severity of a TBI ranges from mild to severe, and ~75% of TBIs are classified as mild TBIs (Centers for Disease Control and Prevention (CDC), 2003). Mild TBI has received much attention recently because of its high prevalence in contact sports ((CDC), 1997; Pellman et al., 2004). Additionally, TBI that occurs during combat, caused by a blast or explosion, often leads to a sudden loss of consciousness and is classified as mild TBI (Hoge et al., 2008; Terrio et al., 2009). Apart from the at-risk populations, mild TBI commonly occurs in the general population (Centers for Disease Control and Prevention (CDC), 2003), and it has become a major issue because of the unknown, yet potential long-term consequences such as neurodegenerative changes in the brain (Loane et al., 2014; Pellman et al., 2004).
While victims of mild TBI often show physical recovery within a few months, psychological symptoms such as heightened irritability, anxiety, apathy, or depression may develop and linger for a longer period of time (Carroll et al., 2004; Hibbard et al., 2004; Whyte et al., 1996). The development of post-traumatic stress disorder (PTSD) is particularly relevant to military personnel following a combat-acquired TBI (Jacobson et al., 2008; Morissette et al., 2011). Combat TBI victims are reported to consume alcohol in an attempt to alleviate post-concussion symptoms (Adams et al., 2012c; Dikmen et al., 1995; Eisen et al., 2012; Jorge et al., 2005; Miller et al., 2013). In fact, up to one fourth of TBI victims consume alcohol following injury, with binge drinking being a common presentation in individuals experiencing depression, PTSD, or other stressors during the recovery period (Adams et al., 2012a; Adams et al., 2012b; Bombardier et al., 2003; Dikmen et al., 1995; Hibbard et al., 1998; Jacobson et al., 2008; Kreutzer et al., 1996; Ponsford et al., 2007; Ramchand R, 2011; Simpson and Tate, 2002). The high prevalence of alcohol use by TBI victims requires particular attention to understand how alcohol use and abuse may affect the recovery from TBI.
Clinical reports show that a history of concussion is associated with impaired cognitive function, predominantly in the areas of executive functioning and memory (Belanger et al., 2010; Bogdanova and Verfaellie, 2012; Guskiewicz et al., 2005). Alcohol exposure can further induce cognitive problems, and neuroimaging studies have revealed persistent brain structural abnormalities in human alcoholics (Oscar-Berman, 2012; Oscar-Berman and Marinkovic, 2007; Ruiz et al., 2013). A clinical study shows that post-injury alcohol use causes deterioration of executive functioning and adversely influences rehabilitation outcomes in TBI survivors (Ponsford et al., 2013). Among 60 TBI patients who participated in that study, cognition and executive functioning tested at 6–9 months after injury was poorer in patients that consumed any alcohol at all during the recovery period (Ponsford et al., 2013). Despite these clinical observations, there has not been sufficient pre-clinical research performed to examine neurological and neurobehavioral outcomes or the underlying neuropathological sequelae resulting from post-TBI alcohol exposure.
Neuroinflammation plays a critical pathophysiological role in TBI-associated morbidity (Lumpkins et al., 2008). A recent clinical study indicates that neuroinflammation and white matter degeneration continue for years after a single TBI episode in humans (Johnson et al., 2013). Sustained neuroinflammation is detrimental and leads to cognitive dysfunction and neuronal damage (Chen and Swanson, 2003; Feuerstein et al., 1998; Johnson et al., 2013; Kabadi and Faden, 2014; Lucas et al., 2006; Nakashima et al., 1995; Niranjan, 2013). Specifically, increased reactive astrocytes and microglial activation with the associated release of pro-inflammatory mediators and reactive oxygen and nitrogen species have been linked to accelerated neuronal loss via mechanisms such as increased glutamate excitotoxicity (Byrnes and Faden, 2007; Byrnes et al., 2007; Eikelenboom et al., 2002; Hewett et al., 1994). Chronic alcohol exposure induces neuroinflammation as evidenced by increased microglial markers detected in the postmortem brains of human alcoholics (He and Crews, 2008). Additionally, increased microglial activation and astrocyte expression have been observed in the parietal association cortex, entorhinal cortex, and hippocampus following chronic binge alcohol administration in rodents (Franke et al., 1997; Gonca et al., 2005; Zhao et al., 2013). Several studies have identified that high mobility group box 1 (HMGB1) has potent pro-inflammatory effects when secreted by activated immune cells (Abraham et al., 2000; Bustin, 1999; Kim et al., 2006; Yang et al., 2010a). HMGB1 is considered a danger signaling cytokine that is critical in alcoholinduced neuroinflammation (Crews et al., 2013). Therefore, together with astrocyte and microglial activation, enhanced HMGB1 expression contributes to a chronic neuroinflammatory milieu that affects the brain’s structural and functional integrity. How chronic alcohol exposure mediates TBI-induced neuroinflammation is poorly understood.
Using an established lateral fluid percussion (LFP) model of TBI, we examined the overt functional outcomes, as well as the magnitude of neuroinflammation in rodents exposed to intermittent alcohol vapor during the post-TBI period. Our working hypothesis was that alcohol exposure post-TBI would exacerbate neuroinflammation, which would be linked to impaired neurological and behavioral recovery from TBI.
2. Materials and Methods
2.1. Animals
Specific-pathogen free adult male Sprague-Dawley rats (Charles River, Raleigh, NC) weighing 250 – 300 g were housed in the Division of Animal Care at the Louisiana State University Health Sciences Center for one week of acclimatization prior to all studies. Animals were exposed to a 12 h light / 12 h dark cycle at 22 °C and had free access to water and standard diet (Purina Rat Chow, Ralston Purina, St. Louis, MO). All animal procedures and experiments were in accordance with the guidelines of the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee of the Louisiana State University Health Sciences Center.
2.2. Craniotomy
Approximately one week after arriving at our facilities, animals were anesthetized by intramuscular injection of ketamine/xylazine (90 mg/kg and 9 mg/kg, respectively) and positioned on a stereotaxic apparatus (Model 900 KOPF Instruments, David Kopf Instruments). The shaved surgical area was cleaned by betadine surgical scrub (povidone-iodine, 7.5%) and isopropyl alcohol. An incision (~3 cm) was made between the eyes and over the cranial crown, and excess tissue and blood were removed. Using a 5.0 mm diameter Michele Trephine (Roboz Surgical Instruments, Gaithersburg, MD), a circular track was drilled on top of the left hemisphere at a location 2 mm posterior to bregma and 3 mm lateral from midline to remove the overlying skull. Once the intact dura was exposed, a female Luer Loc was aligned over the drill hole, and the perimeter was sealed with cyanoacrylate glue followed by Jet Denture Repair Acrylic (Lang Dental Manufacturing, Wheeling, IL). One small steel mounting screw 0–80x3/32 (Plastics One, Roanoke, VA) was hand-drilled and placed posterior to lambda to serve as an extra anchor for the dental acrylic. The dura was kept moist by filling the interior of the female Luer Loc with normal saline before capping. At the completion of all procedures, animals were removed from the stereotaxic apparatus and returned to clean cages. Animals were allowed to recover from surgery for one week, with free access to food and water.
2.3. Traumatic Brain Injury
Animals were anesthetized (isoflurane; 4% induction & 3% maintenance) and placed next to the Lateral Fluid Percussion Injury Device (LFP) (Custom Design and Fabrication, Virginia Commonwealth University Model 01-B). The female Luer Loc attached to the animal’s cranium was connected to the male Luer Lock at the end of a high pressure tubing of the device. TBI was produced by a free swing of the pendulum striking the piston, which produced a wave of fluid pressure inside the water tank. The pressure was then transmitted through the high pressure tubing and generated an immediate impact to the exposed dura (~2 atm in 25 ms). The exact pressure produced for each individual animal’s TBI was recorded by Lab Chart 7. Time-matched sham controls were anesthetized with the same amount/duration of isoflurane, but were not subjected to TBI. Once all animals regained consciousness, they were returned to their home cages and provided with food and water and allowed to recover for 4 days.
2.4. Intermittent Alcohol Vapor Exposure
On day 4 after TBI, animals were randomly divided to receive either 10 days of intermittent alcohol vapor exposure or room air as control. The alcohol vapor inhalation system (La Jolla Alcohol Research Inc.) was set to a daily schedule that turned on at 6 PM in the evening and off at 8 AM in the morning (14 h on /10 h off). The alcohol vapor was created by dripping 95% ethanol into 2,000-mL Erlenmeyer flasks maintained at 50 °C. Air passed over the bottom of the flask, and alcohol was vaporized after hitting the warm glass. The air then carried the alcohol vapor into the chamber for inhalation. The rate of alcohol delivery was adjusted to achieve a target blood alcohol level (BAL) of ~200 mg/dL. The primary advantage of using this alcohol administration model was that it allowed for precise controls of dose, duration, and pattern of alcohol delivery (Gilpin et al., 2008). The 10 day duration of alcohol exposure was based on published studies indicating that this is sufficient to achieve neuroinflammatory changes in rodents exposed to binge-like alcohol administration (Crews et al., 2013). The control animals received room air outside the vapor unit, but under the same environmental conditions with the same light/dark cycle. Free access to food and water was provided throughout the 10 day period of exposure.
2.5. Blood Alcohol Level Measurement
Blood samples were collected by tail snip at 8 AM (end of a 14 h alcohol vapor inhalation cycle). Only the tip of the tail (~1 mm) was cut with a disposable scalpel, and blood was collected into a microcentrifuge tube. The same wound area was cut for subsequent bleeds. BALs were determined using an amperometric oxygen electrode and kit (Analox Instruments Limited, London, England) as previously described by our laboratory (Whitaker et al., 2011).
2.6. Neurological Severity Score Assessment
Assessment for neurological severity score (NSS) was conducted before and after TBI and after 10 days of intermittent alcohol vapor exposure. To avoid confounding effects of alcohol intoxication, all behavioral tests were performed at 6 to 8 hours since the last cycle of alcohol vapor exposure, when BALs were no longer detectable. Body weight was measured at the time of assessment, and all behavioral and neurological test scores were routinely cross-validated between multiple observers in our laboratory. NSS evaluates somatomotor and somatosensory functions by testing the animals’ activities in motor, sensory, reflexes, beam walking, and beam balancing tasks. A score of 0 indicates normal sensory and motor function, as well as having the ability to walk and balance on all beams. Total scores ranged from 0 to 25, and an impaired performance on any activities received a score according to its severity. The scoring system was adapted from previously published methods (Boyko et al., 2011; Ling et al., 2004). A detailed description of our NSS testing procedure and the contribution of individual tasks to the composite NSS score was published previously (Teng and Molina, 2014). Animals were returned to their home cages provided with food and water at the completion of NSS test.
2.7. Open Field Exploration
Open field exploration test (OFT) was conducted on day 12 after TBI (day 8 of alcohol vapor inhalation). The testing room was equipped with dim lighting control, and it was isolated from sound and unintentional interruptions. The open field apparatus (72 cm length×72 cm width×36 cm height) was placed in the middle of the room, and the floor of the apparatus was divided by 25 (5×5) squares with grid lines. At each trial, one animal was placed at the center of the field to explore for a total duration of 5 minutes. Behavior was recorded by an overhead video camcorder. The total number of line crossings and the percentage of time spent in center squares were measured for each animal. The open field was cleaned by Quatricide disinfecting solution at the end of each trial and before testing the next animal. All animals were returned to their home cages after testing and provided with food and water ad libitum.
2.8. Novel Object Recognition Test
Novel object recognition (NOR) test was conducted on day 13 after TBI (day 9 of alcohol vapor inhalation). Two objects were placed inside the same open field the animals had explored in the previous day. During the familiarization phase, an animal was placed at the center of the field, and it was exposed to two identical objects placed diagonally inside the apparatus. After allowing the animal to explore the objects for 5 minutes, the animal was returned to its home cage. Following a delay of 5 minutes, the same animal was placed inside the open field once again but this time exposed to two different objects; one object was exchanged with a novel object while the other object stayed the same as the familiar object. A total of 5 minutes was allowed for the exploration of objects during the test phase, and the animal’s behavior was recorded by an overhead video camcorder. The total time spent and the frequency of approaches to each object were measured. After each trial, both the explored area and objects were cleaned using Quatricide disinfecting solution. All animals were returned to their home cages after testing and provided with food and water ad libitum.
2.9. Collection of Brain for Analysis
Under deep anesthesia using 5% isoflurane, a midline incision was made across the abdomen and chest to expose the heart and lungs. A 20 G needle connected to an infusion pump (Cole-Palmer’s Masterflex pump) was inserted into the apex of the left ventricle, while the right atrium was cut with small scissors. Perfusion was started with cold 1X PBS for 2 minutes at a rate of 15 ml/minute, which was then followed by 10 minutes of cold 4% paraformaldehyde (Electron Microscopy Sciences) at a rate of 15 ml/minute. At the completion of transcardial perfusion, the animal was decapitated to remove the whole brain. The brain was placed in 4% paraformaldehyde followed by 20% sucrose solution. Fixed brains were gently lowered into a cold 2-methyl butane solution and allowed to transpire until the bubbling subsided. Frozen brains were wrapped in aluminum foil and stored at −80 °C until cryosection.
2.10. Frozen Brain Cryosection
Frozen brain was placed onto a tissue metal disc using Embedding Medium for Frozen Tissue Specimens to ensure Optimal Cutting Temperature (OCT). The tissue embedded with OCT froze rapidly onto the metal disc at −20 °C, and it was mounted on a chuck in a cryostat. The brain was then cut frozen with the microtome portion of the cryostat into 35 µm thick slices at −20 °C. Each microscope slide contained 4 – 6 brain sections, and all slides were left to dry at room temperature prior to storing at 4 °C.
2.11. Immunofluorescence
Using a hydrophobic ImmEdge Pen (Vector Laboratories, Burlingame, CA), a circle was drawn around each brain. This created a hydrophobic barrier that prevented mixing of antibody solutions between adjacent brain sections. Once the hydrophobic barrier was dry, 0.3 % Triton X-100 in 1X PBS was added to the center of each brain section until the solution touched the rim of the barrier. After 30 minutes of permeabilization, blocking buffer (1% albumin, 2 % normal donkey serum, and 0.3 % Triton X-100 in 1X PBS) was added to each section and incubated for 1 hour at room temperature. Next, the brain sections were incubated with primary antibody diluted in blocking buffer for 24 hours at 4 °C in a humidification chamber. The primary antibody was then washed off three times in 1X PBS for 5 minutes each. The secondary antibody diluted in blocking buffer was added to each section and incubated for 2 hours at room temperature in the dark. The slides were then washed three times in 1X PBS for 5 minutes each. Using Prolong Gold Antifade Reagent with DAPI (Life Technologies), cover slides were mounted and allowed to cure overnight at room temperature prior to image capture.
2.12. Image Acquisition and Analysis
For each animal, at least three 35 µm sections (approximately 2 – 4 mm posterior to bregma) representing the injury site were immunostained. Immunofluorescence images were captured using a Nikon Eclipse TE 2000-U inverted microscope (Nikon Instruments, Kawasaki, Japan) equipped with a Nikon color camera and operated by Elements software. Images were captured at 10 × magnification for a maximum representation of the extent of damage. The exposure time was fixed to the same duration as that used for the TBI/alcohol experimental group. Digitized images were converted to 8 bit grayscale for quantification of immunoreactive cells. A fixed threshold was set across all experimental groups. The immunoreactivity of GFAP, ED-1, and HMGB1 was determined as the percent area of positive staining using the NIH Image J software. Results from all sections were averaged for each animal. Each experimental group had 4 to 7 animals, as indicated in the figure legends.
2.13. Statistical Analysis
All data are presented as mean ± standard error of the mean (SEM) with the number of animals per group indicated. An unpaired, two-tailed t test was used to compare NSS in sham vs. TBI prior to alcohol exposure. For all other measures, statistical analysis was accomplished by two-way analysis of variance (ANOVA). When significant interaction effects were found, post-hoc tests using Bonferroni multiple comparisons were performed in GraphPad Prism software version 5.0. Statistical significance was set at p < 0.05.
3. Results
3.1. Neurological Severity Score Assessment (NSS)
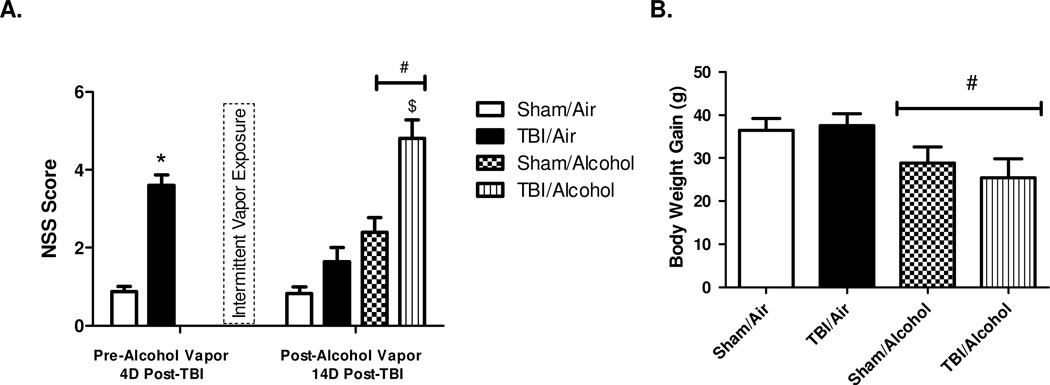
TBI resulted in significant neurological dysfunction at 4 days post-TBI and prior to alcohol exposure (NSS in sham 0.88 ± 0.13 vs. NSS in TBI 3.60 ± 0.26; p < 0.0001). At 14 days post-TBI (10 days post-alcohol vapor exposure), NSS averaged 0.83 ± 0.16 in sham/air, 1.65 ± 0.42 in TBI/air, 2.39 ± 0.43 in sham/alcohol, and 4.80 ± 0.49 in TBI/alcohol. There was a significant main effect of injury on NSS (F(1,82) = 16.44; p = 0.0001) relative to sham controls. There was a significant main effect of alcohol on NSS (F(1,82) = 35.02; p < 0.0001) relative to room air controls. Moreover, there was a significant interaction effect of TBI and alcohol on NSS (F(1,82) = 4.00; p = 0.0487). Bonferroni multiple comparisons revealed that the TBI/alcohol group had significantly increased NSS than TBI/air group (p < 0.0001). The majority of elevated NSS scores come from impaired beam walking and balancing tasks, and the scores of beam tasks alone averaged 0.67 ± 0.16 in sham/air, 1.40 ± 0.30 in TBI/air, 2.22 ± 0.36 in sham/alcohol, and 4.08 ± 0.35 in TBI/alcohol. Therefore, 10 days of post-injury intermittent alcohol vapor exposure impaired neurological recovery at 14 days following TBI (Figure 1 A).
Figure 1. Neurological Severity Scores (NSS) and Body Weight Gain.
Neurological severity scores (NSS) obtained before and after 10 days of intermittent alcohol vapor exposure (1 A) and the body weight gain during exposure (1 B). Values are means ± SEM, (Sham/Air n = 18, Sham/Alcohol n = 23, TBI/Air n = 20, TBI/Alcohol n = 25). NSS before alcohol vapor exposure were analyzed by t-test (Sham vs. TBI). NSS after alcohol vapor exposure and body weight gain were analyzed by two-way ANOVA. * p < 0.05 of the TBI groups vs. time-matched sham controls; # p < 0.05 of the alcohol-exposed groups vs. time-matched room air groups; $ p < 0.05 of the TBI/Alcohol group vs. TBI/Air group.
The body weight gain during the course of exposure averaged 36.4 ± 2.8 g in sham/air, 37.5 ± 2.8 g in TBI/air, 28.9 ± 3.7 in sham/alcohol, and 25.5 ± 4.4 in TBI/alcohol. The main effect of TBI on body weight gain was not significant (F(1,82) = 0.10; p = 0.7499) when compared to sham controls. The main effect of alcohol on body weight gain was significant (F(1,82) = 6.92; p = 0.0102) relative to air controls. However, there was no interaction effect of TBI and alcohol on body weight gain (F(1,82) = 0.36; p = 0.5475) (Figure 1 B).
3.2. Open Field Exploration Test (OFT)
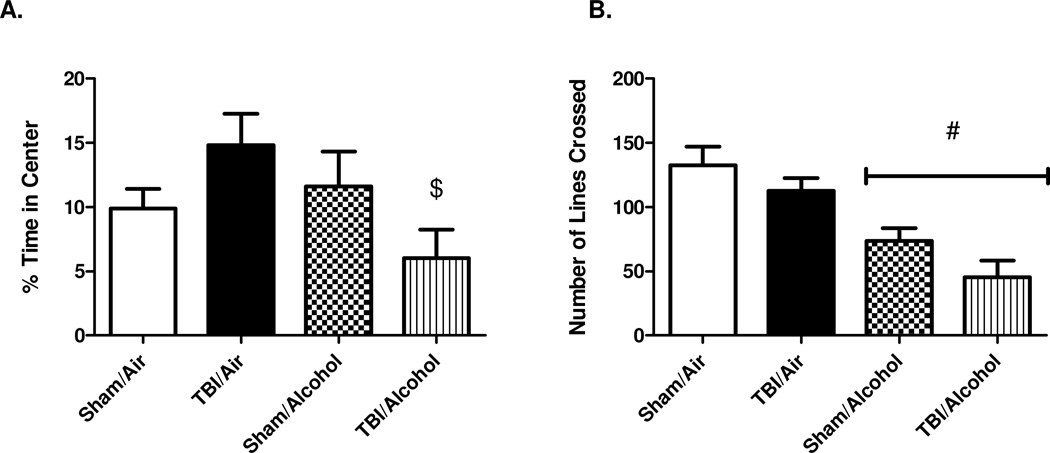
OFT was conducted on day 12 post-TBI (8 days post-alcohol vapor exposure). In a total 5 minutes of field exploration, the percentage of time the animal spent at the center squares was calculated as (time in center (s) / total 300 s)×100%. On average, the percentage of time spent in the central squares was 10 ± 2 % in sham/air, 15 ± 2 % in TBI/air, 12 ± 3 % in sham/alcohol, and 6 ± 2 % in TBI/alcohol group. There was no main effect of TBI (F(1,40) = 0.02; p = 0.8847) relative to sham controls, nor was there a main effect of alcohol (F(1,40) = 2.30; p = 0.1374) relative to room air controls. However, there was a significant interaction effect of TBI and alcohol on the time spent at the center squares (F(1,40) = 5.11; p = 0.0293). Post-hoc analysis by Bonferroni multiple comparisons revealed that TBI/alcohol group had decreased percentage of time spent at the center squares (p < 0.05) when compared to TBI/air group, suggesting a heightened anxiety-like behavior in response to the combined effect of TBI and alcohol (Figure 2 A).
Figure 2. Open Field Exploration Test (OFT).
Open Field Exploration test (OFT) indicating the percentage of time spent at the central squares (2 A) and locomotion with the number of lines crossed (2 B). Values are means ± SEM, (Sham/Air n = 8, Sham/Alcohol n = 12, TBI/Air n = 12, TBI/Alcohol n = 12). # p < 0.05 of the alcohol-exposed groups vs. time-matched room air groups; $ p < 0.05 of the TBI/Alcohol group vs. TBI/Air group, by two-way ANOVA.
Locomotion was assessed by counting the number of horizontal lines each animal crossed during the total 5 minutes of field exploration. The average number of lines crossed was 132 ± 15 in sham/air, 113 ± 10 in TBI/air, 74 ± 10 in sham/alcohol, and 45 ± 13 in TBI/alcohol. The main effect of TBI on locomotion did not reach significance (F(1,40) = 4.07; p = 0.0504) when compared to sham controls. The main effect of alcohol on locomotion was significant (F(1,40) = 28.16; p < 0.0001) relative to air controls. However, there was no interaction effect of TBI and alcohol on locomotion (F(1,40) = 0.13; p = 0.7219) (Figure 2 B).
3.3. Novel Object Recognition (NOR) Test
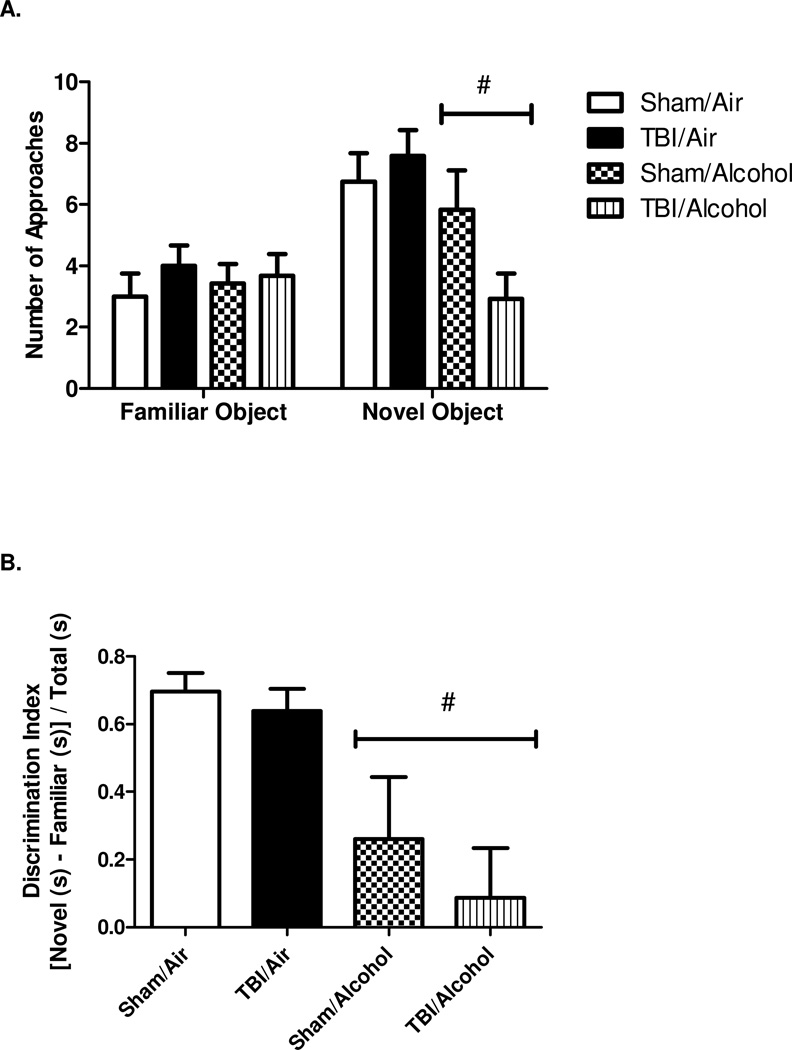
NOR was performed on day 13 post-TBI (day 9 post-alcohol vapor exposure). During the 5 minute test phase, animals were provided with one familiar and one novel object. The frequency of approaches and the time spent on each object were analyzed. The number of approaches to the familiar object averaged 3.0 ± 0.8 in sham/air, 4.0 ± 0.7 in TBI/air, 3.4 ± 0.6 in sham/alcohol, and 3.7 ± 0.7 in TBI/alcohol group. There was no effect of TBI (F(1,40) = 0.77; p = 0.3845), alcohol (F(1,40) = 0.00; p = 0.9535), or an interaction of TBI and alcohol (F(1,40) = 0.28; p = 0.6007) on the approaches to the familiar object. The number of approaches to the novel object averaged 6.8 ± 0.9 in sham/air, 7.6 ± 0.8 in TBI/air, 5.8 ± 1.3 in sham/alcohol, and 2.9 ± 0.8 in TBI/alcohol group. The main effect of injury was not significant (F(1,40) = 1.03; p = 0.3171) relative to sham controls. However, there was a significant main effect of alcohol on the frequency of approaches to the novel object (F(1,40) = 7.37; p = 0.0097) relative to room air controls. The interaction effect of TBI and alcohol was not significant (F(1,40) = 3.33; p = 0.0757). Overall, there were no differences across all groups in the number of approaches to the familiar object, but there was a significant effect of alcohol on the frequency of approaches to the novel object (Figure 3 A).
Figure 3. Novel Object Recognition Test (NOR).
Novel Object Recognition (NOR) test indicating the number of approaches to each object (3 A) and discrimination index reflecting the relative time spent on objects (3 B). Values are means ± SEM, (Sham/Air n = 8, Sham/Alcohol n = 12, TBI/Air n = 12, TBI/Alcohol n = 12). # p < 0.05 of the alcohol-exposed groups vs. time-matched room air groups, by two-way ANOVA.
Discrimination index represents the difference in time spent exploring the novel vs. the familiar object divided by the total time spent exploring both objects. Discrimination index averaged 0.70 ± 0.06 in sham/air, 0.64 ± 0.07 in TBI/air, 0.26 ± 0.18 in sham/alcohol, and 0.09 ± 0.15 in TBI/alcohol groups. There was no main effect of TBI on discrimination index (F(1,40) = 0.72; p = 0.4019) relative to sham controls, but there was a significant effect of alcohol on discrimination index (F(1,40) = 12.97; p = 0.0009) relative to air controls. The interaction effect of TBI and alcohol was not significant (F(1,40) = 0.18; p = 0.6700) (Figure 3 B).
3.4. Ipsilateral Cortex Astrocyte Expression, Microglial Activation, and HMGB1 Expression
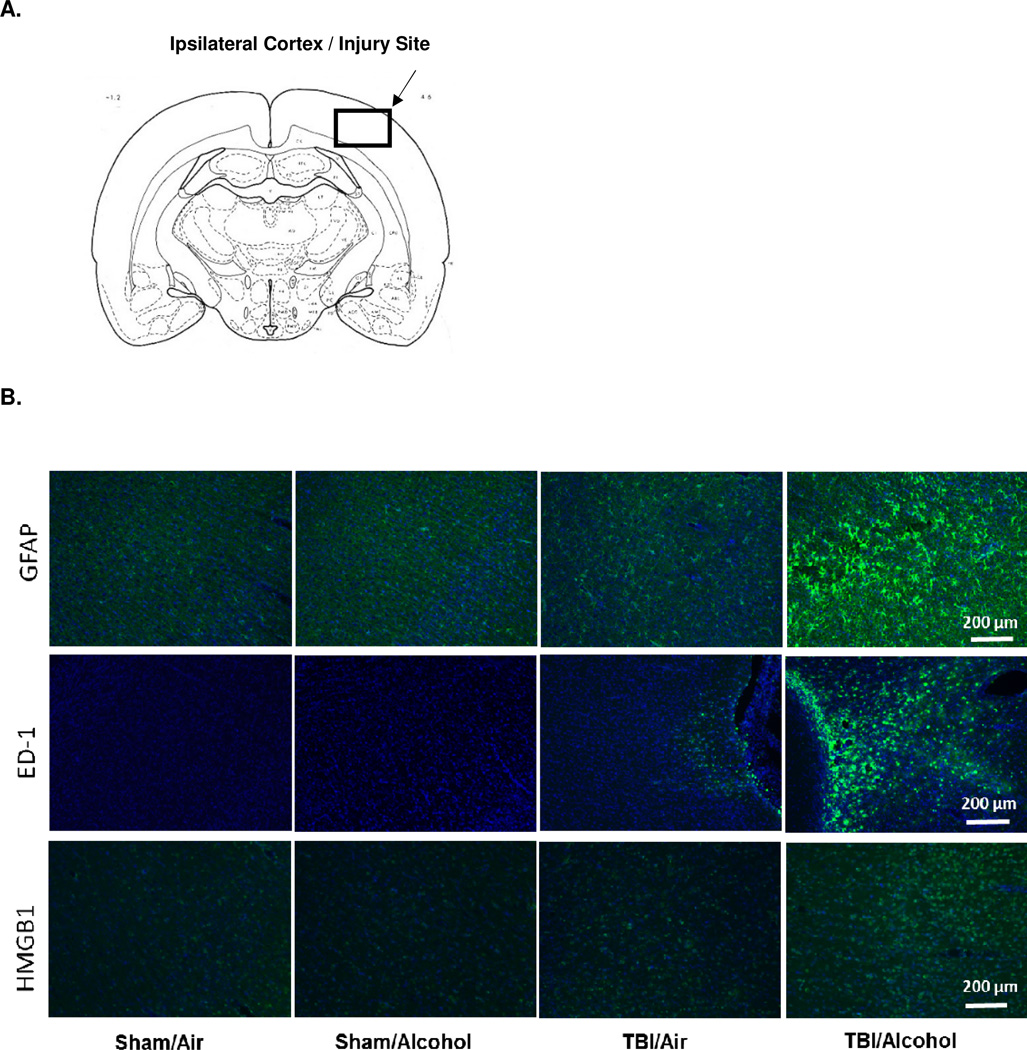
Figure 4 A shows the site of injury in a diagram of a coronal section of a rat brain, where enhanced inflammatory markers were observed under the microscope and immunofluorescence images were captured. No detectable neuroinflammation was observed in the contralateral cortex, nor in areas remote from the injury site in the ipsilateral cortex (data not shown).
Figure 4. Representative Immunofluorescence Images of Astrocyte Expression, Microglial Activation, and HMGB1 Expression.
Diagram of coronal rat brain section showing the site of injury and photographed region (4 A). Representative immunofluorescence images (10 ×) of ipsilateral cortex GFAP, ED-1, and HMGB1 immunoreactivity (green) combined with DAPI (blue) at 14 days post-TBI (10 days post-alcohol exposure) (4 B).
Figure 4 B shows representative immunofluorescence images (10 ×) taken from the ipsilateral cortex 14 days following TBI (end of 10 days alcohol vapor exposure). Images represent the combined staining of DAPI (blue) and GFAP for reactive astrocytes, ED-1 for microglial activation, or HMGB1 (green).
3.5. Immunofluorescence Quantification of Neuroinflammatory Markers and Correlation to Neurological Severity Scores
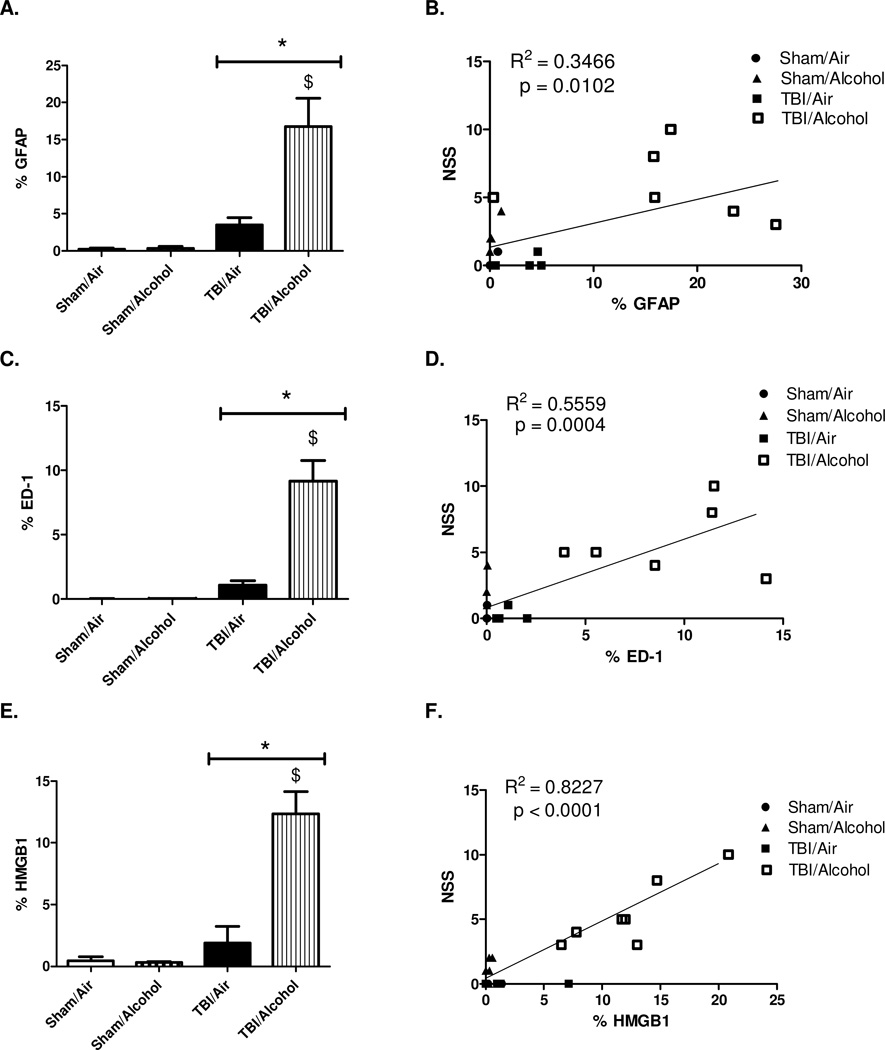
All images were quantified as % area of positive staining in a total area of 1.035 mm2 (10×) using NIH Image J software. The expression of reactive astrocytes is detected by GFAP staining. The percent area of positive staining averaged 0.22 ± 0.17 % in sham/air, 0.33 ± 0.25 % in sham/alcohol, 3.49 ± 1.60 % in TBI/air, and 16.75 ± 2.71 % in TBI/alcohol groups. There was a significant main effect of TBI on GFAP expression (F(1,14) = 13.24; p = 0.0027) relative to time-matched sham controls. There was a significant main effect of alcohol on GFAP expression (F(1,14) = 6.11; p = 0.0269) relative to time-matched air controls. Moreover, there was a significant interaction effect of TBI and alcohol on GFAP expression (F(1,14) = 5.91; p = 0.0290). Post-hoc analysis by Bonferroni multiple comparisons revealed significantly enhanced GFAP immunoreactivity in TBI/alcohol group when compared to TBI/air group (p < 0.01) (Figure 5 A).
Figure 5. Immunofluorescence Quantification of Neuroinflammatory Markers and Correlation to Neurological Severity Scores.
Quantification of ipsilateral cortex GFAP, ED-1, and HMGB1 immunoreactivity at 14 days post-TBI (10 days post-alcohol exposure). Images are quantified as % area of positive staining in 1.035 mm2 (10×). Values are shown as means ± SEM. For GFAP and ED-1, Sham/Air n = 4, Sham/Alcohol n = 4, TBI/Air n = 4, TBI/Alcohol n = 6. For HMGB1, Sham/Air n = 4, Sham/Alcohol n = 5, TBI/Air n = 5, TBI/Alcohol n = 7 (each n represents the average of 3 images taken from each animal) * p < 0.05 of the TBI groups vs. time-matched sham controls; $ p < 0.05 of the TBI/Alcohol group vs. TBI/Air group, by two-way ANOVA (5 A, 5 C, and 5 E).
Scatter plot showing the correlations between the neuroinflammatory markers (GFAP, ED-1, or HMGB1) vs. NSS. Results are analyzed by linear regression. For GFAP or ED-1 vs. NSS, n = 18 (Sham/Air n = 4, Sham/Alcohol n = 4, TBI/Air n = 4, TBI/Alcohol n = 6). For HMGB1 vs. NSS, n = 21 (Sham/Air n = 4, Sham/Alcohol n = 5, TBI/Air n = 5, TBI/Alcohol n = 7) (5 B, 5 D, and 5 F)
Scatter plots were generated to explore the potential association between neuroinflammation and neurological outcomes as determined by NSS following intermittent alcohol vapor exposure in TBI animals. GFAP expression representing reactive astrocytes was significantly (p = 0.0102), weakly (R2 = 0.3466), and positively correlated with NSS (Figure 5 B).
Microglial activation was detected by ED-1 staining. The percent areas of positive ED-1 staining averaged 0.011 ± 0.004 % in sham/air, 0.016 ± 0.007 % in sham/alcohol, 1.07 ± 0.40 % in TBI/air, and 9.17 ± 1.16 % in TBI/alcohol groups. There was a significant main effect of TBI on ED-1 expression (F(1,14) = 20.45; p = 0.0005) relative to time-matched sham controls. There was a significant main effect of alcohol on ED-1 expression (F(1,14) = 12.90; p = 0.0029) relative to time-matched room air controls. Moreover, there was a significant interaction effect of TBI and alcohol on microglial activation (F(1,14) = 12.87; p = 0.0030). Post-hoc analysis by Bonferroni multiple comparisons showed increased ED-1 immunoreactivity in TBI/alcohol group when compared to TBI/air group (p < 0.001) (Figure 5 C).
Scatter plot showing the relationship between ED-1 immunoreactivity and NSS indicated that microglial activation was significantly (p = 0.0004), yet moderately (R2 = 0.5559), and positively correlated with neurological dysfunction (Figure 5 D).
The percent areas of positive HMGB1 staining averaged 0.45 ± 0.26 % in sham/air, 0.30 ± 0.09 % in sham/alcohol, 1.88 ± 0.95 % in TBI/air, and 12.36 ± 1.39 % in TBI/alcohol groups. There was a significant main effect of TBI on HMGB1 expression (F(1,17) = 22.83; p = 0.0002) relative to time-matched sham controls. In addition, there was a significant main effect of alcohol on HMGB1 expression (F(1,17) = 13.42; p = 0.0019) relative to time-matched air controls. A significant interaction effect of TBI and alcohol on HMGB1 expression (F(1,17) = 14.18; p = 0.0015) was also found. Post-hoc analysis by Bonferroni multiple comparisons revealed that the brains of the TBI/alcohol group had significantly increased HMGB1 expression compared to those of the TBI/air group (p < 0.0001) (Figure 5 E).
HMGB1 immunoreactivity showed significant (p < 0.0001), and strong (R2 = 0.8227) positive correlation with neurological dysfunction (Figure 5 F). Moreover, a significant correlation between HMGB1 and NSS was found within the TBI/alcohol group alone (p = 0.0124, R2 = 0.7445). Therefore, expression of all three neuroinflammatory markers (GFAP, ED-1, and HMGB1) was significantly and positively correlated with NSS, strongly suggesting a close association between enhanced neuroinflammation and impaired neurological recovery from post-TBI alcohol exposure.
4. Discussion
In this study, we examined the impact of sub-chronic alcohol exposure on TBI recovery in a rodent model. Our results showed that 10 days of intermittent alcohol vapor inhalation after mild focal TBI significantly impaired neurological recovery and enhanced anxiety-like behavior in the open field test. Alcohol exposure alone was associated with decreased locomotion, as well as an impaired object recognition memory. Immunohistochemistry analysis revealed that alcohol exposure further exacerbated TBI-induced microglial activation, expression of reactive astrocytes, and HMGB1 expression in the ipsilateral cortex at 14 days following the injury. Additionally, we found that the presence of increased neuroinflammatory markers in the brain was closely associated with impaired neurological recovery from post-TBI alcohol exposure.
TBI can be classified as diffuse or focal. Diffuse injuries are associated with axonal injuries, which can be studied by an impact acceleration model that produces a diffuse pattern of brain injury (Andriessen et al., 2010). The model used in our studies, fluid percussion injury, represents a clinically relevant model for mild concussion or a single blast injury (Dewitt et al., 2013; Marmarou et al., 1994). Mild fluid percussion injury is less associated with wide-spread histopathological neuronal injury, thus replicating features of mild focal TBI in humans (Dixon et al., 1987; Gurkoff et al., 2006). We initiated the exposure to intermittent alcohol vapor on day 4 post-TBI. This models the situation where mild TBI victims would be discharged from the hospital and begin alcohol drinking a few days later (Dikmen et al., 1995). The total duration of alcohol exposure was chosen based on literature that showed 10 days of binge alcohol administration is sufficient to induce significant neuroinflammatory changes in rodent’s brain (Crews et al., 2013). Our goal was to examine whether similar duration of sub-chronic alcohol exposure after TBI would lead to further brain damage as determined by both behavioral and neuroinflammatory outcomes. We used an established intermittent alcohol vapor inhalation model to generate moderate to high levels of blood alcohol (150 – 200 mg/dL). This model provides advantages over other traditional alcohol administration methods such that the dose, duration, and pattern of alcohol delivery are easily controlled. In addition, the risk of infection is minimized because of its noninvasive approach (Gilpin et al., 2008). All behavioral assessments were performed when BALs were no longer detectable, at 6 to 8 hours following the completion of one vapor cycle exposure.
To examine the impact of alcohol on cognitive, behavioral, and physical recovery from a mild focal TBI, neurological function was measured before and after TBI and following 10 days of alcohol vapor exposure. Neurological severity scores reflecting measures for reflexes, sensory, and motor functions were markedly increased 4 post-TBI and showed a progressive improvement in TBI/air animals 10 days later (14 days post-TBI). In contrast, TBI/alcohol animals had sustained elevation of NSS score, reflecting impaired neurological function recovery. Because the type of injury in our TBI model produced a mild, focal, and unilateral cortex lesion, no significant impairment in sensory or reflexes was observed. Likewise, no obvious locomotor deficit was observed when the animal walked on a flat surface, as indicated by the lack of TBI effect on number of line crossings in the open field test. However, recognizable deficits in gait and balance were noted when the injured animal traversed on a narrow elevated beam, and most of the elevated NSS score was due to impaired beam walking and balancing tasks. The lack of performance on the beam tasks suggests impaired fine motor coordination, as well as a possible hemiparesis-like effect with weakness in the contralateral side as a result of the unilateral injury to the sensorimotor cortex (Curzon et al., 2009). Previous studies have shown that prolonged alcohol exposure leads to cognitive dysfunction and disturbance in gait and balance (Crews and Nixon, 2009; Zhao et al., 2013). Our study further demonstrated that post-TBI chronic alcohol exposure impaired neurological recovery, predominantly in the area of fine motor coordination as indicated by impaired physical performance on the beams. Whether the outcome is site-specific and injury to a different brain region leads to a different pattern of behavior or NSS impairment remain to be investigated.
Mood and anxiety disorders are frequently observed after mild TBI (Jorge et al., 2004). Clinical reports indicate that the majority of mild TBI patients show signs of physical recovery rather quickly, but psychiatric symptoms such as post-injury stress, depression, and anxiety often linger (Bogdanova and Verfaellie, 2012; Mooney and Speed, 2001; Moore et al., 2006). The open field exploration test is a frequently used paradigm to detect an animal’s anxiety-like behavior (Gould T, 2009; Prut and Belzung, 2003). Our results showed that TBI animals exposed to alcohol during the recovery period spent significantly less time exploring the open field center squares when compared to all of the other experimental groups, suggesting an increased anxiety-like behavior. Additionally, alcohol had a major impact on locomotion, as indicated by a decreased frequency of line crossings in both sham/alcohol and TBI/alcohol groups. This finding agrees with a recent publication that showed increased immobility time and decreased horizontal movement in rodents after 12 days of binge-pattern alcohol administration (Briones and Woods, 2013). Although the withdrawal effect from alcohol can lead to hyperactivity with enhanced movement (Freund, 1969), the length of exposure to alcohol in our study is less than that previously shown to achieve alcohol dependence (Riikonen et al., 2002). No overt clinical signs of withdrawal were observed in our animals. It was recognized however, that two of our TBI/alcohol animals exhibited frequent freezing episodes inside the open field during testing. Similar findings were published in a murine study that 4 cycles of 4-day intermittent alcohol vapor exposure resulted in disrupted fear extinction with higher freezing episodes (Holmes et al., 2012). This further demonstrates the likelihood of increased anxiety-like behavior associated with alcohol exposure after TBI, and alcohol has a major influence on locomotion.
Novel object recognition (NOR) test was performed one day after open field exploration (OFT) test to assess the animal’s recognition memory when provided with a novel and a familiar object. Based on the animal’s natural preference for a novel object, its ability to recognize two different objects and the associated memory function were examined in NOR (Antunes and Biala, 2012; Baxter, 2010; Ennaceur, 2010; Ennaceur and Delacour, 1988). Results showed that alcohol-exposed animals had decreased frequency of approaches to the novel object when compared to alcohol-naïve controls. Discrimination index was decreased in both alcohol groups, indicating that alcoholexposed animals exhibited decreased NOR when compared to the alcohol-naïve controls. This agrees with a recent study, in which four 4-day cycles of binge alcohol administration to rats led to a significant decrease in index for object recognition in NOR (Zhao et al., 2013). Results of NOR can be influenced by both hippocampal and cortical lesions, specifically in the perirhinal cortex and medial temporal lobe (Buckmaster et al., 2004; Clark et al., 2000; Zola et al., 2000). The role of the hippocampus in recognition memory has been controversial, and it is suggested that the interaction between hippocampus and either the perirhinal or medial prefrontal cortices is critical for NOR performance (Barker and Warburton, 2011). Our TBI model produced a mild and focal damage to the sensorimotor cortex, a brain region that has not been typically described to be involved in impaired NOR. This was confirmed by our observation that TBI alone produced no effect on NOR outcomes. Therefore, chronic alcohol exposure led to an impaired recognition memory possibly via other mechanisms such as the modulation of NMDA receptor-mediated synaptic activity (Swartzwelder et al., 1995). Although no interaction effect of TBI and alcohol was determined in this mild focal injury model, the possibility that alcohol affects memory function following a moderate to severe TBI, or to injury produced in a different site remains to be investigated.
Because alcohol exposure had a negative impact on the functional recovery from TBI, we further examined the involvement of the underlying neuroinflammatory changes associated with post-TBI alcohol exposure. Upon injury, resident immune cells such as astrocytes and microglia are activated and promote the release of pro-inflammatory cytokines, reactive oxygen and nitrogen species, and other substances with cytotoxic effects (Czeh et al., 2011). The release of these mediators is essential to clear harmful substances in the brain initially; however, prolonged or imbalanced neuroimmune activation is implicated in the pathophysiological processes of secondary neuronal damage (Czeh et al., 2011; Loane and Byrnes, 2010; Rivest, 2009). A time course of glial activation in response to a controlled cortical impact injury in a rodent study shows that peak microglial and astrocyte activation occurs in the peri-lesion cortex 5 to 7 days after cortical impact injury and remains evident beyond 2 weeks after TBI (d'Avila et al., 2012). Similarly, our study showed a persistent presence of reactive astrocytes and microglial activation detected by GFAP and ED-1 in the ipsilateral cortex of injured animals 14 days after injury. Additionally, 10 days of intermittent alcohol vapor exposure markedly accentuated TBI neuroinflammation with ~ 5-fold increases of GFAP and ~9-fold increases of ED-1 expression at the site of injury. The lack of immunoreactivity for these markers in the sham control brains might be due to the exposure time, which was set equal against the time needed for capturing the TBI/alcohol group. The relatively large differences in immunoreactivity required a short exposure duration for capturing TBI/alcohol images, which could have led to the apparent dim control images. In addition, ED-1 recognizes a glycoprotein on the lysosomal membrane of macrophages and microglia with phagocytic activity, and its expression is expected to be low in uninjured brain (Marshall et al., 2013; Raivich et al., 1999). Several studies have reported that prolonged alcohol exposure is associated with enhanced neuroinflammation (Crews and Nixon, 2009; He and Crews, 2008; Kaltschmidt et al., 1997; Zhao et al., 2013). The postmortem brains of human alcoholics reveal increased markers of microglia and cytokines (He and Crews, 2008). A rodent study showed that binge alcohol administration is linked to microglial activation in parietal association cortex, entorhinal cortex, and hippocampus (Zhao et al., 2013). Our results did not show increased glial activation in response to 10 days of alcohol exposure alone. This could be attributed to the differences in the blood alcohol levels achieved and the duration of alcohol exposure. Other studies that found increased neuroinflammation with alcohol typically utilized BALs of 250 – 400 mg/dL (Crews and Nixon, 2009) or 5 ½ month duration of alcohol exposure (Riikonen et al., 2002). Thus, it is possible that alcohol-induced neuroinflammation requires high blood alcohol levels over prolonged periods of exposure. The maximal BAL achieved in our 10 day period of alcohol vapor exposure was 200 mg/dL. It is likely that this level and duration of alcohol exposure, which we consider to be moderately high, were not sufficient to induce significant neuroinflammatory changes in the brain. Nevertheless, alcohol exposure synergistically enhanced TBI-induced microglial activation and expression of reactive astrocytes, reflecting an exacerbated neuroinflammatory state.
High-mobility group box 1 (HMGB1) is a ubiquitously expressed nuclear DNAbinding protein (Park et al., 2003; Park et al., 2004; Thomas, 2001). Emerging studies suggest that HMGB1 may mediate plasticity in the late remodeling phases of brain injury (Hayakawa et al., 2013). However, during the acute phase after TBI, HMGB1 is released from immune cells and exhibits potent pro-inflammatory effects (Kim et al., 2006). Sustained HMGB1 release can trigger additional microglial activation and cytokine release through binding to receptors such as Toll-like receptors (TLRs) and receptors for advanced glycation endproducts (RAGE) (Asavarut et al., 2013; Fang et al., 2012; Kim et al., 2008; Wang et al., 2011; Yang et al., 2010c). The role of HMGB1 in alcohol-induced neuroinflammation has been recognized in recent years (Crews et al., 2012; Vetreno and Crews, 2012). Enhanced expression of HMGB1 and TLRs are found in the postmortem brains of human alcoholics, as well as in the mouse brains following 10 days of intragastric binge alcohol administration achieving a BAL of 301 ± 19 mg/dL (Crews et al., 2013). Additionally, in vitro alcohol treatment (100 mmol/L) for 10 days is associated with an approximate 4.5 fold-increase in HMGB1 mRNA expression in cultured brain slice supernatant (Crews et al., 2012) These findings support a role for HMGB1 in alcohol-induced neuroinflammation. In addition, HMGB1 has been implicated in the pathophysiology of spinal cord injury, cerebral ischemia, epilepsy, sepsis, and cancer (Karlsson et al., 2008; Kawabata et al., 2010; Maroso et al., 2010; Tang et al., 2010; Yang et al., 2010b; Yang et al., 2010c). Only a few studies have examined the expression of HMGB1 following TBI (Gao et al., 2012). A recent study showed that HMGB1’s binding receptor, RAGE, is increased both at 6 hours and 6 days after TBI in rats (Gao et al., 2012). Results from our study showed that the combination of TBI and alcohol led to a ~ 5 fold increase of cortex HMGB1 expression when compared to either alcohol or TBI alone. We speculate this enhanced HMGB1 expression reflects accentuated neuroinflammation in animals exposed to chronic alcohol exposure after TBI. HMGB1 produces a cascade of feed-forward mechanisms by stimulating astroglial activation and the release of other pro-inflammatory cytokines, ultimately leading to an aggravated pathophysiological process of secondary injury (Kim et al., 2006; Lotze and Tracey, 2005; Luan et al., 2010).
Studies have shown that sustained neuroinflammation is associated with behavioral deficits (Franke et al., 1997; Rodgers et al., 2012). Chronic (36 weeks) alcohol treatment enhanced GFAP immunoreactivity and resulted in a significant loss of neuronal cells in the hippocampus, and deterioration in acquisition of maze performance in rodents (Franke et al., 1997). Furthermore, using a fluid percussion injury, others have found that the development of post-TBI anxiety-like freezing behavior is associated with increased reactive gliosis in key brain structures associated with anxiety including the amygdala, insula, and hippocampus, and that administration of a glial cell activation inhibitor suppresses both reactive gliosis and freezing behavior (Rodgers et al., 2012). Our results showed that alcohol vapor exposure post-TBI dramatically enhanced the expression of GFAP, ED-1, and HMGB1 at the site of injury in the sensorimotor cortex, and the presence of these markers was associated with increased neurological dysfunction predominantly in the area of impaired fine motor coordination. Therefore, our results suggest a close association between elevated neuroinflammatory markers in the brain and impaired neurological recovery, and that factors leading to exacerbated inflammation are detrimental to the overt functional outcomes of TBI.
It is important to mention that when the correlation analysis is performed within TBI/alcohol group alone, there is a significant correlation between HMGB1 and NSS, but not GFAP or ED-1. Nevertheless, our data comprise the outcomes of all experimental groups in order to understand the overall associations between the expression of neuroinflammatory markers and NSS, not necessarily the effect of TBI/Alcohol alone on either of those markers. It is also important to know that this association does not indicate a causal relationship and other potential factors should be considered including acetaldehyde, the metabolic by-product of alcohol metabolism, as it is highly toxic and can activate enzymes such as NADPH oxidase and inducible nitric oxidase synthase, causing oxidative damages on neurovascular cells with subsequent neuroinflammation (Alikunju et al., 2011). Since there is no significant main effect of TBI on body weight gain when compared to sham controls, we conclude that mild TBI produced in our model does not affect subsequent feeding and nutrition. However, we did observe decreased weight gain associated with prolonged alcohol exposure, which could contribute to impaired recovery. Future studies are needed to elucidate a more detailed relationship between neuroinflammation and behavioral recovery, as well as the associated physiological and metabolic changes that may exacerbate TBI recovery.
5. Conclusion
Alcohol exposure after TBI accentuated neuroinflammation, as indicated by enhanced astroglial activation and increased expression of the danger signaling molecule HMGB1. The exacerbated neuroinflammatory state marked by increased cellular reactivity in brain was accompanied by impaired neurological recovery and increased anxiety-like behavior in TBI animals exposed to alcohol vapor during the post-TBI period. The clinical implications of enhanced neuroinflammation for long-term recovery in alcoholic TBI patients warrant further investigation, including the possibility of neurodegenerative changes post-TBI.
Table 1.
Primary and Secondary Antibodies.
| Primary Antibody | Concentration | Secondary Antibody | Concentration |
|---|---|---|---|
| Anti-GFAP Rabbit polyclonal (Abcam, Cambridge, UK) | 1:200 | Donkey anti-Rabbit Alexa Fluor 488 (Invitrogen, Carlsbad, CA) | 1:200 |
| Anti-ED-1 Mouse monoclonal (Abcam, Cambridge, UK) | 1:200 | Donkey anti-Mouse Alexa Fluor 488 (Abcam, Cambridge, UK) | 1:200 |
| Anti-HMGB1 Rabbit polyclonal (Abcam, Cambridge, UK) | 1:100 | Donkey anti-Rabbit Alexa Fluor 488 (Invitrogen, Carlsbad, CA) | 1:200 |
Highlights.
Intermittent alcohol vapor inhalation after mild focal TBI impairs neurological recovery and enhances anxiety-like behavior in the open field test.
Alcohol exposure alone is associated with decreased locomotion, as well as an impaired object recognition memory.
Alcohol exposure further exacerbates TBI-induced microglial activation, expression of reactive astrocytes, and HMGB1 expression in the ipsilateral cortex following the injury.
The presence of increased neuroinflammatory markers in the brain is closely associated with impaired neurological recovery from post-TBI alcohol exposure.
Acknowledgements
The authors would like to thank Drs. Liz Simon, Flavia Souza, Peter Winsauer, Kathleen McDonough, Chu Chen, and Janet Rossi for their scientific guidance and discussions. The authors would also like to thank Renata Impastato for her assistance in surgeries, as well as Brittni Baynes, Drs. Annie Whitaker, Emily Roltsch, and Brandon Baiamonte for assistance with the alcohol vapor chambers.
This research was supported by grants NIAAA-AA7577, AA018400, F30AA022838, and LEQSF-EPS(2012)-PFUND-283.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- (CDC), C.f.D.C.a.P. Sport-related recurrent brain injuries. MMWR Morb Mortal Wkly Rep. 1997 [PubMed] [Google Scholar]
- Abraham E, Arcaroli J, Carmody A, Wang H, Tracey KJ. HMG-1 as a mediator of acute lung inflammation. Journal of immunology. 2000;165:2950–2954. doi: 10.4049/jimmunol.165.6.2950. [DOI] [PubMed] [Google Scholar]
- Adams RS, Corrigan JD, Larson MJ. Alcohol Use after Combat-Acquired Traumatic Brain Injury: What We Know and Don't Know. Journal of social work practice in the addictions. 2012a;12:28–51. doi: 10.1080/1533256X.2012.647580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams RS, Larson MJ, Corrigan JD, Horgan CM, Williams TV. Frequent binge drinking after combat-acquired traumatic brain injury among active duty military personnel with a past year combat deployment. The Journal of head trauma rehabilitation. 2012b;27:349–360. doi: 10.1097/HTR.0b013e318268db94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams ZW, Kaiser AJ, Lynam DR, Charnigo RJ, Milich R. Drinking motives as mediators of the impulsivity-substance use relation: pathways for negative urgency, lack of premeditation, and sensation seeking. Addictive behaviors. 2012c;37:848–855. doi: 10.1016/j.addbeh.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alikunju S, Abdul Muneer PM, Zhang Y, Szlachetka AM, Haorah J. The inflammatory footprints of alcohol-induced oxidative damage in neurovascular components. Brain, behavior, and immunity. 2011;25(Suppl 1):S129–S136. doi: 10.1016/j.bbi.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andriessen TM, Jacobs B, Vos PE. Clinical characteristics and pathophysiological mechanisms of focal and diffuse traumatic brain injury. Journal of cellular and molecular medicine. 2010;14:2381–2392. doi: 10.1111/j.1582-4934.2010.01164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunes M, Biala G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cognitive processing. 2012;13:93–110. doi: 10.1007/s10339-011-0430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asavarut P, Zhao H, Gu J, Ma D. The role of HMGB1 in inflammation-mediated organ injury. Acta anaesthesiologica Taiwanica : official journal of the Taiwan Society of Anesthesiologists. 2013;51:28–33. doi: 10.1016/j.aat.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Barker GR, Warburton EC. When is the hippocampus involved in recognition memory? The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:10721–10731. doi: 10.1523/JNEUROSCI.6413-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter MG. "I've seen it all before": explaining age-related impairments in object recognition. 2010 doi: 10.1037/a0021029. [DOI] [PubMed] [Google Scholar]
- Theoretical comment on Burke et al. Behavioral neuroscience. 2010;124:706–709. doi: 10.1037/a0021029. [DOI] [PubMed] [Google Scholar]
- Belanger HG, Spiegel E, Vanderploeg RD. Neuropsychological performance following a history of multiple self-reported concussions: a meta-analysis. Journal of the International Neuropsychological Society : JINS. 2010;16:262–267. doi: 10.1017/S1355617709991287. [DOI] [PubMed] [Google Scholar]
- Bogdanova Y, Verfaellie M. Cognitive sequelae of blast-induced traumatic brain injury: recovery and rehabilitation. Neuropsychology review. 2012;22:4–20. doi: 10.1007/s11065-012-9192-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bombardier CH, Temkin NR, Machamer J, Dikmen SS. The natural history of drinking and alcohol-related problems after traumatic brain injury. Archives of physical medicine and rehabilitation. 2003;84:185–191. doi: 10.1053/apmr.2003.50002. [DOI] [PubMed] [Google Scholar]
- Boyko M, Ohayon S, Goldsmith T, Novack L, Novack V, Perry ZH, Gruenbaum BF, Gruenbaum SE, Steiner O, Shapira Y, Teichberg VI, Zlotnik A. Morphological and neurobehavioral parallels in the rat model of stroke. Behavioural brain research. 2011;223:17–23. doi: 10.1016/j.bbr.2011.03.019. [DOI] [PubMed] [Google Scholar]
- Briones TL, Woods J. Chronic binge-like alcohol consumption in adolescence causes depression-like symptoms possibly mediated by the effects of BDNF on neurogenesis. Neuroscience. 2013;254:324–334. doi: 10.1016/j.neuroscience.2013.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmaster CA, Eichenbaum H, Amaral DG, Suzuki WA, Rapp PR. Entorhinal cortex lesions disrupt the relational organization of memory in monkeys. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:9811–9825. doi: 10.1523/JNEUROSCI.1532-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin M. Regulation of DNA-dependent activities by the functional motifs of the high-mobilitygroup chromosomal proteins. Molecular and cellular biology. 1999;19:5237–5246. doi: 10.1128/mcb.19.8.5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes KR, Faden AI. Role of cell cycle proteins in CNS injury. Neurochemical research. 2007;32:1799–1807. doi: 10.1007/s11064-007-9312-2. [DOI] [PubMed] [Google Scholar]
- Byrnes KR, Stoica BA, Fricke S, Di Giovanni S, Faden AI. Cell cycle activation contributes to post-mitotic cell death and secondary damage after spinal cord injury. Brain : a journal of neurology. 2007;130:2977–2992. doi: 10.1093/brain/awm179. [DOI] [PubMed] [Google Scholar]
- Carroll LJ, Cassidy JD, Peloso PM, Borg J, von Holst H, Holm L, Paniak C, Pepin M Injury, W.H.O.C.C.T.F.o.M.T.B. Prognosis for mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. Journal of rehabilitation medicine : official journal of the UEMS European Board of Physical and Rehabilitation Medicine. 2004:84–105. doi: 10.1080/16501960410023859. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC), N.C.f.I.P.a.C. Report to Congress on mild traumatic brain injury in the United States: steps to prevent a serious public health problem. Atlanta (GA): Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2003. [Google Scholar]
- Chen Y, Swanson RA. Astrocytes and brain injury. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2003;23:137–149. doi: 10.1097/01.WCB.0000044631.80210.3C. [DOI] [PubMed] [Google Scholar]
- Clark RE, Zola SM, Squire LR. Impaired recognition memory in rats after damage to the hippocampus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:8853–8860. doi: 10.1523/JNEUROSCI.20-23-08853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Nixon K. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol and alcoholism. 2009;44:115–127. doi: 10.1093/alcalc/agn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Qin L, Sheedy D, Vetreno RP, Zou J. High Mobility Group Box 1/Toll-like Receptor Danger Signaling Increases Brain Neuroimmune Activation in Alcohol Dependence. Biological psychiatry. 2012 doi: 10.1016/j.biopsych.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Qin L, Sheedy D, Vetreno RP, Zou J. High mobility group box 1/Toll-like receptor danger signaling increases brain neuroimmune activation in alcohol dependence. Biological psychiatry. 2013;73:602–612. doi: 10.1016/j.biopsych.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curzon P, Zhang M, Radek RJ, Fox GB. The Behavioral Assessment of Sensorimotor Processes in the Mouse: Acoustic Startle, Sensory Gating, Locomotor Activity, Rotarod, and Beam Walking. In: Buccafusco JJ, editor. Methods of Behavior Analysis in Neuroscience. Boca Raton (FL): 2009. [PubMed] [Google Scholar]
- Czeh M, Gressens P, Kaindl AM. The yin and yang of microglia. Developmental neuroscience. 2011;33:199–209. doi: 10.1159/000328989. [DOI] [PubMed] [Google Scholar]
- d'Avila JC, Lam TI, Bingham D, Shi J, Won SJ, Kauppinen TM, Massa S, Liu J, Swanson RA. Microglial activation induced by brain trauma is suppressed by post-injury treatment with a PARP inhibitor. Journal of neuroinflammation. 2012;9:31. doi: 10.1186/1742-2094-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewitt DS, Perez-Polo R, Hulsebosch CE, Dash PK, Robertson CS. Challenges in the development of rodent models of mild traumatic brain injury. Journal of neurotrauma. 2013;30:688–701. doi: 10.1089/neu.2012.2349. [DOI] [PubMed] [Google Scholar]
- Dikmen SS, Machamer JE, Donovan DM, Winn HR, Temkin NR. Alcohol use before and after traumatic head injury. Annals of emergency medicine. 1995;26:167–176. doi: 10.1016/s0196-0644(95)70147-8. [DOI] [PubMed] [Google Scholar]
- Dixon CE, Lyeth BG, Povlishock JT, Findling RL, Hamm RJ, Marmarou A, Young HF, Hayes RL. A fluid percussion model of experimental brain injury in the rat. Journal of neurosurgery. 1987;67:110–119. doi: 10.3171/jns.1987.67.1.0110. [DOI] [PubMed] [Google Scholar]
- Eikelenboom P, Bate C, Van Gool WA, Hoozemans JJ, Rozemuller JM, Veerhuis R, Williams A. Neuroinflammation in Alzheimer's disease and prion disease. Glia. 2002;40:232–239. doi: 10.1002/glia.10146. [DOI] [PubMed] [Google Scholar]
- Eisen SV, Schultz MR, Vogt D, Glickman ME, Elwy AR, Drainoni ML, Osei-Bonsu PE, Martin J. Mental and physical health status and alcohol and drug use following return from deployment to Iraq or Afghanistan. American journal of public health. 2012;102(Suppl 1):S66–S73. doi: 10.2105/AJPH.2011.300609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennaceur A. One-trial object recognition in rats and mice: methodological and theoretical issues. Behavioural brain research. 2010;215:244–254. doi: 10.1016/j.bbr.2009.12.036. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behavioural brain research. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Fang P, Schachner M, Shen YQ. HMGB1 in development and diseases of the central nervous system. Molecular neurobiology. 2012;45:499–506. doi: 10.1007/s12035-012-8264-y. [DOI] [PubMed] [Google Scholar]
- Feuerstein GZ, Wang X, Barone FC. The role of cytokines in the neuropathology of stroke and neurotrauma. Neuroimmunomodulation. 1998;5:143–159. doi: 10.1159/000026331. [DOI] [PubMed] [Google Scholar]
- Franke H, Kittner H, Berger P, Wirkner K, Schramek J. The reaction of astrocytes and neurons in the hippocampus of adult rats during chronic ethanol treatment and correlations to behavioral impairments. Alcohol. 1997;14:445–454. doi: 10.1016/s0741-8329(96)00209-1. [DOI] [PubMed] [Google Scholar]
- Freund G. Alcohol Withdrawal Syndrome in Mice. Archives of neurology. 1969;21:315–320. doi: 10.1001/archneur.1969.00480150105013. [DOI] [PubMed] [Google Scholar]
- Gao TL, Yuan XT, Yang D, Dai HL, Wang WJ, Peng X, Shao HJ, Jin ZF, Fu ZJ. Expression of HMGB1 and RAGE in rat and human brains after traumatic brain injury. The journal of trauma and acute care surgery. 2012;72:643–649. doi: 10.1097/TA.0b013e31823c54a6. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Richardson HN, Cole M, Koob GF. Vapor inhalation of alcohol in rats. Current protocols in neuroscience / editorial board, Jacqueline N. Crawley ... [et al.] Chapter 9, Unit 9 29. 2008 doi: 10.1002/0471142301.ns0929s44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonca S, Filiz S, Dalcik C, Yardimoglu M, Dalcik H, Yazir Y, Erden BF. Effects of chronic ethanol treatment on glial fibrillary acidic protein expression in adult rat optic nerve: an immunocytochemical study. Cell biology international. 2005;29:169–172. doi: 10.1016/j.cellbi.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Gould TDD, Kovacsics C. Mood and Anxiety Related Phenotypes in Mice: The Open Field Test. Neuromethods. 2009:42. [Google Scholar]
- Gurkoff GG, Giza CC, Hovda DA. Lateral fluid percussion injury in the developing rat causes an acute, mild behavioral dysfunction in the absence of significant cell death. Brain research. 2006;1077:24–36. doi: 10.1016/j.brainres.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Guskiewicz KM, Marshall SW, Bailes J, McCrea M, Cantu RC, Randolph C, Jordan BD. Association between recurrent concussion and late-life cognitive impairment in retired professional football players. Neurosurgery. 2005;57:719–726. doi: 10.1093/neurosurgery/57.4.719. discussion 719–726. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Pham LD, Arai K, Lo EH. High-mobility group box 1: an amplifier of stem and progenitor cell activity after stroke. Acta neurochirurgica. Supplement. 2013;118:31–38. doi: 10.1007/978-3-7091-1434-6_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Crews FT. Increased MCP-1 and microglia in various regions of the human alcoholic brain. Experimental neurology. 2008;210:349–358. doi: 10.1016/j.expneurol.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewett SJ, Csernansky CA, Choi DW. Selective potentiation of NMDA-induced neuronal injury following induction of astrocytic iNOS. Neuron. 1994;13:487–494. doi: 10.1016/0896-6273(94)90362-x. [DOI] [PubMed] [Google Scholar]
- Hibbard MR, Ashman TA, Spielman LA, Chun D, Charatz HJ, Melvin S. Relationship between depression and psychosocial functioning after traumatic brain injury. Archives of physical medicine and rehabilitation. 2004;85:S43–S53. doi: 10.1016/j.apmr.2003.08.116. [DOI] [PubMed] [Google Scholar]
- Hibbard MR, Uysal S, Kepler K, Bogdany J, Silver J. Axis I psychopathology in individuals with traumatic brain injury. The Journal of head trauma rehabilitation. 1998;13:24–39. doi: 10.1097/00001199-199808000-00003. [DOI] [PubMed] [Google Scholar]
- Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA. Mild traumatic brain injury in U.S. Soldiers returning from Iraq. The New England journal of medicine. 2008;358:453–463. doi: 10.1056/NEJMoa072972. [DOI] [PubMed] [Google Scholar]
- Holmes A, Fitzgerald PJ, MacPherson KP, DeBrouse L, Colacicco G, Flynn SM, Masneuf S, Pleil KE, Li C, Marcinkiewcz CA, Kash TL, Gunduz-Cinar O, Camp M. Chronic alcohol remodels prefrontal neurons and disrupts NMDAR-mediated fear extinction encoding. Nature neuroscience. 2012;15:1359–1361. doi: 10.1038/nn.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttunen HJ, Kuja-Panula J, Rauvala H. Receptor for advanced glycation end products (RAGE) signaling induces CREB-dependent chromogranin expression during neuronal differentiation. The Journal of biological chemistry. 2002;277:38635–38646. doi: 10.1074/jbc.M202515200. [DOI] [PubMed] [Google Scholar]
- Jacobson IG, Ryan MA, Hooper TI, Smith TC, Amoroso PJ, Boyko EJ, Gackstetter GD, Wells TS, Bell NS. Alcohol use and alcohol-related problems before and after military combat deployment. JAMA : the journal of the American Medical Association. 2008;300:663–675. doi: 10.1001/jama.300.6.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson VE, Stewart JE, Begbie FD, Trojanowski JQ, Smith DH, Stewart W. Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain : a journal of neurology. 2013;136:28–42. doi: 10.1093/brain/aws322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorge RE, Robinson RG, Moser D, Tateno A, Crespo-Facorro B, Arndt S. Major depression following traumatic brain injury. Archives of general psychiatry. 2004;61:42–50. doi: 10.1001/archpsyc.61.1.42. [DOI] [PubMed] [Google Scholar]
- Jorge RE, Starkstein SE, Arndt S, Moser D, Crespo-Facorro B, Robinson RG. Alcohol misuse and mood disorders following traumatic brain injury. Archives of general psychiatry. 2005;62:742–749. doi: 10.1001/archpsyc.62.7.742. [DOI] [PubMed] [Google Scholar]
- Kabadi SV, Faden AI. Neuroprotective strategies for traumatic brain injury: improving clinical translation. International journal of molecular sciences. 2014;15:1216–1236. doi: 10.3390/ijms15011216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltschmidt B, Uherek M, Volk B, Baeuerle PA, Kaltschmidt C. Transcription factor NF-kappaB is activated in primary neurons by amyloid beta peptides and in neurons surrounding early plaques from patients with Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:2642–2647. doi: 10.1073/pnas.94.6.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson S, Pettila V, Tenhunen J, Laru-Sompa R, Hynninen M, Ruokonen E. HMGB1 as a predictor of organ dysfunction and outcome in patients with severe sepsis. Intensive care medicine. 2008;34:1046–1053. doi: 10.1007/s00134-008-1032-9. [DOI] [PubMed] [Google Scholar]
- Kawabata H, Setoguchi T, Yone K, Souda M, Yoshida H, Kawahara K, Maruyama I, Komiya S. High mobility group box 1 is upregulated after spinal cord injury and is associated with neuronal cell apoptosis. Spine. 2010;35:1109–1115. doi: 10.1097/BRS.0b013e3181bd14b6. [DOI] [PubMed] [Google Scholar]
- Kim JB, Lim CM, Yu YM, Lee JK. Induction and subcellular localization of high-mobility group box-1 (HMGB1) in the postischemic rat brain. Journal of neuroscience research. 2008;86:1125–1131. doi: 10.1002/jnr.21555. [DOI] [PubMed] [Google Scholar]
- Kim JB, Sig Choi J, Yu YM, Nam K, Piao CS, Kim SW, Lee MH, Han PL, Park JS, Lee JK. HMGB1, a novel cytokine-like mediator linking acute neuronal death and delayed neuroinflammation in the postischemic brain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:6413–6421. doi: 10.1523/JNEUROSCI.3815-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzer JS, Witol AD, Marwitz JH. Alcohol and drug use among young persons with traumatic brain injury. Journal of learning disabilities. 1996;29:643–651. doi: 10.1177/002221949602900608. [DOI] [PubMed] [Google Scholar]
- Ling GS, Lee EY, Kalehua AN. Traumatic brain injury in the rat using the fluid-percussion model. Current protocols in neuroscience / editorial board, Jacqueline N. Crawley ... [et al.] Chapter 9, Unit 9 2. 2004 doi: 10.1002/0471142301.ns0902s28. [DOI] [PubMed] [Google Scholar]
- Loane DJ, Byrnes KR. Role of microglia in neurotrauma. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2010;7:366–377. doi: 10.1016/j.nurt.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loane DJ, Kumar A, Stoica BA, Cabatbat R, Faden AI. Progressive neurodegeneration after experimental brain trauma: association with chronic microglial activation. Journal of neuropathology and experimental neurology. 2014;73:14–29. doi: 10.1097/NEN.0000000000000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nature reviews. Immunology. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- Luan ZG, Zhang H, Yang PT, Ma XC, Zhang C, Guo RX. HMGB1 activates nuclear factor-kappaB signaling by RAGE and increases the production of TNF-alpha in human umbilical vein endothelial cells. Immunobiology. 2010;215:956–962. doi: 10.1016/j.imbio.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Lucas SM, Rothwell NJ, Gibson RM. The role of inflammation in CNS injury and disease. British journal of pharmacology. 2006;147(Suppl 1):S232–S240. doi: 10.1038/sj.bjp.0706400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumpkins KM, Bochicchio GV, Keledjian K, Simard JM, McCunn M, Scalea T. Glial fibrillary acidic protein is highly correlated with brain injury. The Journal of trauma. 2008;65:778–782. doi: 10.1097/TA.0b013e318185db2d. discussion 782-774. [DOI] [PubMed] [Google Scholar]
- Marmarou A, Foda MA, van den Brink W, Campbell J, Kita H, Demetriadou K. A new model of diffuse brain injury in rats. Part I: Pathophysiology and biomechanics. Journal of neurosurgery. 1994;80:291–300. doi: 10.3171/jns.1994.80.2.0291. [DOI] [PubMed] [Google Scholar]
- Maroso M, Balosso S, Ravizza T, Liu J, Aronica E, Iyer AM, Rossetti C, Molteni M, Casalgrandi M, Manfredi AA, Bianchi ME, Vezzani A. Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nature medicine. 2010;16:413–419. doi: 10.1038/nm.2127. [DOI] [PubMed] [Google Scholar]
- Marshall SA, McClain JA, Kelso ML, Hopkins DM, Pauly JR, Nixon K. Microglial activation is not equivalent to neuroinflammation in alcohol-induced neurodegeneration: The importance of microglia phenotype. Neurobiology of disease. 2013;54:239–251. doi: 10.1016/j.nbd.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SC, Baktash SH, Webb TS, Whitehead CR, Maynard C, Wells TS, Otte CN, Gore RK. Risk for addiction-related disorders following mild traumatic brain injury in a large cohort of active-duty U.S. airmen. The American journal of psychiatry. 2013;170:383–390. doi: 10.1176/appi.ajp.2012.12010126. [DOI] [PubMed] [Google Scholar]
- Mooney G, Speed J. The association between mild traumatic brain injury and psychiatric conditions. Brain injury : [BI] 2001;15:865–877. doi: 10.1080/02699050110065286. [DOI] [PubMed] [Google Scholar]
- Moore EL, Terryberry-Spohr L, Hope DA. Mild traumatic brain injury and anxiety sequelae: a review of the literature. Brain injury : [BI] 2006;20:117–132. doi: 10.1080/02699050500443558. [DOI] [PubMed] [Google Scholar]
- Morissette SB, Woodward M, Kimbrel NA, Meyer EC, Kruse MI, Dolan S, Gulliver SB. Deployment-related TBI, persistent postconcussive symptoms, PTSD, and depression in OEF/OIF veterans. Rehabilitation psychology. 2011;56:340–350. doi: 10.1037/a0025462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima MN, Yamashita K, Kataoka Y, Yamashita YS, Niwa M. Time course of nitric oxide synthase activity in neuronal, glial, and endothelial cells of rat striatum following focal cerebral ischemia. Cellular and molecular neurobiology. 1995;15:341–349. doi: 10.1007/BF02089944. [DOI] [PubMed] [Google Scholar]
- Niranjan R. Molecular Basis of Etiological Implications in Alzheimer's Disease: Focus on Neuroinflammation. Molecular neurobiology. 2013 doi: 10.1007/s12035-013-8428-4. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M. Function and dysfunction of prefrontal brain circuitry in alcoholic Korsakoff's syndrome. Neuropsychology review. 2012;22:154–169. doi: 10.1007/s11065-012-9198-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M, Marinkovic K. Alcohol: effects on neurobehavioral functions and the brain. Neuropsychology review. 2007;17:239–257. doi: 10.1007/s11065-007-9038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Arcaroli J, Yum HK, Yang H, Wang H, Yang KY, Choe KH, Strassheim D, Pitts TM, Tracey KJ, Abraham E. Activation of gene expression in human neutrophils by high mobility group box 1 protein. American journal of physiology. Cell physiology. 2003;284:C870–C879. doi: 10.1152/ajpcell.00322.2002. [DOI] [PubMed] [Google Scholar]
- Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, Abraham E. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. The Journal of biological chemistry. 2004;279:7370–7377. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- Passalacqua M, Patrone M, Picotti GB, Del Rio M, Sparatore B, Melloni E, Pontremoli S. Stimulated astrocytes release high-mobility group 1 protein, an inducer of LAN-5 neuroblastoma cell differentiation. Neuroscience. 1998;82:1021–1028. doi: 10.1016/s0306-4522(97)00352-7. [DOI] [PubMed] [Google Scholar]
- Pellman EJ, Powell JW, Viano DC, Casson IR, Tucker AM, Feuer H, Lovell M, Waeckerle JF, Robertson DW. Concussion in professional football: epidemiological features of game injuries and review of the literature--part 3. Neurosurgery. 2004;54:81–94. doi: 10.1227/01.neu.0000097267.54786.54. discussion 94-86. [DOI] [PubMed] [Google Scholar]
- Ponsford J, Tweedly L, Taffe J. The relationship between alcohol and cognitive functioning following traumatic brain injury. Journal of clinical and experimental neuropsychology. 2013 doi: 10.1080/13803395.2012.752437. [DOI] [PubMed] [Google Scholar]
- Ponsford J, Whelan-Goodinson R, Bahar-Fuchs A. Alcohol and drug use following traumatic brain injury: a prospective study. Brain injury : [BI] 2007;21:1385–1392. doi: 10.1080/02699050701796960. [DOI] [PubMed] [Google Scholar]
- Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. European journal of pharmacology. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- Raivich G, Bohatschek M, Kloss CU, Werner A, Jones LL, Kreutzberg GW. Neuroglial activation repertoire in the injured brain: graded response, molecular mechanisms and cues to physiological function. Brain research. Brain research reviews. 1999;30:77–105. doi: 10.1016/s0165-0173(99)00007-7. [DOI] [PubMed] [Google Scholar]
- Ramchand RMJ, Schell T, Jaycox L, Marshall GN, Tanielian T. Prevalence and Correlates of Drinking Behaviors Among Previously Deployed Military and Matched Civilian Populations. Military Psychology. 2011;23(1):6–21. doi: 10.1080/08995605.2011.534407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riikonen J, Jaatinen P, Rintala J, Porsti I, Karjala K, Hervonen A. Intermittent ethanol exposure increases the number of cerebellar microglia. Alcohol and alcoholism. 2002;37:421–426. doi: 10.1093/alcalc/37.5.421. [DOI] [PubMed] [Google Scholar]
- Rivest S. Regulation of innate immune responses in the brain. Nature reviews. Immunology. 2009;9:429–439. doi: 10.1038/nri2565. [DOI] [PubMed] [Google Scholar]
- Rodgers KM, Bercum FM, McCallum DL, Rudy JW, Frey LC, Johnson KW, Watkins LR, Barth DS. Acute neuroimmune modulation attenuates the development of anxiety-like freezing behavior in an animal model of traumatic brain injury. Journal of neurotrauma. 2012;29:1886–1897. doi: 10.1089/neu.2011.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz SM, Oscar-Berman M, Sawyer KS, Valmas MM, Urban T, Harris GJ. Drinking history associations with regional white matter volumes in alcoholic men and women. Alcoholism, clinical and experimental research. 2013;37:110–122. doi: 10.1111/j.1530-0277.2012.01862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson G, Tate R. Suicidality after traumatic brain injury: demographic, injury and clinical correlates. Psychological medicine. 2002;32:687–697. doi: 10.1017/s0033291702005561. [DOI] [PubMed] [Google Scholar]
- Swartzwelder HS, Wilson WA, Tayyeb MI. Differential sensitivity of NMDA receptor-mediated synaptic potentials to ethanol in immature versus mature hippocampus. Alcoholism, clinical and experimental research. 1995;19:320–323. doi: 10.1111/j.1530-0277.1995.tb01509.x. [DOI] [PubMed] [Google Scholar]
- Tang D, Kang R, Zeh HJ, 3rd, Lotze MT. High-mobility group box 1 and cancer. Biochimica et biophysica acta. 2010;1799:131–140. doi: 10.1016/j.bbagrm.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng SX, Molina PE. Acute alcohol intoxication prolongs neuroinflammation without exacerbating neurobehavioral dysfunction following mild traumatic brain injury. Journal of neurotrauma. 2014;31:378–386. doi: 10.1089/neu.2013.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrio H, Brenner LA, Ivins BJ, Cho JM, Helmick K, Schwab K, Scally K, Bretthauer R, Warden D. Traumatic brain injury screening: preliminary findings in a US Army Brigade Combat Team. The Journal of head trauma rehabilitation. 2009;24:14–23. doi: 10.1097/HTR.0b013e31819581d8. [DOI] [PubMed] [Google Scholar]
- Thomas JO. HMG1 and 2: architectural DNA-binding proteins. Biochemical Society transactions. 2001;29:395–401. doi: 10.1042/bst0290395. [DOI] [PubMed] [Google Scholar]
- Vetreno RP, Crews FT. Adolescent binge drinking increases expression of the danger signal receptor agonist HMGB1 and Toll-like receptors in the adult prefrontal cortex. Neuroscience. 2012;226:475–488. doi: 10.1016/j.neuroscience.2012.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YC, Lin S, Yang QW. Toll-like receptors in cerebral ischemic inflammatory injury. Journal of neuroinflammation. 2011;8:134. doi: 10.1186/1742-2094-8-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker AM, Sulzer JK, Molina PE. Augmented central nitric oxide production inhibits vasopressin release during hemorrhage in acute alcohol-intoxicated rodents. American journal of physiology. Regulatory, integrative and comparative physiology. 2011;301:R1529–R1539. doi: 10.1152/ajpregu.00035.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte J, Polansky M, Cavallucci C, Fleming M, Lhulier J, Coslett HB. Inattentive behavior after traumatic brain injury. Journal of the International Neuropsychological Society : JINS. 1996;2:274–281. doi: 10.1017/s1355617700001284. [DOI] [PubMed] [Google Scholar]
- Yang HA, Hreggvidsdottir HS, Palmblad K, Wang HC, Ochani M, Li JH, Lu B, Chavan S, Rosas-Ballina M, Al-Abed Y, Akira S, Bierhaus A, Erlandsson-Harris H, Andersson U, Tracey KJ. A critical cysteine is required for HMGB1 binding to Toll-like receptor 4 and activation of macrophage cytokine release. Proceedings of the National Academy of Sciences of the United States of America. 2010a;107:11942–11947. doi: 10.1073/pnas.1003893107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang QW, Wang JZ, Li JC, Zhou Y, Zhong Q, Lu FL, Xiang J. High-mobility group protein box-1 and its relevance to cerebral ischemia. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2010b;30:243–254. doi: 10.1038/jcbfm.2009.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang QW, Xiang J, Zhou Y, Zhong Q, Li JC. Targeting HMGB1/TLR4 signaling as a novel approach to treatment of cerebral ischemia. Frontiers in bioscience. 2010c;2:1081–1091. doi: 10.2741/s119. [DOI] [PubMed] [Google Scholar]
- Zhao YN, Wang F, Fan YX, Ping GF, Yang JY, Wu CF. Activated microglia are implicated in cognitive deficits, neuronal death, and successful recovery following intermittent ethanol exposure. Behavioural brain research. 2013;236:270–282. doi: 10.1016/j.bbr.2012.08.052. [DOI] [PubMed] [Google Scholar]
- Zola SM, Squire LR, Teng E, Stefanacci L, Buffalo EA, Clark RE. Impaired recognition memory in monkeys after damage limited to the hippocampal region. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:451–463. doi: 10.1523/JNEUROSCI.20-01-00451.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]