Abstract
Seasonal changes in reproductive-related vocal behavior are widespread among fishes. This review highlights recent studies of the vocal plainfin midshipman fish, Porichthys notatus, a neuroethological model system used for the past two decades to explore neural and endocrine mechanisms of vocal-acoustic social behaviors shared with tetrapods. Integrative approaches combining behavior, neurophysiology, neuropharmacology, neuroanatomy, and gene expression methodologies have taken advantage of simple, stereotyped and easily quantifiable behaviors controlled by discrete neural networks in this model system to enable discoveries such as the first demonstration of adaptive seasonal plasticity in the auditory periphery of a vertebrate as well as rapid steroid and neuropeptide effects on vocal physiology and behavior. This simple model system has now revealed cellular and molecular mechanisms underlying seasonal and steroid-driven auditory and vocal plasticity in the vertebrate brain.
Keywords: androgens, estrogen, aromatase, melatonin, photoperiod, potassium channels, hair cells, hearing, vocal pattern generator, auditory
1. Introduction
Vertebrates that synchronize reproductive effort with seasonally-dependent environmental cues provide discrete timepoints to identify how natural cyclical variation in physiological mechanisms coordinate the performance of specific behaviors, such as vocalization, for successful reproduction. Though many species of fish generate sound in reproductive contexts, we focus on the plainfin midshipman fish, Porichthys notatus, which has proven to be an excellent model to identify neuroendocrine mechanisms underlying seasonal plasticity in vocal-acoustic social behavior, largely because the production and perception of sound is essential to its reproductive success during a restricted time of year [1; 2; 3]. This review highlights recent studies that demonstrate neuroendocrine coupling of seasonal reproduction with adaptive plasticity in audition and vocal communication using the midshipman model.
2. Midshipman life history and reproductive behavior
Midshipman fish belong to a single order and family (Batrachoidiformes, Batrachoididae) of teleost fish known commonly as toadfishes [4]. Midshipman can be found along the western coast of the United States from as far north as Sitka, Alaska to Magdalena Bay, Mexico [5]. Intensive neuroethological studies over the last 25 years have focused on populations that reside close to nine months of the year in deep waters off the coasts of northern California and Washington state and then migrate into the rocky intertidal zone to spawn in late May to mid-August (Fig. 1A-C). This seasonal migration is quite remarkable as it requires adaptations to extreme changes in water depth and habitat which includes transitioning from the deep water benthos (down to 300m) to rocky subtidal/ intertidal shoreline (e.g., Tomales Bay, Puget Sound) where fish are often found within rocky nest shelters without water for several hours during low tide [6; 7; 8]. Little is known about specific environmental (abiotic or biotic) cues that induce the migration to shallow waters for spawning, although light/ dark cycle has recently been demonstrated to change neural excitability/ responsiveness of the vocal system (see below; [9; 10]) and is thus a likely cue. Interestingly, Foran et al. [11] showed that gonadotropin-releasing hormone mRNA in neurons of the ventrolateral thalamic nucleus is modulated by retinal input, and these neurons, which project back to the retina may in turn modulate the visual system in response to seasonal changes in environmental light conditions. Other factors, such as seasonal changes in temperature, upwelling and food abundance may also provide salient cues for gonadal recrudescence (below) and migratory behavior.
Fig. 1.
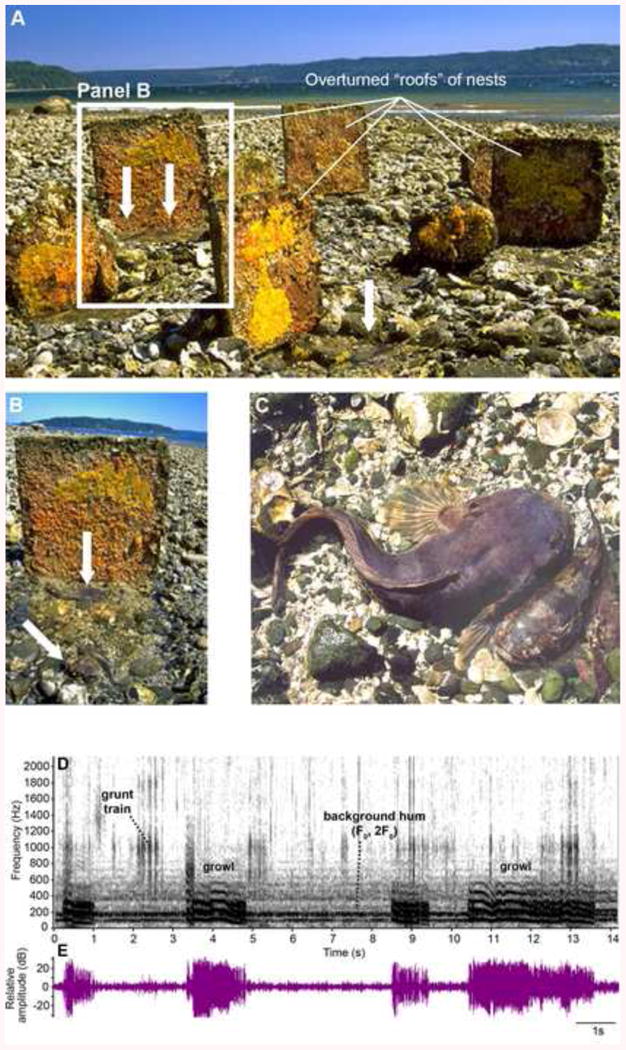
Plainfin midshipman (Porichthys notatus) nests at a site in Puget Sound, WA that was first established by E. DeMartini, who made artificial nests with cement roofs of varying size [7; 134]. Nest covers are apparent in a panoramic view (A) with one of them highlighted (B). The undersides of nest roofs are covered with newly fertilized eggs (bright yellow) and hatched larvae (orange). Nests can contain more than one male (white arrows in A, B). (C) A type I male and female viewed from above after the nest roof is removed; sexual dimorphism in body size is obvious between the larger nesting male and the female. Spectrogram (D) and waveform (E) of a hydrophone recording at 16.29°C of a sequence of type I male growls. In (E), the sound was bandpass filtered between 15-1500 Hz to reduce the incidence of background noise. Four growls are apparent in both the spectrogram and waveform records along with a grunt train and hum (F0, 2F0 indicate fundamental frequency and second harmonic) in the background from other nearby sites. Modified from [8].
Midshipman have three fixed adult reproductive phenotypes, females and two male morphs that exhibit alternative reproductive tactics [6; 12; 13; 14; 15]. Type I males excavate nests under rocks, defend these territories from neighboring males, and court females at night with a long duration (>1 min- hours) advertisement call (“hum”) made by simultaneous contraction of specialized vocal musculature on the sides of the swim bladder [8; 13; 15; 16; 17]. The hum contains a fundamental frequency of ∼100 Hz at 16°C that is positively correlated to ambient temperature (increasing by about 5 Hz per 1°C) and, importantly, is also comprised of prominent harmonics up to 700 Hz [8; 13]. These nesting males also produce agonistic calls known as grunts which are short in duration (50-200 ms) but can be produced at 1-2 Hz in long duration trains, as well as longer, amplitude modulated growls [8; 13; 18] (Fig. 1D). Both types of agonistic calls are thought to be used during competition for nests and territorial defense early in the spring/ summer breeding season [1; 13; 18].
Females find nesting males by localizing the source of the hum, deposit their eggs in a single nest by gluing individual eggs to the ceiling of the nest where fertilization occurs, and then return offshore [13]. Playbacks of tones that mimic the fundamental frequency of the hum matched to the ambient temperature, as well as playbacks of tones at frequencies that mimic a higher harmonic, show that the fundamental is both necessary and sufficient to induce a robust phonotactic response from gravid females in captivity [1; 19; 20; 21]. Importantly, spent females, who have recently spawned (within ∼ 12 hrs), never show this response. Thus, in a short window of time, the female motivation to respond to the hum is no longer evident and assumes that of a non-reproductive female.
Meanwhile, type I males care for the young while continuing to court other females until their nest is full of developing embryos [13]. Since embryos take approximately 40-50 days to absorb their yolk [22], become darkly pigmented and drop off the nest ceiling to swim away as small juveniles, type I males expend a significant amount of energy and time during the latter half of the nesting season devoted to parental care [6; 23]. In contrast, type II males neither defend a nest nor court females, but instead sneak or satellite spawn in the presence of the female to steal fertilizations from type I males [13].
Several studies have shown that type I males differ from type II males in a suite of somatic, neurological and hormonal traits that reflect their divergent reproductive behaviors [6; 12]. Interestingly, in most cases (see below), type II males are more similar to females than to type I males in these traits, suggesting type I males are the derived behavioral and neuroendocrine phenotype [24] (also see section 5.3). Thus, midshipman have also served as an excellent model to investigate both inter- and intrasexual differences in brain and behavior. Phenotypic morph differences are most pronounced during the reproductive, breeding season: type I males, which are twice the length and eight times the mass of type II's, have six times the ratio of vocal muscle to body mass compared to type II males. In contrast, type II males have nine times the ratio of testis to body mass (gonado-somatic index, GSI) compared to type I males [6]. The comparatively reduced vocal muscle seen in type II males and females reflect their vocal ability that is apparently limited to single isolated grunts [13].
3. Seasonal variation in circulating steroid hormones and morphometrics
Like other seasonally breeding vertebrates (e.g., see [25]), midshipman exhibit dramatic changes in circulating steroid hormone levels, and gonadal and somatic morphometrics that parallel changes in reproductive-related behaviors [26; 27]. In addition, as stated above, type I males show a dramatic shift from vocal courtship and territorial aggression to parental care over the course of 2-3 months in the summer which provides an additional opportunity to study neural and endocrine changes as related to reproductive state.
In addition to estrogen and testosterone, teleost fishes express 11-ketotestosterone (11-KT), a non-aromatizable androgen [28]. Sisneros et al. [27] identified natural variation in plasma levels of testosterone (T), 11-KT and estradiol (E2) by sampling adult type I and female midshipman at four different time periods related to reproductive and migratory behavior (Fig. 2). In the winter, non-reproductive period, fish are found at deepest depths and have the smallest GSI (no mature sperm and small, unyolked ova). In the spring, pre-nesting period, fish are found at the shallowest depths offshore, a time when gonadal recrudescence occurs and thus shows the greatest variation in GSI. The late spring and summer comprises the nesting period where fish are found along the rocky shoreline. As expected, when sampled from nests, male testes are filled with mature sperm and gravid females have ovaries filled with large synchronously yolked eggs reaching in total 30% or more of their body mass (also see [29]). In the fall, post-nesting period, fish are again found deep offshore and female ovaries are in a regressed state approaching non-reproductive state. As the chief identifiable characteristic of type II males is their small body size coupled with large gonad size when collected from nests during the summer reproductive period, a significant reduction in GSI in the non-reproductive season might make them indistinguishable from juvenile fish and prevent their identification in the winter non-reproductive season [27]. Therefore, seasonal studies to date have only been carried out with type I males and females. It is still unclear if type II males migrate to deep offshore sites outside of the nesting period or remain in shallower waters close to the intertidal zone.
Fig. 2.
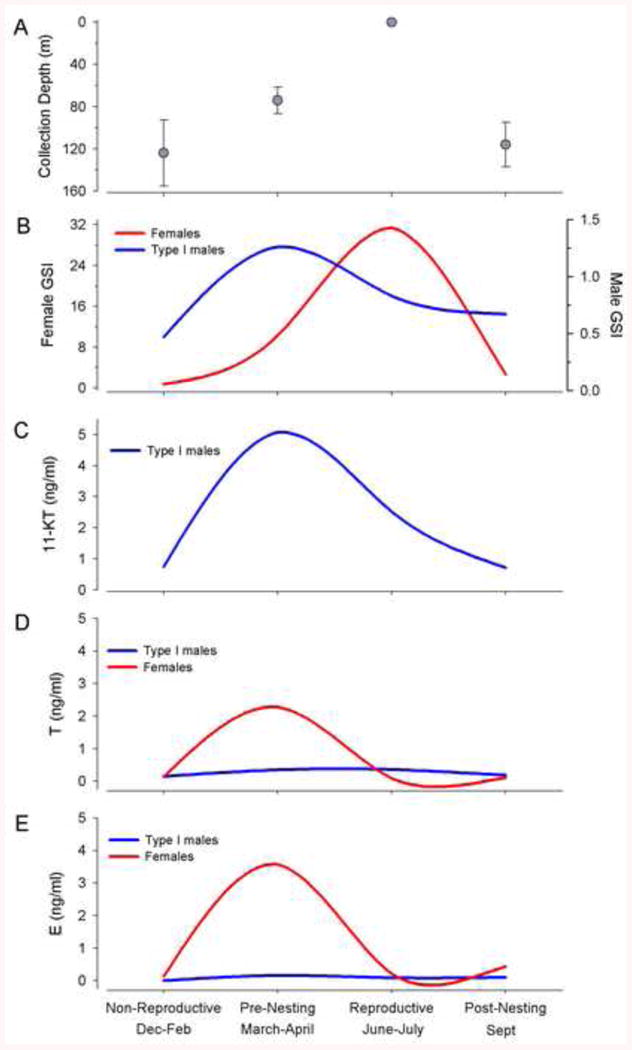
Plasma steroid concentrations for wild-caught female and type I male plainfin midshipman, Porichthys notatus, collected from Monterey Bay and Tomales Bay, CA during the non-reproductive (Dec-Feb), pre-nesting (March-April), reproductive (June-July) and post-nesting (Sept) periods between March 2001 and July 2002. (A) Collection depth and (B) Gonadosomatic index (GSI) profiles of females and type I males collected across the sampling period. (C) 11-ketotestosterone (11-KT), (D) testosterone (T), and (E) estradiol (E) concentrations in the sampled population across the sampling period. Modified from [27].
Sex differences in the temporal cycling of each steroid hormone parallel sex differences in reproductive behavior: females exhibit a single peak in both T and E2 in the pre-nesting period while the remainder of the year (nesting, post nesting and non-reproductive) levels are equivalently low [27]. In contrast, type I males ramp up androgen levels in the pre-nesting period and maintain high levels of 11-KT through the first half of the nesting period (July-August) during courtship and spawning [27; 30; 31]. Indeed, circulating 11-KT is elevated during advertisement calling ([32]; also see [33] for closely related toadfish) and returns to non-reproductive levels at the time of exclusive parental care in the latter half of the nesting period [23; 27]. T shows less variation but is similarly highest in the pre-nesting and nesting period in type I males [27].
To further demonstrate inter and intra-seasonal changes in body condition, vocal muscle and gonadal development associated with the reproductive cycle and behavior of type I males, Sisneros et al. [26] sampled not only non-reproductive and pre-nesting males as described above, but also divided the nesting period into 3 stages (also see [31]): N1 (May), where males only had nests containing fresh eggs; N2 (June-July), where nests had broods of 50% non-pigmented, recently hatched embryos; N3 (July-August), where broods contained at least 75% well-developed embryos. Body condition (measured as Fulton's condition factor) was highest during N1and N2 phase and significantly declined by N3 (Fig. 3A). This is largely attributable to high metabolic expenditure of calling behavior, followed by nest guarding and parental behavior coupled with an anorexic state [26]. Type I male GSI peaked during pre-nesting and N1 stages and steadily declined until GSI values in the non-reproductive period were 1/4 - 1/3 that of the start of the nesting period (Fig. 3B). Finally, vocal musculature was greatest during all phases of the nesting cycle and lowest in the non-reproductive season, strongly suggesting minimal, if any, vocal behavior in the winter when animals are deep offshore (Fig. 3C). The greatest increase in vocal muscle size was seen in the pre-nesting period coincident with the great increase in GSI. It is likely that the initial increase in 11-KT coincident with maximum GSI in the pre-nesting period serves to build vocal muscle (and central vocal motoneurons, see below) in type I males in preparation for intense vocal courtship and territoriality during the initial nesting period. For comparisons to other closely related toadfishes, see [34; 35].
Fig. 3.
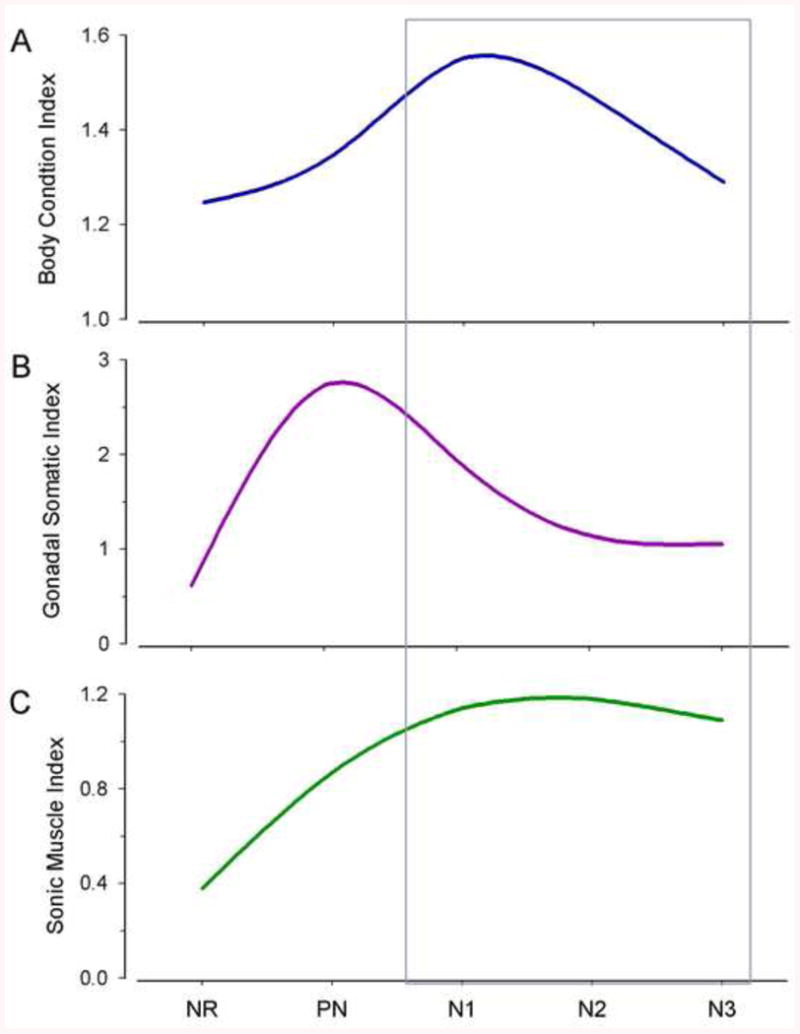
Seasonal variation in body condition (A), gonadosomatic (B) and sonic muscle somatic (C) indices of wild-caught type I male Porichthys notatus collected from Puget Sound and the Hood Canal, WA during the non-reproductive (NR), pre-nesting (PN) and three stages (N1-N3) of the nest cycle during the reproductive period between May 2005 and January 2007. The nesting period was divided into three stages (based on the nest cycle): nesting 1 (N1) defined as an early stage of the nest cycle when type I males had nests with only fresh eggs, nesting 2 (N2) was an intermediate stage of the nest cycle when males had nests with broods of at least 50% non-pigmented, recently hatched embryos, and nesting 3 (N3) was a late stage of the nest cycle when males had nests with broods of at least 75% well-developed pigmented embryos. Modified from [26].
4. Targets of seasonal steroid action in vocal and auditory systems: distribution of aromatase and steroid receptors
As summarized above, sex-specific profiles of circulating steroid hormones vary with sex-specific reproductive behavior that involves vocal courtship in type I males and auditory-driven mate localization in females [1; 2]. In addition to gonadal and adrenal sources of steroid hormones, the brain provides a local source of estrogen to discrete nuclei through the conversion of testosterone to estradiol via the enzyme aromatase that is known to be more highly expressed in the brain of teleost fishes compared to any other vertebrate group [36; 37; 38]. Unlike other vertebrates, aromatase is expressed exclusively in glial cells in the central nervous system (CNS) of teleosts and importantly, this arrangement can function to regulate the amount of testosterone and/ or provide estrogen to specific neuronal populations on a long-term or rapid timescale [38; 39]. Thus, in some instances, seasonal fluctuations in gonadal steroids may affect brain circuitry and behavior by serving as substrates for neurosteroid metabolism.
Both the descending vocal motor and auditory systems, including sites of integration, have been physiologically and anatomically well-characterized in midshipman (Fig. 4) [40; 41; 42; 43]. As a result, this foundational work has facilitated the identification of both androgen (AR) and estrogen receptors (ER) and sites of estrogen synthesis within behaviorally relevant circuitry (Fig. 4) that is differentially activated by season.
Fig. 4.
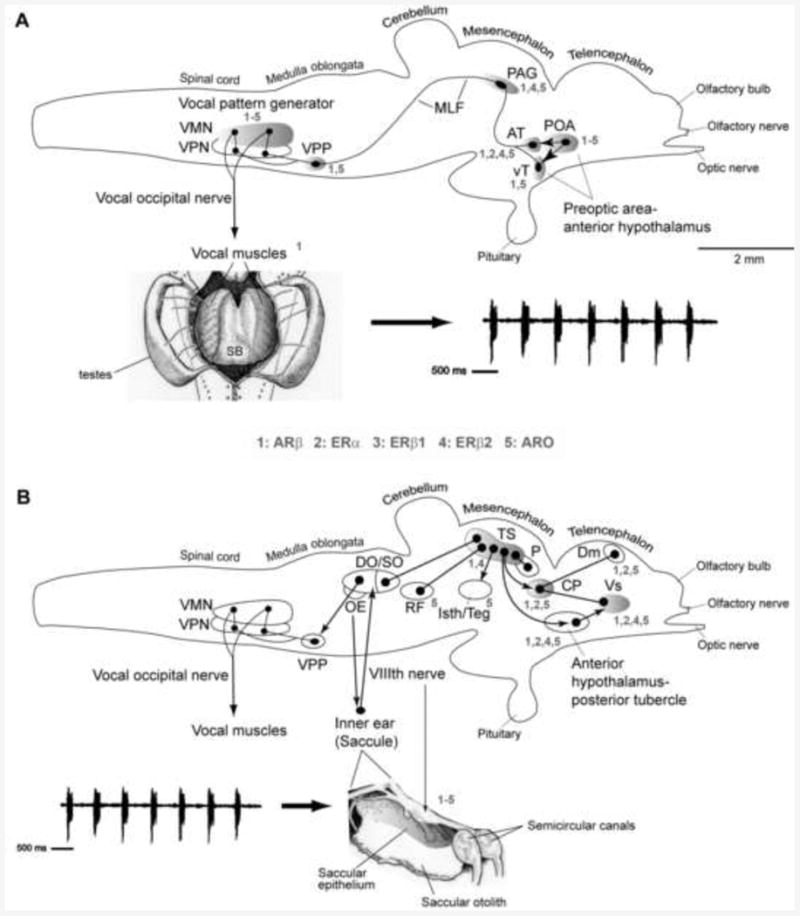
Side views of the brain showing sites of steroid hormone receptor and aromatase (estrogen synthase) in vocal motor (A) and auditory (B) systems in batrachoidid fish (midshipman and toadfish) (modified from [2; 53]). Solid dots represent somata, and lines represent axonal projection pathways. Two connected dots indicate reciprocal connections. Numbers indicate localization of 1) androgen receptor beta (ARβ) mRNA; 2) estrogen receptor alpha (ERα) mRNA; 3) estrogen receptor beta 1 (ERβ1) protein; 4) estrogen receptor beta 2 (ERβ2) protein 5) aromatase mRNA and protein [see 39; 50; 53; 81; 135]. (A) Descending vocal pathways. Preoptic (POA) and hypothalamic ventral (vT) and anterior (AT) tuberal nuclei project to the midbrain periaqueductal gray (PAG) which innervates, in turn, the vocal pattern generator circuit (VPG) in the hindbrain-spinal cord. The VPG consists of vocal prepacemaker (VPP), pacemaker (VPN) and motor (VMN) nuclei. VMN axons exit the brain via occipital nerve roots (likely homologs of hypoglossal) to sound-producing vocal muscle attached to the swim bladder (ventral view). Also shown is a representative “grunt train” vocalization produced by nest guarding male. (B) Central auditory pathways. Vocalizations (e.g., grunt train) are detected by auditory saccule within the inner ear which projects via the VIIIth nerve to auditory nuclei in the hindbrain that innervate the auditory midbrain torus semicircularis (TS). Shown are nuclei interconnected with TS. A dorsal thalamic nucleus (central posterior nucleus, CP) contains reciprocal connections to the telencephalon (dorsomedial/Dm) and ventral supracommissural (Vs) that receives input from anterior hypothalamus-posterior tubercle [for nomenclature see 136]. TS and CP also connect to forebrain (anterior hypothalamus, POA) and midbrain (PAG, isthmal/tegmentum) vocal sites, while auditory-recipient hindbrain nuclei connect to the pattern generating VPG circuit (also see [42; 44]). Modified from [49; 53]; see these reports for additional background references.
4.1. Vocal system
Briefly, the descending vocal pathway of toadfishes consists of forebrain nuclei in the preoptic area and anterior hypothalamus with input to midbrain nuclei that include the periaqueductal gray (PAG), which, in turn, projects to the hindbrain vocal central pattern generator (CPG) (Fig. 4A) [41; 42; 43; 44; 45]. As explained in more detail below in section 5.3, the vocal CPG consists of vocal pre-pacemaker neurons (VPP) that receive input from midbrain vocal centers and hindbrain auditory nuclei, and innervate vocal pacemaker neurons (VPN) and the vocal motor nucleus (VMN) that extends from the caudal hindbrain into the rostral spinal cord and whose axons exit the caudal hindbrain as occipital nerve roots to innervate sound-generating musculature attached to the lateral walls of the swim bladder (Fig. 4A) [41; 42; 44; 46; 47].
Aromatase-containing cell bodies are prominent along ventricular surfaces throughout the brain with radially extending fibers, thus covering forebrain vocal areas including parvocellular preoptic nuclei (PPa/p), anterior tuberal (AT) and ventral tuberal hypothalamus (vT) as well as the PAG and neighboring vocal sites in the midbrain. This pattern extends into the hindbrain where aromatase cell bodies conspicuously line the fourth ventricle with processes coursing ventrolaterally into the reticular formation that includes VPP neurons [39]. Ideally localized to supply vocal motor neurons with local estrogen (or to prevent androgens from reaching the same neurons), the dorsal borders of the extensive VMN are lined with aromatase somata and fibers course ventrally between motor neurons [39].
Due to whole genome duplication events among actinopterygians [48], many teleosts express subtypes of ERs and ARs. ERs (ERα, ERβ1, ERβ2) are found in some combination (largely ERα and ERβ2) in all vocal nuclei, with the exception of the vT and VPP, where aromatase is expressed [49; 50]. In the VMN, all three ER subtypes appear to be expressed in neurons, not glial cells [49; 50]. The levels of ERβ2 expression parallel the levels of aromatase activity and aromatase mRNA expression in the VMN, i.e., greater in type II males and females compared to type I males [12; 49; 51]. It is noteworthy that androgen receptor beta (ARβ) expression is found in virtually all nuclei of the vocal motor pathway including vocal muscle, consistent with expression of androgen receptors in other vocal vertebrates [32; 52; 53]. Unlike ERs, ARβ expression is found in a similar pattern to aromatase, lining the dorsal VMN [53] suggesting colocalization in this area.
Although seasonal changes in steroid hormone receptor expression within vocal circuitry have not been investigated, aromatase mRNA expression in the VMN is greatest in females and type I males in the pre-nesting and nesting period, respectively, when sex-specific circulating steroids are highest, compared to their non-reproductive state [54]. Both testosterone and estradiol upregulate aromatase mRNA in the VMN of females to pre-nesting levels, while ovariectomized females mimic non reproductive levels of aromatase in the VMN [55]. As clearly demonstrated in both females and type II males, testosterone upregulation of brain aromatase expression specifically in the VMN dorsal periphery is consistent with the expression pattern of ARβ in that area that comprises a dense band of glial cells [12; 53; 55]. It is likely that the initial rise in plasma testosterone and estrogen in the pre-nesting period serves to upregulate brain aromatase in and around vocal nuclei (e.g., VMN, PAG), which may be maintained via positive estrogenic feedback, thereby priming vocal-acoustic circuitry for social vocal behavior during the nesting period (see [38] for further discussion).
4.2. Auditory system
Like other teleosts, the saccule, which contains sensory hair cells, is the main end organ of hearing in the inner ear of midshipman [17]. Saccular primary afferents project to hindbrain auditory areas including the descending octaval nucleus and secondary octaval populations, both of which project to the midbrain torus semicircularis (TS), homologous to the mammalian inferior colliculus [40; 56]. Several diencephalic nuclei including AT (above) and the thalamic central posterior nucleus receive input from the TS and relay information to pallial and subpallial nuclei including the supracommissural nucleus of the ventral telencephalon that also receives input from the vocal PAG [40; 42; 44; 57; 58]. A hindbrain octavolateralis efferent nucleus (OE) projects to the inner ear end organs and lateral line system and also receives input from the vocal motor system [40; 41; 44; 59; 60]. Additionally, the auditory system in midshipman is interconnected to vocal nuclei in the forebrain, midbrain and hindbrain (Fig. 4) [40; 42; 45; 61].
With the exception of the forebrain, aromatase is not localized to other central auditory nuclei [39]. The TS contains both ARβ and ERβ2, while hindbrain auditory nuclei do not appear to be direct targets of steroid hormones [49; 50; 53], but may be affected indirectly through hormone-modulated neurotransmitter systems (below). In contrast, ISH, ICC and/or qPCR show that the saccular epithelium contains ARβ and all three ERs and aromatase is found in ganglion cells in the saccular branch of the eighth nerve, providing very strong anatomical evidence for the peripheral auditory system as a target for steroid hormones and site of seasonal auditory plasticity [39; 49; 50; 53]. Interestingly, ERα and ARβ are found adjacent to saccular hair cells (ARα has not been localized, although qPCR indicates its presence; D. Fergus and A. Bass, unpublished observations), while ERβ1/2 are differentially localized within hair cells: ERβ1 is localized at the apical end of hair cells, while ERβ2 is found broadly but variably expressed in the cytoplasm [49].
Seasonal changes in aromatase and steroid receptor expression in central and peripheral auditory system has not been investigated in midshipman. However, steroid hormone receptor mRNA expression has also been identified in the saccule of the cichlid, Astatotilapia burtoni, by qPCR (cellular localization not investigated). This species, although not “vocal” (see below), produces sounds during courtship [62], and receptor expression changes with reproductive state [63].
5. Seasonal enhancement of vocal ability
5.1. Vocal Behavior
Studies of the hormonal control of sound production in teleost fishes date back to 1960 when Winn and Stout [64] reported that testosterone injections in males of the satinfin shiner, Cyprinella analostana (previously Notropis analostanus), induced increases in sound production over a period of 5 to 10 days compared to males injected with sesame oil and “normal control fish”. Later studies of toadfishes including midshipman correlated elevated circulating steroid levels with the seasonal onset of vocal behavior (see section 3). More recent investigations have more directly correlated elevated peripheral steroid levels with calling behaviors. Genova et al. [32] showed that either plasma or testis levels of the androgens 11-KT and testosterone, respectively, are elevated during advertisement calling (“humming”) in a captive population of midshipman fish. Field studies of the closely related Gulf toadfish (Opsanus beta) showed that within two days of being transplanted into a population of advertisement calling males, non-calling males produced advertisement calls and exhibited significantly higher plasma 11-KT levels with a strong trend for higher cortisol levels [33]. These authors also showed that playbacks of advertisement calls, known as boatwhistles, from an underwater speaker to males nesting in their natural habitat led to significant increases in the duration and rate of boatwhistle calling as well as in circulating 11kT levels in the nesting males, A second study at the same study site showed that the administration of 11-KT via food delivery to nesting, advertisement calling males led to an increase in the number, but not duration, of advertisement calling within 10-20 minutes following feeding [65]. A third study at this site demonstrated an acoustic startle response in advertisement calling males that was linked to rapid increases in plasma cortisol levels [66]. Atlantic bottlenose dolphins (Tursiops truncates) prey upon toadfish and other sonic fishes [67]. Following playbacks of dolphin calls known as pops that have a frequency content overlapping the hearing range of toadfish, there was a significant reduction in the number, but not the duration, of boatwhistle calls by males that was paralleled by significantly elevated plasma cortisol levels.
5.2. Vocal Muscle
Several studies have documented the effects of androgens on sonic muscle mass (e.g., [68; 69; 70; 71] consistent with seasonal shifts in circulating androgen levels and sonic muscle mass (e.g., [26; 72; 73]). Most recently, Genova et al. [32] investigated molecular mechanisms that might be mediating the effects of steroids on sonic muscle function by comparing the abundance of mRNA transcripts coding for different receptors and enzymes involved in steroid signaling pathways between advertisement calling (“humming”) and non-calling type I male midshipman. Building upon prior molecular studies of type I male vocal muscle [74; 75], these authors used quantitative real time PCR to measure the relative abundance of mRNA transcripts for two androgen (ARα, ARβ) and two glucocorticoid receptors (GR-1, GR-2), and two enzymes involved in steroid metabolism, namely 11β-hydroxysteroid dehydrogenase (11βHSD) (which converts cortisol to cortisone and 11β-hydroxy (OH)-testosterone (T) to 11-KT) and 11β-hydroxylase (11βH) (which converts 11-deoxycortisol to cortisol and testosterone to 11β-OH-T). The vocal muscle of advertisement calling type I males exhibited higher ARα mRNA transcript levels, while non-hummers showed higher ARβ, GR-1, GR-2, and a strong trend for higher 11βHSD. These authors also measured plasma steroid levels. Humming males showed higher 11-KT levels whereas non-humming males showed higher cortisol levels. Humming males also had higher testis levels of 11-KT as well as testosterone. Together the results show divergent steroid signaling profiles that parallel divergent plasma steroid levels for vocalizing and non-vocalizing males. Higher expression of ARα implies a specific role in supporting the effects of steroids on vocal muscle physiology during advertisement calling, while higher expression of other steroid-related transcripts in the vocal muscle of non-calling males suggests a preparatory role for the physiological demands of long bouts of advertisement calling.
5.3. Central Vocal Mechanisms
5.3.1. Central Vocal Network
Before reviewing the evidence for hormonal modulation of the central vocal network in fishes, we first provide some background in this section explaining why vocal fish are especially good models for demonstrating neurohormonal modulation of neurophysiological mechanisms underlying seasonal shifts in vocal behavior.
Bass and colleagues [44] first used the term vocal to describe sound producing fishes in a neuroanatomical study of brainstem vocal circuitry in the midshipman fish, in part, to draw attention to similarities between the vocal systems of fishes and tetrapods. These authors noted the following characters in making a comparison between the neural circuitry underlying sound production in midshipman to vocal mechanisms among tetrapods (see [76] for more recent review): (1) divergent patterns of acoustic signaling in differing social contexts, (2) a peripheral organ and attached muscles dedicated to sound production, in this case the swim bladder, (3) shared developmental origins of sonic swim bladder, sonic laryngeal and sonic syringeal muscles from occipital somites, (4) innervation of sonic muscles by occipital nerve roots considered as homologues of hypoglossal nerve roots, and (5) a sonic motor nucleus in the same position as the tracheosyringeal division of the hypoglossal motor nucleus in birds. Since that time, a subsequent mapping study of early development in midshipman and other toadfish species has shown that a hindbrain vocal pattern generator (details below) develops in the same neuroepithelial compartment that gives rise to the vocal hindbrain circuitry in tetrapods [77].
Neurophysiological studies of a hindbrain central pattern generator (CPG) in midshipman and the closely related toadfishes have demonstrated a simple translation between vocal network activity and the physical properties of natural vocalization makes (Fig. 5A, B). As noted at the beginning of section 4.1 a vocal CPG includes three anatomically separate hindbrain populations of premotor and motor neurons [44; 46; 77; 78]: VMN, VPN, VPP (originally designated as the ventral medullary nucleus in [44]) (Fig. 5C, D). Chagnaud et al. [41] showed that VPP and VPN code for call duration and pulse repetition rate (PRR, which sets fundamental frequency in fishes), respectively. The VMN plays an essential role in translating the premotor duration-PRR code into a highly synchronous vocal motor output [47]. This output, the vocal motor volley, directly sets the temporal firing properties of the paired vocal muscles that contract simultaneously to vibrate the swim bladder and, in turn, generate a vocalization. VMN output also contributes to patterns of amplitude modulation (AM) of natural calls. When isolated in the in vivo neurophysiological preparation, the hindbrain region containing the vocal CPG network can generate a rhythmic output mimicking the temporal properties of natural calls ([79]; see [80] for corroborative studies in Gulf toadfish). Most recently, Chagnaud and Bass [59] have also identified the synaptic properties of a vocal corollary discharge pathway in midshipman between VPP and the hindbrain nucleus (known as the octavolateralis efferent nucleus in teleosts) that directly innervates the inner ear and lateral line organs (also see [60]). These studies set the stage for a more in depth analysis of how efferent inputs from the hindbrain might play a role in seasonal shifts in the sense of hearing (see section 6).
Fig. 5.
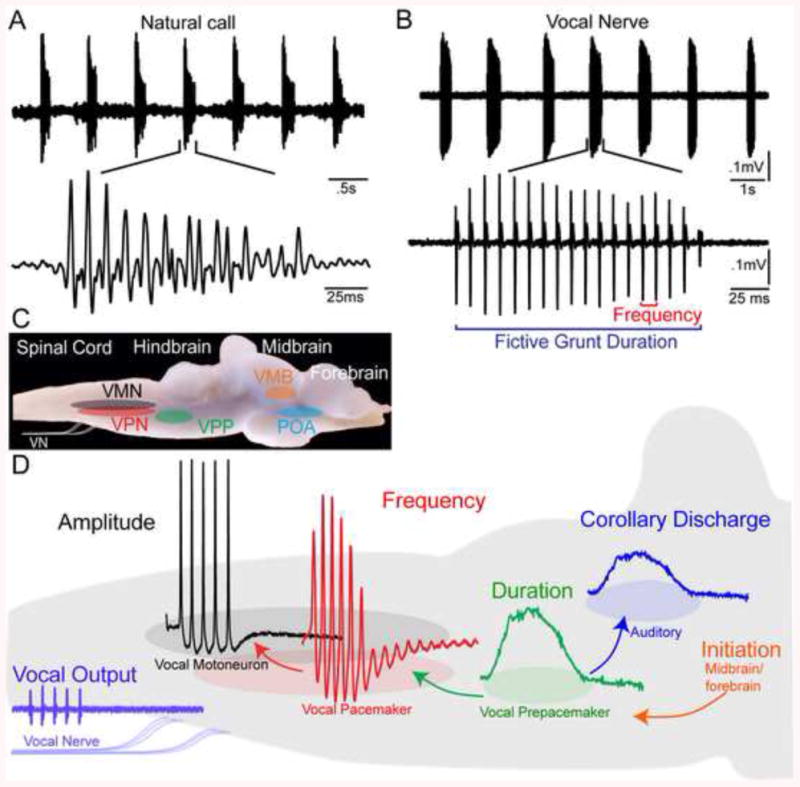
Vocal behavior and central vocal network of fish. (A) Top: Oscillogram trace of hydrophone record of a series (“train”) of agonistic grunts from the nest of a type I male plainfin midshipman fish (Porichthys notatus). Bottom: Single grunt on an expanded time scale. (B) Top: Recording from a type I male of spontaneously generated vocal nerve volley that mimics natural grunt train. Bottom: Single fictive grunt on expanded time scale. Fictive calls are readily evoked using electrical microstimulation from vocally active midbrain sites. (C) Sagittal view of brain of midshipman fish showing the location of regions comprising central vocal motor network. Vocal midbrain regions (VMB) act as a gate for descending forebrain input from the preoptic area (POA) that leads to activation of vocal central pattern generator (CPG). The Vocal CPG includes three distinct cell populations of vocal prepacemaker (VPP), vocal pacemaker (VPN) and vocal motor (VMN) neurons. (D) Schematic sagittal view of hindbrain summarizing the connectivity and neurophysiology of vocal CPG. The vocal CPG generates a vocal nerve motor volley (vocal output) that determines, in turn, natural call properties including duration and pulse repetition rate (PRR, also sets fundamental frequency in fish). Vocalization duration, frequency (PRR) and amplitude are coded by VPP, VPN and VMN neurons, respectively. VPP also transmits a corollary discharge that informs auditory hindbrain populations about the duration of natural vocalizations. Modified from [41; 76].
5.3.2. Hormonal modulation of vocal network
Pilot studies first showed that long term (8-9 weeks), intraperitoneal implants of testosterone proprionate in juvenile male midshipman can induce increased VMN neuron size and increased excitability of the vocal motor system in midshipman fish [12; 52]. Later, more comprehensive studies linked the rapid effects of steroids and neuropeptides to seasonal changes in vocal network excitability and vocal behaviors . The vocal occipital nerve volley described in the previous section is often referred to as a fictive call in an in vivo neurophysiological preparation because it is produced in the absence of muscle activation and directly determines natural call characters such as duration and PRR. Fictive calls are readily evoked following electrical stimulation or localized injections of glutamate in midbrain and forebrain (preoptic area-anterior hypothalamus) sites (e.g., [9; 42; 45; 46]). Several studies in midshipman and Gulf toadfish have used the fictive call in vivo preparation to identify rapid modulation of vocal motor activity by androgens (11-KT, testosterone), estrogens (17beta-estradiol), cortisol, vasoactive intestinal polypeptide (VIP), arginine-vasotocin (AVT; the non-mammalian homolog of arginine-vasopressin, AVP), and isotocin (IT; the non-mammalian homolog of oxytocin) and melatonin ([9; 24; 65; 79; 81; 82; 83; 84]). In general, each of these neurohormones can rapidly (within 5 – 10 minutes) modify fictive call duration when injected either exogenously into dorsal trunk (steroids and receptor antagonists) muscle or directly into the preoptic area-anterior hypothalamus (AVT, IT, and receptor antagonists) or midbrain (VIP and a receptor antagonist). While estradiol is effective in both male morphs and in females, other steroids and neuropeptides show within and between sex-specificity in midshipman fish that have alternative male reproductive morphs. With the exception of estradiol, the distinct vocal repertoire of advertisement calling type I males is paralleled by a neurohormonal sensitivity profile that is equally distinctive from that of type II males and females which are similar to each other in this dimension as well as in their vocal behavior (see [13]). Hence, 11-KT and cortisol potentiate call duration in type I males, while testosterone and IT potentiate it in both type II males and females. Cortisol increases call duration in type I males, but suppresses it in type II males and females. AVT suppresses call duration in type I males, while IT suppresses it in type II males and females.
There also appear to be species differences in the efficacy of rapid steroid modulation of fictive call duration. In Gulf toadfish, 11-KT and cortisol, but neither estradiol nor testosterone, induces rapid increases in fictive call duration [80]. Importantly, rapid steroid effects in Gulf toadfish are supported by behavioral field studies in this species that were reviewed above.
5.3.3. Diurnal rhythms in vocal mechanisms
Type I male midshipman mainly vocalize at night from nests that they build under rocky shelters either in the natural habitat, the intertidal zone, or under captive conditions during the late spring-summer reproductive season [8; 13; 16; 18; 32]. As summarized in the Introduction, type I males in reproductive condition produce grunt trains and growls that can last for seconds in duration and function in agonistic contexts, and much longer duration (mins - >1 h) hum advertisement calls that type I males use to acoustically court females (also [8; 13; 19]). Prior to 2009, studies of type I male fictive calling were conducted only during the day, irrespective of an animal's reproductive state and amount of time held in captivity. Rubow and Bass [10] investigated seasonal and daily changes in excitability of the central vocal motor network. They found that midbrain and rostral hindbrain electrical stimuli evoked fictive calls that resembled hums (e.g., Fig. 6A), growls and grunt trains (e.g., Fig. 5A, B) mainly at night from type I males maintained on long day photoperiods mimicking those during the reproductive season. These authors also showed that type I males were far more responsive to midbrain stimulation when they were in reproductive condition and tested during the dark phase of their daily cycle which is the main time of day that they produce long duration hums. Midbrain-evoked fictive calls were 60-100% longer in duration in these males compared to reproductive males tested during the light phase of their cycle and non-reproductive males tested during either portion of the photoperiod. Vocal excitability was enhanced several fold in reproductive type I males housed for five days in constant dark (5DD) as opposed to constant light (5LL).
Fig. 6.
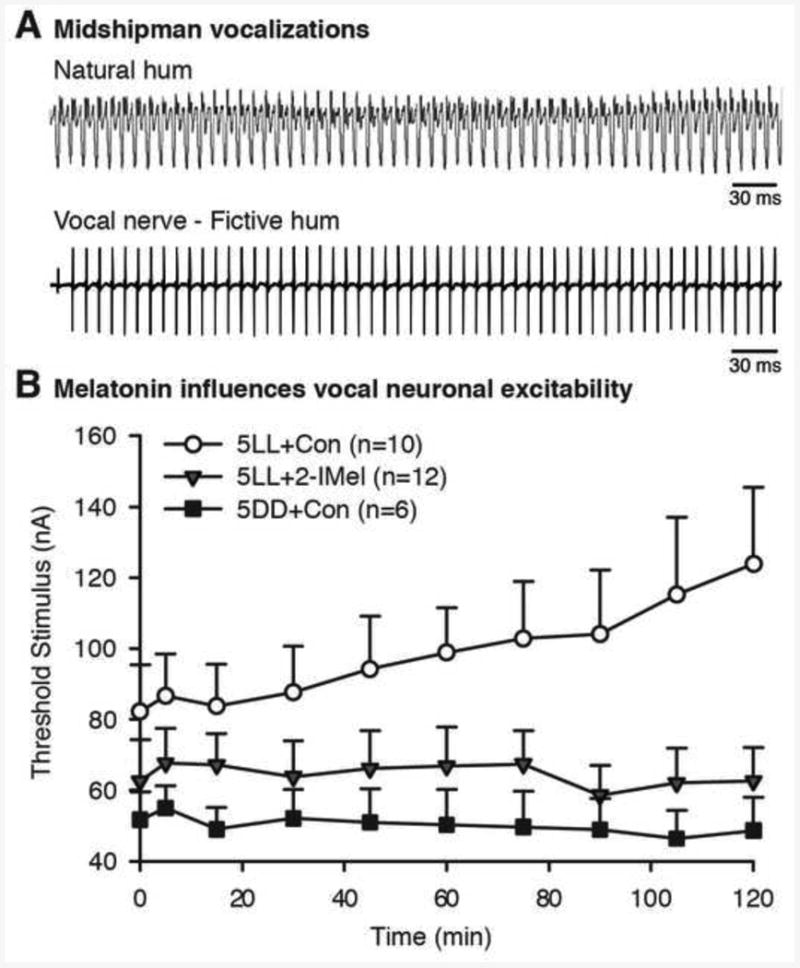
Melatonin influences vocal neuron excitability. (A) Top: Oscillogram trace of hydrophone record of advertisement call (“hum”) recorded from the nest of a type I male plainfin midshipman fish (Porichthys notatus). Bottom: Recording from a type I male of spontaneously generated vocal nerve volley that mimics natural hum. Long-duration, hum-like fictive calls are evoked using electrical microstimulation at midbrain sites from type I males during the reproductive season at nighttime. Brief, grunt-like fictive calls can be evoked at any time of day or year. (B) Summary of experimental results supporting a role for melatonin in the nocturnal rise in vocal excitability among type I males in reproductive condition (see [9] for complete documentation). Vocal excitability measured in terms of threshold, the minimum current of midbrain electrical stimuli needed to evoke a fictive call at 10 time points over the course of 120 min sessions. Type I males received an intraperitoneal implant of either 2-iodomelatonin (2-IMel), a high affinity melatonin analogue that binds to melatonin receptors, or sesame oil (implant control, CON) before subjective lights-off and were then moved to 5 days of constant light (5LL). 5LL+2-IMel males showed fictive call thresholds comparable to males with a control implant and then moved to 5 days of constant darkness (5DD+Con). Compared to these two groups, 5LL+Con fish exhibited higher thresholds at baseline (0 min) that increased over the course of the 120 min session. Adopted from [9].
The discovery of robust daily variation in the excitability of the vocal motor network led to investigations of the potential role of melatonin in modulating seasonal enhancement of vocal output. Pilot studies showed that melatonin did not exert rapid effects on fictive call properties as shown for steroids and the neuropeptides AVT and IT (see above). Adopting the experimental design of Rubow and Bass [10], Feng and Bass [9] showed that five day treatments with either melatonin (via a single intraperitoneal implants) or melatonin receptor antagonists (via daily intramuscular injections into the body wall) could essentially antagonize the effects of housing under 5LL (suppression of vocal motor excitability) or 5DD (potentiation of vocal excitability) conditions, respectively (Fig. 6B). Together, these findings led to the conclusion that melatonin supports nocturnal increments in vocal neuron excitability during the reproductive season in type I male midshipman.
6. Seasonal enhancement of hearing
6.1. Peripheral auditory plasticity
The auditory system of the plainfin midshipman provides a highly tractable model for investigating the related seasonal and steroid-dependent changes in the auditory reception and neural processing of social acoustic signals [1; 3; 6; 18; 85; 86]. The response properties and sensitivity of the midshipman peripheral auditory system, including the saccular hair cells and their afferents, have been extensively investigated and characterized in this highly soniferous fish [86; 87; 88; 89; 90; 91; 92]. The midshipman's inner ear is composed of three semicircular canals with their associated sensory regions (cristae ampullaris) and three otolithic end organs: the saccule, the lagena and the utricle (e.g., Fig. 7A). All three otolithic end organs are innervated by the eighth cranial nerve, but the saccule is the main acoustic end organ used for hearing in the midshipman as in most other teleost fishes [17; 93]. The afferents and hair cells of the saccule are broadly tuned with peak frequency sensitivity near the low frequency components of midshipman vocalizations [87; 88; 89; 91].
Fig. 7.
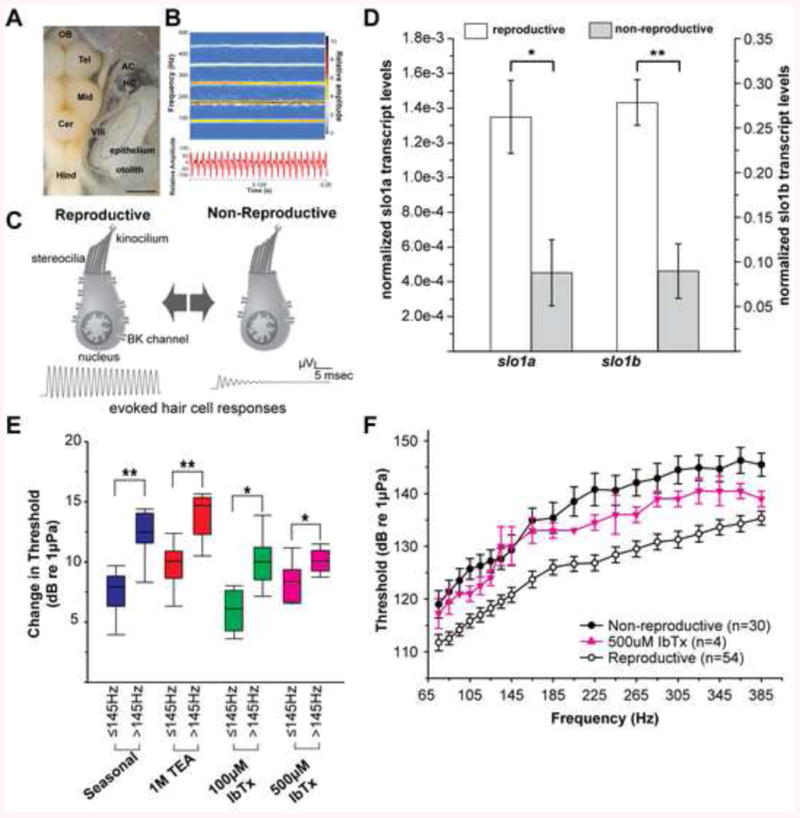
A model for seasonal changes in BK channel expression driving seasonal plasticity of auditory hair cells. A) Dorsal view of brain and inner ear. AC, anterior semicircular canal ampulla; Cer, cerebellum; HC, horizontal canal ampulla; Hind, hindbrain; Mid, midbrain; Tel, telencephalon; VIII, eighth cranial nerve. Portions of semicircular canals were removed to better visualize auditory division of the inner ear, the saccule, and its hair cell epithelium (dotted circle). Scale bar represents 1.5 mm. (B) Spectrogram (top) and waveform (bottom) of a representative midshipman advertisement vocalization with energy concentrated at the fundamental (∼100 Hz) and first two upper harmonics (∼200–300 Hz). (C) Schematic summarizing proposed role of slo1 and BK abundance in seasonal auditory plasticity (adapted from [101]. Increased expression leads to robust encoding of higher frequencies in upper harmonics of advertisement calls. (D) Normalized mRNA expression of slo1a and slo1b, the two genes encoding the pore-forming α-subunit of BK channels. *p<0.05, **p<0.0001). Data plotted as mean ± SEM. (E) Delivery of the broad spectrum potassium channel antagonist tetraethylammonium chloride (TEA) and the specific BK channel antagonist iberiotoxin (IbTx) to the saccule of reproductive animals causes frequency specific shifts in saccular thresholds similar to seasonal plasticity. (F) Injection of IbTx into the saccule of reproductive animals shifts their thresholds to a non-reproductive like state. Adapted from [113].
Previous studies of the midshipman's nocturnal courtship behavior and acoustic playback studies with natural and synthetic advertisement calls showed that “gravid” females (containing mature eggs) exhibited strong phonotaxis to the male advertisement calls, whereas “spent” females (containing little or no eggs) no longer responded to the advertisement call or “hum” [19; 20; 21; 94]. The results from these studies led researchers to investigate whether seasonal variation in reproductive state (gravid vs. non-gravid) influenced midshipman auditory sensitivity. Sisneros and Bass [91] showed that the saccular afferents from reproductive females exhibited higher phase-locking accuracy to a range of frequencies including those associated with the dominant harmonic components of hum (∼ 180-400 Hz; Fig. 7B) compared to non-reproductive females. In addition, Sisneros [90] showed that the hair cells of the saccule in summer reproductive females were approximately 2.5 to 4.5 times more sensitive than non-reproductive females from 75 to 385 Hz. In sum, these studies showed that the auditory sensitivity of saccular hair cells and their afferents changed seasonally with reproductive state and that reproductive females were more optimized than non-reproductive females to encode the dominant harmonic frequencies of the advertisement call [90; 91].
Subsequent studies showed that seasonal auditory plasticity is not limited to females but that type I males also exhibit seasonal changes in saccular hair cell sensitivity [88]. Recordings of evoked saccular potentials from nonreproductive and reproductive type I males collected from multiple sites over multiple years revealed that the saccular thresholds of type I males undergo seasonal changes in saccular hair cell physiology statistically indistinguishable from that of females [88]. Furthermore, the saccular hair cell thresholds in both sexes showed seasonal differences that are greater at frequencies above 145 Hz (Fig. 7) [88]. These findings along with those of previous studies [90; 91] strongly affirm seasonal plasticity in the peripheral auditory system as a biological phenomenon that occurs on an annual basis in midshipman fish populations along the Pacific Coast of the United States.
The frequency overlap between saccular hair cell and eighth nerve plasticity suggests that much or perhaps all of the changes observed at the level of the eighth nerve reflect changes at the level of the hair cell. The seasonal change in saccular hair cell sensitivity may be adaptive for type I males to assess other males during the competition and establishment of nest sites during the breeding season. It is not currently known whether type II males also exhibit seasonal auditory plasticity but there is no a priori reason to expect that such plasticity would be limited to the type I male and female morphs since seasonal enhancement in auditory sensitivity would benefit type II males in their ability to detect the calls of type I males and facilitate the selection of cuckoldry sites for sneak or satellite spawning. The presence of seasonal saccular sensitivity at comparable levels between type I males and females should not be surprising as both show sex-specific phonotactic responses to broadcast nesting male courtship vocalizations during the reproductive season [19]. Hence, both sexes may make use of the greater frequency envelope during breeding season for their own sex-specific behaviors [13] and the most parsimonious hypothesis is that the mechanism(s) of seasonal plasticity in the peripheral auditory system is conserved across sexes and possibly even across the different reproductive morphs.
In addition to the seasonal changes in peripheral auditory sensitivity, Coffin et al. [95] showed that females exhibit a seasonal increase in saccular hair cell density that was concurrent with changes in auditory saccular sensitivity. Reproductive females were found to have a seasonal increase in hair cell receptors in the saccule (Fig. 8) that was not dependent on body size because similar changes in hair cell density were not found in the inner ear's other two otolithic end organs, the utricle and the lagena. Coffin et al. [95] also reported seasonal increases in small, potentially immature hair bundles (Fig. 8) and decreased hair cell death in the saccule of reproductive females. These seasonal increases in smaller hair cell bundles and saccular hair cell receptors in reproductive females were paralleled by an increase in the evoked magnitude of the saccular potentials and a corresponding increase in auditory sensitivity recorded from the saccule. This recent demonstration of concurrent seasonal plasticity in saccular sensitivity and hair cell density may in part facilitate the proposed adaptive auditory plasticity of this species to enhance mate detection during the breeding season.
Fig. 8.
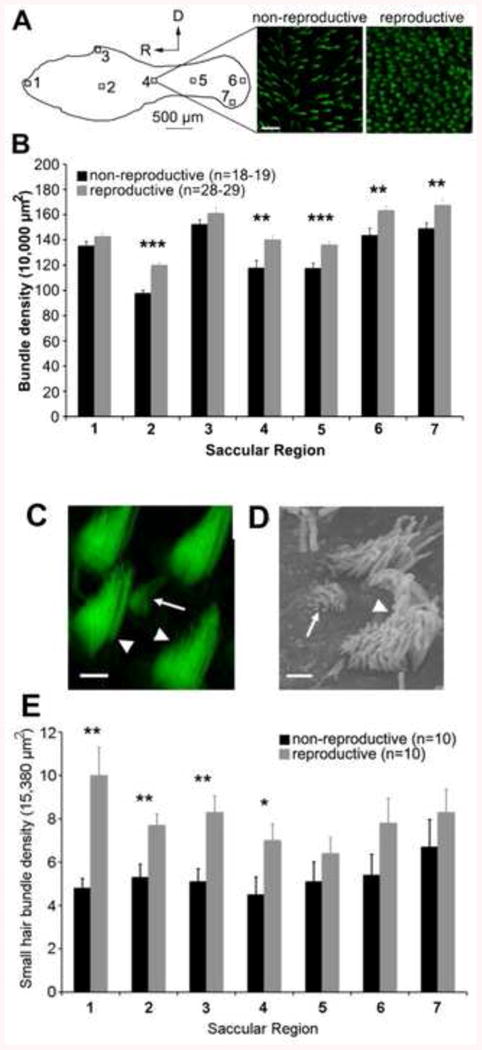
Seasonal differences in hair bundle density and in the number of small hair bundles in the saccule of female midshipman (P. notatus). A) Phalloidin-labeled hair bundles were counted in 10,000 μm2 areas from seven regions across the saccule, as indicated by the numbered boxes on the line drawing (top portion of figure). The micrographs (top right) show representative images from the middle of the saccule from a nonreproductive and reproductive female. B) Hair bundle quantification from these seven saccular regions shows significantly more hair cells in noted regions (**, p <0.01; ***, p<0.001). Note: black bars, nonreproductive females; gray bars, reproductive females. Modified from [95]. C) Confocal brightest-point projection and scanning electron micrograph (B) showing a small, immature-like bundle (arrows) surrounded by a larger, mature hair bundles (arrowheads). Scale bars: C, 2 μm, B, 1 μm. E) Small hair bundles were counted in seven 15,380 μm2 regions of ten saccules from both reproductive (gray bars) and nonreproductive (black bars) females. There were significantly higher densities of small hair bundles between reproductive and nonreproductive females in the noted saccular regions (* p <0.05; **, p<0.01). Modified from [95].
The detection of the higher dominant harmonic components of the male's advertisement call likely increases the probability of conspecific detection and localization. The advertisement call's harmonics have been proposed to provide greater signal detection because the dominant higher harmonics in the call (200-400 Hz) will propagate further than the lower fundamental frequency (∼80-100 Hz) in shallow water due to the inverse relationship between water depth and the cutoff frequency of sound transmission (i.e., as the water depth decreases, the cutoff frequency increases) [91; 96; 97; 98; 99]. In addition, the higher harmonics of the advertisement call may also affect the perception of the call's fundamental frequency when the receiver is near the sound source during localization. McKibben and Bass [86] showed that the encoding of the advertisement call's fundamental frequency by saccular afferents is enhanced when harmonics are added to tonal stimuli.
6.2. Steroid-dependent auditory plasticity
As discussed previously (section 3), female midshipman from natural populations exhibit elevated blood levels of E2 and T approximately one month before the beginning of the summer reproductive season [27]. These observations led us to test the hypothesis that T and E2 could induce seasonal changes in peripheral auditory sensitivity via changes in the phase-locking accuracy and in best frequency of saccular afferents in spring pre-nesting females. Sisneros et al. [100] showed that implanting ovariectomized non-reproductive females with either E2 or T capsules that mimicked pre-nesting steroid levels resulted in increased in phase-locking accuracy of the saccular afferents at frequencies that corresponded to the dominant higher harmonic components of the male's advertisement call (Fig. 9). Midshipman-specific estrogen receptor alpha receptor (ERα) was identified in the sensory epithelium of the saccule by RT-PCR [100]. These results support our hypothesis that the saccule is a primary focal site for estrogen effects on the midshipman auditory system and that these steroid-dependent effects of estrogen act to enhance sensory-motor coupling in this communication system.
Fig. 9.
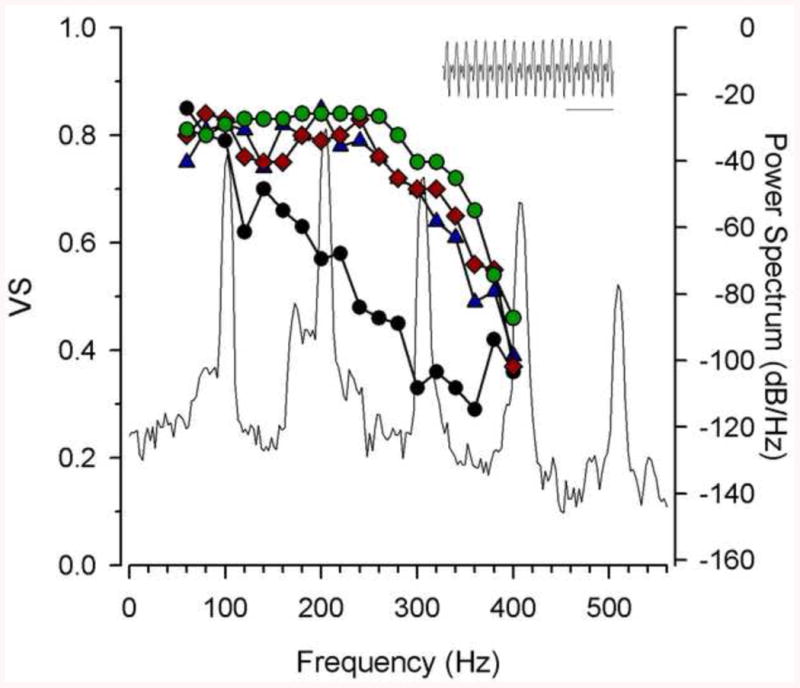
The adaptive coupling between the frequency encoding of saccular afferents and the dominant harmonic components in the male advertisement call of the plainfin midshipman fish (P. notatus). The type I male midshipman advertisement call or “hum” recorded at 16° C is shown in the inset (bar = 10 ms) above the main combined plot of the phase-locking precision of saccular afferents as a function of the vector strength of synchronization (VS, left y-axis) and the power (amplitude) spectrum level of the hum (right y-axis, in relative dB values) from a nesting type I male. Plotted are the median Vector Strength values of the saccular afferents emphasizing the overlap in frequency sensitivity between testosterone-treated (blue triangles) and 17β-estradiol-treated (pink diamonds) non-reproductive females and wild-caught reproductive females (green circles). Steroid-treated and wild-caught reproductive females show robust encoding of the fundamental frequency (∼100 Hz) and the second (∼200 Hz) and third (∼300 Hz) harmonics of the hum. In contrast, winter non-reproductive females (black circles) show comparable encoding only for frequency close to the fundamental frequency at approximately 100 Hz. Adapted from [100].
While circulating steroid hormones have been shown to be sufficient to induce seasonal-like plasticity of auditory afferents [100], the present data for a direct link between seasonal plasticity of auditory hair cells and circulating steroid levels is only correlational. During the conduct of seasonal studies of saccular hair cell physiology of type I males collected over two different winters (non-reproductive condition), it was found that thresholds between the two years varied [88]. Examination of testis size, vocal muscle size and vascularization, and measures of serum testosterone levels revealed that type I males collected in one year had undergone gonadal recrudescence and were in a transitional state between non-reproductive and reproductive season. These animals showed vascularization of the vocal muscle, presence of sperm in enlarged testes, and elevated plasma testosterone levels compared to more basal levels typical of non-reproductive animals. Increases in circulating testosterone and estradiol levels are found in both male and female midshipman during the pre-nesting phase as animals transition from a non-reproductive state to a reproductive one [27]. These transitional males had saccular thresholds that were in between those of breeding animals (both sexes) and those collected from previous non-reproductive (winter) seasons (both sexes) [88]. It would appear that what was captured in this study was the naturally occurring seasonal changes in the reproductive system and the concurrent change in circulating steroid levels correlated with a state of transition for the auditory system as well.
6.3. Molecular basis of seasonal auditory plasticity
As reviewed in the prior section, the seasonal changes in saccular physiology in midshipman fish closely mirror the initial observations of changes in afferents, Given that the expression of steroid receptors in the peripheral auditory system is restricted to hair cells [49; 50; 53] and supporting cells, it is highly likely that the steroid effect observed at the afferent level is due to a steroid effect at the hair cell level. For non-mammalian vertebrate auditory hair cells that encode sound within the frequency range of the midshipman saccule, ion channel expression determines the electrical resonant and best frequency encoded by each hair cell. A key ion channel in this system is the large-conductance, calcium-activated potassium (BK) channel that is expressed in non-mammalian vertebrate hair cells [101] as well as in the more specialized mammalian inner [102] and outer [103] hair cells. In non-mammalian vertebrates, hair cells with greater expression of BK channels are capable of higher resonant frequencies and thus are better at encoding higher frequency sounds [101]. This has been shown to be the case in physiological recordings of dissociated hair cells from the closely related toadfish [104; 105; 106]. This led to the hypothesis that an increase in BK channel expression should be seen in reproductive animals whose saccules are better capable of encoding higher frequency ranges than non-reproductive animals (Fig. 7C). BK channels were an even more compelling target for study because the expression and alternative splicing of the pore-forming α-subunit slo1 gene is regulated by steroid hormones including estradiol, glucocorticoids, and testosterone [107; 108; 109; 110; 111].
Molecular cloning of the slo1 gene in midshipman quickly revealed the presence of duplicate slo1 genes, both of which are expressed in the saccular epithelium of midshipman [112]. Absolute quantitative real-time PCR (qPCR) showed that expression levels of both slo1 transcripts, slo1a and slo1b, are upregulated in the saccular epithelium of reproductive animals compared to non-reproductive (Fig. 7D) [113]. This difference in slo1 transcript abundance was found in both type I males and females [113], consistent with seasonal plasticity that is comparable between sexes. Upregulation of slo1 transcripts during the reproductive season is consistent with higher BK channel expression and improved higher frequency encoding (Fig. 7C).
To determine the functional implications of elevated BK channel expression in reproductive animals, we pharmacologically reduced the number of functional BK channels by delivering channel antagonists to the saccular endolymph [113]. Both the broad spectrum potassium channel antagonist tetraethylammonium chloride (TEA) and the specific BK channel antagonist iberiotoxin (IbTx) caused frequency specific increases in saccular thresholds of reproductive animals compared to vehicle controls. These antagonists replicated the frequency-dependence of natural seasonal plasticity (Fig. 7E). Most importantly, the IbTx shifted thresholds of reproductive animals to the range of non-reproductive animals (Fig. 7F). IbTx was able to replicate the majority of the effects of TEA, indicating that BK channels were the primary effector of the pharmacological manipulation. Most importantly, the IbTx experiments show that by manipulating the action of a single ion channel species, we can replicate the naturally occurring range of seasonal auditory plasticity. BK channels are thus a primary determinant of seasonal auditory plasticity in midshipman saccular hair cells.
Going beyond the realm of seasonal plasticity, when the expression of slo1 genes was examined in individual animals and compared to saccular thresholds of those individuals, it was discovered that variability of slo1 expression within reproductive or non-reproductive seasons accounts for individual differences in auditory thresholds [113]. Differences in the frequency range of sensitivity of individuals has implications for mate choice in a system [113] where increases in the amplitude of call frequency by only a few hertz can determine a positive phonotactic response to playback of recorded or synthesized male courtship calls [114].
While identification of a correlation between season and slo1 expression and the pharmacological manipulation of BK number were the strongest available tests of the hypothesis that changes in BK channel expression in auditory hair cells drives seasonal auditory plasticity, we continue to develop new tools and merge our work in midshipman with other model systems with tools better suited to pursue this mechanism for hair cell plasticity. Zebrafish are a major neurobiological model organism for translational research [115] and have been a major model for study of the molecular genetics of hair cells [116; 117]. Zebrafish provide genetic tools that are not yet applicable to organisms such as midshipman. One such tool is the use of morpholinos, a type of anti-sense technology that allows one to reduce the expression of functional proteins by targeting DNA and pre-mRNA [118]. The first step has now been made in furthering our understanding of the ability of BK channel expression changes to shape auditory tuning by targeting slo1 gene expression in larval zebrafish. Consistent with the midshipman work [119], reduction of BK channel expression via use of morpholinos results in higher saccular thresholds than controls [120].
Another hypothesized mechanism that is likely non-mutually exclusive for seasonal changes in auditory sensitivity may involve the seasonal modulation of efferent activity [91]. The octavolateral efferent nucleus that directly innervates the saccule receives inputs from neurons within the vocal system [44; 60]. More specifically, these neurons are located in the vocal pre-pacemaker (VPP) nucleus which encodes the duration of vocalizations [41] sending a vocal corollary discharge to the saccule [59].
Given the increased excitability of the vocal network of type I males during the reproductive season (see Section 5.3.3), Rohmann and Bass [88] tested the hypothesis that the heightened activity of the vocal-motor network of reproductive males at night would be paralleled by changes in saccular thresholds at night due to inputs from the vocal system via efferent activity. However, no support was found for this hypothesis [88]. Studies similar to Chagnaud and Bass [59], in which there are simultaneous recordings from the vocal and saccular efferent systems, may best address questions of how daily and seasonal changes in the vocal system impact the peripheral auditory system via direct connections to the efferent auditory pathway.
6.4. Diencephalic dopaminergic neurons as potential mediators of steroid-driven seasonal auditory plasticity
While it is well established that estrogen (and testosterone) can induce seasonal peripheral auditory plasticity in midshipman adults in order to better encode the frequency content of the male advertisement call, little is known of the neural substrates that underlie the motivation and coordinated behavioral response to acoustic social signals as seen in female phonotaxis during sound playback experiments, as well as the subsequent change in attention to the call stimulus post-spawning [2]. Catecholamines, which include dopamine and noradrenaline, are good candidate modulators of acoustic-driven social behavior as they are known regulators of motivation, attention and arousal, and hormone regulation of catecholaminergic (CA) activity is proposed to function, in part, to modulate incentive salience of conspecific vocalizations [121; 122; 123; 124]. In order to begin to test the hypothesis that catecholamines are downstream targets of steroid-regulated, seasonal vocal-acoustic behavior, recent studies have examined: 1) direct CA innervation of central and peripheral auditory circuitry, 2) seasonal plasticity of CA innervation of the auditory system, and 3) activation of CA neurons in response to social acoustic signals in midshipman.
Forlano et al. [125] demonstrated direct and robust CA innervation of the auditory system by neurobiotin labeling of the saccule combined with tyrosine hydroxylase immunoreactivity (TH-ir) as a marker of CA synthesis. This study revealed that the saccule contains TH-ir terminals at the base of hair cells and the origin of this innervation are neurons in the periventricular posterior tuberculum (TPp) (Fig. 10) which are proposed homologs of A11 dopaminergic (DA) neurons in tetrapods [126; 127]. These DA neurons have widespread projections and also appear to innervate the cholinergic octavolateral efferent nucleus (OE), hindbrain octaval nuclei and auditory thalamus (CP) as well as the PAG and ventral telencephalon (Fig. 10). Thus, these neurons are in a key position to modulate sensory-motor integration in the context of social auditory cues. Importantly, the TPp is also a major target of steroid hormones, exhibiting robust aromatase mRNA and protein, ERα and ARβ mRNA and ERβ2 protein (Fig. 11) [39; 49; 50; 53].
Fig. 10.
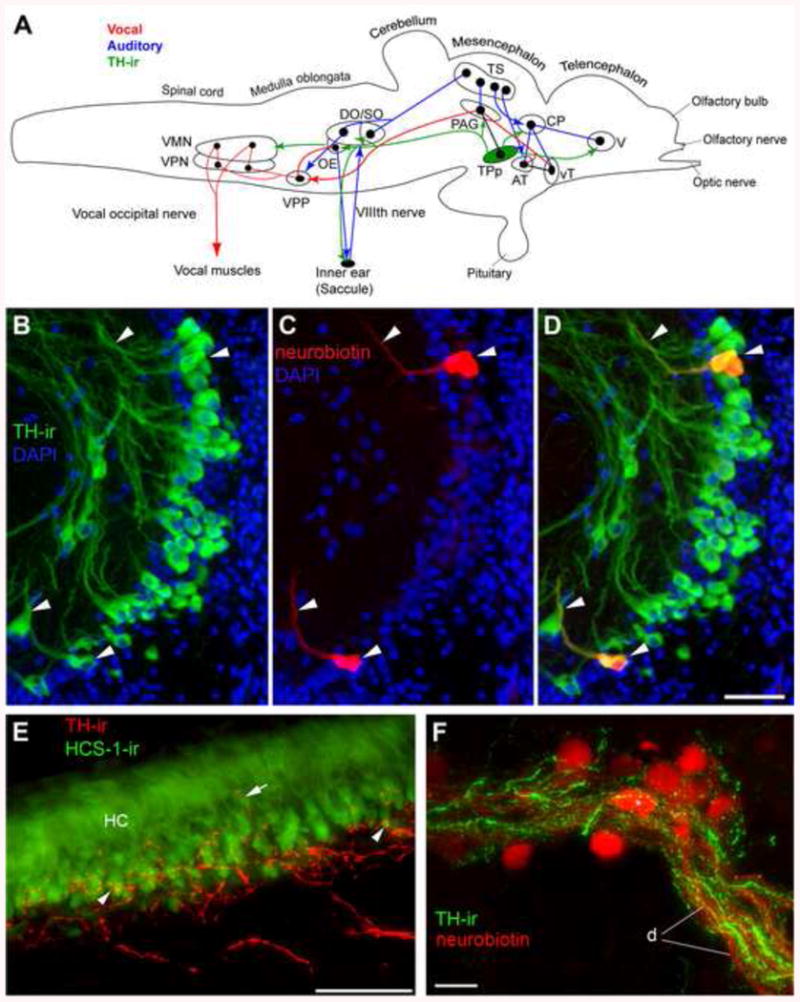
Catecholaminergic innervation of vocal and auditory circuitry in midshipman. (A) Schematic sagittal view of the brain showing both vocal motor (red), and central auditory (blue) systems in batrachoidid fish (midshipman and toadfish) [modified from 1; 45] with connectivity from large tyrosine hydroxylase (TH)-ir neurons within periventricular posterior tuberculum (green, TPp) (adapted from [125]). Solid dots represent somata, and lines represent axonal projection pathways. Two connected dots indicate reciprocal connections. See Fig. 4 for description and definitions of vocal and auditory nuclei. The octavolateralis efferent nucleus (OE) projects to the inner ear which includes the saccule, the main endorgan of hearing [40; 44; 60]. OE contains reciprocal connections with the VPP [41] and receives projections from PAG ([43], not shown). Large, pear-shaped TH-ir neurons from TPp send a massive dorsal turned descending tract into the hindbrain which appears to innervate DO, SO and OE and likely VMN. A branch of this tract exits the brain via the efferent tract in nVIII to the saccule. Other targets of TPp TH-ir neurons include CP and PAG. The PAG and TPp are reciprocally connected [43] but whether PAG projects onto TH-ir cells in TPp is not confirmed. TPp also has a robust ventral ascending TH-ir projection although the exact innervation target in the ventral telencephalon (V) is undetermined. See above references for additional known connectivity. (B-C) Neurobiotin backfills of the saccule combined with TH-ir identify source of TH-ir efferents to the saccular epithelium as a small population of large, pear-shaped cells in the TPp. (B) TH-ir cells in the TPp just medial to the medial forebrain bundle and lateral and dorsal to the paraventricular organ. (C) Three neurobiotin labeled-neurons (two adjacent cells on top) after a saccular backfill. (D) Overlay of images in B and C. Arrowheads indicate same cells and filled axons. All neurobiotin backfilled cells in this region were also TH-ir. (E) TH-ir innervation of the saccule, the main endorgan of hearing. The hair cell layer (HC) is delineated using the hair cell specific antibody (HCS-1, green) which labels HC somata. Thick and smooth TH-ir fibers (red) course through VIII prior to terminating largely at the base of the HC layer. Fine-caliber terminals are found (arrowheads) at the base of the HC and less frequently on the central portion of individual hair cells proximal to the nucleus (arrows). (F) High magnification image of robust TH-ir terminals and varicosities (green) on and around neurobiotin (red) backfilled OE somata (*) and dendrites (d). TH-ir in this area appears highly localized to OE and its dendritic field. Scale bar = 50μm in B-E, 25μm in F.
Fig. 11.
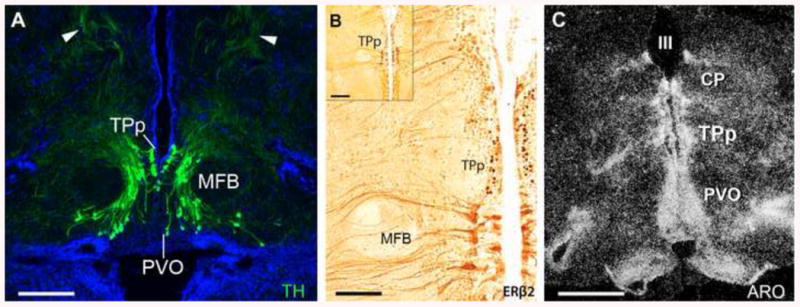
The dopaminergic periventricular posterior tuberculum (TPp) is an estrogen target in the midshipman brain. (A) Large, pear-shaped tyrosine hydroxylase immunoreactive (TH-ir; green) neurons, known to be dopaminergic, are found both in a true periventricular position just dorsal and lateral to the paraventricular organ (PVO), wrapping around the medial forebrain bundle (MFB) in a ventrolateral continuum. Arrowheads indicate thick dorsal projections that turn to descend through the brainstem. Blue is DAPI nuclear stain. For more details see [125]. (B) Robust ERβ2-ir (dark brown) in cells of the TPp. ERβ2-ir cells are found just medial to the MFB, similar to location of TH-ir neurons in A. Adapted from [49]. (C) High expression levels of aromatase mRNA (white grains) in the TPp and along the third ventricle (III) as visualized by dark-field in situ hybridization. CP, central posterior nucleus (auditory thalamus). Adapted from [50]. Scale bar = 250 μm in A, 100 μm in B, 200 μm in B inset, 500 μm in C.
Neuroanatomical evidence suggests TH-ir TPp neurons could modulate the sensitivity of the saccule either directly via efferent innervation of hair cells or indirectly via massive input onto the cholinergic OE, which in turn projects to the saccule [125]. As stated above, seasonal modulation of efferent activity may contribute to seasonal changes in auditory sensitivity [91]. In support of this, a recent study revealed that reproductive females have a significantly greater density of TH-ir terminals on OE somata and dendrites compared to non-reproductive females, while non-reproductive (winter) females contain significantly greater numbers of TH-ir terminals in the saccule [128]. Utilizing double label immunofluorescence for TH and c-Fos as a proxy for neural activity, Petersen et al. [129] demonstrated TH-ir TPp neurons are activated in males exposed to advertisement calls of other males. Taken together, these studies provide evidence that catecholamines, in particular DA neurons in the TPp, may function to seasonally modulate sensitivity of the saccule and ultimately facilitate the appropriate behavioral response to reproductive acoustic signals in midshipman.
7. Concluding comments
Use of the midshipman model to explore the neuroendocrine mechanisms of seasonal changes in the auditory and vocal motor systems of fish has revealed that reproductive state and steroid hormones can profoundly influence an individual's ability to produce, detect and perceive social acoustic signals. While the related seasonal and steroid-mediated plasticity of vocal motor and auditory systems exist in many taxa [84; 122; 130; 131; 132], we have only just begun to determine the mechanisms and adaptive significance of this plasticity. Here, we reviewed the current knowledge on how reproductive state and gonadal steroids can influence vocal and acoustic mechanisms in the plainfin midshipman fish, P. notatus, a highly tractable model for multidisciplinary behavioral, neurophysiological, and cellular-molecular studies.
While this review has concentrated on the influence of seasonal reproductive state and steroid hormones on vocal-acoustic processing, there is also evidence for behaviorally relevant roles for neuropeptides such as gonadotropin-releasing hormone [133] and stress hormones such as cortisol [32; 33; 62; 75] for modulating acoustic communication in fishes that should warrant future attention. The widespread presence of seasonal and steroid-driven vocal and auditory plasticity across vertebrate taxa suggests it is an evolutionary labile solution underlying plasticity of the vertebrate brain.
Highlights for Forlano, Sisneros, Rohmann and Bass.
Seasonal changes in reproductive-related vocal behavior are widespread among fishes.
Vocal fish provide models for elucidating mechanisms underlying seasonal behavior.
Vocal fish exhibit adaptive seasonal plasticity in the auditory periphery.
Steroids drive seasonal plasticity in hearing sensitivity.
Steroids and peptides rapidly modulate vocal neurophysiology and behavior.
Acknowledgments
Research by the authors that is reviewed here was supported, in part, by NIH SC2DA034996 (PMF), Virginia Merrill Bloedel Research Center grant 65-6172 (JAS), NSF IOS-1120925 and NIH DC00092 (AHB), and a postdoctoral training grant NIDCD T32DC000023 (KNR). Thanks to N. Y. Feng and B.P. Chagnaud for help with the figures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bass AH, McKibben JR. Neural mechanisms and behaviors for acoustic communication in teleost fish. Prog Neurobiol. 2003;69:1–26. doi: 10.1016/s0301-0082(03)00004-2. [DOI] [PubMed] [Google Scholar]
- 2.Forlano PM, Bass AH. Neural and hormonal mechanisms of reproductive-related arousal in fishes. Horm Behav. 2011;59:616–29. doi: 10.1016/j.yhbeh.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sisneros JA. Steroid-dependent auditory plasticity for the enhancement of acoustic communication: recent insights from a vocal teleost fish. Hear Res. 2009;252:9–14. doi: 10.1016/j.heares.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenfield DW, Winterbottom R, Collette BB. Review of the toadfish genera (Teleostei: Batrachoididae) Proc Cal Acad Sci. 2008;59:665–710. [Google Scholar]
- 5.Walker HJ, Rosenblatt RH. Pacific toadfishes of the genus Porichthys (Batrachoididae) with descriptions of the three new species. Copeia. 1988:887–904. [Google Scholar]
- 6.Bass AH. Shaping brain sexuality. American Scientist. 1996;84:352–363. [Google Scholar]
- 7.DeMartini EE. Spawning success of the male plainfin midshipman. I. Influences of male body size and are of spawning. Journal of Experimental Marine Biology and Ecology. 1988;121:177–192. [Google Scholar]
- 8.McIver EL, Marchaterre MA, Rice AN, Bass AH. Novel underwater soundscape: acoustic repertoire of plainfin midshipman fish. J Exp Biol. 2014;217:2377–2389. doi: 10.1242/jeb.102772. [DOI] [PubMed] [Google Scholar]
- 9.Feng NY, Bass AH. Melatonin action in a midbrain vocal-acoustic network. J Exp Biol. 2014;217:1046–57. doi: 10.1242/jeb.096669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rubow TK, Bass AH. Reproductive and diurnal rhythms regulate vocal motor plasticity in a teleost fish. J Exp Biol. 2009;212:3252–62. doi: 10.1242/jeb.032748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foran CM, Myers DA, Bass AH. Modification of gonadotropin releasing hormone (GnRH) mRNA expression in the retinal-recipient thalamus. Gen Comp Endocrinol. 1997;106:251–264. doi: 10.1006/gcen.1997.6875. [DOI] [PubMed] [Google Scholar]
- 12.Bass AH, Forlano PM. Neuroendocrine mechanisms of alternative reproductive tactics: the chemical language of social plasticity. In: Oliveira R, Taborsky M, Brockmann J, editors. Alternative Reproductive Tactics: An Integrative Approach. Cambridge University Press; Cambridge, UK: 2008. [Google Scholar]
- 13.Brantley RK, Bass AH. Alternative male spawning tactics and acoustic-signals in the plainfin midshipman fish Porichthys notatus Girard (Teleostei, Batrachoididae) Ethology. 1994;96:213–232. [Google Scholar]
- 14.Lee JSF, Bass AH. Dimorphic male midshipman fish: reduced sexual selection or sexual selection for reduced characters? Behavioral Ecology. 2006;17:670–675. [Google Scholar]
- 15.Bass AH, Marchaterre MA. Sound-generating (sonic) motor system in a teleost fish (Porichthys notatus): Sexual polymorphisms and general synaptology of sonic motor nucleus. Journal of Comparative Neurology. 1989;286:154–169. doi: 10.1002/cne.902860203. [DOI] [PubMed] [Google Scholar]
- 16.Ibara RM, Penny LT, Ebeling AW, van Dykhuizen D, Cailliet G. The mating call of the plainfin midshipman fish, Porichthys notatus. In: Noakes DLG, Lundquist DG, Helfman GS, Ward JA, editors. Predators and Prey in Fishes. Dr. W. Junk Publishers; The Hague: 1983. pp. 205–212. [Google Scholar]
- 17.Cohen MJ, Winn HE. Electrophysiological observations on hearing and sound production in the fish, Porichthys notatus. J Exp Zool. 1967;165:355–370. doi: 10.1002/jez.1401650305. [DOI] [PubMed] [Google Scholar]
- 18.Bass AH, Bodnar DA, Marchaterre MA. Complementary explanations for existing phenotypes in an acoustic communication system. In: Hauser M, Konishi M, editors. Neural Mechanisms of Communication. MIT Press; Cambridge: 1999. pp. 493–514. [Google Scholar]
- 19.McKibben JR, Bass AH. Behavioral assessment of acoustic parameters relevant to signal recognition and preference in a vocal fish. J Acoustic Soc Am. 1998;104:3520–3533. doi: 10.1121/1.423938. [DOI] [PubMed] [Google Scholar]
- 20.McKibben JR, Bass AH. Effects of temporal envelope modulation on acoustic signal recognition in a vocal fish, the plainfin midshipman. J Acoustic Soc Am. 2001;109:2934–2943. doi: 10.1121/1.1373441. [DOI] [PubMed] [Google Scholar]
- 21.Zeddies DG, Fay RR, Alderks PW, Shaub KS, Sisneros JA. Sound source localization by the plainfin midshipman fish, Porichthys notatus. J Acoust Soc Am. 2010;127:3104–13. doi: 10.1121/1.3365261. [DOI] [PubMed] [Google Scholar]
- 22.Alderks PW, Sisneros JA. Development of the Acoustically Evoked Behavioral Response in Larval Plainfin Midshipman Fish, Porichthys notatus. PLoS One. 2013;8:e82182. doi: 10.1371/journal.pone.0082182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knapp R, Marchaterre MA, Bass AH. Early development of the motor and premotor circuitry of a sexually dimorphic vocal pathway in a teleost fish. J Neurobiol. 1999;38:475–490. doi: 10.1002/(sici)1097-4695(199903)38:4<475::aid-neu4>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 24.Remage-Healey L, Bass AH. Plasticity in brain sexuality is revealed by the rapid actions of steroid hormones. J Neurosci. 2007;27:1114–22. doi: 10.1523/JNEUROSCI.4282-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tramontin AD, Brenowitz EA. Seasonal plasticity in the adult brain. Trends Neurosci. 2000;23:251–8. doi: 10.1016/s0166-2236(00)01558-7. [DOI] [PubMed] [Google Scholar]
- 26.Sisneros JA, Alderks PW, Leon K, Sniffen B. Morphometric changes associated with the reproductive cycle and behaviour of the intertidal-nesting, male plainfin midshipman Porichthys notatus. J Fish Biol. 2009;74:18–36. doi: 10.1111/j.1095-8649.2008.02104.x. [DOI] [PubMed] [Google Scholar]
- 27.Sisneros JA, Forlano PM, Knapp R, Bass AH. Seasonal variation of steroid hormone levels in an intertidal-nesting fish, the vocal plainfin midshipman. Gen Comp Endocrinol. 2004;136:101–116. doi: 10.1016/j.ygcen.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 28.Bentley PJ. Comparative Vertebrate Endocrinology. Cambridge University Press; Cambridge: 1998. [Google Scholar]
- 29.Bass A, Andersen K. Intersexual and intrasexual dimorphisms in the vocal control-system of a teleost fish: Motor axon number and size. Brain Behavior and Evolution. 1991;37:204–214. doi: 10.1159/000114359. [DOI] [PubMed] [Google Scholar]
- 30.Brantley RK, Wingfield JC, Bass AH. Sex steroid levels in Porichthys notatus, a fish with alternative reproductive tactics, and a review of the hormonal bases for male dimorphism among teleost fishes. Hormones and Behavior. 1993;27:332–347. doi: 10.1006/hbeh.1993.1025. [DOI] [PubMed] [Google Scholar]
- 31.Knapp R, Wingfield JC, Bass AH. Steroid hormones and paternal care in the plainfin midshipman fish (Porichthys notatus) Hormones and Behavior. 1999;35:81–89. doi: 10.1006/hbeh.1998.1499. [DOI] [PubMed] [Google Scholar]
- 32.Genova RM, Marchaterre MA, Knapp R, Fergus D, Bass AH. Glucocorticoid and androgen signaling pathways diverge between advertisement calling and non-calling fish. Horm Behav. 2013;62:426–32. doi: 10.1016/j.yhbeh.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 33.Remage-Healey L, Bass AH. Rapid elevations in both steroid hormones and vocal signaling during playback challenge: a field experiment in Gulf toadfish. Horm Behav. 2005;47:297–305. doi: 10.1016/j.yhbeh.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 34.Fine ML, Johnson MS, Matt DW. Seasonal variation in androgen levels in the oyster toadfish. Copeia. 2004;2004:235–244. [Google Scholar]
- 35.Modesto T, Canario AV. Morphometric changes and sex steroid levels during the annual reproductive cycle of the Lusitanian toadfish, Halobatrachus didactylus. Gen Comp Endocrinol. 2003;131:220–31. doi: 10.1016/s0016-6480(03)00027-3. [DOI] [PubMed] [Google Scholar]
- 36.Callard G, Schlinger B, Pasmanik M. Nonmammalian vertebrate models in studies of brain-steroid interactions. J Exp Zool Suppl. 1990;4:6–16. doi: 10.1002/jez.1402560404. [DOI] [PubMed] [Google Scholar]
- 37.Diotel N, Le Page Y, Mouriec K, Tong SK, Pellegrini E, Vaillant C, Anglade I, Brion F, Pakdel F, Chung BC, Kah O. Aromatase in the brain of teleost fish: expression, regulation and putative functions. Front Neuroendocrinol. 2010;31:172–92. doi: 10.1016/j.yfrne.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 38.Forlano PM, Schlinger BA, Bass AH. Brain aromatase: new lessons from non-mammalian model systems. Front Neuroendocrinol. 2006;27:247–74. doi: 10.1016/j.yfrne.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 39.Forlano PM, Deitcher DL, Myers DA, Bass AH. Anatomical distribution and cellular basis for high levels of aromatase activity in the brain of teleost fish: aromatase enzyme and mRNA expression identify glia as source. J Neurosci. 2001;21:8943–55. doi: 10.1523/JNEUROSCI.21-22-08943.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bass AH, Bodnar DA, Marchaterre MA. Midbrain acoustic circuitry in a vocalizing fish. J Comp Neurol. 2000;419:505–531. doi: 10.1002/(sici)1096-9861(20000417)419:4<505::aid-cne7>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 41.Chagnaud BP, Baker R, Bass AH. Vocalization frequency and duration are coded in separate hindbrain nuclei. Nat Commun. 2011;2:346. doi: 10.1038/ncomms1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goodson JL, Bass AH. Vocal-acoustic circuitry and descending vocal pathways in teleost fish: Convergence with terrestrial vertebrates reveals conserved traits. J Comp Neurol. 2002;448:298–322. doi: 10.1002/cne.10258. [DOI] [PubMed] [Google Scholar]
- 43.Kittelberger JM, Bass AH. Vocal-motor and auditory connectivity of the midbrain periaqueductal gray in a teleost fish. J Comp Neurol. 2013;521:791–812. doi: 10.1002/cne.23202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bass AH, Marchaterre MA, Baker R. Vocal-acoustic pathways in a teleost fish. J Neurosci. 1994;14:4025–4039. doi: 10.1523/JNEUROSCI.14-07-04025.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kittelberger M, Land BR, Bass AH. Midbrain periaqueductal gray and vocal pattering in a teleost fish. J Neurophysiol. 2006;96:71–85. doi: 10.1152/jn.00067.2006. [DOI] [PubMed] [Google Scholar]
- 46.Bass AH, Baker R. Sexual dimorphisms in the vocal control system of a teleost fish: morphology of physiologically identified neurons. J Neurobiol. 1990;21:1155–1168. doi: 10.1002/neu.480210802. [DOI] [PubMed] [Google Scholar]
- 47.Chagnaud BP, Zee MC, Baker R, Bass AH. Innovations in motoneuron synchrony drive rapid temporal modulations in vertebrate acoustic signaling. J Neurophysiol. 2012;107:3528–42. doi: 10.1152/jn.00030.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taylor JS, Van de Peer Y, Braasch I, Meyer A. Comparative genomics provides evidence for an ancient genome duplication event in fish. Philos Trans R Soc Lond B Biol Sci. 2001;356:1661–79. doi: 10.1098/rstb.2001.0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fergus DJ, Bass AH. Localization and divergent profiles of estrogen receptors and aromatase in the vocal and auditory networks of a fish with alternative mating tactics. J Comp Neurol. 2013;521:2850–69. doi: 10.1002/cne.23320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Forlano PM, Deitcher DL, Bass AH. Distribution of estrogen receptor alpha mRNA in the brain and inner ear of a vocal fish with comparisons to sites of aromatase expression. J Comp Neurol. 2005;483:91–113. doi: 10.1002/cne.20397. [DOI] [PubMed] [Google Scholar]
- 51.Schlinger BA, Greco C, Bass AH. Aromatase activity in the hindbrain vocal control region of a teleost fish: divergence among males with alternative reproductive tactics. Proceedings of the Royal Society of London Series B-Biological Sciences. 1999;266:131–136. [Google Scholar]
- 52.Bass AH, Remage-Healey L. Central pattern generators for social vocalization: androgen-dependent neurophysiological mechanisms. Horm Behav. 2008;53:659–72. doi: 10.1016/j.yhbeh.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Forlano PM, Marchaterre M, Deitcher DL, Bass AH. Distribution of androgen receptor mRNA expression in vocal, auditory, and neuroendocrine circuits in a teleost fish. J Comp Neurol. 2010;518:493–512. doi: 10.1002/cne.22233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Forlano PM, Bass AH. Seasonal plasticity of brain aromatase mRNA expression in glia: Divergence across sex and vocal phenotypes. J Neurobiol. 2005;65:37–49. doi: 10.1002/neu.20179. [DOI] [PubMed] [Google Scholar]
- 55.Forlano PM, Bass AH. Steroid regulation of brain aromatase expression in glia: Female preoptic and vocal motor nuclei. J Neurobiol. 2005;65:50–8. doi: 10.1002/neu.20178. [DOI] [PubMed] [Google Scholar]
- 56.Bass AH, Rose GJ, Pritz MB. Auditory midbrain of fish, amphibians and reptiles: models systems for understanding auditory function. In: Winer JA, Schreiner CE, editors. The Inferior Colliculus. Springer; New York: 2005. pp. 459–492. [Google Scholar]
- 57.McCormick CA. Anatomy of the central auditory pathways of fish and amphibians. In: Popper A, Fay RR, editors. Comparative hearing: fish and amphibians. Springer; New York: 1999. pp. 155–217. [Google Scholar]
- 58.McCormick CA. Auditory/lateral line CNS: Anatomy. In: Farrell AP, editor. Encyclopedia of Fish Physiology: From Genome to Environment. Academic Press; San Diego: 2011. [Google Scholar]
- 59.Chagnaud BP, Bass AH. Vocal corollary discharge communicates call duration to vertebrate auditory system. J Neurosci. 2013;33:18775–80. doi: 10.1523/JNEUROSCI.3140-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weeg MS, Land BR, Bass AH. Vocal pathways modulate efferent neurons to the inner ear and lateral line. J Neurosci. 2005;25:5967–74. doi: 10.1523/JNEUROSCI.0019-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bass AH, Bodnar DA, Marchaterre MA. Acoustic nuclei in the medulla and midbrain of the vocalizing gulf toadfish (Opsanus beta) Brain Behav and Evol. 2001;57:63–79. doi: 10.1159/000047226. [DOI] [PubMed] [Google Scholar]
- 62.Maruska KP, Ung US, Fernald RD. The African cichlid fish Astatotilapia burtoni uses acoustic communication for reproduction: sound production, hearing, and behavioral significance. PLoS One. 2012;7:e37612. doi: 10.1371/journal.pone.0037612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maruska KP, Fernald RD. Steroid receptor expression in the fish inner ear varies with sex, social status, and reproductive state. BMC Neurosci. 2010;11:58. doi: 10.1186/1471-2202-11-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Winn HE, Stout JF. Sound Production by the Satinfin Shiner, Notropis analostanus, and Related Fishes. Science. 1960;132:222–3. doi: 10.1126/science.132.3421.222. [DOI] [PubMed] [Google Scholar]
- 65.Remage-Healey L, Bass AH. From social behavior to neural circuitry: Steroid hormones rapidly modulate advertisement calling via a vocal pattern generator. Horm Behav. 2006;50:432–441. doi: 10.1016/j.yhbeh.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 66.Remage-Healey L, Nowacek DP, Bass AH. Dolphin foraging sounds suppress calling and elevate stress hormone levels in a prey species, the Gulf toadfish. J Exp Biol. 2006;209:4444–51. doi: 10.1242/jeb.02525. [DOI] [PubMed] [Google Scholar]
- 67.Barros NB. Feeding Ecology and Foraging Strategies of Bottlenose Dolphins on the Central East Coast of Florida. University of Miami; Miami: 1993. [Google Scholar]
- 68.Brantley RK, Marchaterre MA, Bass AH. Androgen effects on vocal muscle structure in a teleost fish with inter-sexual and intra-sexual dimorphism. Journal of Morphology. 1993;216:305–318. doi: 10.1002/jmor.1052160306. [DOI] [PubMed] [Google Scholar]
- 69.Connaughton MA, Taylor MH. Effects of exogenous testosterone on sonic muscle mass in the weakfish, Cynoscion regalis. Gen Comp Endocrinol. 1995;100:238–45. doi: 10.1006/gcen.1995.1153. [DOI] [PubMed] [Google Scholar]
- 70.Fine ML, Pennypacker KR. Hormonal basis for sexual dimorphism of the sound-producing apparatus of the oyster toadfish. Exp Neurol. 1986;92:289–98. doi: 10.1016/0014-4886(86)90081-6. [DOI] [PubMed] [Google Scholar]
- 71.Lee JSF, Bass AH. Differential effects of 11-ketotestosterone on dimorphic traits in a teleost with alternative male reproductive morphs. Hormones and Behavior. 2005;47:523–531. doi: 10.1016/j.yhbeh.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 72.Nguyen TK, Lin H, Parmentier E, Fine ML. Seasonal variation in sonic muscles in the fawn cusk-eel Lepophidium profundorum. Biol Lett. 2008;4:707–10. doi: 10.1098/rsbl.2008.0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Walsh PW, Mommsen TP, Bass AH. Biochemical and molecular aspects of singing in batrachoidid fishes. In: Hochachka PW, Mommsen TP, editors. Biochemistry and Molecular Biology of Fishes. 1995. pp. 279–289. [Google Scholar]
- 74.Arterbery AS, Deitcher DL, Bass AH. Divergent expression of 11beta-hydroxysteroid dehydrogenase and 11beta-hydroxylase genes between male morphs in the central nervous system, sonic muscle and testis of a vocal fish. Gen Comp Endocrinol. 2010;167:44–50. doi: 10.1016/j.ygcen.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Arterbery AS, Deitcher DL, Bass AH. Corticosteroid receptor expression in a teleost fish that displays alternative male reproductive tactics. Gen Comp Endocrinol. 2010;165:83–90. doi: 10.1016/j.ygcen.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bass AH, Chagnaud BP. Shared developmental and evolutionary origins for neural basis of vocal-acoustic and pectoral-gestural signaling. Proc Natl Acad Sci U S A. 2012;109(Suppl 1):10677–84. doi: 10.1073/pnas.1201886109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bass AH, Gilland EH, Baker R. Evolutionary origins for social vocalization in a vertebrate hindbrain-spinal compartment. Science. 2008;321:417–21. doi: 10.1126/science.1157632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chagnaud BP, Bass AH. Vocal behavior and vocal central pattern generator organization diverge among toadfishes. Brain, Behavior and Evolution. 2014 doi: 10.1159/000362916. in press. [DOI] [PubMed] [Google Scholar]
- 79.Remage-Healey L, Bass AH. Rapid, hierarchical modulation of vocal patterning by steroid hormones. J Neurosci. 2004;24:5892–900. doi: 10.1523/JNEUROSCI.1220-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Remage-Healey L, Bass AH. From social behavior to neural circuitry: steroid hormones rapidly modulate advertisement calling via a vocal pattern generator. Horm Behav. 2006;50:432–41. doi: 10.1016/j.yhbeh.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 81.Goodson JL, Bass AH. Vasotocin innervation and modulation of vocal-acoustic circuitry in the teleost Porichthys notatus. J Comp Neurol. 2000;422:363–379. doi: 10.1002/1096-9861(20000703)422:3<363::aid-cne4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 82.Goodson JL, Bass AH. Forebrain peptides modulate sexually polymorphic vocal circuitry. Nature. 2000;403:769–772. doi: 10.1038/35001581. [DOI] [PubMed] [Google Scholar]
- 83.Goodson JL, Bass AH. Rhythmic midbrain-evoked vocalization is inhibited by vasoactive intestinal polypeptide in the teleost Porichthys notatus. Brain Res. 2000;865:107–11. doi: 10.1016/s0006-8993(00)02232-0. [DOI] [PubMed] [Google Scholar]
- 84.Remage-Healey L, Bass AH. Estradiol interacts with an opioidergic network to achieve rapid modulation of a vocal pattern generator. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2010;196:137–46. doi: 10.1007/s00359-009-0500-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Forlano PM, Remage-Healey L, Sisneros JA, Bass AH. Steroid-induced plasticity in the auditory and vocal motor system: Recent studies in a teleost fish. In: Canonaco M, Facciolo RM, editors. Evolutionary Molecular Strategies and Plasticity. Research Signpost; Kerala, India: 2007. [Google Scholar]
- 86.McKibben JR, Bass AH. Peripheral encoding of behaviorally relevant acoustic signals in a vocal fish: harmonic and beat stimuli. Journal of Comparative Physiology a-Sensory Neural and Behavioral Physiology. 2001;187:271–285. doi: 10.1007/s003590100199. [DOI] [PubMed] [Google Scholar]
- 87.McKibben JR, Bass AH. Peripheral encoding of behaviorally relevant acoustic signals in a vocal fish: single tones. Journal of Comparative Physiology a-Sensory Neural and Behavioral Physiology. 1999;184:563–576. doi: 10.1007/s003590050356. [DOI] [PubMed] [Google Scholar]
- 88.Rohmann KN, Bass AH. Seasonal plasticity of auditory hair cell frequency sensitivity correlates with plasma steroid levels in vocal fish. J Exp Biol. 2011;214:1931–42. doi: 10.1242/jeb.054114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sisneros JA. Saccular potentials of the vocal plainfin midshipman fish, Porichthys notatus. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2007;193:413–24. doi: 10.1007/s00359-006-0195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sisneros JA. Seasonal plasticity of auditory saccular sensitivity in the vocal plainfin midshipman fish, Porichthys notatus. J Neurophysiol. 2009;102:1121–31. doi: 10.1152/jn.00236.2009. [DOI] [PubMed] [Google Scholar]
- 91.Sisneros JA, Bass AH. Seasonal plasticity of peripheral auditory frequency sensitivity. J Neurosci. 2003;23:1049–1058. doi: 10.1523/JNEUROSCI.23-03-01049.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sisneros JA, Bass AH. Ontogenetic changes in the response properties of individual, primary auditory afferents in the vocal plainfin midshipman fish Porichthys notatus Girard. J Exp Biol. 2005;208:3121–31. doi: 10.1242/jeb.01742. [DOI] [PubMed] [Google Scholar]
- 93.Popper AN. Auditory System Morphology. In: F AP, editor. Encyclopedia of Fish Physiology: From Genome to Environment. Academic Press; San Diego: 2011. pp. 252–261. [Google Scholar]
- 94.Zeddies DG, Fay RR, Gray MD, Alderks PW, Acob A, Sisneros JA. Local acoustic particle motion guides sound-source localization behavior in the plainfin midshipman fish, Porichthys notatus. J Exp Biol. 2012;215:152–60. doi: 10.1242/jeb.064998. [DOI] [PubMed] [Google Scholar]
- 95.Coffin AB, Mohr RA, Sisneros JA. Saccular-specific hair cell addition correlates with reproductive state-dependent changes in the auditory saccular sensitivity of a vocal fish. J Neurosci. 2012;32:1366–76. doi: 10.1523/JNEUROSCI.4928-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fine ML, Lenhardt ML. Shallow-water propagation of the toadfish mating call. Comp Biochem Physiol A. 1983;76:225–31. doi: 10.1016/0300-9629(83)90319-5. [DOI] [PubMed] [Google Scholar]
- 97.Rogers PH, Popper AN, Hastings MC, Saidel WM. Processing of acoustic signals in the auditory system of bony fish. J Acoust Soc Am. 1988;83:338–49. doi: 10.1121/1.396444. [DOI] [PubMed] [Google Scholar]
- 98.Sisneros JA. Adaptive hearing in the vocal plainfin midshipman fish: getting in tune for the breeding season and implications for acoustic communication. Integr Zool. 2009;4:33–42. doi: 10.1111/j.1749-4877.2008.00133.x. [DOI] [PubMed] [Google Scholar]
- 99.Bass AH, Clark C. The physical acoustics of underwater sound communication. In: Simmons AM, Popper AN, Fay RR, editors. Handbook of Auditory Research. Springer-Verlag; New York: 2003. pp. 15–64. [Google Scholar]
- 100.Sisneros JA, Forlano PM, Deitcher DL, Bass AH. Steroid-dependent auditory plasticity leads to adaptive coupling of sender and receiver. Science. 2004;305:404–7. doi: 10.1126/science.1097218. [DOI] [PubMed] [Google Scholar]
- 101.Fettiplace R, Fuchs PA. Mechanisms of hair cell tuning. Annu Rev Physiol. 1999;61:809–34. doi: 10.1146/annurev.physiol.61.1.809. [DOI] [PubMed] [Google Scholar]
- 102.Kros CJ, Ruppersberg JP, Rusch A. Expression of a potassium current in inner hair cells during development of hearing in mice. Nature. 1998;394:281–4. doi: 10.1038/28401. [DOI] [PubMed] [Google Scholar]
- 103.Wersinger E, McLean WJ, Fuchs PA, Pyott SJ. BK channels mediate cholinergic inhibition of high frequency cochlear hair cells. PLoS ONE. 2010;5:e13836. doi: 10.1371/journal.pone.0013836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Steinacker A, Monterrubio J, Perez R, Mensinger AF, Marin A. Electrophysiology and pharmacology of outward potassium currents in semicircular canal hair cells of toadfish, Opsanus tau. Hear Res. 1997;109:11–20. doi: 10.1016/s0378-5955(97)00038-5. [DOI] [PubMed] [Google Scholar]
- 105.Steinacker A, Romero A. Characterization of voltage-gated and calcium-activated potassium currents in toadfish saccular hair cells. Brain Res. 1991;556:22–32. doi: 10.1016/0006-8993(91)90543-5. [DOI] [PubMed] [Google Scholar]
- 106.Steinacker A, Romero A. Voltage-gated potassium current and resonance in the toadfish saccular hair cell. Brain Res. 1992;574:229–36. doi: 10.1016/0006-8993(92)90821-p. [DOI] [PubMed] [Google Scholar]
- 107.Xie J, McCobb DP. Control of alternative splicing of potassium channels by stress hormones. Science. 1998;280:443–6. doi: 10.1126/science.280.5362.443. [DOI] [PubMed] [Google Scholar]
- 108.Mahmoud SF, McCobb DP. Regulation of Slo potassium channel alternative splicing in the pituitary by gonadal testosterone. J Neuroendocrinol. 2004;16:237–43. doi: 10.1111/j.0953-8194.2004.01154.x. [DOI] [PubMed] [Google Scholar]
- 109.Zhu N, Eghbali M, Helguera G, Song M, Stefani E, Toro L. Alternative splicing of Slo channel gene programmed by estrogen, progesterone and pregnancy. FEBS Lett. 2005;579:4856–60. doi: 10.1016/j.febslet.2005.07.069. [DOI] [PubMed] [Google Scholar]
- 110.Holdiman AJ, Fergus DJ, England SK. 17beta-Estradiol upregulates distinct maxi-K channel transcripts in mouse uterus. Mol Cell Endocrinol. 2002;192:1–6. doi: 10.1016/s0303-7207(02)00136-3. [DOI] [PubMed] [Google Scholar]
- 111.Kundu P, Alioua A, Stefani E, Toro L. Regulation of mouse Slo gene expression: multiple promoters, transcription start sites, and genomic action of estrogen. J Biol Chem. 2007;282:27478–92. doi: 10.1074/jbc.M704777200. [DOI] [PubMed] [Google Scholar]
- 112.Rohmann KN, Deitcher DL, Bass AH. Calcium-activated potassium (BK) channels are encoded by duplicate slo1 genes in teleost fishes. Mol Biol Evol. 2009;26:1509–1521. doi: 10.1093/molbev/msp060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rohmann KN, Fergus DJ, Bass AH. Plasticity in Ion Channel Expression Underlies Variation in Hearing during Reproductive Cycles. Curr Biol. 2013;23:678–683. doi: 10.1016/j.cub.2013.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.McKibben JR, Bass AH. Behavioral assessment of acoustic parameters relevant to signal recognition and preference in a vocal fish. J Acoust Soc Am. 1998;104:3520–33. doi: 10.1121/1.423938. [DOI] [PubMed] [Google Scholar]
- 115.Stewart AM, Braubach O, Spitsbergen J, Gerlai R, Kalueff AV. Zebrafish models for translational neuroscience research: from tank to bedside. Trends in Neurosciences. 2014;37:264–278. doi: 10.1016/j.tins.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nicolson T. Fishing for key players in mechanotransduction. Trends Neurosci. 2005;28:140–4. doi: 10.1016/j.tins.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 117.Nicolson T. The genetics of hearing and balance in zebrafish. Annu Rev Genet. 2005;39:9–22. doi: 10.1146/annurev.genet.39.073003.105049. [DOI] [PubMed] [Google Scholar]
- 118.Corey DR, Abrams JM. Morpholino antisense oligonucleotides: tools for investigating vertebrate development. Genome Biol. 2001;2 doi: 10.1186/gb-2001-2-5-reviews1015. REVIEWS1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rohmann KN, Fergus DJ, Bass AH. Plasticity in ion channel expression underlies variation in hearing during reproductive cycles. Curr Biol. 2013;23:678–83. doi: 10.1016/j.cub.2013.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rohmann KN, Tripp JA, Genova RM, Bass AH. Manipulation of BK channel expression is sufficient to alter auditory hair cell thresholds in larval zebrafish. J Exp Biol. 2014;217:2531–2539. doi: 10.1242/jeb.103093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Berridge CW. Noradrenergic modulation of arousal. Brain Res Rev. 2008;58:1–17. doi: 10.1016/j.brainresrev.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Caras ML. Estrogenic modulation of auditory processing: a vertebrate comparison. Front Neuroendocrinol. 2013;34:285–99. doi: 10.1016/j.yfrne.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Maney DL, Pinaud R. Estradiol-dependent modulation of auditory processing and selectivity in songbirds. Front Neuroendocrinol. 2011;32:287–302. doi: 10.1016/j.yfrne.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Riters LV. The role of motivation and reward neural systems in vocal communication in songbirds. Front Neuroendocrinol. 2012;33:194–209. doi: 10.1016/j.yfrne.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Forlano PM, Kim SD, Krzyminska ZM, Sisneros JA. Catecholaminergic connectivity to the inner ear, central auditory, and vocal motor circuitry in the plainfin midshipman fish Porichthys notatus. J Comp Neurol. 2014 doi: 10.1002/cne.23596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Schweitzer J, Lohr H, Filippi A, Driever W. Dopaminergic and noradrenergic circuit development in zebrafish. Dev Neurobiol. 2012;72:256–68. doi: 10.1002/dneu.20911. [DOI] [PubMed] [Google Scholar]
- 127.Tay TL, Ronneberger O, Ryu S, Nitschke R, Driever W. Comprehensive catecholaminergic projectome analysis reveals single-neuron integration of zebrafish ascending and descending dopaminergic systems. Nat Commun. 2011;2:171. doi: 10.1038/ncomms1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Forlano PM, Ghahramani ZN, Monestime C, Kurochkin P, Chernenko A, Milkis D. Seasonal plasticity of catecholaminergic innervation of central and peripheral auditory circuitry in a vocal fish. International Congress for Neuroendocrinology/ Society for Behavioral Neuroendocrinology, Hormones and Behavior; Sydney, Australia. 2014. [Google Scholar]
- 129.Petersen CL, Timothy M, Kim DS, Bhandiwad AA, Mohr RA, Sisneros JA, Forlano PM. Exposure to advertisement calls of reproductive competitors activates vocal-acoustic and catecholaminergic neurons in the plainfin midshipman fish, Porichthys notatus. PLoS One. 2013;8:e70474. doi: 10.1371/journal.pone.0070474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Arch VS, Narins PM. Sexual hearing: the influence of sex hormones on acoustic communication in frogs. Hear Res. 2009;252:15–20. doi: 10.1016/j.heares.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Brenowitz EA. Plasticity of the adult avian song control system. Ann N Y Acad Sci. 2004;1016:560–85. doi: 10.1196/annals.1298.006. [DOI] [PubMed] [Google Scholar]
- 132.Lucas JR, Freeberg TM, Krishnan A, Long GR. A comparative study of avian auditory brainstem responses: correlations with phylogeny and vocal complexity, and seasonal effects. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2002;188:981–92. doi: 10.1007/s00359-002-0359-x. [DOI] [PubMed] [Google Scholar]
- 133.Maruska KP, Tricas TC. Gonadotropin-releasing hormone (GnRH) modulates auditory processing in the fish brain. Horm Behav. 2011;59:451–64. doi: 10.1016/j.yhbeh.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 134.DeMartini EE. Spawning success of the male plainfin midshipman. II. Substratum as a limiting spatial resource. Journal of Experimental Marine Biology and Ecology. 1991;146:235–251. [Google Scholar]
- 135.Goodson JL, Evans AK, Bass AH. Putative isotocin distributions in sonic fish: Relation to vasotocin and vocal-acoustic circuitry. J Comp Neurol. 2003;462:1–14. doi: 10.1002/cne.10679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Braford MR, Jr, Northcutt RG. Organization of the diencephalon and pretectum of the ray-finned fishes. In: Davis RE, Northcutt RG, editors. Fish neurobiology. University of Michigan Press; Ann Arbor: 1983. pp. 117–164. [Google Scholar]


