Abstract
Background
The cardiac cytoskeleton plays key roles in maintaining myocyte structural integrity in health and disease. In fact, human mutations in cardiac cytoskeletal elements are tightly linked with cardiac pathologies including myopathies, aortopathies, and dystrophies. Conversely, the link between cytoskeletal protein dysfunction in cardiac electrical activity is not well understood, and often overlooked in the cardiac arrhythmia field.
Methods and Results
Here, we uncover a new mechanism for the regulation of cardiac membrane excitability. We report that βII spectrin, an actin-associated molecule, is essential for the post-translational targeting and localization of critical membrane proteins in heart. βII spectrin recruits ankyrin-B to the cardiac dyad, and a novel human mutation in the ankyrin-B gene disrupts the ankyrin-B/βII spectrin interaction leading to severe human arrhythmia phenotypes. Mice lacking cardiac βII spectrin display lethal arrhythmias, aberrant electrical and calcium handling phenotypes, and abnormal expression/localization of cardiac membrane proteins. Mechanistically, βII spectrin regulates the localization of cytoskeletal and plasma membrane/sarcoplasmic reticulum protein complexes that include the Na/Ca exchanger, RyR2, ankyrin-B, actin, and αII spectrin. Finally, we observe accelerated heart failure phenotypes in βII spectrin-deficient mice.
Conclusions
Our findings identify βII spectrin as critical for normal myocyte electrical activity, link this molecule to human disease, and provide new insight into the mechanisms underlying cardiac myocyte biology.
Keywords: protein trafficking arrhythmia, cytoskeleton, arrhythmia (heart rhythm disorders), cytoskeletal dynamics, cardiovascular physiology, catecholaminergic polymorphic ventricular tachycardia
Introduction
Life for the vertebrate requires sustained and rhythmic beating of the heart for delivery of oxygen and nutrients to the brain and other organs. The vertebrate cardiomyocyte has evolved elegant membrane regulatory pathways to maintain efficient excitation-contraction coupling at baseline or in the face of acute and chronic stress. Central to this membrane regulation is the cardiac cytoskeleton. In the heart, the cytoskeletal network is comprised of a highly ordered array of structural and accessory proteins spanning from the plasma membrane to the nucleus.
Over the past two decades, the cardiac cytoskeleton has emerged as a central governing factor in the control of cardiac membrane integrity, and dysfunction in cytoskeleton and cytoskeletal-associated proteins has been directly linked with a host of human cardiac pathologies, most notably cardiac myopathies and dystrophies. In fact, human mutations in cardiac cytoskeletal or cytoskeletal-associated genes that alter myocyte signal transduction, myocardial mechanics, and force transmission are now directly linked with dilated cardiomyopathy, muscular dystrophy, and arrhythmogenic cardiomyopathy.1–4
In contrast to myopathy and dystrophy fields, the role of the cytoskeleton in normal cardiac electrical function is not well resolved. Further, until the past decade, human arrhythmia mechanisms were primarily limited to mutations in cardiac ion channels.5 However, while literally hundreds of human variants in cardiac Na+, K+, and Ca2+ channel alpha- and beta-subunits have been linked with sinus node disease, atrial fibrillation, conduction disease, and ventricular fibrillation, a second class of human arrhythmias has emerged due to mutations in ion channel-associated proteins including α-syntrophin, ankyrin-G, caveolin-3, FGF12, and ankyrin-B.6 Mechanistically, dysfunction in these proteins is linked with diverse cellular pathologies including defects in channel synthesis and membrane targeting, channel gating, and channel post-translational modifications. While this information has been important for new disease diagnosis and fundamental cardiac cell biology, there remain large cohorts of phenotype positive/genotype negative patients with familial forms of cardiac arrhythmia. Further, there remain pressing unanswered questions regarding the role for the cardiac cytoskeleton for the local organization of membrane ion channel complexes in vivo.
Based on genetic findings in a proband with severe ventricular arrhythmia and cardiac arrest, we uncovered a new and essential cytoskeletal-based pathway critical for cardiac electrical function. We identify βII spectrin as an integral regulatory node for the organization of critical myocyte membrane and membrane-associated proteins. βII spectrin is critical for the regulation of ankyrin-B and αII spectrin, and defects in this assembly result in severe arrhythmia associated with aberrant calcium phenotypes. Moreover, we link dysfunction in this pathway with accelerated heart failure phenotypes. In summary, our findings provide a new mechanism for human excitable cell disease, as well as uncover new roles for the cardiac cytoskeleton in human cardiovascular disease.
Methods
Statistics
Data are presented as mean ± SEM. For the comparison of two groups, we performed Wilcoxon-Mann-Whitney U tests. For the comparison of greater than two groups, we applied a Kruskal-Wallis test. When we obtained a significant P value, we continued with pair-wise comparisons utilizing Wilcoxon-Mann-Whitney U tests according to the closed testing principle. For our study, a value of P<0.05 was considered statistically significant.
Human Studies
Approval for use of human subjects was obtained from the Institutional Review Board of Ohio State University, subjects provided informed consent.
Animal Studies
Procedures followed were approved and in accordance with institutional guidelines (Ohio State University)
Additional methods are provided in the Data Supplement.
Results
Identification of a new class of ANK2 human arrhythmia mutation
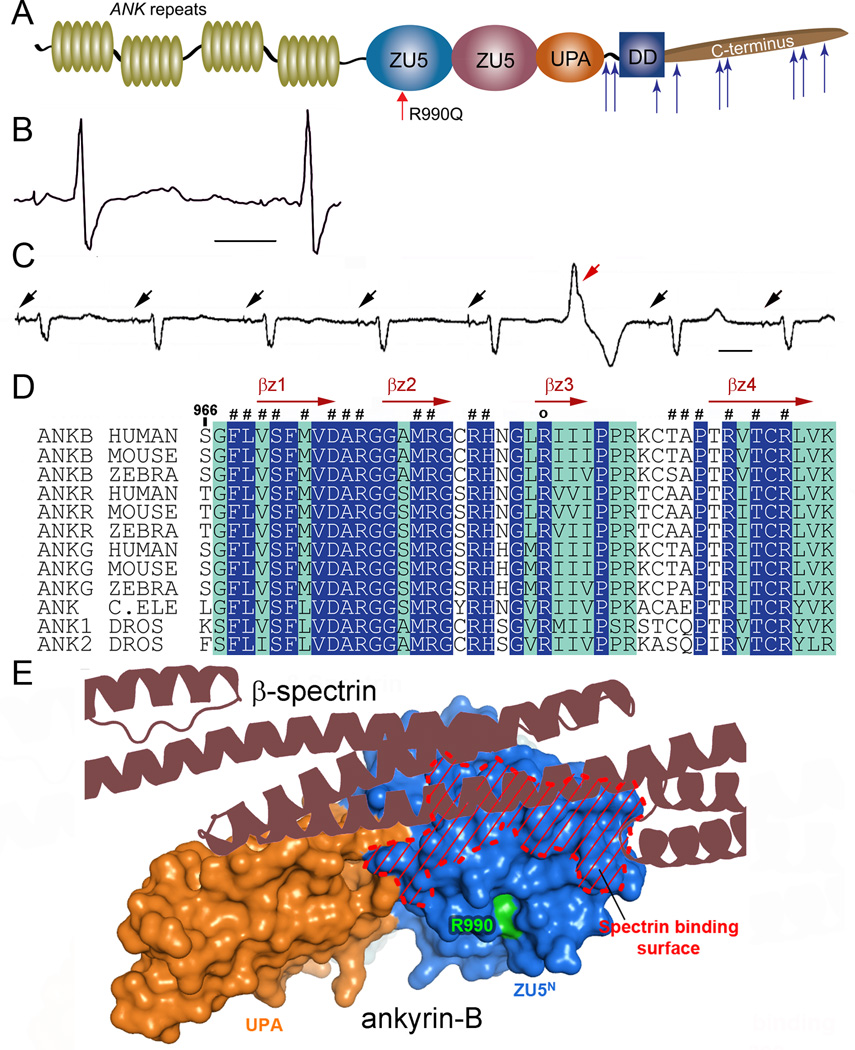
Human ANK2 variants cause cardiac arrhythmia phenotypes including sinus node disease, atrial fibrillation, conduction block, ventricular arrhythmia, syncope, and sudden cardiac death.7–11 We identified a new class of ANK2 variant in a proband with severe history of recurrent sudden cardiac arrest due to ventricular fibrillation (VF) (Figure 1A). The proband is a 36-year-old female with prolonged QTc on her electrocardiogram (ECG) who suffered out of hospital cardiac arrest due to VF. Following resuscitation, she underwent implantation of a transvenous dual chamber implantable cardioverter-defibrillator (ICD). Since her first event, she has had recurrent VF resulting in syncope and ICD shocks, with ICD interrogation demonstrating premature ventricular complexes (PVCs) preceding episodes of VF. In addition to prolonged QTc interval (Figure 1B), the individual displays regular PVCs (Figure 1C), both harbingers of potential arrhythmic events. Initial genetic testing for variants in KCNQ1, KCNH2, SCN5A, KCNE1, and KCNE2 was negative for deleterious mutations. Subsequent genetic testing utilizing an extended sequencing panel including 5 additional genes (ANK2, KCNJ2, CAV3, RYR2, and CASQ2) revealed an ANK2 c.2969G>A change resulting in the substitution of Arg to Gln at position 990 (p.R990Q). Exon array of ANK2 and other genes previously tested by sequencing analysis did not detect any deletions or duplications. The c.2969G>A variant is rare across multiple populations with a minor allele frequency of ~0.007% (0/4406 African-American alleles, 1/8599 European-American alleles; NHLBI ESP). Notably, R990 is highly conserved from human to zebrafish, roundworm, and fruit fly (Figure 1D), and structural modelling reveals that the p.R990Q variant is juxtaposed to the central ZU5 binding surface for βII spectrin (Figure 1A, E12). This region of ankyrin has not previously been linked with disease and in fact is >1,500 base pairs from any previously identified variant (Figure 1A,D-E).
Figure 1.
Ankyrin-B arrhythmia variant identified in conserved spectrin-binding domain. (A) Ankyrin-B includes ANK repeats, a spectrin-binding domain comprised of two ZU5 and one UPA domain, and a regulatory domain comprised of a death and C-terminus. Identified ANK2 loss-of-function mutations are noted by blue arrows and novel p.R990Q variant is indicated in red. (B) ANK2 p.R990Q proband displays QT-prolongation. (C) 10 second rhythm strip in p.R990Q proband demonstrating atrial demand pacing (black arrows) with premature ventricular contraction (red arrow). (D) Sequence alignment of ankyrin-B spectrin-binding sequence. Residues that are absolutely conserved and highly conserved are in blue and green, respectively. Secondary structural elements are indicated above the alignment. p.R990Q is conserved across species and marked with “o”. (E) Structure of the ZU5N-UPA tandem of ankyrin-B SBD reveals the spectrin-binding surface and location of p.R990 (green).
Ankyrin-B and βII spectrin are molecular partners in human heart
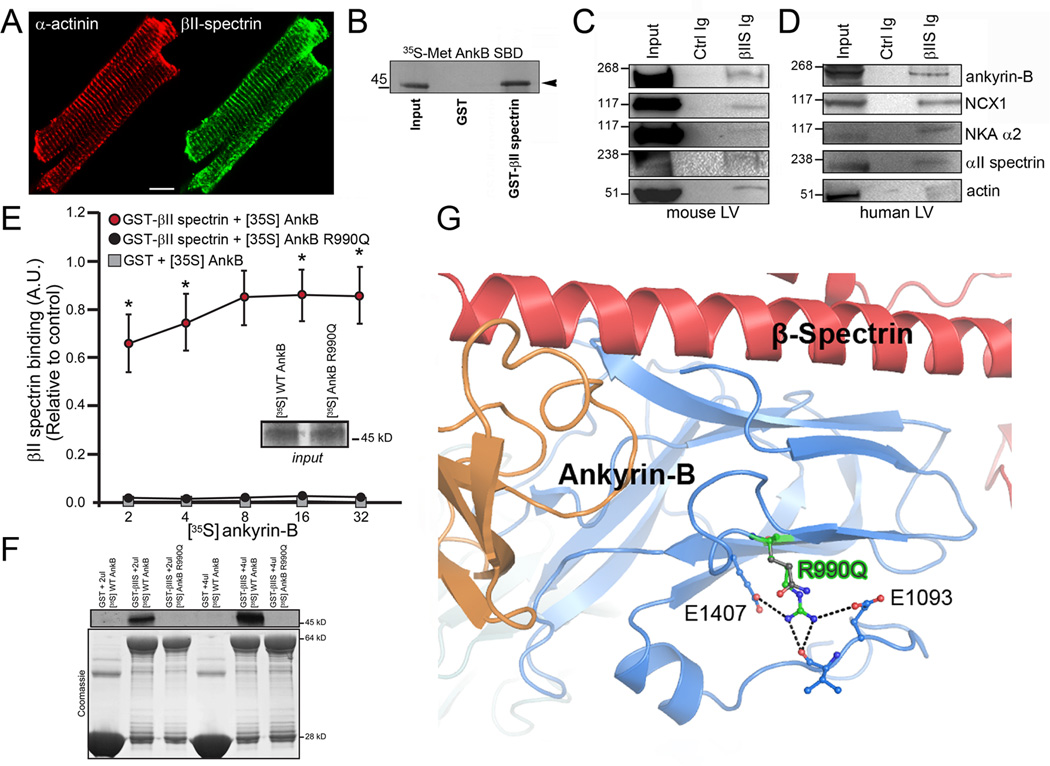
A requisite function of a canonical ankyrin polypeptide is association with the α/β spectrin hetero-tetramer. This complex, by association with actin (via spectrin) functionally couples integral membrane proteins (ion channels, receptors, transporters) with the cytoskeletal infrastructure. Ankyrins associate with β spectrin gene products through conserved residues in the N-terminal ZU5 (Zu5N) domain.12 Based on the location of the human variant, we tested the functional relationship between ankyrin-B and βII spectrin. βII spectrin is normally localized at myocyte T-tubules (Figure 2A and Supplemental Figure 1) with ankyrin-B.13 Ankyrin-B directly associates with radiolabelled βII spectrin (Figure 2B), and βII spectrin co-immunoprecipitates with ankyrin-B from detergent-soluble lysates from adult mouse heart as well as non-failing human heart (Figure 2C-D). We observed a larger macromolecular complex between ankyrin-B and βII spectrin with membrane proteins including the Na+/K+ ATPase and Na+/Ca2+ exchanger (ankyrin-binding partners) as well as the cytoskeletal element actin by co-immunoprecipitation experiments from both mouse and human heart (Figure 2C-D). These data demonstrate the presence of a βII spectrin/ankyrin-B cytoskeletal complex in heart.
Figure 2.
Ankyrin-B/βII spectrin complex is blocked by human disease mutation. (A) βII spectrin (green) is localized in a striated pattern in isolated mouse myocytes (co-labelled with α-actinin, red). Scale=10 μm. (B) Radiolabelled ankyrin-B (spectrin-binding domain) associates with GST βII spectrin fusion protein, but not GST. (C-D) βII spectrin (βIIS) Ig co-immunoprecipitates ankyrin-B, NCX1, Na/K ATPase, αII spectrin, and actin from detergent soluble lysates of non-failing mouse and human LV. (E) Human ANK2 p.R990Q arrhythmia variant displays aberrant βII spectrin-binding. Data in inset represents equal inputs for experiments. Curves denote binding for GST-βII spectrin or GST alone with a concentration range of radiolabelled ankyrin-B or radiolabelled ankyrin-B R990Q (n=5/group; * represents p<0.05). Panel F represents primary binding data and Coomassie Blue-stained gels for fusion proteins in E. (G) Ribbon structure from co-crystal structure of ankyrin-B/βII spectrin. Human p.R990Q variant shown in green.
We hypothesized that the human p.R990Q variant confers susceptibility to cardiac arrhythmia by altering ankyrin-B-binding for βII spectrin, thus affecting integration of membrane proteins with the cytoskeleton.12, 14 While wild-type ankyrin-B robustly associated with βII spectrin, we observed a significant decrease in binding of ankyrin-B p.R990Q with βII spectrin (Figure 2E-F). In silico modelling for the human p.R990Q variant using the co-structure for ankyrin-B and βII spectrin revealed that the R990 forms several hydrogen bonds and salt bridges with surrounding residues, a property strictly conserved in all ankyrins (Figure 2G). Moreover, modelling revealed that p.R990Q results in loss of salt bridge interactions with surrounding residues (Figure 2G). Ankyrin-B p.R990Q may directly disrupt βII spectrin-binding. Alternatively, based on the localization of the residue, this mutation may destabilize the folding of the ZU5 domain, resulting indirectly in the loss of spectrin-binding and overall loss of ankyrin-B function.
Analysis of human variants that block βII spectrin-binding in myocytes
We tested the relationship between human ankyrin-B variants and βII spectrin in primary myocytes. As mice lacking global ankyrin-B expression die in utero or shortly after birth thus limiting the ability to study ankyrin-dependent mechanisms past post-natal day 2, we generated a new mouse model homozygous for a conditional cardiac ankyrin-B null allele (αMHC-Cre; ankyrin-B conditional knockout (cKO) mouse; Supplemental Figure 2). The null allele was created by targeting Ank2 exon 24 disrupting all known Ank2 splice products. The mutant allele was confirmed by Southern blot and PCR strategies (Supplemental Figure 2B), and myocytes from ankyrin-B cKO mice display loss of ankyrin-B protein by immunoblot and immunostaining (Supplemental Figure 2C-F). Using well-differentiated primary neonatal ankyrin-B cKO myocytes (post-natal day 7), we tested activity of the p.R990Q variant. WT ankyrin-B-GFP or human ankyrin-B variant p.R990Q were introduced into ankyrin-B cKO myocytes. Notably, compared with control WT myocytes (Supplemental Figure 3A-B), ankyrin-B cKO myocytes display loss of targeting of Na+/Ca2+ exchanger, a known binding partner in heart (Supplemental Figure 3C-D). GFP-ankyrin-B was striated when expressed in ankyrin-B cKO myocytes, and was sufficient to rescue the localization of the Na+/Ca2+ exchanger (Supplemental Figure 3E-F). In contrast, ankyrin-B p.R990Q, similar to two ankyrin-B mutants previously identified to lack spectrin-binding (A1000P, DAR976AAA)13, was not appropriately targeted (diffuse cytoplasmic expression) and failed to rescue the localization of the Na+/Ca2+ exchanger (Supplemental Figure 3G-L).
βII spectrin cKO mice display sinus node dysfunction and ventricular arrhythmia
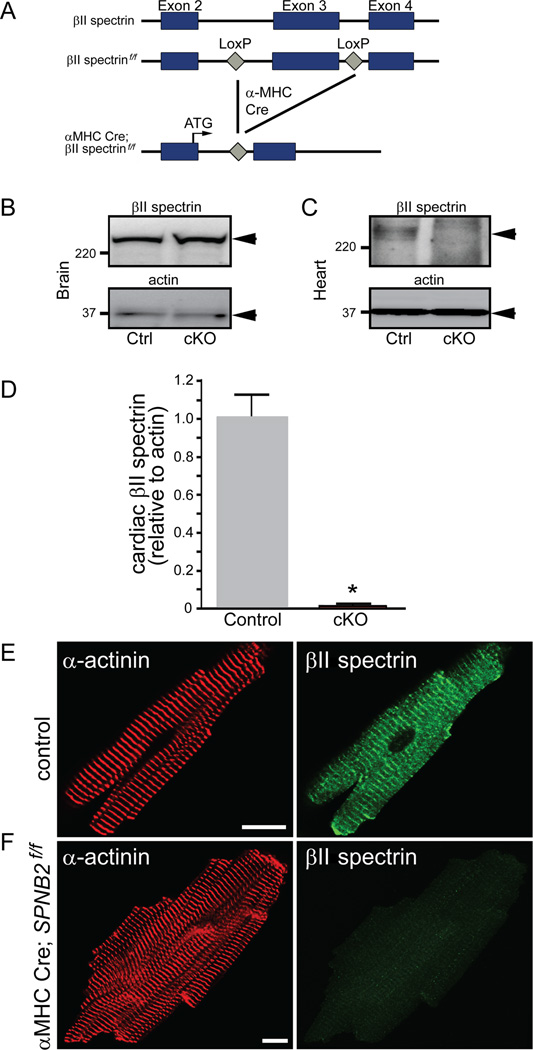
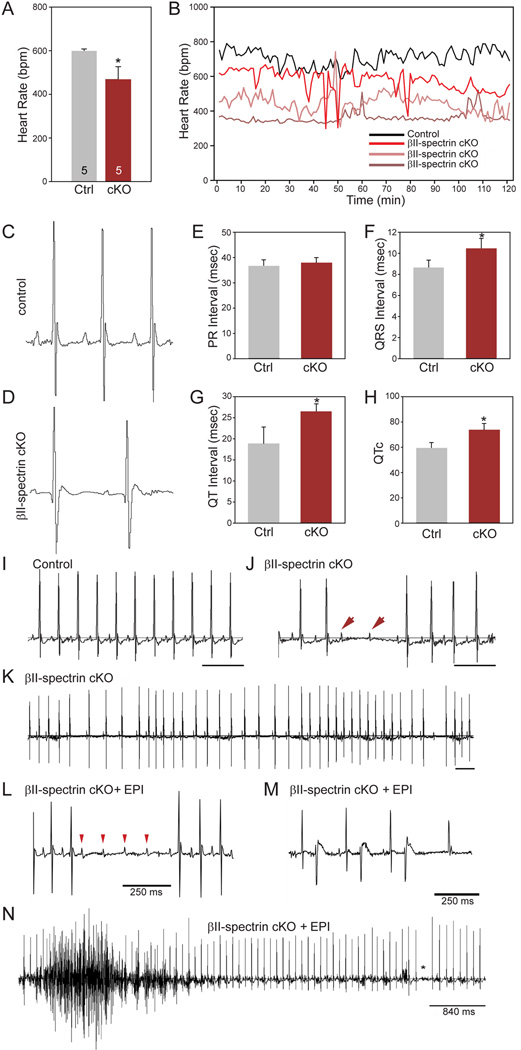
The functional role for βII spectrin for post-natal cardiac function in vivo is unknown and untested. We therefore examined cardiac electrical phenotypes in a new mouse model with conditional deletion of cardiac βII spectrin (Figure 3A; βII spectrin cKO). βII spectrin cKO mice lack βII spectrin in heart but not in other tissues (Figure 3B-F). Telemetry studies of conscious mice show reduced heart rate, increased heart rate variability, atrioventricular (AV) block, and pro-arrhythmic ECG phenotypes (widened QRS complexes, prolonged QT interval) in βII spectrin cKO mice compared with WT littermates at baseline (Figure 4A-H). Notably, catecholaminergic stress (exercise or low dose epinephrine) resulted in pronounced ventricular arrhythmia (both non-sustained and sustained episodes) and death (Figure 4L-N). We observed no difference in maximal heart rate in response to low or high dose epinephrine (Supplemental Figure 4) and under no circumstances were ECG or arrhythmic anomalies observed in control littermates. While cardiac electrical dysfunction may arise secondary to structural heart disease (hypertrophy, heart failure), we observed no significant differences in mean left ventricular ejection fraction (LVEF), cardiac output, wall thickness, or other significant structural phenotypes between control and βII spectrin cKO mice (Supplemental Figure 5A-B and Supplemental Figure 6A-M).
Figure 3.
Generation and validation of mice lacking βII spectrin in cardiomyocytes. (A) Targeting strategy to generate Cre-dependent loss of cardiac βII spectrin. βII spectrin in brain (B) and heart (C) of control and βII spectrin cKO mice. (D) βII spectrin levels in control (n=5) versus βII spectrin cKO hearts (n=5; p<0.05). (E) βII spectrin localization in control and βII spectrin cKO adult ventricular myocytes. Bar= 10 μm.
Figure 4.
Loss of βII spectrin causes bradycardia, rate variability, and arrhythmia. (A-B) βII spectrin cKO mice display reduced heart rate (n=5) as assessed by telemetry compared to control mice (n=5, p<0.05). (B) Heart rates of control and three βII spectrin cKO mice that show bradycardia and rate variability. (C-D) ECGs from control and βII spectrin cKO mouse showing increased RR, QRS, and QT intervals. Mean data for parameters are shown inE-H (n=5 mice/genotype; p<0.05). (I-J) Control ECG recording over 1-second demonstrating no R-R variability or heart block vs. ECG recording from βII spectrin cKO littermate demonstrating type II heart block, confirmed by P-waves (arrow heads) without ventricular conduction. (K) 3-second ECG recording of βII spectrin cKO mouse demonstrating significant R-R variability with heart block. (L-N) βII spectrin cKO mice demonstrate severe arrhythmia phenotypes and death following injection of epinephrine. Examples include (L) four sinus P-waves (arrow heads) without ventricular conduction consistent with type II AV block, (M) bigeminy, and (N) polymorphic ventricular arrhythmia.
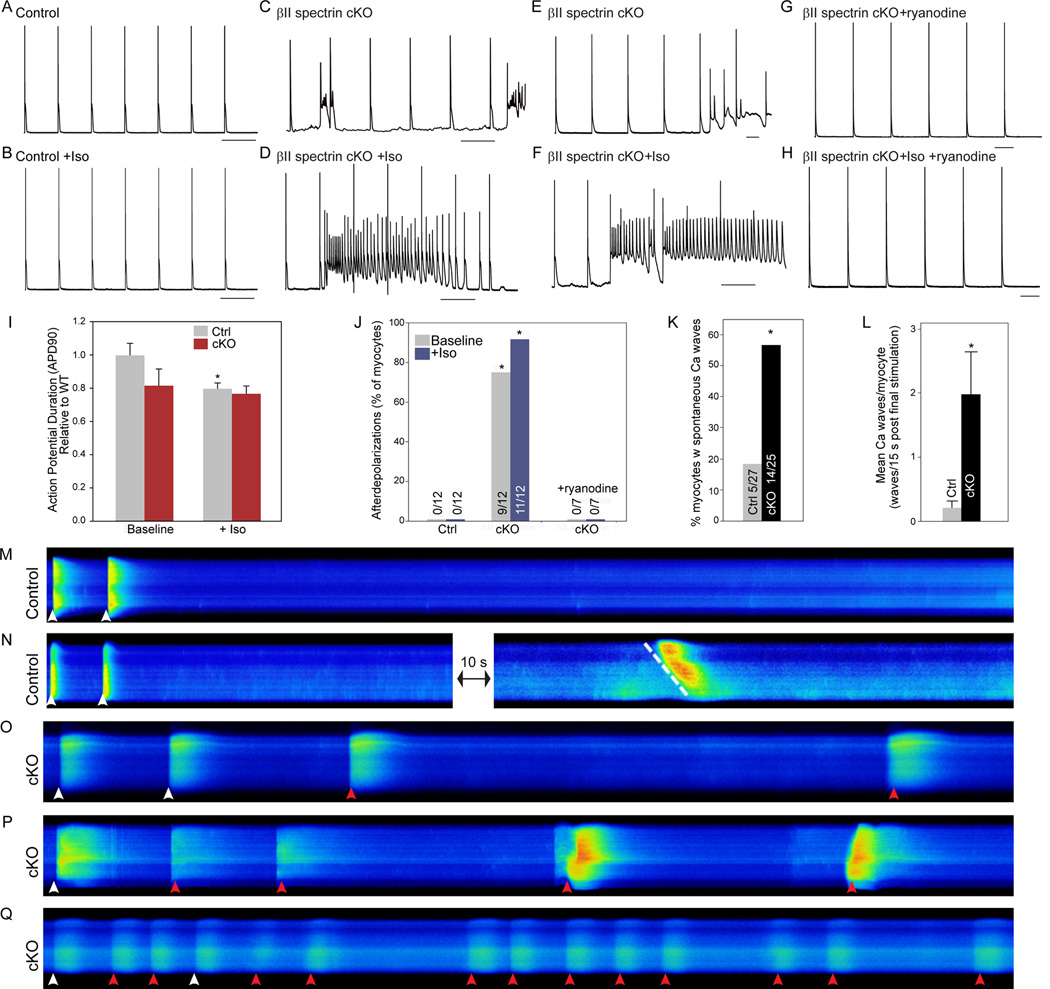
βII spectrin cKO myocytes display afterdepolarizations and abnormal calcium waves
Based on severity of observed arrhythmias in βII spectrin cKO mice, we examined βII spectrin cKO mouse ventricular myocyte action potentials. Unlike myocytes from control littermates, βII spectrin cKO myocytes displayed chaotic electrical behavior even in the absence of catecholamine stimulation (Figure 5A,C). Mean APD90 was not different between genotypes (Figure 5I). However, we observed frequent spontaneous afterdepolarizations in βII spectrin cKO myocytes at baseline (Figure 5C-D). Afterdepolarizations were present independent of pacing frequency and increased in both frequency and duration following superfusion with isoproterenol (Figure 5B, D, F). We tested whether spontaneous calcium release potentially underlies the chaotic electrical behavior of βII spectrin cKO myocytes by examining action potentials in the presence of ryanodine, an inhibitor of Ca2+ release from the SR. Notably, ryanodine (100 nM) prevented afterdepolarizations in myocytes ±isoproterenol (Figure 5G-H, J). Together, these data strongly support a critical role for βII spectrin in regulating myocyte electrical behavior and suggest a role of intracellular Ca2+ as a potential mechanism underlying the electrical arrhythmogenic phenotypes.
Figure 5.
βII spectrin cKO myocytes display electrical instability, afterdepolarizations, and aberrant Ca waves. Action potential measurements of (A-B) control and (C-D) βII spectrin cKO myocytes measured at baseline at 0.5 Hz pacing protocol ± 1μM Iso. (E-F) Electrical instability was present in βII spectrin cKO myocytes independent of pacing frequency (shown at 1 Hz). (G-H) Ryanodine (100 nM) blocked abnormal electrical instability of βII spectrin cKO myocytes ± Iso. (I) Mean APD90 of control and βII spectrin cKO myocytes ±Iso (n> 10 myocytes genotype; p<0.05 for control vs. control + Iso). (J) Prevalence of afterdepolarizations for control and βII spectrin cKO myocytes ±Iso and in the presence of ryanodine (n>10 myocytes/treatment; p<0.05 for control versus cKO at baseline; p<0.05 for control +Iso versus cKO +Iso). (K-L) βII spectrin cKO myocytes were more likely to form spontaneous Ca2+ waves (n=27 control, n=25 βII spectrin cKO; p<0.05). (M-Q) Linescan images of fluo-4-loaded myocytes field stimulated at 0.5 Hz. Following stimulation, myocytes were continuously monitored for spontaneous Ca2+ wave formation for 15 sec. (M-N) Control myocytes with no spontaneous wave activity. When waves formed they were slow moving (N, dashed white line). (O-Q) Spontaneous waves in βII spectrin cKO myocytes (red arrowheads). Stimulated transients are indicated by white arrowheads.
Spontaneous Ca2+ waves (SCaW) at the level of the myocyte may mediate Ca2+ -dependent afterdepolarizations, known cellular triggers for arrhythmia.15, 16 Therefore, we compared the relative propensity of control and βII spectrin cKO myocytes to generate SCaWs. βII spectrin cKO myocytes were significantly more likely to generate SCaWs compared with control myocytes (Figure 5M-Q). SCaWs were observed in ~56% of βII spectrin cKO myocytes versus only ~18% of control myocytes (Figure 5K), and we observed <0.2 waves/WT myocyte versus an average of ~2 waves/βII spectrin cKO myocyte (p<0.05). Importantly, spontaneous unified Ca2+ release events (indicated by a perpendicular spontaneous dye front in the confocal line-scans) were frequently observed in βII spectrin cKO myocytes but not in control myocytes (Figure 5O-Q). Such perpendicular waves are indicative of a unified Ca2+ release within the myocyte, and provide evidence that the cell spontaneously reached threshold that initiated an action potential. Such a unified spontaneous Ca2+ release was not observed in control myocytes; rather only slowly propagating waves were observed (Figure 5M). This SCaW-dependent phenotype provides a mechanism for the afterdepolarizations observed in βII spectrin cKO myocytes.
βII spectrin is required for organization of cardiac membrane proteins
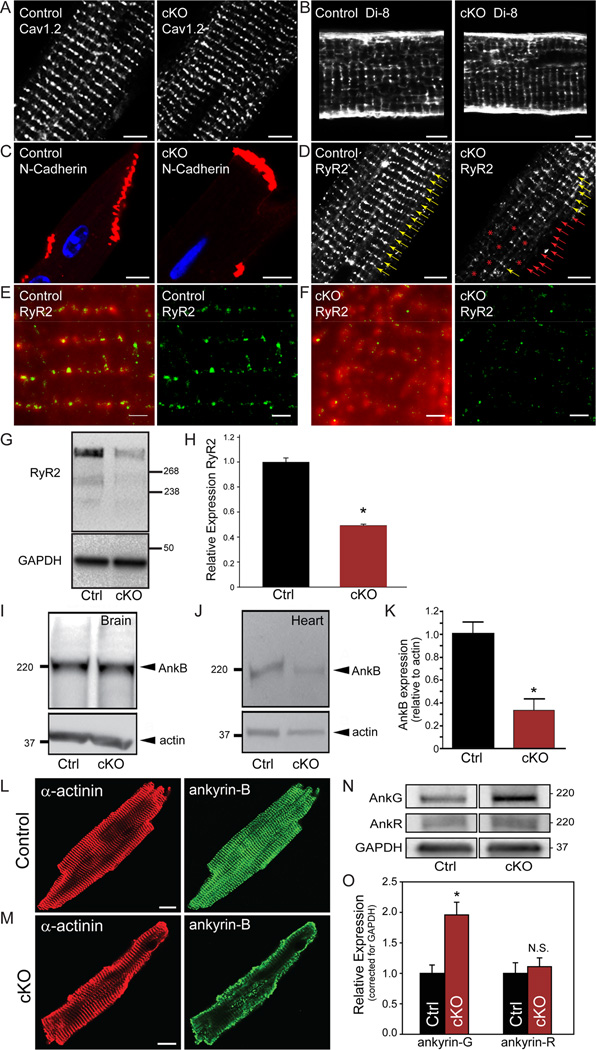
We hypothesized that loss of normal calcium cycling and arrhythmias in βII spectrin cKO mice reflected the central role for βII spectrin in organizing cardiac membrane proteins. To test this hypothesis, we first examined the status of T-tubule and SR membrane proteins critical for calcium release. While we observed no difference in T-tubule L-type calcium channel localization or T-tubule structure between genotypes (Figure 6A-B), we observed heterogeneity in the subcellular localization of ryanodine receptor 2 (RyR2), the primary cardiac SR calcium release channel in βII spectrin cKO myocytes (Figure 6D and Supplemental Figure 7). To further examine potential RyR2 heterogeneity, we analysed RyR2 localization by total internal reflection fluorescence (TIRF) and super-resolution imaging (see Supplemental Methods). In agreement with previous super-resolution imaging of Soeller and colleagues17, RyR2 is localized by these techniques to discrete clusters of irregular shapes/sizes (Figure 6E). In contrast, RyR2 imaging of heterogeneous regions of interest (i.e. red * in Figure 6D, right) in βII spectrin cKO myocytes showed reduced size/intensity of RyR2 clusters as well as irregular intensity patterning compared with control myocytes (Figure 6F). Consistent with these findings RyR2 levels were significantly reduced in βII spectrin cKO hearts (Figure 6G-H). This loss was selective for RyR2 versus other SR proteins as we observed no difference in the expression or localization of the SR calcium ATPase (SERCA2) or in the intercalated disc protein N-cadherin (Figure 6C). In summary, these data define βII spectrin as required for the selective local organization of RyR2 calcium release channels. Of note, defects in local RyR2 organization have been linked with aberrant calcium-dependent release, arrhythmia, and heart failure phenotypes in humans and animal models.18–20
Figure 6.
βII spectrin is required for organization of calcium release units and ankyrin-B. Like control mouse myocytes, βII spectrin cKO myocytes display normal localization of transverse-tubule Cav1.2 (A), transverse-tubule organization (B, visualized by Di-8-ANEPPs), and intercalated disc N-cadherin (C). In contrast, RyR2 expression was reduced and heterogeneous in βII spectrin cKO myocytes (D, red *). (E-F) RyR2 expression was further analyzed by total internal reflection fluorescence (TIRF) and super-resolution imaging. (E-F, left) Overlay of a TIRF image (red) and the corresponding super-resolution image (green) of RyR2 in control (E) and βII spectrin cKO (F) cardiomyocyte. (E-F, right) Super-resolution images of RyR2 in control and βII spectrin cKO myocytes. Note that data in F was collected from area of RyR2 heterogeneity (red * cKO panel D, right) that illustrates reduced RyR2 cluster size and intensity. Scale bar for E-F: 1000 nm. (G-H) RyR2 levels were significantly reduced in βII spectrin cKO myocytes (n=4 hearts/genotype, p<0.05). (I-K) Ankyrin-B levels are decreased in the heart but not brain of βII spectrin cKO mice (n=5 hearts/genotype; p<0.05). (L-M) Ankyrin-B shows reduced expression and abnormal targeting in βII spectrin cKO versus control myocytes (Bar=10 microns). (N-O) Ankyrin-G, but not ankyrin-R levels are decreased in the heart of βII spectrin cKO mice (n=5) versus control mice (n=6; p<0.05).
βII spectrin is required for expression and targeting of ankyrin-B
In addition to RyR2, βII spectrin cKO mice displayed loss of expression and localization of ankyrin-B in adult heart (Figure 6I-M). These findings were initially unanticipated, as prior work implicates ankyrin-B as critical for βII spectrin targeting and localization in post-natal day two neonatal cardiomyocytes.13 To define the functional relationship of ankyrin-B and βII spectrin in myocyte localization, we evaluated βII spectrin and ankyrin-B localization in wild-type neonatal cardiomyocytes. As shown in Supplemental Figure 8, striated expression of ankyrin-B precedes βII spectrin at post-natal day 1 in mice. However, both proteins are expressed and co-localized by post-natal day three (Supplemental Figure 8). In support of a role of ankyrin-B in the targeting of βII spectrin and in agreement with prior work13, ankyrin-B cKO neonatal cardiomyocytes lack βII spectrin expression or striation at post-natal day 1 or day 3 (Supplemental Figure 8). However, we were surprised to observe that later in myocyte maturation (post-natal day 7), ankyrin-B expression was not requisite for βII spectrin expression. In fact, βII spectrin expression in post-natal day myocytes is equivalent to expression/staining observed in wild-type cardiomyocytes (Supplemental Figure 8). This is further illustrated in adult myocytes where we observe no difference in βII spectrin expression or localization in ankyrin-B cKO hearts (Supplemental Figures 9-10). Finally, βII spectrin cKO cardiomyocytes demonstrate normal expression and localization of ankyrin-B at post-natal day 1 and 3, but loss of ankyrin-B expression and targeting at post-natal day 7 (Supplemental Figure 8). Together, these findings demonstrate a complex relationship between ankyrin and /spectrin in heart. More specifically, our data show that ankyrin-B plays a dominant role for βII spectrin targeting in immature, developing myocytes whereas in maturing and mature myocytes, βII spectrin assumes the dominant targeting role for ankyrin-B. While we assume this transition likely represents a shift in the maturity of the membrane/cytoskeletal network (i.e. myocyte transverse-tubules begin to develop ~7 days in culture21), future work will be important to better define the underlying mechanisms. Notably, while βII spectrin cKO mice displayed reduced ankyrin-B expression, we observed no change in the expression of ankyrin-R (Ank1) in βII spectrin cKO adult heart compared to control (Figure 6N-O). However, ankyrin-G (Ank3), a third ankyrin gene product expressed in heart22, showed elevated expression in βII spectrin cKO hearts (Figure 6N-O), likely as a compensatory mechanism in response to reduced ankyrin-B levels.
βII spectrin is required for organization of ankyrin-B-associated membrane proteins
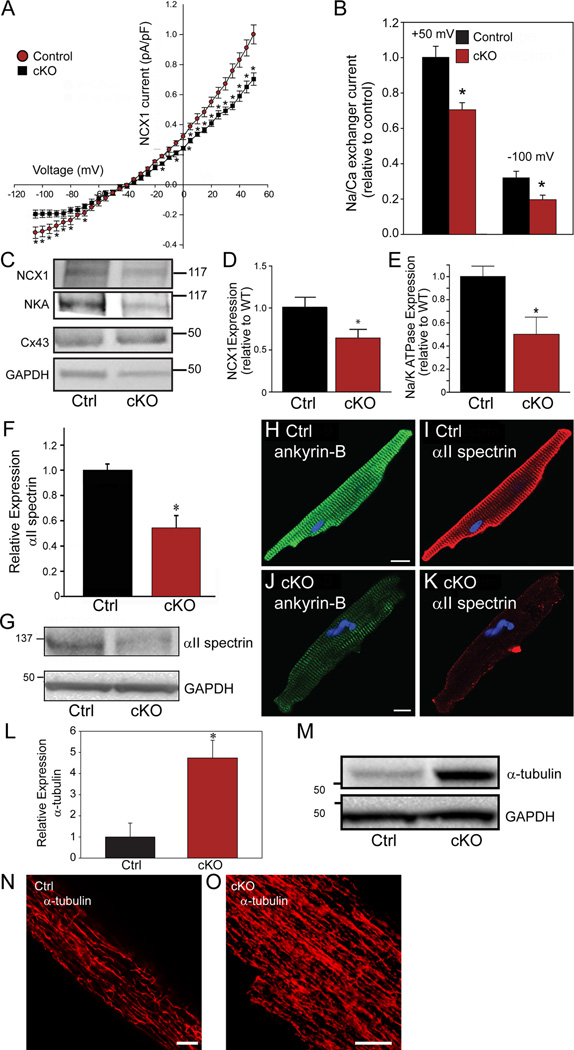
Ankyrin-B targets Na+/Ca2+ exchanger and Na+/K+ ATPase and controls regulation of dyadic proteins, including RyR2.23 Consistent with our above findings, we observed a significant decrease in INCX in βII spectrin cKO myocytes (Figure 7A-B). These changes were confirmed by both immunoblot and immunostaining where we observed decreased expression of Na/Ca exchanger as well as Na/K ATPase (Figure 7C-E). Notably, despite the major alterations in electrical activity in βII spectrin cKO myocytes, we did not observe differences in INa current (peak, activation/ inactivation/late current) and Nav1.5 and connexin43 protein levels were unchanged in βII spectrin cKO hearts (Figure 7C and Supplemental Figure 11). Together with our data on RyR2, these findings identify βII spectrin as essential for the localization of multiple membrane proteins required for myocyte Ca2+ regulation.
Figure 7.
βII spectrin deficiency results in reduced expression of ankyrin-B-associated membrane proteins and abnormal myocyte cytoskeletal organization. (A-B) βII spectrin cKO myocytes (n=12) display reduced INCX compared with control mouse myocytes (n=14; p<0.05). (C-E) βII spectrin cKO hearts display reduced NCX and Na/K ATPase expression but normal expression of Cx43 compared with control hearts (n=4 hearts/genotype; p<0.05). (F-K) βII spectrin cKO hearts (n=5) display reduced αII spectrin expression and localization compared with control hearts (n=5) by immunoblot and immunostaining (p<0.05). (L-O) βII spectrin cKO hearts display significant increase in α-tubulin expression by immunoblot and immunostaining compared to control hearts (n=5 hearts/genotype for panel L; p<0.05). Bar=10 μM for panels N-O.
βII spectrin regulates αII spectrin and the myocyte microtubule network
While Na/Ca exchanger and Na/K ATPase loss is likely related to loss of ankyrin-B targeting in βII spectrin cKO mice, the mechanism for RyR2 dysfunction is less clear. We hypothesized that this defect reflects an important role for βII spectrin in organizing local cardiac cytoskeleton. In metazoans, α- and β-spectrin form a submembrane lattice through formation of hetero-tetramers via anti-parallel N- and C-terminal interactions. However, the requirement of βII spectrin expression for the targeting and formation of the hetero-tetramer in heart is unknown. We examined the abundance of αII spectrin, an in vivo partner for cardiac βII spectrin. Consistent with prior reports demonstrating degradation of α-spectrin in the absence of adequate erythroid β-spectrin24–26, αII spectrin levels were reduced nearly 50% in βII spectrin cKO hearts (Figure 7F-G). In line with these data, we observed reduced abundance of αII spectrin in βII spectrin cKO myocytes by immunostaining (Figure 7H-K). Based on these data, we further investigated the integrity of the cytoskeleton in βII spectrin cKO myocytes. While actin was unchanged in expression or localization in βII spectrin cKO mice, we observed significant increases in levels of βI spectrin and α- and β-tubulin in βII spectrin cKO hearts by both immunoblot and/or immunostaining (Figure 7L-O and Supplemental Figure 12), likely as a compensatory response to βII spectrin deficiency, and consistent with prior findings of tubulin remodeling in failing hearts.27 However, levels of desmin, an intermediate filament protein critical for myocyte cytoskeletal infrastructure, were unchanged between control and βII spectrin cKO hearts (Supplemental Figure 12). In summary, these data demonstrate that myocytes lacking βII spectrin display significant, yet selective cytoskeletal remodeling.
βII spectrin cKO mice display accelerated heart failure phenotypes
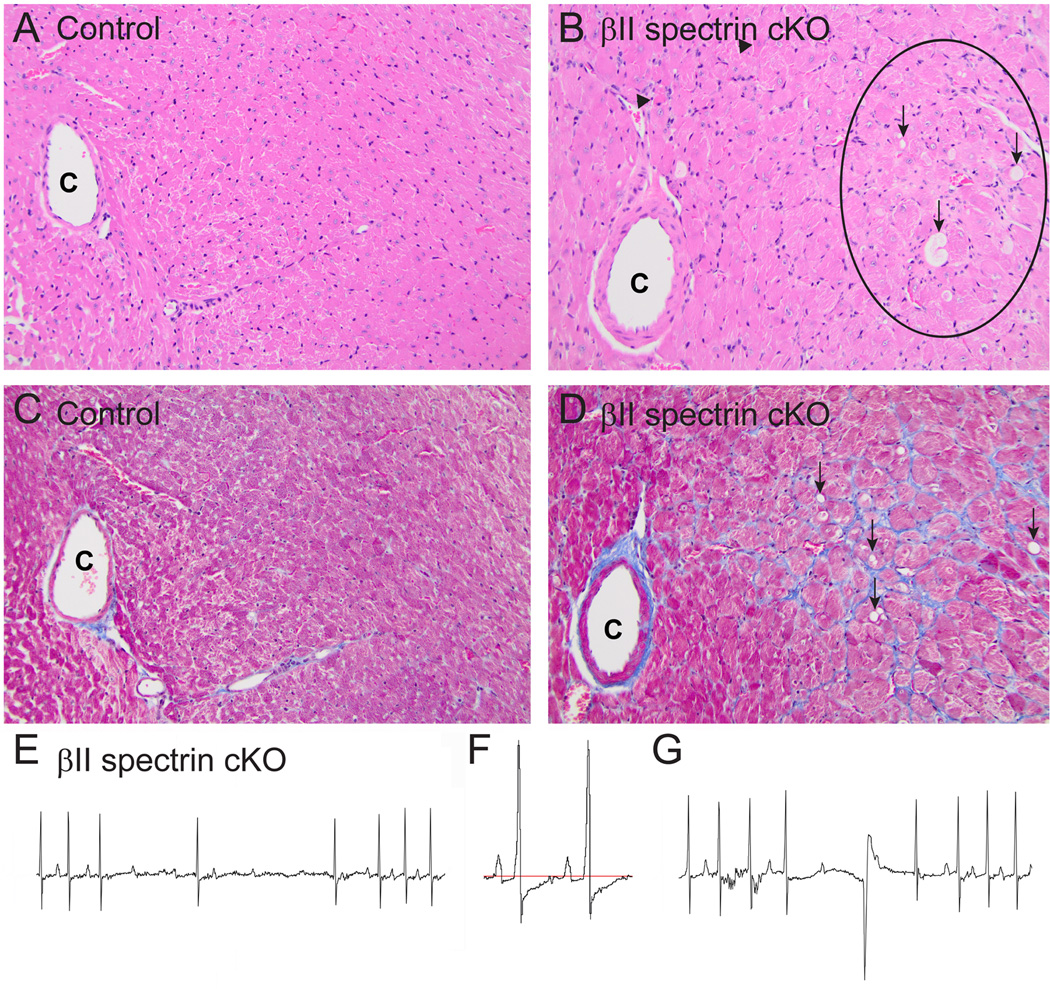
Heart failure is characterized by significant electrical and structural remodelling. Based on severe electrical and structural phenotypes present in βII spectrin cKO mice at baseline, we hypothesized that βII spectrin cKO mice would display accelerated and more pronounced cardiac damage following induction of heart failure through transverse aortic constriction (TAC). Notably, unlike control littermates or sham βII spectrin cKO mice, βII spectrin cKO mice displayed cardiac arrhythmia and death associated with major structural remodelling following six weeks of banding (Figure 8A-G). Examination of βII spectrin cKO mouse banded sections revealed high prevalence of widespread myocardial degeneration of the LV free wall and septum that was characterized by vacuolation, pallor, interstitial and ventricular myocyte necrosis (Figure 8A-D). Further, at six weeks post-banding βII spectrin cKO mice displayed severe AV block, ST segment depression, frequent PVCs, and junctional rhythms (Figure 8E-G). While we observed standard pre-heart failure phenotypes in control mice in response to the TAC protocol, we did not observe the extensive electrical or structural phenotypes found in βII spectrin cKO littermates.
Figure 8.
βII spectrin cKO mice show severe damage and electrical phenotypes following aortic banding. (A-B) Unlike control TAC mice, βII spectrin cKO mice displayed widespread myocardial degeneration of the LV free wall and septum, characterized by vacuolation (arrows), pallor (inside circle), and necrosis of myocytes (C=coronary artery; H&E staining; 200x). (C-D) βII spectrin cKO hearts further displayed increased interstitial fibrosis (blue) of connective tissue compared with control sections (vacuolation noted by arrows; 200x). Electrical phenotypes observed in βII spectrin cKO and not control mice included (E) AV conduction defects, (F) ST segment depression, and (G) intermittent PVCs and junctional beats.
Discussion
The spectrin superfamily is comprised of two α- and five β- spectrin genes. Most information regarding spectrin function comes from the erythrocyte, although a growing body of literature points to key roles of spectrin family members in cytoskeletal infrastructure in complex cells. In the red blood cell, α- and β-spectrin tetramerize and form the basis of the submembrane ultrastructure through interactions with membrane proteins (e.g. anion exchanger) and cytoskeleton (actin). Mutations in α- or β-spectrin in humans or animals result in loss of membrane integrity, spherocytosis, and hemolytic anemia.28 In fact, spectrin mutations are the cause of the most common forms of hereditary spherocytosis in the Caucasian population. In complex cells, spectrins are critical for membrane assembly and maintenance and both αII and βII spectrin deficient mice are embryonic lethal.29, 30 βII spectrin is required for lateral membrane formation of columnar epithelial cells in the lung31 and βII and βIV spectrin are critical for development and maintenance of the axon initial segment and nodes of Ranvier in the central nervous system.32–34 Little is known on the role of βII spectrin in heart as mice homozygous for βII spectrin allele deficiency die in utero.30 However, data from the βIII spectrin literature offers insight into the likely mechanisms for βII spectrin function in heart. For example, work from Stankewich, Morrow, Ranum, and colleagues demonstrate that βIII spectrin is essential for membrane protein targeting in the nervous system.35, 36 Similar to our findings in heart, targeted deletion of βIII spectrin in brain results in impaired assembly of the post-synaptic membrane, endomembrane retention of multiple synaptic proteins, as well as ataxia and seizure phenotypes.36 Moreover, human βIII spectrin gene mutations found in the region where βIII spectrin associates with the dynactin subunit Arp137 cause human spinocerebellar ataxia (SCA5) due in to defects in membrane protein (glutamate transporter EAAT4, metabotropic glutamate receptor 1α) targeting.35, 38 Thus, based on βIII spectrin data, as well as our new findings, we hypothesize that βII spectrin is a critical player in membrane protein sorting, versus simply a static membrane structural protein. In fact, the demonstrated links between spectrins and dynactin37 provide a logical rationale for the cell and molecular phenotypes observed in βII spectrin cKO animals. While this study focused on the relationship between βII spectrin and ankyrin-B, based on our new data (Figure 6N-O), it will be important to identify the relationship of βII spectrin with other cardiac ankyrin and spectrin gene products.
An important, but unexpected finding of this study is the essential role of βII spectrin for the local organization of RyR2. The cardiac RyR2 is a central player for myocyte calcium release and RyR2 dysfunction has been linked with a broad spectrum of heart failure and arrhythmia phenotypes in human and animal models.39–42 Our data demonstrate a role of βII spectrin for both the expression and targeting of RyR2. However, potentially more significant, remaining RyR2 populations in βII spectrin cKO myocytes are disorganized, displaying a heterogeneous pattern. In parallel, loss of βII spectrin results in reorganization of the microtubule network that may underlie the heterogeneous RyR2 distribution. Alternatively, microtubule reorganization may be a compensatory response of the cell to βII spectrin loss, as we also identified increased expression of βI spectrin (Supplemental Figure 12). Notably, abnormal organization of the calcium release structure was also identified in a mutant mouse model harboring a calsequestrin mutation linked with human catecholaminergic polymorphic ventricular tachycardia (CPVT).42 These mice, like the βII spectrin cKO mice, display unstable electrical events and significant cytosolic calcium management phenotypes. Beyond spectrin, junctophilin, a lipophilic molecule anchored to the junctional SR, has also been linked with the organization of the ryanodine receptor in heart. In fact, JPH2−/− mice are embryonic lethal and display an enlarged dyadic cleft.43 While organization of the RyR2 is altered in βII spectrin cKO mice, we observed normal localization and expression of T-tubule L-type calcium channels (Cav1.2). Further, we observed no difference in T-tubule morphology between control and βII spectrin cKO mice through Di-8-ANEPPS imaging. Therefore, the βII spectrin pathway appears to regulate select populations of membrane proteins without altering membrane morphology. It will be critical for future experiments to define the specific pathways underlying βII spectrin-dependent regulation of RyR2 clusters. Importantly, βII spectrin-dependent regulation of RyR2 clusters is likely independent of ankyrin-B, as ankyrin-B+/- mice display no defects in RyR2 expression or localization.10 Finally, our work supports a relationship between βII spectrin and the microtubule system. As spectrins have previously been linked with microtubule-based proteins including kinesin II and the dynein complex44–46, and spectrin-associated proteins including protein 4.1R have been linked with arrhythmia in animals47, work to integrate the role of the βII spectrin pathway with intracellular targeting versus cytoskeletal organization will be an important future area of research. Likewise, as our data show accelerated heart failure phenotypes in βII spectrin cKO mice (Figure 8), it will be important to investigate the long-term function of βII spectrin in well-phenotyped human and animal disease models.
Supplementary Material
Acknowledgments
Funding Sources: NIH HL114893 (TJH), HL084583, HL083422, HL114383 (PJM), James S. McDonell Foundation (TJH), American Heart Association (PJM), NCATS TL1TR001069 (SAS), The Indiana University Health – Indiana University School of Medicine Strategic Research Initiative (KGS and MV), South University of Science and Technology of China (WZ), the European–North American Atrial Fibrillation Research Alliance (07CVD03, DD) grant of Fondation Leducq.
Footnotes
Disclosures: None.
References
- 1.Moulik M, Vatta M, Witt SH, Arola AM, Murphy RT, McKenna WJ, Boriek AM, Oka K, Labeit S, Bowles NE, Arimura T, Kimura A, Towbin JA. Ankrd1, the gene encoding cardiac ankyrin repeat protein, is a novel dilated cardiomyopathy gene. J Am Coll Cardiol. 2009;54:325–333. doi: 10.1016/j.jacc.2009.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lammerding J, Schulze PC, Takahashi T, Kozlov S, Sullivan T, Kamm RD, Stewart CL, Lee RT. Lamin a/c deficiency causes defective nuclear mechanics and mechanotransduction. J Clin Invest. 2004;113:370–378. doi: 10.1172/JCI19670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Tintelen JP, Entius MM, Bhuiyan ZA, Jongbloed R, Wiesfeld AC, Wilde AA, van der Smagt J, Boven LG, Mannens MM, van Langen IM, Hofstra RM, Otterspoor LC, Doevendans PA, Rodriguez LM, van Gelder IC, Hauer RN. Plakophilin-2 mutations are the major determinant of familial arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circulation. 2006;113:1650–1658. doi: 10.1161/CIRCULATIONAHA.105.609719. [DOI] [PubMed] [Google Scholar]
- 4.Stroud MJ, Banerjee I, Veevers J, Chen J. Linker of nucleoskeleton and cytoskeleton complex proteins in cardiac structure, function, and disease. Circ Res. 2014;114:538–548. doi: 10.1161/CIRCRESAHA.114.301236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwartz PJ, Ackerman MJ, George AL, Jr, Wilde AA. Impact of genetics on the clinical management of channelopathies. J Am Coll Cardiol. 2013;62:169–180. doi: 10.1016/j.jacc.2013.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ackerman MJ, Mohler PJ. Defining a new paradigm for human arrhythmia syndromes: Phenotypic manifestations of gene mutations in ion channel- and transporter-associated proteins. Circ Res. 2010;107:457–465. doi: 10.1161/CIRCRESAHA.110.224592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cunha SR, Hund TJ, Hashemi S, Voigt N, Li N, Wright P, Koval O, Li J, Gudmundsson H, Gumina RJ, Karck M, Schott JJ, Probst V, Le Marec H, Anderson ME, Dobrev D, Wehrens XH, Mohler PJ. Defects in ankyrin-based membrane protein targeting pathways underlie atrial fibrillation. Circulation. 2011;124:1212–1222. doi: 10.1161/CIRCULATIONAHA.111.023986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le Scouarnec S, Bhasin N, Vieyres C, Hund TJ, Cunha SR, Koval O, Marionneau C, Chen B, Wu Y, Demolombe S, Song LS, Le Marec H, Probst V, Schott JJ, Anderson ME, Mohler PJ. Dysfunction in ankyrin-b-dependent ion channel and transporter targeting causes human sinus node disease. Proc Natl Acad Sci U S A. 2008;105:15617–15622. doi: 10.1073/pnas.0805500105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohler PJ, Le Scouarnec S, Denjoy I, Lowe JS, Guicheney P, Caron L, Driskell IM, Schott JJ, Norris K, Leenhardt A, Kim RB, Escande D, Roden DM. Defining the cellular phenotype of "ankyrin-b syndrome" variants: Human ank2 variants associated with clinical phenotypes display a spectrum of activities in cardiomyocytes. Circulation. 2007;115:432–441. doi: 10.1161/CIRCULATIONAHA.106.656512. [DOI] [PubMed] [Google Scholar]
- 10.Mohler PJ, Schott JJ, Gramolini AO, Dilly KW, Guatimosim S, duBell WH, Song LS, Haurogne K, Kyndt F, Ali ME, Rogers TB, Lederer WJ, Escande D, Le Marec H, Bennett V. Ankyrin-b mutation causes type 4 long-qt cardiac arrhythmia and sudden cardiac death. Nature. 2003;421:634–639. doi: 10.1038/nature01335. [DOI] [PubMed] [Google Scholar]
- 11.Mohler PJ, Splawski I, Napolitano C, Bottelli G, Sharpe L, Timothy K, Priori SG, Keating MT, Bennett V. A cardiac arrhythmia syndrome caused by loss of ankyrin-b function. Proc Natl Acad Sci U S A. 2004;101:9137–9142. doi: 10.1073/pnas.0402546101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ipsaro JJ, Mondragon A. Structural basis for spectrin recognition by ankyrin. Blood. 2010;115:4093–4101. doi: 10.1182/blood-2009-11-255604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohler PJ, Yoon W, Bennett V. Ankyrin-b targets beta2-spectrin to an intracellular compartment in neonatal cardiomyocytes. J Biol Chem. 2004;279:40185–40193. doi: 10.1074/jbc.M406018200. [DOI] [PubMed] [Google Scholar]
- 14.Wang C, Yu C, Ye F, Wei Z, Zhang M. Structure of the zu5-zu5-upa-dd tandem of ankyrin-b reveals interaction surfaces necessary for ankyrin function. Proc Natl Acad Sci U S A. 2012;109:4822–4827. doi: 10.1073/pnas.1200613109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pogwizd SM. Focal mechanisms underlying ventricular tachycardia during prolonged ischemic cardiomyopathy. Circulation. 1994;90:1441–1458. doi: 10.1161/01.cir.90.3.1441. [DOI] [PubMed] [Google Scholar]
- 16.Pogwizd SM, Qi M, Yuan W, Samarel AM, Bers DM. Upregulation of na(+)/ca(2+) exchanger expression and function in an arrhythmogenic rabbit model of heart failure. Circ Res. 1999;85:1009–1019. doi: 10.1161/01.res.85.11.1009. [DOI] [PubMed] [Google Scholar]
- 17.Baddeley D, Jayasinghe ID, Lam L, Rossberger S, Cannell MB, Soeller C. Optical single-channel resolution imaging of the ryanodine receptor distribution in rat cardiac myocytes. Proc Natl Acad Sci U S A. 2009;106:22275–22280. doi: 10.1073/pnas.0908971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Go LO, Moschella MC, Watras J, Handa KK, Fyfe BS, Marks AR. Differential regulation of two types of intracellular calcium release channels during end-stage heart failure. J Clin Invest. 1995;95:888–894. doi: 10.1172/JCI117739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Oort RJ, Garbino A, Wang W, Dixit SS, Landstrom AP, Gaur N, De Almeida AC, Skapura DG, Rudy Y, Burns AR, Ackerman MJ, Wehrens XH. Disrupted junctional membrane complexes and hyperactive ryanodine receptors after acute junctophilin knockdown in mice. Circulation. 2011;123:979–988. doi: 10.1161/CIRCULATIONAHA.110.006437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Terentyev D, Nori A, Santoro M, Viatchenko-Karpinski S, Kubalova Z, Gyorke I, Terentyeva R, Vedamoorthyrao S, Blom NA, Valle G, Napolitano C, Williams SC, Volpe P, Priori SG, Gyorke S. Abnormal interactions of calsequestrin with the ryanodine receptor calcium release channel complex linked to exercise-induced sudden cardiac death. Circ Res. 2006;98:1151–1158. doi: 10.1161/01.RES.0000220647.93982.08. [DOI] [PubMed] [Google Scholar]
- 21.Mohler PJ, Gramolini AO, Bennett V. The ankyrin-b c-terminal domain determines activity of ankyrin-b/g chimeras in rescue of abnormal inositol 1,4,5-trisphosphate and ryanodine receptor distribution in ankyrin-b (−/−) neonatal cardiomyocytes. J Biol Chem. 2002;277:10599–10607. doi: 10.1074/jbc.M110958200. [DOI] [PubMed] [Google Scholar]
- 22.Makara MA, Curran J, Little SC, Musa H, Polina I, Smith SA, Wright PJ, Unudurthi SD, Snyder J, Bennett V, Hund TJ, Mohler PJ. Ankyrin-g coordinates intercalated disc signaling platform to regulate cardiac excitability in vivo. Circ Res. 2014;115:929–938. doi: 10.1161/CIRCRESAHA.115.305154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeGrande S, Nixon D, Koval O, Curran JW, Wright P, Wang Q, Kashef F, Chiang D, Li N, Wehrens XH, Anderson ME, Hund TJ, Mohler PJ. Camkii inhibition rescues proarrhythmic phenotypes in the model of human ankyrin-b syndrome. Heart Rhythm. 2012;9:2034–2041. doi: 10.1016/j.hrthm.2012.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blikstad I, Nelson WJ, Moon RT, Lazarides E. Synthesis and assembly of spectrin during avian erythropoiesis: Stoichiometric assembly but unequal synthesis of alpha and beta spectrin. Cell. 1983;32:1081–1091. doi: 10.1016/0092-8674(83)90292-1. [DOI] [PubMed] [Google Scholar]
- 25.Moon RT, Lazarides E. Biogenesis of the avian erythroid membrane skeleton: Receptor-mediated assembly and stabilization of ankyrin (goblin) and spectrin. J Cell Biol. 1984;98:1899–1904. doi: 10.1083/jcb.98.5.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woods CM, Lazarides E. Degradation of unassembled alpha- and beta-spectrin by distinct intracellular pathways: Regulation of spectrin topogenesis by beta-spectrin degradation. Cell. 1985;40:959–969. doi: 10.1016/0092-8674(85)90356-3. [DOI] [PubMed] [Google Scholar]
- 27.Heling A, Zimmermann R, Kostin S, Maeno Y, Hein S, Devaux B, Bauer E, Klovekorn WP, Schlepper M, Schaper W, Schaper J. Increased expression of cytoskeletal, linkage, and extracellular proteins in failing human myocardium. Circ Res. 2000;86:846–853. doi: 10.1161/01.res.86.8.846. [DOI] [PubMed] [Google Scholar]
- 28.Agre P, Orringer EP, Bennett V. Deficient red-cell spectrin in severe, recessively inherited spherocytosis. N Engl J Med. 1982;306:1155–1161. doi: 10.1056/NEJM198205133061906. [DOI] [PubMed] [Google Scholar]
- 29.Stankewich MC, Cianci CD, Stabach PR, Ji L, Nath A, Morrow JS. Cell organization, growth, and neural and cardiac development require alphaii-spectrin. J Cell Sci. 2011;124:3956–3966. doi: 10.1242/jcs.080374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang Y, Katuri V, Dillner A, Mishra B, Deng CX, Mishra L. Disruption of transforming growth factor-beta signaling in elf beta-spectrin-deficient mice. Science. 2003;299:574–577. doi: 10.1126/science.1075994. [DOI] [PubMed] [Google Scholar]
- 31.Kizhatil K, Bennett V. Lateral membrane biogenesis in human bronchial epithelial cells requires 190-kda ankyrin-g. J Biol Chem. 2004;279:16706–16714. doi: 10.1074/jbc.M314296200. [DOI] [PubMed] [Google Scholar]
- 32.Yang Y, Lacas-Gervais S, Morest DK, Solimena M, Rasband MN. Betaiv spectrins are essential for membrane stability and the molecular organization of nodes of ranvier. J Neurosci. 2004;24:7230–7240. doi: 10.1523/JNEUROSCI.2125-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galiano MR, Jha S, Ho TS, Zhang C, Ogawa Y, Chang KJ, Stankewich MC, Mohler PJ, Rasband MN. A distal axonal cytoskeleton forms an intra-axonal boundary that controls axon initial segment assembly. Cell. 2012;149:1125–1139. doi: 10.1016/j.cell.2012.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang C, Susuki K, Zollinger DR, Dupree JL, Rasband MN. Membrane domain organization of myelinated axons requires betaii spectrin. J Cell Biol. 2013;203:437–443. doi: 10.1083/jcb.201308116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Armbrust KR, Wang X, Hathorn TJ, Cramer SW, Chen G, Zu T, Kangas T, Zink AN, Oz G, Ebner TJ, Ranum LP. Mutant beta-iii spectrin causes mglur1alpha mislocalization and functional deficits in a mouse model of spinocerebellar ataxia type 5. J Neurosci. 2014;34:9891–9904. doi: 10.1523/JNEUROSCI.0876-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stankewich MC, Gwynn B, Ardito T, Ji L, Kim J, Robledo RF, Lux SE, Peters LL, Morrow JS. Targeted deletion of betaiii spectrin impairs synaptogenesis and generates ataxic and seizure phenotypes. Proc Natl Acad Sci U S A. 2010;107:6022–6027. doi: 10.1073/pnas.1001522107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holleran EA, Ligon LA, Tokito M, Stankewich MC, Morrow JS, Holzbaur EL. Beta iii spectrin binds to the arp1 subunit of dynactin. J Biol Chem. 2001;276:36598–36605. doi: 10.1074/jbc.M104838200. [DOI] [PubMed] [Google Scholar]
- 38.Ikeda Y, Dick KA, Weatherspoon MR, Gincel D, Armbrust KR, Dalton JC, Stevanin G, Durr A, Zuhlke C, Burk K, Clark HB, Brice A, Rothstein JD, Schut LJ, Day JW, Ranum LP. Spectrin mutations cause spinocerebellar ataxia type 5. Nat Genet. 2006;38:184–190. doi: 10.1038/ng1728. [DOI] [PubMed] [Google Scholar]
- 39.Shan J, Kushnir A, Betzenhauser MJ, Reiken S, Li J, Lehnart SE, Lindegger N, Mongillo M, Mohler PJ, Marks AR. Phosphorylation of the ryanodine receptor mediates the cardiac fight or flight response in mice. J Clin Invest. 2010;120:4388–4398. doi: 10.1172/JCI32726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Oort RJ, McCauley MD, Dixit SS, Pereira L, Yang Y, Respress JL, Wang Q, De Almeida AC, Skapura DG, Anderson ME, Bers DM, Wehrens XH. Ryanodine receptor phosphorylation by calcium/calmodulin-dependent protein kinase ii promotes life-threatening ventricular arrhythmias in mice with heart failure. Circulation. 2010;122:2669–2679. doi: 10.1161/CIRCULATIONAHA.110.982298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kannankeril PJ, Mitchell BM, Goonasekera SA, Chelu MG, Zhang W, Sood S, Kearney DL, Danila CI, De Biasi M, Wehrens XHT, Pautler RG, Roden DM, Taffet GE, Dirksen RT, Anderson ME, Hamilton SL. Mice with the r176q cardiac ryanodine receptor mutation exhibit catecholamine-induced ventricular tachycardia and cardiomyopathy. P Natl Acad Sci USA. 2006;103:12179–12184. doi: 10.1073/pnas.0600268103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu N, Denegri M, Dun W, Boncompagni S, Lodola F, Protasi F, Napolitano C, Boyden PA, Priori SG. Abnormal propagation of calcium waves and ultrastructural remodeling in recessive catecholaminergic polymorphic ventricular tachycardia. Circ Res. 2013;113:142–152. doi: 10.1161/CIRCRESAHA.113.301783. [DOI] [PubMed] [Google Scholar]
- 43.Takeshima H, Komazaki S, Nishi M, Iino M, Kangawa K. Junctophilins: A novel family of junctional membrane complex proteins. Mol Cell. 2000;6:11–22. doi: 10.1016/s1097-2765(00)00003-4. [DOI] [PubMed] [Google Scholar]
- 44.Papal S, Cortese M, Legendre K, Sorusch N, Dragavon J, Sahly I, Shorte S, Wolfrum U, Petit C, El-Amraoui A. The giant spectrin betav couples the molecular motors to phototransduction and usher syndrome type i proteins along their trafficking route. Hum Mol Genet. 2013;22:3773–3788. doi: 10.1093/hmg/ddt228. [DOI] [PubMed] [Google Scholar]
- 45.Ayalon G, Davis JQ, Scotland PB, Bennett V. An ankyrin-based mechanism for functional organization of dystrophin and dystroglycan. Cell. 2008;135:1189–1200. doi: 10.1016/j.cell.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 46.Pielage J, Cheng L, Fetter RD, Carlton PM, Sedat JW, Davis GW. A presynaptic giant ankyrin stabilizes the nmj through regulation of presynaptic microtubules and transsynaptic cell adhesion. Neuron. 2008;58:195–209. doi: 10.1016/j.neuron.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stagg MA, Carter E, Sohrabi N, Siedlecka U, Soppa GK, Mead F, Mohandas N, Taylor-Harris P, Baines A, Bennett P, Yacoub MH, Pinder JC, Terracciano CM. Cytoskeletal protein 4.1r affects repolarization and regulates calcium handling in the heart. Circ Res. 2008;103:855–863. doi: 10.1161/CIRCRESAHA.108.176461. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.