Abstract
Both maternal exposure to stressors and exposure of offspring to stressors during early life can have lifelong effects on the physiology and behavior of offspring. Stress exposure can permanently shape an individual’s phenotype by influencing the development of the hypothalamic-pituitary-adrenal (HPA) axis, which is responsible for the production and regulation of glucocorticoids such as corticosterone (CORT). In this study we used captive zebra finches (Taeniopygia guttata) to examine the effects of matching and mismatching maternal and early post-natal exposure to one of two types of antigens or a control on HPA axis reactivity in adult offspring. Prior to breeding, adult females were injected with lipopolysaccharide (LPS), keyhole limpet hemocyanin (KLH) or a control. Offspring of females in each of the three treatments were themselves exposed to LPS, KLH or a control injection at 5 and 28 days post-hatch. When offspring were at least 18 months of age, standardized capture and restraint stress tests were conducted to determine the impact of the treatments on adult stress responsiveness. We found significant interaction effects between maternal and offspring treatments on stress-induced CORT levels, and evidence in support of the environment matching hypothesis for KLH-treated birds not LPS-treated birds. KLH-treated offspring of KLH-treated mothers exhibited reduced stress-induced CORT levels, whereas LPS-treated or control offspring of KLH-treated mothers exhibited elevated stress-induced CORT levels. Although the treatment effects on baseline CORT were non-significant, the overall pattern was similar to the effects observed on stress-induced CORT levels. Our results highlight the complex nature of HPA axis programming, and to our knowledge, provide the first evidence that a match or mismatch between pre and post-natal antigen exposure can have life-long consequences for HPA axis function.
Keywords: Corticosterone, development, environment matching, hypothalamic-pituitary-adrenal axis, keyhole limpet hemocyanin, lipopolysaccharide, maternal effects, maternal immune activation, stressors
Introduction
Adverse early life environments in humans can elevate the risk of both physical and psychiatric disorders in adulthood (e.g., Batten et al., 2004; Goodwin and Stein, 2004; Phillips et al., 2005; Read et al., 2005; Anda et al., 2006). The developmental origins of health and disease hypothesis proposes that pre-natal and post-natal environments play unique roles in influencing adult health and the risk of disease development (Godfrey, 2006). Early life experiences can permanently alter the organizational structure and sensitivity of physiological systems, thereby affecting the manner in which an individual responds to both intrinsic and extrinsic changes. For example, there is evidence that pre-natal and post-natal environments can program the sensitivity of the hypothalamic-pituitary-adrenal (HPA) axis (Shanks et al., 1995; Meaney, 2001; Karrow, 2006; Champagne et al., 2008; Love et al., 2013). The HPA axis is responsible for the production of glucocorticoids (GCs), which are involved in a suite of physiological and behavioral processes. Baseline levels of GCs are important for the maintenance of homeostasis, whereas the release of elevated levels of GCs in response to stressors initiates a cascade of physiological and behavioral changes to help organisms cope with environmental challenges (Breuner et al., 2008).
The sensitivity of the HPA axis to pre-natal and post-natal experiences is thought to be an adaptive mechanism that allows individuals to cope with current environmental conditions (Karrow, 2006; Love et al., 2013), and to potentially prepare for expected future conditions (Gluckman et al., 2005; Love and Williams 2008a; Zimmer et al., 2013). This programming effect may represent a “predictive adaptive response” (PAR- Gluckman et al., 2005). PARs are thought to prepare an individual to cope with anticipated high or low levels of stressors (based on conditions experienced during development) via attenuated or heightened sensitivity, respectively, to those stress levels (sensu Love et al., 2013). This “environmental matching” (Monaghan, 2008) or “maternal matching” (Love et al., 2005) strategy may become maladaptive if the early developmental environment does not match later environments (sensu Hales and Barker, 1992; Bateson et al., 2004; Sheriff et al., 2009).
Although previous studies have documented programming effects of the maternal (Takahashi and Kalin, 1991; Reul et al., 1994; Welberg and Seckl, 2001; Yehuda et al., 2007; Perroud et al., 2014) or neonatal (Plotsky and Meaney, 1993; Shanks et al., 1995; Shanks et al., 2000) environments on HPA axis sensitivity, few studies have examined the combined long-term impact of both pre-natal and early post-natal exposure to stressors on HPA axis function in mammals (e.g., Vallée et al., 1999; Koo et al., 2003) or birds (e.g., Love and Williams 2008b; Zimmer et al., 2013), and fewer still have examined the effects of the same stressor in both pre-natal and post-natal environments (e.g., in rats; Koo et al., 2003). Early life adversity is linked to psychopathological disorders such as depression, conduct disorders, autism, and schizophrenia (Glover, 2011; Pechtel and Pizzagalli, 2011; Carr et al., 2013), but some of the effects of early life stress exposure that appear maladaptive, such as anxiety, aggression, and risk-taking, may improve fitness in adverse environments. Adaptive models of early stress exposure propose that early life adversity adaptively modulates developmental trajectories in ways that enhance survival and reproductive success in adverse environments (Flinn, 2006; Glover, 2011; Del Giudice et al., 2011; Cameron et al., 2005; Del Giudice, 2014). Maladaptive outcomes may be most likely to occur when there are mismatches between predicted and actual environmental conditions (Del Giudice, 2014; Low et al., 2012). Recognition of the adaptive effects of early life stress exposure provides a theoretical framework to understand interactions between maternal and post-natal environmental conditions and opens new areas of inquiry to test interventions that can mitigate the maladaptive effects of early life stress exposure and the adaptive, but psychologically and socially undesirable effects.
Both mothers and offspring may be exposed to pathogens or parasites, and maternal and neonatal immune activation have documented effects on HPA axis sensitivity (Shanks et al., 1995; Howerton and Bale, 2012). Activation of the immune system can also have long-lasting effects on numerous other traits, including learning and behavior (Bilbo and Schwarz, 2009; Meyer et al., 2009; Grindstaff et al., 2012), growth rates (Klasing and Leshchinsky, 1999; Grindstaff, 2008), immune function (Lemke and Lange, 1999; Grindstaff et al., 2006; Pihlaja et al., 2006; Merrill and Grindstaff, 2014), and endocrine function (Karrow, 2006; Hsiao and Patterson, 2011). One way in which immune activation can affect endocrine function is through the release of cytokines such as IL-1, IL-6, and TNF (Turnbull and Rivier, 1995). These cytokines have demonstrated effects on HPA axis activity in adults, as well as on HPA axis programming in neonates (Karrow, 2006; Pechnick et al., 2006), and in the fetuses of immune challenged mothers (Hsiao and Patterson, 2011; Howerton and Bale, 2012).
In this study we were interested in the long-term effects of matching and mismatching between pre-natal and post-natal immune challenges on HPA axis functioning. We manipulated antigen exposure by injecting breeding female zebra finches (Taeniopygia guttata Vieillot) and their offspring with one of two antigen treatments (lipopolysaccharide (LPS) or keyhole limpet hemocyanin (KLH)) or a control (phosphate buffered saline (PBS)). We then measured baseline and stress-induced levels of the GC stress hormone corticosterone (CORT) at approximately 1.5 y of age to examine the long-term effects of the treatments on stress reactivity. LPS is a major component of gram-negative bacteria cell walls and is targeted by the host immune system. When LPS is injected into a vertebrate, it induces a systemic, febrile response (Hart, 1988), which activates the HPA axis and results in elevated CORT levels, as well as increases in pro-inflammatory cytokines (e.g., IL-6) (Karrow, 2006). The organism also produces anti-LPS antibodies as part of an adaptive, secondary response to the infection (Grindstaff, 2008). KLH is a large, immunogenic oxygen-binding protein that results in the production of anti-KLH antibodies but should not activate the HPA axis when injected without dinitrophenyl (DNP) or adjuvants in small to moderate doses (Stenzel-Poore et al., 1993), although this has not yet been examined in birds. There is some evidence, however, that KLH may have a larger impact on HPA axis functioning (sensu Gaillard et al., 1998). LPS is a thymus-independent type 1 antigen, meaning that antibody production is not dependent upon T-cell assistance (Janeway et al., 2001), whereas KLH is a thymus-dependent antigen and production of KLH-reactive antibodies requires the presence of armed helper T-cells (Janeway et al., 2001). T-cells can synthesize adrenocorticotropic hormone (Gaillard, 1994), which is involved in the production and release of CORT, and these immune cells are thought to play a role in regulating the HPA axis. It is not clear whether production of T-cells as part of a primary or secondary response to KLH would be sufficient to activate the HPA axis (Stenzel-Poore et al., 1993). Nonetheless, the use of LPS and KLH offers insight into how activation of T-cells in females during the egg-laying period, and in the offspring during the early post-natal stage, might impact HPA axis functioning in adult offspring.
We were interested in four questions for this study: 1) is there an effect of maternal antigen exposure on adult offspring CORT levels, 2) is there an effect of offspring antigen exposure on adult offspring CORT levels, 3) are there environment matching effects of maternal and offspring antigen exposure on adult offspring CORT levels, and if so, 4) are these effects found for both T-dependent and T-independent antigens?
Methods
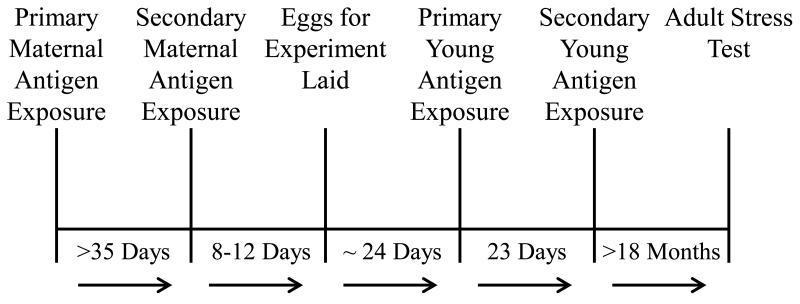
For a detailed description of the experimental set-up and design, see Grindstaff et al., (2012). In short, captive adult zebra finches were housed as breeding pairs in cages visually isolated from one another in an indoor aviary facility. Twenty females were randomly assigned to each treatment group to receive LPS, KLH, or phosphate buffered saline (PBS) as the control. Females in the KLH treatment were injected with 50 μg of KLH (EMD Millipore Bioscience, Billerica, MA, USA, 374817) in 50 μl of sterilized PBS (Sigma-Aldrich, St. Louis, MO, USA, P5368) (n = 20) (Hasselquist et al., 1999). Birds in the LPS treatment were injected with 1.0 mg of LPS/kg body weight (Sigma L7261) in 50 μl of sterilized PBS (n = 20) (Owen-Ashley et al., 2006). These doses have been demonstrated to elicit robust immune responses without leading to prolonged morbidity or mortality (Grindstaff et al, 2012; Merrill and Grindstaff, 2014). Birds in the control treatment were injected with 50 μl of sterilized PBS only. All birds were injected intra-abdominally after cleaning the site of injection with a 70% isopropyl alcohol swab. Females were injected once prior to the production of their first clutch, and given a booster injection at least 35 days after the first injection (Fig. 1). This was long enough to ensure that the primary immune response had occurred and subsided. First clutch eggs were collected within 24 h of laying and replaced with dummy eggs. Once enough birds for each treatment had completed their first clutch, birds were given their booster injection and the dummy eggs were removed 7 days later. Females then laid replacement clutches and each egg was weighed to the nearest 0.01 grams using a digital scale, and individually marked on the day it was laid. All analyses are for the offspring resulting from replacement clutches.
Figure 1.
(from Merrill and Grindstaff, 2014). Timeline for pre-natal and post-natal experimental procedures. Adult female zebra finches were exposed to one of three experimental treatments (keyhole limpet hemocyanin (KLH), lipopolysaccharide (LPS), or phosphate buffered saline (control)) prior to egg laying. Females were then given a secondary injection of the same treatment and allowed to lay a clutch of eggs that were replaced with dummy eggs. Dummy egg clutches were removed 7 days after secondary injection and females laid replacement clutches over the subsequent 4-5 days. Eggs hatched approx. 19 days later, and the offspring were injected with a primary injection on day 5, and secondary injection on day 28. Adult stress tests were conducted when birds were at least 18 months of age.
Young from a natal nest were divided across the three treatments, and within 72 h of hatching were cross-fostered into age, clutch and brood size-matched nests (+/− 1) to that of the natal nest. On day 5, nestlings received a primary injection of KLH, LPS, or PBS. Young within a foster nest received the same treatment as the foster mother. Doses of KLH, LPS and PBS were half those in the adult treatments so as to not overwhelm the chicks’ immune systems and reduce the risk of mortality. On day 28, the young received a booster injection with the adult doses.
Stress Test
Once the birds were old enough to live independently (approximately 40 days), they were housed in same-sex group cages (n=2-10 birds/cage) until the stress test. When the birds were at least 18 months old, they were subjected to a standardized capture and restraint stress protocol to assess baseline CORT and stress-induced levels of CORT at two time points; 10 and 40 min post-stress initiation. By examining CORT at 10 and 40 min post-stressor, we were able to acquire information on the magnitude of the stress response as well as some insight into the duration of the stress response, thus providing a more robust picture of the CORT profile. Same-sex pairs were placed in cages (45W × 45D × 40H cm) and allowed to acclimate for 24 h prior to the stress test. Birds had ad libitum access to food and water during this acclimation period. Stress tests occurred between 1035 and 1400 h to control for diel variation in CORT levels (Breuner et al., 1999). On the day of the test, we captured both birds from the cage at the same time and collected approximately 50 μl of blood from the jugular vein using a sterile syringe within 3 min of capture (Romero and Reed, 2005). The bird was then placed in a cloth bag for 10 min, at which point another blood sample was collected. The bird was then returned to the cloth bag for another 30 min before a final blood sample was collected. Following completion of the stress test, blood was spun down in a centrifuge at 1,845 g for 7 min. Plasma was pulled off the sample using a Hamilton syringe and stored at −80° C until assay. All work was approved by the Oklahoma State University Institutional Animal Care and Use Committee, under protocol AS107.
Plasma corticosterone assessment
We used a commercially available EIA kit (catalog no. ADI-901-097, Enzo Life Sciences International Inc., Plymouth Meeting, PA, USA) to measure plasma CORT. The kit was validated for use with zebra finches by testing the parallelism of a series of plasma dilutions against the standard curve. We also optimized the assay in our lab for zebra finches following Wada et al., (2007) prior to sample quantification. In short, we prepared a 1:40 sample dilution by adding 1% steroid displacement reagent (15 μl) to 10 μl of plasma, and then adding 375 μl of assay buffer to each sample 5 min later. Samples were then vortexed and aliquoted in duplicate (100 μl per well) to assay plates. A standard curve ranging from 20,000 to 32 pg/mL was run in triplicate for each plate. Samples and standards were then incubated with 50 μl conjugated CORT and 50 μl antibody for 2 h at room temperature while being shaken at 0.84 g. Following incubation, wells were washed, filled with 200 μl of substrate, and then incubated for 1 h at room temperature without shaking. 50 μl of stop solution was then added to each well, and each plate was read on a spectrophotometer at 405 nm. The detection limit for the assay is 27 pg (Enzo Life Sciences), and one sample fell below this detection limit and was removed from analyses. Intra-plate variation ranged from 4.54% to 11.14%, and inter-plate variation was 8.33%. There is some cross-reactivity for detection of other steroid hormones, namely deoxycorticosterone (cross-reactivity=28.6%). All other steroid hormones have less than 2% cross-reactivity with the kit components (Enzo Life Sciences).
Statistics
To determine whether antigen challenge affected egg mass, we constructed a GLMM with maternal ID as a random effect, maternal treatment as a fixed effect, and egg mass as the dependent variable. We did not find an effect of maternal challenge (F 2,34.99 = 2.46, P = 0.1), so we did not include egg mass in the models examining CORT production. We also looked for an effect of maternal treatment on latency to lay, but found no effect (F 2,38 = 0.07, P = 0.93) so did not include it in any models.
To examine the impact of early life experience on baseline and stress-induced levels of CORT, we ran mixed models with six or seven independent variables of interest: maternal treatment, young treatment, maternal ID, time (for repeated measures), sex, hatch order, and foster nest hatch order. Hatch order coincided with lay order in over 95% of the eggs laid (unpublished data). Foster nest hatch order is the age of the cross-fostered young relative to other young in the foster nest. All variables were checked for normality of residuals and homogeneity of variance prior to analyses. CORT data were log transformed to achieve normality prior to analysis. Antigen challenge was successful in stimulating the production of antigen-reactive antibody in females and transmission of KLH and LPS specific antibodies to offspring. Furthermore, maternal antigen challenge significantly impacted antibody production by offspring (Merrill and Grindstaff, 2014).
Baseline levels of CORT are important for modulating homeostatic processes and were analyzed independently and as part of the stress-response. To examine baseline CORT independently, we used linear mixed models (Proc Mixed) in which maternal ID was included as a random effect, and the denominator degrees of freedom were approximated using the containment method (Littell et al., 2006). For stress-induced levels of CORT we used repeated measures mixed models analyses incorporating baseline CORT, CORT 10, and CORT 40 values. Maternal ID and time by maternal ID were included as random factors, and maternal treatment, young treatment, time, sex, hatch order, foster nest hatch order, the interaction between maternal treatment and young treatment, the interaction between time and young treatment as well as the interaction between time and maternal treatment were included as fixed effects. The covariance structures between repeated measurements of the same individuals were explicitly modeled. A spatial power law covariance model was used to account for unequally spaced longitudinal measurements in which correlations were expected to decline as a function of time (Littell et al., 2006). The spatial power law covariance model is a generalization of an autoregressive error model (Littell et al., 2006), which allows for accurate assessment of within subject slopes (Schielzeth and Forstmeier, 2009). Denominator degrees of freedom were approximated using the Kenward-Rodgers method (Littell et al., 2006). We used post-hoc pair-wise t-tests of least squares means to compare significant terms generated in the full models and alpha was set at 0.05 for all analyses. Correcting for the number of pairwise comparisons would result in very small alpha levels, so we present raw p-values for each post-hoc pairwise comparison. Finally, we examined the relationships among CORT levels for each sampling period (baseline, CORT at 10 min post-stressor (CORT 10), and CORT at 40 min post-stressor (CORT 40)) using linear mixed models with maternal ID and young ID as random effects. Analyses were run in SAS (v. 9.3, SAS Institute, Cary, North Carolina, 2010) or JMP (v. 10.0, SAS Institute, Cary, North Carolina, 2010).
Results
Baseline CORT
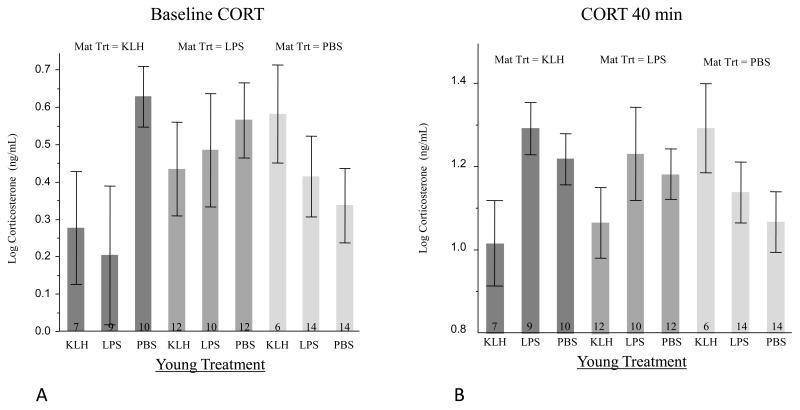
None of the model parameters were significant in explaining baseline CORT levels (Table 1). There was a non-significant trend for an interaction between maternal and young treatment, suggesting that the interplay between maternal treatment and offspring treatment may have moderately impacted baseline levels of CORT (Figure 2A).
Table 1.
Results of the mixed model analyses for baseline CORT. The model included maternal ID as a random effect. Bolded values are for fixed effects that were significant at alpha < 0.05.
| Variable | DF | F-value | P |
|---|---|---|---|
| Mat Trt | 2, 33 | 0.22 | 0.8 |
| Yng Trt | 2, 33 | 0.42 | 0.66 |
| Mat Trt × Yng Trt | 4, 33 | 2.45 | 0.07 |
| Sex | 1, 33 | 0.11 | 0.74 |
| Foster Nest Hatch Order | 5, 33 | 1.75 | 0.15 |
| Hatch Order | 5, 33 | 1.11 | 0.37 |
Figure 2.
Baseline (A) and stress-induced (B) corticosterone (CORT) levels by young and maternal treatment group. Numbers at the bottom of the bars represent the sample size for each young and maternal treatment group, and bars represent mean ± s.e.m. Darkest colored bars are for offspring of females exposed to KLH, intermediate bars are for offspring of females exposed to LPS, and lightest bars are for offspring of females given a control injection.
Stress-induced CORT levels
There was a significant effect of time in which all treatments exhibited an increase from baseline to stress-induced CORT levels (Table 2). There was no effect of maternal or young treatment independently on stress-induced CORT levels, but there was a significant interaction effect between maternal and offspring treatments (Table 2). KLH-treated offspring of KLH-treated mothers had significantly lower stress-induced CORT levels than control offspring of KLH-treated mothers or KLH-treated offspring of control mothers. KLH-treated offspring of KLH-treated mothers also had significantly lower stress-induced CORT levels than LPS-treated offspring of KLH-treated mothers or control offspring of LPS-treated mothers. Additionally, control offspring of control mothers had significantly lower stress-induced CORT levels than both LPS and control offspring of KLH-treated mothers and borderline lower levels than KLH-treated offspring of control-treated mothers (Figure 2B).
Table 2.
Results of the repeated measures mixed model for stress-induced CORT. The model included maternal ID and the interaction between time and maternal ID as random effects. Bolded values are for fixed effects that were significant at alpha < 0.05.
| Variable | DF | F Value | P |
|---|---|---|---|
| Mat Trt | 2. 68.4 | 0.17 | 0.85 |
| Yng Trt | 2, 68.5 | 0.4 | 0.67 |
| Time | 2,70.1 | 264.13 | <.0001 |
| Mat Trt × Yng Trt | 4, 68.9 | 3.04 | 0.02 |
| Sex | 1, 69.4 | 0.32 | 0.57 |
| Foster Nest Hatch Order | 5, 69.6 | 1.66 | 0.15 |
| Hatch Order | 5, 69 | 1.98 | 0.11 |
| Mat Trt × Time | 4, 71.8 | 0.37 | 0.83 |
| Yng Trt × Time | 4, 147 | 0.61 | 0.66 |
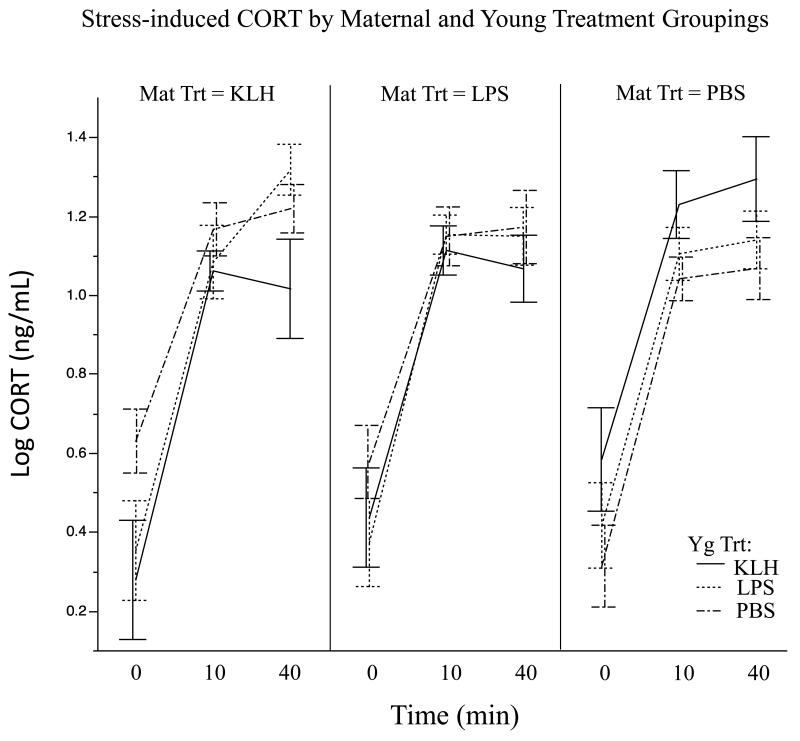
Two of the treatment group combinations exhibited a decline in mean CORT from 10 to 40 min post stressor and these were both KLH-treated offspring of antigen-treated mothers (Figure 3). In contrast, KLH-treated offspring of control mothers continued to increase from 10 to 40 min post-stressor and were the only group to exhibit a significant increase over this time (Figure 3).
Figure 3.
CORT levels over time for each treatment pairing, grouped by maternal treatment. Bars represent mean ± s.e.m.
To further examine the significant interaction effect between maternal and offspring treatments on sensitivity of the offspring HPA axis, we broke the analyses down by maternal treatment. Within the maternal KLH treatment group, there was a significant effect of time on offspring stress-induced CORT levels (F 2,38.4 = 77.78, P < 0.001) and a significant effect of young treatment (F 2,15.2 = 4.97, P = 0.02). Post-hoc analyses showed that KLH-treated offspring had significantly lower stress-induced CORT levels than LPS-treated offspring (t 1,15.1 = 2.6, P = 0.02) as well as control offspring (t 1,15.2 = 3.0, P < 0.01) (Figure 2B). Within the maternal LPS treatment group, there was a significant effect of time (F 2,56.6 = 135.48, P < 0.001) on offspring CORT levels, but no other significant effects (P > 0.28). Within the control maternal group, restraint time again impacted offspring CORT levels (F 2,53.9 = 88.18, P < 0.001) and there was a non-significant trend for an effect of young treatment on CORT levels (F 2,21.2 = 1.83, P = 0.19) with KLH-treated young having higher CORT levels.
Relationships among CORT measurements
Baseline CORT and CORT 10 min were significantly positively related (estimate ± s.e.m. = 0.37 ± 0.05, F 1,93 = 62.36, P < 0.001), as were baseline CORT and CORT 40 min (estimate ± s.e.m. = 0.28 ± 0.07, F 1,93 = 14.4, P < 0.001) and CORT 10 min and CORT 40 min (estimate ± s.e.m. = 0.44 ± 0.12, F 1,95 = 13.05, P < 0.001). CORT 10 and 40 were both significantly higher than baseline CORT (CORT 10 and baseline; t 1,93= 22.86, P < 0.001; CORT 40 and baseline; t 1,93= 18.51, P < 0.001), but did not differ significantly from each other (t 2,95 = 1.18, P = 0.23).
Discussion
We manipulated both the pre-natal and post-natal antigenic environments to determine if mismatches between the environments can program the sensitivity of the offspring HPA axis. We found a significant interaction effect between maternal and neonatal antigen treatments on stress-induced levels of CORT in adult zebra finches, which provides evidence for a programming effect of mismatches in the antigenic environment. Zebra finches produced elevated levels of CORT at 10 and 40 minutes post-restraint during adulthood. This supports prior work on avian species that has documented increases in circulating CORT levels as a result of restraint stress over this time period (e.g., Romero and Reed, 2005). In our study, neither maternal nor young treatment alone resulted in significant differences in baseline or stress-induced CORT levels, but the interaction between the two may have led to distinct strategies for coping with different levels and/or types of stressors. Baseline CORT levels appear to have been less impacted by the treatments than stress-induced CORT, but the trends generally mirror those seen in stress-induced levels with the exception of LPS-treated offspring of KLH-treated mothers (Figure 2). Treatment with KLH had a more pronounced effect on shaping the HPA axis than treatment with LPS in which birds exposed to KLH exhibited an environment matching relationship between pre-natal and post-natal treatments. When both mothers and offspring were challenged with KLH, the offspring had lower HPA axis reactivity than when either only the mother or only the offspring were challenged with KLH. Similarly, control offspring of control mothers exhibited lower HPA axis reactivity than control offspring of KLH-challenged mothers. These data indicate that the combination of pre-natal and early post-natal exposure to stressors such as pathogens and parasites can program the HPA axis, but that programming may be specific to the type of antigen.
Stress-induced CORT
The adrenocortical stress response is an adaptive mechanism for coping with challenging stimuli, and affects numerous physiological and behavioral processes (Breuner et al., 2008). Early life experiences are known to impact the short and long-term functioning of the HPA axis, but previous work on the organizational effects of exposure to glucocorticoids during development has yielded mixed findings. Some work indicates that exposure to elevated levels of glucocorticoids during development results in heightened response to stressors in adulthood (e.g., Hayward and Wingfield, 2004; Chung et al., 2005; Banerjee et al., 2012), whereas other work has found that exposure to elevated glucocorticoids during development results in an attenuated stress response later in adulthood (e.g., Lingas and Matthews, 2001; Love and Williams, 2008a; Vázquez et al., 2012). In this study, exposure to KLH during both pre-natal and post-natal development resulted in relatively low stress-induced CORT levels, and a more rapid cessation of CORT production in the 40 min following the stressor, both of which may be beneficial traits for an individual exposed to many such stressors (sensu Love and Williams, 2008a; Weinstock, 2008; Love et al., 2013). In contrast, birds exposed to KLH during the pre-natal or post-natal environment experienced higher levels of stress-induced CORT, suggesting that the match or mismatch between pre-natal and post-natal environments was critical for shaping HPA axis function in adulthood (sensu Hales and Barker, 1992; Bateson et al., 2004), but only for KLH-treated birds.
Exposure to stressors during the hypo-responsive period early in life may induce altered sensitivity of the HPA axis in adulthood through altered expression of glucocorticoid receptors (GR) and mineralocorticoid receptors (MR) in key regions of the brain (Meaney et al., 1996; Banerjee et al., 2012). Zebra finches do not produce a detectable increase in CORT levels as a result of restraint stress during at least the first 10 days post-hatch and have been suggested to exhibit a stress hypo-responsive period early in life (Wada et al., 2009). Thus, we hypothesize that the immune challenge of nestlings on day 5 post-hatch was responsible for the effect we found on the adult response to capture and restraint stress. Both GR and MR are found in the zebra finch brain and loss of maternal care early in life alters adult sensitivity to stressors and decreases receptor expression (Banerjee et al., 2012). However, the mechanism linking the loss of maternal care in zebra finches with effects on the HPA axis has not been identified. In Long Evans rats, offspring of mothers with high levels of maternal care have decreased methylation in the promoter region of the GR (Weaver et al., 2004) and elevated levels of GR mRNA in the hippocampus (Liu et al., 1997). Thus, epigenetic mechanisms may be responsible for the persistent effects of maternal and developmental immune challenges on the stress responsiveness of zebra finch offspring. Epigenetic modifications as a result of mismatches between environmental conditions in the maternal and offspring generations have been implicated as a mechanism underlying the link between the early life environment and adult risk of disease (Godfrey et al., 2007).
Baseline CORT
We did not find significant effects of maternal or young treatment, or a significant interaction effect between the two on baseline CORT levels. There was a significant positive correlation between baseline and stress-induced CORT levels, however, and the interaction effect between maternal and young treatments was nearly significant. As was observed for stress-induced CORT levels, KLH treatment appeared to have a larger impact on baseline CORT levels than LPS treatment. Also control offspring of control mothers had lower baseline CORT levels than control offspring of KLH-challenged mothers as we found for stress-induced CORT levels. Both KLH and LPS challenged offspring of KLH challenged mothers, had lower baseline CORT levels than control offspring of KLH challenged mothers. This suggests that the interaction between pre-natal and post-natal environments influenced baseline CORT levels, although not to the extent that it affected stress-induced responses.
Baseline CORT levels are thought to act in a permissive manner on physiological and behavioral processes via Type I receptors located in the brain (Sapolsky et al., 2000; Romero, 2004). These receptors likely regulate daily and seasonal CORT cycles and the organism’s energetic state (McEwen and Wingfield, 2003; Romero, 2004; Crespi et al., 2013). Elevated baseline CORT may be indicative of an individual that lives in a state of high preparedness for occasional exposure to stressors, whereas low baseline CORT may be adaptive for an individual that is likely to experience either many or very few such events during its life (sensu Love et al., 2013). There is some indication that baseline CORT levels may exhibit less repeatability than stress-induced CORT levels (Rensel and Schoech, 2011 and references therein), and it is possible that with a larger sample size, we would have been able to capture a stronger effect of antigen treatment on baseline CORT levels.
KLH versus LPS injections
As described above, KLH is a thymus-dependent antigen and requires activation of helper T-cells, and LPS is a thymus-independent antigen and does not require helper T-cells (Janeway et al., 2001). It is likely that the differences we have documented between KLH and LPS are related to the manner in which the antigens activate the immune system. In a related study, Grindstaff et al., (2012) found significantly reduced growth rates in KLH-treated offspring compared to LPS-treated or control zebra finch offspring. Taken together with the data here, these results suggest that the costs of a specific immune response to KLH were high for developing zebra finches (Demas et al., 1997; Klasing and Leshchinksy, 1999). T-cells have the capacity to impact HPA axis functioning through the release of adrenocorticotropic hormone (Gaillard, 1994), and it is possible that activation of helper T-cells in response to KLH exposure resulted in increased production of CORT. The match or mismatch of high CORT production in KLH-treated birds may have led to the attenuated response of baseline and stress-induced CORT in KLH-KLH treated birds, and a heightened response in control-KLH and KLH-control birds. To our knowledge, no studies have examined the long-term effects of T-cell activity during development on HPA-axis programming, but this could be an important avenue to explore.
Another possibility is that the differences between LPS and KLH are related to the timing of immunological response relative to egg production. There is good evidence that exposure to LPS induces an energetically costly immune response (Hart, 1988), but the duration of the response may be significantly shorter than that of the response to KLH (Jurincic-Winkler et al., 1995). This difference in duration could explain some of our results. Females were challenged with a booster injection 7 days prior to the removal of the dummy egg clutch, meaning that females would not have initiated clutch-replacement until at least 7 days post-injection. If females shape their offspring’s development via maternal effects, they may do so by manipulating the relative and total abundance of resources and hormones in the egg. Consequently, if mothers exposed to LPS were no longer in the energetically expensive immune response phase by the time they were allocating resources to their eggs, the impact of LPS may have been lessened compared to KLH. Similarly, because the offspring response to KLH was likely of longer duration than the response to LPS the two injections of KLH on days 5 and 28 post-hatch may have mimicked chronic inflammatory conditions, rather than acute exposure to inflammation.
Conclusions
Changes in HPA axis activity may be adaptive responses for anticipating future events (Mousseau and Fox, 1998; Gluckman et al., 2005; Love et al., 2008a), or may be maladaptive if early environmental conditions are not predictive of later conditions (Hales and Barker, 1992; Bateson et al. 2004). In many species, there is great variation in HPA axis sensitivity to stress, both among individuals and within individuals over time. This variation may be a result of early developmental experiences, because exposure to pre-natal and post-natal stressors can permanently shape HPA axis functioning (Welberg and Seckl, 2001; Champagne and Curley, 2008; Love et al., 2013). These changes may shift the range of potential developmental pathways the offspring can then take. In this study, we examined the long-term effects of maternal and early life exposure to different antigens and documented a significant interaction effect between maternal and offspring treatment on stress-induced levels of CORT. We were explicitly interested in whether exposure to any antigen, the same antigen, or a mismatch between antigens or between control treatment and antigen treatment would result in differential HPA axis programming in the adult bird. It is unclear in this situation whether HPA axis programming due to antigen exposure is beneficial for the adult birds (sensu Gluckman et al., 2005), but it is likely context dependent. For example, low levels of maternal care in rats are linked to increased corticosterone levels in pups and decreased spine density in hippocampal neurons (Bagot et al., 2009). These effects seem maladaptive but the phenotype is associated with enhanced learning and memory in stressful conditions (Champagne et al., 2008). The changes in stress physiology and brain morphology induced by low levels of maternal care, therefore, are adaptive responses to an adverse early life environment if later life conditions are also adverse (Meaney, 2010; Del Giudice et al., 2011).
Environment matching effects mediated through interactions between the immune and endocrine systems have clinical relevance for humans by highlighting novel interventions to reduce maladaptive effects of early life adversity and any potentially adaptive, but psychologically undesirable effects (Del Giudice, 2014). Although mismatches between the maternal and developmental environments may induce increased sensitivity in the HPA axis to adaptively regulate the inflammatory response early in life, hyper-sensitivity in the long-term may increase the risk of developing stress-related diseases, particularly if individuals are also genetically more susceptible to metabolic or psychiatric illnesses (Shanks and Lightman, 2001; Meyer et al., 2009). This work suggests that interventions aimed at decreasing the prevalence and impact of stress-related diseases may be achieved by reducing the risk of infection, and by providing anti-inflammatory treatment after infection early in life, especially in susceptible populations (Meyer et al., 2009).
Our results highlight the complex nature of HPA axis programming, and that differences in the type of antigen an individual is exposed to during development can have life-long consequences for HPA axis function. The data also underscore the need to examine both maternal and neonatal experiences in understanding the long-term impacts of early life stressors.
Highlights.
We examined effects of pre and postnatal antigen exposure on HPA axis function
Treatments resulted in a match or mismatch between pre and postnatal environments
Prenatal exposure to KLH (but not LPS) led to environment-matching effects
Pre and postnatal KLH exposure resulted in an attenuated stress response
Prenatal KLH and postnatal LPS or control exposure led to elevated stress response
Acknowledgements
Matt Anderson, Kent Andersson, Courtney Cupps, Sara Friedemann, Kaylee Hollingsworth, Lisa Hughes, Madeleine Naylor, Amanda Neujahr, Alecia Rains, Arielle Shanahan, and Matt Waselik provided invaluable assistance with zebra finch care and blood sampling. Thanks to Tara Stewart for feedback on the manuscript. Funding for the project was provided by NIH grant 1R15HD066378-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There are no conflicts of interest for either of the authors.
References
- Anda RF, Felitti VJ, Bremner JD, Walker JD, Whitfield C, Perry BD, Dube SR, Giles WH. The enduring effects of abuse and related adverse experiences in childhood - A convergence of evidence from neurobiology and epidemiology. Eur. Arch. Psych. Clin. Neurosci. 2006;256 doi: 10.1007/s00406-005-0624-4. doi:10.1007/s00406-005-0624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagot RC, van Hasselt FN, Champagne DL, Meaney MJ, Krugers HJ, Joels M. Maternal care determines rapid effects of stress mediators on synaptic plasticity in adult rat hippocampal dentate gyrus. Neurobiol. Learn. Mem. 2009;92:292–300. doi: 10.1016/j.nlm.2009.03.004. doi:10.1016/j.nlm.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Banerjee SB, Arterbery AS, Fergus DJ, Adkins-Regan E. Deprivation of maternal care has long-lasting consequences for the hypothalamic-pituitary-adrenal axis of zebra finches. Proc. R. Soc. B-Biol. Sci. 2012;279:759–766. doi: 10.1098/rspb.2011.1265. doi:10.1098/rspb.2011.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateson P, Barker D, Clutton-Brock T, Deb D, D’Udine B, Foley RA, Gluckman P, Godfrey K, Kirkwood T, Lahr MM, McNamara J, Metcalfe NB, Monaghan P, Spencer HG, Sultan SE. Developmental plasticity and human health. Nature. 2004;430:419–421. doi: 10.1038/nature02725. doi:10.1038/nature02725. [DOI] [PubMed] [Google Scholar]
- Batten SV, Aslan M, Maciejewski PK, Mazure CM. Childhood maltreatment as a risk factor for adult cardiovascular disease and depression. J. Clin. Psychiatry. 2004;65:249–254. doi: 10.4088/jcp.v65n0217. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Schwarz JM. Early-life programming of later-life brain and behavior: a critical role for the immune system. Front. Behav. Neurosci. 2009;3:14. doi: 10.3389/neuro.08.014.2009. doi:10.3389/neuro.08.014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuner CW, Patterson SH, Hahn TP. search of relationships between the acute adrenocortical response and fitness. Gen. Comp. Endocrinol. 2008;157:288–295. doi: 10.1016/j.ygcen.2008.05.017. doi:10.1016/j.ygcen.2008.05.017. [DOI] [PubMed] [Google Scholar]
- Breuner CW, Wingfield JC, Romero LM. Diel rhythms of basal and stress-induced corticosterone in a wild, seasonal vertebrate, Gambel’s white-crowned sparrow. J. Exp. Zool. 1999;284:334–342. doi:10.1002/(SICI)1097-010X(19990801)284:3<334::AID-JEZ11>3.0.CO;2-#. [PubMed] [Google Scholar]
- Cameron NM, Champagne FA, Fish C, Ozaki-Kuroda K, Meaney MJ. The programming of individual differences in defensive responses and reproductive strategies in the rat through variations in maternal care. Neurosci. Biobehav. Rev. 2005;29:843–865. doi: 10.1016/j.neubiorev.2005.03.022. doi:10.1016/j.neubiorev.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Carr CP, Severi Martins CM, Stingel AM, Lemgruber VB, Juruena MF. The Role of Early Life Stress in Adult Psychiatric Disorders A Systematic Review According to Childhood Trauma Subtypes. J. Nerv. Ment. Dis. 2013;201:1007–1020. doi: 10.1097/NMD.0000000000000049. doi:10.1097/NMD.0000000000000049. [DOI] [PubMed] [Google Scholar]
- Champagne DL, Bagot RC, Hasselt F, van, Ramakers G, Meaney MJ, Kloet ER, de, Joëls M, Krugers H. Maternal Care and Hippocampal Plasticity: Evidence for Experience-Dependent Structural Plasticity, Altered Synaptic Functioning, and Differential Responsiveness to Glucocorticoids and Stress. J. Neurosci. 2008;28:6037–6045. doi: 10.1523/JNEUROSCI.0526-08.2008. doi:10.1523/JNEUROSCI.0526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA, Curley JP. Maternal regulation of estrogen receptor a methylation. Curr. Opin. Pharmacol. 2008;8:735–739. doi: 10.1016/j.coph.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S, Son GH, Park SH, Park E, Lee KH, Geum D, Kim K. Differential adaptive responses to chronic stress of maternally stressed male mice offspring. Endocrinology. 2005;146:3202–3210. doi: 10.1210/en.2004-1458. doi:10.1210/en.2004-1458. [DOI] [PubMed] [Google Scholar]
- Crespi EJ, Williams TD, Jessop TS, Delehanty B. Life history and the ecology of stress: how do glucocorticoid hormones influence life-history variation in animals? Funct. Ecol. 2013;27:93–106. doi:10.1111/1365-2435.12009. [Google Scholar]
- Del Giudice M. Early stress and human behavioral development: emerging evolutionary perspectives. J. Dev. Orig. Health Dis. 2014;5:270–280. doi: 10.1017/S2040174414000257. doi:10.1017/S2040174414000257. [DOI] [PubMed] [Google Scholar]
- Del Giudice M, Ellis BJ, Shirtcliff EA. The Adaptive Calibration Model of stress responsivity. Neurosci. Biobehav. Rev. 2011;35:1562–1592. doi: 10.1016/j.neubiorev.2010.11.007. doi:10.1016/j.neubiorev.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demas GE, Chefer V, Talan MI, Nelson RJ. Metabolic costs of mounting an antigen-stimulated immune response in adult and aged C57BL/6J mice. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1997;273:R1631–R1637. doi: 10.1152/ajpregu.1997.273.5.R1631. [DOI] [PubMed] [Google Scholar]
- Flinn MV. Evolution and ontogeny of stress response to social challenges in the human child. Dev. Rev. 2006;26:138–174. doi:10.1016/j.dr.2006.02.003. [Google Scholar]
- Gaillard R. Neuroendocrine-Immune System Interactions - the Immune-Hypothalamo-Pituitary-Adrenal Axis. Trends Endocrinol. Metab. 1994;5:303–309. doi: 10.1016/1043-2760(94)p3206-m. doi:10.1016/1043-2760(94)P3206-M. [DOI] [PubMed] [Google Scholar]
- Gaillard RC, Daneva T, Hadid R, Muller K, Spinedi E. The hypothalamo-pituitary-adrenal axis of athymic Swiss nude mice - The implications of T lymphocytes in the ACTH release from immune cells. In: McCann SM, Lipton JM, Sternberg EM, Chrousos GP, Gold PW, Smith CC, editors. Neuroimmunomodulation: Molecular Aspects, Integrative Systems, and Clinical Advances. New York Acad Sciences; New York; 1998. pp. 480–490. [DOI] [PubMed] [Google Scholar]
- Glover V. Prenatal stress and the origins of psychopathology: an evolutionary perspective. J. Child Psychol. Psychiatry. 2011;52:356–367. doi: 10.1111/j.1469-7610.2011.02371.x. doi:10.1111/j.1469-7610.2011.02371.x. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Spencer HG, Bateson P. Environmental influences during development and their later consequences for health and disease: implications for the interpretation of empirical studies. Proc. R. Soc. B. 2005;272:671–677. doi: 10.1098/rspb.2004.3001. doi:10.1098/rspb.2004.3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey KM, Lillycrop KA, Burdge GC, Gluckman PD, Hanson MA. Epigenetic mechanisms and the mismatch concept of the developmental origins of health and disease. Pediatr. Res. 2007;61:5R–10R. doi: 10.1203/pdr.0b013e318045bedb. doi:10.1203/pdr.0b013e318045bedb. [DOI] [PubMed] [Google Scholar]
- Goodwin RD, Stein MB. Association between childhood trauma and physical disorders among adults in the United States. Psychol. Med. 2004;34:509–520. doi: 10.1017/s003329170300134x. doi:10.1017/S003329170300134X. [DOI] [PubMed] [Google Scholar]
- Grindstaff JL. Maternal antibodies reduce costs of an immune response during development. J. Exp. Biol. 2008;211:654–660. doi: 10.1242/jeb.012344. doi:10.1242/jeb.012344. [DOI] [PubMed] [Google Scholar]
- Grindstaff JL, Hasselquist D, Nilsson J-A, Sandell M, Smith HG, Stjernman M. Transgenerational priming of immunity: maternal exposure to a bacterial antigen enhances offspring humoral immunity. Proc. R. Soc. B-Biol. Sci. 2006;273:2551–2557. doi: 10.1098/rspb.2006.3608. doi:10.1098/rspb.2006.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grindstaff JL, Hunsaker VR, Cox SN. Maternal and developmental immune challenges alter behavior and learning ability of offspring. Horm. Behav. 2012;62:337–344. doi: 10.1016/j.yhbeh.2012.04.005. doi:10.1016/j.yhbeh.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales C, Barker D. Type-2 (on-Insulin-Dependent) Diabetes-Mellitus - the Thrifty Phenotype Hypothesis. Diabetologia. 1992;35:595–601. doi: 10.1007/BF00400248. doi:10.1007/BF00400248. [DOI] [PubMed] [Google Scholar]
- Hart B. Biological Basis of the Behavior of Sick Animals. Neurosci. Biobehav. Rev. 1988;12:123–137. doi: 10.1016/s0149-7634(88)80004-6. doi:10.1016/S0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- Hasselquist D, Marsh JA, Sherman PW, Wingfield JC. Is avian humoral immunocompetence suppressed by testosterone? Behav. Ecol. Sociobiol. 1999;45:167–175. doi:10.1007/s002650050550. [Google Scholar]
- Hayward LS, Wingfield JC. Maternal corticosterone is transferred to avian yolk and may alter offspring growth and adult phenotype. Gen. Comp. Endocrinol. 2004;135:365–371. doi: 10.1016/j.ygcen.2003.11.002. doi:10.1016/j.ygcen.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Howerton CL, Bale TL. Prenatal programing: At the intersection of maternal stress and immune activation. Horm. Behav. 2012;62:237–242. doi: 10.1016/j.yhbeh.2012.03.007. doi:10.1016/j.yhbeh.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao EY, Patterson PH. Activation of the maternal immune system induces endocrine changes in the placenta via IL-6. Brain Behav. Immun. 2011;25:604–615. doi: 10.1016/j.bbi.2010.12.017. doi:10.1016/j.bbi.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway CA, Travers P, Walport M, Shlomchik M. Immunobiology: the immune system in health and disease. Garland Publishing, NY; NY: 2001. [Google Scholar]
- Jurincic-Winkler C, Klippel K, Beuth J, Markl J. Value of Keyhole Limpet Hemocyanin in Prevention of Superficial Bladder-Cancer Recurrence. Onkologie. 1995;18:41–50. [Google Scholar]
- Karrow NA. Activation of the hypothalamic-pituitary-adrenal axis and autonomic nervous system during inflammation and altered programming of the neuroendocrine-immune axis during fetal and neonatal development: Lessons learned from the model inflammagen, lipopolysaccharide. Brain Behav. Immun. 2006;20:144–158. doi: 10.1016/j.bbi.2005.05.003. doi:10.1016/j.bbi.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Klasing KC, Leshchinsky TV. In: Adams NJ, Slowtow RH, editors. Functions, costs, and benefits of the immune system during development and growth; In 22nd International Ornithological Congress, Birdlife South Africa, Durban South Africa; Johannesburg. Birdlife; 1999. pp. 2817–2835. [Google Scholar]
- Koo JW, Park CH, Choi SH, Kim NJ, Kim H-S, Choe JC, Suh Y-H. Postnatal environment can counteract prenatal effects on cognitive ability, cell proliferation, and synaptic protein expression. FASEB J. 2003 doi: 10.1096/fj.02-1032fje. doi:10.1096/fj.02-1032fje. [DOI] [PubMed] [Google Scholar]
- Lemke H, Lange H. Is there a maternally induced immunological imprinting phase a la Konrad Lorenz? Scand. J. Immunol. 1999;50:348–354. doi: 10.1046/j.1365-3083.1999.00620.x. doi:10.1046/j.1365-3083.1999.00620.x. [DOI] [PubMed] [Google Scholar]
- Lingas RI, Matthews SG. A short period of maternal nutrient restriction in late gestation modifies pituitary-adrenal function in adult guinea pig offspring. Neuroendocrinology. 2001;73:302–311. doi: 10.1159/000054647. doi:10.1159/000054647. [DOI] [PubMed] [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, Wolfinger K. SAS system for mixed models. SAS Institute Inc.; Cary, NC: 1996. [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. doi:10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Love OP, Chin EH, Wynne-Edwards KE, Williams TD. Stress hormones: A link between maternal condition and sex-biased reproductive investment. Am. Nat. 2005;166:751–766. doi: 10.1086/497440. [DOI] [PubMed] [Google Scholar]
- Love OP, McGowan PO, Sheriff MJ. Maternal adversity and ecological stressors in natural populations: the role of stress axis programming in individuals, with implications for populations and communities. Funct. Ecol. 2013;27:81–92. doi:10.1111/j.1365-2435.2012.02040.x. [Google Scholar]
- Love OP, Williams TD. Plasticity in the adrenocortical response of a free-living vertebrate: The role of pre- and post-natal developmental stress. Horm. Behav. 2008a;54:496–505. doi: 10.1016/j.yhbeh.2008.01.006. doi:10.1016/j.yhbeh.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Love OP, Williams TD. The adaptive value of stress-induced phenotypes: Effects of maternally derived corticosterone on sex-biased investment, cost of reproduction, and maternal fitness. Am. Nat. 2008b;172:E135–E149. doi: 10.1086/590959. doi:10.1086/590959. [DOI] [PubMed] [Google Scholar]
- Low FM, Gluckman PD, Hanson MA. Developmental plasticity, epigenetics and human health. J. Evol. Biol. 2012;39:650–665. doi: 10.1002/bdrc.20198. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Horm. Behav. 2003;43:2–15. doi: 10.1016/s0018-506x(02)00024-7. doi:10.1016/S0018-506X(02)00024-7. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu. Rev. Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. doi:10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Epigenetics and the Biological Definition of Gene x Environment Interactions. Child Dev. 2010;81:41–79. doi: 10.1111/j.1467-8624.2009.01381.x. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Diorio J, Francis D, Widdowson J, LaPlante P, Caldji C, Sharma S, Seckl JR, Plotsky PM. Early environmental regulation of forebrain glucocorticoid receptor gene expression: Implications for adrenocortical responses to stress. Dev. Neurosci. 1996;18:49–72. doi: 10.1159/000111395. doi:10.1159/000111395. [DOI] [PubMed] [Google Scholar]
- Merrill L, Grindstaff JL. Maternal Antibody Transfer Can Lead to Suppression of Humoral Immunity in Developing Zebra Finches (Taeniopygia guttata) Physiol. Biochem. Zool. 2014;87:740–751. doi: 10.1086/677218. doi:10.1086/677218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Fatemi SH. In-vivo rodent models for the experimental investigation of prenatal immune activation effects in neurodevelopmental brain disorders. Neurosci. Biobehav. Rev. 2009;33:1061–1079. doi: 10.1016/j.neubiorev.2009.05.001. doi:10.1016/j.neubiorev.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Monaghan P. Early growth conditions, phenotypic development and environmental change. Phil. Trans. R. Soc. B. 2008;363:1635–1645. doi: 10.1098/rstb.2007.0011. doi:10.1098/rstb.2007.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousseau TA, Fox CW. The adaptive significance of maternal effects. Trends Ecol. Evol. 1998;13:403–407. doi: 10.1016/s0169-5347(98)01472-4. doi:10.1016/S0169-5347(98)01472-4. [DOI] [PubMed] [Google Scholar]
- Pechnick RN, Kariagina A, Hartvig E, Bresee CJ, Poland RE, Chesnokova VM. Developmental exposure to corticosterone: behavioral changes and differential effects on leukemia inhibitory factor (LIF) and corticotropin-releasing hormone (CRH) gene expression in the mouse. Psychopharmacology. 2006;185:76–83. doi: 10.1007/s00213-005-0258-2. doi:10.1007/s00213-005-0258-2. [DOI] [PubMed] [Google Scholar]
- Pechtel P, Pizzagalli DA. Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology. 2011;214:55–70. doi: 10.1007/s00213-010-2009-2. doi:10.1007/s00213-010-2009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perroud N, Rutembesa E, Paoloni-Giacobino A, Mutabaruka J, Mutesa L, Stenz L, Malafosse A, Karege F. The Tutsi genocide and transgenerational transmission of maternal stress: epigenetics and biology of the HPA axis. World J. Biol. Psychiatry. 2014;15:334–345. doi: 10.3109/15622975.2013.866693. doi:10.3109/15622975.2013.866693. [DOI] [PubMed] [Google Scholar]
- Phillips NK, Hammen CL, Brennan PA, Najman JM, Bor W. Early adversity and the prospective prediction of depressive and anxiety disorders in adolescents. J. Abnorm. Child Psychol. 2005;33:13–24. doi: 10.1007/s10802-005-0930-3. doi:10.1007/s10802-005-0930-3. [DOI] [PubMed] [Google Scholar]
- Pihlaja M, Siitari H, Alatalo RV. Maternal antibodies in a wild altricial bird: effects on offspring immunity, growth and survival. J. Anim. Ecol. 2006;75:1154–1164. doi: 10.1111/j.1365-2656.2006.01136.x. doi:10.1111/j.1365-2656.2006.01136.x. [DOI] [PubMed] [Google Scholar]
- Plotsky P, Meaney M. Early, Postnatal Experience Alters Hypothalamic Corticotropin-Releasing Factor (crf) Messenger-Rna, Median-Eminence Crf Content and Stress-Induced Release in Adult-Rats. Mol. Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. doi:10.1016/0169-328X(93)90189-V. [DOI] [PubMed] [Google Scholar]
- Read J, van Os J, Morrison AP, Ross CA. Childhood trauma, psychosis and schizophrenia: a literature review with theoretical and clinical implications. Acta Psychiatr. Scand. 2005;112:330–350. doi: 10.1111/j.1600-0447.2005.00634.x. doi:10.1111/j.1600-0447.2005.00634.x. [DOI] [PubMed] [Google Scholar]
- Rensel MA, Schoech SJ. Repeatability of baseline and stress-induced corticosterone levels across early life stages in the Florida scrub-jay (Aphelocoma coerulescens) Hormones and Behavior. 2011;59:497–502. doi: 10.1016/j.yhbeh.2011.01.010. doi:10.1016/j.yhbeh.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Reul J, Stec I, Wiegers G, Labeur M, Linthorst A, Arzt E, Holsboer F. Prenatal Immune Challenge Alters the Hypothalamic-Pituitary-Adrenocortical Axis in Adult-Rats. J. Clin. Invest. 1994;93:2600–2607. doi: 10.1172/JCI117272. doi:10.1172/JCI117272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero LM. Physiological stress in ecology: lessons from biomedical research. Trends Ecol. Evol. 2004;19:249–255. doi: 10.1016/j.tree.2004.03.008. doi:10.1016/j.tree.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Romero LM, Reed JM. Collecting baseline corticosterone samples in the field: is under 3 min good enough? Comp. Biochem. Physiol. A-Mol. Integr. Physiol. 2005;140:73–79. doi: 10.1016/j.cbpb.2004.11.004. doi:10.1016/j.cbpb.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. doi:10.1210/er.21.1.55. [DOI] [PubMed] [Google Scholar]
- Schielzeth H, Forstmeier W. Conclusions beyond support: overconfident estimates in mixed models. Behav. Ecol. 2009;20:416–420. doi: 10.1093/beheco/arn145. doi:10.1093/beheco/arn145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanks N, Larocque S, Meaney M. Neonatal Endotoxin Exposure Alters the Development of the Hypothalamic-Pituitary-Adrenal Axis - Early Illness and Later Responsivity to Stress. J. Neurosci. 1995;15:376–384. doi: 10.1523/JNEUROSCI.15-01-00376.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanks N, Lightman SL. The maternal-neonatal neuro-immune interface: Are there long-term implications for inflammatory or stress-related disease? J. Clin. Invest. 2001;108:1567–1573. doi: 10.1172/JCI14592. doi:10.1172/JCI14592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanks N, Windle RJ, Perks PA, Harbuz MS, Jessop DS, Ingram CD, Lightman SL. Early-life exposure to endotoxin alters hypothalamic-pituitary-adrenal function and predisposition to inflammation. Proc. Natl. Acad. Sci. U. S. A. 2000;97:5645–5650. doi: 10.1073/pnas.090571897. doi:10.1073/pnas.090571897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheriff MJ, Krebs CJ, Boonstra R. The sensitive hare: sublethal effects of predator stress on reproduction in snowshoe hares. J. Anim. Ecol. 2009;78:1249–1258. doi: 10.1111/j.1365-2656.2009.01552.x. doi:10.1111/j.1365-2656.2009.01552.x. [DOI] [PubMed] [Google Scholar]
- Stenzel-Poore M, Vale W, Rivier C. Relationship Between Antigen-Induced Immune Stimulation and Activation. Endocrinology. 1993;132:1313–1318. doi: 10.1210/endo.132.3.8382598. doi:10.1210/en.132.3.1313. [DOI] [PubMed] [Google Scholar]
- Takahashi L, Kalin N. Early Developmental and Temporal Characteristics of Stress-Induced Secretion of Pituitary-Adrenal Hormones in Prenatally Stressed Rat Pups. Brain Res. 1991;558:75–78. doi: 10.1016/0006-8993(91)90715-8. doi:10.1016/0006-8993(91)90715-8. [DOI] [PubMed] [Google Scholar]
- Turnbull AV, Rivier C. Regulation of the HPA Axis by Cytokines. Brain, Behavior, and Immunity. 1995;9:253–275. doi: 10.1006/brbi.1995.1026. doi:10.1006/brbi.1995.1026. [DOI] [PubMed] [Google Scholar]
- Vallée M, Maccari S, Dellu F, Simon H, Le Moal M, Mayo W. Long-term effects of prenatal stress and postnatal handling on age-related glucocorticoid secretion and cognitive performance: a longitudinal study in the rat. Eur. J. Neurosci. 1999;11:2906–2916. doi: 10.1046/j.1460-9568.1999.00705.x. doi:10.1046/j.1460-9568.1999.00705.x. [DOI] [PubMed] [Google Scholar]
- Vázquez DM, Neal CR, Patel PD, Kaciroti N, Lopez JF. Regulation of corticoid and serotonin receptor brain system following early life exposure of glucocorticoids: Long term implications for the neurobiology of mood. Psychoneuroendocrinology. 2012;37:421–437. doi: 10.1016/j.psyneuen.2011.07.012. doi:10.1016/j.psyneuen.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada H, Hahn TP, Breuner CW. Development of stress reactivity in white-crowned sparrow nestlings: Total corticosterone response increases with age, while free corticosterone response remains low. Gen. Comp. Endocrinol. 2007;150:405–413. doi: 10.1016/j.ygcen.2006.10.002. doi:10.1016/j.ygcen.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Wada H, Salvante KG, Wagner E, Williams TD, Breuner CW. Ontogeny and Individual Variation in the Adrenocortical Response of Zebra Finch (Taeniopygia guttata) Nestlings. Physiol. Biochem. Zool. 2009;82:325–331. doi: 10.1086/599320. doi:10.1086/599320. [DOI] [PubMed] [Google Scholar]
- Weaver ICG, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat. Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. doi:10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Weinstock M. The long-term behavioural consequences of prenatal stress. Neurosci. Biobehav. Rev. 2008;32:1073–1086. doi: 10.1016/j.neubiorev.2008.03.002. doi:10.1016/j.neubiorev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Welberg L. a. M., Seckl JR. Prenatal stress, glucocorticoids and the programming of the brain. J. Neuroendocrinol. 2001;13:113–128. doi: 10.1046/j.1365-2826.2001.00601.x. doi:10.1046/j.1365-2826.2001.00601.x. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Teicher MH, Seckl JR, Grossman RA, Morris A, Bierer LM. Parental posttraumatic stress disorder as a vulnerability factor for low cortisol trait in offspring of holocaust survivors. Arch. Gen. Psychiatry. 2007;64:1040–1048. doi: 10.1001/archpsyc.64.9.1040. doi:10.1001/archpsyc.64.9.1040. [DOI] [PubMed] [Google Scholar]
- Zimmer C, Boogert NJ, Spencer KA. Developmental programming: Cumulative effects of increased pre-hatching corticosterone levels and post-hatching unpredictable food availability on physiology and behaviour in adulthood. Horm. Behav. 2013;64:494–500. doi: 10.1016/j.yhbeh.2013.07.002. doi:10.1016/j.yhbeh.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]