Abstract
In a new strategy, we sought to determine whether topically applied vasoconstrictor, with its accompanying transient skin hypoxia and exclusion of systemic drug, would prevent or suppress radiotherapy or chemotherapy-induced alopecia. Topical vasoconstrictor was applied to 1-cm2 skin patches on the backs of 10-day-old rats and minutes later they received either 7.1 gray (Gy) whole-body radiation or systemic N-nitroso-N-methylurea (MNU) or Cytoxan. The degree of alopecia was scored 10 days later by visual assessment (% coat retention) and hair follicle histologic analysis. Topical application of epinephrine or norepinephrine in an alcohol:water delivery vehicle induced clear skin blanch, and in a dose-dependent manner, topical epinephrine or norepinephrine (20–1,000 mM) applied before 7.1 Gy irradiation conferred 95% of coat retention in the treated skin patches versus 0% coat retention in vehicle controls, or in skin outside the treated patches. By histology, small numbers of dystrophic hair follicles were observed in hairless skin versus the normal density of anagen follicles in the immediately adjacent, drug-protected skin patches at day 20; protected coats were retained into adulthood. Topical epinephrine or norepinephrine before systemic MNU (30 ug/gm body weight) conferred up to 95% of coat retention in treated skin patches versus 0% coat retention elsewhere. Epinephrine-conferred % coat retention dropped to 16% in rats that received systemic Cytoxan, a drug whose plasma half-life is at least 8- to 10-fold longer than MNU. A general strategy is discussed for the use of topical epinephrine or norepinephrine in the clinic to provide an inexpensive and convenient strategy to prevent cancer therapy-induced alopecia.
Keywords: alpha adrenergic vasoconstrictor, epinephrine, norepinephrine, topical formulation
Of the 1.3 million new cases of cancer in the United States each year, more than half of these patients will receive chemotherapy, and of these, about 65% will develop chemotherapy-induced alopecia. In an earlier “quality of life” study of cancer therapy patients,1 alopecia was ranked second in severity out of 72 side effects of chemotherapy. In a related study,2 alopecia was cited as the worst anticipated side effect by 58% of women preparing for chemotherapy, and 8% of these patients were viewed “at risk” for avoiding treatment because of the anticipated alopecia. Hoffman et al.3 found similar expectations among cancer patients entering chemotherapy. Alopecia is also a common outcome for both children and adults who receive cranial radiotherapy for primary or metastatic cancer to the brain and meninges. Nonetheless, systemic chemotherapy and cranial radiotherapy remain standard and essential parts of cancer treatment.
Existing clinical strategies to manage alopecia have been largely ineffective. Mechanical scalp cooling,4,5 when used against a short plasma half-life drug like doxorubicin, has shown protective efficacy, but less so when used against longer plasma half-life drugs, particularly when the chemotherapy is administered in extended infusions; extended patient discomfort and extended clinic stays by patients while attached to the cooling device were not easily sustained. Some pharmacologic strategies have also been described6,7 to alleviate this alopecia.
Rat8,9 and mouse7,10 models have been developed to study the histopathologic progression of hair follicle dystrophy11 after systemic chemotherapy or irradiation of rodents and to test the efficacy of topical or systemic therapies to prevent or suppress alopecia.12–14 A systemic chemotherapy insult to neonatal 10-day-old rat pups, when they are growing their coat at maximum rate, leads to complete alopecia by day 20.8,9 In adult rats or mice, clipping the coats generally results in synchronized anagen induction, and after systemic chemotherapy, alopecia develops in the clipped area only.7,9,10
A new strategy from our laboratory involving topical application of a vasoconstrictor to at-risk tissue has been used to suppress radiation dermatitis15,16 and oral mucositis17 in clinical and preclinical settings. Originally added to a topical skin formulation to prevent departure of a radioprotective aminothiol from its topical application site, we found that topical epinephrine, alone, completely prevented radiation dermatitis, presumably by suppressing the formation of reactive oxygen species formed during tissue irradiation. We wondered (i) could the same topical vasoconstrictor strategy protect hair follicles in irradiated skin and (ii) if topical vasoconstrictor efficiently prevented departure of topically applied, radiolabeled aminothiol from skin, could it also prevent arrival or delivery of systemic chemotherapy to the skin organelles that lie within the 1 millimeter (mm) of skin above the constricted dermal blood vessels?
Our goals in this study were (i) to adapt the neonate rat alopecia model to study radiation-induced alopecia and determine if topical vasoconstrictor conferred protection, (ii) to determine if topical vasoconstrictor prevented chemotherapy-induced alopecia and (iii) to identify a topical vasoconstrictor formulation and application schedule that could be tested in a follow-up clinical trial in humans of alopecia prevention. The data presented here indicate highly significant efficacy of topically applied epinephrine or norepinephrine in preventing both radiation and chemotherapy-induced alopecia and support further study in humans.
Material and Methods
Material
(±)-Epinephrine HCl, (L)-norepinephrine bitartrate, (R)-phenylephrine HCl, sodium pentobarbital, N-nitroso-N-methylurea (MNU), resveratrol, Nile Red dye, spermine and solvents for drug delivery were all obtained from Sigma (St. Louis, MO). L-norepinephrine–HCl was obtained from ChemPacific (Baltimore, MD). Cyclophosphamide for injection was from Baxter (Deerfield, IL). Coal Black dye (#5006) was from Byrnes & Kiefer (Evans City, PA). The PrC-210 aminothiol was synthesized as described previously.18 Lactating female Sprague–Dawley rats with litters of 10-day-old pups were obtained from Harlan (Indianapolis, IN). Rats were maintained on 12-hr light/dark cycle and provided ad lib water and lab chow. All animal procedures were conducted according to a protocol (#M0476) approved by the University of Wisconsin Institutional Animal Care and Use Committee.
Rat pup alopecia assay
In a standard alopecia assay,8 10- to 11-day-old rat pups received two to four topical applications of vehicle containing test drug to a 1-cm2 area on their dorsal backs 10–120 min before irradiation or chemotherapy. During the topical treatment period, rat pups were maintained in compartmentalized cages on warming pads to prevent oral consumption of drug from each other. Table 1 indicates the application times, vehicle composition, volumes, and doses of each applied drug. In two experiments, instead of topical application of drug to their backs, rat pups received a single, subcutaneous, 100 µL bolus injection of 100 millimolar (mM) spermine dissolved in saline in the center of their dorsal backs. For whole-body irradiation, rat pups received intraperitoneal sodium pentobarbital (30 ug/gm body weight [bw]) with a 30-g needle 5–10 min before being placed in irradiator. Rats were irradiated in a J.L. Shepherd 137Cs irradiator. The radiation dose rate (1.9 gray [Gy]/min) was previously calibrated using thermoluminescent dosimeters. Cytoxan (32 ug/gm bw) or MNU (30 ug/gm bw) was administered as a single intraperitoneal injection with a 30-g needle to unanesthetized rats. Following the procedures, 10–12 rat pups (toe-clipped to mark) were housed with one lactating mother rat through day 20 of life. Cages were changed particularly on days 15– 20 of life to remove the shed coat hair. On day 20, rat pups were anesthetized with 3% isoflurane and photographed. Coat density was scored by visual assessment.
Table 1.
Topical drug-conferred prevention of alopecia in rat pup model
| Topical drug | Cages | Drug conc. (mM) |
Vehicle1 | Topical application schedule (min) |
Total volume applied (µL) |
Hair follicle insult2 |
n | % Coat retention mean ± SEM |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vehicle | 647–650 | 0 | 50:25:25 | −120, −60, −30, −10 | 115 | 7.1 Gy | 4 | 0 | ||||
| Epinephrine HCl | 647–650 | 20 | 50:25:25 | −120, −60, −30, −10 | 115 | 7.1 Gy | 4 | 18 ± 6 | ||||
| Epinephrine HCl | 647–650 | 100 | 50:25:25 | −120, −60, −30, −10 | 115 | 7.1 Gy | 4 | 40 ± 17 | ||||
| Epinephrine HCl | 647–650 | 500 | 50:25:25 | −120, −60, −30, −10 | 115 | 7.1 Gy | 4 | 42 ± 17 | ||||
| Epinephrine HCl | 647–650 | 1,000 | 50:25:25 | −120, −60, −30, −10 | 115 | 7.1 Gy | 4 | 87 ± 9 | ||||
| Vehicle | 838–839 | 0 | 50:25:25 | −120, −60, −30, −10 | 230 | 7.1 Gy | 4 | 0 | ||||
| Epinephrine HCl | 838–839 | 1,000 | 50:25:25 | −120, −60, −30, −10 | 230 | 7.1 Gy | 5 | 94 ± 1 | ||||
| Norepinephrine tartrate | 838–839 | 1,050 | 50:25:25 | −120, −60, −30, −10 | 230 | 7.1 Gy | 7 | 49 ± 9 | ||||
| Phenylephrine HCl | 821–824 | 1,000 | 50:25:25 | −120, −60, −30, −10 | 115 | 7.1 Gy | 4 | 0 | ||||
| Epinephrine HCl | 772 | 4,000 | 50:25:25 | −45, −15 | 75 | 7.1 Gy | 2 | 75 | ||||
| Vehicle | CIA-4 | 0 | 70:0:30 | −120, −60, −30, −4, +45 | 140 | MNU3 | 0 | |||||
| Epinephrine | CIA-4 | 1,000 | 50:25:25 | −120, −60, −30, −4, +45 | 140 | MNU | 11 | 95 ± 1 | ||||
| Epinephrine | CIA-5 | 1,000 | 50:25:25 | −60, −30, −4, +45 | 230 | MNU | 3 | 92 ± 6 | ||||
| Epinephrine | CIA-6 | 500 | 50:25:25 | −120, −60, −30, −4, +45 | 140 | MNU | 3 | 95 ± 2 | ||||
| Vehicle | 757–758 | 0 | 50:25:25 | −120, −60, −30, −10, 0 | 140 | Cytoxan4 | 4 | 0 | ||||
| Epinephrine | 838–839 | 1,000 | 50:25:25 | −120, −60, −30, −10, 0 | 140 | Cytoxan | 4 | 16 ± 9 | ||||
| Resveratrol+ PrC-210 | 583–586 |
|
|
|
|
Cytoxan | 3 | 70 ± 13 |
Ethanol:propylene glycol:water (vol:vol:vol).
Whole-body radiation or systemic (IP) chemotherapy was administered at 0 min.
30 µg/g bw.
30 µg/g bw.
Additional procedures
To assess hair follicle and skin histology, skin samples were excised and fixed in 10% formalin. Skin sections were stained with hematoxylin and eosin. Initially, to screen for the efficiency with which different delivery vehicles could deliver an organic molecule similar to norepinephrine (FW: 206) to hair follicles and dermal vasculature, Nile Red (FW: 318) was applied topically to rat pup skin in several vehicles, and skin samples were excised 30 min or 24 hr later for fluorescence microscopy. Unfixed skin samples were embedded in OCT (Tissue-Tek™, Fisher) and sectioned.
Statistical analysis
Graphpad Prism software, typically using Student t-test, was used for analysis of differences between treatment groups.
Results
Topical delivery and skin blanch
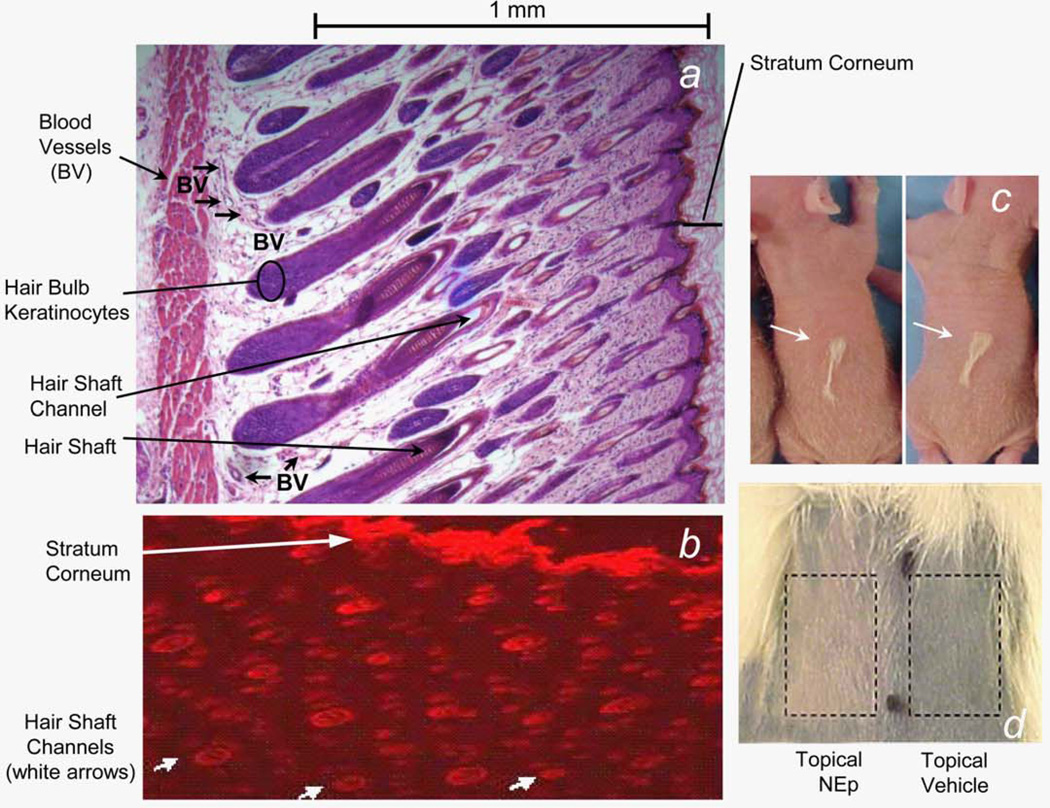
Histologic analysis of dorsal back skin from the neonate 10-day-old rats used in the alopecia assay (Fig. 1a) shows normal hair follicle architecture with interdigitated dermal blood vessels located about 1 mm beneath the skin surface. Topical application of Nile Red dye dissolved in an ethanol:-water (50:50) delivery vehicle to the skin of 10-day rats resulted in similar dye deposition within the stratum corneum and the hair follicle channels (Fig. 1b). Equivalent dye delivery was observed at all follicle channel depths, as evidenced by wide versus narrow channel diameters. As the Nile Red formula weight (318) is greater than that of norepinephrine (206), and they have similar hydrophobicities, we concluded that topically applied adrenergic vasoconstrictors in an alcohol:water vehicle would be delivered in a similar manner throughout the hair follicle.
Figure 1.
(a) Histology cross-section (magnification, 50×) of dorsal back skin of 10-day-old rat stained with hematoxylin and eosin. Note that dermal blood vessels are about 1 mm beneath the skin surface interdigitated around hair bulbs. (b) Fluorescence microscopy cross-section (magnification, 50×) of dorsal back rat skin to which Nile red dye was applied in an ethanol:water (50:50) topical delivery vehicle. (c) Dorsal backs of 20-day-old rat pups that received 7.1 Gy whole-body radiation on day 10 of life. Sixty minutes before irradiation, rats received a subcutaneous injection of a 100 mM of spermine solution in the center of their back. Representative two rats with normal coat retention seen above the subcutaneous needle track (white arrows). (d) In brief, 750 mM of norepinephrine in an ethanol:water (70:30) topical delivery vehicle was applied once to the indicated ~2 cm2 shaved skin patch on dorsal back of a 100 g rat; topical vehicle alone was applied to the adjacent skin patch. Ten minutes later, 150 µL of Coal Black 5006 dye (dilution in saline, 5×) was injected intraperitoneally to rat. Photograph of the back was taken 10 min after the intraperitoneal injection. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Besides identifying what appeared to be an acceptable plan to deliver topical vasoconstrictor, a separate, serendipitous event supported the idea that transient vasoconstriction of skin could prevent alopecia induced by systemic chemotherapy. In the study shown in Figure 1c, a 25-g needle was inserted subcutaneously in the dorsal caudal back and threaded anteriorly beneath the skin to enable subcutaneous injection of a 100 µL bolus of 100 mM spermine, in an attempt to confer chemoprotection to the overlying hair follicles against a subsequent systemic Cytoxan dose administered a few minutes later. Ten days later, there was no evidence of protection above or around the spermine bolus, but each of the six 20-day-old rats had ~normal coat density directly above the subcutaneous needle tracks that had presumably severed or at least transiently disrupted some of the dermal blood vessels that delivered systemic Cytoxan to hair follicles.
In an initial test of the efficacy of a topically applied vasoconstrictor to exclude systemic small molecule delivery to the skin, norepinephrine was applied to a shaved dorsal back skin patch of a 100-g rat, and vehicle alone was applied next to it (Fig. 1d). Ten minutes later, the rat received an intraperitoneal systemic dose of a black dye, and 10 min later, the two skin patches were photographed. The image shows clear exclusion of the dye from the norepinephrine skin patch and uniform skin distribution of dye within the topical vehicle patch.
Topical epinephrine prevents alopecia and protects hair follicle cells
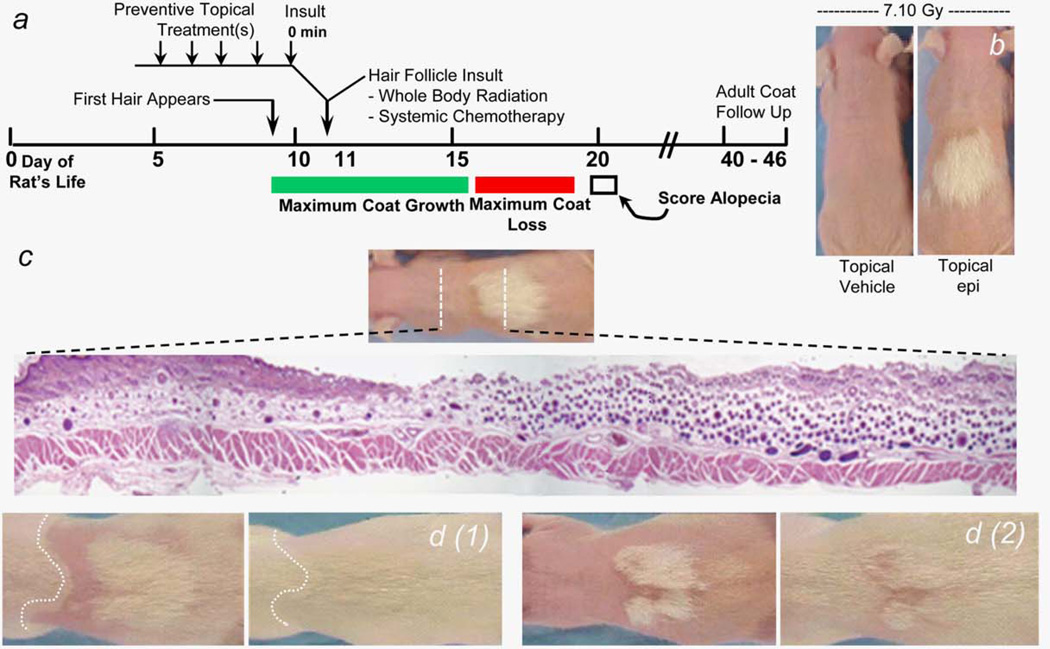
The design of the neonate 10-day-old rat alopecia model8 is shown in Figure 2a along with the addition of topical treatments that were identified empirically in our experiments. Initially, 10-day-old rats received increasing doses of whole-body radiation and were scored for alopecia on day 20. We saw 50% of coat loss at 5.6 Gy and >95% of coat loss at 7.1 Gy, which was chosen for later experiments. To check if topical epinephrine could prevent radiation-induced alopecia, we initially used the schedule of four topical epinephrine applications in 2 hr before radiation that we had identified and used to prevent radiation dermatitis19 (Table 1, Cages 647–650; and Fig. 2b), and we saw clear epinephrine dose-dependent coat protection in the treated skin. Histologic analysis of the hair follicles in skin that traversed the transition from unprotected to protected coat (Fig. 2c) showed only a few dystrophic hair follicles in the unprotected skin but the normal density of anagen hair follicles within the protected coat skin. Rats that were maintained well past the day 20 scoring (Fig. 2d) showed complete retention of the “protected coat” patches into adult life as well as regrowth of the coat in the previously unprotected areas.
Figure 2.
(a) Timeline schematic of events in the rat pup alopecia model. (b) Images of 20-day-old rat pups that received 7.1 Gy wholebody radiation on day 10 of life. The “epi” pup received topical 1,000 mM of epinephrine in water:alcohol vehicle to 1 cm2 patch on dorsal back prior to irradiation (for application schedule, see Materials and Methods section) and other pup received topical vehicle alone. (c) Histology cross-section (magnification, 15×) of dorsal back rat skin. Skin sample from the indicated site on a 20-day-old rat shows largely apoptotic hair bulbs in skin not exposed to topical epinephrine before irradiation and basically normal hair bulb density and architecture in epinephrine-treated skin. (d1, d2) Epinephrine-protected coat in 20-day-old rats (d1, d2 left panels) is retained into adulthood (age, >50 days) in right panels. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Topical vasoconstrictor induces skin blanch
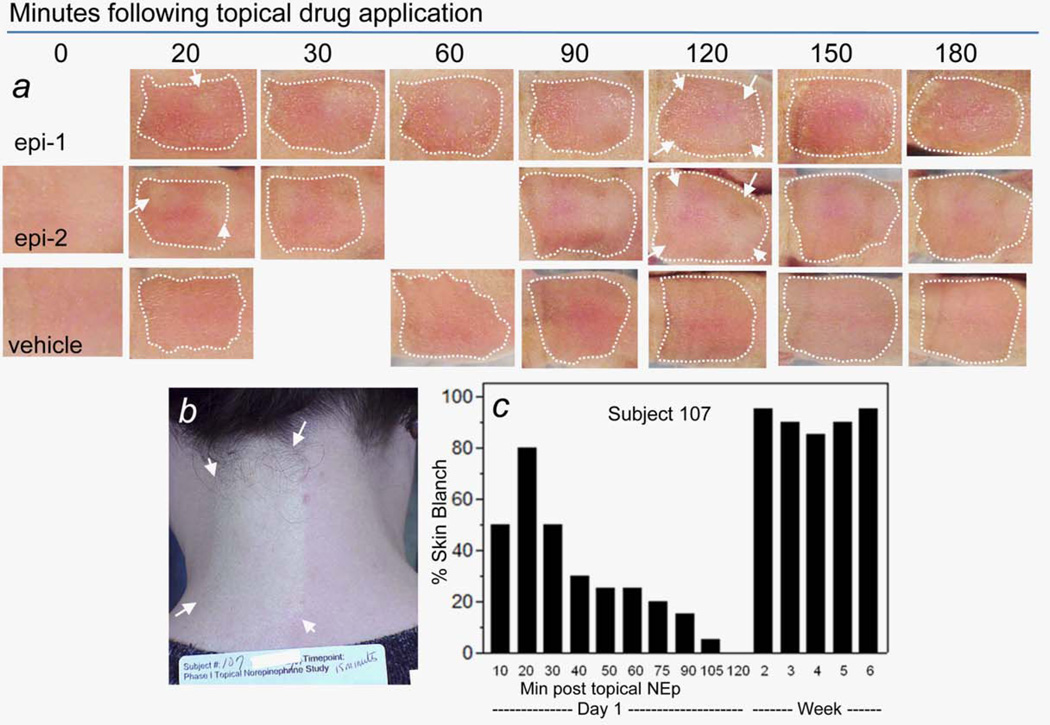
A single topical application of 1,000 mM epinephrine in an ethanol:propylene glycol:water (50:25:25) vehicle to the backs of 7-day-old rat pups, who were still hairless, induced symmetric, circular skin blanch patches (Fig. 3a) within the wet, treated skin. With time and three more topical epinephrine applications over 180 min, the isolated blanches merged into a single blanched field that covered the wet, topical treatment site, and which did not extend beyond. In a few rats, the circular “blanches” did not all fuse, but rather one to two remained as circular symmetric blanches even into the irradiation or chemotherapy insult stage of the experiment.
Figure 3.
(a) Topical 1,000 mM epinephrine (“epi”) in vehicle or vehicle alone (“vehicle,” 50:25:25; ethanol:propylene glycol:water) were applied to ~1 cm2 areas on backs of 7-day-old rat pups (last day before white fur appears and obliterates skin blanch), and images were recorded for 180 min (topical application sites are indicated with dashed white lines). Discrete, circular skin blanches were visible (epi) as early as 5–10 min postapplication (e.g., arrows in 20-min panels), and from 30 to 90 min, discrete blanches fused into a single large blanch that covered the entire epi application site (see arrows surrounding fused blanch). Fused blanches over the entire site were maintained through the 180-min observation period. (b) Area (inside arrows) of skin blanch covering the entire 50 cm2 area of human subject’s neck to which 450 mM of norepinephrine in alcohol:water vehicle (70:30) had been applied 15 min before. Vehicle alone was applied to the 50-cm2 skin site on right side of the subject’s neck. (c) (Left) Scored skin blanch within norepinephrine application site versus time after application on first application day in normal Subject 107. (Right) Scored skin blanch within norepinephrine application site (only the 20-min time point is shown) in normal Subject 107 on representative treatment days from each of the next 5 weeks. (Panel b is copied with permission from Ref. 16). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
For comparison to the neonate rat model skin blanch, the image shown in Figure 3b is copied from a report of a Phase I human clinical trial.15,16 Briefly, healthy subjects received daily, topical applications of norepinephrine in an ethanol:-water (70:30) vehicle to a 50-cm2 area on their neck for 30 successive days to mimic anticipated norepinephrine application in a subsequent study to suppress radiation dermatitis in postlumpectomy breast cancer patients who received 50–60 Gy of radiotherapy to the breast divided over 30 daily doses. Topical 450-mM norepinephrine (Fig. 3b) induced 80–90% skin blanch within 10–20 min in Subject 107, with similar results in 15 other subjects (norepinephrine, 150–600 mM). For Subject 107 (Fig. 3c), and for all other subjects (data not shown), the blanch response, within scoring capabilities, was the same on each of the 30 topical treatment days.
Topical vasoconstrictor confers radioprotection and chemoprotection
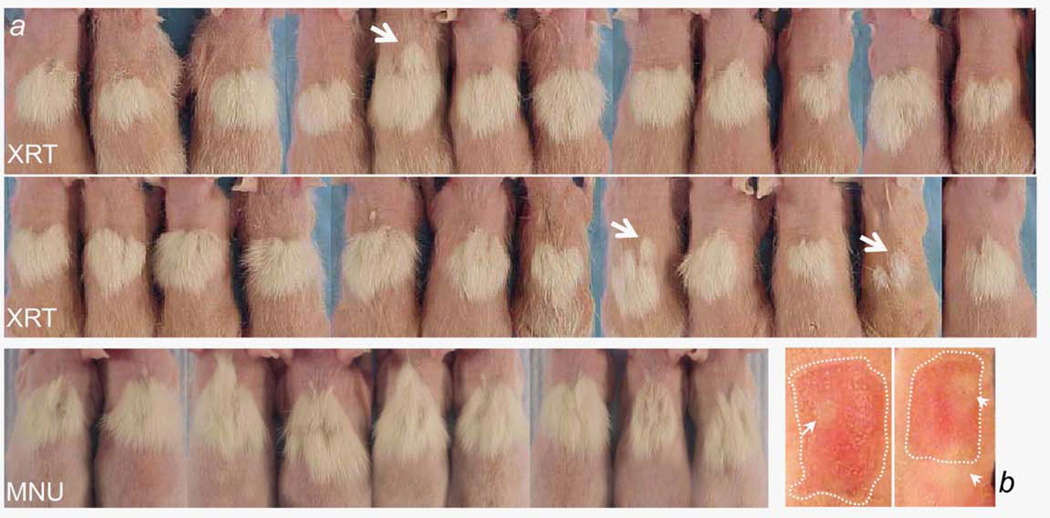
To determine the consistency of the vasoconstrictor-conferred hair follicle protection, at least in a sampling of 24 outbred, 10-day-old Sprague–Dawley rats, rats were treated with topical 950 mM of epinephrine followed by a 7.1 Gy radiation insult. Separately, nine rats treated with topical 950 mM of epinephrine received a 30 µg/g bw systemic MNU insult. Figure 4 shows a very consistent protective response against both insults. One type of variation that is seen is the occasional lack of complete fusion of each individual blanch event (white arrows). These “failures” to fuse illustrate that a circular blanch field in the skin can confer a circular protected coat field; this provides a useful corroboration of the mechanism of protection.
Figure 4.
Ten-day-old rats were treated topically four times with 950 mM of epinephrine in an alcohol:water delivery vehicle for more than 2 hr, and they then received 7.1 Gy whole-body radiation (a, XRT panels) or 30 ug/gm bw MNU (a, MNU panel). Occasional circular fields of protected coat (a arrows) correspond in size and location to circular areas of skin blanch that are seen after topical epinephrine application (b arrows). In most cases, but not all cases, multiple, overlapping, circular skin blanch areas eventually merge to provide a single-protected area covering the entire topical drug treatment area. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Partial skin blanch during chemotherapy sufficient for alopecia prevention
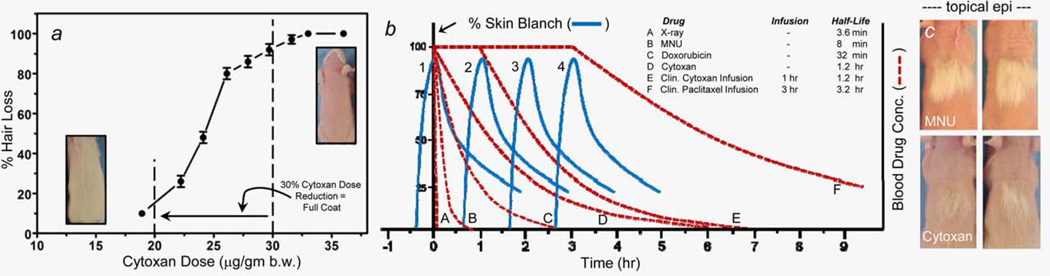
When initially titrating the intraperitoneal Cytoxan dose to determine the dose needed to induce >95% hair loss in this neonate rat model, we observed (Fig. 5a) that 30 µg/g bw induced >95% hair loss. But significantly, a 33% reduction in Cytoxan dose to 20 µg/g bw resulted in an almost complete retention of coat hair.
Figure 5.
(a) % Hair Loss in 20-day-old rat pups versus single intraperitoneal doses of Cytoxan administered on day 10 of life. Day 20 coat density was scored by visual assessment. (b) Curves a–f (dashed lines) show hypothetical human blood clearances for the chemotherapy drug examples listed in the inset of Table 1. The drug clearance half-lives in Table 1 are taken from published reports. Curves 1–4 (solid lines) are exact recreations of the “% Skin Blanch versus Minutes Post Topical Norepinephrine” bar chart shown for Subject 107 in Figure 3c. The Subject 107 skin blanch data are used here as a representative example of the percent and duration of skin blanch achieved in humans after topical application of a vasoconstrictor. (c) Each rat pup received topical epinephrine on day 10 followed by a single intraperitoneal injection of either MNU (30 µg/g bw; top) or Cytoxan (32 µg/g bw; bottom) panels. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Topical epinephrine efficacy in suppressing radiation-induced alopecia was dose dependent (Table 1, Cages 647–650), and the rank-ordered efficacies of epinephrine > norepinephrine >>> phenylephrine suppression of alopecia (Table 1) directly reflect their rank-ordered affinities for alpha adrenergic receptors.20
A 95% effective dose of topical epinephrine against MNU coat loss (Table 1, Cage CIA-6) provided only 16% of coat retention against systemic Cytoxan (Fig. 5c and Table 1, Cages 757–758). The >10-fold longer plasma half-life of Cytoxan and its phosphoramide mustard active metabolite provide the most likely explanation for this reduced efficacy.
Topical application of the antioxidant resveratrol for 3 days combined with topical application of the aminothiol PrC-21021 for 2 hr before Cytoxan had a clear protective effect (Table 1, Cages 583–586).
Discussion
Alopecia is a common and distressing outcome for a large percentage of both chemotherapy and cranial radiotherapy patients. It will cause some patients, estimated to be 8% in one study,2 to forgo therapies that would improve their cancer survival. Scalp cooling by mechanical devices has been used with some success to suppress alopecia, but (i) it has not been very successful against longer drug infusions and/or longer plasma half-life drugs and (ii) it has no utility in the cranial radiotherapy setting.
Topically applied epinephrine or norepinephrine very effectively suppressed alopecia induced by either radiation or chemotherapy. Topical delivery of vasoconstrictor to the subcutaneous blood vessels surrounding hair follicles about 1 mm below the skin surface, with the transient vasoconstriction and local tissue hypoxia that follows, presumably suppressed most or all of the ~70% of radiation-induced cell killing in skin cells that results from X-ray photons striking water molecules and generating •OH that attacks DNA. Hall22 explains that molecular oxygen (O2) is required to permanently “fix” (R• + O2 → RO2•) as a DNA base adduct the initial transient damage done when •OH attacks a site on a deoxyguanosine base in DNA. Without the O2-enabled second reaction, the activated deoxyguanosine base decays back to its basal state, leaving no new DNA adduct. This therapeutic strategy using transient hypoxia to protect skin organelles recreates the known observation that indolent, hypoxic, solid tumor cores are much more resistant to radiotherapy than well-oxygenated tumor periphery cells.23
Topically induced skin vasoconstriction, illustrated by exclusion of a systemic dye in Figure 1d, was associated with 95% suppression of the alopecia induced by the very short half-life drug MNU but only 16% suppression of alopecia from Cytoxan, which has an at least 8- to 10-fold longer presence in plasma, and thus delivery time to hair follicle cells. The neonate rat model, though acceptable here to achieve proof of concept, has an apparent mechanistic artifact. That is, the blanch response (onset, degree and duration) induced in the skin of 7-day-old rats with 1,000 mM of epinephrine (Fig. 3a) could be exceeded in human skin by applying 150 mM of norepinephrine (Fig. 3b and other images not shown). As epinephrine is 2.2-fold more potent as an alpha-adrenergic agonist than norepinephrine,20 this means that adult human skin is about 15-fold more responsive ([1,000/150 mM] × 2.2 = 14.6) to topical vasoconstriction than the skin of 7-day-old rats. This also explains the reason why though topical phenylephrine was nonprotective here against alopecia (Table 1) it does induce some skin blanch when applied topically to human skin (data not shown).
The capacity to topically induce, and sustain, skin blanch in the human scalp, and by so doing suppress delivery of systemic chemotherapy to skin hair follicles will ultimately determine the success of this approach in suppressing chemotherapy-induced alopecia. Figure 5a shows that a 33% reduction in systemic Cytoxan dose, with a presumed 33% reduction in hair follicle Cytoxan dose, caused a nearly complete retention of coat. Thus, in Figure 5b, we propose that topical vasoconstrictor need only achieve a ≥30% of reduction in the AUC dose of “insult” delivered to hair follicles during a single cancer therapy session to achieve apparent efficacy. For a 1–2 min beam-on radiotherapy session, this is simple because of its short duration. For a bolus injection of MNU or doxorubicin, with their short plasma half-lives, it is also simple, and the efficacy against MNU in this neonate rat model is apparent (Fig. 4 and Table 1). A ≥30% reduction in AUC “insult” dose for longer infusions of one or combined chemotherapy drugs, then followed by longer plasma half-lives, is a greater technical challenge. Scalp cooling does not succeed well here because of patient’s discomfort and the need to be physically attached to the cooling machine in the clinic for extended hours. Topical vasoconstrictor, (i) applied and then worn under a sealed “bathing” cap, (ii) possibly “redosed” through a port in the top of the bathing cap, (iii) then washed down the drain by washing one’s hair hours later at home, long after leaving the chemotherapy session or (iv) chemically neutralized by adding, for example, dilute, over-the-counter hydrogen peroxide to the bathing cap port and then removing and washing one’s hair, would be relatively easy to comply with as a patient.
Different from scalp cooling machines, such bathing caps would be fully compatible with standard cranial radiotherapy settings.
A significant part of a Phase I safety study for a topical vasoconstrictor alopecia preventive would be determining what adverse side effects were associated with an extended, hours-long skin blanch that would be needed for at least some chemotherapy infusion and long half-life drug regimens. As with any drug, there would likely be patient’s exclusion criteria and therapy settings where this drug would not be used.
Though vasoconstrictor-induced blanching of a patient’s scalp for hours as a strategy to prevent the alopecia induced by long half-life drugs may sound unusual, much more severe skin blanch, over much larger skin surface areas, is commonly practiced each day in humans in hundreds-thousands of outpatient settings in the world.24 Tumescent liposuction involves extensive subcutaneous injection of lidocaine: epinephrine over the entire area which will be surgically manipulated, and this may involve up to 30% or more of a patient’s entire body surface area being vasoconstricted at one time, for an extended period of hours. The skin blanching, used to prevent systemic distribution of subcutaneous lidocaine, is also the result of subcutaneous epinephrine injection rather than the much safer, less intrusive approach for alopecia prevention of topically applying epinephrine or norepinephrine and achieving delivery to dermal vasculature by passive diffusion through hair follicle channels.
The use of scalp cooling to reduce chemotherapy-induced alopecia4,5 is in itself an acknowledgement of the skin vasoconstriction strategy, and the absence of increased scalp metastases in breast cancer patients who used scalp cooling during chemotherapy, as measured by the incidence of scalp metastases 5.8 years after chemotherapy,25 supports skin vasoconstriction as a safe strategy. Presumably, the tissue depth of mechanical chiller-conferred, scalp vasoconstriction would be greater than what you would anticipate for the vasoconstriction conferred by a limiting dose of a topically applied vasoconstrictor. A recent report from our laboratory26 also showed no radiation protection of three, histologically diverse, xenograft tumor populations that received direct topical application of norepinephrine or phenylephrine 15 min before receiving tumoricidal radiotherapy. This outcome was consistent with the historical observation that human tumor vasculature, in general, lacks the smooth muscle cells with adrenergic receptors that are required to enable a response to an α-adrenergic vasoconstrictor. In the end, only clinical follow-up studies in cancer patients will be able to ascertain (i) whether topical epinephrine or norepinephrine caused an increase in scalp metastases and (ii) what clinical exclusion criteria would be appropriate for its use.
Conclusions
In conclusion, the data presented here demonstrate that topical application of α-adrenergic vasoconstrictors to skin in neonate rats before a large radiation insult or large systemic chemotherapy insult can prevent up to 100% of the alopecia that was seen in vehicle control animals. Variations of this assay provide a straightforward way to further test this strategy that could provide a simple and inexpensive way to prevent or suppress alopecia in human cancer patients.
What’s new?
Nearly two-thirds of cancer patients who receive chemotherapy experience chemotherapy-induced hair loss, or alopecia. To improve clinical management of alopecia, the present study explored a new strategy, based on topical application of a vasoconstrictor prior to radiation or chemotherapy. In a rat alopecia model, application of epinephrine or norepinephrine before radiation or chemotherapy was found to confer up to 95% coat retention. Topical application of the vasoconstrictors induced a visible skin blanch in neonate rats, with protected coat growth occurring at the blanched sites. The results support further development of a topical vasoconstrictor strategy for clinical use.
Acknowledgement
Fahl is a paid consultant to ProCertus BioPharm Inc.
Grant sponsors: The Wisconsin Alumni Research Foundation (WARF, University of Wisconsin-Madison), ProCertus BioPharm, Inc. (Madison, WI)
Abbreviations
- bw
body weight
- Gy
gray
- mm
millimeter
- mM
millimolar
References
- 1.Carelle N, Piotto E, Bellanger A, et al. Changing patient perceptions of the side effects of cancer chemotherapy. Cancer. 2002;95:155–163. doi: 10.1002/cncr.10630. [DOI] [PubMed] [Google Scholar]
- 2.McGarvey EL, Baum LD, Pinkerton RC, et al. Psychological sequelae and alopecia among women with cancer. Cancer Pract. 2001;9:283–289. doi: 10.1046/j.1523-5394.2001.96007.x. [DOI] [PubMed] [Google Scholar]
- 3.Hoffman M, Morrow GR, Roscoe JA, et al. Cancer patients’ expectations of experiencing treatment-related side effects: a University of Rochester Cancer Center-Community Clinical Oncology Program study of 938 patients from community practices. Cancer. 2004;101:851–857. doi: 10.1002/cncr.20423. [DOI] [PubMed] [Google Scholar]
- 4.Ridderheim M, Bjurberg M, Gustavsson A. Scalp hypothermia to prevent chemotherapy-induced alopecia is effective and safe: a pilot study of a new digitized scalp-cooling system used in 74 patients. Support Care Cancer. 2003;11:371–377. doi: 10.1007/s00520-003-0451-y. [DOI] [PubMed] [Google Scholar]
- 5.Christodoulou C, Klouvas G, Efstathiou E, et al. Effectiveness of the MSC cold cap system in the prevention of chemotherapy-induced alopecia. Oncology. 2002;62:97–102. doi: 10.1159/000048253. [DOI] [PubMed] [Google Scholar]
- 6.Sredni B, Xu RH, Albeck M, et al. The protective role of the immunomodulator AS101 against chemotherapy-induced alopecia studies on human and animal models. Int J Cancer. 1996;65:97–103. doi: 10.1002/(SICI)1097-0215(19960103)65:1<97::AID-IJC17>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 7.Katikaneni R, Ponnapakkam T, Matsushita O, et al. Treatment and prevention of chemotherapy-induced alopecia with PTH-CBD, a collagen-targeted parathyroid hormone analog, in a non-depilated mouse model. Anticancer Drugs. 2014;25:30–38. doi: 10.1097/CAD.0b013e3283650bff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hussein AM, Jimenez JJ, McCall CA, et al. Protection from chemotherapy-induced alopecia in a rat model. Science. 1990;249:1564–1566. doi: 10.1126/science.2218498. [DOI] [PubMed] [Google Scholar]
- 9.Wikramanayake TC, Amini S, Simon J, et al. A novel rat model for chemotherapy-induced alopecia. Clin Exp Dermatol. 2012;37:284–289. doi: 10.1111/j.1365-2230.2011.04239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paus R, Schilli MB, Handjiski B, et al. Topical calcitriol enhances normal hair regrowth but does not prevent chemotherapy-induced alopecia in mice. Cancer Res. 1996;56:4438–4443. [PubMed] [Google Scholar]
- 11.Hendrix S, Handjiski B, Peters EM, et al. A guide to assessing damage response pathways of the hair follicle: lessons from cyclophosphamide-induced alopecia in mice. J Invest Dermatol. 2005;125:42–51. doi: 10.1111/j.0022-202X.2005.23787.x. [DOI] [PubMed] [Google Scholar]
- 12.Paik SH, Yoon JS, Ryu HH, et al. Pretreatment of epidermal growth factor promotes primary hair recovery via the dystrophic anagen pathway after chemotherapy-induced alopecia. Exp Dermatol. 2013;22:496–499. doi: 10.1111/exd.12182. [DOI] [PubMed] [Google Scholar]
- 13.Haslam IS, Pitre A, Schuetz JD, et al. Protection against chemotherapy-induced alopecia: targeting ATP-binding cassette transporters in the hair follicle? Trends Pharmacol Sci. 2013;34:599–604. doi: 10.1016/j.tips.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Tsuruki T, Ito A, Takaha K, et al. FPRL1 receptor agonist peptides prevent etoposide-induced alopecia in neonatal rats. J Invest Dermatol. 2004;123:242–243. doi: 10.1111/j.0022-202X.2004.22714.x. [DOI] [PubMed] [Google Scholar]
- 15.Cleary J, Cannon G, Howard S, et al. Radioprotection study of topical norepinephrine in postsurgical breast cancer patients. Available from: http://meetinglibrary.asco.org/content/100998-114. [Google Scholar]
- 16.Cleary JF, Anderson BM, Eickhoff JC, et al. Exploratory study of topical norepinephrine for the prevention of radiodermatitis in post-surgical breast cancer patients. Int J Rad Oncol Biol Phys. submitted. [Google Scholar]
- 17.Soref CM, Fahl WE. A new topical vasoconstrictor-based strategy for prevention of oral mucositis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117:454–461. doi: 10.1016/j.oooo.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Copp RR, Peebles DD, Fahl WE. Synthesis and growth regulatory activity of a prototype member of a new family of aminothiol radioprotectors. Bioorg Med Chem Lett. 2011;21:7427–7430. doi: 10.1016/j.bmcl.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fahl WE, Ruoho AE, Mehta MP. Topical vasoconstrictor preparations and methods for protecting cells during cancer chemotherapy and radiotherapy. 8,114,914. US Pat. Available from: http://patft.uspto.gov.
- 20.Lazou A, Fuller SJ, Bogoyevitch MA, et al. Characterization of stimulation of phosphoinositide hydrolysis by α1-adrenergic agonists in adult rat hearts. Am J Physiol. 1994;267:H970–H978. doi: 10.1152/ajpheart.1994.267.3.H970. [DOI] [PubMed] [Google Scholar]
- 21.Peebles DD, Soref CM, Fahl WE. ROS-scavenger and radioprotective efficacy of the new PrC-210 aminothiol. Radiat Res. 2012;178:57–68. doi: 10.1667/rr2806.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall EJ, Giaccia AJ, editors. Radiobiology for the radiologist. 7th edn. Philadelphia: Lippincott Williams & Wilkins; 2012. p. 312. [Google Scholar]
- 23.Cooper RA, Carrington BM, Loncaster JA, et al. Tumour oxygenation levels correlate with dynamic contrast-enhanced magnetic resonance imaging parameters in carcinoma of the cervix. Radiother Oncol. 2000;97:53–59. doi: 10.1016/s0167-8140(00)00259-0. [DOI] [PubMed] [Google Scholar]
- 24.Boeni R. Safety of tumescent liposuction under local anesthesia in a series of 4,380 patients. Dermatology. 2011;222:278–281. doi: 10.1159/000327375. [DOI] [PubMed] [Google Scholar]
- 25.Lemieux J, Amireault C, Provencher L, et al. Incidence of scalp metastases in breast cancer: a retrospective cohort study in women who were offered scalp cooling. Breast Cancer Res Treat. 2009;118:547–552. doi: 10.1007/s10549-009-0342-0. [DOI] [PubMed] [Google Scholar]
- 26.Fahl WE. Effect of topical vasoconstrictor exposure upon tumoricidal radiotherapy. Int J Cancer. doi: 10.1002/ijc.28739. in press. [DOI] [PubMed] [Google Scholar]