Abstract
The amygdala is part of a neural network that contributes to the regulation of emotional behaviors. Rodents, especially rats, are used extensively as model organisms to decipher the functions of specific amygdala nuclei, in particular in relation to fear and emotional learning. Analysis of the role of the nonhuman primate amygdala in these functions has lagged work in the rodent but provides evidence for conservation of basic functions across species. Here we provide quantitative information regarding the morphological characteristics of the main amygdala nuclei in rats and monkeys, including neuron and glial cell numbers, neuronal soma size, and individual nuclei volumes. The volumes of the lateral, basal, and accessory basal nuclei were, respectively, 32, 39, and 39 times larger in monkeys than in rats. In contrast, the central and medial nuclei were only 8 and 4 times larger in monkeys than in rats. The numbers of neurons in the lateral, basal, and accessory basal nuclei were 14, 11, and 16 times greater in monkeys than in rats, whereas the numbers of neurons in the central and medial nuclei were only 2.3 and 1.5 times greater in monkeys than in rats. Neuron density was between 2.4 and 3.7 times lower in monkeys than in rats, whereas glial density was only between 1.1 and 1.7 times lower in monkeys than in rats. We compare our data in rats and monkeys with those previously published in humans and discuss the theoretical and functional implications that derive from our quantitative structural findings.
INDEXING TERMS: amygdaloid complex, neuron, astrocyte, oligodendrocyte, neuropil, human
The amygdala, also known as the amygdaloid complex, is a brain region located in the rostral portion of the human medial temporal lobe (Freese and Amaral, 2009). In rodents and primates including humans the amygdala consists of 13 nuclei and cortical areas demarcated into several subdivisions (Table 1) (Amaral et al., 1992; Sorvari et al., 1996; Pitkäen, 2000). Amygdala function has long been associated with emotional behavior, particularly fear (Davis, 2000; LeDoux, 2003; Feinstein et al., 2011), stress (Roozendaal et al., 2009), anxiety (Rauch et al., 2003), and appetitive and aversive conditioning (Shabel and Janak, 2009). The amygdala has also been linked to the neural systems that underlie normal social behavior (Adolphs, 2003), essentially via the modulation of fear (Emery et al., 2001; Prather et al., 2001; Amaral et al., 2003; Bauman et al., 2004). In addition, the amygdala has been shown to be important for the modulation of memory processes, particularly under arousing or emotionally salient conditions (McGaugh, 2004; Roozendaal et al., 2009).
TABLE 1.
Nomenclature of the Different Subdivisions of the Amygdaloid Complex in the Rat (Rattus norvegicus), Monkey (Macaca mulatta), and Human (Homo sapiens)
| Rat | Monkey | Human |
|---|---|---|
| lateral nucleus (L) | lateral nucleus (L) | lateral nucleus (L) |
| dorsolateral division (Ldl) | dorsal division (Ld) | lateral division (Ll) |
| ventrolateral division (Lvl) | dorsal intermediate division (Ldi) | medial division (Lm) |
| medial division (Lm) | ventral intermediate division (Lvi) | |
| ventral division (Lv) | ||
| basal nucleus (B) | basal nucleus (B) | basal nucleus (B) |
| magnocellular division(Bmc) | magnocellular division (Bmc) | magnocellular division (Bmc) |
| intermediate division (Bi) | intermediate division (Bi) | intermediate division (Bi) |
| parvicellular division (Bpc) | parvicellular division (Bpc) | parvicellular division (Bpc) |
| accessory basal nucleus (AB) | accessory basal nucleus (AB) | accessory basal nucleus (AB) |
| magnocellular division (Abmc) | magnocellular division (ABmc) | magnocellular division (ABmc) |
| parvicellular division (ABpc) | parvicellular division (ABpc) | parvicellular division (ABpc) |
| ventromedial division (ABvm) | ventromedial division (ABvm) | |
| central nucleus (CE) | central nucleus (CE) | central nucleus (CE) |
| capsular division (CEc) | medial division (CEm) | medial division (CEm) |
| lateral division (CEl) | lateral division (CEl) | lateral division (CEl) |
| intermediate division (CEi) | ||
| medial division (CEm) | ||
| medial nucleus (M) | medial nucleus (M) | medial nucleus (M) |
| rostral division (Mr) | ||
| central division | ||
| dorsal part (Mcd) | ||
| ventral part (Mcv) | ||
| caudal division (Mc) | ||
| – | paralaminar nucleus (PL) | paralaminar nucleus (PL) |
| intercalated nuclei (I) | intercalated nuclei (I) | intercalated nuclei (I) |
| anterior amygdaloid area (AAA) | anterior amygdaloid area (AAA) | anterior amygdaloid area (AAA) |
| nucleus of the lateral olfactory tract (NLOT) | nucleus of the lateral olfactory tract (NLOT) | nucleus of the lateral olfactory tract (NLOT) |
| anterior cortical nucleus (COa) | anterior cortical nucleus (COa) | anterior cortical nucleus (COa) |
| periamygdaloid cortex (PAC) | periamygdaloid cortex (PAC) | periamygdaloid cortex (PAC) |
| medial division (PACm) | (PAC2) | (PAC1) |
| sulcal division (PACs) | (PAC3) | (PAC3) |
| sulcal division (PACs) | sulcal division (PACs) | |
| oral portion (PACo) | oral portion (PACo) | |
| posterior cortical nucleus (COp) | posterior cortical nucleus (COp) | posterior cortical nucleus (COp) |
| amygdalo-hippocampal area (AHA) | amygdalo-hippocampal area (AHA) | amygdalo-hippocampal area (AHA) |
| medial division (AHAm) | ventral division (AHAv) | |
| lateral division (AHAl) | dorsal division (AHAd) | |
| bed nucleus of the accessory | – | – |
| olfactory tract (BAOT) | ||
| Pitkänen (2000) | Amaral et al. (1992) | Sorvari et al. (1996) |
Rodents, especially rats, are used extensively as model organisms to study the structural organization of the amygdala and to decipher the functions of specific amygdala nuclei (Pitkänen et al., 1997; Balleine and Killcross, 2006), in particular with respect to fear and emotional learning (Sigurdsson et al., 2007). In contrast, primates have been the model of choice for studies of the role of the amygdala in social behavior (Amaral et al., 2003; Adolphs, 2010). Several neuroanatomical studies, however, have already pointed out striking differences in the organization of the amygdaloid complex in rats, monkeys, and humans at the cellular level. Differences include, for example, the distribution of calcium-binding proteins (McDonald, 1994a; Pitkänen and Kemppainen, 2002), chondroitin sulfate proteoglycans (Pantazopoulos et al., 2008), GABAA receptors (McDonald and Mascagni, 1996), corticosteroid receptors (Pryce, 2008), and glutamate receptor subunits (McDonald, 1994b). Similarly, a relatively comprehensive description of the fundamental morphological characteristics and connectivity of the amygdaloid complex in rats, cats, and monkeys (Price et al., 1987) also summarized and suggested a number of potentially important species differences.
Stephan and Andy (1977) performed an informative comparative study of the volume of the amygdala in a large number of species. However, there was only limited information regarding the connectivity and neurochemical characteristics of the amygdaloid complex at that time and the nomenclature used in that study did not correspond to our current understanding of the structural organization of the amygdala (Table 1) (Amaral et al., 1992; Sorvari et al., 1996; Pitkänen, 2000). Moreover, it is now clear that although a first step, the simple measure of the volume of a brain structure is insufficient, by itself, to make clear interpretations regarding species differences in brain structure and function (Williams and Herrup, 1988). Indeed, there is not necessarily a direct link between the volume of a structure and the number of neurons in that structure, especially between species that might be only distantly related (Herculano-Houzel et al., 2007). It is therefore critically important to obtain reliable, quantitative information regarding the number of neurons, which are the fundamental functional units of the nervous system, in order to make inferences about the structural organization and putative functional processes subserved by defined nuclei of the amygdaloid complex in different species.
Although a cursory examination of Nissl-stained, coronal brain sections is sufficient to recognize that the amygdala differs between rodents and primates, little reliable quantitative information defining the number of neurons and glial cells, neuronal soma size, and individual nuclei volumes exists. The aim of this study was to provide these normative data in rats and monkeys. We therefore implemented modern, design-based stereological techniques to count neuron and glia numbers, measure neuronal soma size and the volume of the main amygdala nuclei (lateral, basal, paralaminar (in monkeys), accessory basal, medial and central nuclei) in mature rats (at 7 months of age) and monkeys (at 5–9 years of age). We further compared our quantitative data with previously acquired stereological data from the human amygdala (Schumann and Amaral, 2005), in order to provide a critical perspective on the relative development and structural organization of the main amygdala nuclei in two model organisms widely used to decipher the basic principles of amygdala functions in humans.
MATERIALS AND METHODS
Experimental animals
Monkeys
Four rhesus monkeys, Macaca mulatta, (two males: 5.3 and 9.4 years of age; two females: 7.7 and 9.3 years of age) were used for this study. Monkeys were born from multiparous mothers and raised at the California National Primate Research Center (CNPRC). They were maternally reared in 2,000 m2 outdoor enclosures and lived in large social groups until they were killed. These monkeys were the same animals used in quantitative studies of the monkey hippocampal formation (Jabès et al., 2010, 2011).
Rats
Four Sprague-Dawley rats, Rattus norvegicus, two males and two females at 7 months of age, were used for this study. All rats were retired breeders obtained from a commercial supplier (Charles River, San Diego, CA). Animals were perfused, as described below, within 24 hours of their arrival in our laboratory. In the meantime they were housed singly in standard laboratory cages with food and water ad libitum.
All experimental procedures were approved by either the Fribourg Veterinary Commission or the Institutional Animal Care and Use Committee of the University of California, Davis, and were conducted in accordance with the National Institutes of Health guidelines for the use of animals in research.
Brain acquisition
Monkeys
Each monkey was injected with the cell-division marker, 5′-bromo-2-deoxyuridine (BrdU, 150 mg/kg intraperitoneally; Boehringer Mannheim, Germany), 4 weeks prior to death in the context of another experiment (Jabès et al., 2010). Monkeys were deeply anesthetized with an intravenous injection of sodium pentobarbital (50 mg/kg, Fatal-Plus, Vortech Pharmaceuticals, Dearborn, MI) and perfused transcardially with 1% and then 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer (PB; pH 7.4) following protocols previously described (Lavenex et al., 2009a). Coronal sections were cut using a freezing, sliding microtome in six series at 30 μm and one series at 60 μm (Microm HM 450, Microm International, Germany). The 60-μm sections were collected in 10% formaldehyde solution in 0.1 M PB (pH 7.4) and postfixed at 4°C for 4 weeks prior to Nissl staining with thionin. All other series were collected in tissue collection solution and kept at −70°C until further processing (Lavenex et al., 2009a).
Rats
Animals were deeply anesthetized with pentobarbital (Nembutal, 50 mg/kg i.v.) and perfused transcardially with 1% and 4% PFA in 0.1 M PB (pH 7.4). The brains were postfixed for 6 hours in the same fixative, cryoprotected in 10% and 20% glycerol solutions in 0.1 M PB (pH 7.4; for 24 and 72 hours, respectively), frozen in powdered dry ice, and stored at −70°C until sectioning. Coronal sections were cut using a freezing, sliding microtome in four series at 40 μm (Microm HM 450, Microm International, Germany). One series was collected in 10% formaldehyde solution in 0.1 M PB (pH 7.4) and postfixed at 4°C for 4 weeks prior to Nissl staining with thionin. All other series were collected in tissue collection solution and kept at −70°C until further processing.
Histological processing
The procedure for Nissl-stained sections followed our standard laboratory protocol described previously (Lavenex et al., 2009). Briefly, sections were taken out of the 10% formaldehyde solution, thoroughly washed, mounted on gelatin-coated slides, and air-dried overnight at 37°C. Sections were then defatted 2 × 2 hours for monkeys and 2 × 1 hour for rats in a mixture of chloroform/ethanol (1:1, vol), partially rehydrated, and air-dried overnight at 37°C. Sections were then fully rehydrated and stained 40 seconds for rats and 20 seconds for monkeys in a 0.25% thionin solution (Fisher Scientific, Waltham, MA, cat. no. T-409), dehydrated, and coverslipped with DPX (BDH Laboratories, Poole, UK).
Anatomical boundaries of the amygdala
Rats
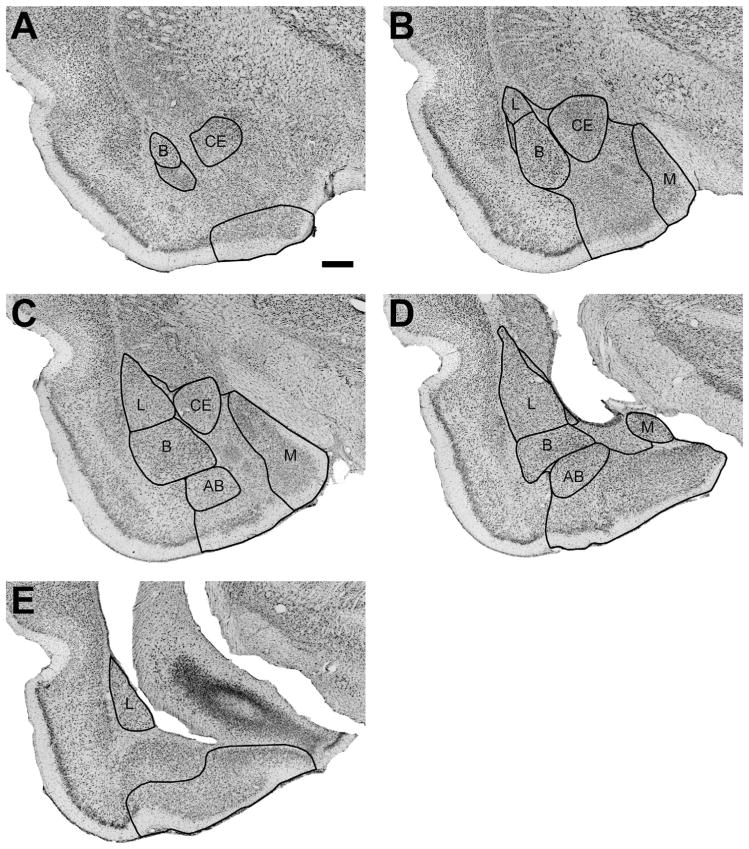
The cytoarchitectonic organization of the rat amygdala has been described in detail previously (Krettek and Price, 1978; McDonald, 1982; Cassell et al., 1986; Price et al., 1987; Pitkänen et al., 1995; Savander et al., 1995, 1996; Jolkkonen and Pitkäen, 1998), and a nomenclature congruent with that used for monkeys and humans has been provided by Pitkänen (2000) for defining the rat amygdala nuclei (Table 1). We thus delineated the five main nuclei of the rat amygdala (i.e., lateral, basal, accessory basal, central, and medial) according to these descriptions for all subsequent analyses (Fig. 1).
Figure 1.
Low-magnification photomicrographs of representative coronal sections through the rat amygdala illustrating the locations of the main nuclei. L, lateral; B, basal; AB, accessory basal; CE, central; M, medial. Nonlabeled areas represent remaining nuclei of the amygdala; see list in Table 1. Scale bar = 500 μm in A (applies to all panels).
Monkeys
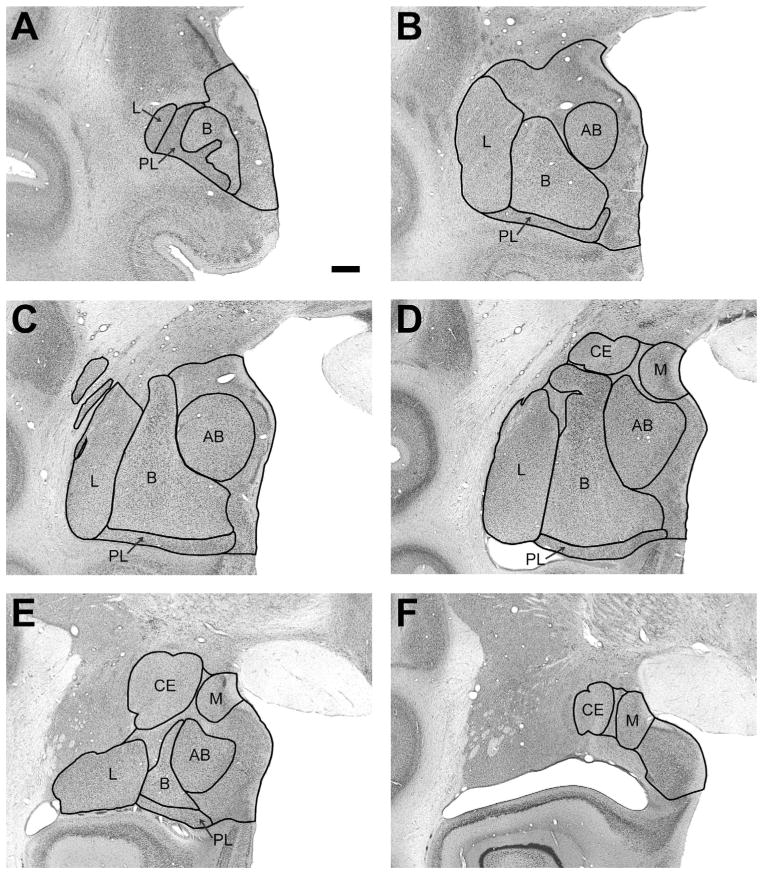
The nomenclature and the basic description of the morphological characteristics of the amygdala nuclei have been described in detail previously by Price et al. (1987), Amaral et al. (1992), and Pitkänen and Amaral (1998) for the cynomolgus monkey (Macaca fascicularis). We used these descriptions to determine the boundaries of the six main nuclei (i.e., lateral, basal, accessory basal, paralaminar, central, and medial) of the Macaca mulatta amygdala (Table 1; Fig. 2).
Figure 2.
Low-magnification photomicrographs of representative coronal sections through the monkey amygdala illustrating the locations of the main nuclei. L, lateral; B, basal; PL, paralaminar; AB, accessory basal; CE, central; M, medial. Nonlabeled areas represent remaining nuclei of the amygdala; see list in Table 1. Scale bar = 1 mm in A (applies to all panels).
Stereological analyses
Volume measurements and neuron and glia counts were performed with StereoInvestigator 7.0 (MicroBright-Field, Williston, VT). We estimated the volume of the brain (telencephalon and diencephalon), the volume of the whole amygdala, the volume of the main amygdala nuclei (lateral, basal, paralaminar (in monkeys), accessory basal, central, and medial) according to the Cavalieri principle on Nissl-stained sections cut at 60 μm in monkeys and 40 μm in rats (Gundersen and Jensen, 1987; West and Gundersen, 1990; Lavenex et al., 2000a,b). An average of 15 sections per animal for monkeys (480 μm apart) and an average of 28 sections per animal for rats (160 μm apart) were used to measure the volume of the whole amygdala. We estimated total amygdala volumes in the left and the right hemisphere and did not find any lateralization in rats (t(3) = −0.606, P = 0.587) or in monkeys (t(3) = −1.222, P = 0.309). We therefore performed estimates unilaterally for the other parameters (see below).
About 29 sections per animal for monkeys (240 μm apart) and about 21 sections per animal for rats (160 μm apart) were used for volume measurements of the main amygdala nuclei. As there was no lateralization for overall amygdala size, we estimated the volumes of individual amygdala nuclei in the left hemisphere for half of the animals, and in the right hemisphere for the other half. Brain volume refers to the volume of the telencephalon and diencephalon bilaterally. Thirty-four to 38 sections per animal for monkeys (1,920 μm apart), with the first section selected randomly within the first seven sections through the brain, and 29 to 32 sections per animal for rats (480 μm apart), with the first section selected randomly within the first three sections through the brain, were used for brain volume measurements. As for brain volume measurements, we used the section cutting thickness (60 μm in monkeys, 40 μm in rats) in order to calculate the volume of individual amygdala nuclei.
The total number of neurons in the main amygdaloid nuclei was determined using the optical fractionator method (Gundersen 1986; West et al., 1991). This design-based method enables the estimation of the number of cells that is independent of volume estimates. Neuron number was estimated in the right or in the left amygdala only, as for volume measurements. About 11 sections per animal for monkeys (480 μm apart), with the first section selected randomly within the first two sections through the nucleus of interest, and about 13 sections per animal for rats (160 μm apart) were used for neuron counts (Supporting Information 1). We used a 100× PlanFluor oil objective (N.A. 1.30) on a Nikon Eclipse 80i microscope (Nikon Instruments, Melville, NY) linked to PC-based StereoInvestigator 7.0. The sampling scheme was established to obtain individual estimates of neuron number with estimated coefficients of error (CE) around 0.10 (CE average (neurons) = 0.107). Section thickness was measured at each counting site in rats and at every other counting site in monkeys (average thickness: 11.89 μm in rats and 13.20 μm in monkeys). The thickness of processed sections was thus 30% that of the cutting thickness in rats and 22% that of the cutting thickness in monkeys, due to the shrinkage induced by the different steps of tissue processing including different defatting times for rats and monkeys.
The volume of neuronal somas was determined using the Nucleator method (Gundersen, 1988). We measured an average of 260 neurons per nucleus, sampled at every counting site during the optical fractionator analysis. Briefly, the nucleator can be used to estimate the mean cross-sectional area and volume of cells. A set of rays emanating from a point randomly chosen within the nucleus is drawn and oriented randomly. The length of the intercept from the point to the cell boundary (l) is measured and the cell volume is obtained by V = (4/3) × 3.1416 × (mean l)3. Essentially, this is the formula used to determine the volume of a sphere with a known radius. Note that the nucleator method provides accurate estimates of neuron size when isotropic-uniform-random sectioning of brain structures is employed (Gundersen, 1988). In our study all brains were cut in the coronal plane. Estimates of cell size might therefore be impacted by the nonrandom orientation of neurons in the different amygdala nuclei, which could lead to a systematic over- or underestimation of cell size in any given structure.
The total number of glial cells in the amygdala nuclei was determined using the optical fractionator method during the neuron counting. Thus, the same sampling scheme as for neuron counts was used for glial cells counts (CE average (glia) = 0.132). In rats and monkeys we differentiated between neurons, glial, and endothelial cells (Konopaske et al., 2007; Morris et al., 2008). In monkeys we were able to further distinguish oligodendrocytes and astrocytes based on morphological criteria identifiable in Nissl preparations (Fig. 3). We refer the reader to the original publications by (Palackal et al., 1993; Grady et al., 2003; Hamidi et al., 2004; Fitting et al., 2008) for detailed descriptions. Neurons are heavily stained with a large and single nucleolus (Fig. 3). Astrocytes display relatively smaller size and pale staining of the nucleus with a thin rim of Nissl-stained cytoplasm immediately adjacent to it (Fig. 3). Oligodendrocytes are smaller than astrocytes and can be identified by round, darker nuclei more densely packed with chromatin (Fig. 3). Microglia were not counted but were characterized in rats and monkeys by their smallest nucleus, darkest, irregular shape, sometimes rod-shaped, oval, or bent (Morris et al., 2008). The differentiation between oligodendrocytes and astrocytes was not sufficiently reliable in rats due to their overall smaller cell size and the lack of clear morphological criteria in Nissl preparations.
Figure 3.
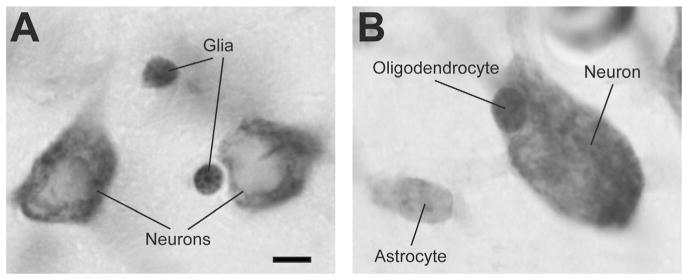
Classification and identification of different cell types in rats and monkeys. A: Typical neuron and glial cells viewed with a 100× objective in the rat amygdala basal nucleus as revealed in Nissl-stained sections cut at 40 μm. B: Typical neuron and glial cells viewed with a 100× objective in the monkey amygdala basal nucleus as revealed in Nissl-stained sections cut at 60 μm. Note that we were able to reliably distinguish between astrocytes and oligodendrocytes in monkeys, but not in rats, based on morphological criteria (see main text for details). Scale bar = 5 μm in A (applies to B).
No gender difference was found for any of the estimated parameters, so data from both genders were combined for presentation. We also evaluated both left and right amygdaloid nuclei in a systematic manner (as described above) and no lateralization was found (data not shown). Thus, our findings and all subsequent considerations are valid for, and can be generalized to, both left and right amygdala in both males and females.
3D reconstructions
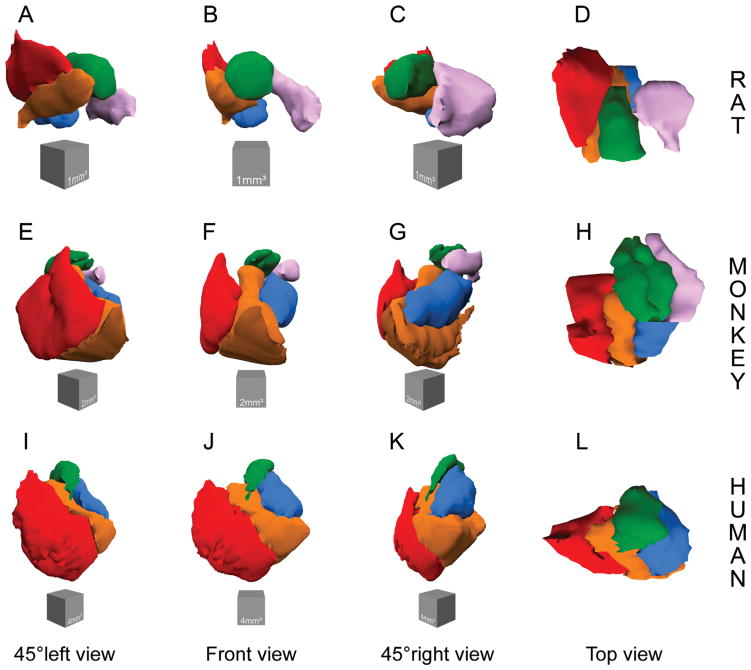
We performed 3D reconstructions of the amygdala nuclei in order to better appreciate the morphological and topological differences between rats, monkeys and humans (Fig. 4; Supporting Information 2 and 3). We used every Nissl-stained, coronal brain section, 160 μm apart for rats, 240 μm apart for monkeys to trace the main amygdala nuclei with StereoInvestigator 7.0. Tracings of the human amygdala nuclei were obtained with StereoInvestigator 5.0 (Schumann and Amaral, 2005). We then imported the tracings into the Reconstruct software (http://synapses.clm.utexas.edu/) (Fiala, 2005), and aligned the serial sections manually by using the contour of the brain as a reference between individual sections. The software generated a surface reconstruction of the brain and main amygdala nuclei using the Boissonnat (1988) method. Finally, 3D reconstruction images were rendered in Blender software v. 2.49 (open-source free-ware, http://www.blender.org).
Figure 4.
Three-dimensional reconstruction of the main amygdala nuclei in rat (A–D), monkey (E–H), and human (I–L). Lateral nucleus is in red, basal nucleus is in orange, paralaminar nucleus is in dark orange (in monkeys only), accessory basal nucleus is in blue, central nucleus is in green, and medial nucleus is in pink (not represented in humans). Scales: rat, gray cube is 1 mm3; monkey, gray cube is 2 mm3; human, gray cube is 4 mm3. 3D reconstructions are available in movie format as Supporting Information 2 and 3 online.
Photomicrographic production
Low-magnification photomicrographs were taken with a Leica DFC420 digital camera on a Leica MZ9.5 stereo-microscope (Leica Microsystems, Wetzlar, Germany). High-magnification photomicrographs were taken with a Leica DFC490 digital camera on a Nikon Eclipse 80i microscope (Nikon Instruments, Tokyo, Japan). Artifacts located outside of the sections were removed and gray levels were adjusted in Adobe Photoshop CS4, v. 11.0 (Adobe, San Jose, CA).
RESULTS
Volumes
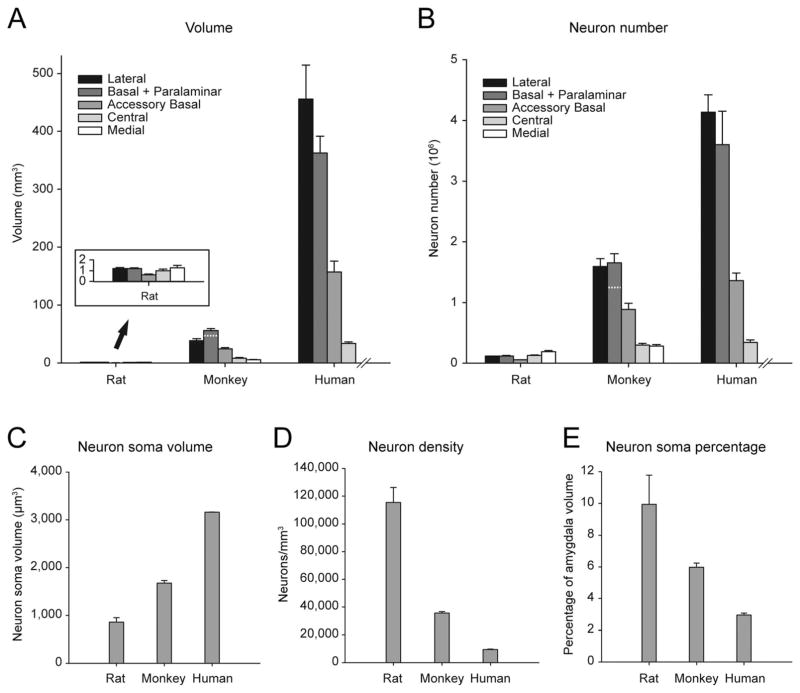
We used the Cavalieri method to estimate the volumes of the brain, the whole amygdala, and the main amygdala nuclei in rats and monkeys (Table 2). We also present human data previously published by Schumann and Amaral (2005) to enable a comparison of the relative size of individual nuclei in the three species. Estimates of the volume of the brain (telencephalon and diencephalon bilaterally) revealed that the monkey brain is about 53 times larger than the rat brain. In comparison, the volume of the whole amygdala (unilaterally) was only 18 times larger in monkeys than in rats. Importantly, this size difference was not homogeneous among amygdala nuclei, and distinct amygdala nuclei exhibited different scaling factors between rats and monkeys (Table 2; Fig. 5A). The lateral, basal, and accessory basal nuclei were, respectively, 32, 39, and 39 times larger in monkeys than in rats. In contrast, the central and medial nuclei were only 8 and 4 times larger, respectively, in monkeys than in rats. The combined volume of the remaining amygdala nuclei (i.e., nuclei other than those described above) was 10 times larger in monkeys than in rats (see Table 2).
TABLE 2.
Volume of the Brain, Amygdala, and Main Amygdala Nuclei in the Rat, Monkey, and Human
| Mean (mm3)
|
Relative2 (%)
|
Ratio
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Rat | Monkey | Human1 | R | M | H | M/R | H/M | H/R | |
| Brain3 | 994 | 52,360 | — | — | — | — | 53 | — | — |
| SD | 110 | 4,875 | — | ||||||
| Amygdala | 10.62 | 192.60 | 1,416.20 | 100 | 100 | 100 | 18 | 7 | 133 |
| SD | 1.02 | 6.26 | 132.49 | ||||||
| Lateral | 1.19 | 38.40 | 455.63 | 11 | 20 | 32 | 32 | 12 | 382 |
| SD | 0.10 | 3.57 | 58.62 | ||||||
| Basal | 1.20 | 47.15 | — | 11 | 24 | — | 39 | — | — |
| SD | 0.06 | 2.67 | — | ||||||
| Basal + Paralaminar | — | 55.98 | 362.33 | — | 29 | 26 | — | 6 | 302 |
| SD | — | 3.36 | 29.22 | ||||||
| Accessory Basal | 0.63 | 24.38 | 157.00 | 6 | 13 | 11 | 39 | 6 | 251 |
| SD | 0.07 | 2.14 | 18.77 | ||||||
| Central | 1.00 | 8.15 | 33.73 | 9 | 4 | 2 | 8 | 4 | 34 |
| SD | 0.14 | 1.44 | 2.84 | ||||||
| Medial | 1.28 | 5.42 | — | 12 | 3 | — | 4 | — | — |
| SD | 0.23 | 0.50 | — | ||||||
| Paralaminar | — | 8.84 | — | — | 5 | — | — | — | — |
| SD | — | 0.90 | — | ||||||
| Remaining4 | 6.60 | 65.70 | 407.53 | 62 | 34 | 29 | 10 | 6 | 62 |
| SD | 0.90 | 14.17 | 40.89 | ||||||
H: human ; M: monkey ; R: rat.
Data reported from Schumann and Amaral (2005).
Refers to the relative percentage of volume occupied by each nucleus in the amygdala. Amygdala volume equals to 100%.
Refers to the volume of the telencephalon and diencephalon bilaterally.
Remaining contains the medial nucleus, intercalated nuclei, anterior amygdaloid area, nucleus of the lateral olfactory tract, anterior cortical nucleus, periamygdaloid cortex, posterior cortical nucleus, amygdalo-hippocampal area and bed nucleus of the accessory olfactory tract (in rat).
Figure 5.
A: Volumes of the main amygdala nuclei in rats, monkeys, and humans. The deep nuclei (lateral, basal, accessory basal) are relatively more developed in monkeys and humans than in rats. Note that the volume of the human medial nucleus was not estimated by Schumann and Amaral (2005). A dashed line in the bar representing the basal nucleus in monkeys indicates the volume of the paralaminar nucleus (upper part of the bar), and is included to enable the comparison with the human data (which included the paralaminar nucleus in the basal nucleus). B: Neuron numbers in the main amygdala nuclei of rats, monkeys, and humans. Note that neuron number in the human medial nucleus was not estimated by Schumann and Amaral (2005). A dashed line in bar representing the basal nucleus in monkeys indicates the number of neurons of the paralaminar nucleus (upper part of the bar), and is included to enable the comparison with the human data. C: Average neuronal soma size in the amygdala of the rat, monkey, and human. D: Average neuron density in the amygdala of the rat, monkey, and human. E: Percentage of total amygdala volume occupied by neuronal somas in the amygdala of the rat, monkey, and human. A lower percentage is associated with greater development of the neuropil. Error bars ± SD.
By way of comparison, the human brain is ≈10 times larger than the monkey brain and the human amygdala is 7 times larger than that of the monkey. Again, however, distinct nuclei exhibited different scaling factors between monkeys and humans (Table 2; Fig. 5A). In particular, the lateral nucleus was 12 times larger in humans than in monkeys. In contrast, the volumes of the basal, accessory basal and central nuclei were respectively 6, 6, and 4 times larger in humans. The combined volume of the remaining amygdala nuclei was 6 times larger in humans than in monkeys.
Altogether, these volumetric data revealed important species differences in the relative size of the main amygdala nuclei in rats, monkeys, and humans. Most interestingly, the lateral, basal, and accessory basal nuclei, which are highly interconnected with the neocortex, were dramatically more developed in monkeys and humans than in rats. Indeed, the summed volume of the lateral, basal (including the paralaminar nucleus in monkeys and humans), and accessory basal nuclei represented 62% of total amygdala volume in monkeys and 69% in humans, whereas it represented only 28% of total amygdala volume in rats. In contrast, the central and medial nuclei exhibited relatively smaller size differences between rats and monkeys. These differences can easily be appreciated in 3D reconstructions of the amygdala in the three species (Fig. 4 and Supporting Information 2 and 3).
Neuron and glial cell numbers
We used the optical fractionator method to estimate the number of neurons and glial cells (astrocytes and oligodendrocytes) in distinct nuclei of the rat and monkey amygdala (Table 3), and compared our results with the number of neurons found in the human amygdala (Schumann and Amaral, 2005). We found that the numbers of neurons in the lateral, basal, and accessory basal nuclei were, respectively, 14, 11, and 16 times greater in monkeys than in rats (Table 3; Fig. 5B). In contrast, the numbers of neurons in the central and medial nuclei were only 2.3 and 1.5 times greater in monkeys than in rats, respectively. Thus, species differences in neuron numbers are substantial but not as striking as species differences in the volume of individual amygdaloid nuclei. This suggests that differences in neuron numbers are accompanied by even more important differences in other cellular components between rats and monkeys.
TABLE 3.
Cell Numbers in the Main Nuclei of the Rat, Monkey, and Human Amygdala
| Mean
|
Ratio
|
||||||
|---|---|---|---|---|---|---|---|
| Rat | Monkey | Human1 | M/R | H/M | H/R | ||
| Lateral | Neuron | 117,486 | 1,592,284 | 4,136,500 | 13.6 | 2.6 | 35.2 |
| SD | 1,229 | 128,381 | 286,150 | ||||
| Glia | 80,744 | 2,156,678 | — | 26.7 | — | — | |
| SD | 7,393 | 189,246 | |||||
| Oligodendrocytes | — | 1,112,480 | — | — | — | — | |
| SD | 131,622 | ||||||
| Astrocytes | — | 1,044,198 | — | — | — | — | |
| SD | 64,819 | ||||||
| Glia/neuron ratio | 0.7 | 1.4 | — | 2.0 | — | — | |
| Basal | Neuron | 115,774 | 1,247,181 | — | 10.8 | ||
| SD | 13,793 | 140,356 | |||||
| *Neuron (B + PL) | — | 1,655,232 | 3,601,500 | — | 2.2 | 31.1 | |
| SD | 148,516 | 552,180 | |||||
| Glia | 106,521 | 2,513,417 | — | 23.6 | — | — | |
| SD | 15,942 | 175,853 | |||||
| Oligodendrocytes | — | 1,326,864 | — | — | — | — | |
| SD | 94,497 | ||||||
| Astrocytes | — | 1,186,553 | — | — | — | — | |
| SD | 82,050 | ||||||
| Glia/neuron ratio | 0.9 | 2.0 | — | 2.2 | — | — | |
| Accessory basal | Neuron | 57,017 | 885,352 | 1,357,250 | 15.5 | 1.5 | 23.8 |
| SD | 1,800 | 101,469 | 125,680 | ||||
| Glia | 35,780 | 1,273,935 | — | 35.6 | — | — | |
| SD | 7,010 | 89,538 | |||||
| Oligodendrocytes | — | 572,196 | — | — | — | — | |
| SD | 82,235 | ||||||
| Astrocytes | — | 701,739 | — | — | — | — | |
| SD | 23,552 | ||||||
| Glia/neuron ratio | 0.6 | 1.4 | — | 2.3 | — | — | |
| Central | Neuron | 127,174 | 297,079 | 344,500 | 2.3 | 1.2 | 2.7 |
| SD | 10,434 | 30,889 | 39,602 | ||||
| Glia | 86,691 | 486,762 | — | 5.6 | — | — | |
| SD | 11,387 | 39,116 | |||||
| Oligodendrocytes | — | 210,656 | — | — | — | — | |
| SD | 28,544 | ||||||
| Astrocytes | — | 276,106 | — | — | — | — | |
| SD | 15,546 | ||||||
| Glia/neuron ratio | 0.7 | 1.6 | — | 2.4 | — | — | |
| Medial | Neuron | 188,742 | 282,622 | — | 1.5 | — | — |
| SD | 19,688 | 25,053 | |||||
| Glia | 103,936 | 338,080 | — | 3.3 | — | — | |
| SD | 9,036 | 26,695 | |||||
| Oligodendrocytes | — | 102,661 | — | — | — | — | |
| SD | 13,889 | ||||||
| Astrocytes | — | 235,419 | — | — | — | — | |
| SD | 17,978 | ||||||
| Glia/neuron ratio | 0.6 | 1.2 | — | 2.2 | — | — | |
| Paralaminar | Neuron | — | 408,051 | — | — | — | — |
| SD | 49,453 | ||||||
| Glia | — | 288,184 | — | — | — | — | |
| SD | 21,186 | ||||||
| Oligodendrocytes | — | 96,422 | — | — | — | — | |
| SD | 23,397 | ||||||
| Astrocytes | — | 191,762 | — | — | — | — | |
| SD | 28,505 | ||||||
| Glia/neuron ratio | — | 0.7 | — | — | — | — | |
H: human ; M: monkey ; R: rat.
Data reported from Schumann and Amaral (2005).
Interestingly, we found that the differences in the number of glial cells between rats and monkeys were about twice as large as differences in neuron number. We found that the numbers of glial cells in the monkey lateral, basal, and accessory basal nuclei were, respectively, 27, 24, and 36 times larger than in the rat. In the central and medial nuclei, there were, respectively, 6 and 3 times more glial cells in monkeys than in rats. Consequently, the ratio between the number of glial cells and the number of neurons was about two times greater in monkeys than in rats; this ratio was relatively constant across all amygdala nuclei (Table 3). Unfortunately, we were unable to reliably distinguish between astrocytes and oligodendrocytes in the rat (see Materials and Methods section). Thus, we could not determine experimentally if one type of glial cells underlies this species difference or if both astrocytes and oligodendrocytes contribute to this difference between rats and monkeys. No data were available for the human amygdala.
Neuronal soma size
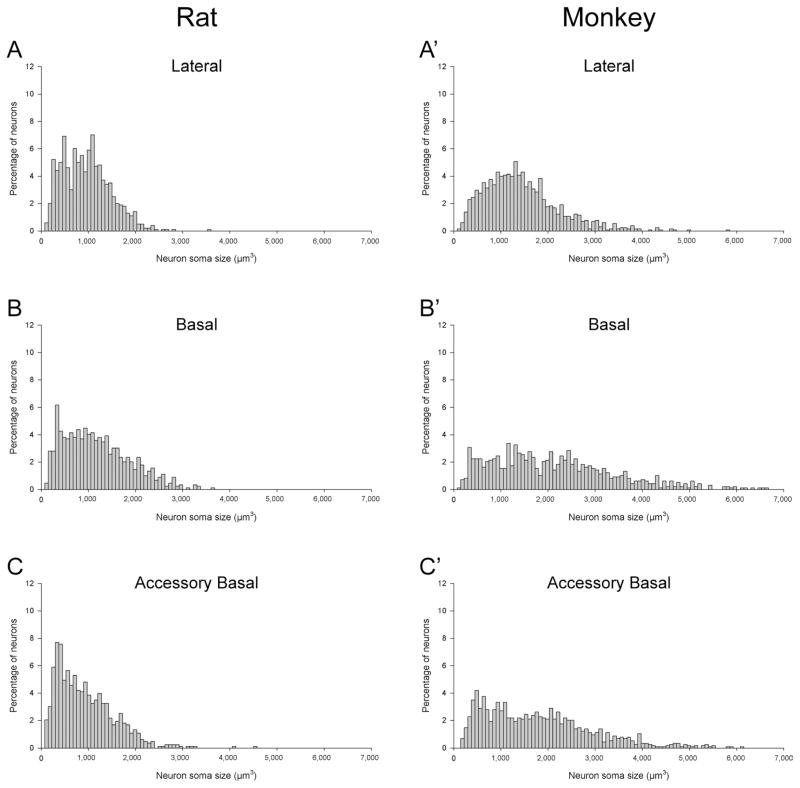
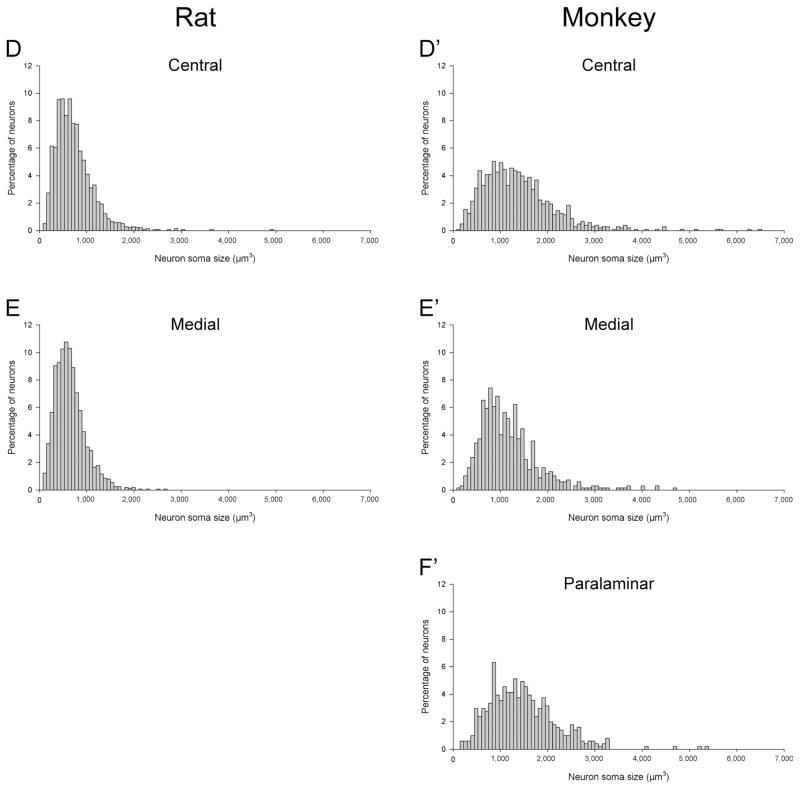
We used the nucleator technique to estimate the volume of neuronal somas in the amygdala nuclei of rats and monkeys; Schumann and Amaral (2006) estimated neuronal somas in human cases with the same method (Table 4). Altogether, these data indicate that the average size of amygdala neurons is progressively larger from rats to monkeys to humans. Across the main amygdala nuclei, neuronal somas were about two times larger in monkeys than in rats and about two times larger in humans than in monkeys (Table 4; Fig. 5C). In rats and monkeys, neurons exhibited distinct size distributions in different amygdala nuclei (Fig. 6). In monkeys, the greater variation in neuronal soma size was clearly indicative of different neuron populations within the basal and accessory basal nuclei. The basal nucleus is well known to have a range of neuronal sizes that goes from larger dorsally to smaller ventrally (Freese and Amaral, 2009). The variation was less obvious in the lateral nucleus, even though this nucleus clearly contains different populations of neurons in both rats (Price et al., 1987) and monkeys (Pitkänen and Amaral, 1998). In general, amygdala neuron populations were more heterogeneous in monkeys than in rats, suggesting a potentially greater diversity of different cell types in primates.
TABLE 4.
Neuron Soma Volume and Cell Density in the Main Nuclei of the Rat, Monkey, and Human Amygdala
| Mean
|
Ratio
|
||||||
|---|---|---|---|---|---|---|---|
| Rat | Monkey | Human1 | M/R | H/M | H/R | ||
| Lateral | Neuron soma volume2 | 963 | 1,433 | 3,099 | 1.49 | 2.16 | 3.22 |
| SD | 76 | 158 | 62 | ||||
| Neuron density3 | 99,000 | 42,000 | 9,000 | 0.42 | 0.21 | 0.09 | |
| SD | 8,400 | 3,700 | 900 | ||||
| Glia density3 | 68,000 | 57,000 | — | 0.84 | — | — | |
| SD | 10,200 | 8,700 | — | ||||
| % of nuclei volume4 | 9.55 | 5.96 | 2.84 | 0.62 | 0.48 | 0.30 | |
| SD | 1.26 | 0.79 | 0.29 | ||||
| Basal | Neuron soma volume | 1,187 | 2,154 | 3,245 | 1.81 | 1.51 | 2.73 |
| SD | 109 | 144 | 88 | ||||
| Neuron density | 97,000 | 26,000 | — | 0.27 | — | — | |
| SD | 13,200 | 1,600 | |||||
| Neuron density (B + PL) | — | 30,000 | 10,000 | — | 0.33 | 0.10 | |
| SD | 1,200 | 700 | |||||
| Glia density | 89,000 | 53,000 | — | 0.60 | — | — | |
| SD | 11,900 | 4,800 | |||||
| % of nuclei volume | 11.44 | 5.69 | 3.21 | 0.50 | 0.56 | 0.28 | |
| SD | 1.39 | 0.61 | 0.23 | ||||
| Accessory basal | Neuron soma volume | 932 | 1,804 | 3,393 | 1.94 | 1.88 | 3.64 |
| SD | 214 | 92 | 72 | ||||
| Neuron density | 92,000 | 36,000 | 9,000 | 0.39 | 0.25 | 0.10 | |
| SD | 11,100 | 1,000 | 500 | ||||
| Glia density | 57,000 | 53,000 | — | 0.93 | — | — | |
| SD | 7,400 | 5,200 | |||||
| % of nuclei volume | 8.74 | 6.54 | 2.94 | 0.75 | 0.45 | 0.34 | |
| SD | 2.82 | 0.41 | 0.19 | ||||
| Central | Neuron soma volume | 726 | 1,374 | 1,893 | 1.89 | — | — |
| SD | 152 | 69 | 44 | ||||
| Neuron density | 130,000 | 37,000 | 10,000 | 0.28 | 0.27 | 0.08 | |
| SD | 24,900 | 3,500 | 1,200 | ||||
| Glia density | 89,000 | 61,000 | — | 0.69 | — | — | |
| SD | 21,500 | 6,200 | |||||
| % of nuclei volume | 9.70 | 5.06 | 1.94 | 0.52 | 0.38 | 0.20 | |
| SD | 3.64 | 0.40 | 0.24 | ||||
| Medial | Neuron soma volume | 651 | 1,176 | — | 1.81 | — | — |
| SD | 60 | 27 | |||||
| Neuron density | 150,000 | 52,000 | — | 0.35 | — | — | |
| SD | 19,300 | 4,700 | |||||
| Glia density | 85,000 | 63,000 | — | 0.74 | — | — | |
| SD | 24,400 | 10,800 | |||||
| % of nuclei volume | 9.81 | 6.16 | — | 0.63 | — | — | |
| SD | 1.71 | 0.63 | |||||
| Paralaminar | Neuron soma volume | — | 1,470 | — | — | — | — |
| SD | 87 | ||||||
| Neuron density | — | 46,000 | — | — | — | — | |
| SD | 5,300 | ||||||
| Glia density | — | 33,000 | — | — | — | — | |
| SD | 5,100 | ||||||
| % of nuclei volume | — | 6.80 | — | — | — | — | |
| SD | 0.70 | ||||||
H: human ; M: monkey ; R: rat.
Data reported from Schumann and Amaral (2005) and (2006).
Neuron soma volume in cubic micrometer.
Cell number per cubic millimeter.
Refers to the fraction of the amygdala nuclei volume occupied by neuronal somas.
Figure 6.
Distributions of neuronal soma size in the main amygdaloid nuclei of rats (A–E) and monkeys (A′–F′). A,A′: Lateral nucleus; B,B′: basal nucleus; C,C′: accessory basal nucleus; D,D′: central nucleus; E,E′: medial nucleus; F′: paralamniar nucleus (only in monkeys).
Cell density and neuropil volume
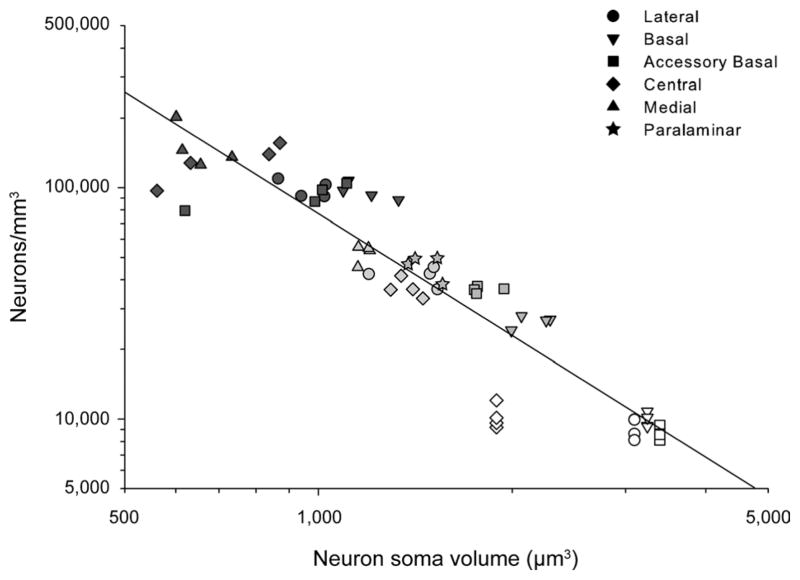
Neuron density and neuropil volume provide additional information about the structural organization of a brain region that neuron number or overall volume measurements alone do not necessarily provide. We therefore calculated neuron density for the main amygdala nuclei (Table 4; Fig. 5D), based on our previous measures of volumes and neuron numbers (Tables 2, 3). Neuron density was between 2.36 (lateral nucleus) and 3.73 (basal nucleus) times lower in monkeys than in rats (average: 3.01), and between 3.00 (basal + paralaminar nuclei) and 4.67 (lateral nucleus) times lower in humans than in monkeys (average: 3.84). In the lateral nucleus, for example, there were on the order of 9,000 neurons per cubic millimeter in the human, 42,000 neurons per cubic millimeter in the monkey, and 99,000 neurons per cubic millimeter in the rat. These data suggest that the volume of the neuropil becomes progressively larger from rats, to monkeys, to humans. We therefore calculated the percentage of the volume occupied by neuronal somas in order to obtain an estimate of neuropil volume in individual amygdala nuclei (Table 4). On average, neuronal somas occupied ≈10% of the volume of the amygdala in rats and ≈6% in monkeys (Fig. 5E). Using data from Schumann and Amaral (2005, 2006), we estimated that the volume occupied by neuronal somas is ≈3% in the human amygdala. Interestingly, the percentage of the volume of individual amygdala nuclei occupied by neuronal somas was inversely related to the size of the neuronal somas (Fig. 5C,E). The increase in neuropil volume was thus proportionally larger than the increase in neuronal soma size. Indeed, the percentage of amygdala volume occupied by the neuropil was progressively larger from rats (90%), to monkeys (94%), to humans (97%). In addition, neuron density in the different amygdala nuclei across the three different species was inversely related to the volume of neuronal somas (Fig. 7; log10(neuron density) = 10.116 – 1.745 × log10(soma size); R2 = 0.85; F(1.59) = 342.444; P < 0.001). Interestingly, glial density was also slightly lower in monkeys than in rats (Table 4), but this difference was more limited than that observed for neuron density (monkey/rat ratios: neuron density, 0.27–0.42; glia density, 0.60–0.93). Altogether, these results suggest that species differences in neuropil volume are likely due to differences in the dendritic and axonal arborizations of amygdala neurons, which might reflect an increased level of connectivity in primates.
Figure 7.
Relationship between neuronal density and neuronal soma size in the main nuclei of the rat, monkey, and human amygdala. Logarithmic regression of neuron density and neuron soma size in the amygdala (log10(neuron density) = 10.116 – 1.745 × log10(soma size); R2 = 0.85; F(1.59) = 342.44; P < 0.001). Rat, dark gray; monkey, gray; human, white. Lateral nucleus, circles; basal nucleus, upside-down triangles; accessory basal nucleus, squares; central nucleus, diamonds; medial nucleus, triangles; paralaminar (only in monkeys), stars.
DISCUSSION
Our stereological data in rats and monkeys provide fundamental, quantitative information regarding the morphological characteristics of the main amygdala nuclei in two species used as model organisms to understand the basic principles of human amygdala functions. We found: 1) that the lateral, basal, and accessory basal nuclei were dramatically more developed in monkeys than in rats, whereas the volume and neuron numbers in the central and medial nuclei differed far less between rats and monkeys; 2) a larger volume of the amygdala is made up of neuropil in monkeys than in rats. The higher percentage of neuropil volume implies a) a greater neuronal arborization and b) a greater number of glial cells relative to neuron number in primates (Fig. 8).
Figure 8.
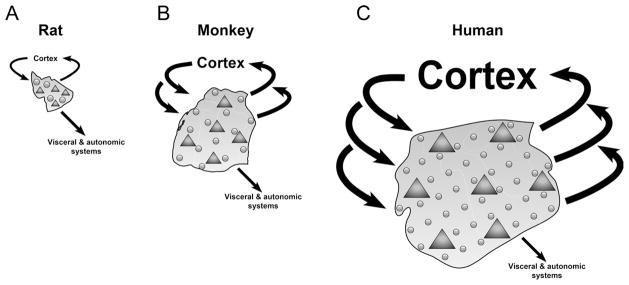
Summary representation of the amygdala in rats (A), monkeys (B), and humans (C). Glial cells, circles; neurons, triangles: rats < monkeys < humans. Neuron density: rats > monkeys > humans. Glia density: rats = monkeys = humans. Glia/neuron ratio: rats < monkeys < humans. Connectivity with visceral and autonomic systems (mainly via the central nucleus): rats = monkeys = humans. Connectivity with cortical systems (between the neocortex and the lateral, basal, and accessory basal nuclei): rats < monkeys < humans. The proportions of the different parameters are not precisely scaled.
In this discussion, we first consider some methodological issues that might impact quantitative analyses of cell numbers and morphological features of the brain. We then evaluate our results in light of previous studies and conclude that our data provide a reliable reference regarding the morphological characteristics of the main amygdala nuclei in rats and monkeys. Finally, we discuss the theoretical and functional implications that derive from our quantitative structural findings.
Methodological considerations
There are a number of methodological issues that must be taken into consideration when evaluating the data we have presented. First, the rats and monkeys used in this study were reared in different environmental conditions. The monkeys were reared by their mothers in 2,000 m2 outdoor naturalistic enclosures and lived in large social groups. In contrast, the rats were retired breeders housed in standard laboratory cages. It is quite possible that different rearing conditions and life experiences might have influenced the development of the amygdala (Joseph, 1999; Okuda et al., 2009). However, the relatively small variations that might be induced by rearing conditions are very unlikely to account for the major differences in the morphological features, such as main amygdala nuclei volumes and neuron and glial cell numbers, observed between species. Moreover, the rearing conditions described here correspond to those typically employed in functional studies of the amygdala in rats (Pitkänen et al., 1997; LeDoux, 2000; Balleine and Killcross, 2006; Sigurdsson et al., 2007) and monkeys (Emery et al., 2001; Prather et al., 2001; Amaral et al., 2003; Bauman et al., 2004).
Second, data on the volume of individual nuclei were not corrected for potential differences in shrinkage of brain tissue during fixation. The rats and monkeys used in the current study were all perfused with the same fixative (4% PFA) following the same protocol used routinely in our laboratory. In contrast, the human brains used previously (Schumann and Amaral, 2005) were fixed by immersion in 10% formalin. We have previously shown, using monkey brains (Lavenex et al., 2009), that different methods of fixation (i.e., perfusion vs. immersion) using the same fixative solution (4% PFA in PB) have a significant impact on the neuroanatomical characteristics of the brain. In brief, neuropil volume is larger in immersion-fixed brains as compared to perfusion-fixed brains. In contrast, neuronal soma volume is smaller in immersion-fixed brains than in perfusion-fixed brains. Moreover, the differential shrinkage observed between immersion-fixed and perfusion-fixed tissue varies considerably between different cell types (Lavenex et al., 2009), so that it is impossible to determine any single correction factor that would enable direct comparison of these parameters for all cell populations. However, this issue is unlikely to affect the conclusions of our study, as we focused our comparisons on the relative rather than absolute volumes of the different amygdala nuclei in the different species. In contrast, the measurements of neuronal soma size in humans (Schumann and Amaral, 2006) might have underestimated true neuronal size, as compared to the perfusion-fixation condition that was used for rats and monkeys. If this hypothesis is true, then the actual differences in neuronal soma size between humans and the other two species might be even greater than that reported in the current study. The determination of the total number of neurons is not sensitive to fixation artifacts and potential differences in shrinkage, as the optical fractionator method is independent of the measure of the volume of the structure (West et al., 1991). Thus, the comparison of total neuron numbers in the main amygdala nuclei of rats and monkeys is valid and reliable.
Interlaboratory differences
Considering the important differences between laboratories that can sometimes outweigh differences between experimental conditions (Scorcioni et al., 2004; Altemus et al., 2005; see also discussion below), it is particularly important to consider that our quantitative data were derived from Nissl-stained, coronal brain sections processed following the same protocol in the laboratories of the two senior investigators (P.L.: rats and two monkeys; D.G.A.: two monkeys). The first author (L.J.C.) performed all quantitative analyses with the same microscope and stereological system in P.L.’s laboratory at the University of Fribourg. We compared the current data in rats and monkeys with those obtained in humans and previously published by Schumann and Amaral (2005). The stereological analysis of human cases was performed by another experimenter (C.M.S.) in D.G.A.’s laboratory at the University of California, Davis. We have thus reduced the potential influence of an experimenter bias to a minimum in order to make sound species comparisons. Moreover, comparison of our comprehensive dataset with those of other studies performed in different laboratories further supports the reliability and generalization of our normative data (see discussion below and Supporting Information 4).
Normalization procedures
Some researchers have considered the need to normalize morphometric data in order to compare different parameters of brain structure that might vary between species or experimental conditions. However, a major hurdle in performing a sound normalization is to find an adequate reference that does not falsely impact the parameter under study (Lavenex et al., 2002, 2000b). For example, it would be easy to conclude that the amygdala is less developed in monkeys, as compared to rats, if one defines the size of the amygdala in relation to the size of the brain. This conclusion is obviously incorrect considering that 1) the actual volume and the numbers of neurons and glial cells are indeed greater in the monkey amygdala, as compared to rats; and 2) we do not know what factors might impact the development of the rest of the brain in different species. This is especially true for species that are only distantly related (Herculano-Houzel et al., 2007). Consequently, we did not use any normalization procedure in the current study. Instead, we compared directly the various parameters that we estimated and considered the implications that could be inferred from defined species differences in our specific estimates.
Comparison with previous studies
Rats
Previous studies of neuron number and nuclei volume in the rat amygdala have reported variable results (Supporting Information 4). Data from two separate studies, which considered the volume and neuron number of at least three of the main amygdala nuclei (Tuunanen and Pitkänen, 2000; Pêgo et al., 2008), differed significantly from each other but in a consistent manner as compared to our own results in the six main amygdala nuclei. The neuron numbers in the lateral, basal, and accessory basal nuclei of 6-month-old Sprague-Dawley rats reported by Tuunanen and Pitkänen (2000) were about 50% lower than our estimates. Similarly, the results on volume and neuron number in the lateral, basal, and central nuclei in 4-month-old Wistar rats reported by Pêgo et al. (2008) were consistently 20–30% below our estimates. As Pêgo et al. (2008) used different criteria to delineate the accessory basal nucleus, it is difficult to compare our estimate to theirs.
In contrast, our estimates of neuron number in the central and medial nuclei were consistent with the results reported by Fan et al. (2008). Our estimate of the volume of the central nucleus was also consistent with their results, but the volume of the medial nucleus that they reported was smaller than our own estimate; this is reasonable, however, since Fan et al. excluded the cell free layer I in their volume measurements. Our estimate of the number of neurons in the basal nucleus is consistent with previous estimates by Rubinow and Juraska (2008). Similarly, our estimates of neuronal soma size are consistent with those reported by Cooke et al. (2000) in the medial nucleus and by Berdel et al. (1997) in the lateral nucleus.
Altogether, the consistency of our results with those of a number of studies performed in different laboratories, together with the reliability of our stereological analysis system established in the monkey amygdala (see below) and hippocampal formation (Jabès et al., 2010, 2011), support the reliability of our stereological data on the volumes and neuron numbers in the main nuclei of the rat amygdala. Our data can therefore be considered a reliable base on which to build models and perform interspecies comparisons of amygdala structure and putative functional circuits.
Monkeys
Our results on nuclei volume and neuron number in the monkey amygdala are consistent with recent data reported by Carlo et al. (2010) (Supporting Information 4). Considering the volume of individual nuclei, the estimates of the paralaminar nucleus are the only ones that differed by more than 20% between the two studies (29%). Considering the number of neurons, only the estimates of the central and medial nuclei differed by more than 10% between the two studies (central: 27%, medial: 17%). Since these differences are relatively small, they do not significantly impact the comparisons with the rat or human amygdala. Indeed, such small differences in the estimations of volume and neuron number performed by different experimenters in different laboratories, using different materials and methods, provide a strong validation of the current results. To our knowledge, there are no other data on neuron size and glia number in the rhesus monkey amygdala.
Humans
The stereological data on the human amygdala reported by Schumann and Amaral (2005) were also supported by those of another study, which found similar results in the lateral nucleus of the human amygdala (Kreczmanski et al., 2007) (Supporting Information 4). Despite differences in the delimitation of individual nuclei, Vereecken et al. (1994) found a total number of neurons in the whole amygdala that was close to that found by Schumann and Amaral (2005). The data on neuronal soma size were consistent with those of Aliashkevich et al. (2003) in the lateral and basal nuclei (Supporting Information 4). Data on neuronal soma size in humans from Berretta et al. (2007) were also consistent for the basal and accessory basal nuclei but not in the lateral nucleus, where the data reported by Berretta were 28% below those reported by Schumann and Amaral (2005). Bezchlibnyk et al. (2007) reported neuronal soma sizes twice as big as those found by Schumann and Amaral (2005). These differences could be due to the use and calibration of different analysis systems, or the use of paraffin-embedded brains by Bezchlibnyk et al. (2007) and the use of frozen sections by Schumann and Amaral (2005). Nevertheless, the data previously reported by Schumann and Amaral (2005) are largely consistent with the results of other studies and can thus be used as a reliable reference to establish sound comparisons with our current data in rats and monkeys.
Cell density and glia/neuron ratio
Our data suggested that the greater glia/neuron ratio in the primate amygdala (i.e., in both monkeys and humans) is mainly due to a lower density of neurons in primates as compared to rats. The amygdala glia/neuron ratio (total number of glia divided by the total number of neurons) is greater in humans (5.36, derived from the data on glia density published by Hamidi et al. (2004) and the volume measurements of Schumann and Amaral (2005)) than in monkeys (1.50) or rats (0.68). An increase in the glia/neuron ratio with increased complexity of the brain between species has previously been reported in the cerebral cortex (Friede and Van Houten, 1962), as well as more specifically in frontal cortical areas within primates (Sherwood et al., 2006). In the human amygdala, Hamidi et al. (2004) estimated a glia density (oligodendrocytes + astrocytes; 50,254 cells/mm3) that is close to what we calculated in the monkey amygdala (53,591 cells/mm3). Although our study found glial density to be higher in the rat (78,819 cells/mm3), what is important is that the differences in glial density between species (1.55 times higher in rats than monkeys, and 1.55 times higher in rats than humans) are not nearly as pronounced as the differences in neuron density (3.2 times higher in rats than monkeys, and 12 times higher in rats than humans). These results indicate that, in contrast to neuron density, glial density is relatively constant across species.
The glia/neuron ratio that we determined in the monkey amygdala (1.50) was much higher than previous estimates of glia/neuron ratio (including microglia) from several other regions of the Macaca mulatta rhesus monkeys brain. Previously reported glia/neuron ratios were: 1.0 in the prefrontal cortex (Dombrowski et al., 2001), 0.46 in the visual cortex (O’Kusky and Colonnier, 1982), 0.56 in various frontal, parietal, temporal, and occipital regions (Christensen et al., 2007), and 0.82 across all cortical areas (Lidow and Song, 2001). As glia density is relatively constant across the brain, the higher glia/neuron ratio observed in the amygdala is more likely linked to a lower neuron density (34,000 neurons/mm3) as compared to neocortical areas (prefrontal cortex: 38,569–58,708 neurons/mm3 (Dombrowski et al., 2001); visual cortex: 120,000 neurons/mm3 (O’Kusky and Colonnier, 1982)). Similarly, neuron density in the human amygdala (9,000 neurons/mm3) appears to be markedly lower than neuron density in the human posterior orbitofrontal (area 13; 30,000 neurons/mm3) (Semendeferi et al., 1998) and prefrontal (area 10; 34,000 neurons/mm3) (Semendeferi et al., 2001) cortices. These differences in glia/neuron ratios reveal important structural and cellular variations in the organization of the different gray matter areas in the primate brain. The low neuron density of the amygdala, as compared to neocortical areas, could reflect a higher degree of intrinsic and extrinsic interconnectivity, and thus the functional specificity of this structure.
Neuropil
The lower neuronal density in the primate amygdala, as compared to rodents, is related to a larger neuropil volume, which is likely associated with greater complexity in the dendritic and axonal arborizations of individual amygdala neurons. This likely suggests that primates have a greater capacity to integrate information in specific amygdala circuits than do rats. A larger volume of neuropil in humans compared to monkeys has been shown in several cortical regions: Broca’s area (Schenker et al., 2008), the face area of the primary motor cortex (Sherwood et al., 2003), area 10 of the prefrontal cortex (Semendeferi et al., 2001), and the visual cortex (Zilles et al., 1982). Accordingly, a smaller neuronal density in humans as compared to monkeys has been shown in the posterior orbitofrontal cortex (area 13) (Semendeferi et al., 1998) and prefrontal cortex (area 10) (Semendeferi et al., 2001). In the rat amygdala, dendrites and axons represent more than 80% of the neuropil volume, glial cells 14% and synapses only 2% (Cooke et al., 2007). Although largely similar, glial density is slightly lower in monkeys and humans than in rats. The proportion of neuropil occupied by glial cells might thus be somehow compensated for by the greater size of glial cells in humans than in monkeys, and in monkeys than in rats (Oberheim et al., 2006, 2009). Nevertheless, we also found in the stratum radiatum of the CA1 region of the adult monkey hippocampus that glial processes occupy less then 10% of the neuropil (Lavenex et al., 2011). The larger neuropil volume observed in primates therefore most likely reflects an increase in dendrites and axons. Accordingly, the complexity of the dendritic arborization of amygdala neurons is greater in monkeys than in rats (Rai et al., 2005; Mohandas Rao et al., 2009). Indeed, assumption-based 2D Scholl analyses of amygdala neurons reported between 22 and 29 intersections (between dendrites and the concentric analysis circles centered on the soma) for neurons randomly selected in the rat amygdala (Rai et al., 2005; Mohandas Rao et al., 2009), and between 125 to 210 dendritic intersections for neurons randomly selected in the monkey amygdala (Herzog, 1982; see Altemus et al., 2005 for data on the dendritic arborization of pyramidal neurons in the CA1 field of the rat and monkey hippocampus). Taken together, these results suggest a greater complexity of amygdala networks that might contribute to a greater integration of inputs reaching the amygdala in primates. This hypothesis is supported by the higher convergence of information through the cascade of intrinsic amygdala circuits.
Input convergence and integration of information in amygdala circuits
One obvious interpretation of our data is that the differences in the relative size and neuron numbers of the main amygdala nuclei observed between rats and monkeys are linked to their degree of connectivity with other brain structures. This hypothesis is in agreement with the theory that brain structures with major anatomical and functional links evolve together independently of evolutionary changes in other unrelated structures (Barton and Harvey, 2000). The lateral, basal, and accessory basal nuclei are more developed in primates than in rodents, and parallel the greater development of the neocortical areas with which these nuclei are interconnected in primates. As discussed above, the larger neuropil and greater neuronal soma size observed in the primate amygdala is associated with a greater complexity of the dendritic and local axonal arborizations, which could subsequently have a direct impact on the integration capacity of the functional network that includes this brain region. Here we consider the major connections of the main amygdala nuclei (Freese and Amaral, 2009), in rats and monkeys (the connectivity of the human amygdala remains largely unknown), in order to evaluate the hypothesis of a higher convergence and integration of information in the primate amygdala.
As discussed above, the relative development of the main amygdala nuclei might be influenced by their interconnections with other brain structures, i.e., their afferent and efferent connections (Stephan et al., 1987). In rats and monkeys the lateral, basal, and accessory basal nuclei are the main recipients and originators of neocortical-amygdala connections (Pitkänen, 2000; Freese and Amaral, 2009). Cortical projections to the amygdala are organized similarly in rats and monkeys (McDonald, 1998) but these connections are far more substantial in monkeys than in rats due to the dramatic expansion of the neocortex in primates (Super and Uylings, 2001). Similarly, the efferent projections from the amygdala to the neocortex are more substantial in primates. For example, the amygdala projects to primary sensory areas in monkeys (Amaral and Price, 1984; Iwai and Yukie, 1987; Amaral et al., 1992; Yukie, 2002) and in humans (Catani et al., 2003), but these connections are apparently absent in rats (Krettek and Price, 1977). Consequently, and in contrast to rodents, the primate amygdala could influence the sensory processing at primary cortical stages (Freese and Amaral, 2009). The expansion of cortical areas and the greater complexity of cortical information reaching the amygdala are thus associated with a greater development of the amygdala nuclei interconnected with the neocortex.
In contrast, the central nucleus is connected mainly to visceral and autonomic systems (Pitkänen, 2000; Freese and Amaral, 2009). These systems are presumed to have been conserved throughout the course of evolution, thus leading to more limited species differences in the size and neuron number of the central nucleus. Similarly, the relatively small species differences in the volume and neuron number of the medial nucleus is paralleled by the highly conserved organization of the olfactory cortex with which it is interconnected (Crosby and Humphrey, 1944). Projections from the olfactory cortical areas are essentially the same in rats and monkeys, except that it represents a greater fraction of the total sensory inputs in rats than in monkeys, in which other sensory modalities have become dominant (Price, 1973; Carmichael et al., 1994).
The main amygdala nuclei, including the lateral, basal, accessory basal, central, and medial nuclei, are also characterized by their unique intraamygdala connections (see Freese and Amaral, 2009, for a comprehensive description). Within the amygdala circuitry, the connections between these different nuclei are highly unidirectional. The lateral nucleus projects to the basal, accessory basal, medial, and central nuclei, whereas the return projections are either very meager or totally nonexistent. The basal nucleus projects to the accessory basal, medial, and central nuclei, but the return projections are also either meager or nonexistent. The accessory basal nucleus projects most strongly to the central nucleus and sends moderate projections to the medial nucleus, whereas it returns only light projections to the lateral and basal nuclei. The medial nucleus receives it strongest afferents from the lateral nucleus and moderate projections from the accessory basal nucleus. The medial nucleus projects most heavily to the central nucleus and returns only light projections to the basal and accessory basal nuclei, but not to the lateral nucleus. Finally, the central nucleus is the site of convergence of projections originating in all the other main amygdala nuclei. In contrast, the central nucleus projects only lightly to the lateral, basal, and accessory basal nuclei.
Interestingly, we found the largest species differences in neuron numbers at the earliest stages of the intrinsic amygdala circuitry, that is, within the lateral, basal, and accessory basal nuclei. Indeed, there were about 13 times more neurons in these three nuclei combined in monkeys (3.73 million) than in rats (290 thousand). In contrast, the central nucleus, upon which inputs from the other amygdaloid nuclei converge, exhibit relatively small species differences. There were only 2.3 times more neurons in monkeys (297 thousand) than in rats (127 thousand). Although fundamental amygdala functions are likely conserved across species, the relative importance of the functional processes carried out by distinct amygdalar circuits might differ between rodents and primates. Specifically, the functions subserved by the interconnections of the lateral, basal, and accessory basal nuclei with the neocortex in primates might be relatively preponderant compared to the functions subserved by the circuitry involving the central nucleus. Cortical information reaching the primate amygdala via the lateral, basal, and accessory basal nuclei can be processed within these nuclei and sent back to the neocortex to modulate cortical activity and cognitive processes. Intrinsic amygdala projections can further process these inputs and forward highly integrated information in order to influence the function of the central nucleus. In addition, direct projections from the lateral, basal, and accessory basal nuclei can also directly contribute to the modulation of neuronal activity in various subcortical structures (see Freese and Amaral, 2009, for details regarding these connections). In sum, the basic functions carried out by the amygdala are likely conserved between species, even though the processes carried out by the central and medial nuclei might be under greater influence by cortical inputs in primates, via projections from the lateral, basal, and accessory basal nuclei. Thus, to illustrate the functional impact of these morphometric differences between species, let us consider that the amygdala serves essentially as a danger detector, in both rodents and primates, thus facilitating the survival of the individual animal. Although this survival function is essentially conserved in primates, because the primate amygdala can integrate additional contextual information, this additional information can further lead to the regulation of more complex behaviors such as the modulation of social interactions (Amaral et al., 2003). Going one step farther, as in the case of the human, where integration may be even greater, the amygdala is also activated when subjects are presented with untrustworthy faces (Adolphs et al., 1998; Winston et al., 2002), positive words (Hamann and Mao, 2002), happy faces (Canli et al., 2002), amusement-inducing movies (Aalto et al., 2002), and other complex social stimuli that might not have an equivalent in rodents. Thus, the highly developed lateral nucleus of the monkey and human amygdala has greater computational resources to carry out learning and template matching for a wide range of environmental stimuli.
CONCLUSION
This quantitative study has revealed important differences in the structural organization and cellular components of the main amygdala nuclei in rats, monkeys, and humans. First, the primate amygdala is characterized by a lower neuron density, larger neuropil volume, and greater glia/neuron ratio than the rat amygdala. These fundamental differences in the structural organization of the amygdala could be linked to a greater complexity and integration capacity of sensory information within the intrinsic amygdala circuits in primates. Second, the lateral, basal, and accessory basal nuclei were dramatically more developed in monkeys than in rats, whereas the volume and neuron numbers in the central and medial nuclei differed relatively little between rats and monkeys. This suggests that although the fundamental function of the amygdala, to regulate fear and emotional learning, is conserved across species, amygdala function might be under greater influence of cortical activity in primates, and therefore integrate additional contextual information that influences the regulation of more complex behaviors such as social interactions.
Supplementary Material
Acknowledgments
Grant sponsor: Swiss National Science Foundation; Grant numbers: PP00A-106701, PP00P3-124536; Grant sponsor: National Institutes of Health (NIH); Grant number: RO1-MH041479; and was conducted, in part, at the California National Primate Research Center (RR00169); Grant sponsor: Swiss National Science Foundation; Grant numbers: PMPDP3_122844, PMPDP3_128996 (to P.B.L.).
We thank the CNPRC staff, Jeff Bennett, Grégoire Favre, Jane Favre, Adeline Jabès, Danièle Uldry, and K.C. Wells for technical assistance at various stages of the project.
Footnotes
Additional Supporting Information may be found in the online version of this article.
LITERATURE CITED
- Aalto S, Naatanen P, Wallius E, Metsahonkala L, Stenman H, Niem PM, Karlsson H. Neuroanatomical substrata of amusement and sadness: a PET activation study using film stimuli. Neuroreport. 2002;13:67–73. doi: 10.1097/00001756-200201210-00018. [DOI] [PubMed] [Google Scholar]
- Adolphs R. Is the human amygdala specialized for processing social information? Ann N Y Acad Sci. 2003;985:326–340. doi: 10.1111/j.1749-6632.2003.tb07091.x. [DOI] [PubMed] [Google Scholar]
- Adolphs R. What does the amygdala contribute to social cognition? Ann N Y Acad Sci. 2010;1191:42–61. doi: 10.1111/j.1749-6632.2010.05445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio AR. The human amygdala in social judgment. Nature. 1998;393:470–474. doi: 10.1038/30982. [DOI] [PubMed] [Google Scholar]
- Aliashkevich AF, Yilmazer-Hanke D, Van Roost D, Mundhenk B, Schramm J, Blumcke I. Cellular pathology of amygdala neurons in human temporal lobe epilepsy. Acta Neuropathol. 2003;106:99–106. doi: 10.1007/s00401-003-0707-0. [DOI] [PubMed] [Google Scholar]
- Altemus KL, Lavenex P, Ishizuka N, Amaral DG. Morphological characteristics and electrophysiological properties of CA1 pyramidal neurons in macaque monkeys. Neuroscience. 2005;136:741–756. doi: 10.1016/j.neuroscience.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Price JL. Amygdalo-cortical projections in the monkey (Macaca fascicularis) J Comp Neurol. 1984;230:465–496. doi: 10.1002/cne.902300402. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Price JL, Pitkänen A, Carmichael ST. Anatomical organization of the primate amygdaloid complex. In: Aggleton J, editor. The amygdala: neurobiological aspects of emotion, memory, and mental dysfunction. New York: Wiley-Liss; 1992. pp. 1–66. [Google Scholar]
- Amaral DG, Bauman MD, Capitanio JP, Lavenex P, Mason WA, Mauldin-Jourdain ML, Mendoza SP. The amygdala: is it an essential component of the neural network for social cognition? Neuropsychologia. 2003;41:517–522. doi: 10.1016/s0028-3932(02)00310-x. [DOI] [PubMed] [Google Scholar]
- Balleine BW, Killcross S. Parallel incentive processing: an integrated view of amygdala function. Trends Neurosci. 2006;29:272–279. doi: 10.1016/j.tins.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Barton RA, Harvey PH. Mosaic evolution of brain structure in mammals. Nature. 2000;405:1055–1058. doi: 10.1038/35016580. [DOI] [PubMed] [Google Scholar]
- Bauman MD, Lavenex P, Mason WA, Capitanio JP, Amaral DG. The development of mother-infant interactions after neonatal amygdala lesions in rhesus monkeys. J Neurosci. 2004;24:711–721. doi: 10.1523/JNEUROSCI.3263-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdel B, Morys J, Maciejewska B. Neuronal changes in the basolateral complex during development of the amygdala of the rat. Int J Dev Neurosci. 1997;15:755–765. doi: 10.1016/s0736-5748(97)00022-1. [DOI] [PubMed] [Google Scholar]
- Berretta S, Pantazopoulos H, Lange N. Neuron numbers and volume of the amygdala in subjects diagnosed with bipolar disorder or schizophrenia. Biol Psychiatry. 2007;62:884–893. doi: 10.1016/j.biopsych.2007.04.023. [DOI] [PubMed] [Google Scholar]
- Bezchlibnyk YB, Sun X, Wang JF, MacQueen GM, McEwen BS, Young LT. Neuron somal size is decreased in the lateral amygdalar nucleus of subjects with bipolar disorder. J Psychiatry Neurosci. 2007;32:203–210. [PMC free article] [PubMed] [Google Scholar]
- Boissonnat JD. Shape reconstruction from planar cross sections. Comput Vision Graph. 1988;44:1–29. [Google Scholar]
- Canli T, Sivers H, Whitfield SL, Gotlib IH, Gabrieli JD. Amygdala response to happy faces as a function of extra-version. Science. 2002;296:2191. doi: 10.1126/science.1068749. [DOI] [PubMed] [Google Scholar]
- Carlo CN, Stefanacci L, Semendeferi K, Stevens CF. Comparative analyses of the neuron numbers and volumes of the amygdaloid complex in old and new world primates. J Comp Neurol. 2010;518:1176–1198. doi: 10.1002/cne.22264. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Clugnet MC, Price JL. Central olfactory connections in the macaque monkey. J Comp Neurol. 1994;346:403–434. doi: 10.1002/cne.903460306. [DOI] [PubMed] [Google Scholar]
- Cassell MD, Gray TS, Kiss JZ. Neuronal architecture in the rat central nucleus of the amygdala: a cytological, hodological, and immunocytochemical study. J Comp Neurol. 1986;246:478–499. doi: 10.1002/cne.902460406. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, Donato R, Ffytche DH. Occipito-temporal connections in the human brain. Brain. 2003;126(Pt 9):2093–2107. doi: 10.1093/brain/awg203. [DOI] [PubMed] [Google Scholar]
- Christensen JR, Larsen KB, Lisanby SH, Scalia J, Arango V, Dwork AJ, Pakkenberg B. Neocortical and hippocampal neuron and glial cell numbers in the rhesus monkey. Anat Rec (Hoboken) 2007;290:330–340. doi: 10.1002/ar.20504. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Chowanadisai W, Breedlove SM. Post-weaning social isolation of male rats reduces the volume of the medial amygdala and leads to deficits in adult sexual behavior. Behav Brain Res. 2000;117:107–113. doi: 10.1016/s0166-4328(00)00301-6. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Stokas MR, Woolley CS. Morphological sex differences and laterality in the prepubertal medial amygdala. J Comp Neurol. 2007;501:904–915. doi: 10.1002/cne.21281. [DOI] [PubMed] [Google Scholar]
- Crosby EC, Humphrey T. Studies of the vertebrate telencephalon. III. The amygdaloid complex in the shrew (Blarina brevicauda) J Comp Neurol. 1944;81:285–305. [Google Scholar]
- Davis M. The role of the amygdala in conditioned and unconditioned fear and anxiety. In: Aggleton J, editor. The amygdala: the functional analysis of the amygdala. New York: Wiley-Liss; 2000. pp. 31–115. [Google Scholar]
- Dombrowski SM, Hilgetag CC, Barbas H. Quantitative architecture distinguishes prefrontal cortical systems in the rhesus monkey. Cereb Cortex. 2001;11:975–988. doi: 10.1093/cercor/11.10.975. [DOI] [PubMed] [Google Scholar]
- Emery NJ, Capitanio JP, Mason WA, Machado CJ, Mendoza SP, Amaral DG. The effects of bilateral lesions of the amygdala on dyadic social interactions in rhesus monkeys (Macaca mulatta) Behav Neurosci. 2001;115:515–544. [PubMed] [Google Scholar]
- Fan L, Hanbury R, Pandey SC, Cohen RS. Dose and time effects of estrogen on expression of neuron-specific protein and cyclic AMP response element-binding protein and brain region volume in the medial amygdala of ovariectomized rats. Neuroendocrinology. 2008;88:111–126. doi: 10.1159/000129498. [DOI] [PubMed] [Google Scholar]
- Feinstein JS, Adolphs R, Damasio A, Tranel D. The human amygdala and the induction and experience of fear. Curr Biol. 2011;21:34–38. doi: 10.1016/j.cub.2010.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala JC. Reconstruct: a free editor for serial section microscopy. J Microsc. 2005;218(Pt 1):52–61. doi: 10.1111/j.1365-2818.2005.01466.x. [DOI] [PubMed] [Google Scholar]
- Fitting S, Booze RM, Hasselrot U, Mactutus CF. Differential long-term neurotoxicity of HIV-1 proteins in the rat hippocampal formation: a design-based stereological study. Hippocampus. 2008;18:135–147. doi: 10.1002/hipo.20376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese JL, Amaral DG. Neuroanatomy of the primate amygdala. In: Whalen PJ, Phelps EA, editors. The human amygdala. New York: Guilford; 2009. pp. 3–42. [Google Scholar]
- Friede RL, Van Houten WH. Neuronal extension and glial supply: functional significance of glia. Proc Natl Acad Sci U S A. 1962;48:817–821. doi: 10.1073/pnas.48.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady MS, Charleston JS, Maris D, Witgen BM, Lifshitz J. Neuronal and glial cell number in the hippocampus after experimental traumatic brain injury: analysis by stereological estimation. J Neurotrauma. 2003;20:929–941. doi: 10.1089/089771503770195786. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ. Stereology of arbitrary particles. A review of unbiased number and size estimators and the presentation of some new ones, in memory of William R. Thompson. J Microsc. 1986;143(Pt1):3–45. [PubMed] [Google Scholar]
- Gundersen HJ. The nucleator. J Microsc. 1988;151(Pt 1):3–21. doi: 10.1111/j.1365-2818.1988.tb04609.x. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987;147(Pt 3):229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Hamann S, Mao H. Positive and negative emotional verbal stimuli elicit activity in the left amygdala. Neuroreport. 2002;13:15–19. doi: 10.1097/00001756-200201210-00008. [DOI] [PubMed] [Google Scholar]
- Hamidi M, Drevets WC, Price JL. Glial reduction in amygdala in major depressive disorder is due to oligodendrocytes. Biol Psychiatry. 2004;55:563–569. doi: 10.1016/j.biopsych.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Herculano-Houzel S, Collins CE, Wong P, Kaas JH. Cellular scaling rules for primate brains. Proc Natl Acad Sci U S A. 2007;104:3562–3567. doi: 10.1073/pnas.0611396104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog AG. The relationship of dendritic branching complexity to ontogeny and cortical connectivity in the pyramidal cells of the monkey amygdala: a Golgi study. Brain Res. 1982;256:73–77. doi: 10.1016/0165-3806(82)90098-0. [DOI] [PubMed] [Google Scholar]
- Iwai E, Yukie M. Amygdalofugal and amygdalopetal connections with modality-specific visual cortical areas in macaques (Macaca fuscata, M. mulatta, and M. fascicularis) J Comp Neurol. 1987;261:362–387. doi: 10.1002/cne.902610304. [DOI] [PubMed] [Google Scholar]
- Jabès A, Lavenex PB, Amaral DG, Lavenex P. Quantitative analysis of postnatal neurogenesis and neuron number in the macaque monkey dentate gyrus. Eur J Neurosci. 2010;31:273–285. doi: 10.1111/j.1460-9568.2009.07061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabès A, Lavenex PB, Amaral DG, Lavenex P. Postnatal development of the hippocampal formation: a stereological study in macaque monkeys. J Comp Neurol. 2011;519:1051–1070. doi: 10.1002/cne.22549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolkkonen E, Pitkanen A. Intrinsic connections of the rat amygdaloid complex: projections originating in the central nucleus. J Comp Neurol. 1998;395:53–72. doi: 10.1002/(sici)1096-9861(19980525)395:1<53::aid-cne5>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Joseph R. Environmental influences on neural plasticity, the limbic system, emotional development and attachment: a review. Child Psychiatry Hum Dev. 1999;29:189–208. doi: 10.1023/a:1022660923605. [DOI] [PubMed] [Google Scholar]
- Konopaske GT, Dorph-Petersen KA, Pierri JN, Wu Q, Sampson AR, Lewis DA. Effect of chronic exposure to antipsychotic medication on cell numbers in the parietal cortex of macaque monkeys. Neuropsychopharmacology. 2007;32:1216–1223. doi: 10.1038/sj.npp.1301233. [DOI] [PubMed] [Google Scholar]
- Kreczmanski P, Heinsen H, Mantua V, Woltersdorf F, Masson T, Ulfig N, Schmidt-Kastner R, Korr H, Steinbusch HW, Hof PR, Schmitz C. Volume, neuron density and total neuron number in five subcortical regions in schizophrenia. Brain. 2007;130(Pt 3):678–692. doi: 10.1093/brain/awl386. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. Projections from the amygdaloid complex to the cerebral cortex and thalamus in the rat and cat. J Comp Neurol. 1977;172:687–722. doi: 10.1002/cne.901720408. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. A description of the amygdaloid complex in the rat and cat with observations on intra-amygdaloid axonal connections. J Comp Neurol. 1978;178:255–280. doi: 10.1002/cne.901780205. [DOI] [PubMed] [Google Scholar]
- Lavenex P, Steele MA, Jacobs LF. The seasonal pattern of cell proliferation and neuron number in the dentate gyrus of wild adult eastern grey squirrels. Eur J Neurosci. 2000a;12:1–6. doi: 10.1046/j.1460-9568.2000.00949.x. [DOI] [PubMed] [Google Scholar]
- Lavenex P, Steele MA, Jacobs LF. Sex differences, but no seasonal variations in the hippocampus of food-caching squirrels: a stereological study. J Comp Neurol. 2000b;425:152–166. [PubMed] [Google Scholar]
- Lavenex P, Steele MA, Jacobs LF. Lack of seasonal variation in the hippocampus: statistics is not the issue. Anim Behav. 2002;64:15–18. [Google Scholar]
- Lavenex P, Banta Lavenex P, Bennett JL, Amaral DG. Postmortem changes in the neuroanatomical characteristics of the primate brain: hippocampal formation. J Comp Neurol. 2009;512:27–51. doi: 10.1002/cne.21906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavenex P, Sugden SG, Davis RR, Gregg JP, Banta Lavenex P. Developmental regulation of gene expression and astrocytic processes may explain selective hippocampal vulnerability. Hippocampus. 2011;21:142–149. doi: 10.1002/hipo.20730. [DOI] [PubMed] [Google Scholar]
- LeDoux J. The amygdala and emotion: a view through fear. In: Aggleton J, editor. The amygdala: the functional analysis of the amygdala. New York: Wiley-Liss; 2000. pp. 289–310. [Google Scholar]
- LeDoux J. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol. 2003;23:727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidow MS, Song ZM. Primates exposed to cocaine in utero display reduced density and number of cerebral cortical neurons. J Comp Neurol. 2001;435:263–275. doi: 10.1002/cne.1028. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Cytoarchitecture of the central amygdaloid nucleus of the rat. J Comp Neurol. 1982;208:401–418. doi: 10.1002/cne.902080409. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Calretinin immunoreactive neurons in the basolateral amygdala of the rat and monkey. Brain Res. 1994a;667:238–242. doi: 10.1016/0006-8993(94)91501-6. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Neuronal localization of glutamate receptor subunits in the basolateral amygdala. Neuroreport. 1994b;6:13–16. doi: 10.1097/00001756-199412300-00005. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Cortical pathways to the mammalian amygdala. Prog Neurobiol. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Immunohistochemical localization of the beta 2 and beta 3 subunits of the GABAA receptor in the basolateral amygdala of the rat and monkey. Neuroscience. 1996;75:407–419. doi: 10.1016/0306-4522(96)00269-2. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- Mohandas Rao KG, Muddanna Rao S, Gurumadhva Rao S. Enhancement of amygdaloid neuronal dendritic arborization by fresh leaf juice of Centella asiatica (Linn) during growth spurt period in rats. Evid Based Complement Alternat Med. 2009;6:203–210. doi: 10.1093/ecam/nem079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JA, Jordan CL, Breedlove SM. Sexual dimorphism in neuronal number of the posterodorsal medial amygdala is independent of circulating androgens and regional volume in adult rats. J Comp Neurol. 2008;506:851–859. doi: 10.1002/cne.21536. [DOI] [PubMed] [Google Scholar]
- O’Kusky J, Colonnier M. A laminar analysis of the number of neurons, glia, and synapses in the adult cortex (area 17) of adult macaque monkeys. J Comp Neurol. 1982;210:278–290. doi: 10.1002/cne.902100307. [DOI] [PubMed] [Google Scholar]
- Oberheim NA, Wang X, Goldman S, Nedergaard M. Astrocytic complexity distinguishes the human brain. Trends Neurosci. 2006;29:547–553. doi: 10.1016/j.tins.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Oberheim NA, Takano T, Han X, He W, Lin JH, Wang F, Xu Q, Wyatt JD, Pilcher W, Ojemann JG, Ransom BR, Goldman SA, Nedergaard M. Uniquely hominid features of adult human astrocytes. J Neurosci. 2009;29:3276–3287. doi: 10.1523/JNEUROSCI.4707-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda H, Tatsumi K, Makinodan M, Yamauchi T, Kishimoto T, Wanaka A. Environmental enrichment stimulates progenitor cell proliferation in the amygdala. J Neurosci Res. 2009;87:3546–3553. doi: 10.1002/jnr.22160. [DOI] [PubMed] [Google Scholar]
- Palackal T, Neuringer M, Sturman J. Laminar analysis of the number of neurons, astrocytes, oligodendrocytes and microglia in the visual cortex (area 17) of 6- and 12-month-old rhesus monkeys fed a human infant soy-protein formula with or without taurine supplementation from birth. Dev Neurosci. 1993;15:54–67. doi: 10.1159/000111317. [DOI] [PubMed] [Google Scholar]
- Pantazopoulos H, Murray EA, Berretta S. Total number, distribution, and phenotype of cells expressing chondroitin sulfate proteoglycans in the normal human amygdala. Brain Res. 2008;1207:84–95. doi: 10.1016/j.brainres.2008.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pêgo JM, Morgado P, Pinto LG, Cerqueira JJ, Almeida OF, Sousa N. Dissociation of the morphological correlates of stress-induced anxiety and fear. Eur J Neurosci. 2008;27:1503–1516. doi: 10.1111/j.1460-9568.2008.06112.x. [DOI] [PubMed] [Google Scholar]
- Pitkänen A. Connectivity of the rat amygdaloid complex. In: Aggleton J, editor. The amygdala: the functional analysis of the amygdala. New York: Wiley-Liss; 2000. pp. 31–115. [Google Scholar]
- Pitkänen A, Amaral DG. Organization of the intrinsic connections of the monkey amygdaloid complex: projections originating in the lateral nucleus. J Comp Neurol. 1998;398:431–458. doi: 10.1002/(sici)1096-9861(19980831)398:3<431::aid-cne9>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Kemppainen S. Comparison of the distribution of calcium-binding proteins and intrinsic connectivity in the lateral nucleus of the rat, monkey, and human amygdala. Pharmacol Biochem Behav. 2002;71:369–377. doi: 10.1016/s0091-3057(01)00690-6. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Stefanacci L, Farb CR, Go GG, LeDoux JE, Amaral DG. Intrinsic connections of the rat amygdaloid complex: projections originating in the lateral nucleus. J Comp Neurol. 1995;356:288–310. doi: 10.1002/cne.903560211. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci. 1997;20:517–523. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- Prather MD, Lavenex P, Mauldin-Jourdain ML, Mason WA, Capitanio JP, Mendoza SP, Amaral DG. Increased social fear and decreased fear of objects in monkeys with neonatal amygdala lesions. Neuroscience. 2001;106:653–658. doi: 10.1016/s0306-4522(01)00445-6. [DOI] [PubMed] [Google Scholar]
- Price JL. An autoradiographic study of complementary laminar patterns of termination of afferent fibers to the olfactory cortex. J Comp Neurol. 1973;150:87–108. doi: 10.1002/cne.901500105. [DOI] [PubMed] [Google Scholar]
- Price JL, Russchen FT, Amaral DG. The limbic region. II: The amygdaloid complex. In: Björklund A, Hökfelt T, Swanson LW, editors. Handbook of chemical neuroanatomy, vol. 5. Integrated systems of the CNS, Part I. Amsterdam: Elsevier; 1987. pp. 279–388. [Google Scholar]
- Pryce CR. Postnatal ontogeny of expression of the corticosteroid receptor genes in mammalian brains: inter-species and intra-species differences. Brain Res Rev. 2008;57:596–605. doi: 10.1016/j.brainresrev.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Rai KS, Murthy KD, Rao MS, Karanth KS. Altered dendritic arborization of amygdala neurons in young adult rats orally intubated with Clitorea ternatea aqueous root extract. Phytother Res. 2005;19:592–598. doi: 10.1002/ptr.1657. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Wright CI. Neuroimaging studies of amygdala function in anxiety disorders. Ann N Y Acad Sci. 2003;985:389–410. doi: 10.1111/j.1749-6632.2003.tb07096.x. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci. 2009;10:423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- Rubinow MJ, Juraska JM. Neuron and glia numbers in the basolateral nucleus of the amygdala from preweaning through old age in male and female rats: a stereological study. J Comp Neurol. 2008;512:717–725. doi: 10.1002/cne.21924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savander V, Go CG, LeDoux JE, Pitkanen A. Intrinsic connections of the rat amygdaloid complex: projections originating in the basal nucleus. J Comp Neurol. 1995;361:345–368. doi: 10.1002/cne.903610211. [DOI] [PubMed] [Google Scholar]
- Savander V, Go CG, Ledoux JE, Pitkanen A. Intrinsic connections of the rat amygdaloid complex: projections originating in the accessory basal nucleus. J Comp Neurol. 1996;374:291–313. doi: 10.1002/(SICI)1096-9861(19961014)374:2<291::AID-CNE10>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Schenker NM, Buxhoeveden DP, Blackmon WL, Amunts K, Zilles K, Semendeferi K. A comparative quantitative analysis of cytoarchitecture and minicolumnar organization in Broca’s area in humans and great apes. J Comp Neurol. 2008;510:117–128. doi: 10.1002/cne.21792. [DOI] [PubMed] [Google Scholar]
- Schumann CM, Amaral DG. Stereological estimation of the number of neurons in the human amygdaloid complex. J Comp Neurol. 2005;491:320–329. doi: 10.1002/cne.20704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann CM, Amaral DG. Stereological analysis of amygdala neuron number in autism. J Neurosci. 2006;26:7674–7679. doi: 10.1523/JNEUROSCI.1285-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scorcioni R, Lazarewicz MT, Ascoli GA. Quantitative morphometry of hippocampal pyramidal cells: differences between anatomical classes and reconstructing laboratories. J Comp Neurol. 2004;473:177–193. doi: 10.1002/cne.20067. [DOI] [PubMed] [Google Scholar]
- Semendeferi K, Armstrong E, Schleicher A, Zilles K, Van Hoesen GW. Limbic frontal cortex in hominoids: a comparative study of area 13. Am J Phys Anthropol. 1998;106:129–155. doi: 10.1002/(SICI)1096-8644(199806)106:2<129::AID-AJPA3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Semendeferi K, Armstrong E, Schleicher A, Zilles K, Van Hoesen GW. Prefrontal cortex in humans and apes: a comparative study of area 10. Am J Phys Anthropol. 2001;114:224–241. doi: 10.1002/1096-8644(200103)114:3<224::AID-AJPA1022>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Shabel SJ, Janak PH. Substantial similarity in amygdala neuronal activity during conditioned appetitive and aversive emotional arousal. Proc Natl Acad Sci U S A. 2009;106:15031–15036. doi: 10.1073/pnas.0905580106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood CC, Holloway RL, Gannon PJ, Semendeferi K, Erwin JM, Zilles K, Hof PR. Neuroanatomical basis of facial expression in monkeys, apes, and humans. Ann N Y Acad Sci. 2003;1000:99–103. doi: 10.1196/annals.1280.021. [DOI] [PubMed] [Google Scholar]
- Sherwood CC, Stimpson CD, Raghanti MA, Wildman DE, Uddin M, Grossman LI, Goodman M, Redmond JC, Bonar CJ, Erwin JM, Hof PR. Evolution of increased glia-neuron ratios in the human frontal cortex. Proc Natl Acad Sci U S A. 2006;103:13606–13611. doi: 10.1073/pnas.0605843103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdsson T, Doyere V, Cain CK, LeDoux JE. Long-term potentiation in the amygdala: a cellular mechanism of fear learning and memory. Neuropharmacology. 2007;52:215–227. doi: 10.1016/j.neuropharm.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Sorvari H, Soininen H, Pitkanen A. Calretinin-immunoreactive cells and fibers in the human amygdaloid complex. J Comp Neurol. 1996;369:188–208. doi: 10.1002/(SICI)1096-9861(19960527)369:2<188::AID-CNE2>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Stephan H, Andy OJ. Quantitative comparison of the amygdala in insectivores and primates. Acta Anat (Basel) 1977;98:130–153. doi: 10.1159/000144789. [DOI] [PubMed] [Google Scholar]
- Stephan H, Frahm HD, Baron G. Comparison of brain structure volumes in Insectivora and primates. VII. Amygdaloid components. J Hirnforsch. 1987;28:571–584. [PubMed] [Google Scholar]
- Super H, Uylings HB. The early differentiation of the neocortex: a hypothesis on neocortical evolution. Cereb Cortex. 2001;11:1101–1109. doi: 10.1093/cercor/11.12.1101. [DOI] [PubMed] [Google Scholar]
- Tuunanen J, Pitkanen A. Do seizures cause neuronal damage in rat amygdala kindling? Epilepsy Res. 2000;39:171–176. doi: 10.1016/s0920-1211(99)00123-0. [DOI] [PubMed] [Google Scholar]
- Vereecken TH, Vogels OJ, Nieuwenhuys R. Neuron loss and shrinkage in the amygdala in Alzheimer’s disease. Neurobiol Aging. 1994;15:45–54. doi: 10.1016/0197-4580(94)90143-0. [DOI] [PubMed] [Google Scholar]
- West MJ, Gundersen HJG. Unbiased stereological estimation of the number of neurons in the human hippocampus. J Comp Neurol. 1990;296:1–22. doi: 10.1002/cne.902960102. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Williams RW, Herrup K. The control of neuron number. Annu Rev Neurosci. 1988;11:423–453. doi: 10.1146/annurev.ne.11.030188.002231. [DOI] [PubMed] [Google Scholar]
- Winston JS, Strange BA, O’Doherty J, Dolan RJ. Automatic and intentional brain responses during evaluation of trustworthiness of faces. Nat Neurosci. 2002;5:277–283. doi: 10.1038/nn816. [DOI] [PubMed] [Google Scholar]
- Yukie M. Connections between the amygdala and auditory cortical areas in the macaque monkey. Neurosci Res. 2002;42:219–229. doi: 10.1016/s0168-0102(01)00325-x. [DOI] [PubMed] [Google Scholar]
- Zilles KH, Stephan H, Schleicher A. Quantitative cytoarchitectonics of the cerebral cortices of several prosimian species. In: Armstrong E, Falk D, editors. Primate brain evolution: methods and concepts. New York: Plenum Press; 1982. pp. 177–201. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.