Key Points
Conditional TR2/TR4 knockout leads to induction of murine embryonic globin genes.
Abstract
The orphan nuclear receptors TR2 and TR4 have been shown to play key roles in repressing the embryonic and fetal globin genes in erythroid cells. However, combined germline inactivation of Tr2 and Tr4 leads to periimplantation lethal demise in inbred mice. Hence, we have previously been unable to examine the consequences of their dual loss of function in adult definitive erythroid cells. To circumvent this issue, we generated conditional null mutants in both genes and performed gene inactivation in vitro in adult bone marrow cells. Compound Tr2/Tr4 loss of function led to induced expression of the embryonic εy and βh1 globins (murine counterparts of the human ε- and γ-globin genes). Additionally, TR2/TR4 function is required for terminal erythroid cell maturation. Loss of TR2/TR4 abolished their occupancy on the εy and βh1 gene promoters, and concurrently impaired co-occupancy by interacting corepressors. These data strongly support the hypothesis that the TR2/TR4 core complex is an adult stage-specific, gene-selective repressor of the embryonic globin genes. Detailed mechanistic understanding of the roles of TR2/TR4 and their cofactors in embryonic and fetal globin gene repression may ultimately enhance the discovery of novel therapeutic agents that can effectively inhibit their transcriptional activity and be safely applied to the treatment of β-globinopathies.
Introduction
High levels of fetal hemoglobin (HbF) in adult definitive hematopoietic cells have been demonstrated to be clinically beneficial for patients with sickle cell disease (SCD) and β-thalassemia1-3 because increased HbF levels can disrupt sickle hemoglobin polymerization4 or can compensate for the underproduction of β-globin chains in β-thalassemia,5 respectively. Thus, for the past few decades, concerted efforts have focused on identifying pharmacologic agents that can effectively induce HbF synthesis in adult erythroid cells of patients with β-globinopathies.
The human embryonic ε- and fetal γ-globin, but not the adult β-globin, genes have direct repeat (DR) elements in their promoters.6 These DR motifs, which are also conserved in the murine εy- and βh1-globin (homologs of the human ε- and γ-globin) gene promoters,7 are the consensus-binding sites for nonsteroidal nuclear receptors (NRs).8 Mutation of these DR sequences leads to the derepression of ε- and γ-globin transcription.9-12 Specifically, naturally occurring or genetically engineered mutations in the DR element of either the Gγ or Aγ promoter lead to hereditary persistence of fetal hemoglobin (HPFH), wherein only the cis-affected γ-globin gene continues to be expressed at high levels into adulthood.12-14 These observations suggested that the binding of an NR complex to these DR sites might play an important role in the stage-specific silencing of the ε- and γ-globin genes.
Among the 48 known human NRs,15 TR2/TR4 (NR2C1/NR2C2) and COUP-TFII (NR2F2) are both capable of binding in vitro to the DR elements in the ε- and γ-globin promoters.9,10 However, additional genetic evidence substantiating a role for other DR element-binding protein(s) in γ-globin silencing has not been forthcoming.
It was reported earlier that short hairpin RNA (shRNA)-mediated knockdown of COUP-TFII in cultured human CD34+ cells resulted in induction of γ-globin expression.16 Analysis of outbred, embryonic day (E) 14.5 embryos bearing targeted germline null mutations in Tr2 and Tr4, and a human globin locus YAC transgene, has demonstrated the role of both NRs in repressing the mouse (εy- and βh1-) and human (ε- and γ-) globin genes.7,17 However, compound null mutations in the Tr2 and Tr4 genes lead to partially penetrant embryonic demise prior to implantation in outbred mice, whereas in C57Bl/6J congenic animals, the penetrance is complete.7,18,19 Hence, the predicted roles for TR2 and TR4 as adult-stage repressors of the embryonic and fetal globin genes have not been readily testable by detailed genetic analysis in an inbred genetic background.
In this study, we quantitatively assessed the relative concentrations of all 48 known human NRs during erythroid terminal differentiation of CD34+ cells isolated from mobilized adult peripheral blood. The data show that TR2 and TR4 are among the most abundant NRs in definitive erythroid cells at all developmental stages, whereas other nuclear receptors that have been implicated as γ-globin repressors are not expressed.
We further assessed the roles of TR2 and TR4 in adult-staged globin gene regulation and in erythropoiesis by generating conditional (floxed) mutations in the Tr2 and Tr4 genes and then deleting both genes simultaneously in adult bone marrow (BM) cells that were induced to undergo terminal erythroid proliferation and differentiation.20 We found that compound depletion of TR2 and TR4 led to a substantial increase of εy- and βh1-globin transcripts (3.6- and 8.2-fold, respectively). Furthermore, the loss of TR2/TR4 resulted in blocking the differentiation, and impairing the maturation, of erythroid cells as anticipated from an earlier RNA sequencing (RNA-seq) analysis showing that the majority of genes affected by TR4 loss of function are intimately linked to vital cellular metabolic functions,21 indicating that TR2/TR4 are required for erythroid cell survival. Further detailed mechanistic analysis demonstrated that the depletion of both TR2 and TR4 in differentiating erythroid cells abolished their chromatin occupancy, as well as that of interacting corepressors, on the promoters of the εy- and βh1-globin genes.
In light of the protective effects of elevated HbF levels against the consequences of SCD, it becomes important to unravel the mechanisms underlying the regulatory repression of embryonic and fetal globin genes in adult erythroid cells. The present studies further substantiate the role of TR2/TR4 as an adult-staged repressor complex of the embryonic and fetal globin genes. Additional protein domain dissection studies of TR2/TR4 and their interacting corepressor partners could reveal drug-targetable features of this adult-staged repressor complex, which in turn could potentially lead to important therapeutic target development for disease management of β-globinopathies.
Materials and methods
Mice
We constructed Tr2 and Tr4 targeting constructs (inserted loxP sites into introns flanking the exon(s) encoding the DNA-binding domain) for homologous recombination in embryonic stem (ES) cells. Tr2 and Tr4 null or floxed mice were generated by intercrossing mice with the Tr2 or Tr4 targeted allele with Cre recombinase- or Flp recombinase-expressing transgenic mice. Homozygous Lsd1 floxed (Lsd1f/f) and Dnmt1 floxed (Dnmt1f/f) mice were previously described.22,23 All of the animal experiments were approved by the University Committee on Use and Care of Animals at the University of Michigan.
Cell culture and viral infection
Human CD34+ cells were cultured to induce erythroid lineage differentiation as previously described.16,24 Murine Lin− BM cells were cultured and induced to undergo terminal erythroid differentiation.20,25 After 1 day of culture, Lin− cells were infected with an adenovirus that expressed Cre recombinase (Ad-Cre). After 48 hours, the cells were reinfected. Infected cells were harvested at the indicated time points for flow cytometric, chromatin immunoprecipitation (ChIP), reverse transcription (RT)–quantitative polymerase chain reaction (qPCR), genomic DNA qPCR analyses.
Quantitative RT-PCR, genomic DNA qPCR, and western blotting analyses
Complementary DNAs (cDNAs) were reverse transcribed from total RNAs of cultured cells and SYBR Green-based qPCRs were performed on an ABI SteponePlus instrument.
Purified genomic DNAs from cultured cells were used to determine gene-specific deletion efficiency for each gene locus by 2 primer pairs designed to specifically amplify the genomic DNA sequences flanked by tandem loxP sites as well as an adjacent genomic DNA region.
Protein extracts from cultured and primary cells were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting with primary antibodies specific for β-actin (Millipore), COUP-TFII (Abcam), TR2 and TR4 as previously described.26 Positive immunoreactivity to TR4, COUP-TFII, and β-actin was detected using the appropriate species-specific IRDye secondary antibodies and quantified using the Odyssey Imaging System (Licor).
Flow cytometry
Lin− cells were stained with anti-mouse CD71 (BioLegend) and anti-mouse Ter119 (BioLegend) antibodies for 15 minutes on ice and were then washed twice with phosphate-buffered saline containing 0.5% bovine serum albumin. Analytical flow cytometry was carried out on a FACSCanto II instrument (BD).27
ChIP assay
Lin− cells isolated from mice of each desired genotype were harvested 4 days after the first adenovirus infection for ChIP assays, which were performed as previously described.28 Anti-lysine-specific demethylase 1 (LSD1), anti-DNA methyltransferase 1 (DNMT1), anti-corepressor for element-1-silencing transcription factor (CoREST), anti-transcription intermediary factor-1beta (TIF1β) antibodies, and control immunoglobulin G (IgG) were purchased from Abcam, whereas anti-TR2 and -TR4 antibodies were previously generated in our laboratory.7
Results
Expression of all human NRs during human erythroid differentiation of primary CD34+ cells
Historically, various members of the NR family, including NF-E3, COUP-TFII, and TR2/TR4, have been tentatively identified as the genuine γ-globin repressor because they can bind to the γ-globin DR motifs in vitro.9,10,16,29,30 However, no comprehensive NR expression profiling analysis of human erythroid cells as they differentiate from stem cells into erythrocytes has been reported. To possibly exclude at least some of these DR element-binding regulatory proteins as candidate γ-globin repressors, we documented the expression of all 48 known human NRs15 in early human progenitors differentiated along the erythroid pathway.
We recently reported RNA sequencing (RNA-seq) on primary human CD34+ cells induced to differentiate ex vivo using a serum-free culture condition that reportedly achieves 1000-fold cell proliferation and nearly synchronous erythroid differentiation.21,31 Our studies were performed using an Illumina platform starting with erythroid lineage cells harvested after 4, 8, 11, or 14 days of induced CD34+ cell differentiation.
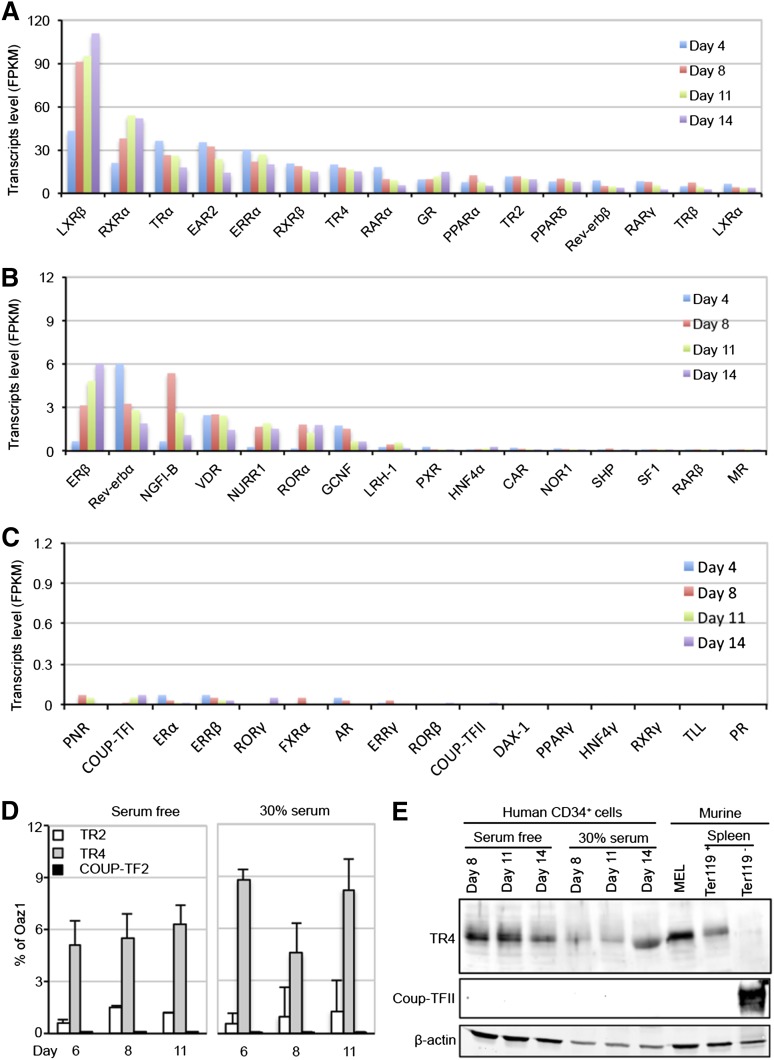
The expression profiles of the 48 known human NRs from differentiation days 4 to 14 are schematically summarized in Figure 1 and tabulated in supplemental Table 1 (see supplemental Data available on the Blood Web site). Of all NRs, Liver X receptor-β (LXRβ), Retinoid X receptor-α (RXRα), V-erbA-related receptor (EAR2), Estrogen-related receptor-α (ERRα), and Thyroid hormone receptor-α (TRα) messenger RNAs (mRNAs) were the most abundant NRs at almost all developmental stages in differentiating human erythroid cells (Figure 1A). TR2 and TR4 were expressed at relatively high and constant abundance throughout differentiation (Figure 1A); notably, COUP-TFII expression was undetectable (Figure 1C).
Figure 1.
Expression of NRs in differentiating human CD34+ cells. (A-C) RNA-seq analyses were performed on primary human CD34+ cells induced to differentiate ex vivo under serum-free culture conditions.21,31 The relative abundances (FKPM) of all 48 known NRs on differentiation days 4, 8, 11, and 14 are graphed in order of decreasing abundance. Note that the ordinate axis decreases by10-fold consecutively from the top panel down. (D-E) TR2/TR4, but not COUP-TFII, mRNAs and proteins were readily detected by qRT-PCR and western blotting assays in human CD34+ cells subjected to 2 different erythroid differentiation conditions.16,31 Similarly, TR4, but not COUP-TFII, proteins were detected in MELs and flow-sorted Ter119+ (erythroid) cells from adult mouse spleen. Abundant COUP-TFII protein, on the other hand, were detected in Ter119− (nonerythroid) adult mouse splenocytes. OAZ1 mRNA and anti-β-actin immunoreactivity were used as normalization controls in qRT-PCR and immunoblotting assays.
Recently, Aerbajinai et al reported that shRNA-mediated knockdown of COUP-TFII in human CD34+ cells cultured in media containing serum and growth factor additives resulted in induction of γ-globin expression.16 In an attempt to resolve the discrepancy between COUP-TFII expression in human erythroid cells differentiated from CD34+ cells in the reported studies and those presented here, we prepared cDNAs from erythroid cells differentiated under both culture conditions and reinvestigated the relative abundance of all 48 human NRs, this time by real-time qPCR (qRT-PCR). Regardless of the culture conditions, we routinely detected higher TR4 transcript levels than TR2, but both species remained relatively unchanged throughout the time course of differentiation (Figure 1D). In contrast, only an extremely low level of COUP-TFII expression, which was 5000- to 9000-fold lower than TR4 and not statistically different from background, was detected in erythroid cells grown under either tissue culture condition (Figure 1D).
Western blots further demonstrated that TR4 was expressed throughout erythroid development (Figure 1E). TR4 was also detected in murine erythroleukemia (MEL) cells, and in Ter119+ erythroid cells, but not in the Ter119− cell fraction, from adult mouse spleens (Figure 1E). In contrast, COUP-TFII was not detected in human or mouse erythroid cells, but was quite abundant in Ter119− (nonerythroid) splenocytes (Figure 1E). Hence, we were unable to provide evidence for COUP-TFII expression under any in vivo or in vitro culture condition from human or mouse adult erythroid cells, confirming previous microarray32,33 as well as RNA-seq studies.21 Based on the fact that TR2 and TR4 were the only NRs that were originally recovered from MEL cells using a proteomics purification and site-specific binding affinity strategy,28 available evidence continues to support the contention that TR2/TR4 is the adult-stage, DR element-binding repressor of embryonic and fetal β-type globin gene transcription.
Generation and analysis of Tr2 and Tr4 germline conditional mutant mice
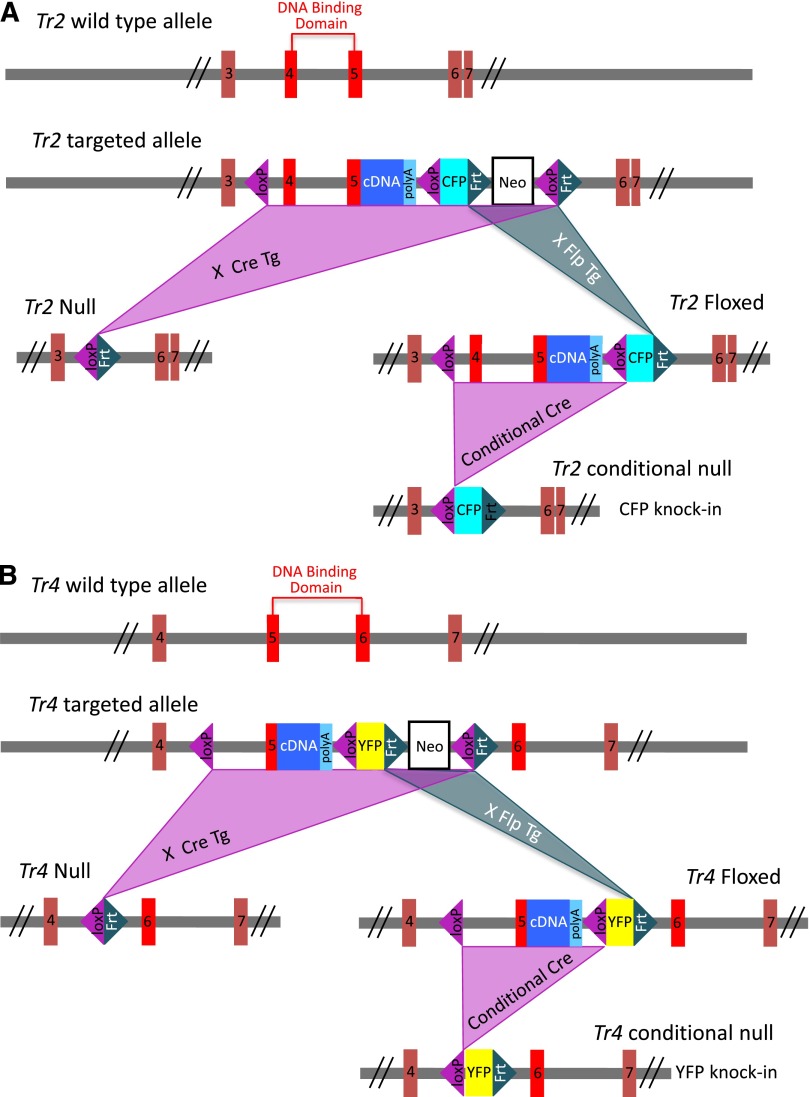
Previously, we and others observed that Tr2:Tr4 compound homozygous null embryos (Tr2−/−:Tr4−/−) died at various early gestational days according to strain background.7,18,19 To circumvent the inability to examine the effect of the compound mutations during erythropoiesis, we generated Tr2 and Tr4 conditionally mutant (Tr2 floxed and Tr4 floxed) null alleles by inserting loxP sequences at sites flanking exons 4 and 5, or exon 5, respectively, which fully or partially encode the DNA-binding domain of TR2 or TR4 (Figure 2). After conditional deletion, homozygous null Tr2 adult mutant animals (Tr2−/−) exhibited no overt phenotypes, whereas Tr4−/− mice displayed growth retardation and behavioral defects, in accord with those described for independently reported Tr2 and Tr4 constitutive null mutant alleles.34,35 Because erythroid differentiation and globin transcripts in the Tr2−/− and Tr4−/− mice were unaltered relative to wild type (supplemental Figure 1) and because the compound mutants die at a periimplantation stage, the data strongly suggest that the 2 proteins share largely overlapping, reciprocal functional compensation.
Figure 2.
Generation of Tr2 and Tr4 null and conditionally mutant mice. Schematic illustrations of the wild-type, targeted, null, floxed, and conditional null alleles of Tr2 (A) and Tr4 (B). The null and floxed alleles of Tr2 or Tr4 were generated by intercrossing the respective gene targeted mice with Cre- or Flp-expressing transgenic lines, respectively. To generate the Tr2 null allele, exons 4 and 5 encoding the DNA binding domain were excised, whereas the Tr4 null allele was deleted for exon 5 encoding part of the DNA binding domain. In both floxed alleles, a DNA fragment containing the rest of the coding exons for each gene and the SV40 polyadenylation sequence was fused in-frame to the respective exons 5. The Tr2 or Tr4 conditional null allele was generated by Cre-mediated excision of the respective floxed allele, leaving behind a fluorescent reporter gene (CFP or YFP) flanked by 1 loxP and 1 Frt site. CFP, cyan fluorescent protein; YFP, yellow fluorescent protein.
TR2/TR4 combined loss reactivates embryonic and fetal globin gene expression
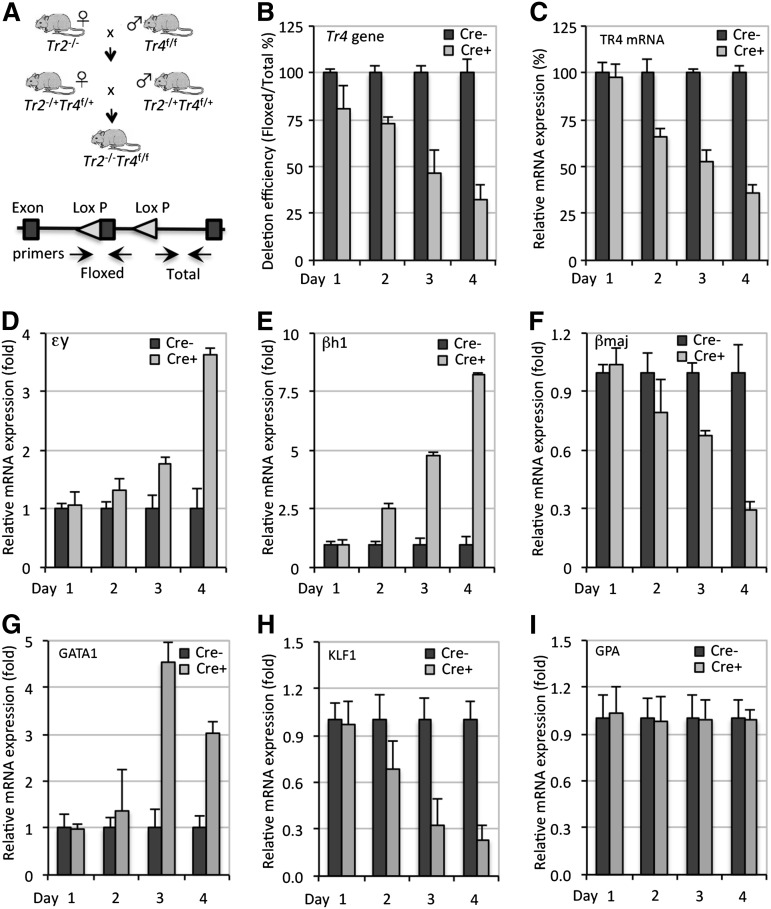
To investigate the effects of compound Tr2 and Tr4 loss of function on globin gene expression during adult erythropoiesis, Tr2−/− mice were interbred with Tr4 floxed (Tr4f/f) mice to finally generate Tr2−/−:Tr4f/f compound mutants (Figure 3A). We isolated lineage marker-negative (Lin−) cells (containing a mixture of hematopoietic and mesenchymal stem and progenitor cells) from the BM of Tr2−/−:Tr4f/f and control mice. Lin− BM cells were cultured in medium supplemented with erythropoietin (Epo) to support terminal erythroid differentiation.20 Cells were infected twice (on days 0 and 2) during the culture period with a Cre-expressing adenovirus (Ad-Cre), from which Cre was transcriptionally directed by the cytomegalovirus (CMV) promoter.36
Figure 3.
Combinatorial genetic loss of Tr2/Tr4 leads to elevated expression of the murine embryonic β-type globin genes. (A) Breeding strategy to generate conditional tandem loss of Tr2 and Tr4 function and schematic showing the location of the primers used in qPCR for detection of the floxed (unrecombined) and total DNA. (B-C) Time-course analyses of Cre-mediated Tr4f/f deletion and TR4 mRNA change in Tr2−/−:Tr4f/f Lin− BM cells, either mock-infected (Cre−) or infected with Ad-Cre (Cre+), on days 0 and 2 of culture. Tr4f/f genomic DNA and TR4 mRNA quantification were performed using SBYR Green-based qPCR. (D-I) Combined loss of TR2/TR4 leads to elevated embryonic β-type globin gene expression. Values of tested genes are first expressed relative to the expression of housekeeping gene Oaz1, and then relative to the transcript of interest in Cre− cells (arbitrarily set at 100% or onefold).
To assess the deletion efficiency at the Tr4 floxed locus, we performed qPCR using 2 primer pairs that specifically detected the unrecombined Tr4 floxed allele and an adjacent genomic region as a normalization control (Figure 3A). By 4 days postinfection, the recombination efficiency had reached ∼70% (Figure 3B). The reduction in TR4 mRNAs showed an initial 24-hour lag time, and was down to 25% by day 4 of culture (Figure 3C). Hence, we confirmed at the genomic DNA and mRNA levels that TR4 conditional inactivation was successful, albeit incomplete.
To ascertain the consequences, if any, of reducing TR4 activity in Tr2−/− erythroid cells on transcription of the β-type globin genes, we performed qRT-PCR using primer pairs that specifically detected embryonic εy-, βh1-, and adult βmajor-globin mRNAs. We observed a gradual initial increase of εy- and βh1-globin transcripts followed by a marked increase on day 4 of culture to 3.6- and 8.2-fold, respectively (Figure 3D-E). Unexpectedly, adult βmajor-globin mRNA decreased during the same culture period (Figure 3F). We found that the level of several important erythroid-specific globin regulatory genes, such as GATA137 and KLF1/EKLF,38 were significantly altered in expression upon TR2/TR4 loss (Figure 3G-H), whereas the erythroid cell surface marker glycophorin A (GPA) was unchanged (Figure 3I). Hence, the combined loss of both NRs in adult erythroid cells led to induced embryonic globin transcript levels, in continuing support of a role for TR2/TR4 as an adult-stage repressor of the embryonic globin genes.
Loss of TR2/TR4 impairs erythroid differentiation
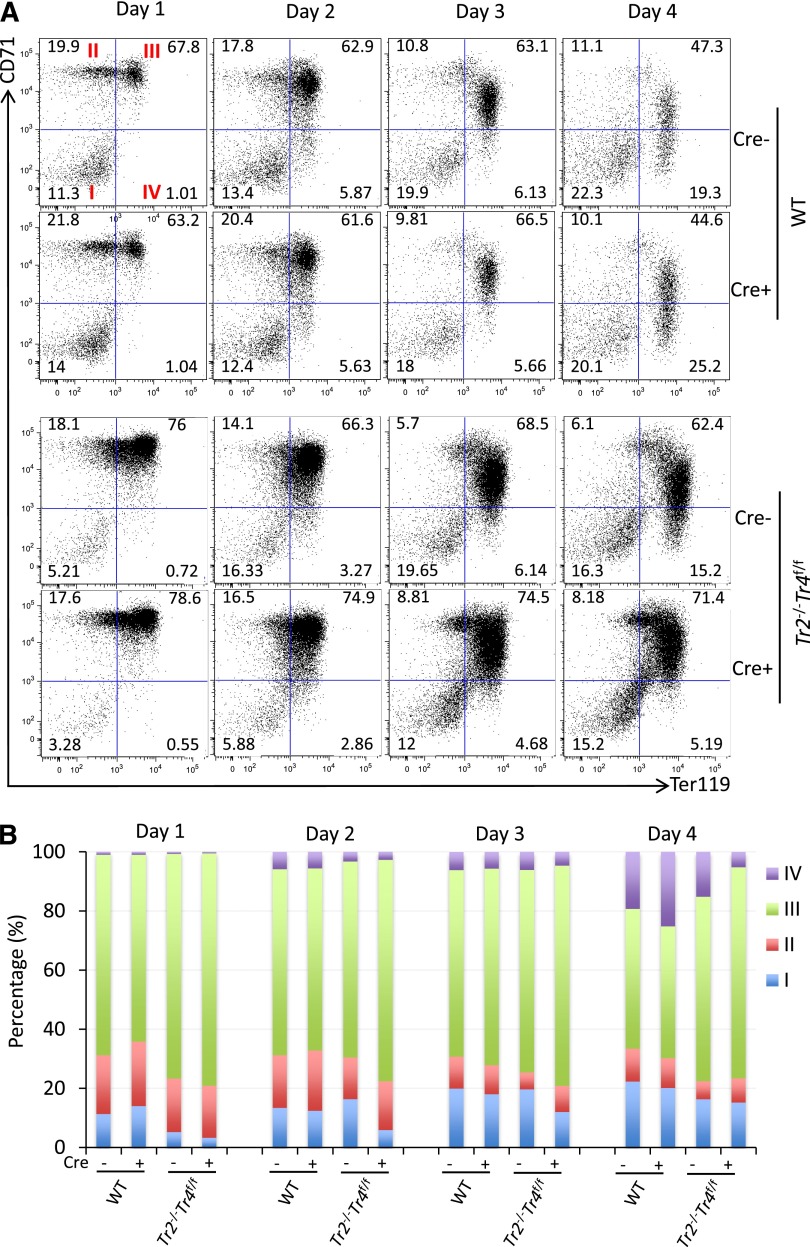
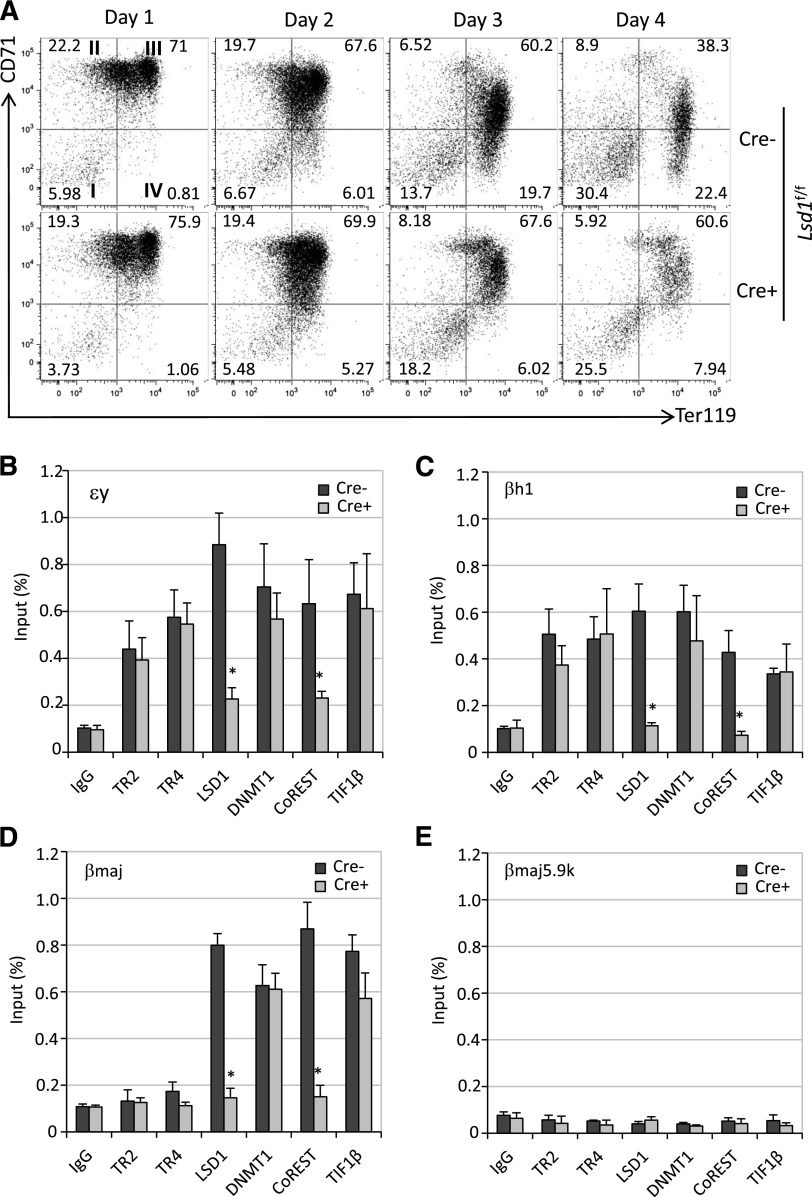
The adult βmajor-globin gene promoter does not contain a DR element.6 However, we observed declining βmajor-globin mRNA accumulation in Tr2−/−:Tr4f/f, but not in wild-type, Tr2−/− or Tr4−/−, cells infected with Ad-Cre virus (compare Figure 3F with supplemental Figure 1B). We therefore addressed whether the onset of TR4 depletion in Ad-Cre–infected Tr2−/−:Tr4f/f Lin− BM cells had any effect on erythroid differentiation per se. The combinatorial expression of the erythroid-specific cell surface marker Ter119 and the transferrin receptor CD71 antigens can be used to subdivide erythroid cells according to their maturational state.39 Differentiating cells were stained with anti-Ter119 and anti-CD71 antibodies prior to flow cytometric analyses. At day 4 postinfection, there were markedly fewer mature CD71−Ter119+ (quadrant IV) and more immature CD71+Ter119+ (quadrant III) erythroid cells in the Tr2−/−:Tr4f/f Lin− BM culture than in wild-type, Tr2−/−, or Tr4−/− Ad-Cre–infected BM cultures (Figure 4, supplemental Figure 1). Hence, these data indicate that TR2 and TR4 play compensatory roles during normal erythropoiesis, and that dual inactivation of both NRs rapidly leads to attenuation of terminal erythroid differentiation.
Figure 4.
Compound loss of TR2/TR4 impairs erythropoiesis. (A) WT and Tr2−/−:Tr4f/f Lin− BM cells were mock infected (Cre−) or infected with Ad-Cre (Cre+) on days 0 and 2 of culture. An aliquot of cells was stained daily with anti-CD71 and anti-Ter119 antibodies prior to flow cytometric analyses. As erythroid differentiation progressed, CD71+Ter119− immature erythroid cells (quadrant II) became CD71+Ter119+ (quadrant III) before becoming CD71−Ter119+ (quadrant IV). Numbers represent the fractional percentages of cells in each quadrant. Only Tr2−/−:Tr4f/f Lin− BM cells, which had been exposed to Ad-Cre infection, had far fewer circulation mature CD71−Ter119+ cells and an increased number of immature CD71+Ter119+ cells. (B) Data from erythroid cells at each maturational state (quadrants I-IV) from 4 to 6 mice of either genotype are summarized in the histogram. WT, wild type.
Loss of TR2/TR4 alters the occupancy of interacting cofactors on embryonic globin gene promoters
To gain possible mechanistic insights into how the loss of TR2 and TR4 activity influences β-type globin gene expression, we performed ChIP assays on Tr2−/−:Tr4f/f Lin− cells at 4 days after infection with the Ad-Cre virus. In Tr2−/−:Tr4f/f erythroid cells with no Ad-Cre infection (loss of TR2 activity alone), we detected substantial chromatin occupancy of TR4 and other DRED corepressor components (LSD1, DNMT1, CoREST, and TIF1β) on the εy- and βh1-globin gene promoters (Figure 5), which, like the corresponding human ε- and γ-globin genes, contain DR elements.7 The additional depletion of TR4 (upon Ad-Cre infection for 4 days) in Tr2−/−:Tr4f/f erythroid cells quantitatively abolished the binding of TR4, LSD1, DNMT1, CoREST, and TIF1β on the DR element-containing εy- and βh1-globin gene promoters (Figure 5A-B). In contrast, the occupancy of LSD1, DNMT1, CoREST, and TIF1β was only modestly diminished on the βmajor-globin promoter (Figure 5C). Because the βmajor-globin gene promoter does not bear a DR element,7 it was not surprising that we observed no TR2/TR4 binding. None of these factors bound to a negative control region (βmajor-5.9k) located 5.9 kbp 5′ to the βmajor-globin gene (Figure 5D). Thus, the binding of TR2/TR4 hetero- or TR4 homodimers is a prerequisite for the binding of other core repressor components to the εy- and βh1-globin promoters. In this way, by recruiting these interacting corepressors, TR2/TR4 elicits adult stage-specific silencing of the embryonic and fetal β-type globin genes.
Figure 5.
Genetic loss of Tr2/Tr4 abolishes the chromatin occupancy of TR2/TR4 and their cofactors at the DR-binding sites in the embryonic globin gene promoters. The binding of TR2/TR4 and other corepressors to select murine β-like globin gene promoters: εy (A), βh1 (B), and βmaj (C) as well as to an irrelevant control sequence located 5.9 kbp 5′ to the βmaj promoter (D) in infected Lin− cells were analyzed in ChIP experiments. Statistically significant enrichment of the orphan receptors and/or the cofactors at the promoters and control sequence is indicated in comparison with the control IgG values (indicated with asterisks; *P < .05).
Loss of LSD1 and DNMT1 alter erythropoiesis, globin transcription, and corepressor binding on globin gene promoters
Previously, we found that TR2/TR4 physically interacts with LSD1 and DNMT1 to form a core tetrameric repressor complex.28 This core complex then (presumably) assumes additional functions as it can independently associate with other coeffector proteins to form even larger complexes.28 To understand the role of each component within the core complex, we examined the effects of Lsd1 or Dnmt1 genetic loss of function on β-type globin gene transcription and erythropoiesis using the same experimental system. Lin− BM cells from mice homozygous for floxed Lsd1 (Lsd1f/f) or Dnmt1 (Dnmt1f/f) conditional mutant allele were infected with Ad-Cre virus.22,23
The conditional loss of LSD1 or DNMT1 function resulted in a decrease in the number of mature CD71−Ter119+ erythroid cells (quadrant IV) 4 days postinfection (Figures 6A and 7A), indicating that LSD1 and DNMT1 activities are required for normal terminal erythroid differentiation. Upon successful Cre-mediated ablation of Lsd1 in Lsd1f/f Lin− BM cells (supplemental Figure 2A-B), embryonic εy-globin mRNA expression was significantly induced (by about 4-old), whereas the adult βmajor-globin mRNA level was diminished (supplemental Figure 2C-D). Similarly, the loss of DNMT1 function (supplemental Figure 3A-B) led to elevated εy-globin expression (a modest 2-fold) accompanied by diminished adult βmajor-globin mRNA (supplemental Figure 3C-D). Interestingly, there was no effect on βh1-globin expression when either Lsd1 or Dnmt1 was conditionally inactivated (data not shown), suggesting that the residual LSD1 or DNMT1 mRNA (25%) is sufficient to maintain the repression of βh1-globin transcription in adult-stage erythroid cells.
Figure 6.
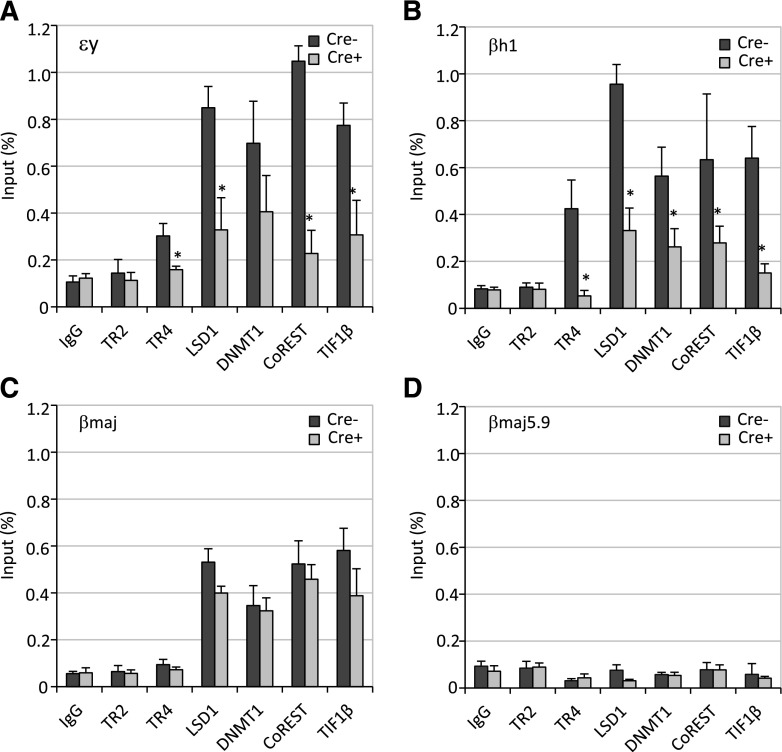
The effects of Lsd1 conditional inactivation on erythropoiesis and chromatin occupancy by TR2 and TR4 and corepressors. (A) Lsd1f/f Lin− BM cells were mock-infected (Cre−) or infected with Ad-Cre (Cre+) on days 0 and 2 of culture. An aliquot of cells was stained daily with anti-CD71 and anti-Ter119 antibodies prior to flow cytometric analyses. Conditional loss of Lsd1 in Lin− BM cells, which had been exposed to Ad-Cre infection, led to far fewer mature CD71−Ter119+ cells and an increased number of immature CD71+Ter119+ cells. (B-E) LSD1 inactivation obliterated its chromatin occupancy, as expected, and of its partner CoREST. The binding of TR2/TR4 and other corepressor components to the β-type globin gene promoters were either unchanged or quite modestly reduced in infected Lsd1f/f Lin− cells.
Figure 7.
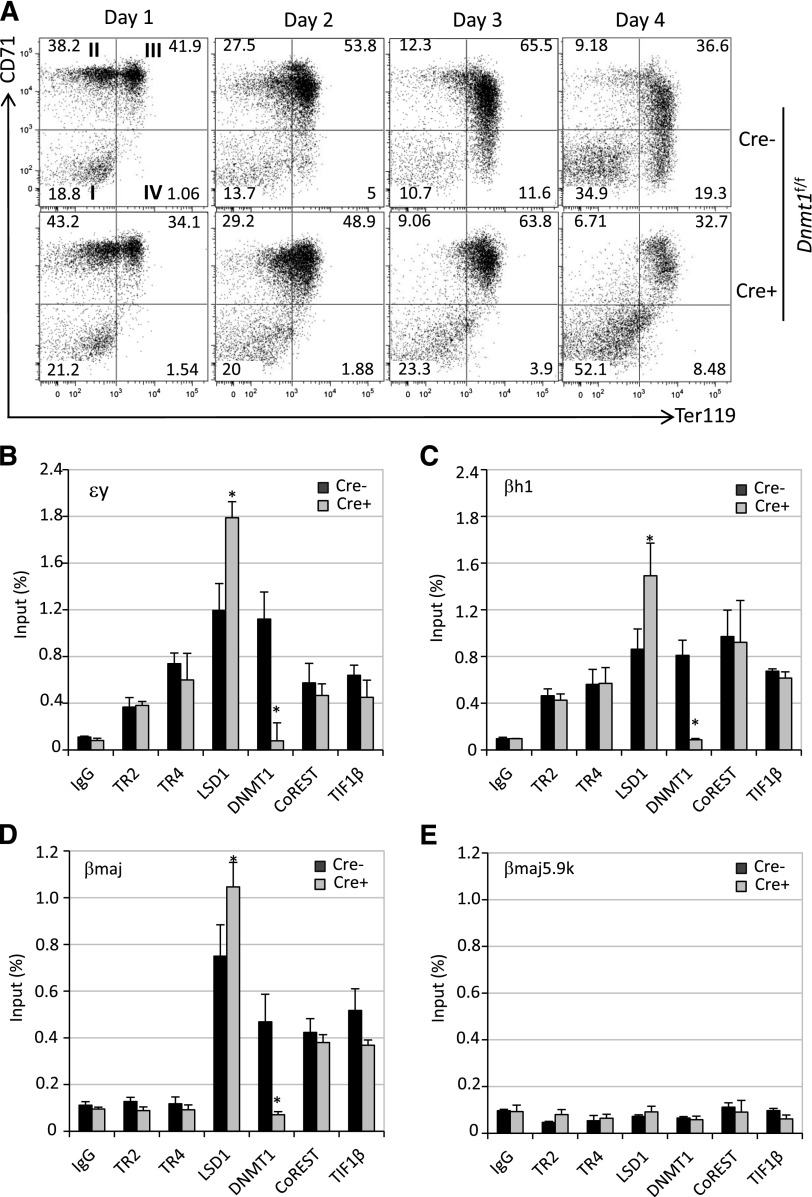
Conditional Dnmt1 ablation alters erythroid differentiation and cofactor chromatin occupancy in globin gene promoters. (A) Flow cytometry following the differentiation of Ad-Cre–infected Lin− cells from Dnmt1f/f mice (see legend to Figure 6A). (B-E) Loss of DNMT1 activity led to enhanced LSD1 chromatin occupancy as detected by ChIP assays in Cre-infected Dnmt1f/f Lin− cells.
We next determined whether LSD1 or DNMT1 is required for the recruitment of other cofactors onto the globin gene promoters by performing additional ChIP assays. The loss of LSD1 (upon Ad-Cre infection in Lsd1f/f erythroid cells) significantly impaired CoREST binding, with little or no effect on the binding of any other corepressor components to the embryonic globin gene promoters (Figure 6B-E). Interestingly, loss of DNMT1 (upon Ad-Cre infection in Dnmt1f/f erythroid cells) selectively and significantly enhanced the chromatin occupancy of LSD1 on the embryonic promoters while the binding of other cofactor components was unaffected (Figure 7B-E). It has been reported that inhibition or knockdown of DNMT1 resulted in demethylation of the globin gene promoters,40,41 which indicates that the binding of other components is not methylation-dependent. Based on these data, we postulate that first, CoREST recruitment is dependent on LSD1 binding and second, the binding of DNMT1 destabilizes LSD1 association, possibly by occluding physical contacts with proteins in the tetrameric core repressor at all 3 globin gene promoters (supplemental Figure 4).
Discussion
NRs COUP-TFII and TR2/TR4 have been shown to bind to the DR elements in the embryonic ε- and fetal γ-globin gene promoters in vitro9,10; this has been proposed to result in silencing of the embryonic ε- and fetal γ-globin genes.7,16,17,42 These discoveries generated significant interest because NRs have long been one of the most exploited categories of therapeutic targets for pharmaceutical development.43 Any DR element-binding protein might be a drug-targetable candidate by exploiting a specific regulatory pathway: inhibiting the repressor activity of NR repressors during adult erythropoiesis would be predicted to increase fetal-globin synthesis, and therefore may be effective in treating SCD.
To better understand the role of TR2/TR4 on globin gene regulation as well as to survey the expression of the other human NRs during adult erythropoiesis, we performed a comprehensive expression analysis of all 48 known NRs, including COUP-TFII and TR2/TR4, during human erythroid differentiation. Our published RNA-seq data indicate that TR2/TR4 is constitutively expressed throughout definitive erythroid differentiation,21 which is consistent with their hypothetical roles as a dimeric repressor that regulates fetal globin gene silencing at the adult stage. In contrast, we were not able to detect COUP-TFII expression at any time during definitive erythroid differentiation under any culture conditions (Figure 1). These data provide additional evidence that the majority of repressor activity acting through the DR element within the γ-globin gene promoter at the adult stage may be attributed to TR2/TR4 (and their corepressor partners).
We showed previously that TR2, TR4, DNMT1, and LSD1 comprise a tetrameric core DR element-binding repressor complex to which additional proteins (NuRD or CoREST complexes, HDAC3 or TIF1β) independently bind, in an apparently mutually exclusive manner, to form at least 4 distinct larger complexes.28 Studies in mice have shown that constitutive systemic loss of TR2/TR4, DNMT1, or LSD1 results in early embryonic lethality.7,18,19,22,44 TR2 and TR4 were differentially expressed when ES cells were induced into different specialized cell types, and TR2 and TR4 may play important roles in early embryonic development by regulating key genes (Oct-3/4 and Nanog) involved in stem cell self-renewal.19 Dnmt1 homozygous null mutant embryos do not survive past midgestation due to a significant reduction of DNA methylation.44 Genetic inactivation of Lsd1 causes early lethality at approximately E6.5. Lsd1 inactivation in ES cells induces a progressive loss of DNA methylation that correlates with diminished levels of DNMT1 as a result of reduced protein stability.45,46
To investigate the effects of manipulating each component of the core complex on embryonic and fetal globin gene silencing and erythroid cell differentiation during adult erythropoiesis, we used a gene deletion strategy using Ad-Cre to conditionally inactivate each gene encoding the core complex factors in an in vitro culture system, which supports terminal erythroblast proliferation and differentiation, and closely recapitulates erythroid development in vivo.20,25 Consistent with a role in silencing of embryonic and fetal globin genes at the adult stage, compound mutant inactivation of TR2 and TR4 in Lin− BM cells leads to activation of the embryonic εy- and βh1-globin genes and at the same time to diminished expression of βmajor (the latter likely as an indirect consequence of reduced expression of KLF138,47,48) (Figure 3, supplemental Figure 4). In addition, TR2/TR4 combined loss blocks erythroid differentiation and attenuates terminal erythroid maturation (Figure 4). Similarly, loss of LSD1 or DNMT1 also impairs erythroid maturation during adult erythropoiesis (Figures 6A and 7A), which is consistent with a prior report that partial inactivation of LSD1 by shRNAs impairs the ex vivo maturation of erythroid cells derived from human adult primary CD34+ progenitor cells.49 Interestingly, deletion of LSD1 or DNMT1 does not induce βh1-globin expression, which is robustly induced (to 8.2-fold) in the case of TR2/TR4 deficiency (Figure 4E). This suggests that depletion of LSD1 or DNMT1 alone cannot overcome the repressed state of the βh1-globin gene and, therefore, that additional corepressors are involved in repression of this gene at the adult stage.50 Alternatively, the residual LSD1 or DNMT1 mRNA (25%) may be sufficient to maintain the repression of βh1-globin transcription.
In the absence of both TR2 and TR4, the binding of LSD1 and DNMT1 as well as other corepressors (CoREST and TIF1β) is markedly decreased at the DR sites of the εy- and βh1-globin gene promoters (Figure 5), suggesting that the recruitment of these cofactors is highly dependent on the presence of the TR2/TR4. Furthermore, we observed that the recruitment of CoREST was critically dependent on the presence of LSD1 in the complex, and that loss of LSD1 significantly reduced CoREST binding to the embryonic εy-, βh1-, and adult βmajor-globin gene promoters (Figure 6). However, a reduction in the abundance of LSD1 only slightly altered the binding of TR4, DNMT1, and TIF1β on the same promoters. Surprisingly, the binding of LSD1 to the various promoters was significantly enhanced in the absence of DNMT1 (Figure 7). This may partially explain why we observe lower (2-fold) or no induction of the εy-globin and βh1-globin genes, respectively, in Dnmt1-depleted Lin− BM cells.
In summary, these data examining the behavior of conditional Tr2 and Tr4 mutants continue to support the hypothesis that TR2/TR4 (or perhaps a TR4 homodimer) leads to adult stage-specific silencing of embryonic (and by extension to the human locus, fetal) globin genes by recruiting multiple corepressors that act through coordinated epigenetic chromatin modifications to silence specific genic domains. These data also demonstrate that inhibition of the binding of at least a subset of these corepressors within the core complex leads to the reversal of embryonic globin gene silencing in definitive adult erythroid cells. Perhaps in addition, altering the activity of TR2/TR4 through mimetic ligand binding51 or posttranscriptional modifications52 might provide additional strategies as avenues to induce fetal globin transcription for the treatment of SCD.
Acknowledgments
The authors are grateful to their colleagues for many insightful comments on the manuscript.
This work was supported by an American Heart Association Scientist Development grant (S.C.), and by National Institutes of Health, National Heart, Lung and Blood Institute research grants R01HL24415 and U01HL117658 (J.D.E.).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: S.C. performed and analyzed experiments and wrote the paper; O.T. designed and performed experiments; M.S. performed and analyzed experiments; L.S. analyzed experiments, contributed vital reagents, and wrote the paper; A.C. wrote the paper; K.-C.L. analyzed experiments and wrote the paper; and J.D.E. designed and analyzed experiments and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: James Douglas Engel, Department of Cell and Developmental Biology, University of Michigan Medical School, 3035 BSRB, 109 Zina Pitcher Pl, Ann Arbor, MI 48109-2200; e-mail: engel@umich.edu.
References
- 1.Higgs DR, Engel JD, Stamatoyannopoulos G. Thalassaemia. Lancet. 2012;379(9813):373–383. doi: 10.1016/S0140-6736(11)60283-3. [DOI] [PubMed] [Google Scholar]
- 2.Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet. 2010;376(9757):2018–2031. doi: 10.1016/S0140-6736(10)61029-X. [DOI] [PubMed] [Google Scholar]
- 3.Serjeant GR. Natural history and determinants of clinical severity of sickle cell disease. Curr Opin Hematol. 1995;2(2):103–108. doi: 10.1097/00062752-199502020-00001. [DOI] [PubMed] [Google Scholar]
- 4.Sunshine HR, Hofrichter J, Eaton WA. Requirement for therapeutic inhibition of sickle haemoglobin gelation. Nature. 1978;275(5677):238–240. doi: 10.1038/275238a0. [DOI] [PubMed] [Google Scholar]
- 5.Stamatoyannopoulos JA, Nienhuis AW. Therapeutic approaches to hemoglobin switching in treatment of hemoglobinopathies. Annu Rev Med. 1992;43:497–521. doi: 10.1146/annurev.me.43.020192.002433. [DOI] [PubMed] [Google Scholar]
- 6.Myers RM, Tilly K, Maniatis T. Fine structure genetic analysis of a beta-globin promoter. Science. 1986;232(4750):613–618. doi: 10.1126/science.3457470. [DOI] [PubMed] [Google Scholar]
- 7.Tanabe O, McPhee D, Kobayashi S, et al. Embryonic and fetal beta-globin gene repression by the orphan nuclear receptors, TR2 and TR4. EMBO J. 2007;26(9):2295–2306. doi: 10.1038/sj.emboj.7601676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mangelsdorf DJ, Evans RM. The RXR heterodimers and orphan receptors. Cell. 1995;83(6):841–850. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- 9.Filipe A, Li Q, Deveaux S, et al. Regulation of embryonic/fetal globin genes by nuclear hormone receptors: a novel perspective on hemoglobin switching. EMBO J. 1999;18(3):687–697. doi: 10.1093/emboj/18.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanimoto K, Liu Q, Grosveld F, Bungert J, Engel JD. Context-dependent EKLF responsiveness defines the developmental specificity of the human epsilon-globin gene in erythroid cells of YAC transgenic mice. Genes Dev. 2000;14(21):2778–2794. doi: 10.1101/gad.822500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berry M, Grosveld F, Dillon N. A single point mutation is the cause of the Greek form of hereditary persistence of fetal haemoglobin. Nature. 1992;358(6386):499–502. doi: 10.1038/358499a0. [DOI] [PubMed] [Google Scholar]
- 12.Omori A, Tanabe O, Engel JD, Fukamizu A, Tanimoto K. Adult stage gamma-globin silencing is mediated by a promoter direct repeat element. Mol Cell Biol. 2005;25(9):3443–3451. doi: 10.1128/MCB.25.9.3443-3451.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gelinas R, Endlich B, Pfeiffer C, Yagi M, Stamatoyannopoulos G. G to A substitution in the distal CCAAT box of the A gamma-globin gene in Greek hereditary persistence of fetal haemoglobin. Nature. 1985;313(6000):323–325. doi: 10.1038/313323a0. [DOI] [PubMed] [Google Scholar]
- 14.Fucharoen S, Shimizu K, Fukumaki Y. A novel C-T transition within the distal CCAAT motif of the G gamma-globin gene in the Japanese HPFH: implication of factor binding in elevated fetal globin expression. Nucleic Acids Res. 1990;18(17):5245–5253. doi: 10.1093/nar/18.17.5245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Z, Burch PE, Cooney AJ, et al. Genomic analysis of the nuclear receptor family: new insights into structure, regulation, and evolution from the rat genome. Genome Res. 2004;14(4):580–590. doi: 10.1101/gr.2160004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aerbajinai W, Zhu J, Kumkhaek C, Chin K, Rodgers GP. SCF induces gamma-globin gene expression by regulating downstream transcription factor COUP-TFII. Blood. 2009;114(1):187–194. doi: 10.1182/blood-2008-07-170712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanabe O, Katsuoka F, Campbell AD, et al. An embryonic/fetal beta-type globin gene repressor contains a nuclear receptor TR2/TR4 heterodimer. EMBO J. 2002;21(13):3434–3442. doi: 10.1093/emboj/cdf340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanabe O, Shen Y, Liu Q, et al. The TR2 and TR4 orphan nuclear receptors repress Gata1 transcription. Genes Dev. 2007;21(21):2832–2844. doi: 10.1101/gad.1593307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shyr CR, Kang HY, Tsai MY, et al. Roles of testicular orphan nuclear receptors 2 and 4 in early embryonic development and embryonic stem cells. Endocrinology. 2009;150(5):2454–2462. doi: 10.1210/en.2008-1165. [DOI] [PubMed] [Google Scholar]
- 20.Shuga J, Zhang J, Samson LD, Lodish HF, Griffith LG. In vitro erythropoiesis from bone marrow-derived progenitors provides a physiological assay for toxic and mutagenic compounds. Proc Natl Acad Sci USA. 2007;104(21):8737–8742. doi: 10.1073/pnas.0701829104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi L, Lin YH, Sierant MC, et al. Developmental transcriptome analysis of human erythropoiesis. Hum Mol Genet. 2014;23(17):4528–4542. doi: 10.1093/hmg/ddu167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J, Scully K, Zhu X, et al. Opposing LSD1 complexes function in developmental gene activation and repression programmes. Nature. 2007;446(7138):882–887. doi: 10.1038/nature05671. [DOI] [PubMed] [Google Scholar]
- 23.Jackson-Grusby L, Beard C, Possemato R, et al. Loss of genomic methylation causes p53-dependent apoptosis and epigenetic deregulation. Nat Genet. 2001;27(1):31–39. doi: 10.1038/83730. [DOI] [PubMed] [Google Scholar]
- 24.Shi L, Cui S, Engel JD, Tanabe O. Lysine-specific demethylase 1 is a therapeutic target for fetal hemoglobin induction. Nat Med. 2013;19(3):291–294. doi: 10.1038/nm.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang J, Socolovsky M, Gross AW, Lodish HF. Role of Ras signaling in erythroid differentiation of mouse fetal liver cells: functional analysis by a flow cytometry-based novel culture system. Blood. 2003;102(12):3938–3946. doi: 10.1182/blood-2003-05-1479. [DOI] [PubMed] [Google Scholar]
- 26.Cui S, Tanabe O, Lim KC, et al. PGC-1 coactivator activity is required for murine erythropoiesis. Mol Cell Biol. 2014;34(11):1956–1965. doi: 10.1128/MCB.00247-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campbell AD, Cui S, Shi L, et al. Forced TR2/TR4 expression in sickle cell disease mice confers enhanced fetal hemoglobin synthesis and alleviated disease phenotypes. Proc Natl Acad Sci USA. 2011;108(46):18808–18813. doi: 10.1073/pnas.1104964108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cui S, Kolodziej KE, Obara N, et al. Nuclear receptors TR2 and TR4 recruit multiple epigenetic transcriptional corepressors that associate specifically with the embryonic β-type globin promoters in differentiated adult erythroid cells. Mol Cell Biol. 2011;31(16):3298–3311. doi: 10.1128/MCB.05310-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mantovani R, Malgaretti N, Nicolis S, et al. An erythroid specific nuclear factor binding to the proximal CACCC box of the beta-globin gene promoter. Nucleic Acids Res. 1988;16(10):4299–4313. doi: 10.1093/nar/16.10.4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mantovani R, Malgaretti N, Nicolis S, Ronchi A, Giglioni B, Ottolenghi S. The effects of HPFH mutations in the human gamma-globin promoter on binding of ubiquitous and erythroid specific nuclear factors. Nucleic Acids Res. 1988;16(16):7783–7797. doi: 10.1093/nar/16.16.7783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giarratana MC, Kobari L, Lapillonne H, et al. Ex vivo generation of fully mature human red blood cells from hematopoietic stem cells. Nat Biotechnol. 2005;23(1):69–74. doi: 10.1038/nbt1047. [DOI] [PubMed] [Google Scholar]
- 32.An X, Schulz VP, Li J, et al. Global transcriptome analyses of human and murine terminal erythroid differentiation. Blood. 2014;123(22):3466–3477. doi: 10.1182/blood-2014-01-548305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sripichai O, Kiefer CM, Bhanu NV, et al. Cytokine-mediated increases in fetal hemoglobin are associated with globin gene histone modification and transcription factor reprogramming. Blood. 2009;114(11):2299–2306. doi: 10.1182/blood-2009-05-219386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shyr CR, Collins LL, Mu XM, Platt KA, Chang C. Spermatogenesis and testis development are normal in mice lacking testicular orphan nuclear receptor 2. Mol Cell Biol. 2002;22(13):4661–4666. doi: 10.1128/MCB.22.13.4661-4666.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Collins LL, Lee YF, Heinlein CA, et al. Growth retardation and abnormal maternal behavior in mice lacking testicular orphan nuclear receptor 4. Proc Natl Acad Sci USA. 2004;101(42):15058–15063. doi: 10.1073/pnas.0405700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee JH, Yi SM, Anderson ME, et al. Propagation of infectious human papillomavirus type 16 by using an adenovirus and Cre/LoxP mechanism. Proc Natl Acad Sci USA. 2004;101(7):2094–2099. doi: 10.1073/pnas.0308615100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohneda K, Yamamoto M. Roles of hematopoietic transcription factors GATA-1 and GATA-2 in the development of red blood cell lineage. Acta Haematol. 2002;108(4):237–245. doi: 10.1159/000065660. [DOI] [PubMed] [Google Scholar]
- 38.Siatecka M, Bieker JJ. The multifunctional role of EKLF/KLF1 during erythropoiesis. Blood. 2011;118(8):2044–2054. doi: 10.1182/blood-2011-03-331371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Socolovsky M, Nam H, Fleming MD, Haase VH, Brugnara C, Lodish HF. Ineffective erythropoiesis in Stat5a(-/-)5b(-/-) mice due to decreased survival of early erythroblasts. Blood. 2001;98(12):3261–3273. doi: 10.1182/blood.v98.12.3261. [DOI] [PubMed] [Google Scholar]
- 40.Mabaera R, Greene MR, Richardson CA, Conine SJ, Kozul CD, Lowrey CH. Neither DNA hypomethylation nor changes in the kinetics of erythroid differentiation explain 5-azacytidine’s ability to induce human fetal hemoglobin. Blood. 2008;111(1):411–420. doi: 10.1182/blood-2007-06-093948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saunthararajah Y, Lavelle D, DeSimone J. DNA hypo-methylating agents and sickle cell disease. Br J Haematol. 2004;126(5):629–636. doi: 10.1111/j.1365-2141.2004.05064.x. [DOI] [PubMed] [Google Scholar]
- 42.Liberati C, Cera MR, Secco P, et al. Cooperation and competition between the binding of COUP-TFII and NF-Y on human epsilon- and gamma-globin gene promoters. J Biol Chem. 2001;276(45):41700–41709. doi: 10.1074/jbc.M102987200. [DOI] [PubMed] [Google Scholar]
- 43.Overington JP, Al-Lazikani B, Hopkins AL. How many drug targets are there? Nat Rev Drug Discov. 2006;5(12):993–996. doi: 10.1038/nrd2199. [DOI] [PubMed] [Google Scholar]
- 44.Li E, Bestor TH, Jaenisch R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell. 1992;69(6):915–926. doi: 10.1016/0092-8674(92)90611-f. [DOI] [PubMed] [Google Scholar]
- 45.Foster CT, Dovey OM, Lezina L, et al. Lysine-specific demethylase 1 regulates the embryonic transcriptome and CoREST stability. Mol Cell Biol. 2010;30(20):4851–4863. doi: 10.1128/MCB.00521-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang J, Hevi S, Kurash JK, et al. The lysine demethylase LSD1 (KDM1) is required for maintenance of global DNA methylation. Nat Genet. 2009;41(1):125–129. doi: 10.1038/ng.268. [DOI] [PubMed] [Google Scholar]
- 47.Wijgerde M, Gribnau J, Trimborn T, et al. The role of EKLF in human beta-globin gene competition. Genes Dev. 1996;10(22):2894–2902. doi: 10.1101/gad.10.22.2894. [DOI] [PubMed] [Google Scholar]
- 48.Borg J, Papadopoulos P, Georgitsi M, et al. Haploinsufficiency for the erythroid transcription factor KLF1 causes hereditary persistence of fetal hemoglobin. Nat Genet. 2010;42(9):801–805. doi: 10.1038/ng.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu J, Bauer DE, Kerenyi MA, et al. Corepressor-dependent silencing of fetal hemoglobin expression by BCL11A. Proc Natl Acad Sci USA. 2013;110(16):6518–6523. doi: 10.1073/pnas.1303976110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suzuki M, Yamamoto M, Engel JD. Fetal globin gene repressors as drug targets for molecular therapies to treat the β-globinopathies. Mol Cell Biol. 2014;34(19):3560–3569. doi: 10.1128/MCB.00714-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou XE, Suino-Powell KM, Xu Y, et al. The orphan nuclear receptor TR4 is a vitamin A-activated nuclear receptor. J Biol Chem. 2011;286(4):2877–2885. doi: 10.1074/jbc.M110.168740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huq MDM, Gupta P, Tsai NP, Wei LN. Modulation of testicular receptor 4 activity by mitogen-activated protein kinase-mediated phosphorylation. Mol Cell Proteomics. 2006;5(11):2072–2082. doi: 10.1074/mcp.M600180-MCP200. [DOI] [PubMed] [Google Scholar]