Abstract
Objective
To study the clinical and histopathological presentation of ovarian masses.
Method
Retrospective analysis of 205 cases from May 2009 to June 2013.
Results
Incidence of ovarian masses was 6.9 %. Among 205 cases, 68 % were neoplastic. Among the neoplasms, 87.8 % were benign, 10 % malignant, and 2.2 % borderline. Mean ages of malignant and benign neoplasm were 41 and 39 years, respectively. 42.9 % malignant tumors presented with non-specific abdominal and constitutional symptoms. Serous cystadenoma was the commonest benign tumor (67 %) followed by Mucinous (19 %) and Dermoid (11.6 %). Most common malignant ovarian tumor was Serous cystadenocarcinoma (42.9 %). Out of the malignant cases, all were primary except one secondary deposit from Non-Hodgkin’s Lymphoma. Only 28.6 % presented at stage I, remaining presented at stage III/IV.
Conclusion
Ovarian neoplasms have twice the incidence of non-neoplasms. Mean age of malignant tumors is decreased. Rising trend in Mucinous cystadenoma is noted.
Keywords: Ovarian masses, Changing trends, Mucinous cystadenoma
Introduction
Ovarian tumors account for 30 % of all cancers of female genital tract [1]. The total number of Ovarian cancer cases worldwide has been estimated to be 1, 92,000 per year in 2000 [2]. Ovarian malignancy ranks fifth in cancer deaths among worldwide and third among the female genital tract malignancy in India with age-adjusted standardized ratio of 6.7/100,000 [1]. In India, during the period 2004–2005, proportion of Ovarian cancer varied from 1.7 to 8.7 % of all female cancers in various urban and rural population-based registries [3]. The 5-year relative survival rate is around 25.4 % for ovarian malignancy [4]. Higher survival is noted in patients younger than 35 years and with localized early-stage disease. Ovarian cancer origin is still a subject of debate and research. The hypothesis of incessant ovulation as the reason for Ovarian cancer is being questioned by the recent molecular genetic studies which show that the fimbrial end of the fallopian tube is the original site of origin rather than ovary per se. Anatomical location of the ovary and its complex histology is responsible for the late presentation of the Ovarian cancer and its management difficulties. Considering the age-related risk, a high index of suspicion is needed, and biochemical and radiological assistance should always be implemented for earlier diagnosis and thereby to reduce the burden of morbidity and mortality. Understanding the origin of ovarian cancer and the specific histological types is of prime importance in diagnosing as well as offering the specific treatment. So this study has been done to note the changing trends in clinical presentation and histopathological pattern of ovarian masses in our hospital over 4 years.
Methodology
This is a retrospective analysis of 205 patients with ovarian masses from May 2009 to June 2013 at ESIC Medical College and PGIMSR, Chennai, Tamilnadu, South India. Patients with ovarian masses, who were surgically managed, were included in the study. Patients with non-ovarian masses and those who were conservatively managed were excluded. Details regarding the patient such as age, age at menarche, post-menopausal status, obstetric history, presenting symptoms, and surgery details were noted. Histopathological examinations of the excised specimens were conducted by the Pathology department of our hospital by appropriate stains (Hematoxylin and Eosin). Immunohistochemistry was performed as per the need. All patients with benign ovarian masses were followed up till discharge. In case of malignancy, the stage of the disease and treatment details were noted, and patients were followed up to 1 year. The collected data were analysed by descriptive statistics and pearson Chi-square test using SPSS software.
Results
Out of 2940 admissions in the gynecology ward, Incidence of ovarian masses was 6.9 % (n = 205) and that of ovarian neoplasms was 4.7 %. Among the ovarian masses, neoplasms accounted for 68 % (140/205) and non-neoplastic lesions 32 % (65/205). Out of the neoplastic lesions, 87.8 % (123/140) were benign, 10 % malignant (14/140), and three had borderline lesion. Mean age for non-neoplastic, benign, and malignant masses were 35, 39, and 41 years, respectively.
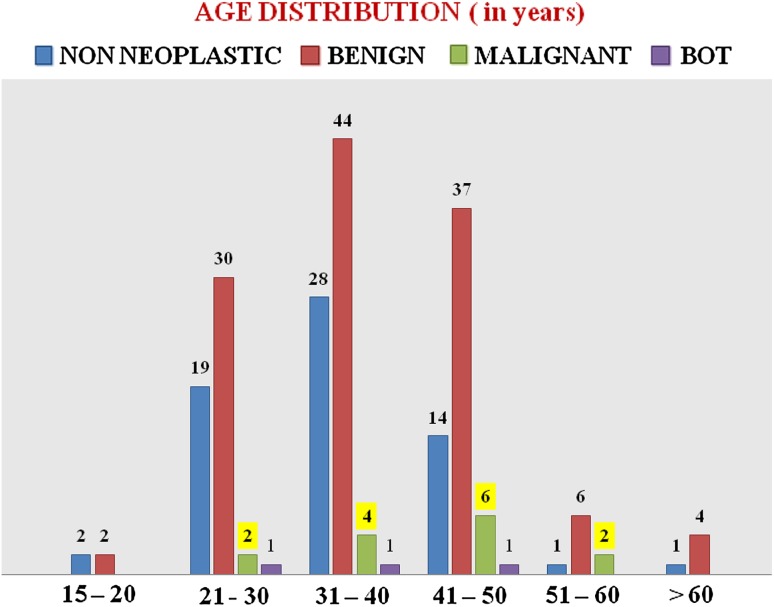
Maximum number of benign cases (44/123) was noted in the 31–40 years age group, and the malignant cases were more common (10/14) in the 31–50 years age group. The distribution of ovarian masses in different age groups is shown in Chart 1. Demographic details, Parity, and history of sterilization of the study group are shown in Table 1. Age at menarche, parity, sterilization, and post-menopausal status were not significantly related with ovarian neoplasms. History of Ovulation induction was present in 12 cases, and all were benign. History of Carcinoma Breast was present in one patient who presented at the age of 38 years with Ovarian Mucinous cystadenocarcinoma.
Chart 1.
Distribution of ovarian masses in various age groups
Table 1.
Demographic and obstetric profile among ovarian masses
| Parameters | Non-neoplastic | Benign | Malignant | Bot |
|---|---|---|---|---|
| Age at menarche in years | ||||
| 11–12 | 31 | 51 | 9 | 2 |
| 13–14 | 26 | 63 | 5 | 1 |
| 15–16 | 8 | 9 | NIL | NIL |
| Age group | ||||
| Reproductive | 60 | 107 | 10 | 2 |
| Post-menopausal | 5 | 16 | 4 | 1 |
| Sterilization | ||||
| Yes | 49 | 98 | 11 | 3 |
| No | 13 | 12 | 1 | NIL |
| Parity | ||||
| Nulliparous | 3 | 3 | NIL | NIL |
| 1, 2 | 49 | 75 | 6 | 1 |
| >3 | 10 | 32 | 5 | 2 |
“p” = ns
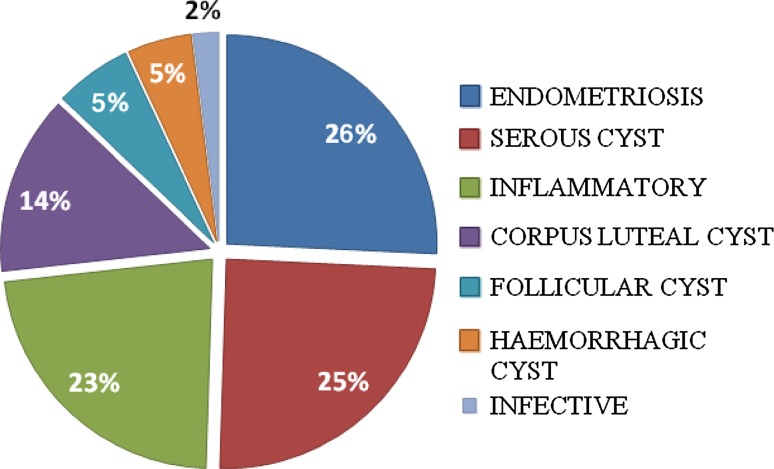
Among the Non-neoplastic masses, the commonest was Endometriotic cyst (26 %) followed by Serous cyst (25 %). Histopathological pattern of non-neoplastic ovarian masses is shown in Chart 2.
Chart 2.
Histopathological pattern of non-neoplastic ovarian masses
Clinical presentation of ovarian masses is shown in Table 2. Abdominal pain followed by abnormal menstrual pattern was the predominant presenting symptom in patients with both non-neoplastic and benign ovarian masses. On the contrary, malignant group predominantly presented with vague abdominal and constitutional symptoms (42.9 %) and with pain abdomen only in 35.7 %.
Table 2.
Clinical presentation of ovarian masses
| Symptoms | Non-neoplastic (in %) | Benign (in %) | Malignant (in %) |
|---|---|---|---|
| Pain abdomen | 66.1 | 61.8 | 35.7 |
| Mass abdomen | NIL | 10.5 | 7.2 |
| Abdominal symptoms | NIL | 1.6 | 28.6 |
| Menstrual symptoms | 30.8 | 18.7 | 7.1 |
| Urinary symptoms | 3.1 | 4.1 | NIL |
| Constitutional | NIL | NIL | 14.3 |
| Asymptomatic | NIL | 3.3 | 7.1 |
“p” value .001
Serous cystadenoma (67 %) followed by Mucinous cystadenoma (19 %) and Benign cystic teratoma (11.6 %) were the commonest benign ovarian tumors. The rest were two cases of Brenner tumor and one mixed tumor, Mucinous cystadenoma with Brenner. Of the three Borderline Ovarian Tumors (BOT), two were Serous and one was Mucinous subtype. Of the 14 malignant ovarian tumors, 10 were Surface epithelial tumors, two were Germ cell tumors, Sex Cord Stromal Tumor and secondary deposit from Non-Hodgkin’s Lymphoma accounted for one each. Among the surface epithelial tumors, Serous cystadenocarcinoma was the most common (42.9 %) followed by Mucinous cystadenocarcinoma (28.6 %).
Out of the malignant tumors, only 28.6 % presented at stage I and majority (71.4 %) at stage III/IV. All the Germ cell tumors were diagnosed at stage I, and all the Surface epithelial tumors were diagnosed only in stage III and IV. Of the 14 patients with malignancy, those presented in stage I were managed with curative surgery, and of the nine patients with stage III/IV, seven underwent Neoadjuvant chemotherapy followed by cytoreductive surgery and two were given palliative chemotherapy. At the end of 1-year follow-up, six patients are on follow-up chemotherapy, eight patients died.
Discussion
In the present study, incidence of ovarian masses is 6.9 % and that of ovarian neoplasms is 4.7 %. Ovarian neoplasms have almost twice the incidence of non-neoplastic lesions. Mean age of malignant tumors is 41 years which is much lower than the studies done by Mondal et al. [5] and Wasim et al. [6] who reported the mean age as 48 and 49.5 years, respectively. The significant reduction in the mean age of malignancy and its proximity with the mean age of benign ovarian tumors (39 years) emphasizes the need for ruling out malignancy in all the age groups. The mean age of malignancy and incidence of benign and malignant ovarian masses of our study is compared with other studies in Table 3. Maximum numbers of ovarian neoplasms were found in the 21–40 years age group which is comparable with Ameena et al. [7] study. Results from European studies show that the Age-specific incidence rates rise sharply from around 40–44 years, peaking among women in their 70s and 80s. The number of cases is highest among women in their 60s and 70s, accounting for almost half the diagnosis [8]. Thus, the increasing trend of ovarian neoplasms in younger age group in our population is noted.
Table 3.
Comparison of mean age of malignancy and incidence of ovarian neoplasms
| Parameters | Our study | Mondal et al. [5] | Ameena et al. [7] | Pilli et al. [12] | Jha et al. [13] |
|---|---|---|---|---|---|
| Period of study | May 2009–June 2013 | Jan 2001–Dec 2010 | Jan 2007–Dec 2010 | 2002 | Apr 2004–Mar 2006 |
| Number of cases | 205 | 957 | 212 | 282 | 164 |
| Place of study | Chennai Tamilnadu | Calcutta | Lahore | Belgaum | Nepal |
| Mean age malignancy (years) | 41 | 48 | – | – | – |
| Benign (%) | 87.8 | 63 | 64.5 | 75.2 | 83.9 |
| Malignant (%) | 10 | 29.6 | 35.4 | 21.9 | 16.1 |
Relation between sterilization and ovarian neoplasms is not statistically significant in our study. Previously, it was hypothesized that tubal ligation is a significant protective factor in ovarian malignancies [9]. But the latest research [10] has made clear that tubal ligation protects only against Clear cell and Endometrioid ovarian cancers as they migrate from the endometrium, whereas the Serous cancers arise from the fimbrial end of the fallopian tube, and Mucinous and Brenner tumors originate from Walthard cell nests which are not protected by tubal ligation. As there are no cases of Clear cell and Endometrioid cancers in our study, exact correlation between tubal ligation and specific histological pattern Ovarian cancer is not possible.
Nulliparity is not a statistically significant risk factor for ovarian neoplasms in our study because of small number. Patients with history of ovulation induction have only benign ovarian tumors in our group. However, results of metaanalysis [11] show a stronger association between fertility drugs usage and Borderline Ovarian Tumors.
Among the ovarian neoplasms, 87.8 % were benign and 10 % were malignant. These findings are comparable with Pilli et al. [12] and Jha et al. [13] study. Pain in abdomen is the commonest presenting symptom both in non-neoplastic and benign ovarian masses, whereas vague abdominal symptoms and constitutional symptoms were predominant in the malignant group. Asymptomatic presentation is more common in malignancy (7.1 %) compared to the benign neoplasms (3.3 %).
Out of benign tumors, Serous cystadenoma accounted for 67 %, Mucinous cystadenoma 19 %, and Mature cystic teratoma 11.6 %. This is comparable with Manivasakan et al. [14] study but differs from Mondal et al. [5], Pilli et al. [12], and Jha et al. [13] study. Histopathological pattern of ovarian masses in our study is compared with other studies in Table 4. Ethnic differences are common among ovarian tumors; in Thanikasalam et al. [12] study, it is shown that Teratomas are the commonest benign ovarian tumors among Malays and Chinese, whereas Serous tumors ranks first among Indians. Now, a rising trend in the Mucinous cystadenomas is noted in our study and South Indian Manivasakan et al. [14] study. This trend is significant as various molecular and histological evidences suggest that Mucinous epithelial ovarian cancers develop via a sequence from benign through borderline tumor to invasive cancer which suggests the potential preventability of borderline and invasive Mucinous Ovarian cancer by surgical excision of identifiable precursor lesions. This mucinous adenoma carcinoma sequence embarks a significant change in the treatment modality.
Table 4.
Comparison of the histopathological pattern of ovarian masses
Of the malignant tumors, Serous cystadenocarcinoma accounted for 42.9 % followed by 28.6 % Mucinous cystadenocarcinoma which is similar to the Jha et al. [13] study. Deposits of Non-Hodgkin’s Lymphoma are noted in 1 patient who presented with orbital lymphoma and enlarged ovary and was staged as IV. Ovaries are not only the site of primary tumors but also the metastatic site for gastrointestinal, breast, and hematological malignancies. Asymptomatic nature and vague constitutional complaints of the malignant ovarian tumors are responsible for their late presentation and thereby its mortality and morbidity. All the Surface epithelial ovarian cancers presented at stage III/IV.
The limitation of the study is that it is a single-center retrospective study. Multicentric studies with large numbers are needed to validate our results.
Conclusion
Ovarian neoplasms are almost double the number of non-neoplastic lesions. Significant reduction of mean age of malignancy and occurrence of maximum number of neoplasms in 21–40 years of age group emphasizes our priority to rule out malignancy in all age groups. Non-specific abdominal symptoms should be given more importance as it may be the only clue to the underlying malignancy. Rising trend in Mucinous cystadenomas in our population is a subject of research and further studies.
Acknowledgement
The authors express their sincere thanks to Dr. Srikumari Damodaram, Dean, ESIC Medical College And PGIMSR, Dr. V. Rajalakshmi, Dr. K. Meenakshi sundaram, Dr. Sathish Selvakumar, Department of Pathology and Dr. P. N. Chandrika, Junior Resident for their immense help in doing this study.
Compliance with ethical requirements and Conflict of interest
SO. Sharadha, T. A. Sridevi, T. K. Renukadevi, R. Gowri, Binayak Debbarman and V. Indra declare that they have no conflict of interest. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. Informed consent was obtained from all the patients for all diagnostic and therapeutic procedures. This article does not contain any studies with animal subjects.
Dr. SO. Sharadha
MBBS, DGO, DNB (OBG), is aged about 31 years. She graduated from Coimbatore Medical College in the year of 2005, Tamil Nadu Dr. MGR Medical University, Tamil Nadu. She completed DGO from Madurai Medical College in 2009, Tamil Nadu Dr. MGR Medical University, Tamil Nadu. She completed DNB OBG from CSI Kalyani Multispeciality Hospital in 2012, Chennai, Tamil Nadu. She has won the following awards and prizes: Best Outgoing Student Medal in MBBS and University Gold Medalist and A.L. Mudaliar Award in DGO. She is currently working as a Senior Resident in ESIC Medical College and PGIMSR, Chennai, Tamil Nadu. She is interested in Gynec-oncology and Endo-gynec surgeries
Footnotes
Ovarian neoplasms are in the rising trend, and ovarian malignancy ranks third among female genital tract malignancies. This is a retrospective analysis of ovarian masses over 4 years to note the changing trends in the clinical and histopathological presentation.
References
- 1.Uma Devi K. Current status of gynecological cancer care in India. J Gynecol Oncol. 2009;20(2):77–80. doi: 10.3802/jgo.2009.20.2.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Murthy NS, Shalini S, Suman G, et al. Changing trends in incidence of ovarian cancer- the Indian Scenario. Asian Pac J Cancer Prev. 2009;10(6):1025–1030. [PubMed] [Google Scholar]
- 4.Yeole BB, Kumar AV, Kurkure A, et al. Population-based survival from cancers of breast, cervix and ovary in women in Mumbai, India. Asian Pac J Cancer Prev. 2004;5(3):308–315. [PubMed] [Google Scholar]
- 5.Mondal SK, Banyopadhayay R, Nag DR, et al. Histologic pattern, bilaterality and clinical evaluation of 957 ovarian neoplasms: a 10 year study in a tertiary hospital of eastern India. J Cancer Res Ther. 2011;7(4):433–437. doi: 10.4103/0973-1482.92011. [DOI] [PubMed] [Google Scholar]
- 6.Wasim T, Majrroh A, Siddiq S. Comparison of clinical presentation of benign and malignant ovarian tumors. J Pak Med Assoc. 2009;59:1. [PubMed] [Google Scholar]
- 7.Ashraf A, Shaikh S, Ishfaq A, et al. The relative frequency and histopathological pattern of ovarian masses. Biomedica. 2013;Vol. 28:98–102. [Google Scholar]
- 8.Poole J, Nordin A. Trent cancer registry. Profile of ovarian cancer in England, 2012.
- 9.Rosenblatt KA, Thomas DB. Reduced risk of ovarian cancer in women with a tubal ligation or hysterectomy. The World Health Organization Collaborative Study of Neoplasia and Steroid Contraceptives. Cancer Epidemiol Biomark Prev. 1996;5:933–935. [PubMed] [Google Scholar]
- 10.Dutta DK, Dutta I. Origin of ovarian cancer: molecular profiling. J Obstet Gynecol India. 2013;63(3):152–157. doi: 10.1007/s13224-013-0419-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahdavi A, Pejovic T, Nezhat F. Induction of ovulation and ovarian cancer: a critical review of literature. Fertil Steril. 2006;Vol. 85:4. doi: 10.1016/j.fertnstert.2005.08.061. [DOI] [PubMed] [Google Scholar]
- 12.Narula R, Arya A. Overview of benign and malignant tumors of female genital tract. J Appl Pharm Sci. 2013;3(01):140–149. [Google Scholar]
- 13.Jha R, Karki S. Histological pattern of ovarian tumors and their age distribution. Nepal Med Coll J. 2008;10:81–85. [PubMed] [Google Scholar]
- 14.Manivasakan J, et al. A study of benign adnexal masses. Int J Reprod Contracept Obstet Gynaecol. 2012;1(1):12–16. doi: 10.5455/2320-1770.ijrcog000812. [DOI] [Google Scholar]