Abstract
Endometriosis is a common condition affecting a significant proportion of women in their reproductive age. Apart from the impact of endometriosis on the quality of life of these patients, it also can have an impact on the potential of these women to have a family. The options for treating women with endometriosis desiring a family include surgery or assisted reproduction techniques. The choice of treatment will depend on the stage of disease and the characteristics of the couple seeking help. We review here the latest evidence on the management of endometriosis in women desiring fertility and describe our current practice.
Keywords: Endometriosis, Fertility, Laparoscopy, IVF
Introduction
Endometriosis is a common finding in couples with infertility. Women diagnosed with endometriosis have one third of the monthly fecundity rate of the general population ranging between 2 and 10 % per month [1]. Even within a population of subfertile women, endometriosis may reduce the chance of spontaneous conception further compared to women with unexplained infertility [2]. Despite this, the optimal treatment strategy in couples with endometriosis desiring fertility remains a subject of controversy. We will review here the evidence supporting various management options in these couples and outline our current practice.
Impact of Endometriosis on Fertility
A hallmark of endometriosis is inflammation and subsequent formation of adhesions in the pelvis. These distort the pelvic anatomy which in turn impacts the female partner’s fertility in a variety of ways. The most direct consequence of adhesions can be disturbance of the anatomical relationship between the ovaries and fallopian tubes. Tubal blockage and formation of hydrosalpinges are usually seen in more advanced stages of endometriosis and these not only cause infertility but may also require excision before assisted conception. The anatomical impact of endometriosis is not the only mechanism through which endometriosis affects fertility. Indeed, minimal or mild endometriosis, where there is little anatomical impact, may still reduce the chance of spontaneous conception. It has been shown that endometriosis alters the composition of the peritoneal fluid deregulating a number of immunologic factors and increasing the level of reactive oxygen species. These changes may alter folliculogenesis and lead to poorer quality oocytes with reduced fertilization rates and poorer embryonic development and implantation [1]. Peritoneal fluid from women with endometriosis may also affect tubal ciliary activity which in combination with anatomical distortion combine to reduce pickup of the ovum [3]. Sperm motility and function is also affected, thus reducing further the chance of fertilization [3, 4]. Even if the oocyte is successfully picked up by the Fallopian tube and fertilized, women with endometriosis have functional alterations in eutopic endometrium which may cause progesterone resistance and reduce implantation [3].
The management strategy of endometriosis in couples desiring fertility should take into account all these mechanisms to achieve optimal results.
Diagnosis of Endometriosis
Workup
Any couple presenting with infertility should have a satisfactory workup before treatment options can be discussed. Past diagnosis of endometriosis and surgical treatment details are particularly important in planning further management. Special consideration should be given to women who report significant dysmenorrhea and dyspareunia, and those who have signs (see below) of endometriosis on pelvic ultrasound [5]. Infertile women have on average a 30 % chance of endometriosis, rising to 50 % if dyspareunia is present. Currently, the gold standard for diagnosis of endometriosis is laparoscopy which should include a dye test if fertility is a consideration. However, this is an invasive procedure with some risks and may not be appropriate for all couples. Ultrasound can reliably diagnose endometriosis especially in the presence of endometriotic cysts [6]. In addition to endometriomas, expert operators can accurately map endometriosis including rectovaginal nodules and bowel nodules. Coupled with an assessment of pelvic organ mobility, it is now possible to reliably diagnose advanced endometriosis on ultrasound [7, 8]. This information can be used to reduce the need for diagnostic laparoscopy and accelerate appropriate treatment for patients. A reliable ultrasound examination can also accelerate scheduling for surgery before assisted conception in cases of hydrosalpinges. Given that an ultrasound examination also establishes ovarian reserve through antral follicle count and the presence/location of uterine fibroids, it becomes clear that this is a pivotal examination to guide subsequent treatment. It therefore should be performed early in the diagnostic process by adequately trained operators. To complete their evaluation, women should also have a complete ovarian reserve test including day 2–5 follicle-stimulating hormone (FSH) and antimullerian hormone (AMH). Before any treatment decisions’ can be undertaken, male factor investigations should also be completed.
Treatment Options
Treatment options for couples center around two questions:
Is surgical treatment of endometriosis advisable to improve fertility?
Should the couple try to conceive spontaneously longer or seek assisted conception?
The most appropriate treatment for each couple will depend on a number of parameters including female partner’s age, duration of infertility, ovarian reserve, sperm analysis, history of past surgery for endometriosis as well as endometriosis severity. Defining the location and severity of endometriosis will help clinicians to estimate the probability of spontaneous conception and appropriately counsel couples. For this purpose, formal classification systems have been developed.
Classification Systems
The most frequently used classification system for endometriosis is the revised American Fertility Society (rAFS) or American Society of Reproductive Medicine (rASRM) score. This system has serious limitations as it is not predictive of pregnancy rates after treatment [9]. Despite this, based on findings at the time of surgery, clinicians usually guide patients to appropriate treatment. For example, if the Fallopian tubes appear clubbed or phimotic despite being patent to dye, clinicians are more likely to refer patients for IVF than advise expectant management after surgery. Similarly, older patients, over 35 years of age, are more likely to be referred for assisted conception to avoid loss of ovarian reserve. Recently, a more formal approach to predicting spontaneous pregnancy after endometriosis surgery has been made with the endometriosis fertility index (EFI) [10]. Based on this, surgeons assess the reproductive function of each adnexa (Fallopian tube, fimbria, and ovary) intraoperatively and assign a score. Further demographic, clinical, and surgical information is then added to calculate a composite score (EFI). This ranges between 0 and 10, with 0 representing the poorest prognosis. The EFI has been externally validated and found to correlate well with the probability of spontaneous (non-ART) conception. For example, women with an EFI < 4 have <20 % chance of spontaneous conception after 1 year of trying, while those with EFI 7–8 have a 40–45 % chance [11]. Even though age is part of the EFI, clinicians should keep ovarian reserve in mind when recommending expectant management. In practice, patients over 37 with endometriosis are more likely to be referred for IVF sooner irrespective of EFI score.
Treatment of Endometriosis in Couples Desiring Fertility
Endometriosis is a highly variable disease ranging from minimal superficial lesions to severe disease involving the bowel and bladder. Surgeons treating endometriosis to improve pain symptoms endeavor to completely remove or destroy visible endometriotic lesions. Depending on disease severity, such surgery can be highly complex and requires significant expertise. From a perspective of fertility, attempting complete surgical removal of endometriosis must be balanced by the inherent surgical risks and the impact of surgery on ovarian reserve. So, in the context women with endometriosis desiring fertility, case selection for endometriosis surgery is critical, and the surgical approach has to be individually tailored.
Stage I and II Endometriosis–Normal Reserve/Sperm Analysis
Good evidence from a meta-analysis suggests that in couples with 12–24 months of infertility with a female partner under 39 years, normal ovulatory cycles, and normal sperm analysis, surgical treatment of stage I or stage II endometriosis improves the chance of spontaneous conception over the year following surgery [11]. The treatment effect is not large and to achieve one additional live birth 12 patients with minimal or mild endometriosis need to be treated. The absolute difference in favor of therapy is 8.6 % [12]. It was previously thought that in women with endometriosis, suppressing ovulation for a period of time with any number of agents (e.g., danazol, medroxyprogesterone acetate, gestrinone, and combined oral contraceptive pills) may improve the chance of conception immediately after cessation of treatment through a reduction in pelvic inflammation. A meta-analysis of all published RCTs concluded that this approach does not improve the chance of spontaneous conception and should not be used as it is associated with considerable side effects [13].
Stage III and IV Endometriosis
Moderate and severe endometriosis presents a more challenging clinical scenario. In couples desiring conception, expectant management is not a viable treatment option as the overall spontaneous pregnancy rate has been reported to vary between 0 and 33 % [5]. In couples with no other infertility factors, surgical treatment of stage III and stage IV endometriosis by expert surgeons, including excision of endometriotic cysts and excision or coagulation of endometriotic peritoneal implants, can restore the chance of spontaneous conception within 3 years after surgery to over 50 % [9]. As expected, age was a significant predictor of successful conception in this study, which must be borne in mind when counseling a couple.
Ovarian Endometriomas
No RCT exists to answer the question whether removing endometriotic ovarian cysts compared to expectant management improves spontaneous pregnancy rates. Several uncontrolled observational studies have reported pregnancy rate of 30–67 % 1 year after surgery. A pooled average spontaneous pregnancy rate of 50 % has been calculated but taking into account confounding factors and publication bias, an absolute benefit increase over background pregnancy rate of 25 % has been suggested [12].
The surgical approach to treating endometriomas also remains controversial. At least some ovarian endometriomas are thought to result from invagination of the ovarian cortex and do not have a true capsule. Inevitably, surgical excision will result in removal of some healthy ovary. Indeed, it has been shown that the mean cyst wall thickness of endometriomas is 1.4 ± 0.6 mm and that when ovarian endometriomas are excised, identifiable healthy ovarian tissue will be present in over 80 % of specimens [14]. For this reason, vaporization or coagulation the cyst’s inner surface has been suggested as preferred treatment. Despite these concerns, a meta-analysis of existing RCTs comparing excision vs. ablation found that the former is preferable both for spontaneous conception and recurrence rates [15]. Nevertheless, ovaries containing an endometrioma that have been subjected to a cystectomy display reduced response after COH. This may be due to the disease itself rather than surgery as a recent meta-analysis confirmed that surgery did not impact the antral follicle count (AFC) of an affected ovary; affected ovaries, however, had lower AFC than contralateral healthy ovaries both pre- and post-surgery [16]. In any case, it would seem prudent that a surgeon planning to perform excision for an ovarian endometrioma in a woman hoping to conceive counsels her regarding the risks of reduced ovarian function after surgery especially in cases of bilateral cysts [5].
Deep Endometriosis
Treatment of endometriosis affecting the rectovaginal septum or bowel requires complex surgery with considerable risk of complications. Limited information exists on the impact of this type on endometriosis on reproductive performance. Theoretically, because the endometriosis is located deep in the pelvis and may be compartmentalized or “pseudoretroperitonealised” [12], its effect on the peritoneal environment may be small. Reports in the literature are generally confounded by concomitant treatment of endometriosis in other locations such as endometriotic cysts and so the effect of treating rectovaginal disease on spontaneous fertility is not well defined [12]. In a non-randomized study comparing the reproductive performance of women with rectovaginal disease who underwent surgery vs. those managed expectantly for 24 months, cumulative pregnancy rate was similar in the two groups (44.9 vs. 46.8 %) [17], arguing against attempting surgery for rectovaginal disease in these patients simply to improve chance of spontaneous conception. However, a review of uncontrolled published reports on surgical treatment of deep endometriosis with colorectal involvement did show postoperative pregnancy rates of 23–57 % [18]. Again a balance must be struck between exposing the patient to surgical risk and improvement in pain and fertility potential.
Controversy surrounds the optimal treatment of bowel endometriosis with some surgeons proposing bowel resection and anastomosis and others shaving of endometriotic lesions. The treatment option adopted does not appear to impact chance of conception after surgery. In a study comparing pregnancy rate after treatment of moderate/severe endometriosis, women with and without bowel resection had similar pregnancy rates (including spontaneous and assisted) of approximately 50 % after 1 year of follow-up [19].
While treatment of deep endometriosis provides benefits in terms of pain relief, it appears that in patients desiring fertility, current evidence does not point to a substantial benefit in their chance of spontaneous conception from surgical treatment of rectovaginal disease. The available literature, however, is limited and it is conceivable that alleviating dyspareunia in a younger woman may have a positive effect on her chance to conceive spontaneously.
Endometriosis and Medically Assisted Reproduction
Superovulation and Intrauterine Insemination (SO+IUI)
A randomized controlled trial of women with mild or minimal endometriosis showed that superovulation with gonadotrophins and intrauterine insemination has an odds ratio of 5.6 (95 % CI 1.8–17.4) for live birth over expectant management [5]. It has also been shown that treating mild or moderate endometriosis surgically restores the chance of success with SO + IUI to the same level as for couples with unexplained infertility [20]. It must be borne in mind, however, that the overall effectiveness of SO + IUI for couples with unexplained infertility has been brought into question [21].
In our practice, young couples with a normal sperm analysis in whom the female partner is found to have stage I or II endometriosis are offered surgical reduction of the disease followed by 3–6 cycles of SO + IUI. This approach would appear to give the highest chance of live birth without resorting to IVF.
In vitro Fertilization
In principle, IVF should preempt the deleterious effects of endometriosis as it removes the oocyte–sperm interaction from the peritoneal cavity and is not dependent on Fallopian tube function. However, controversy exists as to whether surgical treatment of endometriosis prior to IVF improves the chance of success. In a disease so diverse as endometriosis, treating individual components of the disease may have differential impact.
A systematic review and meta-analysis of observational studies showed that women who had an endometrioma previously treated surgically or aspirated will have significantly less oocytes retrieved after ovarian stimulation. The clinical impact of this effect appears to be marginal as the same study showed a similar pregnancy rate after IVF between these patients and women with tubal factor fertility [22]. More recently, a study of 99 infertile couples randomized women to surgical excision of endometriomas prior to IVF–ICSI or immediate IVF–ICSI with the endometrioma aspirated at the time of oocyte collection. The study found that the pregnancy rate was similar between the groups [23].These results argue against excision of an endometrioma in women scheduled for IVF as surgery does not appear to improve the chance of pregnancy. Nevertheless, all endometriomas are not the same, and it appears that cysts over 3 cm have a more severe impact on ovarian performance during COH [24]. Moreover, such large endometriomas may restrict vaginal access to the ovary during retrieval. So, while ovarian cystectomy for an endometrioma should not be routine before IVF, it may be appropriate in selected cases of larger cysts [3, 22]. In patients with history of previous surgery for endometriomas, or with reduced ovarian reserve, drainage of endometriomas prior to IVF may be an alternative to cystectomy to improve accessibility of ovarian follicles.
Recently, a retrospective study of 661 women with stage I and II endometriosis who underwent IVF or IVF/ICSI was published [25]. This showed that treatment of minimal to mild endometriosis with surgical destruction of endometriotic lesions improved the chance of conception and live birth/cycle in women who had their endometriosis surgically treated before IVF (27.6 vs. 20.6 %). However, this was a study over several years (1995–2009) with the diagnostic laparoscopy patients being more frequent in the earlier years of the study. It is possible that improvements in IVF technology may account for the difference observed rather than an effect of surgery.
Adjuvant Treatments
A meta-analysis of three RCTs comparing prolonged pituitary down regulation (GnRHa for 3–6 months) vs. standard treatment in women with endometriosis (all stages) showed a significant improvement in favor of prolonged treatment both in terms of clinical pregnancy (OR 4.28, 95 % CI 2.0–9.2) and live birth (9.2, 95 % CI 1.1–79.2). The mechanism of this effect could be either endometrial or directly on the oocytes by reducing inflammation in the pelvic environment [3]. It is important to note that in these studies, women who did not receive GnRHa had an IVF cycle immediately after surgery. It is possible therefore that the effect observed is partly due to a longer time period from surgery to IVF in the treatment group.
Conclusion
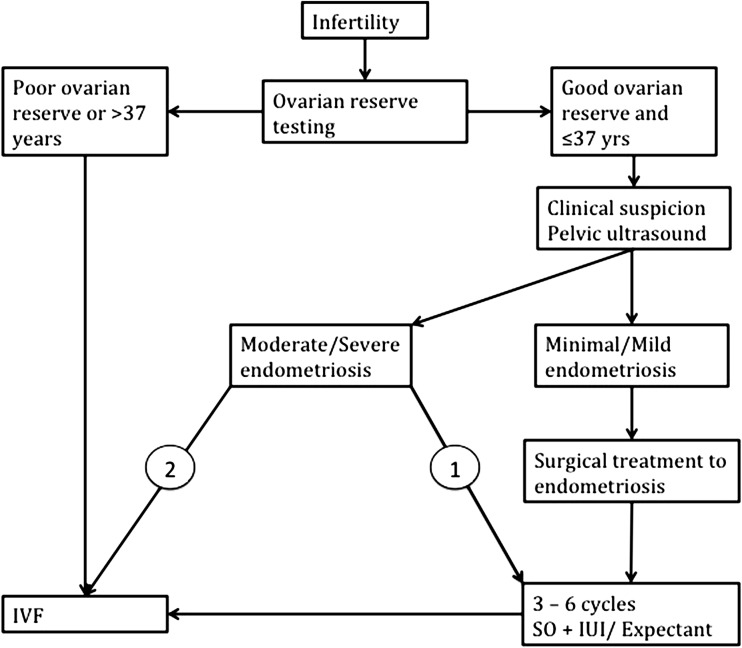
Women with endometriosis desiring fertility present a challenge to treating clinicians as this is a complex disorder with highly variable presentation. Our diagnostic workup centers on ovarian reserve testing and pelvic ultrasound to detect endometriosis (Fig. 1). In young women with minimal or mild disease and good ovarian reserve, we perform surgery with the aim to eradicate the endometriosis and allow sufficient time for conception either spontaneously or through SO + IUI. A similar approach is offered to women with small (<3 cm), unilateral, endometriomas. When we operate on endometriomas, we routinely excise the lesion with minimal resort to diathermy and aim to minimize loss of healthy ovarian tissue by careful manipulation. In older patients with reduced ovarian reserve, surgery does not appear to offer benefits except in those with large endometriomas that compromise access to the ovary during transvaginal oocyte retrieval.
Fig. 1.
Algorithm for diagnosis and treatment of patients with endometriosis desiring fertility. 1 If the pelvic anatomy is restored and Fallopian tubes are found to be patent during laparoscopy 2 if pelvic anatomy is significantly distorted and/or Fallopian tubes found to be affected at laparoscopy
Acknowledgments
Compliance with ethical requirements and Conflict of interest
The manuscript does not contain clinical studies or patient data. Mavrelos D and Saridogan E declare that they have no conflict of interest.
Dimitrios Mavrelos
has been NIHR academic clinical lecturer in Reproductive Medicine at University College London since 2012. He studied medicine at the University of Oxford and Guy’s King’s St Thomas’ School of Medicine. He completed his core Obstetrics and Gynecology training in London and undertook his MD in gynecological ultrasound. He is currently a subspecialty trainee in Reproductive Medicine at University College London Hospital. His research interests include the diagnosis and management of endometriosis, the diagnosis and management of early pregnancy complications, and the impact of gynecological disorders on assisted conception techniques.
References
- 1.Gupta S, Goldberg JM, Aziz N, et al. Pathogenic mechanisms in endometriosis-associated infertility. Fertil Steril. 2008;90:247–257. doi: 10.1016/j.fertnstert.2008.02.093. [DOI] [PubMed] [Google Scholar]
- 2.Akande VA, Hunt LP, Cahill DJ, et al. Differences in time to natural conception between women with unexplained infertility and infertile women with minor endometriosis. Hum Reprod (Oxford, England) 2004;19:96–103. doi: 10.1093/humrep/deh045. [DOI] [PubMed] [Google Scholar]
- 3.De Ziegler D, Borghese B, Chapron C. Endometriosis and infertility: pathophysiology and management. Lancet. 2010;376:730–738. doi: 10.1016/S0140-6736(10)60490-4. [DOI] [PubMed] [Google Scholar]
- 4.Lyons RA, Saridogan E, Djahanbakhch O. The effect of ovarian follicular fluid and peritoneal fluid on Fallopian tube ciliary beat frequency. Hum Reprod. 2006;21:52–56. doi: 10.1093/humrep/dei306. [DOI] [PubMed] [Google Scholar]
- 5.Dunselman GA, Vermeulen N, Becker C, et al. ESHRE guideline: management of women with endometriosis. Hum Reprod (Oxford, England) 2014;29:400–412. doi: 10.1093/humrep/det457. [DOI] [PubMed] [Google Scholar]
- 6.Hudelist G, English J, Thomas AE, et al. Diagnostic accuracy of transvaginal ultrasound for non-invasive diagnosis of bowel endometriosis: systematic review and meta-analysis. Ultrasound Obstet Gynecol Off J Int Soc Ultrasound Obstet Gynecol. 2011;37:257–263. doi: 10.1002/uog.8858. [DOI] [PubMed] [Google Scholar]
- 7.Holland TK, Hoo WL, Mavrelos D, et al. Reproducibility of assessment of severity of pelvic endometriosis using transvaginal ultrasound. Ultrasound Obstet Gynecol Off J Int Soc Ultrasound Obstet Gynecol. 2013;41:210–215. doi: 10.1002/uog.11216. [DOI] [PubMed] [Google Scholar]
- 8.Holland TK, Yazbek J, Cutner A, et al. Value of transvaginal ultrasound in assessing severity of pelvic endometriosis. Ultrasound Obstet Gynecol Off J Int Soc Ultrasound Obstet Gynecol. 2010;36:241–248. doi: 10.1002/uog.7689. [DOI] [PubMed] [Google Scholar]
- 9.Vercellini P, Fedele L, Aimi G, et al. Reproductive performance, pain recurrence and disease relapse after conservative surgical treatment for endometriosis: the predictive value of the current classification system. Hum Reprod (Oxford, England) 2006;21:2679–2685. doi: 10.1093/humrep/del230. [DOI] [PubMed] [Google Scholar]
- 10.Adamson GD, Pasta DJ. Endometriosis fertility index: the new, validated endometriosis staging system. Fertil Steril. 2010;94:1609–1615. doi: 10.1016/j.fertnstert.2009.09.035. [DOI] [PubMed] [Google Scholar]
- 11.Jacobson, T. Z., Duffy, J. M., Barlow, D et al. Laparoscopic surgery for subfertility associated with endometriosis. The Cochrane database of systematic reviews CD001398 (2010). doi:10.1002/14651858.CD001398.pub2. [DOI] [PubMed]
- 12.Vercellini P, Somigliana E, Viganò P, et al. Surgery for endometriosis-associated infertility: a pragmatic approach. Hum Reprod (Oxford, England) 2009;24:254–269. doi: 10.1093/humrep/den379. [DOI] [PubMed] [Google Scholar]
- 13.Hughes E, Brown J, Collins JJ, et al. Ovulation suppression for endometriosis for women with subfertility. Cochrane Libr. 2007 doi: 10.1002/14651858.CD000155.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muzii L, Bianchi A, Bellati F, et al. Histologic analysis of endometriomas: what the surgeon needs to know. Fertil Steril. 2007;87:362366. doi: 10.1016/j.fertnstert.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 15.Hart, R. J., Hickey, M., Maouris, P et al. Excisional surgery versus ablative surgery for ovarian endometriomata. The Cochrane database of systematic reviews CD004992 (2008). doi:10.1002/14651858.CD004992.pub3. [DOI] [PubMed]
- 16.Muzii L, Di Tucci C, Di Feliciantonio M, et al. The effect of surgery for endometrioma on ovarian reserve evaluated by antral follicle count: a systematic review and meta-analysis. Hum Reprod. 2014 doi: 10.1093/humrep/deu199. [DOI] [PubMed] [Google Scholar]
- 17.Vercellini P, Pietropaolo G, De Giorgi O, et al. Reproductive performance in infertile women with rectovaginal endometriosis: is surgery worthwhile? Am J Obstet Gynecol. 2006;195:1303–1310. doi: 10.1016/j.ajog.2006.03.068. [DOI] [PubMed] [Google Scholar]
- 18.Meuleman C, Tomassetti C, D’Hoore A, et al. Surgical treatment of deeply infiltrating endometriosis with colorectal involvement. Hum Reprod Update. 2011;17:311–326. doi: 10.1093/humupd/dmq057. [DOI] [PubMed] [Google Scholar]
- 19.Meuleman C, Tomassetti C, Wolthuis A, et al. Clinical outcome after radical excision of moderate—severe endometriosis with or without bowel resection and reanastomosis: a prospective Cohort study. Ann Surg. 2014;259:522–531. doi: 10.1097/SLA.0b013e31828dfc5c. [DOI] [PubMed] [Google Scholar]
- 20.Werbrouck E, Spiessens C, Meuleman C, et al. No difference in cycle pregnancy rate and in cumulative live-birth rate between women with surgically treated minimal to mild endometriosis and women with unexplained infertility after controlled ovarian hyperstimulation and intrauterine insemination. Fertil Steril. 2006;86:566571. doi: 10.1016/j.fertnstert.2006.01.044. [DOI] [PubMed] [Google Scholar]
- 21.Steures P, van der Steeg JW, Hompes PG, et al. Effectiveness of intrauterine insemination in subfertile couples with an isolated cervical factor: a randomized clinical trial. Fertil Steril. 2007;88:1692–1696. doi: 10.1016/j.fertnstert.2007.01.124. [DOI] [PubMed] [Google Scholar]
- 22.Tsoumpou I, Kyrgiou M, Gelbaya T, et al. The effect of surgical treatment for endometrioma on in vitro fertilization outcomes: a systematic review and meta-analysis. Fertil Steril. 2009;92:75–87. doi: 10.1016/j.fertnstert.2008.05.049. [DOI] [PubMed] [Google Scholar]
- 23.Demirol A, Guven S, Baykal C, et al. Effect of endometrioma cystectomy on IVF outcome: a prospective randomized study. Reprod Biomed Online. 2006;12:639–643. doi: 10.1016/S1472-6483(10)61192-3. [DOI] [PubMed] [Google Scholar]
- 24.Coccia M, Rizzello F, Barone S, et al. Is there a critical endometrioma size associated with reduced ovarian responsiveness in assisted reproduction techniques? Reprod BioMed Online. 2014 doi: 10.1016/j.rbmo.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 25.Opøien H, Fedorcsak P, Byholm T, et al. Complete surgical removal of minimal and mild endometriosis improves outcome of subsequent IVF/ICSI treatment. Reprod Biomed Online. 2011;23:389–395. doi: 10.1016/j.rbmo.2011.06.002. [DOI] [PubMed] [Google Scholar]