Abstract
Background
Cervical spondylosis is now recognised as the leading cause of myelopathy and spinal cord dysfunction worldwide. Chronic spinal cord compression results in chronic inflammation, cellular apoptosis, and microvacular insufficiency, which are thought to the biologic basis for cervical spondylotic myelopathy (CSM).
Questions/Purposes
Our purpose was to address the key principles of CSM, including natural history and presentation, pathogenesis, optimal surgical approach, results and complication rates of posterior surgical approaches for CSM so that the rationale for addressing CSM by a posterior approach can be fully understood.
Methods
We conducted a systematic search of PubMed/MEDLINE and the Cochrane Collaboration Library for literature published through February 2014 to identify articles that evaluated CSM and its management. Reasons for exclusion included patients with ossification of the posterior longitudinal ligament (OPLL), patients with degenerative disc disease without CSM, and patients with spine tumor, trauma and infection. Meeting abstracts/proceedings, white articles and editorials were additionally excluded.
Results
The search strategy yielded 1,292 articles, which was reduced to 52 articles, after our exclusion criteria were introduced. CSM is considered to be a surgical disorder due to its progressive nature. There is currently no consensus in the literature whether multilevel spondylotic compression is best treated via an anterior or posterior surgical approach.
Conclusion
Multilevel CSM may be safely and effectively treated using a posterior approach, either by laminoplasty or with a laminectomy and fusion technique.
Electronic supplementary material
The online version of this article (doi:10.1007/s11420-014-9425-5) contains supplementary material, which is available to authorized users.
Keywords: cervical spondylotic myelopathy, natural history, presentation, pathogenesis, optimal management, posterior surgical treatment options
Introduction
Cervical myelopathy was first described by Stookey in 1928, after the cord became compressed by cartilaginous nodules of degenerated disc material [2]. However, it was not until 1952 that the association between cervical spondylosis and myelopathy was established by Brain [4]. Cervical spondylosis is now recognised as the leading cause of myelopathy and spinal cord dysfunction worldwide [37]. However, recent cadaveric and animal studies have suggested that malalignment of the cervical spine may be an important contributing factor to the etiology of cervical myelopathy. In a cadaveric study, Farley et al. found an increase in cord tension with kyphosis greater than 51 [7]. Shimizu et al. demonstrated a significant correlation between the degree of kyphosis and the amount of cord flattening, leading to decreased vascular supply, and ultimately demyelination with neuronal loss in a small animal study [47]. These studies highlight the importance of sagittal alignment and imbalance in the management of cervical spondylotic myelopathy (CSM).
The purpose of this report was to perform a systematic review on the posterior surgical management of CSM. However, this can only be satisfactorily accomplished if one has a thorough understanding of the natural history, presentation, pathophysiology and management options of CSM. We, therefore, addressed the following key clinical questions in adult patients with cervical spondylotic myelopathy: (1) What is the natural history and typical presentation of CSM? (2) What is the pathogenesis and pathobiology of CSM? (3) What is the optimal surgical approach for patients with CSM? (4) What are the results and complication rates of posterior surgical approaches to CSM?
Methods
We conducted a systematic search of PubMed/MEDLINE and the Cochrane Collaboration Library for literature published through February 2014. The search results were limited to human and animal studies in the English language. Reference lists of key articles were also systematically checked to identify additional eligible articles. The focus was on the identification of studies explicitly designed to evaluate CSM and the surgical management of this condition. Terms specific to CSM included the following: cervical spondylotic myelopathy or cervical myelopathy or (cervical and myelopathy). They were combined with terms specifying the surgery (anterior or posterior) and (decompression and fusion or laminoplasty or laminectomy). Reasons for exclusion included patients with ossification of the posterior longitudinal ligament (OPLL), patients with degenerative disc disease without CSM, and patients with spine tumor, trauma and infection. Meeting abstracts/proceedings, white articles and editorials were additionally excluded.
Data Extraction
The following demographic information was extracted: study design, patient demographics, diagnosis, and operated levels, follow-up period and the rate of follow-up for each treatment group. We sought changes in Japanese Orthopedic Association (JOA) scores, postoperative neck pain, changes in sagittal alignment and complication rates. We initially identified 1,292 articles, and this was reduced to 52 articles, after our exclusion criteria were introduced.
Results
While the natural history of CSM is not well known [3], CSM is generally considered to be a surgical disorder due to its progressive nature. If there is a role for nonoperative treatment as a primary treatment modality, it may be in the patient with mild myelopathy. Clark and Robinson reported in 1956 that 5% of patients deteriorate quickly, 20% have a gradual but steady decline in function and 70% have a stepwise progression in their symptoms with variable periods of quiescent disease [10]. More recent studies have highlighted this fact that activities of daily living (ADL) will continue to deteriorate in untreated myelopathic patients, but the time frame in which this occurs is unclear—Kadanka found that 6.3% deteriorated after 1 year, 27.3% after 3 years and 56% after 10 years of follow-up—compared with baseline [21, 22, 24, 42]. The clinical presentation of CSM is varied, with a constellation of symptoms and physical findings [20]. No single finding is pathognomotic of CSM as it typically presents insidiously, and its diagnosis requires a high index of suspicion. The lower extremities tend to be affected first, with spasticity and paresis. The patient often complains of a gait disturbance caused by abnormalities in the corticospinal and spinocerebellar tracts. The upper limbs become involved later, with loss of strength and dexterity [10, 28, 39].
Static and dynamic factors are the main contributing components to compression on the cervical spinal cord [23]. The static factors are the structural spondylotic abnormalities that cause canal stenosis and subsequent cord compression, with the degenerative intervertebral disc being the initiating event for these spondylotic changes [1, 6, 9, 11, 31]. The dynamic factors are characterized by the repetitive injury placed on the already compressed cord during the flexion and extension movements of the cervical spine [44]. Neck extension pinches the cervical cord between the degenerated disc and osteophytes anteriorly, and the hypertrophied facet joints and infolded ligaments posteriorly, while neck flexion forces the cord against the vertebral bodies, inducing anterior cord pathology and limiting blood supply, while increasing the longitudinal cord tension due to the cord being tethered by the dentate ligaments and cervical nerve roots [25, 29]. The biological basis for neural deterioration is thought to be secondary to the disruption of the blood spinal cord barrier by chronic cord compression which results in neuronal and oligodendrocytes apoptosis, microglial infiltration and injury through the CX3CL1/R1 signalling pathway, and microvascular compromise [5, 45, 48, 50, 51].
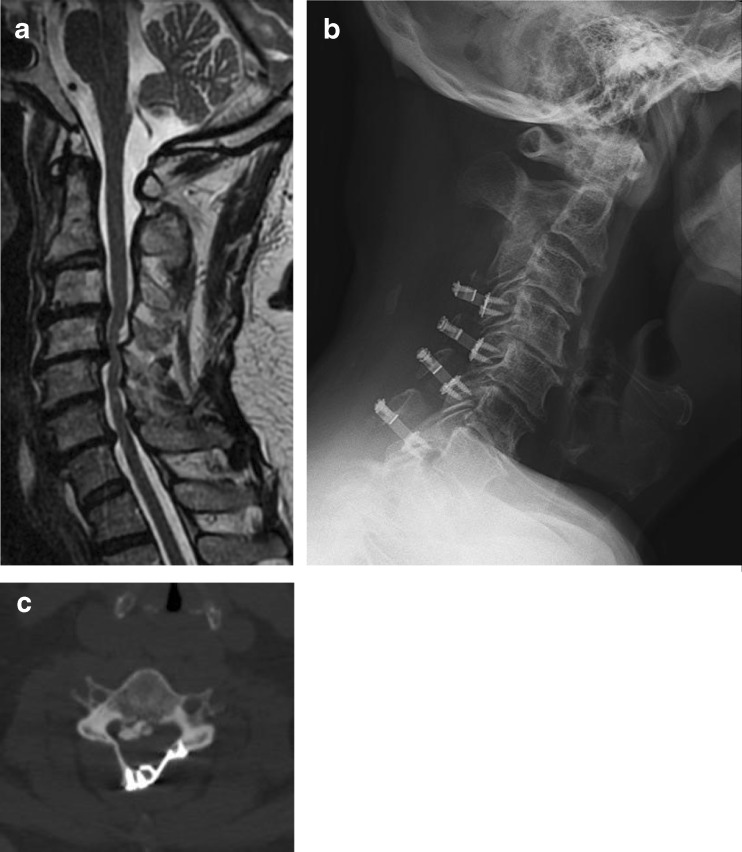
Currently, there is no consensus in the literature whether multilevel spondylotic compression is best treated via an anterior or posterior surgical route [8, 32]. Lawrence et al. suggested adopting an individualized approach when treating patients with CSM accounting for pathoanatomical variations (ventral vs. dorsal, focal vs. diffuse, sagittal, dynamic instability) because they found similar outcomes between approaches with regard to effectiveness and safety [32]. Awareness of preoperative cervical sagittal alignment is crucial in surgical decision making, and the posterior approach is thought to be ideally suited for patients with multilevel CSM, patients with preservation of cervical lordosis (Fig. 1) [10, 19, 36].
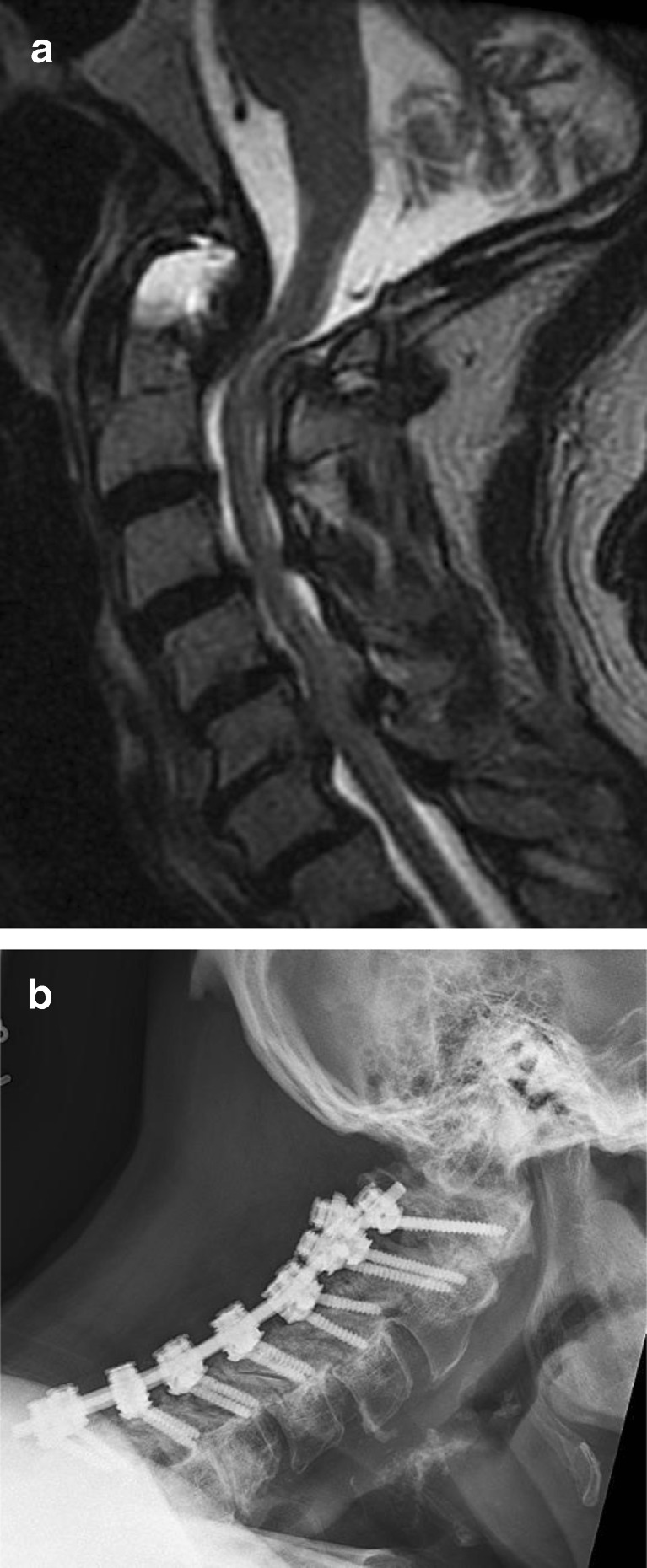
Fig. 1.
a Preoperative T2 sagittal MRI cervical spine demonstrating multilevel spondylosis with dorsal compression. b Postoperative lateral cervical spine radiograph following open door laminoplasty. c Postoperative axial CT scan following open door laminoplasty.
There are more than 300 published studies on the topic of laminoplasty and laminectomy with fusion for CSM [12, 14, 16, 17, 26, 35]. However, there are only, to the best of our knowledge, four studies that compare the two groups within a single study, and all four were retrospective cohort studies. Heller et al. reviewed a matched cohort of 13 patients who underwent laminectomy and fusion versus 13 patients who underwent laminoplasty [14]. After a mean follow-up of 26 months, they found greater improvement in objective (Nurick scores) and subjective findings (strength, dexterity, sensation and gait) in the laminoplasty-treated group. They also reported no complications in the laminoplasty group, while the laminectomy and fusion group had 14 complications in nine patients, including increasing myelopathy, increased kyphosis, instrumental failure, non-union, persistent graft site donor pain, adjacent segment disease and infection. In a larger matched cohort study involving 121 patients over a 5-year period, Woods et al. reported on 39 patients who underwent laminoplasty and on 82 patients who underwent laminectomy and fusion, with a mean follow-up of 24 months [49]. Patient-reported outcomes were similar between the two groups, as were the complication rates, with 2 and 5 % requiring reoperation in the laminoplasty and laminectomy/fusion group, respectively.
Discussion
Cervical spondylosis is now recognised as the leading cause of myelopathy and spinal cord dysfunction worldwide [37]. Chronic spinal cord compression can result in chronic inflammation, cellular apoptosis and microvacular insufficiency, which are thought to the biologic basis for CSM [5, 45, 48, 50, 51]. Unfortunately, there is no effective nonoperative treatment for symptomatic myelopathic patients. In a systematic review in 2013, Karadimas et al. found that 20 to 60% of patients with CSM will deteriorate neurologically over time without surgical intervention at 3 to 6 years of follow-up [24]. In this review paper, an attempt has been made to address the key principles of CSM, including natural history and presentation, pathogenesis of CSM, optimal surgical approach for CSM management and results, and complication rates of posterior surgical approaches for CSM so that the rationale for addressing CSM by a posterior approach can be fully understood.
There is currently no consensus in the literature whether multilevel spondylotic compression is best treated via an anterior or posterior surgical route. Shamji et al. showed no difference between anterior and posterior surgery for patients with CSM in a large administrative database [46]. However, Fehlings et al. described the complications of surgical treatment in patients with CSM and found that the posterior surgical group had a higher incidence of postoperative wound infection but the overall complication rates, C5 palsy and the incidence of dysphagia were similar [8]. Our review supports using a customized approach for each individual patient, given the similarity with regard to outcome and safety, and highlights that, at a minimum, surgery not only halts the progression of symptoms but can promote meaningful functional recovery in a significant portion of treated individuals [8, 32, 33].
The posterior approach is thought to be ideally suited for patients with multilevel CSM, patients with preservation of cervical lordosis and for patients with alkylosed spines [10, 19, 36]. Awareness of preoperative cervical sagittal alignment is crucial in surgical decision making, as a posterior decompression in a kyphotic spine does not allow the cord to migrate posteriorly and, if the kyphosis progresses, further compression on the cord may ensue causing worsening neurologic decline [30]. The two most common posterior cervical surgical procedures for the treatment of CSM are cervical laminoplasty and cervical laminectomy and fusion. Decompressive laminectomy alone has fallen out of favour because of postlaminectomy kyphosis, which may occur in 10 to 45% of patients [26]. Matsunaga and colleagues compared 37 patients treated by laminectomy alone with 64 patients who underwent laminoplasty, with a mean follow-up of greater than 5 years. They found postoperative kyphosis rates of 35% in the laminectomy group and only 7% in the laminoplasty group [35]. Likewise, Kato et al. found 47% of patients developed postoperative kyphosis, although this did not correlate with neurologic deterioration [26].
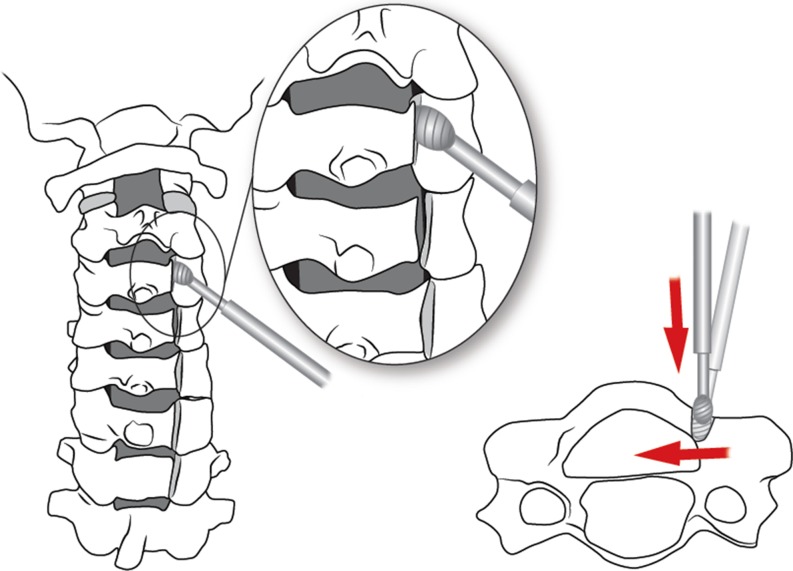
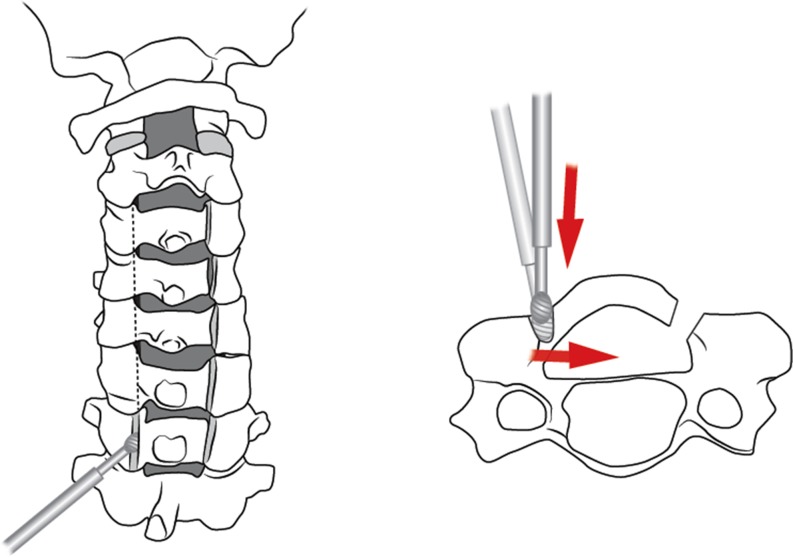
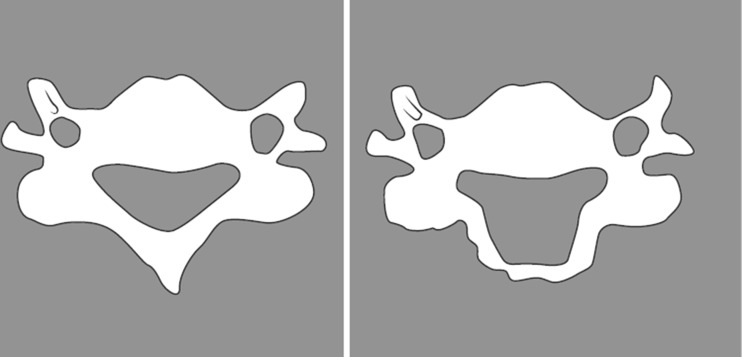
Laminoplasty was developed primarily to avoid the issue of postoperative kyphosis, but also postoperative instability, postoperative laminectomy membrane and late neurological deterioration [12]. Laminoplasty may be performed by three techniques: the open door technique, the French door or sagittal spinous process splitting technique, and the expansive midline threadwire swa (T-saw) technique, which is a modification of the French door technique. Hirabayashi originally described the technically demanding, open door technique in 1978 [16]. This technique requires performing a bicortical trough on the open door side and a unilateral trough on the contralateral, hinge, side (Figs. 2 and 3). The bicortical trough is then opened and hinges on the unicortical trough, allowing for an increased spinal canal diameter (Fig. 4). The lamina is then held open with a cortical bone graft spacer with or without specially designed laminoplasty plastes (Fig. 5).
Fig. 2.
Creation of bicortical trough is performed first using a high speed burr positioned medially at an angle perpendicular to the lamina to avoid entry into the lateral mass/facet joint.
Fig. 3.
Creation of the contralateral, hinge side, is performed second. Similarly angulation of the burr is directed medially at the junction of the lamina and lateral mass. Once hinge side is thin enough, the hinge may be gently opened.
Fig. 4.
Increase in spinal canal diameter post open door laminoplasty.
Fig. 5.
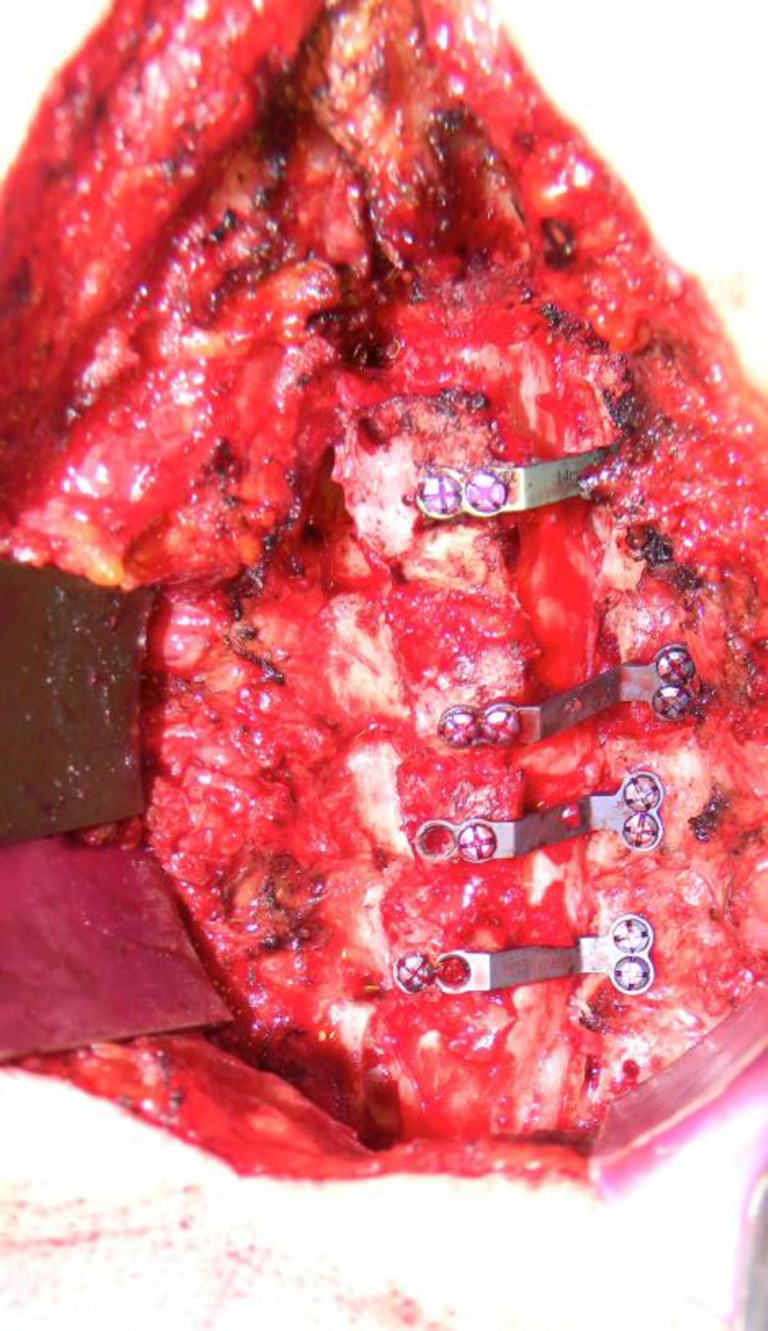
Intraoperative picture demonstrating laminae held open by specially designed laminoplasty plates during the open door laminoplasty technique.
The French door technique was described by Kurokawa et al. in 1982, and involves a high speed burr to develop a sagittal split in the spinous process, creating 2 hemilaminae [17]. Bilateral unicortical troughs are then developed at the edge of the lamina-lateral mass border, and then the hemilaminae are separated, like the opening of a French door, and held open with cortical bone grafts, that are secured with wire to the laminae. The third technique is a modification of the French door technique, and uses the same concept as the sagittal split, but uses a T-saw instead of a high speed burr.
In a cost and outcome comparison study, Highsmith et al. analysed the records of 56 patients, 30 of whom underwent laminoplasty and 26 who underwent laminectomy and fusion, and found that both groups had similar improvements in Nurick scores, mJOA scores and Odom scores [15]. Patients who underwent fusion had significant improvement in VAS postoperative pain scores (5.8 ± 3.2 to 3.0 ± 2.3, p < 0.01) in comparison to laminoplasty patients (3.2 ± 2.8 to 3.4 ± 2.6, p < 0.01). However, complications were twice as common in the fusion group, and implant costs were nearly three times as high as in the laminoplasty group.
Recently, Manzano et al. performed a small prospective randomised trial in which they randomised 16 patients to laminoplasty or laminectomy and fusion, with 12 month follow-up, including data on Nurick scores, mJOA scores, Neck Disability Index and Short Form 36 [34]. They reported similar outcome measures in each group, but the laminoplasty group had significantly improved Nurick scores at 1 year. The laminectomy and fusion group had a 75% decrease in cervical range of motion between C2 and C7, whereas the laminoplasty group had only a 20% reduction.
Complications
One significant complication of laminoplasty is axial symptoms, including shoulder pain and spasm, and neck pain. Hosono et al. reported a 60% incidence of postoperative axial symptoms in laminoplasty patients [18]. In their meta-analysis of cervical laminoplasty, Ratliff and Cooper concurred with Honsono’s findings, when they found a 25 to 60% incidence of axial neck pain [38]. Lawrence and Brodke have suggested that postoperative axial neck pain may be reduced by the preservation of the C2 and C7 muscle attachments during laminoplasty [31]. Sakura et al. supported this observation by finding a 11% reduction in neck pain in patients that had preservation of their superior and inferior cervical spine muscle attachments [40]. Progressive kyphosis (10%) and decreased cervical range of motion (15 to 50%) are also associated with laminoplasty. In contrast to laminoplasty in which early mobility, avoidance of bleeding bone surfaces intraoperatively, and other techniques are employed to avoid a fusion, in patients undergoing multilevel spinal fusion, non-union may occur at a rate reported between 1-38% depending on a number of factors (Fig. 6).
Fig. 6.
a Preoperative MRI demonstrated multilevel spondylosis, crowned dens syndrome, myelomalacia and compression both dorsally and ventrally. b Postoperative lateral cervical radiograph following laminectomy and fusion with positive sagittal balance but excellent clinical result and improvement of neurological function.
Another complication common to both techniques is C5 nerve root paresis, with deltoid paralysis and biceps weakness. The incidence varies between 3 and 11%, and is thought to result from the acute posterior translation of the spinal cord causing a traction palsy of the C5 nerve root [41, 27]. Satomi found a 7.8% incidence of biceps weakness in 206 patients that had undergone an open door laminoplasty [43]. Hatta et al. demonstrated that selective laminoplasties reduced the migration of the cord posteriorly from 2.7 to 1.1 mm and, concomitantly, decreased the incidence from 8 to 0% [13].
In summary, moderate and severe CSM may be effectively and safely treated using a posterior surgical decompression approach, either with a laminoplasty or laminectomy and fusion technique. Cervical sagittal alignment must be considered preoperatively when surgical strategies are being considered for CSM to prevent postoperative kyphosis and sagittal imbalance, which are now known to contribute to the progression of CSM.
Electronic supplementary material
(PDF 1224 kb)
Disclosures
Conflict of Interest
Paul D. Kiely, MD, John C. Quinn, MD and Jerry Y. Du, BS, have declared that they have no conflict of interest. Darren R. Lebl, MD reports consulting fees from Medtronic; outside the submitted work; reviewer for The Spine Journal, Global Spine Journal, Journal of Spinal Disorders & Techniques, HSS Journal; Clinical Advisor - Spine Frontier.
Human/Animal Rights
This article does not contain any studies with human or animal subjects performed by the any of the authors.
Informed Consent
N/A.
References
- 1.Ames CP, Blondel B, Scheer JK, et al. Cervical radiographical alignment: comprehensive assessment techniques and potential importance in cervical myelopathy. Spine (Phila Pa 1976) 2013;38(22 Suppl 1):S149–S160. doi: 10.1097/BRS.0b013e3182a7f449. [DOI] [PubMed] [Google Scholar]
- 2.Baptiste DC, Fehlings MG. Pathophysiology of cervical myelopathy. Spine J. 2006;6(6 Suppl):190S–197S. doi: 10.1016/j.spinee.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 3.Boogaarts HD, Bartels RH. Prevalence of cervical spondylotic myelopathy. Eur Spine J. 2013;25:E10. doi: 10.1007/s00586-013-2781-x. [DOI] [PubMed] [Google Scholar]
- 4.Brain WR, Northfield D, Wilkinson M. The neurological manifestations of cervical spondylosis. Brain. 1952;75(2):187–225. doi: 10.1093/brain/75.2.187. [DOI] [PubMed] [Google Scholar]
- 5.Donnelly DJ, Longbrake EE, Shawler TM, et al. Deficient CX3CR1 signaling promotes recovery after mouse spinal cord injury by limiting the recruitment and activation of Ly6Clo/iNOS+ macrophages. J Neurosci. 2011;31(27):9910–9922. doi: 10.1523/JNEUROSCI.2114-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Epstein NE. Laminectomy for cervical myelopathy. Spinal Cord. 2003;41(6):317–327. doi: 10.1038/sj.sc.3101477. [DOI] [PubMed] [Google Scholar]
- 7.Farley CW, Curt BA, Pettigrew DB, et al. Spinal cord intramedullary pressure in thoracic kyphotic deformity: a cadaveric study. Spine (Phila Pa 1976) 2012;37(4):E224–E230. doi: 10.1097/BRS.0b013e31822dd69b. [DOI] [PubMed] [Google Scholar]
- 8.Fehlings MG, Smith JS, Kopjar B, et al. Perioperative and delayed complications associated with the surgical treatment of cervical spondylotic myelopathy based on 302 patients from the AOSpine North America Cervical Spondylotic Myelopathy Study. J Neurosurg Spine. 2012;16(5):425–432. doi: 10.3171/2012.1.SPINE11467. [DOI] [PubMed] [Google Scholar]
- 9.Fehlings MG, Wilson JR, Karadimas SK, et al. Clinical evaluation of a neuroprotective drug in patients with cervical spondylotic myelopathy undergoing surgical treatment: design and rationale for the CSM-Protect Trial. Spine (Phila Pa 1976) 2013;38(22 Suppl 1):S68–S75. doi: 10.1097/BRS.0b013e3182a7e9b0. [DOI] [PubMed] [Google Scholar]
- 10.Geck MJ, Eismont FJ. Surgical options for the treatment of cervical spondylotic myelopathy. Orthop Clin North Am. 2002;33(2):329–348. doi: 10.1016/S0030-5898(02)00002-0. [DOI] [PubMed] [Google Scholar]
- 11.Goya T, Morita Y. Chronological changes in the operative indications and approaches for the treatment of spondylosis deformans of the spine. Brain Nerve. 2009;61(6):627–635. [PubMed] [Google Scholar]
- 12.Hale JJ, Gruson KI, Spivak JM. Laminoplasty: a review of its role in compressive cervical myelopathy. Spine J. 2006;6(6 Suppl):289S–298S. doi: 10.1016/j.spinee.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 13.Hatta Y, Shiraishi T, Hase H, et al. Is posterior spinal cord shifting by extensive posterior decompression clinically significant for multisegmental cervical spondylotic myelopathy? Spine (Phila Pa 1976) 2005;30(21):241–249. doi: 10.1097/01.brs.0000184751.80857.3e. [DOI] [PubMed] [Google Scholar]
- 14.Heller JG, 2nd, Edwards CC, Murakami H, et al. Laminoplasty versus laminectomy and fusion for multilevel cervical myelopathy: an independent matched cohort analysis. Spine (Phila Pa 1976) 2001;26(12):1330–1336. doi: 10.1097/00007632-200106150-00013. [DOI] [PubMed] [Google Scholar]
- 15.Highsmith JM, Dhall SS, Haid RW, Jr, et al. Treatment of cervical stenotic myelopathy: a cost and outcome comparison of laminoplasty versus laminectomy and lateral mass fusion. J Neurosurg Spine. 2011;14(5):619–625. doi: 10.3171/2011.1.SPINE10206. [DOI] [PubMed] [Google Scholar]
- 16.Hirabayashi K, Watanabe K, Wakano K, et al. Expansive open-door laminoplasty for cervical spinal stenotic myelopathy. Spine (Phila Pa 1976) 1983;8(7):693–699. doi: 10.1097/00007632-198310000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Hoshi K, Kurokawa T, Nakamura K, et al. Expansive cervical laminoplasties–observations on comparative changes in spinous process lengths following longitudinal laminal divisions using autogenous bone or hydroxyapatite spacers. Spinal Cord. 1996;34(12):725–728. doi: 10.1038/sc.1996.132. [DOI] [PubMed] [Google Scholar]
- 18.Hosono N, Yonenobu K, Ono K. Neck and shoulder pain after laminoplasty. A noticeable complication. Spine (Phila Pa 1976) 1996;21(17):1969–1973. doi: 10.1097/00007632-199609010-00005. [DOI] [PubMed] [Google Scholar]
- 19.Hukuda S, Mochizuki T, Ogata M, et al. Operations for cervical spondylotic myelopathy. A comparison of the results of anterior and posterior procedures. J Bone Joint Surg Br. 1985;67(4):609–615. doi: 10.1302/0301-620X.67B4.4030860. [DOI] [PubMed] [Google Scholar]
- 20.Iwasaki M, Okuda S, Miyauchi A, et al. Surgical strategy for cervical myelopathy due to ossification of the posterior longitudinal ligament: part 2: advantages of anterior decompression and fusion over laminoplasty. Spine (Phila Pa 1976) 2007;32(6):654–660. doi: 10.1097/01.brs.0000257566.91177.cb. [DOI] [PubMed] [Google Scholar]
- 21.Kadanka Z, Bednarik J, Novotny O, et al. Cervical spondylotic myelopathy: conservative versus surgical treatment after 10 years. Eur Spine J. 2011;20(9):1533–1538. doi: 10.1007/s00586-011-1811-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalsi-Ryan S, Karadimas SK, Fehlings MG. Cervical spondylotic myelopathy: the clinical phenomenon and the current pathobiology of an increasingly prevalent and devastating disorder. Neuroscientist. 2013;19(4):409–421. doi: 10.1177/1073858412467377. [DOI] [PubMed] [Google Scholar]
- 23.Kalsi-Ryan S, Singh A, Massicotte EM, et al. Ancillary outcome measures for assessment of individuals with cervical spondylotic myelopathy. Spine (Phila Pa 1976) 2013;38(22 Suppl 1):S111–S122. doi: 10.1097/BRS.0b013e3182a7f499. [DOI] [PubMed] [Google Scholar]
- 24.Karadimas SK, Erwin WM, Ely CG, et al. Pathophysiology and natural history of cervical spondylotic myelopathy. Spine (Phila Pa 1976) 2013;38(22 Suppl 1):S21–S36. doi: 10.1097/BRS.0b013e3182a7f2c3. [DOI] [PubMed] [Google Scholar]
- 25.Karadimas SK, Moon ES, Yu WR, et al. A novel experimental model of cervical spondylotic myelopathy (CSM) to facilitate translational research. Neurobiol Dis. 2013;54:43–58. doi: 10.1016/j.nbd.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 26.Kato Y, Iwasaki M, Fuji T, et al. Long-term follow-up results of laminectomy for cervical myelopathy caused by ossification of the posterior longitudinal ligament. J Neurosurg. 1998;89(2):217–223. doi: 10.3171/jns.1998.89.2.0217. [DOI] [PubMed] [Google Scholar]
- 27.Kawai S, Sunago K, Doi K, et al. Cervical laminoplasty (Hattori’s method). Procedure and follow-up results. Spine (Phila Pa 1976) 1988;13(11):1245–1250. doi: 10.1097/00007632-198811000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Kiely P, Baker JF, O'hEireamhoin S, et al. The evaluation of the inverted supinator reflex in asymptomatic patients. Spine (Phila Pa 1976) 2010;35(9):955–957. doi: 10.1097/BRS.0b013e3181bccf0f. [DOI] [PubMed] [Google Scholar]
- 29.Kim TH, Lee SY, Kim YC, et al. T1 slope as a predictor of kyphotic alignment change after laminoplasty in patients with cervical myelopathy. Spine (Phila Pa 1976) 2013;38(16):E992–E997. doi: 10.1097/BRS.0b013e3182972e1b. [DOI] [PubMed] [Google Scholar]
- 30.Klineberg E. Cervical spondylotic myelopathy: a review of the evidence. Orthop Clin North Am. 2010;41(2):193–202. doi: 10.1016/j.ocl.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 31.Lawrence BD, Brodke DS. Posterior surgery for cervical myelopathy: indications, techniques, and outcomes. Orthop Clin North Am. 2012;43(1):29–40. doi: 10.1016/j.ocl.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 32.Lawrence BD, Jacobs WB, Norvell DC, et al. Anterior versus posterior approach for treatment of cervical spondylotic myelopathy: a systematic review. Spine (Phila Pa 1976) 2013;38(22 Suppl 1):S173–S182. doi: 10.1097/BRS.0b013e3182a7eaaf. [DOI] [PubMed] [Google Scholar]
- 33.Lunsford LD, Bissonette DJ, Zorub DS. Anterior surgery for cervical disc disease. Part 2: treatment of cervical spondylotic myelopathy in 32 cases. J Neurosurg. 1980; 53(1): 12-19. [DOI] [PubMed]
- 34.Manzano GR, Casella G, Wang MY, et al. A prospective, randomized trial comparing expansile cervical laminoplasty and cervical laminectomy and fusion for multilevel cervical myelopathy. Neurosurgery. 2012;70(2):264–277. doi: 10.1227/NEU.0b013e3182305669. [DOI] [PubMed] [Google Scholar]
- 35.Matsunaga S, Sakou T, Nakanisi K. Analysis of the cervical spine alignment following laminoplasty and laminectomy. Spinal Cord. 1999;37(1):20–24. doi: 10.1038/sj.sc.3100749. [DOI] [PubMed] [Google Scholar]
- 36.Menezes AH. Decision making. Childs Nerv Syst. 2008;24(10):1147–1153. doi: 10.1007/s00381-008-0604-x. [DOI] [PubMed] [Google Scholar]
- 37.Rao R. Neck pain, cervical radiculopathy, and cervical myelopathy: pathophysiology, natural history, and clinical evaluation. J Bone Joint Surg Am. 2002;84-A(10):1872–1881. doi: 10.2106/00004623-200210000-00021. [DOI] [PubMed] [Google Scholar]
- 38.Ratliff JK, Cooper PR. Cervical laminoplasty: a critical review. J Neurosurg. 2003;98(3 Suppl):230–238. doi: 10.3171/spi.2003.98.3.0230. [DOI] [PubMed] [Google Scholar]
- 39.Rhee JM, Shamji MF, Erwin WM, et al. Nonoperative management of cervical myelopathy: a systematic review. Spine (Phila Pa 1976) 2013;38(22 Suppl 1):S55–S67. doi: 10.1097/BRS.0b013e3182a7f41d. [DOI] [PubMed] [Google Scholar]
- 40.Sakaura H, Hosono N, Mukai Y, et al. Preservation of muscles attached to the C2 and C7 spinous processes rather than subaxial deep extensors reduces adverse effects after cervical laminoplasty. Spine (Phila Pa 1976) 2010;35(16):E782–E786. doi: 10.1097/BRS.0b013e3181d34614. [DOI] [PubMed] [Google Scholar]
- 41.Sakaura H, Hosono N, Mukai Y, et al. C5 palsy after decompression surgery for cervical myelopathy: review of the literature. Spine (Phila Pa 1976) 2003;28(21):2447–2451. doi: 10.1097/01.BRS.0000090833.96168.3F. [DOI] [PubMed] [Google Scholar]
- 42.Sampath P, Bendebba M, Davis JD, et al. Outcome of patients treated for cervical myelopathy. A prospective, multicenter study with independent clinical review. Spine (Phila Pa 1976) 2000;25(6):670–676. doi: 10.1097/00007632-200003150-00004. [DOI] [PubMed] [Google Scholar]
- 43.Satomi K, Nishu Y, Kohno T, et al. Long-term follow-up studies of open-door expansive laminoplasty for cervical stenotic myelopathy. Spine (Phila Pa 1976) 1994;19(5):507–510. doi: 10.1097/00007632-199403000-00003. [DOI] [PubMed] [Google Scholar]
- 44.Scheer JK, Tang JA, Smith JS, et al. Cervical spine alignment, sagittal deformity, and clinical implications: a review. J Neurosurg Spine. 2013;19(2):141–159. doi: 10.3171/2013.4.SPINE12838. [DOI] [PubMed] [Google Scholar]
- 45.Sekhon LH. Posterior cervical decompression and fusion for circumferential spondylotic cervical stenosis: review of 50 consecutive cases. J Clin Neurosci. 2006;13(1):23–30. doi: 10.1016/j.jocn.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 46.Shamji MF, Cook C, Tackett S, et al. Impact of preoperative neurological status on perioperative morbidity associated with anterior and posterior cervical fusion. J Neurosurg Spine. 2008;9(1):10–16. doi: 10.3171/SPI/2008/9/7/010. [DOI] [PubMed] [Google Scholar]
- 47.Shimizu K, Nakamura M, Nishikawa Y, et al. Spinal kyphosis causes demyelination and neuronal loss in the spinal cord: a new model of kyphotic deformity using juvenile Japanese small game fowls. Spine (Phila Pa 1976) 2005;30(21):2388–2392. doi: 10.1097/01.brs.0000184378.67465.5c. [DOI] [PubMed] [Google Scholar]
- 48.Takenouchi T, Setoguchi T, Yone K, et al. Expression of apoptosis signal-regulating kinase 1 in mouse spinal cord under chronic mechanical compression: possible involvement of the stress-activated mitogen-activated protein kinase pathways in spinal cord cell apoptosis. Spine (Phila Pa 1976) 2008;33(18):1943–1950. doi: 10.1097/BRS.0b013e3181822ed7. [DOI] [PubMed] [Google Scholar]
- 49.Woods BI, Hohl J, Lee J, et al. Laminoplasty versus laminectomy and fusion for multilevel cervical spondylotic myelopathy. Clin Orthop Relat Res. 2011;469(3):688–695. doi: 10.1007/s11999-010-1653-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu WR, Baptiste DC, Liu T, et al. Molecular mechanisms of spinal cord dysfunction and cell death in the spinal hyperostotic mouse: implications for the pathophysiology of human cervical spondylotic myelopathy. Neurobiol Dis. 2009;33(2):149–163. doi: 10.1016/j.nbd.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 51.Yu WR, Liu T, Fehlings TK, et al. Involvement of mitochondrial signaling pathways in the mechanism of Fas-mediated apoptosis after spinal cord injury. Eur J Neurosci. 2009;29(1):114–131. doi: 10.1111/j.1460-9568.2008.06555.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1224 kb)