Abstract
Pathological contraction bands (CBs) are a type of necrosis pattern found in the myocardium. The composition of CB is not well studied. This is because CBs have diverse forms and can be observed in various causes of death. In pathology fields, CBs are classified artifactual CB and CB necrosis. We have identified different forms of CB by examining the expression and distribution of complement component C9 (CCC9) and Sirtuin1 by immunohistochemistry in the myocardium of patients who died because of different causes of death. We used cardiac tissues with CB from 30 forensic autopsy cases in our department from the last 2 years. We excluded the cases that had very little CB. We found that our CB classification based on expression levels of both CCC9 and Sirtuin1 correlated well with the agonal situation, including high temperature, myocardial infarction, cardiopulmonary resuscitation, and hypothermia. On the basis of these results, we here advocate a classification scheme based on immunohistochemistry. Furthermore, we found that CB necrosis could be detected using immunostaining with CCC9. Using our classification scheme, it will be possible to more accurately research each type of CB and the causative mechanisms.
Key Words: contraction bands, contraction band necrosis, immunohistochemistry, forensic pathology, cardiopulmonary resuscitation
Pathological contraction bands (CB) are the characteristic necrosis pattern in myocardium and involve hypercontraction and lysis of small groups of myocardial cells. Myocardial CB changes surrounding an acute myocardial infarction (AMI) were reported in 1939.1 Since then, many terms have been used to describe this change, such as either myofibrillar degeneration, coagulative myocytolysis, or Zenker necrosis. The CBs are also found in many cases that had different agonal situations, such as coronary occlusion,2 resuscitation attempts,3 drowning,4 burning,5 hypothermia,6 and so forth.
The composition of CB is not well studied. This is because CBs have diverse forms and can be observed in various causes of death. In pathological fields, artifactual CBs are indicated. Artifactual CBs are seen in myocardial biopsy specimen, especially in the specimen that is immersed in fixative chilled rather than at room temperature.7,8 The Society of Cardiovascular Pathology defines artifactual CB as being different from CB necrosis (CBN). In contrast to artifactual CB, CBN tissues are surrounded by normal-appearing myocardium and often have a granular basophilic hue because of mineralization due to intracellular calcium deposition.8 The pathological differences described previously are not often recognized by many researchers. In fact, artifactual CB and CBN are often not distinguished in forensic examinations. Previously, we found a difference using immunohistchemistry between focal CB and diffuse CB. Focal CB is stained with complement component C9 (CCC9), but diffuse CB did not.9 We also found that certain CB lesions are stained with Sirtuin1 (SIRT1).
Here, we advocate for a classification of CB using CCC9 and SIRT1 expression levels. This classification of CB will help to investigate the mechanisms of CB development in the future.
MATERIALS AND METHODS
We used 30 cardiac tissues taken from forensic autopsy cases in our department from the last 2 years in which we could find CB. We stained all cardiac tissues with both hematoxylin-eosin and Azan and looked for CB by microscopy. Azan staining visualized CB easily. We excluded the cases that had CB lesions that were too small to evaluate with immunohistochemistry. Prepared 4-μm paraffin sections were stained by standard immunohistochemical techniques using antibodies against both CCC9 and SIRT1. The staining procedure was based on the linked streptavidin-biotin method.
The ethics committee waivered the need for consent from patient’s next of kin because autopsy was dictated by law.
RESULTS
We found that our classification of CB correlated well with the agonal situation (Table 1).
TABLE 1.
Results of Immunohistchemistry and Cause of Death

CCC9+/SIRT1− staining: This CB staining pattern occurred in ischemic myocardium and in myocarditis. The ischemic area was clearly stained with CCC9, and SIRT1 staining was clearly absent (Fig. 1).
CCC9−/SIRT1+ staining: This CB staining pattern occurred in the cases with various causes of death, but every case involved a cardiopulmonary resuscitation (CPR) attempt. These CBs were scattered in the tissue and often localized at the edge of the left ventricle (Fig. 2). With SIRT1, the borders were clearly defined between the positive areas and the background cells. By comparison, SIRT1-negative CBs, which were found in other types of patient deaths, had weakly stained CB or CB, which did not show a difference between the background cells and the CB.
CCC9−/SIRT1− staining: This CB staining pattern occurred in the cases that had been exposed to low temperatures. All 4 drowning cases, which had occurred in winter, showed this staining pattern. However, only 1 burn case showed these CB staining patterns. The CBs were clearly negative for both proteins, whereas by comparison, the background cells stained well with SIRT1 (Fig. 3). These CBs are often found at the septum.
CCC9+/SIRT1+ staining: This CB staining pattern occurred in the cases exposed to elevated temperatures, but immunohistochemistry is not suitable for all cases because the high temperatures denatured the proteins. It is not correct to conclude that both SIRT1 and CCC9 appear in high-temperature cases. On the basis of our previous study, we concluded that SIRT1 and CCC9 basically had a mutually exclusive relationship in the myocardium. The CCC9-positive cells stained more weakly than that in the CB found in the AMI cases.
FIGURE 1.
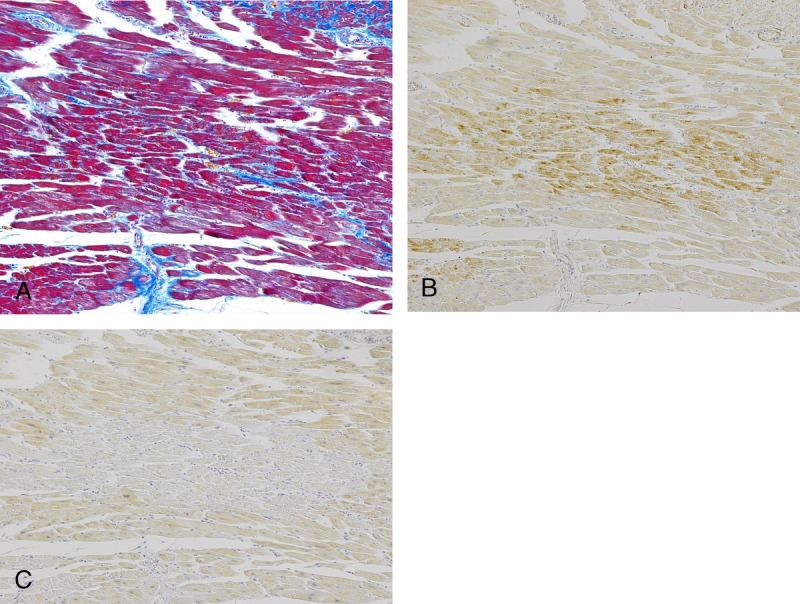
CCC9+/SIRT1− staining: CB was identified with Azan staining (A). The area that had CB clearly stained with CCC9 (B) and lacked staining with SIRT1 (C). The cause of death of this case was AMI, and no CPR was performed. Figure 1 can be viewed online in color at www.amjforensicmedicine.com.
FIGURE 2.
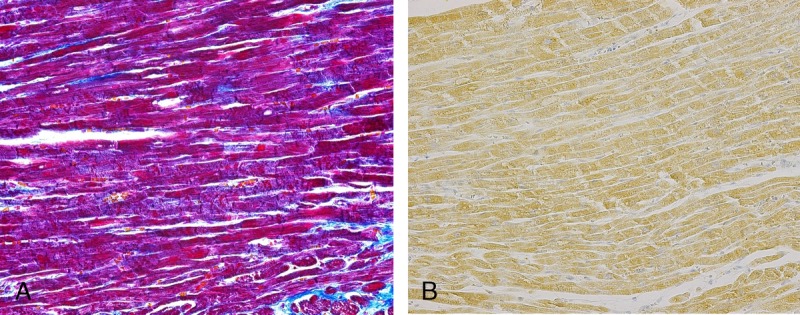
CCC9−/SIRT1+ staining: The borders were not very clear between the positive SIRT1-stained areas and the background cells (B). In comparison with SIRT1-negative CB (which was classified into other types), this CB was clearly positive for SIRT1. The cause of death of this case was a thoracic aneurism rapture. The patient did receive thoracotomy CRP. Figure 2 can be viewed online in color at www.amjforensicmedicine.com.
FIGURE 3.
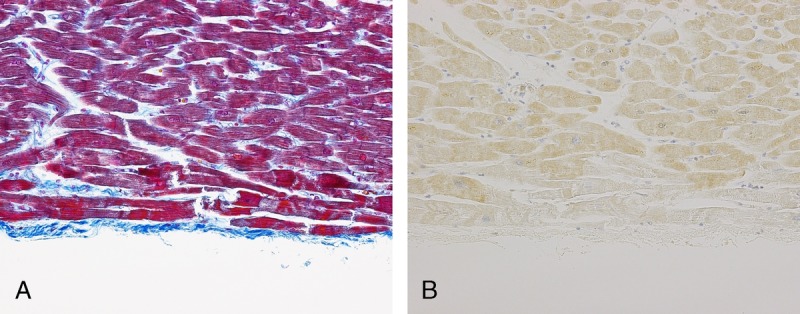
CCC9−/SIRT1− staining: In comparison with the staining of the background cells, this CB was clearly negative for SIRT1 (B). The cause of death of this case was drowning after falling into a river in the winter. Figure 3 can be viewed online in color at www.amjforensicmedicine.com.
DISCUSSION
Previously, many researchers have reported various cases that showed CB. Some pathologist tried to distinguish artifactual CB and CBN, but the distinction actually depends on the situation of specimen because it is too difficult to distinguish by pathological findings. By forensic researches, no attempt has been made to classify CB because it was assumed that CBs were the same in all cases. CBNs are composed of hypercontracted myocytes in which the sarcolemma is disrupted and the cell geometry is dramatically distorted as a consequence of extreme cell shortening.10 It was generally known that CBN can be observed in the hearts of reperfusion injury cases and quite early in AMI cases. The CBN induced by reperfusion has been reported by 1 group who evaluated the pathological findings after pig coronary artery ligation for 20, 30, 60, and 120 minutes followed by reperfusion for 8 hours. This research group found many CBNs in the 20- and 30-minute ligated hearts, and massive necrosis areas and less CBN were found in both the 60- and 120-minute ligated hearts.11
Immunostaining with CCC9 is a simple and useful way of defining myocardial damage. In very early AMI cases, the standard test includes hematoxylin-eosin staining, but no abnormality is observed, whereas CCC9 is detectable.12 The CCC9 can be detected in the AMI cases where the patient died within 6 hours.13 The CBN is also a well-established feature of early myocardial ischemia.12 On the basis of these reports and our studies, we concluded that CBN appeared in the very early phases of ischemia and that CBN would become coagulation necrosis in prolonged ischemia. Immunostaining with CCC9 could discriminate CBN from the other CB.
The SIRT1 deacetylase acts as a redox and oxygen sensor. The SIRT1 modulates cellular adaptation to hypoxia by targeting hypoxia-inducible factor 1α.14 We previously studied the relationship between hypoxia markers and agonal situations.9 We found that some CB lesions were stained with SIRT1. The CB positive for SIRT1 had weak staining, ambiguous borders, and negative areas. In contrast, CB negative for SIRT1 had clear borders and positive areas outside the CB. We compared CB with the background and determined that CBN, which lacked staining, was clearly negative and that CB, which was weakly stained or did not have apparent differences in staining from that of the background, was positive. We emphasize that a negative finding with SIRT1 is more important than a positive appearance. As we previously reported, the myocardium from hypothermia death cases was stained with SIRT1,9 whereas we found no SIRT1 staining in CB from hypothermia cases.
On the basis of these results, we here advocate the classification scheme that is shown in Table 2. As described previously, the cases with fever are not suitable for classification using immunohistochemistry. We classify the fever-induced CB as type 1. We classify AMI-induced CB that is clearly positive for CCC9 as type 2, which is called CBN in pathological fields. Because SIRT1 and CCC9 basically have a mutually exclusive relationship in the myocardium, type 2 CBs are never positive for SIRT1. Therefore, one should stain with SIRT1 when it is difficult to classify type 2 CB. For type 2, the CB should be checked to be sure that it is negative for CCC9. By staining with SIRT1, the CB can be classified as type 3, which is clearly negative for SIRT1 compared with that of the background. Type 3 CB is induced by cold temperatures at the time of death. The remaining CB is classified as type 4. Type 4 CB was all found in the cases with a CPR attempt.
TABLE 2.
The Classification Scheme
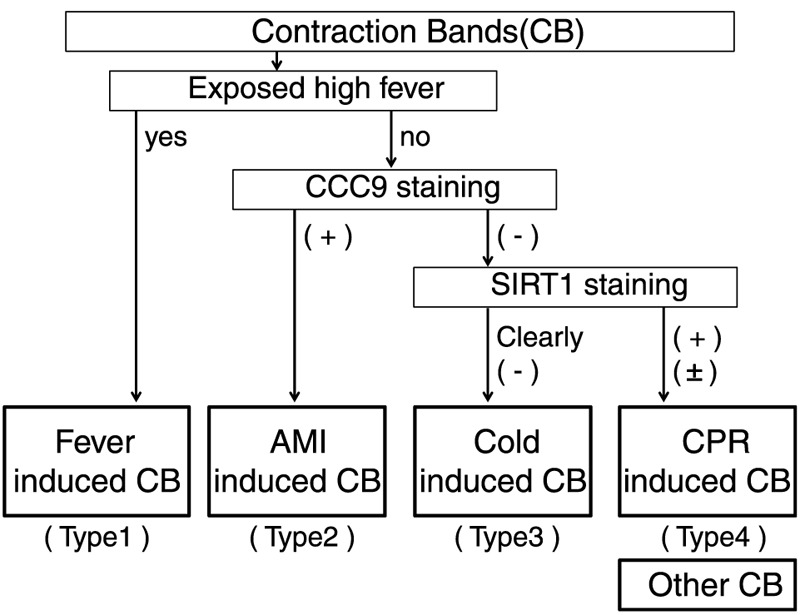
We could not study all types of CB. Classification of the CB cases, which we did not study or those that will be found in the future, could be investigated using an approach similar to our classification scheme. As new types of CB are identified in the future, we advocate that CPR attempt–induced CBN be classified as type 4. Certain CBN is classified into type 2, and artifactual CBs are classified into the other type. If one only needs the detection of CBN, one solely uses CCC9 staining after excluding fever-induced CB. Here, we advocate the classification of CB including artifactual CB because the situation and surroundings of the victim are very important in forensic fields.
Some researchers reported that CB was induced by a catecholamine, such as in pheochromocytoma.2 After subarachnoid hemorrhage (SAH), excessive release of catecholamine makes neurogenic pulmonary edema.15 We evaluated 1 SAH case, which had CPR attempt. Excessive release of catecholamine was suggested because pulmonary edema was found in this case, and any CCC9-positive type 2 CB could not be found. Of course, intravenous catecholamine infusion is done during a CPR attempt, but we did not analyze any cases of known high endogenous catecholamine production without CPR attempt. Thus, we presume that CBs induced by a catecholamine were CCC9 negative, but we certainly could not classify this type of CB with CCC9 and SIRT1.
As with pheochromocytoma, not all types of death that made CB were studied. Furthermore, we studied small sample size of each cause of death. Thus, our classification scheme was based on our limited data. To establish correct classification scheme, future research and more discussion are necessary. However, it is a very important point in our study that CBs are probably made by some mechanism and can be classified using immunohistchemistry. Using our classification scheme, we actually could distinguish cases with multiple types of CB. For example, in a case of death due to AMI where CPR was administered, we could clearly detect both type 2 CB and type 4 CB.
Our data show that multiple mechanisms contribute to CB. We here advocate a classification scheme for CB. Our scheme is compatible to the classification of Society of Cardiovascular Pathology, CBN, or artifactual CB. Previously, the composition of CB and mechanisms of development were not well studied because all CB lesions were regarded as the same in forensic fields. Using our classification scheme, for pathologists, it will be possible to distinguish CBN very easily. For forensic scientists, it will be possible to research correctly each CB type and better define the mechanisms of induction of CB.
Footnotes
Manuscript received February 11, 2014; accepted June 9, 2014.
The authors report no conflict of interest.
REFERENCES
- 1. Mallory GK, White PD, Salcedo-Salgar J. The speed of healing of myocardial infarction. A study of the pathologic anatomy of seventy two cases. Am Heart J. 1939; 18: 647– 671. [Google Scholar]
- 2. Karch SB, Billingham ME. Myocardial contraction bands revisited. Hum Pathol. 1986; 17: 9– 13. [DOI] [PubMed] [Google Scholar]
- 3. Karch SB. Resuscitation-induced myocardial necrosis. Catecholamine and defibrillation. Am J Forensic Med Pathol. 1987; 8: 3– 8. [DOI] [PubMed] [Google Scholar]
- 4. Lunt DWR, Rose AG. Pathology of the human heart in drowning. Arch Pathol Lab Med. 1987; 111: 939– 942. [PubMed] [Google Scholar]
- 5. Morita S, Furukawa S, Wingenfeld L, et al. Pathological findings of myocardium in carbon monoxide poisoning are basically similar to those in hypothermic deaths. Anil Aggrawal Internet J Forensic Med Toxicol. 2013; 14 (2): 12. [Google Scholar]
- 6. Furukawa S, Wingenfeld L, Sakaguchi I, et al. Histopathological characteristics of human cardiac tissues in accidental hypothermia using conventional staining techniques. Rom J Leg Med. 2012; 20: 241– 246. [Google Scholar]
- 7. Billingham ME. Role of endomyocardial biopsy in diagnosis and treatment of heart disease. In: Silver MD, ed. Cardiovascular Pathology. 2nd ed Michigan, PA: Churchill Livingstone; 1991: 1465– 1487. [Google Scholar]
- 8. SCVP Artifacts [SCVP Web Site]. 2013. Available at: http://scvp.net/acr/arti.html Accessed March 1, 2014.
- 9. Morita S, Furukawa S, Nishi K. Immunohistochemical evaluation of hypoxia markers in the myocardium. Aust J Forensic Sci. 2014doi:10.1080/00450618.2014.906653. [Google Scholar]
- 10. Rodriguez-Sinovas A, Abdallah Y, Piper HM, et al. Reperfusion injury as a therapeutic challenge in patients with acute myocardial infarction. Heart Fail Rev. 2007; 12: 207– 216. [DOI] [PubMed] [Google Scholar]
- 11. Miyazaki S, Fujiwara H, Onodera T, et al. Quantitative analysis of contraction band and coagulation necrosis after ischemia and reperfusion in the porcine heart. Circulation. 1987; 75 (5): 1074– 1082. [DOI] [PubMed] [Google Scholar]
- 12. Ferreira MAS, Owen HE, Howie AJ. High prevalence of acute myocardial damage in a hospital necropsy series, shown by C9 immunohistology. J Clin Pathol. 1998; 51: 548– 551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jasra SK, Badian C, Macri I, et al. Recognition of early myocardial infarction by immunohistochemical staining with cardiac troponin-I and complement C9. J Forensic Sci. 2012; 57 (6): 1595– 1600. [DOI] [PubMed] [Google Scholar]
- 14. Lim JH, Lee YM, Chun YS, et al. Sirtuin 1 modulates cellular responses to hypoxia by deacetylating hypoxia inducible factor 1α. Mol Cell. 2010; 38: 864– 878. [DOI] [PubMed] [Google Scholar]
- 15. Inamasu J, Sugimoto K, Yamada Y, et al. The role of catecholamines in the pathogenesis of neurogenic pulmonary edema associated with subarachnoid hemorrhage. Acta Neurochir. 2012; 154: 2179– 2185. [DOI] [PubMed] [Google Scholar]


