Abstract
Background and Purpose
Many previous studies on dementia in stroke have restrictive inclusion criteria which may result in under-estimation of dementia rates. We undertook a large prospective population-based study of all TIA and stroke to determine the impact of study entry criteria on measured rates of pre- and post-event dementia.
Methods
All patients with acute TIA or stroke from a defined population of 92 728 are referred from primary care or at hospital admission to the Oxford Vascular Study (2002-2007) and have baseline clinical and cognitive assessment and follow-up. We examined the impact of early death, other non-availability, and commonly-used selection criteria, on measured rates of dementia.
Results
Among 1236 patients (mean age/sd 75.2/12.1 years, 582 male, 403 TIA), 139 died or were otherwise unavailable for baseline assessment, 224 were dysphasic, 319 had prior dependency, 425 had co-morbidity, 512 were aged ≥80 years and 502 were hospitalised. Pre-event dementia was threefold higher in patients dying pre-ascertainment (10/47, 21%) and twice as high in other non-assessed (14/92,15%) vs assessed patients (69/1097, 6%; p=0.0006 and p=0.002) and was several-fold higher in those with prior functional impairment (24% vs 3%,p<0.0001), age>80 years (13% vs 3%,p<0.0001), dysphasia (11% vs 7%,p<0.0001) and co-morbidity (10% vs 6%,p=0.04). Findings for post-event dementia were similar: prior functional impairment (40% vs 13%,p<0.0001), age>80 years (28% vs 10%,p<0.0001), dysphasia (22% vs 15%,p=0.02) and co-morbidity (25% vs 15%,p=0.005).
Conclusions
Exclusion of patients unavailable for assessment, and other widely used selection criteria, results in underestimation of the measured rate of dementia associated with TIA and stroke.
Keywords: selection bias, dementia, TIA, stroke
Introduction
Stroke and dementia share similar risk factors and frequently co-exist.1 We have previously shown that rates of dementia in the first year after stroke are dependent on case-mix with lowest rates after first ever stroke in population-based studies and highest rates in hospital-based studies of major and recurrent stroke but there are few data on other sources of inclusion bias.2,3 A better understanding of the effects of selection on the measured cognitive impairment rate is required for understanding the biological mechanisms underpinning the relationship between stroke and dementia, for planning clinical trials and other large pragmatic studies and for calculating the overall cognitive burden attributable to symptomatic cerebrovascular disease. This paper is the first of three to examine methodological issues in measuring rates of TIA and stroke-associated dementia, subsequent studies will examine attrition and applicability of short cognitive tests.
We undertook a large prospective population-based study of dementia associated with all TIA and stroke. Study interview was used in all available patients together with hand-searching of primary care, hospital and mortality records to identify dementia in non-available patients. We then determined the impact of various indirect and specific selection criteria applied at study entry on measured rates of pre- and post-event dementia.
Methods
Patients with TIA or stroke were prospectively recruited from 1st April 2002-31st March 2007 into the Oxford Vascular Study (OXVASC), a prospective population-based cohort study of all acute vascular events occurring within a defined population of 92 728 covered by around 100 general practitioners (GPs-primary care) in nine GP practices in Oxfordshire, UK.4,5 The study was approved by the local research ethics committee. Informed written consent (or assent from relatives) was obtained for study interview and follow-up either in person or where not possible, by telephone, and also consent/assent for indirect follow-up using primary care physician records, hospital records and death certificate data. Where patients died before first assessment or where assent from a family member could not be obtained in patients lacking capacity (eg owing to dysphasia or dementia) the ethics committee approved review of the patient’s medical records.
Patients were ascertained as soon as possible after the initial TIA or stroke by study clinicians through a combination of multiple methods of hot and cold pursuit which has been shown to achieve near-complete ascertainment of TIA and stroke presenting to medical attention in this population.6 TIA and stroke were defined clinically by WHO criteria.7 Major stroke was defined as National Institute of Stroke Scale (NIHSS >3). Baseline brain and vascular imaging was performed and all cases were reviewed by a senior vascular neurologist (PMR). Stroke sub-type was determined according to Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria.8 Leukoaraiosis was defined as absent, mild, moderate or severe using a qualitative scale based on the severity score (absent, mild, moderate, or severe) of the Blennow scale9 for CT scans, and a modified version of the Fazekas scale10 for MRI scans as described previously.11
Patient data were collected by interview using a standardised form and general practitioner records and entered onto a custom-built database.4-8 Risk factors were recorded at study entry. Hypertension, diabetes and hypercholesterolaemia were defined on the basis of history and/or use of relevant medication and smoking as current smoking at the time of study entry. Pre-morbid functional status was assessed using modified Rankin12 and Barthel scores.13 The Informant questionnaire for cognitive decline in the elderly (IQCODE)14 was administered to an informant from 2002-2003 in a pilot study. However, owing to absence of a reliable informant in many patients and redundancy, in that data on pre-morbid function were available from primary care, it was discontinued thereafter and data were only available for 101 subjects.”
Follow-up interviews were done by trained research nurses at 1, 3 and 6 months and 1, 5 and 10 years either in the out-patient clinic or by home visit where hospital clinic visit was not possible. Telephone follow-up was performed where face-to-face follow-up was not possible (eg because the patient had moved away from the area). Functional status, assessed using modified Rankin12 and Barthel13 scores and Nottingham extended ADL15 index, was done at 1 month and 1, 5 and 10 years.
Cognitive testing was done at all follow-ups using the MMSE,16 TICSm17 and MoCA18 all of which have been validated against the National Institute of Neurological Disorders and Stroke-Canadian Stroke Network (NINDS-CSN) Vascular Cognitive Impairment Harmonisation Standards Neuropsychological Battery.19-22 The MMSE was done at all time-points until 1st April 2005 when the baseline MMSE was replaced by the 10 point Abbreviated Mental Test Score (AMTS).23 From April 2007, the MoCA was introduced for the 6 month, 1, 5 and 10 year follow-ups as recommended by the National Institute of Neurological Disorders and Stroke-Canadian Stroke Network (NINDS-CSN) Vascular Cognitive Impairment Harmonisation Standards Working Group.19 The TICSm or telephone MoCA (out of 12)21 was done by telephone where possible when face-to-face follow-up was not feasible. Reasons for lack of study interview and/or lack of cognitive test and problems with cognitive testing including visual impairment, hemiparesis, and dysphasia were recorded as described previously.22
Dementia was defined as pre- or post-event according to whether the diagnosis was made before or after the index event. Pre-event dementia was recorded if dementia was a listed diagnosis in the primary care record at the time of the index event. Where there was no listed dementia diagnosis and baseline cognitive testing was above the cut-off for dementia (see later), pre-event dementia was excluded. For remaining cases, pre-event dementia diagnosis was made by STP (a senior physician/geriatrician with expertise in dementia) after review of all study assessment data where available and hand-searching of the entire primary care record including individual consultation records, all hospital out-patient clinic letters, and hospitalisation documentation to establish pre-event dementia diagnosis on the basis of the DSM-IV criteria and/or by IQCODE score ≥3.6.
Of the 95 patients with pre-event dementia, dementia was present in the primary care problem list for 41 (43%). A further 22 had a non-specific cognitive problem listed including “memory loss symptom”, “mental disorder”, “cognitive decline”, “confusional state”, “chronic confusion”, “memory disturbance” and “memory loss of elderly” and the remaining 32 had no record of cognitive impairment in the primary care problem list but evidence of dementia was obtained from individual primary care consultations, hospital records, and or clinic letters.
Post-event dementia diagnosis was made after exclusion of patients with pre-event dementia. Post-event dementia diagnosis required MMSE<2424 and remaining <24 for all subsequent follow-ups or MoCA<2014 or TICSm<22 or TMoCA<9.21 For subjects with an incomplete test (ie testing was done but there was a problem such as dysphasia, visual impairment, inability to use the dominant arm, English as a second language), individual patient study records including that from primary care and information from informant were used to determine whether the DSM-IV criteria were met thus avoiding patients being spuriously classed as impaired on the basis of a low cognitive score. For patients without a direct study assessment, post-event dementia was diagnosed if there was a recorded diagnosis of dementia in the primary care record or if the DSM-IV25 criteria were met after from hand-searching of the entire primary care record as for pre-event dementia as described by Kokmen et al26 and/or dementia was listed on the death certificate.
Amongst the 61 patients diagnosed with post-event dementia after being lost to study-follow-up or who never had a study assessment, 30 had dementia diagnosis recorded in the primary care summary or from other physicians and 31 were made by STP after review of all available information from the primary care record.
To establish that there was no under-diagnosis of dementia in the study, death certificate data were examined for patients dying by 1st April 2013. In 36 patients with death certification of dementia, three had not been picked up by either study assessment or STP-primary care search. One died six years after completing study follow-up and was diagnosed with dementia in the post-study period, one moved away and was lost to follow-up, and one had a diagnosis of cognitive impairment at 1 year but moved away without forwarding contact and died before 5 year follow-up.
Statistical Analysis
Patients with index stroke who had a recurrent stroke did not re-enter the study and were only included once for the purposes of data analysis. Patients with index TIA who had a subsequent stroke (n=32) re-entered the study with the time of stroke defined as the new baseline. Demographic and clinical differences between dead and surviving, assessed and not-assessed patients were compared using analysis of variance (ANOVA) or χ2 test as appropriate. Post-event dementia rate was calculated as an actuarial rate (the proportion of dementia cases in the number at risk during the first year (the denominator)). The actuarial method is a way of allowing for withdrawals (deaths) that would only be at risk for part of the year; half the number censored is subtracted from the denominator at the start of the interval in the actuarial risk estimate.
The effects of application of various commonly used baseline selection factors on the number of included patients, case-mix and measured pre- and post-event dementia rate were examined. Indirect selection factors included early death before ascertainment and declining study interview at baseline. Direct inclusion/exclusion criteria were chosen on the basis of their use in previous studies of pre- and post-stroke dementia: age >80 years, premorbid functional dependency (mRS>3), co-morbidity (any significant co-morbidity recorded at study entry), dysphasia (NIHSS language score>0) and hospitalisation.
Results
1236 patients (mean age/sd 75.2/12.1 years, 582 (47%) male and 403 (33%) TIA, 463 (37%) minor stroke, 370 (30%) major stroke, 65 (5%) primary intracerebral haemorrhage) were ascertained (table 1), of which 992 (80.1%) were first ever events. Only 23/1236 (<2%) patients had no study assessment or GP records review at 5 years (n=18) or within 1-year of death (n=5) resulting in direct or indirect follow-up for over 98% of patients.
Table 1.
Impact of different baseline selection criteria on cohort numbers, demographics, case mix and rates of pre- and post-event dementia
| Population-based, all | Alive at ascertainment | Alive and study assessment | No comorbidity | No premorbid dependency | Age<=80 | Non-dysphasic | Non-hospitalised | |
|---|---|---|---|---|---|---|---|---|
| Number in study | 1236 | 1189 | 1097 | 811 | 917 | 724 | 1012 | 734 |
| Mean/sd age | 75.2/12.1 | 75.0/12.1 | 74.8/12.1 | 74.1/12.4 | 72.9/12.1 | 67.7/10.1 | 74.5/12.2 | 73.9/12.4 |
| Male sex | 520(48) | 558(47) | 520(47) | 405(50) | 468(51) | 386(53) | 484(48) | 339(46) |
| Education <12 yrs | 645(59) | 403(34) | 659(60) | 425(52) | 535(58) | 406(56) | 605(60) | 424(58) |
| TIA | 403(33) | 403(34) | 378(34) | 285(35) | 338(37) | 257(35) | 377(37) | 343(47) |
| Ischaemic stroke | 768(62) | 736(62) | 679(62) | 467(58) | 544(59) | 424(59) | 600(59) | 378(51) |
| PICH | 65(5) | 50(4) | 40(4) | 59(7) | 35(4) | 43(6) | 35(3) | 13(2) |
| Minor stroke | 463(37) | 461(39) | 429(39) | 297(37) | 360(39) | 287(40) | 416(41) | 303(41) |
| Major stroke | 370(30) | 325(27) | 290(26) | 229(28) | 219(24) | 180(25) | 219(22) | 88(12) |
| Rankin≥3 † | 221(19) | 212(19) | 192(18) | 116(16) | --- | 72(10) | 172(18) | 109(16) |
| Barthel<20 † | 292(26) | 283(26) | 273(26) | 154(21) | 120(13) | 108(16) | 246(25) | 158(23) |
| Pre-event dementia | 93(8) | 83(7) | 69(6) | 52(6) | 23(3) | 24(3) | 60(6) | 44(6) |
| 1-year post-event dementia * | 16.4/1.1 (173/1143) | 16.7/1.2 (173/1106) | 17.0/1.2 (165/1028) | 14.5/1.3 (103/759) | 12.7/1.1 (90/894) | 9.5/1.1 (63/700) | 15.4/1.2 (139/952) | 11.1/1.2 (74/690) |
Numbers are n (%).
actuarial risk/standard error (n/total), excluding pre-event dementia. PICH=primary intracerebral haemorrhage.
Pre-morbid values.
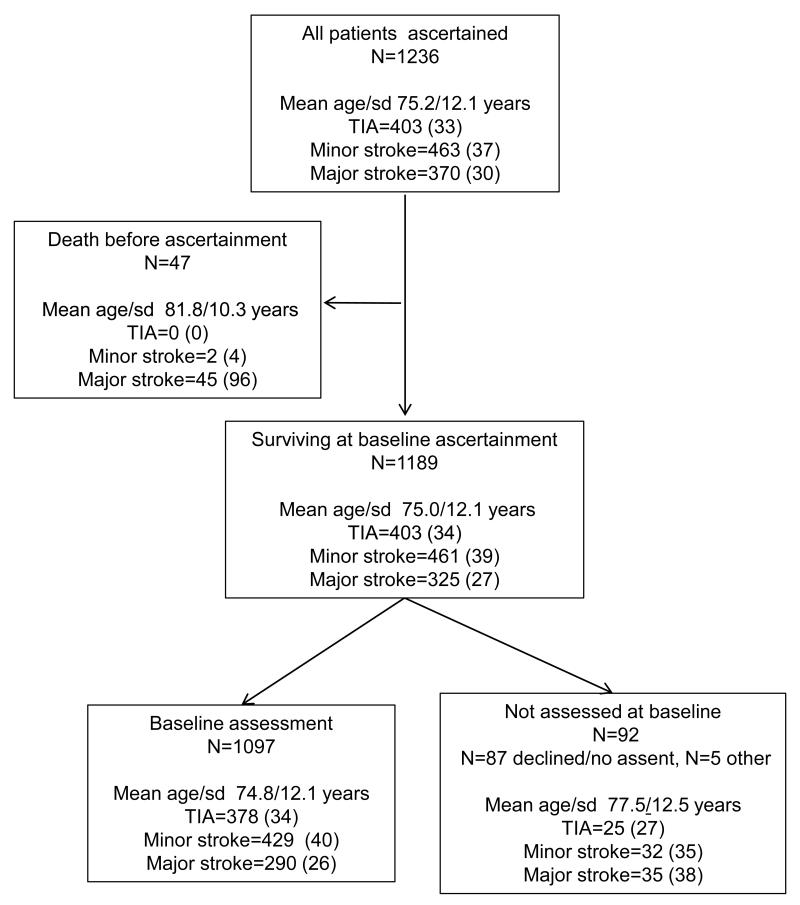
As a result of death and non-availability for interview, indirect selection at study entry occurred. Of the 1189 (92%) patients alive at ascertainment, 1097 (92%) had a study interview at baseline, with lack of consent or assent preventing assessment in most of the remainder (figure 1). Patients dying before ascertainment or who were unavailable for baseline study assessment were older (mean/sd age 81.8/10.3 years and 77.5/12.5 years) and had more major stroke (88% and 38%) and less TIA (2% and 27%) than assessed patients (mean/sd age 74.8/12.1 years, 26% major stroke, 35% TIA; all p<0.01) (figure 1, table 1, please see http://stroke.ahajournals.org for first-ever strokes only). These indirect selection effects had a major impact on the measured rate of dementia. Pre-stroke dementia in those assessed at baseline was 6% (69/1097) but was over three times higher in patients who died in the hyper-acute phase before ascertainment (10/47 (21%), p<0.001) and over twice as high in those who survived to baseline but did not have a study assessment (14/92 (15%), p=0.002 (figure 1)). The true population-based pre-event dementia rate was therefore about a third higher at 93/1236 (8%) than when measured in the 1097 patients surviving and assessed at baseline (6%, n=69) table 1). In contrast, the post-event dementia rate in those not-assessed versus assessed at baseline was lower at 8/78 (10%) vs 186/1097 (17%), probably owing to high rates of early death in the not-assessed group.
Figure 1.
Flow chart showing the numbers and characteristics of all patients in the population, of those dying versus surviving to ascertainment and amongst survivors, those with versus without baseline assessment.
Specific inclusion criteria also had a major impact on sample size and case-mix (tables 1 and 2, please see http://stroke.ahajournals.org for first-ever strokes only). Exclusion of older (>80 years), previously dependent, dysphasic and co-morbid patients resulted in cohorts that were unrepresentative both of the total population and within the hospitalised group. Non-hospitalised and hospitalised patients had very different demographic make-up, functional dependency and cerebrovascular disease burden: age (mean age/sd 73.1/12.1 vs 77.2/11.3 years), pre-morbid dependency (Rankin ≥3 86 (12%) vs 112 (25%)), TIA (289 (41%) vs 60 (12%)), major stroke (94 (13%) vs 282 (56%)), moderate/severe leukoaraiosis (105 (17%) vs 110 (25%)), all p<0.01.
Table 2.
Impact of different baseline selection criteria on cohort numbers, demographics, case mix and rates of pre- and post-event dementia for hospitalised patients only.
| Hospitalised, all | No comorbidity | No premorbid dependency | Age<=80 | Non-dysphasic | |
|---|---|---|---|---|---|
| Number in study | 502 | 313 | 330 | 259 | 437 |
| Mean age/sd | 77.2/11.3 | 76.3/11.8 | 75.0/11.7 | 69.0/9.7 | 77.1/11.6 |
| Male sex | 243(48) | 167(53) | 175(53) | 154(59) | 218(50) |
| Education (<12 yrs) | 244(49) | 143(46) | 183(55) | 134(52) | 203(46) |
| TIA | 60(12) | 37(12) | 44(13) | 32(12) | 60(14) |
| Ischaemic stroke | 390(78) | 227(73) | 259(78) | 194(75) | 330(76) |
| PICH | 52(10) | 49(16) | 27(8) | 33(13) | 47(11) |
| Minor stroke | 160(32) | 94(30) | 114(35) | 92(36) | 153(35) |
| Major stroke | 282(56) | 182(58) | 172(52) | 135(52) | 224(51) |
| Rankin≥3 † | 112(25) | 59(22) | - | 35(15) | 96(25) |
| Barthel<20 † | 134(31) | 67(25) | 49/32 (15) | 44(19) | 110(30) |
| Pre-event dementia | 49(10) | 30(10) | 12(4) | 13(5) | 42(10) |
| 1-year post-event dementia * | 25.4/2.2 (99/453) | 24.0/2.7 (59/283) | 22.6/2.5 (65/318) | 16.8/2.5 (37/246) | 22.5/2.3 (76/395) |
Numbers are n (%).
actuarial risk/standard error (n/total), excluding pre-event dementia. PICH=primary intracerebral haemorrhage.
Pre-morbid values.
The variations in case-mix resulting from the application of different selection criteria resulted in a wide range of measured dementia rates (tables 1 and 2). Exclusion of older or functionally impaired patients from the population resulted in a halving (p<0.001) of pre-event dementia from 93/1236 (8%) to 23/917 (3%), whereas exclusion of co-morbid and dysphasic patients produced reductions of around a quarter (table 1) with qualitatively similar although smaller effects in the group of hospitalised patients (table 2). Exclusion criteria also had significant impact on post-event dementia rates with the greatest effects for age. Rates of pre- and post-event dementia were around twice as high in hospitalised compared to non-hospitalised patients: pre-event dementia (49/502 (10%) vs 44/734 (6%), p=0.01) and post-event dementia (99/416 (24%) vs 74/690 (11%), p<0.0001).
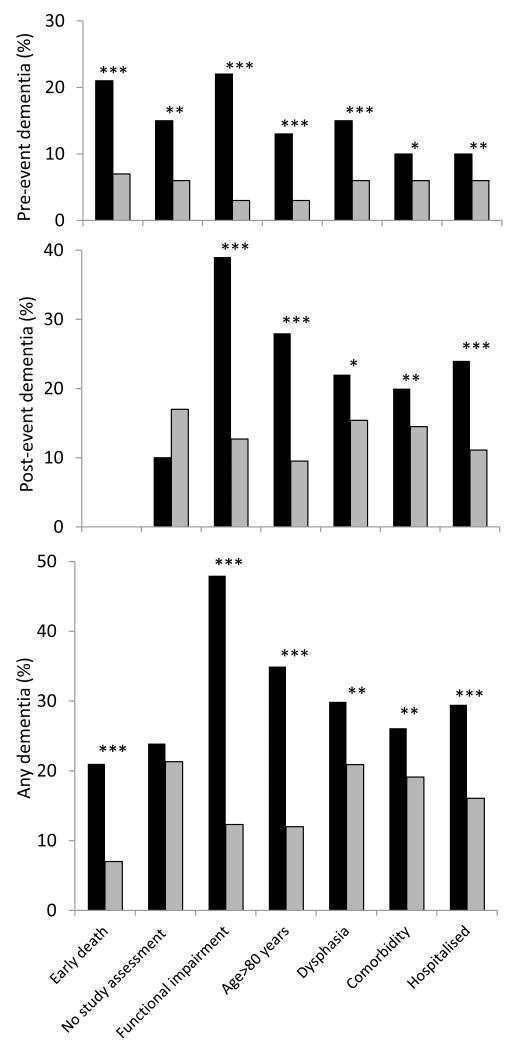
The impact of selection is unsurprising given the markedly higher dementia rates in those with specific exclusion criteria (figure 2). Pre-event dementia was seven times more prevalent in those with versus without prior functional impairment (70/319 (22%) vs 23/917 (3%) p<0.0001)) and four times as common in those aged >80 versus ≤80 years (69/512 (13%) vs 24/724 (3%), p<0.0001). In patients surviving to ascertainment, new post-event dementia (excluding pre-event dementia) was around three times as common in those with functional impairment (83/212 (39%) vs 90/894 (10%), p<0.0001) or age >80 years (110/406 (27%) vs 63/700 (9%), p<0.0001). Overall, any dementia (pre- or post-event) was most common in those with pre-morbid functional impairment followed by those ≥80 years, with dysphasia or who were hospitalised for their event (figure 2).
Figure 2.
Top graph: percentage of population with pre-event dementia. Middle graph: percentage of patients surviving to ascertainment with post-event dementia to 1-year (cumulative incidence excluding pre-event dementia). Bottom graph: percentage of population with any (pre and post-event) dementia to 1-year. Black bars indicate those with versus without (grey bars) specific baseline exclusion criteria, ***p<0.001, **p<0.01, *p<0.05.
Discussion
In our study of over 1200 patients with TIA and stroke from a defined population, sample size, case-mix and measured rates of pre- and post-event dementia were substantially impacted by the application of different baseline selection criteria. Rates of dementia were several fold higher in groups excluded in previous studies including in those non-available for direct study assessment (for pre-event dementia), and with older age, dysphasia and prior functional impairment. Dementia rates were twice as high in hospitalised patients, consistent with fact that they were older and had more major stroke than non-hospitalised patients.
Even in the absence of restrictive criteria, older, more impaired patients were more likely to die before ascertainment and less likely to undergo formal study interview in keeping with epidemiological observations from non-stroke populations.3 Previous inclusive studies of pre-stroke dementia note difficulties in applying informant-based assessments of pre-morbid cognitive function in patients with early death in hospital-based studies27 and before assessment/ascertainment in population-based studies.28 Our findings show that indirect exclusion of such patients results in underestimation by one-third of pre-event dementia since rates were over threefold higher in those dying before versus surviving to assessment and over two-fold higher in those not assessed. Post-event dementia rates are less impacted by indirect selective baseline assessment since such patients often die early on follow-up.
Besides being subject to unavoidable indirect baseline selection, many previous studies of stroke-associated dementia also employed specific exclusion criteria, commonly including dysphasia, but also older age and co-morbidity, dependency or a combination of these.2,3 Exclusion of dysphasia patients is often undertaken as there are difficult methodological issues in assessing cognition in such patients. If dysphasic patients are not excluded, it is important to ensure that low cognitive scores are evaluated in the light of other available clinical information. In our large pragmatic study, all cognitive tests from testable dysphasic patients were coded. Where the cognitive scores fell below the dementia threshold, the patient’s study assessment (where available) and all available clinical records were reviewed to establish whether the DSM criteria were satisfied. For untestable dysphasic patients, diagnosis of dementia was made if the criteria were satisfied after review of all available data including information from carers and primary care.
Our findings show the extent to which such selection criteria impact on case-mix and thus on measured rates of both pre- and post-event dementia. All selection criteria resulted in unrepresentative cohorts of younger, fitter patients with less severe stroke and dementia rates that were up to seven-fold lower than in the corresponding excluded group. The effect of selection can also be seen when comparing the relatively young mean age (~69-70 years) of subjects included in previously reported hospitalised cohorts with that seen in our study (>77 years).3
Both pre- and post-event dementia were more common in hospitalised patients versus in the total population with symptomatic cerebrovascular disease and hospitalisation was associated with a greater prevalence of major stroke as seen in previous studies.2,3 However, the current study demonstrates that hospitalised patients also have a greater prevalence of non-stroke factors associated with dementia including older age, premorbid functional dependency and severe leukoaraiosis.
There are some limitations to our study. We relied on the primary care record to inform pre- and post-event dementia status for those without direct study data. Only 41% of patients with pre-event dementia had a formal diagnosis of the condition listed in the primary care record in keeping with reported under-recording of dementia diagnosis in primary care.29 However, we tried to correct for this by hand searching of the entire GP consultation record including individual consultation records and all hospital clinic and discharge letters to look for evidence of cognitive impairment satisfying the DSM-IV criteria for dementia. Moreover, any under-estimation of dementia by this method in non-assessed cases will have resulted in conservative estimates of the impact of bias. Primary care records are of particular value in the UK where patients have a single primary care provider holding a continuous life-long record for that individual but this may not be available in other healthcare systems. Second, we used MMSE<24 to diagnose dementia in those with study assessment rather than clinical diagnosis using established dementia criteria which may have underestimated mild dementia in this cohort with cerebrovascular disease or over-estimated it in subjects with low education although recent studies suggest the MMSE is reliable for detecting multi-domain cognitive impairment and dementia in this population.1,20,23, 30,31
In conclusion, different baseline selection criteria had a major impact on case-mix and measured pre- and post-event dementia rates in patients with TIA and stroke. The majority of previous studies were subject to these selection biases, likely accounting for heterogeneity in reported dementia rates which may have been under-estimated. Future studies on pre- and post TIA/stroke cognitive impairment should be as inclusive as possible: interventions to prevent dementia are likely to be ineffective at the population level if developed in response to results from highly selected groups. In particular, older or functionally impaired patients should not be excluded and data should be reported on unavailable patients in whom the use of indirect assessment should be considered.
Acknowledgements
We would like to acknowledge the use of the facilities of the Acute Vascular Imaging Centre, Oxford.
Sources of Funding The Oxford Vascular Study has been funded by the Wellcome Trust, Wolfson Foundation, UK Stroke Association, British Heart Foundation, Dunhill Medical Trust, National Institute of Health Research (NIHR), Medical Research Council, and the NIHR Oxford Biomedical Research Centre. Dr Pendlebury is supported by the NIHR Oxford Biomedical Research Centre. Professor Rothwell is an NIHR Senior investigator and a Wellcome Trust Senior Investigator.
Footnotes
Disclosures/ Competing interests: None declared.
References
- 1.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, et al. Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42:2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: A systematic review and meta-analysis. Lancet Neurol. 2009;8:1006–1018. doi: 10.1016/S1474-4422(09)70236-4. [DOI] [PubMed] [Google Scholar]
- 3.Pendlebury ST. Dementia in patients hospitalized with stroke: rates, time course, and clinico-pathologic factors. Int J Stroke. 2012;7:570–81. doi: 10.1111/j.1747-4949.2012.00837.x. [DOI] [PubMed] [Google Scholar]
- 4.Rothwell PM, Coull AJ, Giles MF, Howard SC, Silver LE, Bull LM, et al. Oxford Vascular Study Change in stroke incidence, mortality, case-fatality, severity, and risk factors in Oxfordshire, UK from 1981 to 2004 (Oxford Vascular Study) Lancet. 2004;363:1925–33. doi: 10.1016/S0140-6736(04)16405-2. [DOI] [PubMed] [Google Scholar]
- 5.Rothwell PM, Coull AJ, Silver LE, Fairhead JF, Giles MF, Lovelock CE, et al. Oxford Vascular Study Population-based study of event-rate, incidence, case fatality, and mortality for all acute vascular events in all arterial territories (Oxford Vascular Study) Lancet. 2005;366:1773–83. doi: 10.1016/S0140-6736(05)67702-1. [DOI] [PubMed] [Google Scholar]
- 6.Coull AJ, Silver LE, Bull LM, Giles MF, Rothwell PM, Oxford Vascular (OXVASC) Study Direct assessment of completeness of ascertainment in a stroke incidence study. Stroke. 2004;35:2041–5. doi: 10.1161/01.STR.0000137605.48864.2f. [DOI] [PubMed] [Google Scholar]
- 7.Hatano S. Experience from a multicentre stroke register: A preliminary report. Bull World Health Organ. 1976;54:541–553. [PMC free article] [PubMed] [Google Scholar]
- 8.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. 3rd. [DOI] [PubMed] [Google Scholar]
- 9.Blennow K, Wallin A, Uhlemann C, Gottfries GC. White matter lesions on CT in Alzheimer patients: relation to clinical symptomatology and vascular factors. Acta Neurol Scand. 1991;83:187–193. doi: 10.1111/j.1600-0404.1991.tb04675.x. [DOI] [PubMed] [Google Scholar]
- 10.Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol. 1987;149:351–6. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- 11.Simoni M, Li L, Paul NL, Gruter BE, Schulz UG, Küker W, et al. Age- and sex-specific rates of leukoaraiosis in TIA and stroke patients: population-based study. Neurology. 2012;79:1215–22. doi: 10.1212/WNL.0b013e31826b951e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rankin J. Cerebrovascular accidents in patients over the age of 60:2 Prognosis. Scott Med J. 1957;2:200–215. doi: 10.1177/003693305700200504. [DOI] [PubMed] [Google Scholar]
- 13.Mahoney FI, Barthel DW. Functional Evaluation: The Barthel Index. Md State Med J. 1965;14:61–5. [PubMed] [Google Scholar]
- 14.Jorm AF. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross-validation. Psychol Med. 1994;24:145–53. doi: 10.1017/s003329170002691x. [DOI] [PubMed] [Google Scholar]; Psychol Med. 1995;25:437. Erratum in: [Google Scholar]
- 15.Lincoln NB, Gladman JR. The Extended Activities of Daily Living scale: a further validation. Disabil Rehabil. 1992;14:41–3. doi: 10.3109/09638289209166426. [DOI] [PubMed] [Google Scholar]
- 16.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 17.Brandt J, Spencer M, Folstein M. The Telephone Interview for Cognitive Status. Neuropsychiatry Neuropsychol Behavioral Neurol. 1988;1:111–117. [Google Scholar]
- 18.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. The montreal cognitive assessment, moca: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 19.Hachinski V, Iadecola C, Petersen RC, Breteler MM, Nyenhuis DL, Black SE, et al. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke. 2006;37:2220–2241. doi: 10.1161/01.STR.0000237236.88823.47. [DOI] [PubMed] [Google Scholar]
- 20.Pendlebury ST, Mariz J, Bull L, Mehta Z, Rothwell PM. Moca, ACE-R and MMSE versus the national institute of neurological disorders and stroke-canadian stroke network vascular cognitive impairment harmonization standards neuropsychological battery after TIA and stroke. Stroke. 2012;43:464–469. doi: 10.1161/STROKEAHA.111.633586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pendlebury ST, Welch SJ, Cuthbertson FC, Mariz J, Mehta Z, Rothwell PM. Telephone assessment of cognition after TIA and stroke: TICSm and telephone MoCA vs face-to-face MoCA and neuropsychological battery. Stroke. 2013;44:227–9. doi: 10.1161/STROKEAHA.112.673384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hodkinson HM. Evaluation of a mental test score for assessment of mental impairment in the elderly. Age Ageing. 1972;1:233–8. doi: 10.1093/ageing/1.4.233. [DOI] [PubMed] [Google Scholar]
- 23.Pendlebury ST, Cuthbertson FC, Welch SJ, Mehta Z, Rothwell PM. Underestimation of cognitive impairment by mini-mental state examination versus the montreal cognitive assessment in patients with transient ischemic attack and stroke: A population-based study. Stroke. 2010;41:1290–1293. doi: 10.1161/STROKEAHA.110.579888. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell AJ. A meta-analysis of the accuracy of the mini-mental state examination in the detection of dementia and mild cognitive impairment. J Psychiatr Res. 2009;43:411–431. doi: 10.1016/j.jpsychires.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 25.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) ed 4 American Psychiatric Association, 24 International standard classification; Washington: 1994. [Google Scholar]
- 26.Kokmen E, Whisnant JP, O’Fallon WM, Chu CP, Beard CM. Dementia after ischemic stroke: a population-based study in Rochester, Minnesota (1960-1984) Neurology. 1996;46:154–9. doi: 10.1212/wnl.46.1.154. [DOI] [PubMed] [Google Scholar]
- 27.Barba R, Castro MD, Del Mar Morin M, Rodriguez-Romero R, Rodriguez-Garcia E, Canton R, et al. Prestroke dementia. Cerebrovascular Diseases. 2001;11:216–24. doi: 10.1159/000047642. [DOI] [PubMed] [Google Scholar]
- 28.Appelros P, Nydevik I, Seiger A, Terént A. Predictors of severe stroke: influence of preexisting dementia and cardiac disorders. Stroke. 2002;33:2357–62. doi: 10.1161/01.str.0000030318.99727.fa. [DOI] [PubMed] [Google Scholar]
- 29.Iliffe S, Robinson L, Brayne C, Goodman C, Rait G, Manthorpe J, et al. DeNDRoN Primary Care Clinical Studies Group. Primary care and dementia: 1. diagnosis, screening and disclosure. Int J Geriatr Psychiatry. 2009;24:895–901. doi: 10.1002/gps.2204. [DOI] [PubMed] [Google Scholar]
- 30.Pendlebury ST, Mariz J, Bull L, Mehta Z, Rothwell PM. Impact of different operational definitions on mild cognitive impairment rate and MMSE and MoCA performance in transient ischaemic attack and stroke. Cerebrovasc Dis. 2013;36:355–62. doi: 10.1159/000355496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lees R, Selvarajah J, Fenton C, Pendlebury ST, Langhorne P, Stott DJ, et al. Test accuracy of cognitive screening tests for diagnosis of dementia and multidomain cognitive impairment in stroke. Stroke. 2014;45:3008–18. doi: 10.1161/STROKEAHA.114.005842. [DOI] [PubMed] [Google Scholar]