Abstract
Myocardin (MYOCD) is a potent transcriptional coactivator that functions primarily in cardiac muscle and smooth muscle through direct contacts with serum response factor (SRF) over cis elements known as CArG boxes found near a number of genes encoding for contractile, ion channel, cytoskeletal, and calcium handling proteins. Since its discovery more than 10 years ago, new insights have been obtained regarding the diverse isoforms of MYOCD expressed in cells as well as the regulation of MYOCD expression and activity through transcriptional, post-transcriptional, and post-translational processes. Curiously, there are a number of functions associated with MYOCD that appear to be independent of contractile gene expression and the CArG-SRF nucleoprotein complex. Further, perturbations in MYOCD gene expression are associated with an increasing number of diseases including heart failure, cancer, acute vessel disease, and diabetes. This review summarizes the various biological and pathological processes associated with MYOCD and offers perspectives to several challenges and future directions for further study of this formidable transcriptional coactivator.
Cell lineage determination and differentiation require context-dependent extracellular and intracellular signaling events that converge upon the nuclear genome to coordinate specific patterns of gene expression requisite for normal cellular homeostasis. Such programs of gene transcription require cell-restricted and more widely expressed DNA binding transcription factors and their attending co-regulators that fashion the epigenome for appropriate control of gene expression. Skeletal muscle cells were the first cell type shown to arise through the activity of a single DNA binding transcription factor[1]. This factor, named MYOD1, represented a paradigm of cell specification[2], and its discovery triggered a surge in interest to identify similarly acting transcription factors that could contribute to the identity of other distinct cell types such as neurons, cardiac muscle cells, beta cells of the pancreas, and various hematopoietic cells[3]-[5]. These and other seminal studies further indicate that a cell's phenotype is not fixed; rather, under appropriate conditions, cells may undergo transdifferentiation from one cell type into another[6].
Unlike most of the ∼250 distinct cell types that arise from discrete regions of the developing embryo, vascular smooth muscle cell (SMC) types originate from multiple points in the embryo and exhibit considerable phenotypic plasticity during development and disease[7]-[11]. Throughout the 1990s and early 2000s, investigators utilized such techniques as low stringency degenerate oligonucleotide screening[12],[13], mRNA differential display[14],[15], and interaction cloning[16]-[18] to discover transcription factors that could program SMC differentiation in a manner similar to MYOD1; however, none of the identified factors exhibited a MYOD1-like function. A major breakthrough occurred in 2001 when Da-Zhi Wang in Eric Olson's laboratory discovered myocardin[19]. As detailed below, myocardin (MYOCD) is both necessary and sufficient for the development and differentiation of most SMC, suggesting it has some features in common with MYOD1. The purpose of this review is to summarize the initial findings related to MYOCD as a powerful co-activator of serum response factor (SRF)-dependent gene expression in cardiac muscle and SMC and the subsequent literature on its expression, regulation, and function in molecular and pathological processes that extend beyond the cardiovascular system. Part one summarizes the initial discovery, mRNA expression, and major function of MYOCD as a vital component to a molecular switch for SMC differentiation. Part two addresses the transcriptional, post-transcriptional, and post-translational control of MYOCD as well as the growing number of regulatory proteins that alter its expression or activity. In part three, the role of MYOCD in other aspects of cell biology is synopsized including suppression of cell growth, modulation of microRNAs and ion channels, repression of skeletal muscle differentiation, and several CArG-independent functions. Part four will highlight a number of pathological disorders in which MYOCD is suspected to play some role. Throughout each section, future directions for the study of this amazing co-activator of gene expression are offered.
Discovery, expression, and initial characterization of myocardin function
The discovery of MYOCD is a lesson in applying computational biology to gene discovery. Exploiting the wealth of data accumulating from global work on transcriptomics, Wang et al. used the simple BLAST search algorithm to compare expressed sequence tags from embryonic cardiac muscle cDNA libraries with existing sequence data and found one of the 20 novel sequences to correspond to the 3′ untranslated region of MYOCD[19]. Myocardin was so named because of its primarily cardiac muscle-restricted pattern of expression during embryogenesis and in the adult[19]. Initial construction of the mouse Myocd cDNA revealed an open reading frame of 807 amino acids with the amino-terminal 300 amino acids consisting of a basic domain, a SAP domain common to transcription factors mediating changes in genomic architecture, and a polyglutamine domain found commonly in transcription factors. Interestingly, expansion of polyglutamine domains in certain transcription factors is implicated in neurodegenerative diseases[20] though such augmentation of polyglutamine tracts in the human MYOCD gene have not been reported in neurons. The carboxy-terminal domain of MYOCD shows little homology to other proteins except for a leucine zipper motif that appears to mediate MYOCD homodimerization[21]. Nuclear localization of ectopically expressed MYOCD and GAL4 reporter assays supported the idea that MYOCD exhibits transcriptional activity[19]. Deletion analysis indicated that most of the transcriptional activity of MYOCD is mediated through the carboxy-terminal 300 amino acids. Reporter assays confirmed MYOCD to be a strong transactivator of promoters containing CArG boxes, which are found predominantly in muscle and cytoskeletal genes[22],[23]. Depending upon context, the degree of MYOCD transactivation was more than three orders of magnitude above baseline making MYOCD one of nature's titanic transcriptional co-activators. Finer mapping studies showed that the basic and polyglutamine domain were essential for MYOCD transcriptional activity. Gel shift assays demonstrated MYOCD was unable to bind to a CArG box from the Tagln promoter; however, MYOCD was shown to physically bind serum response factor (SRF), a ubiquitously expressed DNA binding transcription factor[24], and together SRF-MYOCD formed a ternary complex over the Tagln CArG box[19]. Apparently, MYOCD does not directly contact DNA, though X-ray crystallography experiments of MYOCD bound to SRF over a CArG box should be done to formally prove this point. The association of MYOCD with SRF requires the basic and polyglutamine domains of MYOCD coming into contact with the amino-terminal MADS domain of SRF[19]. The results of this elegant series of experiments proved that MYOCD, like many transcription factors, is modular with respect to its functionality; the amino-terminus serves to bind to SRF and the carboxy-terminus mediates strong transcriptional activation. Deletion of the carboxy-terminal domain of MYOCD resulted in a dominant negative protein that could, if ectopically expressed in developing Xenopus embryos, inhibit endogenous cardiac muscle gene expression[19]. This finding suggested that MYOCD was a critical cofactor for the cardiac muscle program and might be necessary for normal cardiogenesis. Two other myocardin-related factors were subsequently discovered and have been reviewed elsewhere[25]-[28].
Expression profile of myocardin
Initial Northern blotting showed Myocd mRNA to be restricted to adult heart tissue and in situ hybridization of developing mouse embryos confirmed robust expression in the cardiac crescent as early as embryonic day 7.75[19]. Levels of Myocd mRNA persist in the developing mouse heart over later stages of development and could also be detected in SMC of gut and vasculature[19],[29]. Myocd mRNA expression could not be detected in skeletal muscle, either in vivo or in vitro[19],[30]. Further, in situ hybridization failed to detect Myocd mRNA in vascular SMC prior to embryonic day 11.5 in the mouse[29]. Lineage tracing data, however, suggest that Myocd is present in the dorsal aorta as early as embryonic day 10.0 of development[31] (Fig. 1). This suggests that Myocd mRNA may exist in early vascular SMC lineages, but is below the level of detection using standard methods. Expression of Myocd mRNA in heart and SMC-containing tissues was confirmed in developing Xenopus[32] and chicken[33]. RNase protection assay showed robust expression of Myocd mRNA in adult rat aortic SMC, with levels approximating those seen in adult heart tissue[30]. More extensive mRNA profiling revealed human and mouse Myocd mRNA are expressed highly in many adult SMC-containing tissues[29],[34]. Myocd mRNA is not detectable in endothelial cells but is induced in a multipotent stem cell line stimulated to become SMC[34]. Moreover, Myocd mRNA is elevated following stimulation with all-trans retinoic acid[35] (a potent phenotypic modulator of SMC in vitro and in vivo[36]), potassium chloride[37], stretch[38], thrombin[39], NOX4[40], NRF3[41], and TGFβ1[42]. As discussed below, levels of MYOCD mRNA are modulated in association with various human diseases. While many commercial antibodies to MYOCD are available, they do not appear to reliably detect endogenous MYOCD protein, perhaps due to low-level expression. Thus, until a more widely accepted and validated antibody to MYOCD is developed, assessing endogenous MYOCD will be largely restricted to mRNA levels. Utilization of proximity ligation assay or knocking in an epitope tag (such as 3xFLAG) into the endogenous mouse Myocd locus could circumvent challenges presented with existing antibodies to this cofactor.
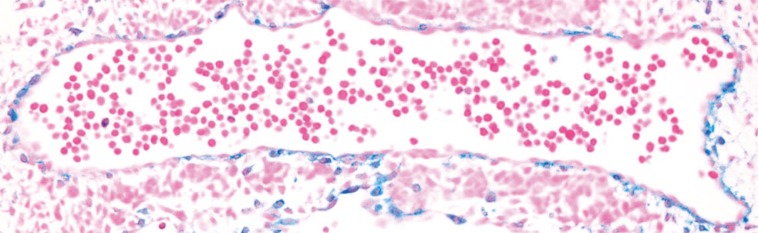
Fig. 1. Lineage tracing of myocardin in developing mouse aorta.
Beta galactosidase staining (blue SMC) of embryonic day 10.0 mouse aorta. Result was obtained in embryos carrying the R26R reporter gene and Cre recombinase knocked into the endogenous Myocd locus.
Myocardin is an essential cofactor for the SMC differentiated phenotype
SMC are notorious for exhibiting a range of phenotypes both in vitro and in vivo[10]. In general, the contractile phenotype of SMC is reduced when they are cultured or following injury to the vessel wall. This process was originally described as de-differentiation[43], but is more often referred to as phenotypic modulation[44], phenotypic plasticity[45] or phenotypic switching[46]. For decades, the transcriptional processes associated with this change in SMC phenotype remained a mystery. The initial reporting of MYOCD acting with SRF to strongly induce cardiac gene expression[19] launched an effort to define the role of this factor in SMC. The first preliminary study in early 2002 indicated that MYOCD could serve a MYOD1-like role in SMC[47]. A subsequent paper published in the same year reported four novel findings pertaining to MYOCD and the SMC differentiation program[30]. First, levels of Myocd mRNA were shown in an RNase protection assay to be lower in immortalized or primary-derived cultures of SMC as compared to SMC within aortic tissue. This result suggested that MYOCD is part of the biochemical de-differentiation program that occurs when SMC are removed from their native milieu and induced to proliferate in a culture dish. Second, overexpression of MYOCD induced the activity of several SMC promoters in a luciferase assay, including the highly specific promoter, Myh11. Several other SMC-restricted genes were later shown to exhibit MYOCD-dependent expression and promoter activity including the potassium channel, Kcnmb1[48], Lmod1[49], and telokin[50]. Third, ectopic expression of MYOCD in a cell line that exhibits no detectable expression of Myocd mRNA resulted in the stimulation of SMC marker genes such as Acta2 and Cnn1. This MYOD1-like gain-of-function study was the first to clearly demonstrate a role for MYOCD in directing an endogenous SMC differentiation program. Finally, overexpression of MYOCD could blunt cell growth, a key characteristic of differentiated, contractile SMC in the vessel wall. Collectively, these findings – the reduced expression of Myocd mRNA in de-differentiated SMC, MYOCD-induced activation of SMC promoters and endogenous SMC marker genes, and the attenuated growth of cells with overexpression of MYOCD – supported a new concept for MYOCD function, namely, its primacy in directing a SMC differentiation program[30]. These findings were subsequently confirmed and extended in important ways by several independent groups[21],[29],[34]. MYOCD also appears sufficient for directing a functionally competent SMC phenotype based on ultrastructural and physiological experiments (e.g., calcium flux and slow wave contraction)[51]-[53]. Interestingly, there is in vivo evidence to support ectopic MYOCD converting hepatocytes to a SMC-like state[54]. Thus, whereas the transdifferentiation of a cell type into another distinct cell type often requires a cocktail of transcription factors[5],[55], it appears that ectopic expression of a single factor (i.e., MYOCD) is adequate for the conversion of a non-SMC into an SMC-like phenotype. Whether cells transduced with MYOCD first revert to a pluripotency state before differentiating into SMC is unclear. Collectively, in vitro studies have established MYOCD as the principal mediator of the normal vascular SMC contractile phenotype.
Further evidence for MYOCD functioning to direct the SMC differentiation program comes from mouse genetic studies. Wang et al. showed first that a pan-knockout of Myocd resulted in mid-gestation arrest with little evidence of aortic SMC differentiation; interestingly, however, embryonic vessels were patterned normally and the heart did not appear to display any obvious defect, though there were signs of defects in the yolk sac vasculature[56]. Subsequent studies indicated a strong heart phenotype in mice lacking normal MYOCD[57],[58]. The reasons for such disparate cardiac data are unclear, but could relate to the strain of mouse studied. It is also relevant to point out that evidence supports some SMC differentiation in vessels where MYOCD is knocked out, suggesting that parallel pathways exist to direct the SMC differentiation program[59]. The weight of evidence, however, clearly supports a dominant role for MYOCD in the establishment and maintenance of a SMC contractile phenotype in vivo[60].
Regulatory control of myocardin expression and activity
Several levels of regulation in MYOCD expression and activity have been studied over the last 10 years, and these can be broadly defined in terms of transcriptional, post-transcriptional, and post-translational control processes. Transcriptional processes include promoter studies and the identification of cis-acting elements either activating or inhibiting gene transcription. Post-transcriptional processes encompass splicing and stability with the latter governed by a growing number of microRNAs. Post-translational processes involve various modifications to the MYOCD protein (e.g., phosphorylation) that modulate its expression or activity. Each of these control processes will be reviewed briefly next.
Transcriptional control of myocardin expression
Several in vitro studies have defined regulatory elements controlling Myocd promoter activity and, by extension, Myocd mRNA expression. The first positive-acting element described was a proximal binding site for the cardiac muscle-restricted transcription factor, NKX2-5[61]. The effect of NKX2-5 on Myocd promoter activity was further shown to be modulated either positively or negatively through direct interactions between NKX2-5 and either CDC7[62] or SMAD3[63], respectively. An upstream NFATc3 binding site was demonstrated in the rat Myocd promoter using ChIP assays and forced expression of constitutively active NFATc3 could stimulate both Myocd promoter activity and mRNA expression[64]. Interestingly, cyclosporine A, which inhibits NFAT activity, reduces Myocd mRNA and SMC markers both in vitro and in vivo upon delivery to the vessel wall[65]. Another putative activator of Myocd expression is the RNA-binding heterogeneous nuclear ribonucleoprotein A1 (HNRNPA1) factor, which binds to a proximal region of the mouse Myocd promoter and stimulates both promoter activity and endogenous mRNA expression in embryonic stem cells[66]. Of note, HNRNPA1 could also bind and stimulate the Srf and Mef2c promoters and physically interacted with SRF to enhance SMC contractile gene expression[66]. Levels of Myocd mRNA are reduced upon inactivation of SRF in a knockout mouse model[67]; however, extensive luciferase assays of conserved CArG elements around the Myocd locus have failed to demonstrate significant SRF-dependent activation (author's unpublished data). Nevertheless, a recent study showed TET2 binding around a CArG box in the human MYOCD promoter with expected demethylation as shown by ChIP assays for the repressive methylation mark, H3K27me3[68]. TET2 was proposed to act upstream of the SRF-MYOCD switch, suggesting that it could be a master orchestrator of the vascular SMC contractile phenotype[68]. FOXO3A[69] and KLF4/KLF5[70] bind to regions of the human MYOCD promoter and appear to mediate transcriptional repression. Further, embryonic fibroblasts derived from p53 knockout mice showed elevated Myocd mRNA expression, and wildtype p53 could dose-dependently repress a 1-kb human MYOCD promoter-driven luciferase reporter[71]. A short hairpin RNA to p53 blunted TGFβ1-induced Myocd and its target genes in SMC[71]. In all of the aforementioned studies, experiments were limited to in vitro analyses and in most cases, the conservation of the putative regulatory element was either not defined or poorly conserved, indicating need for further validation studies.
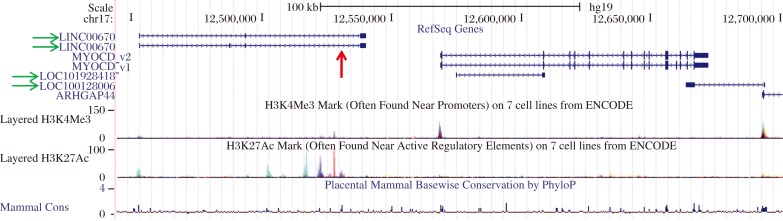
Remarkably, only one publication has examined Myocd promoter activity in the context of a living animal. Creemers et al. performed an elegant series of reporter tiling experiments in mice to show that a distal (∼30 kb upstream) enhancer module recapitulated endogenous Myocd expression during embryonic and postnatal development[72]. Beta galactosidase reporter gene activity was noted as early as embryonic day 10.0 in the dorsal aorta, consistent with lineage tracing data (Fig. 1). The enhancer region was shown by gel shift, expression screening, and luciferase activities to harbor positively-acting binding sites for MEF2C (whose inactivation results in defective SMC differentiation[73]), FOXO4, and TEAD2[72]. No SRF-binding CArG boxes were present in this upstream enhancer region. In this context, MYOCD could activate its own distal enhancer in a MEF2-dependent manner providing the first demonstration of MYOCD acting in a CArG-SRF-independent fashion[72]. It is also interesting to note that the orthologous human distal enhancer lies within an intron of a conserved long intervening noncoding RNA (red arrow in Fig. 2). The functional relationship of the MYOCD enhancer module to this uncharacterized transcript is presently unknown.
Fig. 2. Human MYOCD locus.
Screenshot of UCSC Genome Browser showing the two human cardiac muscle MYOCD isoforms (MYOCD_v1 and MYOCD_v2), plus surrounding long noncoding RNA genes (green horizontal arrows) and the approximate location of the human MYOCD enhancer (red vertical arrow). Note the prominent H3K27 acetylation peaks that mark regulatory elements in close proximity to the enhancer and long noncoding RNA and the smaller H3K4 trimethylation peaks that mark promoters at the 5′ ends of both the MYOCD and ARHGAP44 genes.
The study of the transcriptional control of MYOCD is still in its embryonic stages. Several future studies are needed including in vivo validation of key regulatory elements through either BAC recombineering, as shown for the CNN1 gene[74] or through CRISPR/Cas9-mediated genome editing[75]. Second, several long noncoding RNA genes overlap or are in close proximity to MYOCD (green arrows in Fig. 2), and these could act in cis to affect MYOCD expression. In this context, a recent study proposed that SENCR, a long noncoding RNA expressed abundantly in vascular endothelial cells and SMC, contributes to a SMC differentiated state through undefined effects on MYOCD mRNA expression[76]. Finally, it will be instructive to determine whether any SNPs exist in validated regulatory elements that could compromise MYOCD expression and the normal SMC contractile phenotype.
Post-transcriptional control of myocardin expression
Essentially all protein-coding genes undergo alternative splicing and MYOCD is no exception. Initial reports defined two isoforms of MYOCD that differed by inclusion of a 48 amino acid exon 10a (originally referred to as MYOCD-A)[29],[61]. siRNA knockdown studies targeting this alternate exon suggest a repressor function[77]. Of note, ERK1/2 mediated phosphorylation of MYOCD was shown previously to diminish MYOCD transactivation[78], though none of the phosphorylation sites map to this exon. However, a putative ERK1/2 phosphorylation site was found in exon 10a, suggesting that there may be novel functions yet to be defined[79]. Subsequent work described inclusion of an exon (exon 2a) containing a premature stop codon resulting in downstream usage of a methionine within exon 4 that generates a SMC-restricted MYOCD isoform[80]. Excluding exon 2a results in a longer isoform of MYOCD that exhibits a more cardiac muscle-restricted pattern of expression[80]. Thus, there are at least 4 isoforms of MYOCD (v1-v4) with MYOCD_v1 and MYOCD_v2 encoding longer, cardiac muscle-restricted isoforms of amino acid lengths 983 and 935 (Fig. 2) and MYOCD_v3 and MYOCD_v4 encoding shorter, SMC-restricted isoforms of amino acid lengths 904 and 856[79]. Similar splice variants were described in human tissues[81].
Recently, an RNA-binding protein known as Quaking (QK1) was shown to regulate the balance of MYOCD isoforms in vascular SMC through direct interactions with the pre-mRNA of MYOCD[82]. Normal contractile SMC exhibit little expression of QK1 and express the dominant MYOCD_v3 isoform; however, QK1 expression was elevated in vascular lesions and shown to promote expression of the longer, cardiac muscle isoform of MYOCD (MYOCD_v1)[82]. Interestingly, the MYOCD_v3 isoform was more effective in blocking proliferation of SMC and promoting the contractile state than MYOCD_v1 suggesting that a delicate balance of MYOCD isoforms exists, in vascular SMC to maintain normal vessel wall homeostasis[82]. Formal definition of the function of each MYOCD isoform will require precision-guided genome editing using the CRISPR/Cas9 methodology[75].
Another mechanism for the post-transcriptional control of MYOCD expression is through microRNA-mediated mRNA deadenylation and degradation through miR-binding sites in the ∼5-kb 3′ un-translated region of MYOCD. The miR-143/145 cluster is a major mediator of the SMC contractile phenotype, in part, through its direct or indirect effect on levels of MYOCD[83]. There is a miR-145 binding site in the 3′ un-translated region of MYOCD that somehow augments its stability and or translation[83]. Of note, miR-143/145 expression proceeds in a SRF/MYOCD-dependent manner via an upstream CArG box[83],[84] and studies in human coronary artery SMC have shown that TGFβ1, a potent stimulus for the SMC differentiated phenotype[85], signals to induce miR143/145 expression via CArG and adjacent SMAD binding sites[86]. Binding sites for miR-145 also exist in the KLF4 and KLF5 transcripts, and evidence exists showing conventional repression of these known antagonists of MYOCD expression[83],[84]. KLF4 is also targeted by miR-146a[87], miR-25[88], and miR-1[89], leading to an indirect repressive action on MYOCD expression. Another mechanism of post-transcriptional control of MYOCD is through miR-221-mediated repression of KIT, which apparently can activate MYOCD gene expression[90].
Recently, a miR-1 binding site was demonstrated in the 3′ un-translated region of MYOCD, and levels of MYOCD_v3 (SMC enriched) were higher in miR-1 null mice as were several SMC-restricted genes[91],[92]. Thus, miR-1 is a negative regulator of SMC gene expression in the heart. This post-transcriptional process of MYOCD regulation could explain why there is only transient expression of many SMC-restricted genes in the embryonic heart, only to re-emerge in adult hearts undergoing failure. There are other miR-binding sites in the MYOCD 3′ un-translated region including miR-9[64] and miR-135b[93] that effect changes in cardiac hypertrophy and cellular growth, respectively. It will be important to define the dynamic interplay among the known and yet-to-be-defined microRNAs that bind and regulate MYOCD expression. In particular, it will be instructive to study the functionality of these binding sites in proper context, such as in a living animal.
Post-translational control of myocardin expression and activity
More than 200 post-translational modifications have been defined that mediate a protein's stability, localization or functional activity, often times through direct protein-protein interactions. A recent review summarized some of the chemical modifications of MYOCD protein[94], and a partial listing of protein-protein interactions associated with MYOCD post-translational modifications or attending activity is provided in the Table. As the Table indicates, in most cases, MYOCD association with another protein alters activity through changes in MYOCD-SRF binding. GSK3B mediates MYOCD phosphorylation at several serine residues; inhibiting GSK3B accentuated MYOCD-dependent cardiac gene activation and hypertrophy of cultured cardiomyocytes, suggesting that MYOCD phosphorylation may be an important control mechanism for pathophysiological cardiac hypertrophy[95]. MYOCD undergoes sumoylation on K445 and this modification appears to increase its transactivation over cardiac muscle-specific genes since a K445R mutant exhibits reduced activity[96]. MYOCD can also be ubiquitinylated and this post-translational change has been linked to reduced levels of MYOCD via the proteasome[97]. On the other hand, UBR5, an ubiquitin E3 ligase, interacts with MYOCD and stabilizes its protein expression, thereby enhancing transactivation of SMC-restricted genes; down-regulation of UBR5 resulted in a decrease in MYOCD-induced SMC gene expression[98]. There may be other ubiquitin ligases that interact with MYOCD in certain contexts to enhance or attenuate expression and/or activity.
Table 1. Myocardin (MYOCD)-interacting proteins and functional consequence.
| Interacting protein | Function | Reference |
| ACTR5 (ARP5) | Displace SRF binding; reduced MYOCD activity | [162] |
| EP300 (p300) | Acetylation; increased MYOCD activity | [100] |
| EPC1 | Increased MYOCD activity; reduced neointimal formation | [163] |
| FOXF1 | Enhances SRF binding; increased MYOCD activity | [164] |
| FOXO4 | Displace SRF binding; reduced MYOCD activity | [165] |
| GATA4 | Specifies MYOCD-dependent cardiac muscle cell target gene expression | [103] |
| GATA6 | Specifies MYOCD-dependent SMC target gene expression | [166] |
| GSK3B | Phosphorylation of MYOCD; reduced MYOCD activity | [78],[95] |
| HDAC5 | Repress SMC gene expression | [99] |
| HMGXB4 (HMG2L1) | Displace SRF binding; reduced MYOCD activity | [167] |
| IRF8 | Displace p300 binding; reduced MYOCD activity | [168] |
| IRF9 | Displace p300 binding; reduced MYOCD activity | [169] |
| JUN | Displace SRF binding; reduced MYOCD activity | [170] |
| KDM3A (JMJD1A) | H3K9 demethylase; increased MYOCD activity | [171] |
| KPNB1 (Importin β1) | Nuclear localization of MYOCD | [172] |
| KLF15 | Displace SRF binding; reduced MYOCD activity | [173] |
| NCOA3 (SRC3) | Cofactor of MYOCD; increased MYOCD activity | [174] |
| PDX1 | Increases insulin gene expression in pancreatic β cells | [101] |
| PLAUR (UPAR) | Proteasomal degradation; reduced MYOCD activity | [175] |
| RELA (p65) | Displace SRF binding; reduced MYOCD activity | [106] |
| RUNX2 | Displace SRF binding; reduced MYOCD activity | [176] |
| SMAD3 | Increased MYOCD activity in SMC; CArG-independent | [102] |
| SMARCA4 (BRG1) | Enhance SMC gene expression | [177] |
| SMARCD3 (BRM) | Enhance SMC gene expression | [177] |
| SOX9 | Displace SRF binding; reduced MYOCD activity | [178] |
| STUB1 (CHIP) | Ubiquitinylation of MYOCD; reduced MYOCD activity | [97] |
| TBX5 | Increased MYOCD activity in cardiac muscle; CArG-independent | [104] |
| TDG | Displace SRF binding; reduced MYOCD activity | [179] |
| TERT | Enhance cardiac and SMC gene expression | [180] |
| TSHZ3 | Displace SRF binding; reduced MYOCD activity | [181] |
| UBR5 | Ubiquitinylation of MYOCD; increased MYOCD activity | [98] |
| YAP1 | Displace SRF binding; reduced MYOCD activity | [182] |
MYOCD is known to recruit chromatin remodeling factors such as p300 that acetylate lysine residues in histones tails, leading to augmented gene transcription[99]. More recently, an elegant study demonstrated p300-dependent acetylation of the MYOCD protein. Acetylation occurs at the amino-terminus and appears to enhance the association of MYOCD with SRF over CArG boxes, thereby increasing SMC target gene expression. Acetylation of MYOCD was also associated with displacement of HDAC5, a repressor of gene expression[100]. Thus, we see how a factor (p300) serves a dual role of acetylating both local chromatin and the MYOCD cofactor itself to mediate gene expression. Whether these processes are mutually exclusive or facultative remains to be sorted out. Interestingly, ERK1/2 phosphorylation of MYOCD as well as serine-to-aspartic acid phosphomimetics of MYOCD reduce the interaction between MYOCD and p300 showing that different modifications of MYOCD can exhibit opposing activities[78]. This implies complex and dynamic, context-dependent changes in MYOCD modifications and subsequent activities necessary to effect moment-to-moment cellular changes in SMC and cardiac muscle cell phenotype. Since MYOCD undergoes phosphorylation, it must also interact with phosphatases that remove phosphate groups from key amino acid residues; however, no studies to date have formally demonstrated dephosphorylation of MYOCD through a specific phosphatase. There are a number of other regulatory post-translational modifications such as methylation, hydroxylation, oxidation, sulfation, and glycosylation (to name just a few) that have yet to be demonstrated in MYOCD. Post-translational modification of MYOCD in normal developmental and pathological conditions is an open area of future investigation and will be important to fully elucidate the biology of this important cofactor of gene expression.
Diversity in function of myocardin
Although much of the literature on MYOCD reports its role as a strong cofactor of SRF directing SMC and cardiac muscle gene expression, there is increasing recognition of its biological activities that are independent of CArG-SRF in these muscle cell types or dependent on CArG-SRF in a non-muscle cell type. For example, a recent study showed that MYOCD interacts with the pancreatic beta cell-specific transcription factor, PDX1, to synergistically activate insulin expression in human mesenchymal stem cells, suggesting that an improved method may exist to generate insulin-expressing beta cells for type I diabetes[101]. Further, a number of unanticipated functions of MYOCD have emerged such as its role in blocking cell growth and inhibiting the skeletal muscle program of differentiation. Thus, like many proteins, MYOCD exhibits a number of disparate biological activities and a brief summary of these follows.
CArG-independent stimulatory functions of myocardin on muscle gene expression
As discussed above, MYOCD appears to enhance its own expression through association with MEF2 over a MEF2-binding site located in an upstream (CArG-less) enhancer region, presumably through the well-defined MEF2 interaction site located in the amino-terminal RPEL domain of MYOCD[72]. MYOCD binds to SMAD3 over a SMAD response element and increases Tagln (aka Sm22α) promoter activity independent of the upstream CArG elements in this promoter[102]. MYOCD can interact with the DNA-binding domain of GATA4 independent of SRF and stimulate cardiac muscle gene expression. Interestingly, the stimulatory effect of MYOCD-GATA4 does not require the transactivation domain of GATA4, which when present acts to repress cardiac muscle genes[103]. Another report showed that MYOCD can interact with the TBX5 transcription factor to direct cardiac muscle (but not SMC) gene expression through the TBX-binding site in cardiac muscle gene promoters[104]. This study provides some insight into how MYOCD distinguishes between target genes associated with cardiac muscle versus SMC gene programs. ITGA8, an integrin alpha subunit highly enriched in SMC, is stimulated by MYOCD independent of SRF or a CArG element[105]. It is unclear as to how MYOCD stimulates this and perhaps other genes that do not otherwise require the CArG-SRF binary complex for gene activation. Further, it will be important to assess the full complement of MYOCD-dependent, CArG-SRF-independent genes in cells where SRF is lacking using ChIP-seq and RNA-seq following MYOCD overexpression. This will require new antibodies to MYOCD such as one recognizing an endogenous, epitope-tagged MYOCD protein.
Growth inhibitory action of myocardin
Given the dominant role of MYOCD in establishing a SMC contractile phenotype, it was perhaps not surprising to find that its overexpression resulted in reduced proliferation of stably-transfected cells[30]. This early finding was corroborated by a series of subsequent studies showing MYOCD-dependent growth inhibition in SMC associated with blunted action of NFκB[106], reduced cyclin D1 expression[51], and miR-1 induction[107]. Interestingly, there is also evidence for loss in MYOCD expression in certain neoplasms, and the expression of MYOCD has been shown to block cancer cell phenotype through its powerful orchestration of the SMC contractile state[108],[109]. Future challenges include the elucidation of MYOCD-dependent cyclin D1 repression as well as other genes that are likely to be influenced by the MYOCD cofactor (e.g., induction of tumor suppressor genes).
Repression of skeletal muscle differentiation
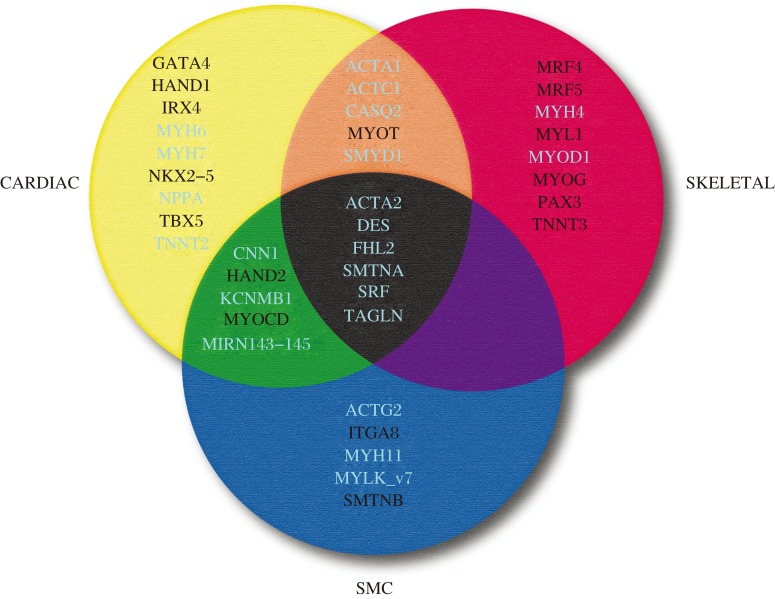
SRF is most abundant in all three muscle types where high level expression of SRF-dependent contractile genes exist[110]. In addition to SRF, there are some SRF-dependent genes that are expressed transiently in each of the three muscle cell types during development (e.g., Acta2 and Tagln)[111]. Importantly, whereas embryonic cardiac muscle and SMC lineages exhibit overlapping patterns of SMC gene expression, there is no such overlap in gene expression between SMC and skeletal muscle (Fig. 3). The molecular basis for this lack of overlap in gene expression between skeletal muscle and SMC was not known until the serendipitous finding that MYOCD completely blocks expression of myogenin, a skeletal muscle master regulatory gene[31]. Repression occurred at the level of the myogenin promoter via MYOCD binding MYOD1, thereby competitively inhibiting MYOD1 binding to an E-box in the myogenin promoter[31]. Consistent with these findings, MYOCD overexpression was shown to inhibit atrogin expression and the skeletal muscle phenotype while knockdown of atrogin elevated MYOCD in the L6 skeletal muscle cell precursor[112]. The incompatibility of the SMC and skeletal muscle gene programs is consistent with developmental lineage decisions where common progenitors in the somite give rise to either skeletal muscle or vascular SMC[113]. In this context, lineage tracing studies suggest that MYOCD is transiently expressed within the somite invoking the hypothesis that somitic progenitors with MYOCD expression may be fated for vascular SMC[31],[114].
Fig. 3. 3-set Venn diagram of genes across the three muscle cell types.
Shown are the most specific genes unique to each of the indicated muscle cell types. White-labeled genes are those that lack functionally-validated CArG boxes while the genes labeled in light color harbor functional CArG boxes. Genes common to the three muscle cell types during development (center) each have at least one functional CArG box. Whereas several genes are present at the intersection of skeletal and cardiac muscle (orange) and cardiac and SMC (green), no genes are found at the intersection of SMC and skeletal muscle (purple). See the text for discussion.
Miscellaneous functions of myocardin
In order for cells to contract, there must be careful transcriptional control of ion currents across cell membranes. Surprisingly, little has been done to link MYOCD to specific activation of ion channel genes. The KCNMB1 gene, which is highly restricted to SMC, is a direct target of MYOCD though two CArG-SRF sites in 5′ untranslated and proximal intronic regions[48]. Genetic proof for the function of these CArG elements was demonstrated in transgenic mouse experiments[48]. Remarkably, ectopic expression of MYOCD can elicit ion current activity in cultured cells[48],[53]. Interestingly, ion currents, particularly voltage-dependent calcium entry, can elicit MYOCD expression and various SMC contractile genes[37],[115]. These intriguing findings underscore the important concept of excitation-transcription coupling[116], and highlight the gap in understanding how channel activity mediates MYOCD gene transcription or stability[117]. Given the wide number of ion channels expressed in both cardiac muscle and SMC[118],[119], it will be important to delineate those channels whose expression requires MYOCD and whether their expression proceeds in a CArG-SRF-dependent or CArG-SRF–independent manner. There is also a growing appreciation for the role of MYOCD in activation of microRNAs such as miR-145[83] and miR-1[107]. Of note, some microRNAs may be repressed by ectopic MYOCD expression, including miR-199a-3p and miR-214[120]. That MYOCD may repress gene transcription (miRs, myogenin), emphasizing the need for more study of the binding partners of MYOCD in the nucleus that directly or indirectly influence chromatin architecture as well as RNA polymerase II-dependent transcription. Finally, as more RNA-seq experiments are undertaken, it will be informative to further advance our understanding of MYOCD-dependent gene expression.
Pathological processes linked to myocardin
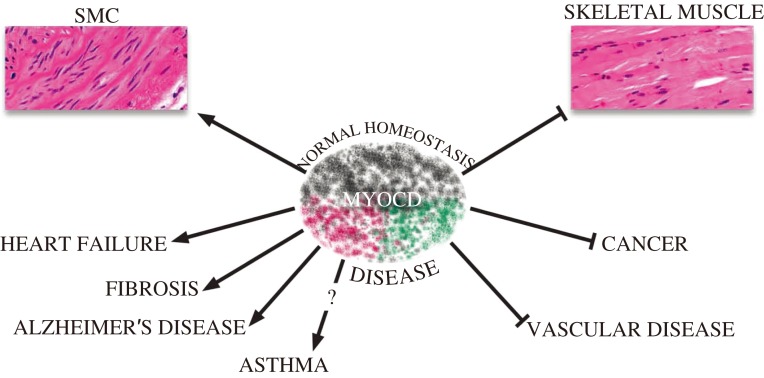
There is a growing literature documenting changes in MYOCD expression in association with a number of diseases. In addition, MYOCD has been shown to attenuate or exacerbate disease phenotypes, highlighting its potential as a therapeutic agent or target for disease prevention (Fig. 4). Much of the literature describes altered expression of MYOCD in diseases of the heart or vasculature, but there are other disease states (both clinical and experimental) linked or correlated with changes in MYOCD expression.
Fig. 4. Diverse roles of MYOCD in normal homeostasis and disease.
Shaded colors of MYOCD icon reflect its diversity in activity, most of which relates to the maintenance of normal homeostasis (SMC differentiation and repression of the skeletal muscle phenotype). MYOCD may contribute to diseases (shade of red) or attenuate diseases (shade of green). See the text for details.
Cardiovascular diseases
Heart failure is a pervasive clinical problem in Western countries and is a leading cause of morbidity and mortality. There are several causes of heart failure including ischemic heart disease and hypertension with the terminal stages characterized as dilated cardiomyopathy (DCM). Levels of MYOCD mRNA were first shown to be elevated in humans with DCM as compared to normal hearts[81]. Moreover, there have been a series of clinical studies showing increases in MYOCD mRNA in circulating cells or cardiac tissue from patients with idiopathic cardiomyopathy[121],[122], hypertrophic cardiomyopathy[123], and essential hypertension[124]. Interestingly, variants in the 5′ promoter region of human MYOCD were associated with reduced levels of circulating MYOCD, which correlated with attenuated left ventricular mass in hypertrophic cardiomyopathy patients[125]. The function of these promoter variants is unknown at this time, but they presumably interfere with the transcriptional regulation of MYOCD. A rare missense mutation (K259R) of MYOCD has been reported in a congenital heart disease patient, and this mutation alters SRF binding to the cardiac, but not the SMC, isoforms of MYOCD[126]. Increases in MYOCD transcripts were observed with elevations in GATA4 and NKX2-5 in patients with 3-vessel coronary artery disease[127]. The latter clinical data are congruent with experimental studies showing forced expression of MYOCD provoking impaired left ventricular systolic function and electrical activity in the piglet[128] as well as hypertrophy of cardiomyocytes[122]. Importantly, silencing MYOCD via intramyocardial delivery of a short hairpin RNA in a doxorubicin-induced model of heart failure, normalized increases in MYOCD and several fetal genes (including various SMC genes) and attenuated cardiac muscle dysfunction and death[129]. Collectively, these clinical and experimental data suggest that MYOCD represents a viable therapeutic target/biomarker in the setting of cardiac hypertrophy and failure.
Whereas MYOCD levels increase in association with cardiac disease, a number of reports have found MYOCD mRNA to be transiently down-regulated following acute injuries to the blood vessel wall in different animal models[130]-[134]. A recent elegant study showed that MYOCD delivery to the vessel wall blocked the neointimal response to vascular injury through microRNA-mediated suppression of the Pdgfrb gene[132]. This result suggests therapeutic potential for enhanced MYOCD expression in the context of acute injuries such as those encountered in the clinic where there remains some residual restenosis rates following balloon angioplasty of atherosclerotic lesions. MYOCD levels are also reduced in experimental models of atherosclerosis[135] and hypertension[136]. It remains to be studied whether ectopic MYOCD exerts any beneficial effect in these chronic conditions of vascular disease. MYOCD mRNA levels were reported to be reduced in venular SMC of experimentally-induced and clinically-diagnosed variocose veins[137]. Interestingly, treatment with Bortezomib, (a proteasome inhibitor) enhanced MYOCD expression and reduced the proliferation and migration of SMC as well as the varicosity of veins in a mouse model[137].
Pulmonary hypertension is associated with hypoxia and the formation of so-called plexiform lesions. Hypoxia induces Midkine which has been linked to increases in MYOCD in the setting of pulmonary hypertension[138], and these findings are congruent with hypoxia-mediated increases in MYOCD in other models of pulmonary hypertension[139],[140]. Hypoxia has also been shown to induce MYOCD in human cerebral SMC as well as rodent SMC[141]. Hypoxia-induced MYOCD in this setting appears to be linked to faulty amyloid beta clearance and the emergence of cerebral amyloid angiopathy, which is pathognomonic of Alzheimer's disease[141]. Of note, a putative hypoxia response element was found in the 5′ promoter region of Myocardin[141]; however, formal proof that this element binds to HIF1A and underlies the induction of MYOCD by hypoxia awaits further study. Interestingly, increases in MYOCD and the SMC contractile program have also been observed in Alzheimer's disease patient-derived cerebral SMC with consequent hyper-contractility and hypoperfusion, which are additional features of Alzheimer's disease in humans[142]. Thus, Alzheimer's disease and attending neurodegeneration and cognitive decline may result from elevated MYOCD expression with poor amyloid clearance and hypoperfusion due to hypercontractile vascular SMC[143]. This would suggest a more vascular-centric (as opposed to neuro-centric) view for the pathogenesis of this devastating neurological disorder. Although the weight of evidence supports hypoxia as a stimulus for MYOCD induction, there are studies that show a negative correlation between MYOCD levels and hypoxia, highlighting the context-dependent nature of these responses[144],[145]. Finally, there is one report of increased MYOCD in calcified aortic valve cusps with accompanying elevations in SMC markers, suggesting that SMC-derived MYOCD could play a role in the pathogenesis of calcific aortic stenosis[146]. There remain other vascular disorders where altered MYOCD may be of functional consequence, including peripheral artery disease, aneurysm formation, and transplant arteriopathy. The integration of inducible isoforms of MYOCD in animal genomes using CRISPR-Cas9 technology will be of major interest to further assess the efficacy of MYOCD in thwarting a number of vascular disorders.
Cancer
The consistent finding that MYOCD suppresses normal cell growth is consonant with the increasing evidence supporting a tumor suppressor role for this SRF cofactor in the setting of cancer[147]. One clinical study has shown attenuated MYOCD in several nasopharyngeal carcinoma cell lines, and the decrease in MYOCD correlated with hypermethylation of its promoter[148]. Notably, treatment of these cancer cells with the de-methylating agent, 5-azacytidine, increased MYOCD expression, further suggesting the tumor suppressive nature of MYOCD[148]. Sometimes, the tumor suppressor effect of MYOCD may be indirect. For example, Maspin is transcriptionally induced by MYOCD and the up-regulation of Maspin leads to apoptosis of breast cancer cells[149]. In uterine leiomyosarcoma cell lines, MYOCD shows clear growth suppressive properties, in part through the activation of the p21 growth inhibitor via SRF-binding CArG element[109], but also because of the induction of several contractile genes such as CNN1, which itself has been labeled a tumor suppressor gene[150]. In this context, CNN1 and other SMC contractile genes have been demonstrated to be reduced in metastatic human tumors and form part of a molecular signature for the metastatic phenotype[151]. Whether reductions in these genes, all of which carry SRF-binding CArG boxes, stem from reduced levels of MYOCD is presently unknown. It must be stressed, however, that there are some instances where levels of MYOCD are elevated in tumor cells[152], further underscoring the complexity of human disease and the need to exercise circumspection when making broad assumptions over the importance of this or any other protein in human pathology. On one final note, it is intriguing to consider the fact that while vascular SMC exhibit phenotypic plasticity, vascular leiomyosarcomas are exceedingly rare cancers[153]. The molecular basis for such scarce tumors is completely unknown, but may well relate to the SMC-restricted isoforms of MYOCD that appear to exert greater growth inhibitory action than the longer, cardiac muscle-enriched isoforms[91].
Diabetes
Type 2 diabetes has ascended to epidemic proportions and will increasingly strain Western health care systems. One of the manifestations of type 2 diabetes is a spike in RAGE with inflammation and calcification. Overexpression of RAGE suppresses MYOCD and SMC contractile genes and favors an osteogenic phenotype, which can lead to calcification of arteries[154]. In addition to vascular complications, diabetic patients often present with erectile dysfunction. Experimental models of diabetes have consistently shown a reduced expression of MYOCD in the corpus cavernosum[155],[156]. From a therapeutic standpoint, ectopic MYOCD was shown to reconstitute normal erectile function in diabetic rats through the conversion of proliferating SMC within the corpus cavernosum to a contractile state[157]. Other complications of diabetes include accelerated atherosclerosis, retinal angiopathy, and peripheral artery disease all of which have yet to be examined in the context of MYOCD.
Miscellaneous diseases
There are several other diseases in which MYOCD has been studied. For example, in a model of intestinal obstruction, MYOCD mRNA levels were shown to be reduced concomitant with several MYOCD-dependent SMC contractile genes[158]. In one of the first examples of Myocd haploinsufficiency, mice with only one functional Myocd allele, exhibited bladder SMC hypersensitivity. This was surmised to result from the reduced expression of miR-1 and a corresponding increase in a known miR-1 target mRNA, Gja1 or connexin 43. Adult mice with one copy of Myocd showed lower bladder capacity consistent with a hypersensitive phenotype[159]. Hepatic stellate cells can undergo transdifferentiation to a myofibroblast-like phenotype in liver diseases associated with fibrosis, and studies have shown MYOCD is induced in experimental models of liver fibrosis[160],[161]. Interestingly, reducing MYOCD with siRNA predictably normalized the myofibroblast phenotype, suggesting that MYOCD (and probably its related MRTFs) could be a novel target for the treatment of liver fibrosis[161].
Future perspectives
The last 10 years of research on MYOCD have focused mainly on its well accepted role as the principal component to a switch for the SMC differentiated state. There have been new insights into MYOCD regulation, its association with other proteins, as well as its expression and potential utility as a marker or target of therapy for several diseases. Work in the next decade should be focused on the development of new reagents (antibodies) and animal models (inducible expression of MYOCD) to further understand this remarkable cofactor's function in normal and pathological processes. In addition, there should be effort devoted towards fully elucidating transcriptomes under control of MYOCD, particularly the expanding class of long non-coding RNAs. Further, we need to define the mechanisms through which MYOCD functions independently of SRF. What other DNA binding factors does MYOCD interact with and in what contexts? There are SMC-associated diseases in which virtually nothing is known regarding expression and functionality of MYOCD (e.g., asthma). Finally, as more and more human genomes are sequenced, it will be informative to define the functionality of sequence variants such as SNPs both in and around MYOCD coding and non-coding sequence space.
Acknowledgments
Dr. Miano is a Fellow at the Collaborative Innovation Center for Cardiovascular Translational Medicine of Jiangsu Province.
References
- 1.Davis RL, Weintraub H, Lassar AB.Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell 198751(6), 987–1000. [DOI] [PubMed] [Google Scholar]
- 2.Olson EN.MyoD family: a paradigm for development. Genes Dev 19904(9), 1454–1461. [DOI] [PubMed] [Google Scholar]
- 3.Lee JE, Hollenberg SM, Snider L, et al. Conversion of Xenopus ectoderm into neurons by NeuroD, a basic helix-loop-helix protein. Science 1995268(5212), 836–844. [DOI] [PubMed] [Google Scholar]
- 4.Komuro I, Izumo S.Csx: A murine homeobox-containing gene specifically expressed in the developing heart. Proc Natl Acad Sci U S A 199390(17), 8145–8149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graf T, Enver T.Forcing cells to change lineages. Nature 2009462(7273), 587–594. [DOI] [PubMed] [Google Scholar]
- 6.Graf T.Historical origins of transdifferentiation and reprogramming. Cell Stem Cell 20119(6), 504–16. [DOI] [PubMed] [Google Scholar]
- 7.Majesky MW.Developmental basis of vascular smooth muscle diversity. Arterioscler Thromb Vasc Biol 200727(6), 1248–1258. [DOI] [PubMed] [Google Scholar]
- 8.Campbell GR, Campbell JH.Smooth muscle phenotypic changes in arterial wall homeostasis: Implications for the pathogenesis of atherosclerosis. Exp Mol Pathol 198542(2), 139–162. [DOI] [PubMed] [Google Scholar]
- 9.Halayko AJ, Solway J.Molecular mechanisms of phenotypic plasticity in smooth muscle cells. J Appl Physiol 200190(1), 358–368. [DOI] [PubMed] [Google Scholar]
- 10.Owens GK, Kumar MS, Wamhoff BR.Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev 200484(3), 767–801. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen AT, Gomez D, Bell RD, et al. Smooth muscle cell plasticity: fact or fiction? Circ Res 2013112(1), 17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel CV, Gorski DH, LePage DF, et al. Molecular cloning of a homeobox transcription factor from adult aortic smooth muscle. J Biol Chem 1992267(36), 26085–26090. [PubMed] [Google Scholar]
- 13.Miano JM, Firulli AB, Olson EN, et al. Restricted expression of homeobox genes distinguishes fetal from adult human smooth muscle cells. Proc Natl Acad Sci U S A 199693(2), 900–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsieh CM, Yoshizumi M, Endege WO, et al. APEG-1, a novel gene preferentially expressed in aortic smooth muscle cells, is down-regulated by vascular injury. J Biol Chem 1996271(29), 17354–17359. [DOI] [PubMed] [Google Scholar]
- 15.Watanabe M, Layne MD, Hsieh CM, et al. Regulation of smooth muscle cell differentiation by AT-rich interaction domain transcription factors Mrf2alpha and Mrf2beta. Circ Res 200291(5), 382–389. [DOI] [PubMed] [Google Scholar]
- 16.Layne MD, Endege WO, Jain MK, et al. Aortic carboxypeptidase-like protein, a novel protein with discoidin and carboxypeptidase-like domains, is up-regulated during vascular smooth muscle cell differentiation. J Biol Chem 1998273(25), 15654–15660. [DOI] [PubMed] [Google Scholar]
- 17.Chin MT, Maemura K, Fukumoto S, et al. Cardiovascular basic helix loop helix factor 1, a novel transcriptional repressor expressed preferentially in the developing and adult cardiovascular system. J Biol Chem 2000275(9), 6381–6387. [DOI] [PubMed] [Google Scholar]
- 18.Kawai-Kowase K, Kumar MS, Hoofnagle MH, et al. PIAS1 activates the expression of smooth muscle cell differentiation marker genes by interacting with serum response factor and class I basic helix-loop-helix proteins. Mol Cell Biol 200525(18), 8009–8023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang DZ, Chang PS, Wang Z, et al. Activation of cardiac gene expression by myocardin, a transcriptional cofactor for serum response factor. Cell 2001105(7), 851–862. [DOI] [PubMed] [Google Scholar]
- 20.Yamada M, Tsuji S, Takahashi H.Pathology of CAG repeat diseases. Neuropathology 200020(4), 319–325. [DOI] [PubMed] [Google Scholar]
- 21.Wang Z, Wang DZ, Pipes GCT, et al. Myocardin is a master regulator of smooth muscle gene expression. Proc Natl Acad Sci U S A 2003100(12), 7129–7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Minty A, Kedes L.Upstream regions of the human cardiac actin gene that modulate its transcription in muscle cells: presence of an evolutionarily conserved repeated motif. Mol Cell Biol 19866(6), 2125–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miano JM.Serum response factor: toggling between disparate programs of gene expression. J Mol Cell Cardiol 200335(6), 577–593. [DOI] [PubMed] [Google Scholar]
- 24.Norman C, Runswick M, Pollock R, et al. Isolation and properties of cDNA clones encoding SRF, a transcription factor that binds to the c-fos serum response element. Cell 198855(6), 989–1003. [DOI] [PubMed] [Google Scholar]
- 25.Cen B, Selvaraj A, Prywes R.Myocardin/MKL family of SRF coactivators: key regulators of immediate early and muscle specific gene expression. J Cell Biochem 200493(1), 74–82. [DOI] [PubMed] [Google Scholar]
- 26.Wang DZ, Olson EN.Control of smooth muscle development by the myocardin family of transcriptional coactivators. Curr Opin Genet Dev 200414(5), 558–566. [DOI] [PubMed] [Google Scholar]
- 27.Pipes GCT, Creemers EE, Olson EN.The myocardin family of transcriptional coactivators: versatile regulators of cell growth, migration, and myogenesis. Genes Dev 200620(12), 1545–1556. [DOI] [PubMed] [Google Scholar]
- 28.Parmacek MS.Myocardin-related transcription factors: critical coactivators regulating cardiovascular development and adaptation. Circ Res 2007100(5), 633–644. [DOI] [PubMed] [Google Scholar]
- 29.Du K, Ip HS, Li J, et al. Myocardin is a critical serum response factor cofactor in the transcriptional program regulating smooth muscle cell differentiation. Mol Cell Biol 200323(7), 2425–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen J, Kitchen CM, Streb JW, et al. Myocardin: a component of a molecular switch for smooth muscle differentiation. J Mol Cell Cardiol 200234(10), 1345–1356. [DOI] [PubMed] [Google Scholar]
- 31.Long X, Creemers EE, Wang DZ, et al. Myocardin is a bifunctional switch for smooth versus skeletal muscle differentiation. Proc Natl Acad Sci U S A 2007104(42), 16570–16575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Small EM, Warkman AS, Wang DZ, et al. Myocardin is sufficient and necessary for cardiac gene expression in Xenopus. Development 2005132(:987–997). [DOI] [PubMed] [Google Scholar]
- 33.Warkman AS, Yatskievych TA, Hardy KM, et al. Myocardin expression during avian embryonic heart development requires the endoderm but is independent of BMP signaling. Dev Dyn 2008237(1), 216–221. [DOI] [PubMed] [Google Scholar]
- 34.Yoshida T, Sinha S, Dandre F, et al. Myocardin is a key regulator of CArG-dependent transcription of multiple smooth muscle marker genes. Circ Res 200392(8), 856–864. [DOI] [PubMed] [Google Scholar]
- 35.Yoshida T, Kawai-Kowase K, Owens GK.Forced expression of myocardin is not sufficient for induction of smooth muscle differentiation in multipotential cells. Arterioscler Thromb Vasc Biol 200424(9), 1596–1601. [DOI] [PubMed] [Google Scholar]
- 36.Miano JM, Berk BC.Retinoids: Versatile biological response modifiers of vascular smooth muscle phenotype. Circ Res 200087(5), 355–362. [DOI] [PubMed] [Google Scholar]
- 37.Wamhoff BR, Bowles DK, McDonald OG, et al. L-type voltage-gated Ca 2+ channels modulate expression of smooth muscle differentiation marker genes via a Rho kinase/myocardin/SRF-dependent mechanism. Circ Res 200495(4), 406–414. [DOI] [PubMed] [Google Scholar]
- 38.Chiu CZ, Wang BW, Shyu KG.Effects of cyclic stretch on the molecular regulation of myocardin in rat aortic vascular smooth muscle cells. J Biomed Sci 201320(1), 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martin K, Weiss S, Metharom P, et al. Thrombin stimulates smooth muscle cell differentiation from peripheral blood mononuclear cells via protease-activated receptor-1, RhoA, and myocardin. Circ Res 2009105(3), 214–218. [DOI] [PubMed] [Google Scholar]
- 40.Xiao Q, Luo Z, Pepe AE, et al. Embryonic stem cell differentiation into smooth muscle cells is mediated by Nox4-produced H2O2. Am.J.Physiol Cell Physiol 2009296(4), C711–C723. [DOI] [PubMed] [Google Scholar]
- 41.Pepe AE, Xiao Q, Zampetaki A, et al. Crucial role of Nrf3 in smooth muscle cell differentiation from stem cells. Circ Res 2010106(5), 870–879. [DOI] [PubMed] [Google Scholar]
- 42.Kurpinski K, Lam H, Chu J, et al. Transforming growth factor-beta and notch signaling mediate stem cell differentiation into smooth muscle cells. Stem Cells 201028(4), 734–742. [DOI] [PubMed] [Google Scholar]
- 43.Fritz KE, Jarmolych J, Daoud AS.Association of DNA synthesis and apparent dedifferentiation of aortic smooth muscle cells in vitro Exp Mol Pathol 197012(3), 354–362. [DOI] [PubMed] [Google Scholar]
- 44.Chamley-Campbell J, Campbell GR, Ross R.The smooth muscle cell in culture. Physiol Rev 197959(1), 1–61. [DOI] [PubMed] [Google Scholar]
- 45.Iyemere VP, Proudfoot D, Weissberg PL, et al. Vascular smooth muscle cell phenotypic plasticity and the regulation of vascular calcification. J Intern Med 2006260(3), 192–210. [DOI] [PubMed] [Google Scholar]
- 46.Alexander MR, Owens GK.Epigenetic control of smooth muscle cell differentiation and phenotypic switching in vascular development and disease. Annu Rev Physiol 201274(:13–40). [DOI] [PubMed] [Google Scholar]
- 47.Miano JM, Kitchen CM, Chen JY, et al. Myocardin is expressed in adult aorta and activates a smooth muscle cell differentiation program. Arterioscler Thromb Vasc Biol 200222(5), A2–A2. [Google Scholar]
- 48.Long X, Tharp DL, Georger MA, et al. The smooth muscle cell-restricted KCNMB1 ion channel subunit is a direct transcriptional target of serum response factor and myocardin. J Biol Chem 2009284(48), 33671–33682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nanda V, Miano JM.Leiomodin 1: A new serum response factor-dependent target gene expressed preferentially in differentiated smooth muscle cells. J Biol Chem 2012287(4), 2459–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou J, Herring BP.Mechanisms responsible for the promoter-specific effects of myocardin. J Biol Chem 2005280(11), 10861–10869. [DOI] [PubMed] [Google Scholar]
- 51.Long X, Bell RD, Gerthoffer WT, et al. Myocardin is sufficient for a SMC-like contractile phenotype. Arterioscler Thromb Vasc Biol 200828(8), 1505–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jiang Y, Yin H, Zheng XL.MicroRNA-1 inhibits myocardin-induced contractility of human vascular smooth muscle cells. J Cell Physiol 2010225(2), 506–511. [DOI] [PubMed] [Google Scholar]
- 53.Raphel L, Talasila A, Cheung C, et al. Myocardin overexpression is sufficient for promoting the development of a mature smooth muscle cell-like phenotype from human embryonic stem cells. PLoS One 20127(8), e44052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McDonald OG, Wamhoff BR, Hoofnagle MH, et al. Control of SRF binding to CArG box chromatin regulates smooth muscle gene expression in vivo. J Clin Invest 2006116(1), 36–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ieda M, Fu JD, Delgado-Olguin P, et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell 2010142(3), 375–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li S, Wang DZ, Richardson JA, et al. The serum response factor coactivator myocardin is required for vascular smooth muscle development. Proc Natl Acad Sci U S A 2003100(16), 9366–9370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang J, Lu MM, Cheng L, et al. Myocardin is required for cardiomyocyte survival and maintenance of heart function. Proc Natl Acad Sci U S A 2009106(44), 18734–18739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang J, Elicker J, Bowens N, et al. Myocardin regulates BMP10 expression and is required for heart development. J Clin Invest 2012122(10), 3678–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hoofnagle MH, Neppl RL, Berzin EL, et al. Myocardin is differentially required for the development of smooth muscle cells and cardiomyocytes. Am J Physiol Heart Circ Physiol 2011300(5), H1707–H1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang J, Cheng L, Li J, et al. Myocardin regulates expression of contractile genes in smooth muscle cells and is required for closure of the ductus arteriosus in mice. J Clin Invest 2008118(2), 515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ueyama T, Kasahara H, Ishiwata T, et al. Myocardin expression is regulated by Nkx2.5, and its function is required for cardiomyogenesis. Mol Cell Biol 200323(24), 9222–9232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shi N, Chen SY.Cell division cycle 7 mediates transforming growth factor-beta-induced smooth muscle maturation through activation of myocardin gene transcription. J Biol Chem 2013288(48), 34336–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xie WB, Li Z, Miano JM, et al. Smad3-mediated myocardin silencing: a novel mechanism governing the initiation of smooth muscle differentiation. J Biol Chem 2011286(17), 15050–15057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang K, Long B, Zhou J, et al. miR-9 and NFATc3 regulate myocardin in cardiac hypertrophy. J Biol Chem 2010285(16), 11903–11912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Garvey SM, Sinden DS, Schoppee Bortz PD, et al. Cyclosporine up-regulates Kruppel-like factor-4 (KLF4) in vascular smooth muscle cells and drives phenotypic modulation in vivo. J Pharmacol Exp Ther 2010333(1), 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang Y, Lin L, Yu X, et al. Functional involvements of heterogeneous nuclear ribonucleoprotein A1 in smooth muscle differentiation from stem cells in vitro and in vivo. Stem Cells 201331(5), 906–17. [DOI] [PubMed] [Google Scholar]
- 67.Miano JM, Ramanan N, Georger MA, et al. Restricted inactivation of serum response factor to the cardiovascular system. Proc Natl Acad Sci U S A 2004101(49), 17132–17137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu R, Jin Y, Tang WH, et al. Ten-eleven translocation-2 (TET2) is a master regulator of smooth muscle cell plasticity. Circulation 2013128(18), 2047–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang X, Gong Y, Tang Y, et al. Spry1 and Spry4 differentially regulate human aortic smooth muscle cell phenotype via Akt/FoxO/myocardin signaling. PLoS One 20138(3), e58746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Turner EC, Huang CL, Govindarajan K, et al. Identification of a Klf4-dependent upstream repressor region mediating transcriptional regulation of the myocardin gene in human smooth muscle cells. Biochim Biophys Acta 20131829(11), 1191–201. [DOI] [PubMed] [Google Scholar]
- 71.Molchadsky A, Shats I, Goldfinger N, et al. p53 plays a role in mesenchymal differentiation programs, in a cell fate dependent manner. PLoS One 20083(11), e3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Creemers EE, Sutherland LB, McNally J, et al. Myocardin is a direct transcriptional target of Mef2, Tead and Foxo proteins during cardiovascular development. Development 2006133(21), 4245–4256. [DOI] [PubMed] [Google Scholar]
- 73.Lin Q, Lu J, Yanagisawa H, et al. Requirement of the MADS-box transcription factor MEF2C for vascular development. Development 1998125(22), 4565–4574. [DOI] [PubMed] [Google Scholar]
- 74.Long X, Slivano OJ, Cowan SL, et al. Smooth muscle calponin: an unconventional CArG-dependent gene that antagonizes neointimal formation. Arterioscler Thromb Vasc Biol 201131(10), 2172–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang H, Yang H, Shivalila CS, et al. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell 2013153(4), 910–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bell RD, Long X, Lin M, et al. Identification and initial functional characterization of a human vascular cell-enriched long noncoding RNA. Arterioscler Thromb Vasc Biol 201434(6), 1249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ilagan RM, Genheimer CW, Quinlan SF, et al. Smooth muscle phenotypic diversity is mediated through alterations in myocardin gene splicing. J Cell Physiol 2011226(10), 2702–2711. [DOI] [PubMed] [Google Scholar]
- 78.Taurin S, Sandbo N, Yau DM, et al. Phosphorylation of myocardin by extracellular signal regulated protein kinase. J Biol Chem 2009284(49), 33789–33794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Imamura M, Long X, Nanda V, et al. Expression and functional activity of four myocardin isoforms. Gene 2010464(1–2), 1–10. [DOI] [PubMed] [Google Scholar]
- 80.Creemers EE, Sutherland LB, Oh J, et al. Coactivation of MEF2 by the SAP domain proteins myocardin and MASTR. Mol Cell 200623(1), 83–96. [DOI] [PubMed] [Google Scholar]
- 81.Torrado M, L¢pez E, Centeno A, et al. Myocardin mRNA is augmented in the failing myocardium: expression profiling in the porcine model and human dilated cardiomyopathy. J Mol Med 200381(9), 566–577. [DOI] [PubMed] [Google Scholar]
- 82.van der Veer EP, de Bruin RG, Kraaijeveld AO, et al. Quaking, an RNA-binding protein, is a critical regulator of vascular smooth muscle cell phenotype. Circ Res 2013113(9), 1065–75. [DOI] [PubMed] [Google Scholar]
- 83.Cordes KR, Sheehy NT, White MP, et al. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature 2009460(7256), 705–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xin M, Small EM, Sutherland LB, et al. MicroRNAs miR-143 and miR-145 modulate cytoskeletal dynamics and responsiveness of smooth muscle cells to injury. Genes Dev 200923(18), 2166–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tang Y, Yang X, Friesel RE, et al. Mechanisms of TGF-beta-induced differentiation in human vascular smooth muscle cells. J Vasc Res 201148(6), 485–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Long X, Miano JM.Transforming growth factor-b1 (TGF-b1) utilizes distinct pathways for the transcriptional activation of microRNA 143/145 in human coronary artery smooth muscle cells. J Biol Chem 2011286(34), 30119–30129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sun SG, Zheng B, Han M, et al. miR-146a and Kruppel-like factor 4 form a feedback loop to participate in vascular smooth muscle cell proliferation. EMBO Reports 201112(1), 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kuhn AR, Schlauch K, Lao R, et al. MicroRNA expression in human airway smooth muscle cells: role of miR-25 in regulation of airway smooth muscle phenotype. Am J Respir Cell Mol Biol 201042(4), 506–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xie C, Huang H, Sun X, et al. MicroRNA-1 regulates smooth muscle cell differentiation by repressing Kruppel-like factor 4. Stem Cells Dev 201120(2), 205–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Davis BN, Hilyard AC, Nguyen PH, et al. Induction of microRNA-221 by platelet-derived growth factor signaling is critical for modulation of vascular smooth muscle phenotype. J Biol Chem 2009284(6), 3728–3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Heidersbach A, Saxby C, Carver-Moore K, et al. microRNA-1 regulates sarcomere formation and suppresses smooth muscle gene expression in the mammalian heart. Elife 20132(:e01323). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wystub K, Besser J, Bachmann A, et al. miR-1/133a clusters cooperatively specify the cardiomyogenic lineage by adjustment of myocardin levels during embryonic heart development. PLoS Genet 20139(9), e1003793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xu WG, Shang YL, Cong XR, et al. MicroRNA-135b promotes proliferation, invasion and migration of osteosarcoma cells by degrading myocardin. Int J Oncol 201445(5), 2024–32. [DOI] [PubMed] [Google Scholar]
- 94.Zheng XL. Myocardin and smooth muscle differentiation. Arch Biochem Biophys. 2014;543:48–56. doi: 10.1016/j.abb.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 95.Badorff C, Seeger FH, Zeiher AM, et al. Glycogen synthase kinase 3a inhibits myocardin-dependent transcription and hypertrophy induction through site-specific phosphorylation. Circ Res 200597(7), 645–654. [DOI] [PubMed] [Google Scholar]
- 96.Wang J, Li AK, Wang ZG, et al. Myocardin sumoylation transactivates cardiogenic genes in pluripotent 10T1/2 fibroblasts. Mol Cell Biol 200727(2), 622–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xie P, Fan Y, Zhang H, et al. CHIP represses myocardin-induced smooth muscle cell differentiation via ubiquitin-mediated proteasomal degradation. Mol Cell Biol 200929(9), 2398–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hu G, Wang X, Saunders DN, et al. Modulation of myocardin function by the ubiquitin E3 ligase UBR5. J Biol Chem 2010285(16), 11800–11809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cao D, Wang Z, Zhang CL, et al. Modulation of smooth muscle gene expression by association of histone acetyltransferases and deacetylases with myocardin. Mol Cell Biol 200525(1), 364–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cao D, Wang C, Tang R, et al. Acetylation of myocardin is required for the activation of cardiac and smooth muscle genes. J Biol Chem 2012287(46), 38495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li JT, Sun FX.Myocardin and pdx-1 synergistically induce hMSCs to differentiate into insulin secreting cells. Biochem Biophys Res Commun 2014. [DOI] [PubMed] [Google Scholar]
- 102.Qiu P, Ritchie RP, Fu Z, et al. Myocardin enhances Smad3-mediated transforming growth factor-b1 signaling in a CArG box-independent manner: Smad-binding element is an important cis element for SM22a transcription in vivo. Circ Res 200597(10), 983–991. [DOI] [PubMed] [Google Scholar]
- 103.Oh J, Wang Z, Wang DZ, et al. Target gene-specific modulation of myocardin activity by GATA transcription factors. Mol Cell Biol 200424(19), 8519–8528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang C, Cao D, Wang Q, et al. Synergistic activation of cardiac genes by myocardin and Tbx5. PLoS One 20116(8), e24242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kitchen CM, Cowan SL, Long X, et al. Expression and promoter analysis of a highly restricted integrin alpha gene in vascular smooth muscle. Gene 2013513(1), 82–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tang Rh, Zheng XL, Callis TE, et al. Myocardin inhibits cellular proliferation by inhibiting NF- k B(p65)-dependent cell cycle progression. Proc Natl Acad Sci U S A 2008105(9), 3362–3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen J, Yin H, Jiang Y, et al. Induction of microRNA-1 by myocardin in smooth muscle cells inhibits cell proliferation. Arterioscler Thromb Vasc Biol 201131(2), 368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Milyavsky M, Shats I, Cholostoy A, et al. Inactivation of myocardin and p16 during malignant transformation contributes to a differentiation defect. Cancer Cell 200711(2), 133–146. [DOI] [PubMed] [Google Scholar]
- 109.Kimura Y, Morita T, Hayashi K, et al. Myocardin functions as an effective inducer of growth arrest and differentiation in human uterine leiomyosarcoma cells. Cancer Res 201070(2), 501–511. [DOI] [PubMed] [Google Scholar]
- 110.Belaguli NS, Schildmeyer LA, Schwartz RJ.Organization and myogenic restricted expression of the murine serum response factor gene: a role for autoregulation. J Biol Chem 1997272(29), 18222–18231. [DOI] [PubMed] [Google Scholar]
- 111.Li L, Miano JM, Cserjesi P, et al. SM22a, a marker of adult smooth muscle, is expressed in multiple myogenic lineages during embryogenesis. Circ Res 199678(2), 188–195. [DOI] [PubMed] [Google Scholar]
- 112.Jiang Y, Singh P, Yin H, et al. Opposite roles of myocardin and atrogin-1 in L6 myoblast differentiation. J Cell Physiol 2013228(10), 1989–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lagha M, Brunelli S, Messina G, et al. Pax3:Foxc2 reciprocal repression in the somite modulates muscular versus vascular cell fate choice in multipotent progenitors. Dev Cell 200917(6), 892–899. [DOI] [PubMed] [Google Scholar]
- 114.Espinoza-Lewis RA, Wang DZ.Generation of a Cre knock-in into the Myocardin locus to mark early cardiac and smooth muscle cell lineages. Genesis 201452(10), 879–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ren J, Albinsson S, Hellstrand P.Distinct effects of voltage- and store-dependent calcium influx on stretch-induced differentiation and growth in vascular smooth muscle. J Biol Chem 2010285(41), 31829–31839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wamhoff BR, Bowles DK, Owens GK.Excitation-transcription coupling in arterial smooth muscle. Circ Res 200698(7), 868–878. [DOI] [PubMed] [Google Scholar]
- 117.Miano JM. Channeling to myocardin. Circ Res. 2004;95:340–342. doi: 10.1161/01.RES.0000140893.16465.2d. [DOI] [PubMed] [Google Scholar]
- 118.Demolombe S, Marionneau C, Le Bouter S, et al. Functional genomics of cardiac ion channel genes. Cardiovasc Res 200567(3), 438–47. [DOI] [PubMed] [Google Scholar]
- 119.Cidad P, Moreno-Dominguez A, Novensa L, et al. Characterization of ion channels involved in the proliferative response of femoral artery smooth muscle cells. Arterioscler Thromb Vasc Biol 201030(6), 1203–1211. [DOI] [PubMed] [Google Scholar]
- 120.Park C, Hennig GW, Sanders KM, et al. Serum response factor-dependent microRNAs regulate gastrointestinal smooth muscle cell phenotypes. Gastroenterology 2011141(1), 164–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kontaraki JE, Parthenakis FI, Nyktari EG, et al. Myocardial gene expression alterations in peripheral blood mononuclear cells of patients with idiopathic dilated cardiomyopathy. Eur J Heart Fail 201012(6), 541–8. [DOI] [PubMed] [Google Scholar]
- 122.Xing W, Zhang TC, Cao D, et al. Myocardin induces cardiomyocyte hypertrophy. Circ Res 200698(8), 1089–1097. [DOI] [PubMed] [Google Scholar]
- 123.Kontaraki JE, Parthenakis FI, Patrianakos AP, et al. Altered expression of early cardiac marker genes in circulating cells of patients with hypertrophic cardiomyopathy. Cardiovasc Pathol 200716(6), 329–335. [DOI] [PubMed] [Google Scholar]
- 124.Kontaraki JE, Marketou ME, Zacharis EA, et al. Early cardiac gene transcript levels in peripheral blood mononuclear cells in patients with untreated essential hypertension. J Hypertens 201129(4), 791–797. [DOI] [PubMed] [Google Scholar]
- 125.Kontaraki JE, Parthenakis FI, Patrianakos AP, et al. Myocardin gene regulatory variants as surrogate markers of cardiac hypertrophy - study in a genetically homogeneous population. Clin Genet 200773(1), 71–78. [DOI] [PubMed] [Google Scholar]
- 126.Ransom JF, King IN, Garg V, et al. A rare human sequence variant reveals myocardin autoinhibition. J Biol Chem 2008283(51), 35845–35852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kontaraki JE, Kochiadakis GE, Marketou ME, et al. Early cardiac gene transcript levels in peripheral blood mononuclear cells reflect severity in stable coronary artery disease. Hellenic J Cardiol 201455(2), 119–25. [PubMed] [Google Scholar]
- 128.Torrado M, Centeno A, Lopez E, et al. In vivo forced expression of myocardin in ventricular myocardium transiently impairs systolic performance in early neonatal pig heart. Int J Dev Biol 200953(8–10), 1457–1467. [DOI] [PubMed] [Google Scholar]
- 129.Torrado M, Iglesias R, Centeno A, et al. Targeted gene-silencing reveals the functional significance of myocardin signaling in the failing heart. PLoS One 20116(10), e26392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tharp DL, Wamhoff BR, Turk JR, et al. Upregulation of intermediate-conductance Ca 2+ -activated K + channel (IKCa1) mediates phenotypic modulation of coronary smooth muscle. Am J Physiol Heart Circ Physiol 2006291(5), H2493–H2503. [DOI] [PubMed] [Google Scholar]
- 131.Hendrix JA, Wamhoff BR, McDonald OG, et al. 5' CArG degeneracy in smooth muscle alpha -actin is required for injury-induced gene suppression in vivo. J Clin Invest 2005115(2), 418–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Talasila A, Yu H, Ackers-Johnson M, et al. Myocardin regulates vascular response to injury through miR-24/-29a and platelet-derived growth factor receptor-beta. Arterioscler Thromb Vasc Biol 201333(10), 2355–65. [DOI] [PubMed] [Google Scholar]
- 133.Tharp DL, Wamhoff BR, Wulff H, et al. Local delivery of the KCa3.1 blocker, TRAM-34, prevents acute angioplasty-induced coronary smooth muscle phenotypic modulation and limits stenosis. Arterioscler Thromb Vasc Biol 200828(6), 1084–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shen J, Yang M, Jiang H, et al. Arterial injury promotes medial chondrogenesis in Sm22 knockout mice. Cardiovasc Res 201190(1), 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Minami T, Kuwahara K, Nakagawa Y, et al. Reciprocal expression of MRTF-A and myocardin is crucial for pathological vascular remodelling in mice. EMBO J 201231(23), 4428–4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pfisterer L, Feldner A, Hecker M, et al. Hypertension impairs myocardin function: a novel mechanism facilitating arterial remodelling. Cardiovasc Res 201296(1), 120–9. [DOI] [PubMed] [Google Scholar]
- 137.Pfisterer L, Meyer R, Feldner A, et al. Bortezomib protects from varicose-like venous remodeling. FASEB J 201428(8), 3518–27. [DOI] [PubMed] [Google Scholar]
- 138.Reynolds PR, Mucenski ML, Le Cras TD, et al. Midkine is regulated by hypoxia and causes pulmonary vascular remodeling. J Biol Chem 2004279(35), 37124–37132. [DOI] [PubMed] [Google Scholar]
- 139.Jie W, Wang X, Huang L, et al. Contribution of CXCR4(+)/PDGFRbeta(+) progenitor cells in hypoxic alveolar arterioles muscularization: role of myocardin. Cardiovasc Res 201087(4), 740–750. [DOI] [PubMed] [Google Scholar]
- 140.Zhu P, Huang L, Ge X, et al. Transdifferentiation of pulmonary arteriolar endothelial cells into smooth muscle-like cells regulated by myocardin involved in hypoxia-induced pulmonary vascular remodeling. Int J Exp Pathol 200787(6), 463–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bell RD, Deane R, Chow N, et al. SRF and myocardin regulate LRP-mediated amyloid-beta clearance in brain vascular cells. Nat Cell Biol 200911(2), 143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chow N, Bell RD, Deane R, et al. Serum response factor and myocardin mediate arterial hypercontractility and cerebral blood flow dysregulation in Alzheimer's phenotype. Proc Natl Acad Sci U S A 2007104(3), 823–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zlokovic BV.Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat Rev Neurosci 201112(12), 723–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhou W, Negash S, Liu J, et al. Modulation of pulmonary vascular smooth muscle cell phenotype in hypoxia: role of cGMP-dependent protein kinase and myocardin. Am J Physiol Lung Cell Mol Physiol 2009296(5), L780–L789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jie W, Guo J, Shen Z, et al. Contribution of myocardin in the hypoxia-induced phenotypic switching of rat pulmonary arterial smooth muscle cells. Exp Mol Pathol 201089(3), 301–306. [DOI] [PubMed] [Google Scholar]
- 146.Latif N, Sarathchandra P, Chester AH, et al. Expression of smooth muscle cell markers and co-activators in calcified aortic valves. Eur Heart J. 2014 doi: 10.1093/eurheartj/eht547. [DOI] [PubMed] [Google Scholar]
- 147.Shats I, Milyavsky M, Cholostoy A, et al. Myocardin in tumor suppression and myofibroblast differentiation. Cell Cycle 20076(10), 1141–1146. [DOI] [PubMed] [Google Scholar]
- 148.Chen F, Mo Y, Ding H, et al. Frequent epigenetic inactivation of Myocardin in human nasopharyngeal carcinoma. Head Neck 201133(1), 54–59. [DOI] [PubMed] [Google Scholar]
- 149.Liao XH, Li YQ, Wang N, et al. Re-expression and epigenetic modification of maspin induced apoptosis in MCF-7 cells mediated by myocardin. Cell Signal 201426(6), 1335–46. [DOI] [PubMed] [Google Scholar]
- 150.Horiuchi A, Nikaido T, Taniguchi S, et al. Possible role of calponin h1 as a tumor suppressor in human uterine leiomyosarcoma. J Natl Cancer Inst 199991(9), 790–796. [DOI] [PubMed] [Google Scholar]
- 151.Ramaswamy S, Ross KN, Lander ES, et al. A molecular signature of metastasis in primary solid tumors. Nat Genet 200333(1), 49–54. [DOI] [PubMed] [Google Scholar]
- 152.Perot G, Derre J, Coindre JM, et al. Strong smooth muscle differentiation is dependent on myocardin gene amplification in most human retroperitoneal leiomyosarcomas. Cancer Res 200969(6), 2269–2278. [DOI] [PubMed] [Google Scholar]
- 153.Malone MD, Kerr K, Kavanah M, et al. Primary leiomyosarcoma of the abdominal aorta. J Vasc Surg 199624(3), 487–493. [DOI] [PubMed] [Google Scholar]
- 154.Suga T, Iso T, Shimizu T, et al. Activation of receptor for advanced glycation end products induces osteogenic differentiation of vascular smooth muscle cells. J Atheroscler Thromb 201118(8), 670–683. [DOI] [PubMed] [Google Scholar]
- 155.He SH, Wei AY, Yang Y, et al. Reduced expression of myocardin and serum response factor in the cavernous tissue of diabetic rats. Andrologia 201244(Suppl 1), 518–522. [DOI] [PubMed] [Google Scholar]
- 156.Wei AY, He SH, Zhao JF, et al. Characterization of corpus cavernosum smooth muscle cell phenotype in diabetic rats with erectile dysfunction. Int J Impot Res 201224(5), 196–201. [DOI] [PubMed] [Google Scholar]
- 157.He S, Zhang T, Liu Y, et al. Myocardin restores erectile function in diabetic rats: phenotypic modulation of corpus cavernosum smooth muscle cells. Andrologia. 2014 doi: 10.1111/and.12261. [DOI] [PubMed] [Google Scholar]
- 158.Chen J, Chen H, Sanders KM, et al. Regulation of SRF/CArG-dependent gene transcription during chronic partial obstruction of murine small intestine. Neurogastroenterol Motil 200820(7), 829–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Imamura M, Sugino Y, Long X, et al. Myocardin and microRNA-1 modulate bladder activity through connexin 43 expression during post-natal development. J Cell Physiol 2013228(9), 1819–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hermann J, Haas U, Gressner AM, et al. TGFb up-regulates serum response factor in activated hepatic stellate cells. Biochim Biophys Acta 20071772(11–12), 1250–1257. [DOI] [PubMed] [Google Scholar]
- 161.Shimada H, Ochi T, Imasato A, et al. Gene expression profiling and functional assays of activated hepatic stellate cells suggest that myocardin has a role in activation. Liver Int 201030(1), 42–54. [DOI] [PubMed] [Google Scholar]
- 162.Morita T, Hayashi K.Arp5 is a key regulator of myocardin in smooth muscle cells. J Cell Biol 2014204(5), 683–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Joung H, Kwon JS, Kim JR, et al. Enhancer of polycomb1 lessens neointima formation by potentiation of myocardin-induced smooth muscle differentiation. Atherosclerosis 2012222(1), 84–91. [DOI] [PubMed] [Google Scholar]
- 164.Hoggatt AM, Kim JR, Ustiyan V, et al. The transcription factor Foxf1 binds to serum response factor and myocardin to regulate gene transcription in visceral smooth muscle cells. J Biol Chem 2013288(40), 28477–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Liu ZP, Wang Z, Yanagisawa H, et al. Phenotypic modulation of smooth muscle cells through interaction of Foxo4 and myocardin. Dev Cell 20059(2), 261–270. [DOI] [PubMed] [Google Scholar]
- 166.Yin F, Herring BP.GATA-6 can act as a positive or negative regulator of smooth muscle-specific gene expression. J Biol Chem 2005280(6), 4745–4752. [DOI] [PubMed] [Google Scholar]
- 167.Zhou J, Hu G, Wang X.Repression of smooth muscle differentiation by a novel high mobility group box-containing protein, HMG2L1. J Biol Chem 2010285(30), 23177–23185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhang SM, Gao L, Zhang XF, et al. Interferon regulatory factor 8 modulates phenotypic switching of smooth muscle cells by regulating the activity of myocardin. Mol Cell Biol 201434(3), 400–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jiang DS, Luo YX, Zhang R, et al. Interferon regulatory factor 9 protects against cardiac hypertrophy by targeting myocardin. Hypertension 201463(1), 119–27. [DOI] [PubMed] [Google Scholar]
- 170.Gordon JW, Pagiatakis C, Salma J, et al. Protein kinase A-regulated assembly of a MEF2-HDAC4 repressor complex controls c-Jun expression in vascular smooth muscle cells. J Biol Chem 2009284(28), 19027–19042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lockman K, Taylor JM, Mack CP.The histone demethylase, jmjd1a, interacts with the myocardin factors to regulate SMC differentiation marker gene expression. Circ Res 2008101(12), e115–e123. [DOI] [PubMed] [Google Scholar]
- 172.Nakamura S, Hayashi K, Iwasaki K, et al. Nuclear import mechanism for myocardin family members and their correlation with VSMC phenotype. J Biol Chem 2010285(48), 37314–37323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Leenders JJ, Wijnen WJ, Hiller M, et al. Regulation of cardiac gene expression by KLF15, a repressor of myocardin activity. J Biol Chem 2010285(35), 27449–27456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Li HJ, Haque Z, Lu Q, et al. Steroid receptor coactivator 3 is a coactivator for myocardin, the regulator of smooth muscle transcription and differentiation. Proc Natl Acad Sci U S A 2007104(10), 4065–4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kiyan Y, Limbourg A, Kiyan R, et al. Urokinase receptor associates with myocardin to control vascular smooth muscle cells phenotype in vascular disease. Arterioscler Thromb Vasc Biol 201232(2), 449–458. [DOI] [PubMed] [Google Scholar]
- 176.Tanaka T, Sato H, Doi H, et al. Runx2 represses myocardin-mediated differentiation and facilitates osteogenic conversion of vascular smooth muscle cells. Mol Cell Biol 200728(3), 1147–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zhou J, Zhang M, Fang H, et al. The SWI/SNF chromatin remodeling complex regulates myocardin-induced smooth muscle-specific gene expression. Arterioscler Thromb Vasc Biol 200929(6), 921–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Xu Z, Ji G, Shen J, et al. SOX9 and myocardin counteract each other in regulating vascular smooth muscle cell differentiation. Biochem Biophys Res Commun 2012422(2), 285–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zhou J, Blue EK, Hu G, et al. Thymine DNA glycosylase represses myocardin-induced smooth muscle cell differentiation by competing with serum response factor for myocardin binding. J Biol Chem 2008283(51), 35383–35392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Madonna R, Willerson JT, Geng YJ.Myocardin A enhances telomerase activities in adipose tissue mesenchymal cells and embryonic stem cells undergoing cardiovascular myogenic differentiation. Stem Cells 200826(1), 202–211. [DOI] [PubMed] [Google Scholar]
- 181.Martin E, Caubit X, Airik R, et al. TSHZ3 and SOX9 regulate the timing of smooth muscle cell differentiation in the ureter by reducing myocardin activity. PLoS One 20138(5), e63721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Xie C, Guo Y, Zhu T, et al. Yap1 protein regulates vascular smooth muscle cell phenotypic switch by interaction with myocardin. J Biol Chem 2012287(18), 14598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]