Abstract
In the current study, we sought to establish a novel rat model of portal vein arterialization (PVA) and evaluate its impact on liver regeneration after extended partial hepatectomy (PH). A total of 105 Sprague-Dawley rats were randomly assigned to three groups: 68% hepatectomy (the PH group), portal arterialization after 68% hepatectomy (the PVA group), and right nephrectomy only (the control group). Liver regeneration rate (LRR), 5-bromo-2-deoxyuridine (BrdU) labeling index, and liver functions were assessed on postoperative day 2, 7, 14 and 28. The 28-day survival rates were compared among the three groups. The 28-day survival rates were similar in all groups (P = 0.331), and the anastomotic patency was 100%. The LRR in the PVA group was significantly higher than that of the PH group within postoperative 14 days (P < 0.05). The PVA and PH group had increased serum alanine aminotransferase levels (232 ± 61 U/L and 212 ± 53 U/L, respectively) compared with the control group (101 ± 13 U/L) on postoperative day 2, whereas from postoperative day 7 to day 28 there were no differences among the three groups. Serum albumin values were higher after the PVA procedure within postoperative day 14, which gradually became comparable on postoperative day 28 among the three groups. The peaks of BrdU labeling index appeared on postoperative day 2 in all rats, and the PVA procedure was associated with increased BrdU labeling index from postoperative day 7 to 28. The 28-day survival of the PVA rats was comparable. Our findings demonstrate that the PVA procedure utilizing portal vein trunk-renal artery microvascular reconstruction promotes remnant liver regeneration and confers beneficial effects on maintaining and even optimizing liver function after extended partial hepatectomy in rats.
Keywords: portal vein arterialization, microsurgery, hepatectomy, liver regeneration, liver function
INTRODUCTION
Portal vein arterialization (PVA) was initially introduced to improve encephalopathy and prevent liver failure after portacaval shunt in cirrhotic patients[1] and massive necrosis due to obstruction of the hepatic artery after extended hepatopancreatobiliary surgery[2]. Portal vein arterialization combined with portacaval shunt increases blood supply to the liver through the arterialization of the portal stump by an artery[3]-[7]. In orthotopic and heterotopic liver transplantation, permanent portal vein arterialization was performed in cases with insufficient portal flow to the graft and showed beneficial effects on shortening warm ischemia time of the donor liver and consequently reducing the incidence of immediate postoperative graft failure[8],[9]. In the event of an extensive portal vein thrombosis or the absence of a mesenteric vein as an anatomic variant, PVA ensures an adequate blood supply to an orthotopic graft[10]-[12]. In addition, some authors have emphasized a proliferation-promoting influence of PVA on the liver tissue and recommended PVA as a therapeutic option in acute liver failure or after extensive liver resection[13]-[15].
A steady supply of oxygen is necessary for liver regeneration after hepatectomy[16],[17]. The effects of portal arterialization on hepatic regeneration after partial hepatectomy have been investigated by several authors and beneficial effects on hepatic regeneration have been reported[18],[19]. However, other authors suggested that the destruction of hepatic architecture brought about portal hypertension and that mitosis in the regenerating liver was not enhanced[20],[21]. There are no proven answers for regenerative ability of the liver after PVA. Furthermore, due to the various sizes of vessels between portal vein and alternative artery, microsurgical technical obstacles of this direct hand suturing anastomosis were overcome in the last decades. The models presented in literatures were established by various stent cannula techniques, in which the simulation of the PVA is insufficient; thus, the outcomes of different models are not comparable and debatable.
The conflicting evidence prompted us to attempt a more simulative technique of end-to-end arterial-venous anastomoses following an extended partial hepatectomy (68% hepatectomy) to establish a novel PVA model. Liver growth and regeneration rate were compared among rats with either venous portal inflow or arterialized portal inflow after extended partial hepatectomy, and then proliferation of hepatocytes was examined. Therefore, we determined whether this PVA procedure, which increased oxygen concentration in the portal vein, was an effective approach to the promotion of liver regeneration rate (LRR) and the improvement of liver function after extended hepatectomy.
MATERIALS AND METHODS
Experimental animals
Male specific pathogen free (SPF) Sprague-Dawley rats weighting from 240 to 300g were used in this study. The study protocol was approved by the local institutional review board at the authors' affiliated institutions, and animal welfare as well as the experimental procedures was carried out in accordance with the established institutional and state guidelines regarding animal care and use. All rats were housed in environmentally controlled conditions (a temperature-controlled environment, a 12 hours light/dark cycle with the light cycle from 6:00 to 18:00 and the dark cycle from 18:00 to 6:00) with ad libitum access to a rodent chow diet and water. After an acclimation period of 1 week, all rats were fasted for 24 hours before surgery. Sixty rats were randomly assigned into 3 groups with 20 rats per group for specimen harvesting, and another 45 rats were divided into 3 groups for survival data collection and analyses.
Surgical procedure
The procedures were performed under aseptic techniques and ether anesthesia via inhalation. A heating pad was used to maintain a stable body temperature at approximately 36°C throughout the surgery. Through a median laparotomy, the liver was mobilized from its supporting ligaments. The right renal artery was isolated from its origin to the bifurcation, and the right renal vein was separated from the renal pedicle to the vena cava. The ureter was sectioned and nephrectomy was performed in all groups.
In the PH group, a 68% partial hepatectomy was accomplished as described by Higgins and Anderson[22]. The median and left lobes of the liver were excised after placement of a 5-0 silk suture ligature on the corresponding pedicle. Then, the portal triad clamping was performed for 20 minutes (equivalent to the average time needed to perform PVA and porto-renal shunt in rats of the PVA group).
In the PVA group, ligation and division of the pyloric vein was carried out following 68% partial hepatectomy, and then transection at the lower 1/3 of the portal vein trunk was completed after it was gently isolated from the hepatic hilum to the confluence of the superior mesenteric and splenic veins. The right renal artery was sectioned at the bifurcation and reshaped as an artery disc to fix the caliber of the hepatic inflow portal trunk. After irrigation with heparinized solution, an end-to-end anastomotic reconstruction between the right renal artery disc and the hepatic inflow portal trunk was accomplished by a microsurgical semi-continuous suture technique, which 6 stitches were placed on the anterior wall first, and then another 6 stitches were placed on the posterior wall after a 180-grade rotation of the vessel. Finally, an end-to-end porto-renal (confluence of the superior mesenteric and splenic veins-right renal vein) shunt was completed according to the techniques by Lee and Fisher[23]. Then, 10-0 polypropylene non-absorbable monofilament sutures (Surgipro™ II-Surgipro™ Suture, Covidien, MA, USA) were utilized in vascular anastomosis. Anastomotic patency was examined after the vascular procedures were finished. The vascular reconstruction was operated under a microsurgical telescope with 10–16 × magnifications. The peritoneal cavity was closed in 2 layers (Fig. 1).
Fig. 1. Illustration of portal vein arterialization (PVA) associated with porto-renal shunt model.
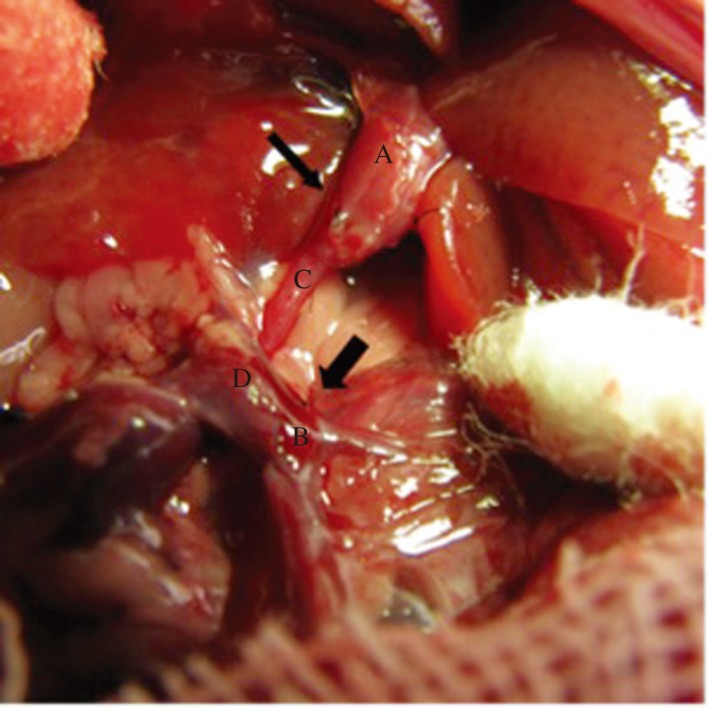
The right kidney was removed after isolating the right renal artery and renal vein, then an end-to-end hepatic inflow portal vein trunk-right renal artery anastomoses (thin arrow) was established after shaping the bifurcation of the right artery, the end-to-end porto-renal shunt (thick arrow) was performed subsequently. A: Hepatic inflow portal vein trunk, B: Confluence of the superior mesenteric and splenic veins, C: Right renal artery, D: Right renal vein, E: Superior mesenteric vein, F: Splenic vein.
At the end of the operation, 1.5 mL of heparinized saline was transfused intravenously. The preoperative diet of food pellets was resumed 2 hours after the operation.
Assessment of liver regeneration
Five rats from each group were sacrificed on postoperative day 2, 7, 14 and 28 to harvest blood and liver specimens for biochemical, histological, and immunohistochemical examinations. All rats were weighed, and then intraperitoneal injection of 5-bromo-2-deoxyuridine (BrdU, Sigma-Aldrich, St. Louis, MO, USA) was given [100 mg/kg per body weight (BW)] 1 hour prior to autopsy. Each liver specimen was removed and immediately weighed. The ratio of the liver weight (LW) to the BW was calculated. From the resected LW at surgery and remnant LW at autopsy, the LRR was determined according to the formula of Child et al.[24]: (LW at autopsy-estimated residual LW at the time of surgery)/resected LW × 100%.
Hepatocyte synthesis evaluation
Liver specimens were fixed in 10% formalin and embedded in paraffin. Five-micron thick sections were stained with hematoxylin and eosin for light microscopic examination. To allow calculation of the BrdU labeling index, an indicator for DNA synthesis in hepatocytes, immunohistochemical staining of incorporated paraffin embedding BrdU was performed with established method as follows: heat-induced antigen retrieval was performed by a microwave with Tris-EDTA buffer for 20 minutes after deparaffinizing by xylene, and then the slides were washed 2 × 5 minutes in TBS plus 0.025% Triton X-100 with gentle agitation, and blocked in 10% goat serum with 1% BSA in TBS for 2 hours at room temperature. The slides were incubated with anti-BrdU antibodies (Abcam, Cambrige, UK) overnight at 4°C afterward, and secondary antibody incubation for 30 minutes at 37°C was performed subsequently. After incubation with 3,3′-diaminobenzidine chromogen for 10 minutes and rinsed in phosphate buffer solution (PBS), the slides were counterstained using hematoxylin for 6 minutes and bluing reagent for 2 minutes. Hepatocytes contained nuclei with brown staining were considered labeled. The number of positive stained cells was counted in randomly selected fields of 10 each in periportal, midzonal, and centrolobular areas using high-power fields (original magnification × 400). The number of BrdU-labeled nuclei per 1,000 hepatocytes was designated the BrdU labeling index.
Liver biochemical tests
Serum alanine aminotransferase (ALT) and albumin (Alb) concentrations were measured by an automatic analyzer (Hitachi Co. Ltd. Tokyo, Japan) for assessment of hepatic parenchymal injury and hepatic anabolism, respectively.
Statistical analysis
Data are expressed as mean ± standard deviation (SD). One-way analysis of variance (Student's t-test) or Mann-Whitney U test was applied for normally or non-normally distributed continuous data, respectively. The Log-rank test was used for survival analyses. P value < 0.05 was set as statistically significant.
RESULTS
Survival rate and anastomosis patency
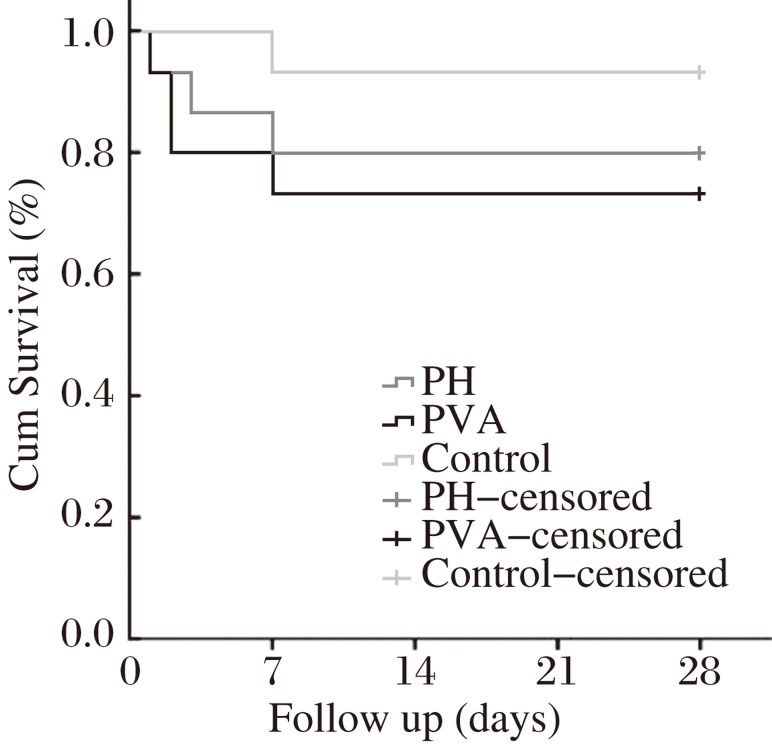
The postoperative 28 day survival rates were 80.0%, 73.3% and 93.3% in the PH group, the PVA group and the control group, respectively (Fig. 2). There were no differences observed in the survival rate among the 3 groups (Log-rank test, P = 0.331). In the PVA group, all the anastomoses remained patent at autopsy, and the rate of the anastomotic patency was 100%.
Fig. 2. The postoperative 28-day survival rates in three groups according to different procedures.
There were no differences observed in survival rates among three groups (Log-rank test, P = 0.331).
Liver regeneration rate
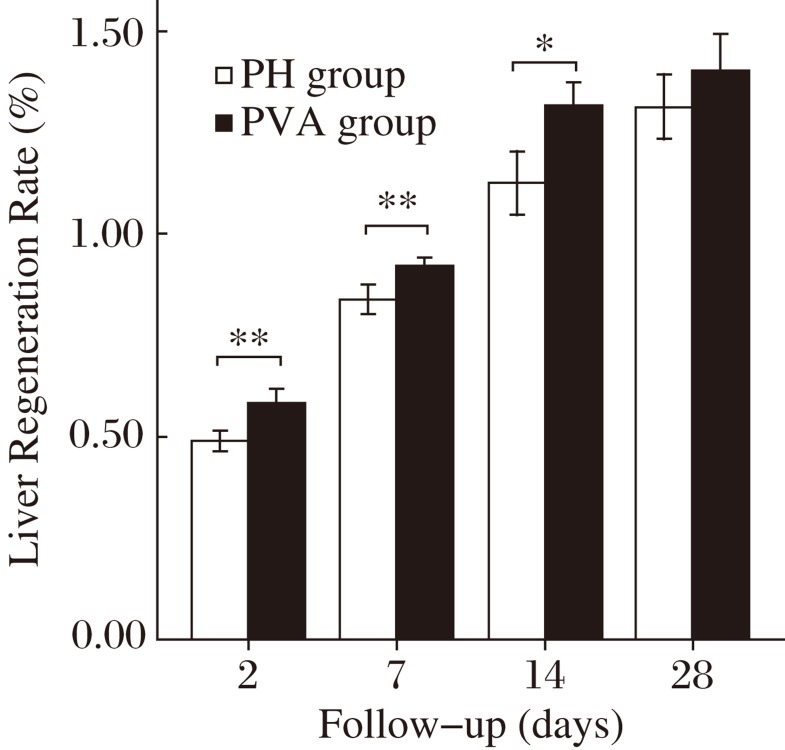
As shown in Fig. 3, the LRR gradually increased after operation in the PH group and the PVA group. The LRR increased from 49.0% ± 2.4% (the PH group) and 58.2% ± 3.7% (the PVA group) on postoperative day 2 to 83.9% ± 3.6% and 92.0% ± 2.2% on postoperative day 7, and 112.5% ± 7.9% and 131.6% ± 5.8% on day postoperative 14, respectively. In comparison, the LRR of the PVA group was significantly higher at all time within 14 days postoperatively (P < 0.05). However, on postoperative day 28, the LRR of the PH group (131.3% ± 8.0%) and the PVA group (140.4% ± 9.0%) became comparable (P = 0.13).
Fig. 3. Liver regeneration rates (LRRs) after surgery.
Rats with PVA procedure (the PVA group, n = 5) presented significantly greater liver growth rate than rats with 68% PH only (the PH group) within the postoperative 14 days. However, on postoperative 28 day, the LRRs between these two groups turned to similar levels. Note: Data are expressed as mean ± SD. *P < 0.05. **P < 0.01.
Liver biochemical tests
The results in serum albumin and ALT are summarized in Table 1. The mean serum albumin concentrations remained significantly higher in the PVA group than either the PH group or the control group within 14 days postoperatively (P < 0.05). Meanwhile, there were no significant differences between the PH group and the control group at all time after operation. On postoperative day 28, serum albumin levels gradually became comparable in all three groups.
Table 1. Postoperative serum albumin and ALT concentrations.
| Day 2 | Day 7 | Day 14 | Day 28 | |
| Alb (g/dL) | ||||
| The PH group | 2.9±0.3 | 3.2±0.1 | 3.3±0.2 | 3.3±0.3 |
| The PVA group | 3.4±0.1* | 3.6±0.3* | 3.5±0.1* | 3.4±0.3 |
| The control group | 3.0±0.3 | 3.1±0.2 | 3.4±0.1 | 3.3±0.3 |
| ALT (U/L) | ||||
| The PH group | 212±53† | 68±31 | 56±15 | 50±17 |
| The PVA group | 232±61† | 72±29 | 63±13 | 49±14 |
| The control group | 101±13 | 56±17 | 50±8 | 51±10 |
NOTE: n = 5. Data expressed as mean ± SD. Alb: albumin; ALT: alanine aminotransferase. *P < 0.05 versus the PH group and the control group, †P < 0.05 versus the control group.
Serum ALT values peaked on postoperative day 2 in all groups, in the rats undergoing 68% hepatectomy in both the PH group (212 ± 53 U/L) and the PVA group (232 ± 61 U/L), and the values were significantly higher than those of the control group (101 ± 13 U/L) (P < 0.05). However, no significant difference was observed between the PH group and the PVA group. The ALT values gradually decreased thereafter, and there were no significant differences from postoperative day 7 to 28 in all groups.
BrdU labeling index
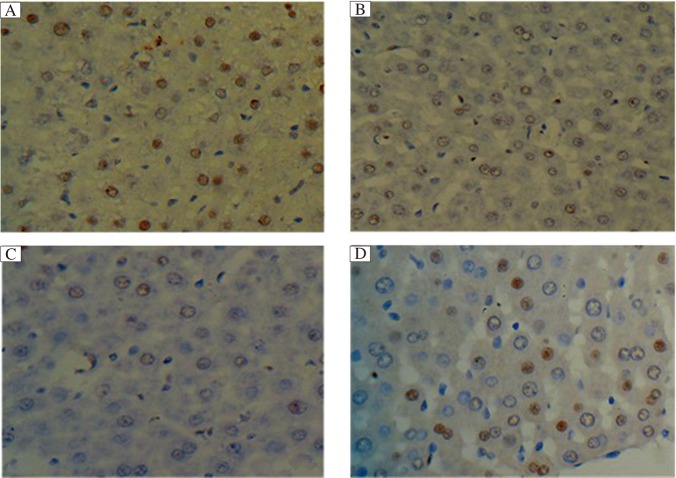
Immunohistochemical staining for BrdU in the liver tissues of the 3 groups is shown in Fig. 4. The BrdU labeling index peaked on postoperative day 2, which then gradually declined within 28 days postoperatively in all rats. The values in the PVA group and the PH group were both significantly higher than those in the control group at all time after operation (P < 0.01). On postoperative day 2, BrdU labeling indexes in the PVA group and the PH group were similar; however, from postoperative 7 to 28 days, BrdU labeling index in the PVA group was significantly higher than that in the PH group (P < 0.05, Table 2).
Fig. 4. The BrdU labeling indexes after surgery (HE×400).
The BrdU labeling indexes peaked on postoperative 2 day, then gradually declined within postoperative 28 days in all rats. On postoperative day 2, BrdU labeling index in the PVA group (A) and the PH group (B) was similar, and both were significantly higher than the control group (C). From postoperative 7 to 28 days, BrdU labeling indexes in the PVA group (D) were maintained significantly higher than either the PH group or the control group (P < 0.01).
Table 2. Postoperative BrdU labeling indexes in the 3 groups.
| Group (n = 5) | Day 2 | Day 7 | Day 14 | Day 28 |
| The PH group | 126±19† | 68±11† | 53±12† | 23±10† |
| The PVA group | 133±21† | 87±13*† | 76±11*† | 39±9*† |
| The control group | 41±9 | 32±8 | 25±9 | 19±6 |
Data were expressed as mean±SD. *P < 0.05 versus the PH group, †P < 0.01 versus the control group.
DISCUSSION
The liver has a dual blood supply, via the hepatic artery and portal vein, and about 80% is poorly oxygenated venous blood delivered through the portal tract. Alternations of oxygen and blood supply underlie pathological changes in a majority of liver diseases. We established this rat model to evaluate the PVA technique, which can effectively enhance oxygen and blood supply to the liver, to verify the benefits and also to investigate the damage to hepatic function. The 28-day survival of the PVA rats were comparable, which demonstrated that the technique of the mcrovascular hand suturing anastomosis was feasible and reliable. PVA helped faster recovery of hepatic function, and ALT level was not deteriorated due to the drastic changes of the hemodynamics of the portal tract. The remnant liver regeneration was optimized and accelerated by the PVA procedure.
Several studies in small and large experimental animal models reported that increasing oxygen delivery to the liver by arterialization of the portal vein enhances the regenerative capacity after extended hepatectomy[25],[26]. Nonami et al.[17] have reported that hepatic oxygen consumption affected by dopamine and dobutamine is associated with an increase in the extraction of lactate and thus hyperdynamic hepatic circulatory support is advantageous to hemodynamics and metabolism in the residual liver after massive hepatectomy. However, the concept of PVA and the outcomes of the animal model have been controversial in decades. The alternation of the reconstructed hepatic hila and the subsequently physiopathologic changes induced by the elevated pressure of the portal tract and increased volume of the blood supply still remain uncertain, and it is still unclear that whether the lack of portal blood including specific hepatic factors would inhibit liver regeneration, or whether an adequate blood supply is adequate for liver growth. Furthermore, microvascular reconstruction by hand suturing technique is very demanding and usually requirs longer learning curve; hence, the models of PVA reported in literatures are commonly established by different artificial polyethylene tube cannula technique in spite of the high risk of stent thrombosis due to its non-endothelialization nature. Besides, inflammation and adhesion caused by external artificial graft might also affect portal tract hemodynamics, hepatic structure and liver function[21]. There still remains a gap between the simulation of animal model and clinical practice. Few literatures have presented the survival rates of the PVA model after partial hepatectomy and checked the patency of the anastomoses at autopsy. Theoretically, when the pressure and inflow volume changed rapidly as a result of portal vein-artery reconstruction, the unalterable and inelastic nature of the stent pipe could not adapt and buffer the hyperdynamic circulation as effectively as autogenous vascular anastomoses. To that extent, the polyethylene tube interposition technique is an adverse factor that may affect the survival rate and the experimental outcomes. To eliminate the shortcomings of sutureless cannula technique, we developed a novel model of PVA by reconstructing the portal vein and renal artery in a microvascular hand suturing anastomotic method after we had shaped and matched the sizes of two different vessel ends. Moreover, the combination of total portocaval shunt was an effective prevention of splanchnic portal tract hypertension and upper digestive bleeding. The survival data revealed that there were no significant differences in all groups according to different procedures, and the anastomoses of the portal vein-artery reconstruction demonstrated reliable patency when the rats were sacrificed, which proved that the microvascular suturing techniques played a beneficial role in survival rates and could be considered an accepted standard. In contrast to the stent cannula anastomosis, our microsurgical end-to-end semi-continuous vascular suturing technique does not require external artificial graft and could maximally eliminate the injury to the vascular wall and endothelium. Hence, owing to its safety and patency, our method was more applicable and feasible for PVA after extended partial hepatectomy.
Investigators have shown that perfusion of systemic venous blood rather than blood from the intestines and pancreas maintains the liver size[24],[26],[27] and regenerative hyperplasia is within the normal range[28]. Rocheleau[29] et al. showed that either portal venous or hepatic arterial flow could maintain viable liver mass in proportion to the amount of blood flow delivered. The present study documented that the liver regeneration rates were significantly higher in rats that underwent arterialization within 14 days after extended partial hepatectomy, which showed that an increase in portal blood flow and oxygen supply was beneficial to liver regeneration after hepatectomy. Besides, the initiation and maintenance of liver regeneration after hepatectomy do not necessarily require highly concentrated hepatotrophic factors; instead, other classic splanchnic factors in the portal blood may be the alternative initial triggers of the regenerative process. The LLR outcome indicated that the sufficiency of the hepatic blood inflow, originated from either the portal tract or artery, play a more important and essential role rather than the presence of the factors in portal blood. The reason is probably that factors and elements involved in liver regeneration will be absorbed and metabolized more efficiently in a hyperdynamic circulating situation.
The limitation of the PVA technique is the negative effects of “overarterialization”, which may result in damage to the parenchyma. Furthermore, the mechanical injury caused by hyperdynamics may lead to hepatic fibrosis. In our study, increasing portal inflow and portal tract pressure was buffered and well adapted, reaching a new hemodaymic balance shortly after operation. However, further research is needed to investigate the anastomoses with inflow control which can ultimately avoid the overflow to the liver. The hand suturing microvascular reconstruction technique utilized in our model is more demanding and frustrating as compared with the sutureless stenting technique. To our knowledge, this limitation can be conquered by sufficient practice and a tacit cooperative surgical team. In addition, longer-term studies are required to evaluate liver function and the effects of the altered hepatic hemodynamics on sinusoidal cell function, and longer-term observations of the ultrastructural pathological changes of the hepatic parenchymal and mesenchymal cell are also desired.
In conclusion, our microvascular hand suturing anastomotic technique for portal vein-renal artery reconstruction is simple, safe and feasible, and the PVA rat model which we established is not only a pathway for improving remnant liver regeneration swiftly, it also maintains and even optimizes the liver function after extended hepatectomy.
References
- 1.Maillard JN, Benhamou JP, Rueff B.Arterialization of the liver with portacaval shunt in the treatment of portal hypertension due to intrahepatic block. Surgery 197067(6), 883–890. [PubMed] [Google Scholar]
- 2.Iseki J, Touyama K, Noie T, et al. Partial portal arterialization for the prevention of massive liver necrosis following extended pancreatobiliary surgery: experience of two cases. Surg Today 199222(6), 568–571. [DOI] [PubMed] [Google Scholar]
- 3.Fritsch A, Funovics J, Gangl A, et al. Controlled arterialization of the liver. II. Clinical results (author's transl). Langenbecks Arch Chir 1974336(1), 67–89. [DOI] [PubMed] [Google Scholar]
- 4.Funovics J, Gangl A, Horak W, et al. et al. Controlled arterialization of the liver. I. Experimental observationes (author's transl). Langenbecks Arch Chir 1974335(4), 339–349. [DOI] [PubMed] [Google Scholar]
- 5.Gigot JF, Otte JB, Lambotte L, et al. Arterialization of the portal vein associated with a portocaval shunt: Long-term results of a controlled prospective study. Acta Gastroenterol Belg 199053(2), 237–247. [PubMed] [Google Scholar]
- 6.Matzander U. Results of veno-venous shunt with arterialization of the liver. Langenbecks Arch Chir. 1976;342:145–151. doi: 10.1007/BF01267361. [DOI] [PubMed] [Google Scholar]
- 7.Voorhees AB, Jr, Price JB, Jr, Britton RC.Portasystemic shunting procedures for portal hypertension. Twenty-six year experience in adults with cirrhosis of the liver. Am J Surg 1970119(5), 501–505. [DOI] [PubMed] [Google Scholar]
- 8.Housari G, Nuño J, Calero P, et al. Portal vein arterialization in liver transplantation: an option to restore arterial flow: a case report. Transplant Proc. 201143(3), 755–757. [DOI] [PubMed] [Google Scholar]
- 9.Bonnet S, Sauvanet A, Bruno O, et al. Long-term survival after portal vein arterialization for portal vein thrombosis in orthotopic liver transplantation. Gastroenterol Clin Biol. 201034(1), 23–28. [DOI] [PubMed] [Google Scholar]
- 10.Erhard J, Lange R, Giebler R, et al. Arterialization of the portal vein in orthotopic and auxiliary liver transplantation. Transplantation 199560(8), 877–879. [PubMed] [Google Scholar]
- 11.Aspinall RJ, Seery JP, Taylor-Robinson SD, et al. Comments on “Arterialization of the portal vein in orthotopic and auxiliary liver transplantation”. Transplantation 199662(9), 1375–1376. [DOI] [PubMed] [Google Scholar]
- 12.Troisi R, Kerremans I, Mortier E, et al. Arterialization of the portal vein in pediatric liver transplantation. A report of two cases. Transpl Int 199811(2), 147–151. [DOI] [PubMed] [Google Scholar]
- 13.Nardo B, Caraceni P, Montalti R, et al. Portal vein arterialization: A new surgical option against acute liver failure?. Transplant Proc 200537(6), 2544–2546. [DOI] [PubMed] [Google Scholar]
- 14.Nardo B, Caraceni P, Puviani L, et al. Successful treatment of CCl(4)-induced acute liver failure with portal vein arterialization in the rat. J Surg Res 2006135(2), 394–401. [DOI] [PubMed] [Google Scholar]
- 15.Nardo B, Vaccarisi S, Pellegrino V, et al. Extracorporeal portal vein arterialization in man after extended hepatectomy to prevent acute liver failure: a case report. Transplant Proc. 201143(4), 1193–1195. [DOI] [PubMed] [Google Scholar]
- 16.Hinkle PC. In: Mitochondria. The Liver: Biology and pathobiology. 3rd ed. Arias I M, Boyer J L, Fausto N, Jakoby W B, Schachter D, Shafritz D A, editors. New York, NY: Raven Press; pp. 323–364. [Google Scholar]
- 17.Nonami T, Asahi K, Harada A, et al. Effect of hyperdynamic circulatory support on hepatic hemodynamics, oxygen supply and demand after massive hepatectomy. Surgery 1991109(3 Pt 1), 277–283. [PubMed] [Google Scholar]
- 18.Fisher B, Russ C, Updegraff H, et al. Effect of increased hepatic blood flow upon liver regeneration. AMA Arch. Surg. 195469(2), 263–272. [DOI] [PubMed] [Google Scholar]
- 19.Nardo B, Puviani L, Prezzi D, et al. Protective effect of portal vein arterialization in acute liver failure induced by hepatectomy in normal and fatty liver rat. Transpl Proc 200638(10), 3249–3250. [DOI] [PubMed] [Google Scholar]
- 20.Fan YD, Praet M, Van Huysse J, et al. Effects of portal vein arterialization on liver regeneration after partial hepatectomy in the rat. Liver Transpl 20028(2), 146–152. [DOI] [PubMed] [Google Scholar]
- 21.Schleimer K1, Stippel DL, Kasper HU, et al. Portal hyperperfusion causes disturbance of microcirculation and increased rate of hepatocellular apoptosis: Investigations in heterotopic rat liver transplantation with portal vein arterialization. Transpl Proc 200638(3), 725–729. [DOI] [PubMed] [Google Scholar]
- 22.Higgins GM, Anderson RM. Experimental pathology of the liver: I. Restoration of the liver of the white rat following partial surgical removal. Arch Pathol. 1931;12:186–202. [Google Scholar]
- 23.Lee S, Fisher B. Porto-caval shunt in the rat. Surgery. 1961;50:668–675. [PubMed] [Google Scholar]
- 24.Child CG, 3rd, Barr D, Halswarde GK.Liver regeneration following portal-caval transposition in dogs. Ann Surg 1953138(4), 600–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogata A, Miyazaki M, Ohtawa S, et al. Short-term effect of portal vein arterialization on hepatic protein synthesis and endotoxaemia after extended hepatectomy in dogs. J Gastroenterol Hepatol 199712(9-10), 633–638. [DOI] [PubMed] [Google Scholar]
- 26.Shimizu Y, Miyazaki M, Shimizu H, et al. Beneficial effects of arterialization of the portal vein on extended hepatectomy. Br J Surg 200087(6), 784–789. [DOI] [PubMed] [Google Scholar]
- 27.de Jonge J, Madern GC, Terpstra OT, et al. Directing portal flow is essential for graft survival in auxiliary partial heterotopic liver transplantation in the dog. J Pediatr Surg 199934(8), 1265–1268. [DOI] [PubMed] [Google Scholar]
- 28.Lee S, Broelsch CE, Flamant YM, et al. Liver regeneration after portacaval transportation in rats. Surgery 197577(1), 144–149. [PubMed] [Google Scholar]
- 29.Rocheleau B, Ethier C, Houle R, et al. Hepatic artery buffer response following left portal vein ligation: Its role in liver tissue homeostasis. Am J Physiol 1999277(5 Pt 1), G1000–1007. [DOI] [PubMed] [Google Scholar]