Background: Systemic lupus erythematosus (SLE)-related autoantibodies are of unknown origin but target multiple apoptotic cell-derived antigens.
Results: T cell responses to multiple epitopes on β2-glycoprotein I (β2GPI), an apoptotic cell-binding protein, were associated with SLE-related autoantibody production.
Conclusion: Distinct β2GPI-reactive T cell responses are associated with SLE-related autoantibodies.
Significance: Factors enabling β2GPI-reactive T cell responses may predispose individuals to SLE.
Keywords: Animal Model, Autoimmune Disease, Epitope Mapping, Major Histocompatibility Complex (MHC), T Cell, β2-Glycoprotein I, Autoantibodies, Epitope Spread, Systemic Lupus Erythematosus
Abstract
Systemic lupus erythematosus (SLE) is a prototypic model for B cell epitope spread in autoimmunity. Autoantibodies to numerous and molecularly distinct self-antigens emerge in a sequential manner over several years, leading to disease manifestations. Among the earliest autoantibodies to appear are those targeting the apoptotic cell-binding protein β2-glycoprotein I (β2GPI). Notably, mice immunized with β2GPI and LPS display a remarkably similar pattern of autoantibody emergence to that seen in human SLE. Here, we used this model to investigate whether epitope spread to SLE-related autoantibodies is associated with a unique or limited β2GPI-specific T cell response. We ask whether MHC class II haplotype and its associated T cell epitope restriction impact epitope spread to SLE-related autoantibodies. We found that β2GPI/LPS-immunized mice produced similar SLE-related autoantibody profiles regardless of their β2GPI T cell epitope specificity or MHC class II haplotype. Although β2GPI T cell epitope specificity was clearly determined by MHC class II haplotype, a number of different β2GPI T cell epitopes were associated with epitope spread to SLE-related autoantibodies. Notably, one β2GPI T cell epitope (peptide 23, NTGFYLNGADSAKCT) was also recognized by T cells from an HLA-DRB1*0403+ autoimmune patient. These data suggest that the generation of a β2GPI-reactive T cell response is associated with epitope spread to SLE-related autoantibodies, independent of epitope specificity or MHC class II restriction. On the basis of these findings, we propose that factors enabling a β2GPI-reactive T cell response may predispose individuals to the development of SLE-related autoantibodies independent of their MHC class II haplotype.
Introduction
Systemic lupus erythematosus (SLE)6 is an autoimmune disease in which autoantibodies to self-antigens, particularly cellular components, appear in a consistent and sequential pattern (1, 2). Autoantibodies to the plasma protein β2-glycoprotein I (β2GPI), either alone (anti-β2GPI) or bound to anionic phospholipid (anti-cardiolipin (anti-CL)), are among the earliest to appear. Other SLE-associated autoantibodies, such as anti-dsDNA, anti-Sm, and anti-nuclear ribonucleoprotein (nRNP) antibodies, emerge somewhat later (1, 3). The accumulation of multiple diverse SLE-related autoantibodies leads eventually to clinically evident disease (1).
Although much is known about the targets and specificities of SLE-related autoantibodies, far less is understood about their etiology. Antibodies to β2GPI are generally included in the category of anti-phospholipid antibodies and are present in ∼20–30% of patients with SLE. Patients who are anti-phospholipid antibody-positive not only develop other SLE-related autoantibodies earlier than anti-phospholipid antibody-negative individuals, but also appear to have a more severe clinical outcome (3). The early appearance of anti-phospholipid antibodies and their association with a premature onset of other SLE-related autoantibodies suggest that these autoantibodies, or the mechanism leading to their formation, may be an initiating event for epitope spread to multiple other autoantibodies in SLE.
Consistent with this theory, we have shown that non-autoimmune mice immunized with β2GPI in the presence of a strong innate immune activator (e.g. LPS) produce SLE-related autoantibodies in a sequential manner recapitulating that seen in human SLE and develop overt SLE-like glomerulonephritis (4). We have proposed that the strong and persistent T cell response to β2GPI observed in these mice (5) is responsible for B cell epitope spread to multiple SLE-related autoantibodies (4). β2GPI binds to apoptotic cells (6), which express many SLE-associated autoantigens (7, 8), and it is this property of β2GPI that we believe underlies the ability of β2GPI-specific T cells to promote intermolecular spread to other SLE autoantigens (4, 9).
Here, we took advantage of the influence of MHC class II background on T cell epitope specificity to test the hypothesis that generation of a β2GPI-specific T cell response enables epitope spread to SLE-related antibodies. Using our model of induced SLE, we first produced a strong T cell response to β2GPI in several non-autoimmune murine strains of varying MHC class II haplotype. We then determined the epitope specificity of the resulting β2GPI-reactive T cell response, and whether MHC class II haplotype, and its associated β2GPI T cell epitope restriction, impact epitope spread to SLE-related autoantibodies. Finally, we investigated whether β2GPI T cell epitopes are shared between murine and human individuals.
Our findings demonstrate that a T cell response to β2GPI alone is associated with B cell epitope spread to SLE-related autoantibodies. Although the epitope specificity of the β2GPI-specific T cell response was determined by the individual's MHC class II haplotype, multiple β2GPI T cell epitopes were associated with the production of SLE-related autoantibodies. One β2GPI T cell epitope was shared by both H-2b-bearing mice and an HLA-DRB1*0403+ autoimmune patient, suggesting that the induced β2GPI-specific T cell response mimics that in autoimmune disease. Together, our data indicate that B cell epitope spread to SLE-related autoantibodies can occur in the context of multiple MHC class II haplotypes and their correspondingly restricted T cell epitopes. We propose that generation of a β2GPI-reactive T cell response may represent a critical initiating event permitting B cell epitope spread and leading ultimately to the production of the full range of SLE-related autoantibodies.
EXPERIMENTAL PROCEDURES
Mice and Immunization
Specific pathogen-free female C57BL/6 and BALB/c mice (8–12 weeks of age) were purchased from Harlan Sprague-Dawley (Indianapolis, IN). Female C3H/HeN and some BALB/c mice were generously provided by Drs. Salman Qureshi and Samuel David, respectively. Female 129S1/SvImJ (129S1), B6.C-H2d/bByJ (B6.C), and C.B10-H2b/LilMcdJ (C.B10) mice were purchased from The Jackson Laboratory (Bar Harbor, Maine). Mice were maintained and bred according to Canadian Council on Animal Care guidelines and were given food and water ad libitum. Animal experiments were approved by the McGill University and the Research Institute of the McGill University Health Centre.
C57BL/6, BALB/c, and C3H/HeN mice were immunized with 20 μg of β2GPI and 10 μg of LPS as described previously (5). Mice were injected every 2 weeks and bled for serum 10 days following the second and third immunizations. For the T cell studies, the number of immunizations required was determined by the levels of anti-β2GPI antibodies observed. C57BL/6 mice received four immunizations (the fourth immunization was with half the dose of β2GPI and LPS), BALB/c mice received three immunizations, and C3H/HeN mice received two immunizations. C3H/HeN mice were immunized only twice, as they did not survive a third immunization. Their antibody levels (after the second immunization) were similar to those of C57BL/6 and BALB/c mice following three immunizations. Two (of four) 129S1 mice died after the third immunization; the post-second immunization serum was used for those mice. Premature death in the C3H/HeN mice and some 129S1 mice may have been due to an accelerated antibody response to the immunogen.
Reagents
Unless stated otherwise, all reagents were obtained commercially from the indicated sources and used without further purification: human β2GPI (≥95% pure; Crystal Chem, Downers Grove, IL); LPS (Escherichia coli-derived, serotype O111:B4; List Biological Laboratories, Campbell, CA); bovine heart cardiolipin (Avanti Polar Lipids, Alabaster, AL); E. coli dsDNA (Worthington); Ro (SS-A), La (SS-B), Smith antigen (Sm), and nRNP (Immunovision, Springdale, AR); recombinant IL-2, rat anti-mouse IL-2, biotinylated rat anti-mouse IL-2, mouse IFN-γ ELISA set (BD OptEIA kit), 3,3′,5,5′-tetramethylbenzidine substrate reagent set (BD OptEIA kit), and hamster anti-mouse CD3e (BD Biosciences); alkaline phosphatase-conjugated goat anti-rabbit IgG and alkaline phosphatase-conjugated streptavidin (SouthernBiotech, Birmingham, AL); and p-nitrophenyl phosphate (Sigma-Aldrich).
β2GPI Recombinant Fragments and Synthetic Peptides
Recombinant maltose-binding protein (MalBP) fusion proteins encoding the following regions of human β2GPI were used as antigens for T cell stimulation as described previously (10). These fusion proteins, which have been described previously (11), included GP-F, encoding the entire amino acid sequence of β2GPI (amino acid residues 1–326); GP-1, encoding Domains I and II (amino acid residues 1–133); GP-2, encoding Domains III and IV (amino acid residues 119–254); and GP-3, encoding Domains IV and V (amino acid residues 182–326) (see Fig. 1A). MalBP was also prepared and used as a control antigen.
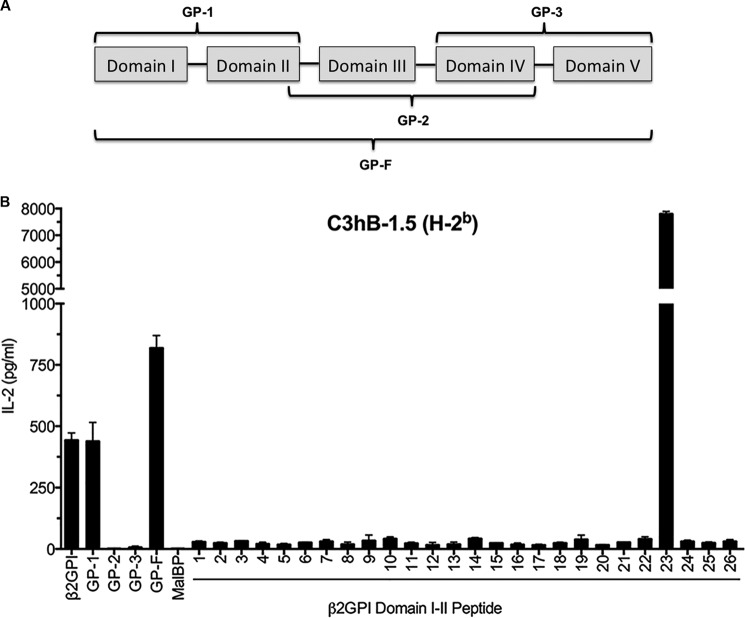
FIGURE 1.
A β2GPI-reactive T cell hybridoma from C57BL/6 (H-2b) mice recognizes peptide 23 in Domain II of β2GPI. A, β2GPI recombinant fragments consisting of different combinations of domains were used to evaluate T cell epitope specificity. The recombinant fragments (shown schematically) are GP-1 (Domains I and II), GP-2 (Domains III and IV), GP-3 (Domains IV and V), and GP-F (full-length β2GPI). B, the C3hB-1.5 β2GPI-specific T cell hybridoma was evaluated for recognition of recombinant fragments of human β2GPI and twenty-six 15-mer peptides with a 10-residue overlap and encompassing Domains I and II of β2GPI. Human β2GPI (20 μg/ml), recombinant fragments (20 μg/ml), or peptides (10 μg/ml) were incubated with C3hB-1.5 cells and C57BL/6-derived APCs for 24 h. The IL-2 concentration in the supernatant was measured by ELISA. Values represent the mean IL-2 concentration (ng/ml) ± S.E. of triplicate samples, and the data shown are representative of three independent experiments.
Twenty-six 15-mer peptides with a 10-residue overlap and spanning Domains I and II of human β2GPI were synthesized, and their purity was determined by HPLC (Sigma-Aldrich). The peptides were dissolved in 200 μl of dimethyl sulfoxide and further diluted in 0.01 m PBS (pH 7.3) in 500-μl stocks. Peptide stock solutions in dimethyl sulfoxide and PBS were stored at −70 °C. The peptides were added to the antigen presentation assays described below at a final concentration of 10 μg/ml in PBS.
Cell Culture
Unless stated otherwise, all cells were cultured in DMEM (4.5 g/liter glucose and 110 mg/ml sodium pyruvate) containing 10% heat-inactivated FBS, 1% penicillin/streptomycin, 1% l-glutamate, 1% HEPES, 1% nonessential amino acids, and 0.1% 2-mercaptoethanol (medium and supplements were from Invitrogen), hereafter referred to as complete DMEM. Splenic T cells from immunized mice were isolated using an EasySep T cell kit (STEMCELL Technologies, Vancouver, British Columbia, Canada) and cultured in complete DMEM containing β2GPI-depleted FBS. FBS was depleted of β2GPI using a HiTrap heparin HP column (GE Healthcare) to eliminate the potential influence of bovine β2GPI. β2GPI-depleted FBS was unable to support binding of a bovine β2GPI-dependent murine monoclonal antibody to CL by ELISA (data not shown). Human T cell clones were cultured in RPMI 1640 medium supplemented with 10% β2GPI-depleted FBS, 2 mm l-glutamine, 10 mm HEPES, 50 units/ml penicillin, and 50 μg/ml streptomycin at 37 °C and 5% CO2.
Generation of β2GPI-specific T Cell Hybridoma C3hB-1.5
The C3hB-1.5 T cell hybridoma was generated from a C57BL/6 mouse that had received four biweekly intravenous immunizations of human β2GPI (20 μg) on Day 1 and LPS (10 μg) on Day 2 as described previously (4, 5) and a fifth injection of β2GPI alone (20 μg) 2 weeks prior to the fusion experiment. Isolated splenic CD4+ T cells (EasySep T cell kit) were plated at 106 cells/well and incubated with human β2GPI (15 μg/ml) at 37 °C and 10% CO2 in the presence of naive C57BL/6 splenocytes (4 × 106 cells/well) as antigen-presenting cells (APCs). IL-2 (20 μg/ml) was added to the culture on Day 5. On Day 11, the cultured T cells (2.9 × 106) were fused with 107 BWα/β-cells as described previously (12). The resulting hybridomas were screened against human β2GPI and human serum albumin. T cell hybridomas that responded to human β2GPI but not human serum albumin were kept and subcloned by limiting dilution. C3hB-1.5 is a subclone that responded strongly to human β2GPI but showed no response to human serum albumin.
Domain and Epitope Specificity of β2GPI-specific T Cell Hybridoma C3hB-1.5
The domain specificity of the C3hB-1.5 T cell hybridoma was determined using recombinant protein fragments of human β2GPI (GP-1, GP-2, GP-3, and GP-F) or MalBP as a negative control. Twenty microliters of recombinant protein fragments (10 μg/ml final concentration in PBS) or commercial human β2GPI (10 or 20 μg/ml final concentration) were added to murine APCs (C57BL/6 splenocytes, 2 × 106 cells/ml, 50 μl/well) in triplicate wells. C3hB-1.5 T cell hybridoma cells (2 × 106 cells/ml, 50 μl/well) were then added to the wells. C3hB-1.5 T cell hybridoma cells stimulated with anti-mouse CD3e antibody (5 μg/ml in PBS) served as a positive control. Supernatants were screened for IL-2 by ELISA as described previously (13). Results are expressed as the mean IL-2 concentration (ng/ml) of triplicate samples as determined from a standard curve using recombinant IL-2. The epitope specificity of the C3hB-1.5 T cell hybridoma was determined using the assay described above for domain specificity, except that peptides (10 μg/ml final concentration) were used in place of recombinant fragments.
Domain and Epitope Specificity of T Cells from Immunized Mice
Strain-matched APCs (splenocytes from naive C57BL/6, BALB/c, or C3H/HeN mice) were plated at 4 × 106 cells/well in complete DMEM containing β2GPI-depleted FBS. Recombinant protein fragments of β2GPI or MalBP, commercial β2GPI, or Domain I-II peptides were added to the APCs in duplicate. Splenic T cells (106 cells/well) isolated from immunized C57BL/6, BALB/c, or C3H/HeN mice were then added to the culture and incubated for 48 h at 37 °C and 5% CO2. T cells stimulated with phorbol 12-myristate 13-acetate (0.02 μg/ml in PBS) and ionomycin (1 μm in PBS) served as a positive control for maximal T cell activity. Cell supernatants were collected, and IFN-γ levels were quantified by ELISA. Results are expressed as the mean IFN-γ concentration (pg/ml) of duplicate samples as determined from a standard curve using recombinant IFN-γ.
Detection of Autoantibodies
Anti-β2GPI, anti-CL, anti-dsDNA, anti-Ro (SS-A), anti-La (SS-B), anti-Sm, and anti-nRNP antibodies were determined by ELISA as described previously (4). Anti-β2GPI domain antibodies were determined in mouse sera by the following ELISA. ELISA high binding plates (Greiner Bio-One, Monroe, NC) were coated with recombinant protein fragments of β2GPI (10 μg/ml in PBS with 0.02% azide (PBS/azide)) for 16 h at 37 °C. The coated plates were blocked with PBS/azide containing 0.5% gelatin and 10% FBS for 2 h at 4 °C and washed three times with 0.01 m TBS (pH 7.4). Sera were diluted 1:100 (unless noted otherwise) in PBS/azide containing 0.3% gelatin and 10% FBS and incubated in duplicate in coated wells for 3 h at 25 °C. Following three washes with TBS, alkaline phosphatase-conjugated goat anti-mouse IgG (diluted 1:1000 in PBS/azide containing 0.4% BSA) was added and incubated for 16 h at 4 °C. Plates were washed with TBS and developed with p-nitrophenyl phosphate, and the absorbance at 405 nm was read using an ELISA reader (BioTek Instruments, Inc., Winooski, VT). Murine hybridoma anti-β2GPI antibodies (with known domain specificity) served as positive controls.
Anti-β2GPI domain antibodies in patient serum were measured using an ELISA in which CL-coated plates were preincubated with purified human native β2GPI, GP-F, GP-1, GP-2, GP-3, and MalBP as described previously (14). Serum samples (diluted at 1:100) were tested in triplicate, and the values represent the mean A405 ± S.D.
β2GPI-reactive Human T Cell Clones
All patient samples were obtained following written informed consent as approved by the Keio University Institutional Review Board. Human CD4+ T cell clones (OM3, OM9, and OM13) reactive with GP-1 (Domains I and II) of β2GPI were derived from a patient (OM) with primary anti-phospholipid syndrome (APS). These T cell clones were generated from peripheral blood T cells by repeated stimulation with GP-F followed by limiting dilution as described previously (10) and were selected based on specific recognition of GP-1 in an HLA-DR-restricted manner and availability. The HLA-DR alleles of patient OM include DRB1*1502, DRB1*0403, DRB4*0103, and DRB5*0101.
T Cell Proliferation Assay
Antigen-specific T cell proliferation in peripheral blood T cells and β2GPI-reactive CD4+ T cell clones was assayed as described previously (10, 11). For peripheral blood T cells, peripheral blood mononuclear cells, isolated from heparinized venous blood by Lymphoprep (Fresenius Kabi Norge AS, Oslo, Norway) density gradient centrifugation, were cultured with or without antigen in 96-well flat-bottomed culture plates for 7 days. GP-F, GP-1, GP-2, GP-3, MalBP, and tetanus toxoid (List Biological Laboratories) were used as antigens at a concentration of 5 μg/ml. Phytohemagglutinin (1 μg/ml) was used to ensure that the T cells were responsive. The T cell clones were cultured with irradiated autologous Epstein-Barr virus-transformed B cells and antigen, including GP-F, GP-1, GP-2, GP-3, MalBP, and a series of synthetic peptides covering Domains I and II of β2GPI (5 μg/ml) for 3 days. L cells transfected with the DRA gene and one of the following DRB genes (DRB1*1501 (LDR2B)), DRB1*0403 (B19), DRB4*0103 (L17.8), and DRB5*0101 (LDR2A)) were used as APCs in place of B cells to evaluate HLA-DR restriction. L cells were irradiated and incubated with synthetic peptides (5 μg/ml) for 2 h before mixing with T cell clones. [3H]Thymidine (0.5 μCi/well) was added to the cultures during the final 16 h of incubation. The cells were harvested, and [3H]thymidine incorporation was measured in a TopCount microplate scintillation counter (Packard Instrument Co., Meriden, CT). All cultures were performed in triplicate, and values represent the mean of triplicate determinations.
Statistical Analysis
Statistical significance was determined by a two-tailed unpaired non-parametric Mann-Whitney test using Prism 6.0 (GraphPad Software, San Diego, CA). The minimal threshold for significance was p < 0.05.
RESULTS
A β2GPI-reactive T Cell Hybridoma from C57BL/6 (H-2b) Mice Recognizes a Peptide (Peptide 23) from Domain II of β2GPI
We have previously shown that C57BL/6 mice immunized with β2GPI and LPS produce a strong T cell response to β2GPI (5). As the first step in investigating the domain and epitope specificity of this T cell response, we evaluated a β2GPI-reactive T cell hybridoma (C3hB-1.5) derived from β2GPI/LPS-immunized C57BL/6 mice. Domain specificity was evaluated using recombinant protein fragments of human β2GPI: GP-1 (Domains I and II), GP-2 (Domains III and IV), and GP-3 (Domains IV and V) (Fig. 1A). Full-length recombinant β2GPI (GP-F) served as a positive control, and the control fusion protein (MalBP) served as a negative control. The β2GPI-specific T cell hybridoma (C3hB-1.5) recognized recombinant fragment GP-1 exclusively (Fig. 1B), indicating recognition of a peptide in Domains I and II. Next, the T cell hybridoma C3hB-1.5 was screened with a peptide library (twenty-six 15-mer peptides) that spanned the entire sequence of Domains I and II. The T cell hybridoma recognized a single peptide (peptide 23, NTGFYLNGADSAKCT) located in Domain II of β2GPI (Fig. 1B).
β2GPI/LPS-immunized C57BL/6 (H-2b) Mice Show a Dominant T cell Response to a Single Peptide (Peptide 23) from Domain II of β2GPI
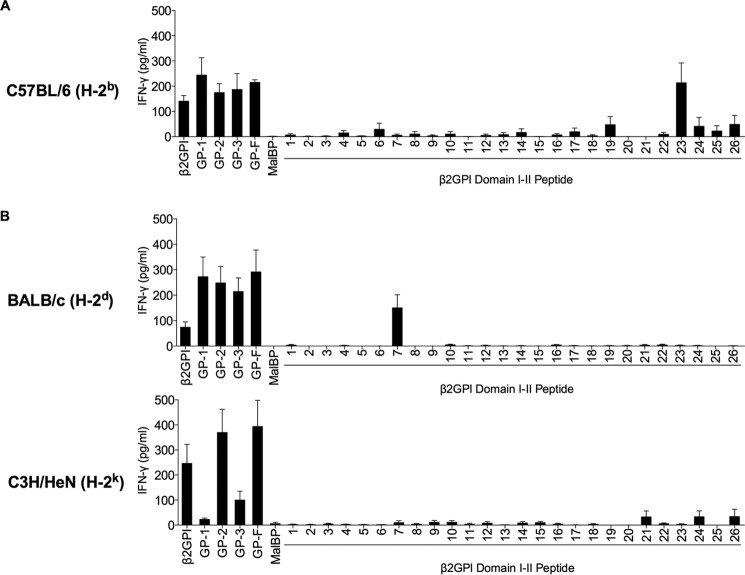
We next investigated whether the domain and epitope specificity of the C57BL/6-derived β2GPI-specific T cell hybridoma (C3hB-1.5) is representative of primary T cells from these mice. Splenic T cells were isolated from C57BL/6 (H-2b) mice immunized with human β2GPI and LPS and evaluated for their response to human β2GPI or its different recombinant fragments. C57BL/6-derived T cells showed strong recognition of GP-1, similar to that of the C3hB-1.5 T cell hybridoma, and also recognized GP-2 and GP-3 (Fig. 2A). These T cells also responded to serum-derived human β2GPI and recombinant full-length β2GPI (GP-F), but not to the control recombinant protein (MalBP). Because C57BL/6-derived T cells recognized GP-1, their epitope specificity was evaluated using the same peptide library comprising the GP-1 sequence that we used to evaluate the C3hB-1.5 T cell hybridoma. Similar to the C3hB-1.5 T cell hybridoma, T cells from β2GPI/LPS-immunized C57BL/6 mice recognized a single peptide (peptide 23) (Fig. 2A). These findings suggest that C57BL/6 (H-2b) mice immunized with β2GPI and LPS have a dominant T cell response to peptide 23 in Domain II of β2GPI.
FIGURE 2.
Domain and epitope specificity of the β2GPI-reactive T cell response varies with MHC class II haplotype. Splenic T cells from β2GPI/LPS-immunized mice (C57BL/6 (H-2b) (A), BALB/c (H-2d) (B), or C3H/HeN (H-2k) (B)) were plated with strain-matched APCs. β2GPI, GP-1 (Domains I and II), GP-2 (Domains III and IV), GP-3 (Domains IV and V), GP-F (full-length β2GPI), or MalBP (control fusion protein) was added to the culture at a concentration of 20 μg/ml, and individual peptides from Domains I and II were added at 10 μg/ml. Cells were incubated for 48 h, and IFN-γ production in the supernatant was measured by ELISA. Values represent the mean IFN-γ concentration (pg/ml) ± S.E. of duplicate samples, and the data shown are pooled from three independent experiments.
Domain and Epitope Specificity of the β2GPI-reactive T Cell Response Varies with MHC Class II Haplotype
To determine whether the T cell response to β2GPI is affected by MHC class II haplotype, we investigated the epitope specificity of the β2GPI-specific T cell response in mice with haplotypes other than H-2b. Like C57BL/6 (H-2b)-derived T cells, T cells from β2GPI/LPS-immunized BALB/c (H-2d) mice recognized all recombinant fragments of β2GPI equally and responded to a single peptide within GP-1 (Fig. 2B). However, T cells from BALB/c (H-2d) mice responded to a different peptide in Domain I (peptide 7, FSTVVPLKTFYEPGE) than that recognized by C57BL/6-derived T cells. We next evaluated T cells from β2GPI/LPS-immunized mice with a third haplotype (H-2k). C3H/HeN (H-2k) mice responded strongly to GP-2 but showed a minimal response to GP-3 and no response to GP-1 (Fig. 2B). Consistent with their lack of response to GP-1, C3H/HeN-derived T cells did not recognize any peptides from GP-1 (Fig. 2B). These data demonstrate that mice with different MHC class II haplotypes produced a strong T cell response to β2GPI, but the domain and epitope specificity of the T cell response varied among these strains.
β2GPI T Cell Epitope Specificity Segregates with MHC Class II Haplotype
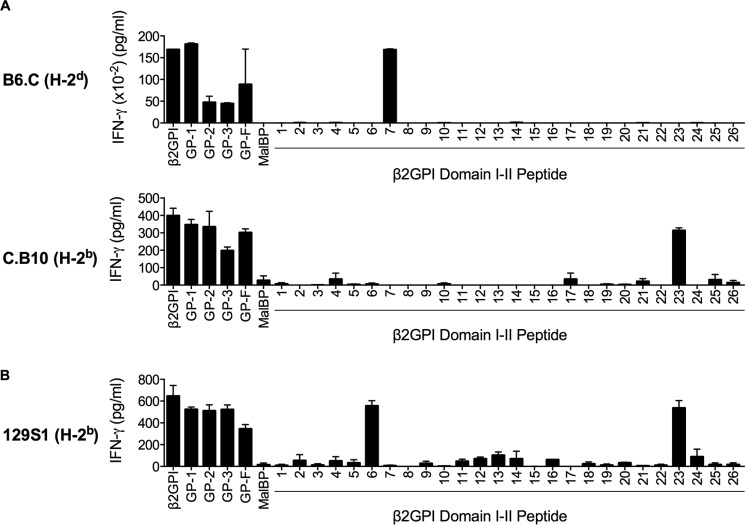
We wondered whether the differences in epitope specificity between β2GPI-reactive T cells from different mouse strains was determined by MHC class II haplotype or by other genetic differences between these strains. To ensure that MHC class II haplotype was the only variable assessed in these experiments, we used congenic strains of mice differing solely in MHC class II haplotype. To complement our earlier experiments, we selected mice in which the C57BL/6 (H-2b) and BALB/c (H-2d) MHC class II haplotypes had effectively been swapped: B6.C (C57BL/6 background with H-2d haplotype) and C.B10 (BALB/c background with H-2b haplotype). In both strains, T cell domain and epitope specificity was strikingly associated with MHC class II haplotype. Like BALB/c-derived T cells, T cells from β2GPI/LPS-immunized B6.C (H-2d) mice recognized GP-1 (Domains I and II) and peptide 7 in Domain I (Fig. 3A). Similarly, T cells from C.B10 (H-2b) mice showed domain and epitope specificity resembling that in C57BL/6 mice (H-2b) (Fig. 3A). Like C57BL/6-derived T cells, C.B10-derived T cells recognized all domains of β2GPI, with specific recognition of peptide 23 in Domain II (Fig. 3A). We also evaluated T cells from 129S1 mice, which have the same MHC class II haplotype (H-2b) as C57BL/6 mice but are otherwise unrelated to this strain. Similar to the other H-2b-bearing murine strains, T cells from β2GPI/LPS-immunized 129S1 mice showed epitope specificity for peptide 23 (Fig. 3B). However, unlike C57BL/6- and C.B10-derived T cells, 129S1-derived T cells also recognized peptide 6 in Domain I of β2GPI. Together, these data demonstrate that β2GPI T cell epitope specificity is strikingly associated with MHC class II haplotype but do not rule out a contribution from non-MHC class II genes within the MHC complex, such as those involved in antigen processing and presentation.
FIGURE 3.
β2GPI T cell epitope specificity segregates with MHC class II haplotype. Splenic T cells from B6.C (C57BL/6 with H-2d) or C.B10 (BALB/c with H-2b) mice (A) or 129S1 (H-2b) mice (B) that had been immunized with β2GPI and LPS were plated with MHC class II haplotype-matched APCs. β2GPI, GP-1 (Domains I and II), GP-2 (Domains III and IV), GP-3 (Domains IV and V), GP-F (full-length β2GPI), or MalBP (control fusion protein) was added to the culture at concentration of 20 μg/ml, and individual peptides from Domains I and II were added at 10 μg/ml. Cells were incubated for 48 h, and IFN-γ production in the supernatant was measured by ELISA. Values represent the mean IFN-γ concentration (pg/ml) ± S.E. of duplicate samples, and the data shown are representative of three independent experiments.
Multiple Distinct MHC Class II-restricted β2GPI T Cell Epitopes Are Associated with B Cell Epitope Spread to SLE Autoantibodies
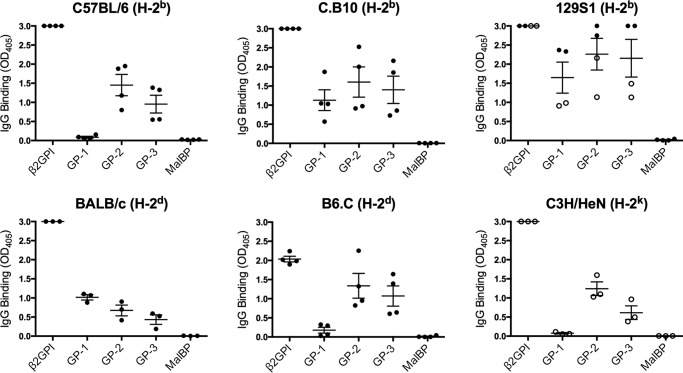
As the specificity of the β2GPI T cell response differed between mice with different MHC class II haplotypes, we wondered whether the difference in T cell specificity and MHC class II haplotype would impact B cell epitope spread to SLE-related autoantibodies. We compared the induction of SLE-related IgG autoantibodies in mice with different β2GPI T cell epitopes and MHC class II haplotypes: C57BL/6 (H-2b), BALB/c (H-2d), and C3H/HeN (H-2k). We first evaluated the antibody response to the immunizing antigen, human β2GPI, either alone (anti-β2GPI) or bound to CL (anti-CL). Notably, β2GPI/LPS-immunized mice from all three strains produced high levels (>1:1000 dilution) of anti-β2GPI (p < 0.03) and anti-CL (p < 0.03) antibodies compared with LPS-immunized mice (Fig. 4A). However, antibody titers differed among strains, particularly when comparing C3H/HeN mice with the other two strains. Following only two immunizations with β2GPI and LPS, C3H/HeN mice had antibody levels that were similar to those of C57BL/6 and BALB/c mice after three immunizations (Fig. 4A). Between C57BL/6 and BALB/c mice, autoantibody titers were generally higher in BALB/c mice following three immunizations (Fig. 4), but we have previously shown that these two strains produced similar levels of autoantibodies over the full course of immunization (4).
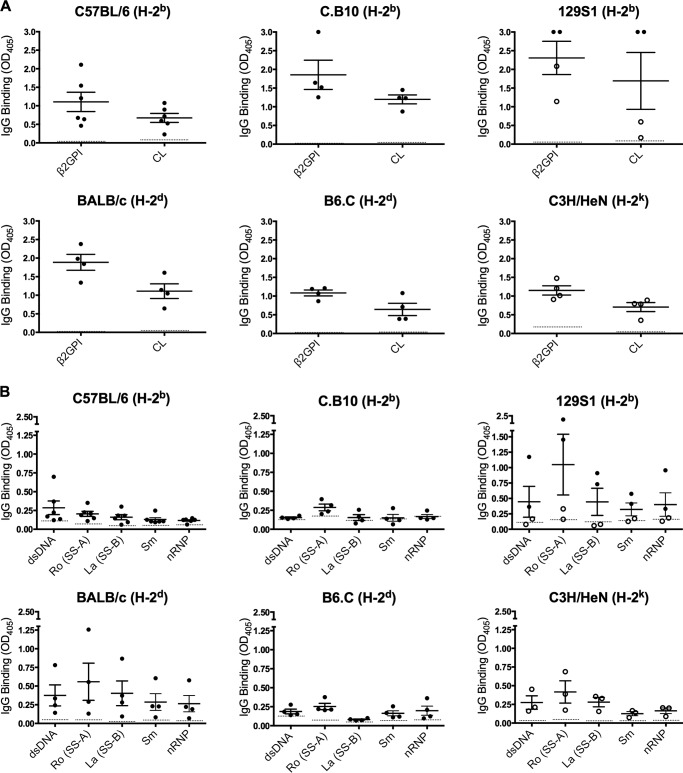
FIGURE 4.
Multiple distinct MHC class II-restricted β2GPI T cell epitopes are associated with B cell epitope spread to SLE autoantibodies. Sera from β2GPI/LPS-immunized mice were tested for IgG antibodies to human β2GPI (1:5000 dilution) and CL (1:1000 dilution) (A) and dsDNA, Ro (SS-A), La (SS-B), Sm, and nRNP (1:50 dilution) (B) by ELISA. The data shown for C57BL/6, C.B10, BALB/c, and B6.C mice are post-third immunization (black circles), whereas the data for all C3H/HeN mice and two 129S1 mice are post-second immunization (white circles) (see “Mice and Immunization”). Sera from mice of the same strain, immunized with PBS and LPS, served as a negative control in these assays. The mean value for PBS/LPS-immunized mice (n = 2 for each strain) is shown as a dotted line for each autoantibody assay. In certain cases, the mean value for the controls is very close to zero and so may be difficult to distinguish from the x axis. Each circle represents the mean IgG antibody binding (A405 ± S.E.) of duplicate samples for an individual mouse (n = three to six mice/group), and the data shown are representative of three independent experiments.
We next looked at whether the β2GPI/LPS-immunized mice developed other SLE-related autoantibodies (Fig. 4B). Anti-dsDNA antibodies, which are considered highly specific for SLE, were found in all strains except C.B10 mice. Levels varied among strains but were significantly elevated compared with the LPS-immunized strain controls in all strains except 129S1 (p < 0.008). In 129S1 mice, variability in the data shown in Fig. 4B is due to the use of sera from different bleeds (post-second or post-third immunization), but both post-third immunization bleeds had elevated levels of anti-dsDNA antibodies (A405 of 0.36 and 1.17 versus 0.11 for the control). All strains had significantly elevated levels of anti-Ro (SS-A) antibodies (p < 0.03), and most (except C.B10, 129S1, and B6.C) had significantly elevated levels of anti-La (SS-B) antibodies (p < 0.008). Anti-Sm (p < 0.008) and anti-nRNP (p < 0.02) antibodies were observed in all strains except C.B10 and 129S1 (Fig. 4B).
Together, these data indicate that despite having different β2GPI T cell epitope specificities and MHC haplotypes, multiple murine strains produced SLE-related autoantibodies following immunization with β2GPI/LPS. Intermolecular B cell epitope spread occurred in all strains, suggesting that a strong β2GPI-specific T cell response, independent of its epitope specificity or MHC class II restriction, can support B cell epitope spread in this model.
To determine whether “intramolecular B cell epitope spread” (i.e. between epitopes within β2GPI) had also occurred in these mice, we evaluated whether antibodies to β2GPI domains other than those recognized by the T cells were present (Fig. 5). This is clearest among strains that did not show T cell responses to all domains. For example, B6.C (H-2d) mice, which had a T cell response predominantly to GP-1, produced antibodies to GP-2 and GP-3. Similarly, C57BL/6 (H-2b) mice had a strong T cell response to GP-1, but the focus of the B cell response was to GP-2 and GP-3. These data indicate that intramolecular and intermolecular B cell epitope spread occurred in the presence of a β2GPI-specific T cell response across different MHC class II haplotypes.
FIGURE 5.
B cell epitope spread to other β2GPI domains occurs in the presence of a β2GPI-specific T cell response. Sera from β2GPI/LPS-immunized mice were tested by ELISA for antibodies to human β2GPI (native protein), recombinant protein fragments of human β2GPI (GP-1, GP-2, and GP-3), or MalBP (control fusion protein). The data shown for C57BL/6, C.B10, BALB/c, and B6.C mice are post-third immunization (black circles), whereas the data for all C3H/HeN mice and two 129S1 mice are post-second immunization (white circles) (see “Mice and Immunization”). Each circle represents the mean IgG binding (A405 ± S.E.) of duplicate samples (1:1000 dilution, except for C57BL/6, with a 1:100 dilution) for an individual mouse (n = three to four mice/group), and the data shown are representative of three independent experiments.
β2GPI-reactive CD4+ T Cell Clones Derived from an Autoimmune Patient Recognize the Same Peptide as H-2b-bearing Mice
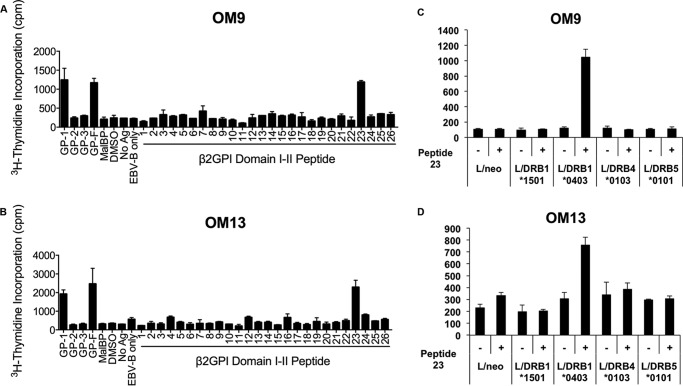
To address whether the T cell epitope response that we observed in β2GPI/LPS-immunized mice also occurs in human autoimmune disease, primary peripheral blood mononuclear cells from a patient (OM) with APS were evaluated for proliferation to the same recombinant fragments of β2GPI. The clinical characteristics of this patient have been described previously (10). T cells and antibodies from this patient were strongly reactive to GP-1 and GP-3 (11) but minimally reactive to GP-2 (Fig. 6, A and B). β2GPI-reactive CD4+ T cell clones from this patient were used for epitope mapping. Two of the clones (OM9 and OM13) specifically recognized peptide 23 in Domain II, similar to splenic T cells from C57BL/6 mice (H-2b) (Fig. 7, A and B). To identify the HLA-DR molecules that present peptide 23 to these T cell clones, L cell transfectants expressing single HLA-DR molecules were used as APCs (Fig. 7, C and D). Both OM9 and OM13 responded to peptide 23 presented selectively by DRB1*0403+ L cells. These findings indicate that peptide 23 is a dominant epitope in Domain II of β2GPI for β2GPI-reactive T cells from this APS patient. The fact that the same T cell epitope specificity occurs as an induced response in H-2b-bearing mice and spontaneously in an HLA-DRB1*0403+ autoimmune patient suggests that the induced β2GPI-specific T cell response in our model mimics that in autoimmune disease.
FIGURE 6.
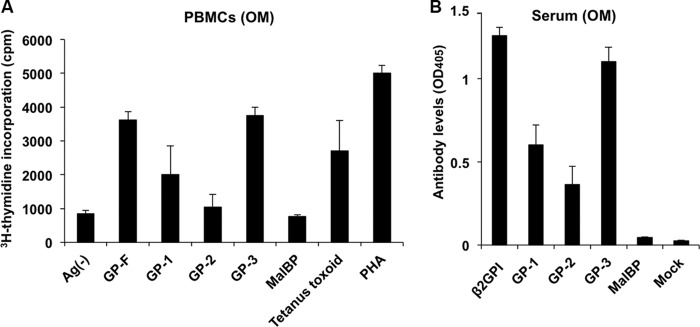
Primary T cells and antibodies from an autoimmune patient react with a recombinant fragment containing Domains I and II. A, an in vitro T cell assay for evaluating proliferative responses to GP-1 (Domains I and II), GP-2 (Domains III-IV), GP-3 (Domains IV-V), GP-F (full-length β2GPI), or MalBP (control fusion protein) (20 μg/ml) or to peptides (10 μg/ml) covering the entire sequence of Domains I and II of β2GPI was done using peripheral blood mononuclear cells (PBMCs) isolated from an APS patient (OM). Tetanus toxoid and phytohemagglutinin (PHA) were used for evaluating T cell viability. Values represent the mean [3H]thymidine incorporation (cpm) ± S.E. of triplicate samples. The data shown are representative of three independent experiments. Ag(−), no antigen. B, ELISAs were performed to detect antibodies to GP-1, GP-2, GP-3, GP-F, and MalBP (control fusion protein) in sera from donor OM (1:100 serum dilution). Values represent mean IgG binding (A405) ± S.D. of triplicate samples. The data shown are representative of three independent experiments.
FIGURE 7.
β2GPI-reactive CD4+ T cell clones derived from an autoimmune patient recognize the same peptide as H-2b-bearing mice. β2GPI-specific T cell clones OM9 (A) and OM13 (B) were evaluated for proliferative responses to GP-1 (Domains I and II), GP-2 (Domains III and IV), GP-3 (Domains IV and V), GP-F (full-length β2GPI), or MalBP (control fusion protein) (20 μg/ml) or to peptides (10 μg/ml) covering the entire sequence of Domains I and II of β2GPI using an in vitro T cell assay. MHC class II haplotype restriction of β2GPI-specific T cell clones OM9 (C) and OM13 (D) was evaluated using a series of L cell transfectants expressing a single human HLA class II molecule in the presence or absence of peptide 23. Cells were incubated for 72 h, and cell proliferation was measured using [3H]thymidine incorporation. Values represent mean [3H]thymidine incorporation (cpm) ± S.E. of triplicate samples. The data shown are representative of two independent experiments. DMSO, dimethyl sulfoxide; Ag, antigen; EBV-B; Epstein-Barr virus-transformed B cells.
DISCUSSION
We have previously proposed that development of a β2GPI-reactive T cell response is a critical early event in the initiation of SLE-like autoantibodies and subsequent disease in mice immunized with β2GPI and LPS (4, 5). Here, we tested the hypothesis that generation of a β2GPI-directed T cell response, independent of its epitope specificity, enables B cell epitope spread to SLE-related antibodies. We demonstrated that mice with different MHC class II haplotypes are capable of developing a strong T cell response to β2GPI, as well as antibodies to β2GPI, CL, Ro (SS-A), and, in most cases, dsDNA. Although T cells from strains with different MHC class II haplotypes displayed very different epitope specificities for β2GPI, the autoantibody profiles of MHC-differing mice looked similar. T cells from β2GPI/LPS-immunized H-2b-bearing mice recognized a peptide (peptide 23, NTGFYLNGADSAKCT) in Domain II, whereas T cells from H-2d-bearing mice responded to a peptide (peptide 7, FSTVVPLKTFYEPGE) in Domain I. T cells from mice of a third MHC class II haplotype (C3H/HeN (H-2k)) failed to recognize either Domain I or II. Overall, our data demonstrate that SLE-related autoantibodies emerge in mice with a strong β2GPI-reactive T cell response, irrespective of MHC class II haplotype and corresponding T cell epitope specificity.
Our findings imply that, regardless of epitope specificity, β2GPI-reactive T cells are able to provide the T cell help needed for B cell epitope spread to multiple SLE-related autoantibodies. In mice, the autoantibody response to β2GPI in the presence of a potent innate stimulus (LPS) occurs within weeks after the first immunization with β2GPI (4). However, the production of anti-CL, anti-dsDNA, and other SLE-related autoantibodies requires multiple immunizations with β2GPI and LPS (4). In the present study, the number of immunizations was determined by the titer of anti-β2GPI antibodies. Once a relatively high titer (>1:5000) of anti-β2GPI antibodies was observed, mice were used for T cell studies. C57BL/6 mice received four immunizations, but BALB/c and C3H/HeN mice received three and two immunizations, respectively. Interestingly, C3H/HeN mice did not survive a third immunization (data not shown), and this premature death of C3H/HeN and some 129S1 mice (see “Experimental Procedures”) was associated with an accelerated antibody response to β2GPI and LPS. The reason for the premature death in these mice is currently under investigation, but premature death was observed only when β2GPI and LPS were administered as immunogen, and not when either was administered alone.
In patients with SLE, autoantibodies to β2GPI also occur relatively early, whereas other SLE-related autoantibodies (e.g. anti-dsDNA, anti-Sm, and anti-nRNP antibodies) appear later (1). Together with our murine model of induced SLE-related autoantibodies (4), these findings suggest that there is epitope spread in the autoantibody response from β2GPI to other SLE-related autoantigens (e.g. dsDNA). Epitope spread between these molecules (“intermolecular epitope spread”) presumably requires the physical association of β2GPI with these autoantigens. The apoptotic or dying cell represents a physiological scaffold upon which such association can occur.
Apoptotic cells express multiple SLE-related autoantigens (7, 8) and thus provide an ideal “scaffold” for epitope spread of the autoantibody response from one autoantigen to another. β2GPI binds to apoptotic cells through interaction with phosphatidylserine (6, 15, 16) or Ro 60 (17) exposed on the surface of these cells. Thus, a B cell specific for other SLE-related autoantigens (e.g. dsDNA) would recognize its cognate antigen on the apoptotic cell surface and therefore ingest this apoptotic cell. The ingesting B cell would then present peptide fragments from both cell-bound β2GPI and other apoptotic cell-derived proteins in the context of MHC class II. The key point is that a B cell presenting β2GPI peptides on its surface could receive help from β2GPI-reactive T cells independent of its autoantigen specificity (e.g. anti-dsDNA) (4). In our model, we use human β2GPI as our immunogen. In addition to being the source of the T cell epitopes that we have identified, the injected β2GPI may also bind to apoptotic cells in the immunized mice. Notably, the human β2GPI-derived epitopes recognized by T cells in human β2GPI/LPS-immunized mice are closely related to comparable sequences within murine β2GPI, as illustrated by a high degree of homology between the sequences (80 and 66.7% identities for peptides 7 and 23, respectively) (Fig. 8). We do not yet know whether murine β2GPI plays a role in the epitope spread observed in our induced model. However, it is clear from our previous studies (4) that epitope spread to antibodies recognizing murine β2GPI does develop in human β2GPI/LPS-immunized mice.
FIGURE 8.
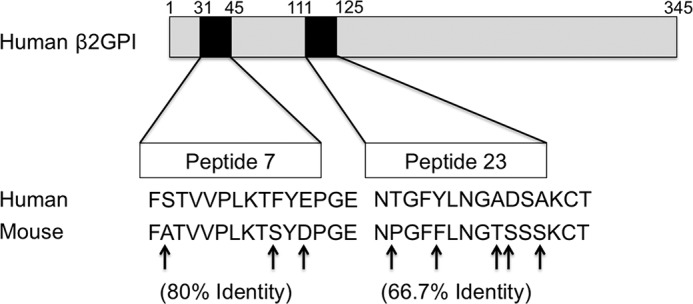
Human β2GPI-derived peptide sequences recognized by β2GPI-reactive T cells share sequence identity with murine β2GPI. Shown is a schematic representation of the full amino acid sequence of human β2GPI (top) and the peptides (black boxes) recognized by T cells from human β2GPI/LPS-immunized mice. The expanded regions show the amino acid sequences of epitopes in Domains I (peptide 7) and II (peptide 23) recognized by T cells from H-2d- and H-2b-bearing mice, respectively. Peptide 23 was also recognized by HLA-DRB1*0403-bearing human CD4+ T cell clones. Human β2GPI-derived sequences for peptides 7 and 23 are aligned with the comparable sequences from murine β2GPI to illustrate the percent identity between the sequences, with amino acid differences indicated by arrows.
We have shown here that β2GPI-reactive T cells with distinct epitope specificity and MHC class II restriction permit B cell epitope spread to SLE-related autoantibodies. Notably, all of the non-autoimmune strains examined here (expressing three different MHC class II haplotypes) developed a similar profile of SLE-related autoantibodies. The extent of B cell epitope spread to different domains of β2GPI varied among strains but did not appear to affect the degree of epitope spread to other autoantigens. Variation in the kinetics and intensity of the autoantibody response among strains may relate to non-MHC class II-related genes, and this area is currently under investigation.
Although SLE-related autoantibodies emerge in a remarkably similar pattern and sequence prior to the onset of clinical SLE disease (1), the mechanism responsible for epitope spread in these patients is not known. Of note, the role of β2GPI-reactive T cells has not yet been adequately evaluated in human and murine SLE. In fact, the limited number of studies of β2GPI-reactive T cells in both mice (18–20) and autoimmune patients (10, 11, 21–23) focused primarily on the association of these cells with thrombosis or atherosclerosis (clinical findings in patients with APS). The majority of human β2GPI-reactive CD4+ T cell clones evaluated in these studies recognized Domain V, and epitope mapping focused solely on this domain (10, 11, 22). Moreover, as most of these human studies showed the presence of β2GPI-reactive CD4+ T cells only in patients with anti-phospholipid antibodies, their association with other SLE-related autoantibodies was not evaluated (10, 11, 22, 23). Notably, a recent study found β2GPI-reactive peripheral blood mononuclear cell responses in 32% of SLE patients compared with 25% of primary APS patients and 0% of control subjects (21).
In this study, we focused on human CD4+ T cell clones recognizing epitopes in Domains I and II to address whether the T cell epitope response that we observed in β2GPI/LPS-immunized mice also occurs in human autoimmune disease. We selected human CD4+ T cell clones recognizing a recombinant fragment containing Domains I and II to compare their epitope specificity with the T cell hybridoma and primary cells from our immunized mice, which showed predominant recognition of Domains I and II. Two human CD4+ T cell clones (OM9 and OM13) from a patient with APS recognized Domains I–II and peptide 23 within that recombinant fragment of β2GPI. This epitope specificity is identical to that recognized by both the murine T cell hybridoma C3hB-1.5 and primary T cells from β2GPI/LPS-immunized mice bearing an H-2b haplotype (C57BL/6, 129S1, and C.B10). Notably, the human T cell clones responded to peptide 23 presented in the context of a single HLA-DR allele, DRB1*0403. Identification of HLA-DRB1*0403 as the allele capable of presenting peptide 23 to β2GPI-reactive T cells is interesting, as this MHC allele has been shown to be strongly associated with the presence of anti-phospholipid antibodies (both anti-CL and anti-β2GPI) in a European cohort of SLE patients (24). Together with our findings in mice, these data suggest that the T cell response to β2GPI is restricted by MHC class II haplotype in both humans and mice. Moreover, the finding of a shared epitope specificity to β2GPI (peptide 23 in Domain II) in an HLA-DRB1*0403+ patient and H-2b haplotype-bearing mice indicates the relevance of our induced model for human autoimmune T cell specificities. Further studies are required to investigate whether the presence of β2GPI-reactive T cells is associated with epitope spread to multiple SLE autoantibodies in patients with SLE.
In summary, we have shown that a strong T cell response to β2GPI is associated with B cell epitope spread to SLE-related autoantibodies in β2GPI/LPS-immunized mice with different MHC class II haplotypes. Although the specific β2GPI T cell epitopes recognized by the different mouse strains were directly linked to MHC class II haplotype, epitope spread to SLE-related autoantibodies occurred in all of the strains developing a strong β2GPI-reactive T cell response regardless of β2GPI-reactive T cell epitope specificity. The dominant T cell epitope recognized by β2GPI-reactive T cells from mice with an H-2b haplotype (e.g. C57BL/6 mice) was also recognized by T cells from a patient with APS and was restricted by a MHC class II allele that has been genetically associated with the presence of autoantibodies to β2GPI and CL in SLE. These findings suggest that generation of a strong β2GPI-reactive T cell response regardless of epitope specificity is a common and decisive step in the initiation of SLE-related autoantibodies across multiple MHC class II backgrounds. We hypothesize that a T cell response to an apoptotic cell-binding protein like β2GPI allows B cell epitope spread of the autoimmune response to other SLE-related autoantigens expressed on the apoptotic cell surface.
Acknowledgments
We are grateful to Drs. Salman Qureshi and Samuel David for providing some of the mice used in this study, Annie Beauchamp for expertise and assistance with some of the mouse strains, and Dr. Sylvie Lesage for reading and providing invaluable input on the manuscript.
This was supported in part by Canadian Institutes of Health Research (CIHR)/Institute of Musculoskeletal Health and Arthritis (IMHA)/Arthritis Society Grant MOP-42391, CIHR Grants MOP-67101 and MOP-97916, and CIHR/IMHA (Fall 2008 Priority Announcement) Grant MUS-67101 (to J. R.); and a research grant on intractable diseases from the Japanese Ministry of Health, Labor and Welfare (to M. K.).
- SLE
- systemic lupus erythematosus
- β2GPI
- β2-glycoprotein I
- anti-CL
- anti-cardiolipin
- anti-nRPP
- anti-nuclear ribonucleoprotein
- MalBP
- maltose-binding protein
- APCs
- antigen-presenting cells
- APS
- anti-phospholipid syndrome.
REFERENCES
- 1. Arbuckle M. R., McClain M. T., Rubertone M. V., Scofield R. H., Dennis G. J., James J. A., Harley J. B. (2003) Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N. Engl. J. Med. 349, 1526–1533 [DOI] [PubMed] [Google Scholar]
- 2. Tsokos G. C. (2011) Systemic lupus erythematosus. N. Engl. J. Med. 365, 2110–2121 [DOI] [PubMed] [Google Scholar]
- 3. McClain M. T., Arbuckle M. R., Heinlen L. D., Dennis G. J., Roebuck J., Rubertone M. V., Harley J. B., James J. A. (2004) The prevalence, onset, and clinical significance of antiphospholipid antibodies prior to diagnosis of systemic lupus erythematosus. Arthritis Rheum. 50, 1226–1232 [DOI] [PubMed] [Google Scholar]
- 4. Levine J. S., Subang R., Nasr S. H., Fournier S., Lajoie G., Wither J., Rauch J. (2006) Immunization with an apoptotic cell-binding protein recapitulates the nephritis and sequential autoantibody emergence of systemic lupus erythematosus. J. Immunol. 177, 6504–6516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tolomeo T., Rico De Souza A., Roter E., Dieudé M., Amireault P., Subang R., Levine J. S., Rauch J. (2009) T cells demonstrate a Th1-biased response to native β2-glycoprotein I in a murine model of anti-phospholipid antibody induction. Autoimmunity 42, 292–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Price B. E., Rauch J., Shia M. A., Walsh M. T., Lieberthal W., Gilligan H. M., O'Laughlin T., Koh J. S., Levine J. S. (1996) Anti-phospholipid autoantibodies bind to apoptotic, but not viable, thymocytes in a beta2-glycoprotein I-dependent manner. J. Immunol. 157, 2201–2208 [PubMed] [Google Scholar]
- 7. Casciola-Rosen L. A., Anhalt G., Rosen A. (1994) Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J. Exp. Med. 179, 1317–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Qian Y., Wang H., Clarke S. H. (2004) Impaired clearance of apoptotic cells induces the activation of autoreactive anti-Sm marginal zone and B-1 B cells. J. Immunol. 172, 625–635 [DOI] [PubMed] [Google Scholar]
- 9. Levine J., Subang R., Setty S., Cabrera J., Laplante P., Fritzler M., Rauch J. (2014) Phospholipid-binding proteins differ in their capacity to induce autoantibodies and murine systemic lupus erythematosus. Lupus 23, 752–768 [DOI] [PubMed] [Google Scholar]
- 10. Arai T., Yoshida K., Kaburaki J., Inoko H., Ikeda Y., Kawakami Y., Kuwana M. (2001) Autoreactive CD4+ T-cell clones to β2-glycoprotein I in patients with antiphospholipid syndrome: preferential recognition of the major phospholipid-binding site. Blood 98, 1889–1896 [DOI] [PubMed] [Google Scholar]
- 11. Hattori N., Kuwana M., Kaburaki J., Mimori T., Ikeda Y., Kawakami Y. (2000) T cells that are autoreactive to β2-glycoprotein I in patients with antiphospholipid syndrome and healthy individuals. Arthritis Rheum. 43, 65–75 [DOI] [PubMed] [Google Scholar]
- 12. McRae B. L., Kennedy M. K., Tan L. J., Dal Canto M. C., Picha K. S., Miller S. D. (1992) Induction of active and adoptive relapsing experimental autoimmune encephalomyelitis (EAE) using an encephalitogenic epitope of proteolipid protein. J. Neuroimmunol. 38, 229–240 [DOI] [PubMed] [Google Scholar]
- 13. Behar S. M., Podrebarac T. A., Roy C. J., Wang C. R., Brenner M. B. (1999) Diverse TCRs recognize murine CD1. J. Immunol. 162, 161–167 [PubMed] [Google Scholar]
- 14. Kaburaki J., Kuwana M., Yamamoto M., Kawai S., Matsuura E., Ikeda Y. (1996) Phospholipid-dependent anti-beta 2-glycoprotein I (beta 2-GPI) antibodies and antiphospholipid syndrome. Intern. Med. 35, 105–110 [DOI] [PubMed] [Google Scholar]
- 15. Balasubramanian K., Chandra J., Schroit A. J. (1997) Immune clearance of phosphatidylserine-expressing cells by phagocytes. The role of β2-glycoprotein I in macrophage recognition. J. Biol. Chem. 272, 31113–31117 [DOI] [PubMed] [Google Scholar]
- 16. Hunt J. E., Simpson R. J., Krilis S. A. (1993) Identification of a region of β2-glycoprotein I critical for lipid binding and anti-cardiolipin antibody cofactor activity. Proc. Natl. Acad. Sci. U.S.A. 90, 2141–2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Reed J. H., Giannakopoulos B., Jackson M. W., Krilis S. A., Gordon T. P. (2009) Ro 60 functions as a receptor for β2-glycoprotein I on apoptotic cells. Arthritis Rheum. 60, 860–869 [DOI] [PubMed] [Google Scholar]
- 18. Blank M., George J., Barak V., Tincani A., Koike T., Shoenfeld Y. (1998) Oral tolerance to low dose β2-glycoprotein I: immunomodulation of experimental antiphospholipid syndrome. J. Immunol. 161, 5303–5312 [PubMed] [Google Scholar]
- 19. Buttari B., Profumo E., Capozzi A., Facchiano F., Saso L., Sorice M., Riganò R. (2011) Advanced glycation end products of human β2 glycoprotein I modulate the maturation and function of DCs. Blood 117, 6152–6161 [DOI] [PubMed] [Google Scholar]
- 20. George J., Harats D., Gilburd B., Afek A., Shaish A., Kopolovic J., Shoenfeld Y. (2000) Adoptive transfer of β2-glycoprotein I-reactive lymphocytes enhances early atherosclerosis in LDL receptor-deficient mice. Circulation 102, 1822–1827 [DOI] [PubMed] [Google Scholar]
- 21. Conti F., Spinelli F. R., Alessandri C., Pacelli M., Ceccarelli F., Marocchi E., Montali A., Capozzi A., Buttari B., Profumo E., Sorice M., Arca M., Valesini G., Riganò R. (2014) Subclinical atherosclerosis in systemic lupus erythematosus and antiphospholipid syndrome: focus on β2GPI-specific T cell response. Arterioscler. Thromb. Vasc. Biol. 34, 661–668 [DOI] [PubMed] [Google Scholar]
- 22. Ito H., Matsushita S., Tokano Y., Nishimura H., Tanaka Y., Fujisao S., Mitsuya H., Hashimoto H., Nishimura Y. (2000) Analysis of T cell responses to the β2-glycoprotein I-derived peptide library in patients with anti-β2-glycoprotein I antibody-associated autoimmunity. Hum. Immunol. 61, 366–377 [DOI] [PubMed] [Google Scholar]
- 23. Visvanathan S., McNeil H. P. (1999) Cellular immunity to β2-glycoprotein-1 in patients with the antiphospholipid syndrome. J. Immunol. 162, 6919–6925 [PubMed] [Google Scholar]
- 24. Galeazzi M., Sebastiani G. D., Tincani A., Piette J. C., Allegri F., Morozzi G., Bellisai F., Scorza R., Ferrara G. B., Carcassi C., Font J., Passiu G., Smolen J., Papasteriades C., Houssiau F., Nebro A. F., Ramon Garrido E. D., Jedryka-Goral A., Marcolongo R. (2000) HLA class II alleles associations of antiCL and anti-β2GPI antibodies in a large series of European patients with systemic lupus erythematosus. Lupus 9, 47–55 [DOI] [PubMed] [Google Scholar]