Background: Recent human and animal studies suggest that gut microbes can influence atherosclerosis via generation of trimethylamine N-oxide (TMAO).
Results: Cecal microbial transplantation from atherosclerosis-prone versus -resistant inbred strains of mice transmitted enhanced choline diet-dependent atherosclerosis and TMAO levels.
Conclusion: Atherosclerosis susceptibility can be transmitted with gut microbial transplantation.
Significance: Gut microbes participate in atherosclerosis susceptibility and are thus a potential therapeutic target.
Keywords: Atherosclerosis, Dyslipidemia, Lipid, Nutrition, Phospholipid, Koch's Postulate, Choline, Gut Microbiota, Trimethylamine N-Oxide (TMAO)
Abstract
Recent studies indicate both clinical and mechanistic links between atherosclerotic heart disease and intestinal microbial metabolism of certain dietary nutrients producing trimethylamine N-oxide (TMAO). Here we test the hypothesis that gut microbial transplantation can transmit choline diet-induced TMAO production and atherosclerosis susceptibility. First, a strong association was noted between atherosclerotic plaque and plasma TMAO levels in a mouse diversity panel (n = 22 strains, r = 0.38; p = 0.0001). An atherosclerosis-prone and high TMAO-producing strain, C57BL/6J, and an atherosclerosis-resistant and low TMAO-producing strain, NZW/LacJ, were selected as donors for cecal microbial transplantation into apolipoprotein e null mice in which resident intestinal microbes were first suppressed with antibiotics. Trimethylamine (TMA) and TMAO levels were initially higher in recipients on choline diet that received cecal microbes from C57BL/6J inbred mice; however, durability of choline diet-dependent differences in TMA/TMAO levels was not maintained to the end of the study. Mice receiving C57BL/6J cecal microbes demonstrated choline diet-dependent enhancement in atherosclerotic plaque burden as compared with recipients of NZW/LacJ microbes. Microbial DNA analyses in feces and cecum revealed transplantation of donor microbial community features into recipients with differences in taxa proportions between donor strains that were transmissible to recipients and that tended to show coincident proportions with TMAO levels. Proportions of specific taxa were also identified that correlated with plasma TMAO levels in donors and recipients and with atherosclerotic lesion area in recipients. Atherosclerosis susceptibility may be transmitted via transplantation of gut microbiota. Gut microbes may thus represent a novel therapeutic target for modulating atherosclerosis susceptibility.
Introduction
It has long been known that trillions of bacteria colonize our intestines, but only recently has the intestinal microbial community been appreciated as a biologically active participant in human health and disease (1–4). Our laboratory has elucidated a link between intestinal microbiota, dietary trimethylamine-containing nutrients (e.g. choline, phosphatidylcholine, and carnitine), and cardiovascular disease (5–7). Using an unbiased metabolomic approach, we initially discovered that phosphatidylcholine, the major dietary source of choline, and the gut microbe-dependent metabolite, trimethylamine N-oxide (TMAO),4 are both mechanistically linked to atherosclerosis development (5, 6, 8) and that plasma levels in subjects are associated with prevalent and incident cardiovascular disease risks (5, 7, 9, 10). Oral dietary supplementation with the trimethylamine (TMA)-containing precursors, choline or carnitine, and dietary TMAO directly, were each shown to enhance aortic root atherosclerotic plaque in the atherosclerosis-prone C57BL/6J apolipoprotein e null (Apoe−/−) mouse model. An obligatory role for gut microbes in TMAO formation in both mice (5, 6) and humans (7) was shown, and the involvement of gut microbes in atherosclerosis susceptibility was suggested by demonstrating that both dietary choline-induced and carnitine-induced enhancement in atherosclerosis in mice is prevented by suppression of gut microbes (and TMAO formation) with poorly absorbed broad spectrum oral antibiotics (5, 6).
Following Koch's postulates (11–13), if the disease-causing entity is a microbe, differences in disease susceptibility should follow transplantation of the microbe into a new host. Here we investigate whether atherosclerosis susceptibility can be transmitted by cecal microbial transplantation. We hypothesized that cecal contents might serve as a relatively stable and transplantable microbial community to permit simultaneous testing of two distinct hypotheses: (i) that choline diet-dependent capacity for TMA and TMAO generation is a transmissible trait; and (ii) that atherosclerosis susceptibility may be transmitted to a recipient host by microbial transplantation. Cecal contents from distinct inbred donor strains with differing atherosclerosis potential and TMAO production capacity were identified and then introduced via gastric gavage into recipient mice in which endogenous gut microbes were initially suppressed through use of an oral poorly absorbed antibiotic mixture. We now show that recipients demonstrated altered TMA and TMAO production capacity and atherosclerosis disease susceptibility, indicating that atherosclerosis susceptibility is a transmissible trait.
EXPERIMENTAL PROCEDURES
General Procedures and Reagents
All procedures were approved by Institutional Animal Care and Use Committees within both the Cleveland Clinic and the University of California in Los Angeles. Glucose, cholesterol, and triglyceride levels were quantified on a Roche Diagnostics Applied Science Cobas 4000 analyzer series instrument as described (6). Internal standards for mass spectrometry studies (d9-TMA and d9-TMAO) were obtained from Cambridge Isotopes Laboratories, Inc., Andover, MA. HDL cholesterol was determined with colorimetric assay of the HDL cholesterol-containing fraction isolated following buoyant density ultracentrifugation as described (6). Flavin-dependent monooxygenase activity was determined from an 8-h reaction of liver homogenate (800 mg/liter of protein final, generated from livers homogenized with a TissueLyser II; Qiagen, Valencia, CA) with 100 μm d9-TMA and 100 μm reduced NADPH in 10 mm HEPES buffer, pH 7.4, as described previously (8). Stable isotope dilution liquid chromatography with on-line tandem mass spectrometry (LC/MS/MS) was used to quantify TMA and TMAO levels in plasma, as well as total choline content in food following base hydrolysis, as described previously (5, 14) using an AB SCIEX API 5000 triple quadrupole mass spectrometer.
Mouse Diversity Panel Studies
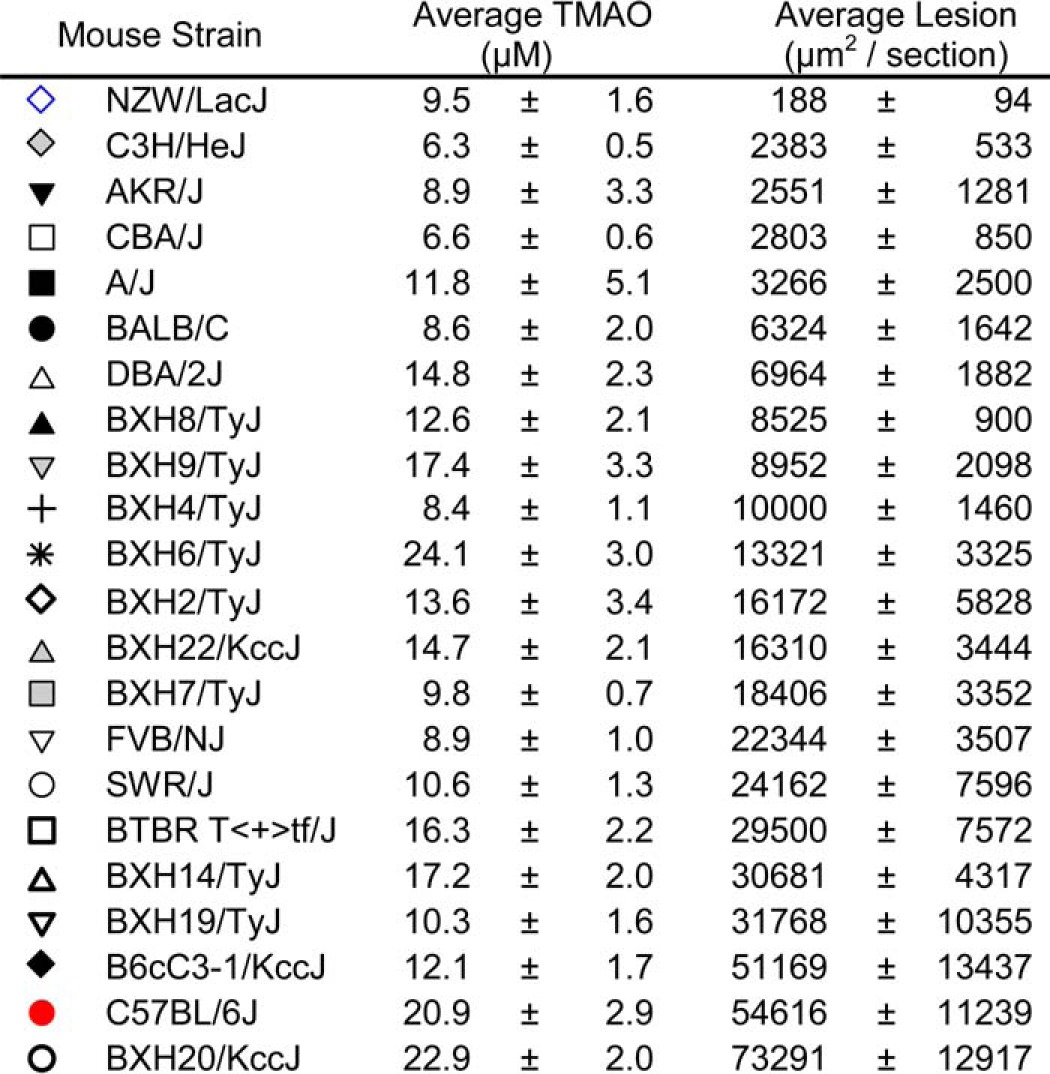
TMAO was measured in plasma recovered from mice from a mouse diversity panel. Briefly, 22 distinct inbred strains (see Table 1) were crossed with C57BL/6J mice homozygous for the human apolipoprotein B transgene. F1 progeny at 9 weeks of age were placed on a Paigen diet (Teklad diet TD.88051) until time of sacrifice at 25 weeks of age when aortic root atherosclerotic plaque was quantified and plasma was recovered, as described previously (15).
TABLE 1.
Relationship between plasma TMAO and aortic lesion area in mouse diversity panel
Plasma TMAO and aortic root atherosclerosis plaque extent quantified in female F1 progeny of the mouse diversity panel as described under “Experimental Procedures.” Data shown represent mean results and S.E. within the female F1 heterozygotes examined (cross between the indicated inbred background strain and homozygote C57BL/6-tg(Apob) mice. Strains appear in rank order of lesion area from least to greatest. Symbols shown correspond to the mouse strains plotted in Fig. 1.
Mouse Cecal Transplant Studies
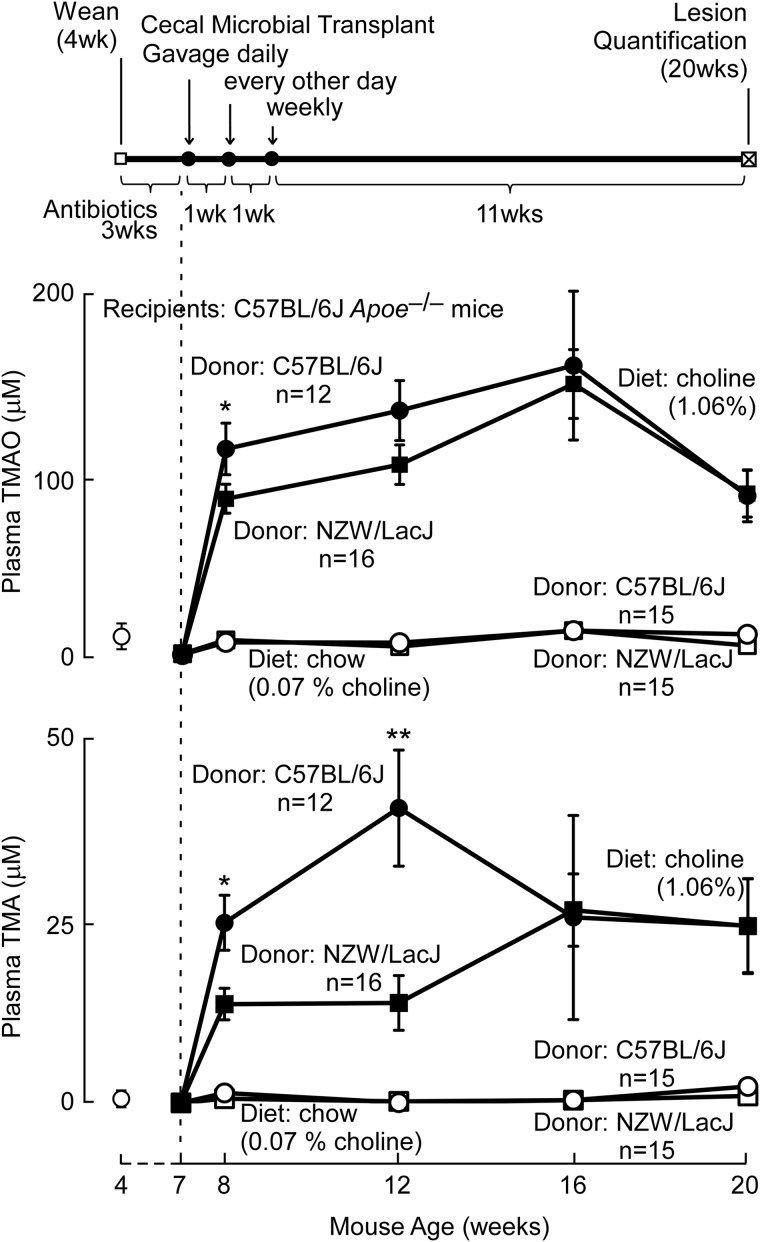
Female C57BL/6J Apoe−/− mice at the time of weaning (4 weeks of age) were placed on a mixture of broad spectrum, poorly absorbed antibiotics (vancomycin, neomycin sulfate, metronidazole, and ampicillin, as described previously (5)) for 3 weeks to suppress intestinal microbes. Mice were then taken off the antibiotics and serially orally gavaged with donor cecal contents from strains identified as atherosclerosis-prone (C57BL/6J) versus atherosclerosis-resistant (NZW/LacJ), as outlined below and in Fig. 2. Mice were placed on either a chemically defined diet comparable in composition with normal “chow” (Teklad diet 2918) or the same diet supplemented with additional choline (1.0%, w/w; Teklad diet TD.09041). All drinking water (+/− antibiotics) and all diets used were sterilized. Control studies examined the choline content in diets following sterilization procedures and showed the total choline content of the chow and choline diets to be 0.07 ± 0.01 and 1.06 ± 0.01%, respectively. Blood was collected via saphenous vein to measure plasma TMAO throughout the experiment in the four Apoe−/− female recipient experimental arms: 1) those receiving donor C57BL/6J cecal microbes and placed on the choline diet; 2) those receiving donor C57BL/6J microbes and placed on the chow diet; 3) those receiving donor NZW/LacJ cecal microbes and placed on the choline diet; and 4) those receiving donor cecal NZW/LacJ microbes and placed on the chow diet.
FIGURE 2.
Cecal transplant experiment design, and plasma TMA and TMAO levels following transplantation of donor cecal microbiota. Top, cecal microbial transplantation study design. The timeline shows that recipient mice upon weaning were administered a mixture of broad spectrum antibiotics for 3 weeks. At 7 weeks of age, administration of antibiotics was terminated, and cecal microbial transplantation began with a gavage of cecal slurry freshly harvested from euthanized donor mice daily the first week, every other day the second week, and then weekly until the 20 weeks of age study end point. Vertical arrows indicate where timing of cecal microbial gavages changed. Middle and bottom, plasma TMAO (middle) and TMA (bottom) levels in the four recipient groups were monitored to track transmissibility of choline diet-dependent donor TMA/TMAO levels. Open symbols at 4 weeks of age represent plasma TMA and TMAO levels in recipients at time of weaning, prior to antibiotic administration. The dotted line at 7 weeks of age represents the end of administration of antibiotics and the beginning of both serial cecal microbial gavages, as well as the indicated diets. *, p < 0.05, **, p < 0.01 for comparison between similar time point recipients on the high choline diet of cecal microbe suspensions from C57BL/6J versus NZW/LacJ donors. Data points represent mean ± S.E.
Cecal microbial contents from donor mice (female, 14 weeks old on average, C57BL/6J or NZW/LacJ) were harvested from euthanized mice by rapidly removing the cecum and then manually extruding the contents into a vial containing sterile argon-sparged PBS (1.5 ml/0.2 ml of cecal volume). Following successive passages through an 18- and then a 20-gauge needle, the suspended cecal contents from one donor mouse were introduced via gastric gavage using a 22-gauge feeding needle into five recipient mice (0.2 ml each). Stable isotope dilution LC/MS/MS analysis of TMAO and TMA was measured in a sampling of cecal slurries across the duration of the study. The TMAO content in cecal donor slurries was below the limit of detection in all samples (∼30 fmol on column, or ≪0.01 μm within the slurry). The median TMA content in donor cecal slurries was 3 nmol (amount within inoculum) for C57BL/6J, and 2.5 nmol for NZW/LacJ mice. Thus, the amounts of TMA and TMAO present in the inocula were negligible (<2% total body pool of TMA + TMAO). Total time between euthanizing of a donor and installation of microbial contents into recipients via gastric gavage was kept as short as possible (15 min). Recipient mice received cecal microbial suspensions via gastric gavage initially daily for 5 successive days starting on the day antibiotics were removed from drinking water and separation into the dietary arms. Cecal transplantation (with freshly harvested cecal contents from new donor mice for each gavage) were then continued on a schedule of every other day for another week, and then weekly thereafter until time of animal sacrifice for atherosclerosis plaque quantification at 20 weeks of age (see Fig. 2). Feces were collected from individual recipient mice at least 48 h after oral gavage (collection day was the day before the new gavage for the subsequent week). Separate control studies showed that waiting 48 h after gavage allowed microbial DNA isolated from feces collected under this schedule to have minimal detectable contamination from DNA in the gavage and to thus arise predominantly from the endogenous microbial community.
Donor mice were maintained on the chemically defined diet with lower choline content (chow) and housed in separate areas. Within the colony of each donor strain (C57BL/6J versus NZW/LacJ), fecal pellets and mice were constantly transferred among cages to help maintain a unified microbial composition among all mice within a given donor inbred strain. Similarly, mice within the recipient C57BL/6J Apoe−/− colony prior to enrollment in studies were maintained on chow diet, continuously cohoused using harem breeding, and with similar continual transfer of fecal pellets among cages within the colony. At time of weaning, cohoused siblings were split into separate cages, housed in a different room, and still maintained on chow diet during the 3-week antibiotic treatment period. On the morning of the first cecal microbial transplant, antibiotic treatment was stopped, and mice were divided into the indicated recipient groups based on diet and donor. All mice were separately housed, and mice within a given group were maintained on separate racks. Husbandry personnel changed protective outerwear and used clean technique between handling the distinct groups of mice. Husbandry chores in the different groups of mice were performed on separate days to further decrease chances of microbial cross dissemination.
Mouse Atherosclerosis Studies
Recipient mice were euthanized and evaluated for aortic root atherosclerosis area quantification at 20 weeks (± 1 day) of age. Hearts were fixed and stored in formalin or frozen in OCT. After sectioning, slides were stained with Oil-Red-O and hematoxylin or with immunohistochemical staining as outlined below. Aortic root lesion area was quantified by computer-assisted planimetry taking the mean value from six sections, as described previously (5, 6). Immunohistochemical staining for Iba1 (ionized calcium-binding adapter molecule 1), a specific marker for macrophages (16, 17), was carried out in parallel on sections adjacent to those used for Oil-Red-O analysis. Before immunohistochemical staining, high temperature antigen retrieval in citrate retrieval buffer, pH 6.0, utilizing the Decloaking Chamber (Biocare Medical, Concord, CA) and the 15-min, 95 °C program, was performed. All materials used for Iba1 staining were from Vector Laboratories (Burlingame, CA) unless otherwise indicated. Sections were blocked in 3% normal goat serum in PBS, as well as avidin D and biotin blocking solution. Slides were incubated at room temperature for 30 min with rabbit anti-Iba1 primary antibody (Wako Chemicals USA, Inc., Richmond, VA) diluted 1:500 with PBS supplemented with 1.5% (v/v) normal goat serum. Biotinylated anti-rabbit secondary antibody (1:200 dilution) was applied for 30 min at room temperature, followed by Vector ABC alkaline phosphatase reagent and then Vector Red substrate for fluorescence visualization. Sections were mounted in VECTASHIELD mounting medium for fluorescence imaging with DAPI staining for nuclei. Images were collected using a Leica SCN 400 slide scanner (Leica Microsystems Inc., Buffalo Grove, IL), and quantification of Iba1-stained area was carried out using ImagePro9 software (Microsoft, Redmond, WA). Results represent mean ± S.E. of computer-assisted planimetry from the indicated numbers of animals (taken at the level of the aortic ring annulus). Images shown are representative pictures from each group (red color channels in gray scale).
Microbiota Studies and Statistical Analyses
Microbial DNA was isolated with PowerSoil DNA isolation kit (MO BIO Laboratories, Inc., Carlsbad, CA) from samples stored at −80 °C. Amplification and sequencing of V4 hypervariable region of the 16 S rRNA gene were performed using the validated, region-specific bacterial primers 515F and 806R according to a previously described method (18, 19). High throughput sequencing analysis of bacterial rRNA genes was performed using the Illumina MiSeq platform (Illumina, Inc., San Diego, CA) at the GenoSeq Core facility, University of California, Los Angeles. De-multiplexing 16 S rRNA gene sequences, quality control, and operational taxonomic unit binning were performed using open source pipeline Quantitative Insights Into Microbial Ecology (QIIME) version 1.7.0 (20) and established guidelines (21). Quality-filtered reads were de-multiplexed, yielding 881,836 sequences total with an average length of 153 bases per read, and an average coverage of 15,204 sequences per sample. Sequences were binned into operational taxonomic units based on 97% identity using UCLUST (22) against the Greengenes reference database (23). The taxonomic composition was assigned to the representative sequence of each operational taxonomic unit using the Greengenes database. The sequences of each sample were rarefied to a depth of 10,500 sequences per sample to reduce the effect of sequencing depth, and then used for downstream analysis. The beta diversity of samples was measured using the weighted UniFrac metric (24), and the dimensionality reduction technique of principal coordinates analysis was used to visualize the community differences between C57BL/6J and NZW/LacJ strains. Additional analyses were carried out on cecal samples to examine success of transplant in recipients at the study end point. The beta diversity distance matrix was calculated for the cecal samples of donors and recipients and visualized using unweighted pair group method with arithmetic mean, which forces the samples into an ultrametric tree. The resulting tree file was visualized by the TopiaryExplorer program (25).
In additional analyses, the LDA effect size (LEfSe) algorithm (26, 27) was used to identify taxa that account for the greatest differences between donor strain microbiota in cecal and fecal sequencing data. The algorithm, which was designed to aid in biomarker discovery from large datasets, performs Kruskal-Wallis rank-sum test between main classes, Wilcoxon on subclasses, followed by linear discriminate analysis (LDA) to assign a signed score. C57BL/6J taxa are indicated with positive LDA score (red), and taxa enriched in NZW/LacJ donors have a negative LDA score (green). Only taxa meeting an LDA significance threshold >2 are shown. Ten characteristic taxa were identified using LEfSe analyses that were characteristic of donor origin whether analyzed in samples from cecum or feces. Then, the proportions of these taxa were examined in feces of recipient mouse groups longitudinally over time as a secondary examination of success of gut microbial transplantation. These 10 taxa also served as the focus of examination for evaluating the relationships between “donor characteristic” taxa proportions in recipient mice and either plasma TMAO levels or aortic root atherosclerosis lesion area. p values reported are false discovery rate adjusted. Adjusted post tests were used to compare two groups at any time. One-way analysis of variance was carried out to determine differences among the four recipient groups. Spearman's rank-order correlations were calculated to determine associations between taxa proportions and TMAO or atherosclerotic plaque levels. All values are expressed as mean ± S.E.
RESULTS
Mouse Diversity Panel Reveals Inbred Strains with Divergent Susceptibility to Atherosclerosis and Plasma TMAO Levels
To identify mice for use as potential donors for cecal microbial transplantation studies, we utilized a previously studied (8, 15) panel of inbred strains of mice (Table 1) crossed with C57BL/6J mice carrying the human apolipoprotein b transgene to accelerate atherosclerosis. Plasma TMAO levels were analyzed in Apob hemizygous female F1 progeny following 16 weeks of atherosclerotic diet and recovered at time of animal sacrifice for aorta harvest (n = 4–13 mice per inbred strain, with most strains n ≥ 8). Examination of the relationship between aortic root atherosclerotic plaque burden and plasma TMAO levels in mice within the majority of each inbred strain (not shown), and across the different inbred strains examined, showed a remarkably strong positive association (r = 0.38, p = 0.0001; Fig. 1A; Table 1). From this panel, we tentatively selected two inbred strains to serve as potential cecal microbial community donors: (i) C57BL/6J mice as an atherosclerosis-prone donor strain, with concurrent high TMAO and high lesion area; and (ii) NZW/LacJ mice as an atherosclerosis-resistant donor strain, with concurrent low TMAO and low lesion area (Fig. 1A; Table 1).
FIGURE 1.
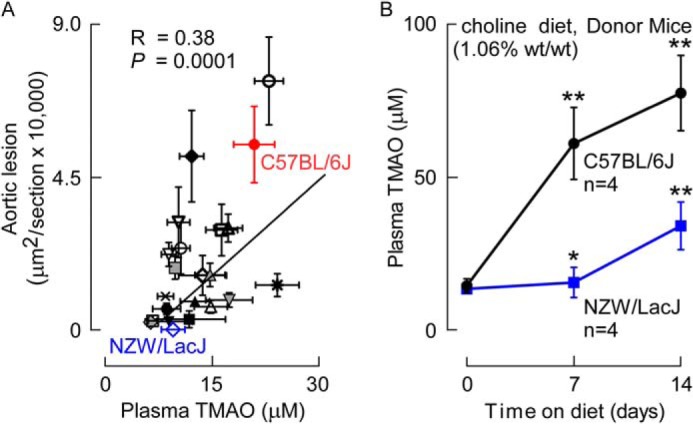
Aortic lesion area and plasma TMAO levels are positively associated across multiple inbred strains of mice. A, atherosclerotic plaque and plasma TMAO levels were determined in female F1 progeny from the cross between C57BL/6 mice homozygous for the human Apob transgene and 22 distinct inbred strains of mice (Table 1) as described under “Experimental Procedures.” Each data point represents the mean ± S.E. of lesion area and terminal plasma TMAO level within each strain, with Spearman correlation across all strains shown. Inbred strains with markedly divergent atherosclerosis susceptibility and TMAO levels selected to serve as cecal microbe donors included the atherosclerosis-prone (large lesion and high plasma TMAO) C57BL/6J (red) and atherosclerosis-resistant (small lesion and low plasma TMAO) NZW/LacJ (blue) strains. The key for symbols, along with mean ± S.E. values for atherosclerosis area and TMAO levels in each strain, are found in Table 1. B, confirmation that selected donor strains, C57BL/6J and NZW/LacJ, demonstrate markedly different TMAO plasma levels on a choline-supplemented diet. Selected donor strains were placed on the choline-supplemented (1.06%, w/w) diet, and plasma TMAO levels were quantified at the indicated times (n = 4/group at each time point). *, p < 0.05 and **, p < 0.01 for comparison with baseline (prior to choline diet) plasma TMAO level in each inbred strain.
The atherosclerosis studies in the above mouse diversity panel examined Apob hemizygous mice on the Paigen diet, an atherogenic diet with moderate and somewhat variable choline content (because it is not one of the defined components in the diet). Before proceeding with cecal microbial transplant studies, we therefore sought to confirm that the selected inbred strains recapitulated a wide divergence in plasma TMAO levels on a less atherogenic but chemically defined high choline content diet. Female mice (8 weeks of age) from each inbred donor strain were placed on a chemically defined diet supplemented with choline chloride (total choline content 1.06%), and plasma levels of TMAO were monitored. Significant (p < 0.05) increases in TMAO levels were observed in both strains of mice. Importantly, whereas only modest increases in plasma TMAO levels were observed in the NZW/LacJ mice, a far greater rise was observed within the C57BL/6J mice (Fig. 1B). Parallel LC/MS/MS analyses similarly showed significantly higher plasma TMA levels within the C57BL/6J versus NZW/LacJ donors (12.7 ± 1.9 μm versus 1.1 ± 0.4 μm, respectively, at 14 day time point; p = 0.006).
Cecal Microbial Transplant Studies Show Transmissibility of Donor Strain Diet-dependent Plasma TMA and TMAO Levels
The overall schedule of the cecal microbial transplantation studies is shown in Fig. 2 (top panel). Recipient Apoe−/− female mice at the time of weaning were placed on a mixture of broad spectrum, poorly absorbed antibiotics for 3 weeks to suppress intestinal microbes. The day antibiotics were stopped, mice were placed on chow- versus choline-supplemented diets, and recolonization efforts were begun with serial gastric gavage with donor cecal microbe suspensions from atherosclerosis-prone versus atherosclerosis-resistant inbred strains (C57BL/6J versus NZW/LacJ, respectively), as described under “Experimental Procedures.” Examination of plasma TMA and TMAO levels over time revealed that both recipient groups on the choline-supplemented diet showed higher TMA and TMAO levels. In addition, comparison between the recipient groups on the choline diet showed that initially following gut microbial transplantation (8 weeks of age), mice receiving cecal microbes from the C57BL/6J donors had higher TMA and TMAO levels than those receiving cecal microbes from the NZW/LacJ mice (e.g. for TMAO, 123.0 ± 15.3 μm versus 93.2 ± 8.6 μm; p < 0.05). At 12 weeks of age on the high choline diet, recipients gavaged with C57BL/6J cecal microbes still showed significantly higher TMA levels (p < 0.01) and a tendency toward increased plasma TMAO levels as compared with the NZW/LacJ group (for TMAO, 145.4 ± 17.8 μm versus 113.4 ± 11.7 μm; p = 0.1). However at 16 and 20 weeks of age, both TMAO and TMA levels in the two high choline diet groups of mice were overlapping (Fig. 2).
Atherosclerosis Susceptibility Is Transmitted via Gut Microbial Transplantation
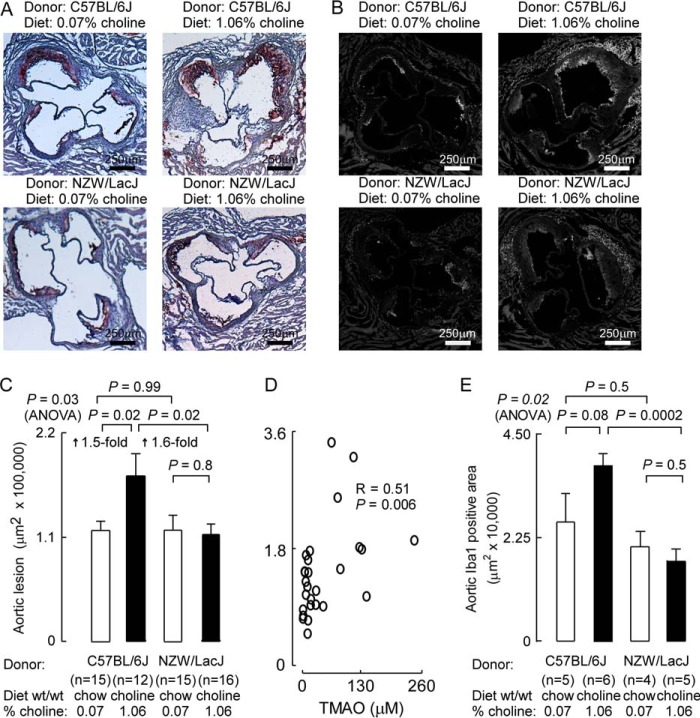
At the study end point (20 weeks of age), aortic root atherosclerotic plaque area was quantified (Fig. 3). Characteristic images from Oil-Red-O staining of each group of mice are illustrated in Fig. 3A. Remarkably, recipients of cecal microbial transplantation from the C57BL/6J donors, but not the NZW/LacJ donors, showed dietary choline-dependent enhancement (1.5-fold, p = 0.02) in atherosclerosis (Fig. 3C). No differences in atherosclerotic plaque area were noted between recipient groups on the low choline (chow) diet. Examination of the association between aortic lesion area and plasma TMAO within the mice receiving cecal microbes from atherosclerosis-prone donors, C57BL/6J, showed a positive correlation (r = 0.51, p = 0.006) (Fig. 3D).
FIGURE 3.
Demonstration of choline diet-dependent transmissibility of atherosclerosis. At 20 weeks of age, the indicated numbers of recipient groups of Apoe−/− female mice were sacrificed, and aortic atherosclerotic plaque was quantified. Groups are defined by the indicated diet arm (chow versus choline) and donor source for cecal microbial suspensions given by gastric gavage (atherosclerosis-prone C57BL/6J versus atherosclerosis-resistant NZW/LacJ). A, representative Oil-Red-O staining images from each group. B, representative images following immunohistochemical staining with antibodies to the macrophage marker Iba1 from each group. C, aortic root lesion area quantified using Oil-Red-O staining, as described under “Experimental Procedures,” from the indicated numbers of mice. ANOVA, analysis of variance. D, relationship between aortic root lesion area (on y axis, in units of μm2 × 100,000) and plasma TMAO levels among recipients of cecal microbes from atherosclerosis-prone C57BL/6J donors, and Spearman rank correlation (r = 0.51, p = 0.006). E, aortic root lesion area quantified using Iba1 staining, as described under “Experimental Procedures,” from the indicated numbers of mice. Data points represent mean ± S.E.
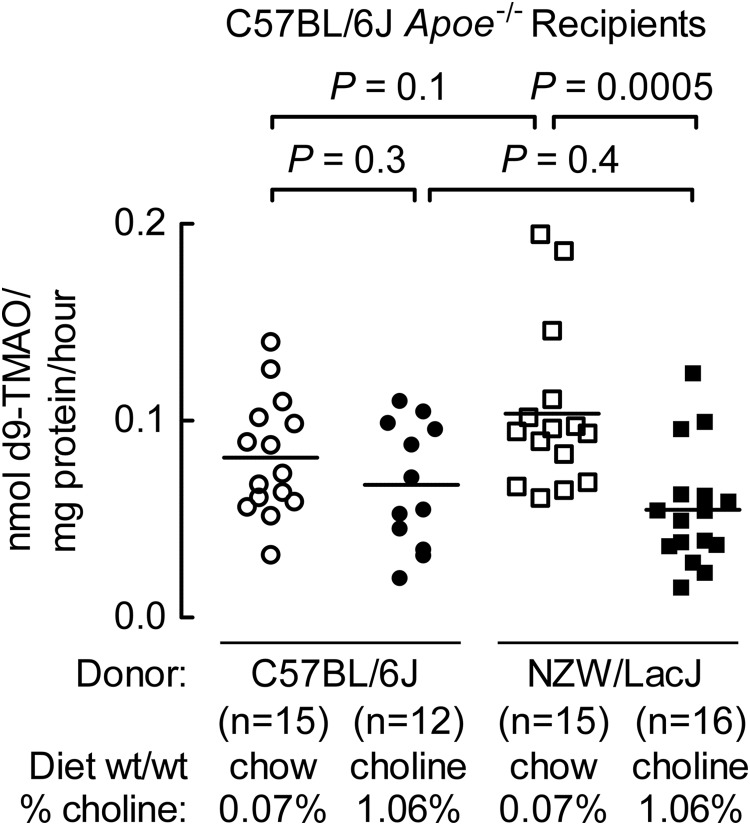
In parallel studies, adjacent aortic root sections were used for macrophage quantification by immunohistochemical staining with anti-Iba1, a macrophage specific cell surface marker (16, 17). Similar results were observed with recipient mice that were gavaged with C57BL/6J cecal microbes and maintained on the high choline diet, showing significantly greater stained area for macrophages within the lesion than similarly treated mice except for receipt of NZW/LacJ donor microbiota (Fig. 3, B and E). Characterization of plasma lipids (total cholesterol, triglycerides, and HDL cholesterol) from the two donor strains at the time of cecal content harvest showed higher HDL cholesterol and total cholesterol in the atherosclerosis-resistant NZW/LacJ inbred strain (Table 2). However, parallel examination among each of the recipient mouse groups from plasma collected at 8 weeks (not shown) and study termination (20 weeks) showed no significant differences (Table 3), indicating no evidence for dyslipidemic changes to account for the observed differences in atherosclerosis. Examination of plasma glucose and weights between the different groups of recipient mice similarly revealed no significant differences (Table 3). Liver flavin-dependent monooxygenase activity was determined in recipient mice at time of organ harvest in both donor (Table 2) and recipient mice (Fig. 4). No significant differences were observed overall among donors and recipients, or between donor groups of mice (Table 2). Among the recipients, those receiving NZW/LacJ cecal microbes and choline diet showed reduced total hepatic FMO activity (Fig. 4).
TABLE 2.
Characterization of traditional risk factors and liver FMO activity in donor strains of mice
Donor plasma and livers were collected at the time of cecal content harvest. Total cholesterol, triglycerides, glucose, and HDL cholesterol were quantified as outlined under “Experimental Procedures.” Liver flavin-dependent monooxygenase activity, expressed in nmol of d9-TMAO formed per mg of protein/hour, was determined as outlined under “Experimental Procedures.” Data shown are mean ± S.E. for each of the groups.
| Plasma chemistry (mg dl−1) | Donor mice |
p value | |
|---|---|---|---|
| C57BL/6J | NZW/LacJ | ||
| Triglyceride | 71 ± 5 | 112 ± 5 | 0.0001 |
| Total Cholesterol | 78 ± 4 | 138 ± 10 | 0.0001 |
| HDL | 41 ± 7 | 71 ± 7 | 0.003 |
| Glucose | 193 ± 17 | 169 ± 16 | 0.3 |
| Liver FMO activity (nmol/mg/h) | 0.036 ± 0.010 | 0.049 ± 0.010 | 0.4 |
TABLE 3.
Characterization of traditional risk factors among recipient groups of mice in cecal microbial transplant studies
Recipient plasma at study termination (20 weeks) was collected. Total cholesterol, triglycerides, glucose, and HDL cholesterol were quantified as outlined under “Experimental Procedures.” Recipient weights shown were collected at the end of the study. Data shown are mean ± S.E. for each of the groups.
| Plasma chemistry (mg dl−1) | C57BL/6J Apoe−/− recipient mice |
p value |
||||||
|---|---|---|---|---|---|---|---|---|
| C57BL/6J donor |
NZW/LacJ donor |
Chow vs. choline |
C57BL/6J vs. NZW/LacJ |
|||||
| Diet: chow (n = 15) | Diet: choline (n = 12) | Diet: chow (n = 15) | Diet: choline (n = 16) | C57BL/6J | NZW/LacJ | Chow | Choline | |
| Triglyceride | 81 ± 7 | 72 ± 6 | 71 ± 3 | 69 ± 10 | 0.2 | 0.8 | 0.2 | 0.6 |
| Total cholesterol | 299 ± 16 | 277 ± 9 | 299 ± 14 | 294 ± 11 | 0.2 | 0.8 | 0.8 | 0.5 |
| HDL | 28 ± 5 | 26 ± 3 | 24 ± 2 | 28 ± 3 | 0.4 | 0.4 | 0.1 | 0.9 |
| Glucose | 236 ± 12 | 215 ± 18 | 204 ± 13 | 195 ± 11 | 0.07 | 0.4 | 0.1 | 0.8 |
| Body weight(g) | 23.8 ± 0.4 | 23.8 ± 0.4 | 23.2 ± 0.5 | 22.3 ± 0.3 | 0.4 | 0.1 | 0.2 | 0.06 |
FIGURE 4.
Hepatic flavin monooxygenase activity among recipient mice. Total FMO activity in liver homogenates was determined as described under “Experimental Procedures” using liver recovered from the indicated recipient Apoe−/− mice included in the aortic atherosclerotic plaque study. As in Fig. 3, groups are defined by the indicated diet arm (chow versus choline) and donor source for cecal microbial suspensions given by gastric gavage (atherosclerosis-prone C57BL/6J versus atherosclerosis-resistant NZW/LacJ).
Characterization of Microbial Communities in Donor and Recipient Mice
We next sought to examine intestinal microbial composition within the donor strains (C57BL/6J versus NZW/LacJ) and to see whether features of the community structure were transplanted to the recipients. Over the course of the studies, the average age of donors from each inbred strain used was 14.8 ± 1.4 weeks for C57BL/6J and 14.0 ± 1.2 for NZW/LacJ. Initial analyses were performed on microbial DNA encoding 16 S rRNA recovered from cecal contents used in gastric gavages. Principal coordinate analyses of donor cecal microbial compositions showed distinct microbial communities for the inbred C57BL/6J and NZW/LacJ strains (Fig. 5A). When beta diversity measures of the cecal samples of the donors and the four recipient groups were examined within a hierarchal tree, microbial compositions of the recipient groups clearly clustered with the compositions of their respective donor (Fig. 5B).
FIGURE 5.
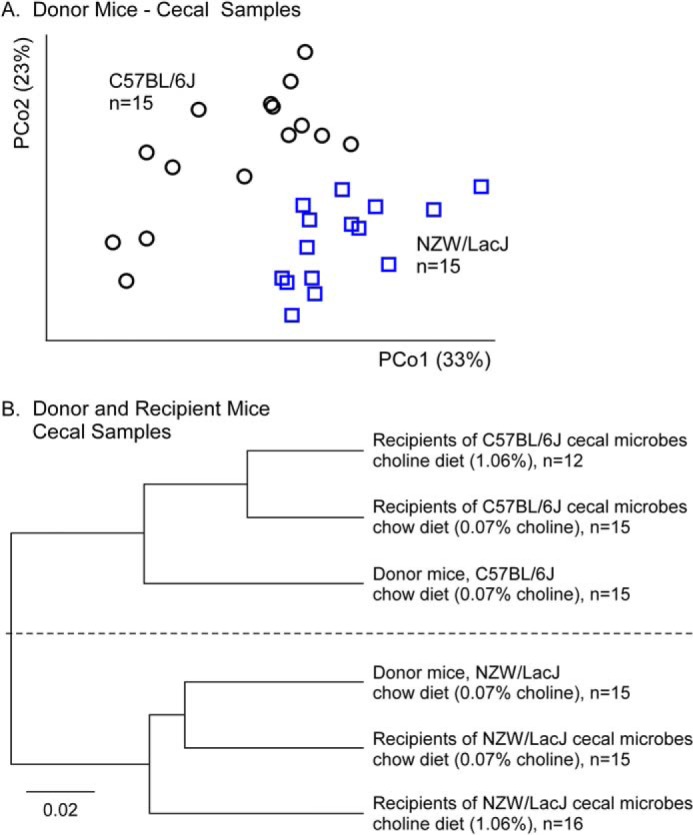
Demonstration of distinct cecal microbial compositions between donor strains and relatedness between donors and their respective recipient groups regardless of dietary arm. A, unweighted UniFrac distances plotted in principal coordinates analysis space comparing cecum microbial communities between two donor strains. Each data point represents a sample from a distinct mouse projected onto the first two principal coordinates (percentage of variation explained by each principal coordinates (PCo) is shown in parentheses). Circles represent C57BL/6J, and squares represent NZW/LacJ. B, unweighted pair group method with arithmetic mean clustering on the cecal samples from the two donor groups and all four recipient groups collected at 20 weeks of age. Beta diversity distance matrix distances are displayed into a hierarchical tree in which branches, and lengths illustrate how closely the groups are related to one another. Recipients of cecal microbes from C57BL/6J donors, regardless of diet, are more closely related to C57BL/6J donors, and conversely, recipients of cecal microbes from NZW/LacJ donors, regardless of diet, are more closely related to NZW/LacJ donors.
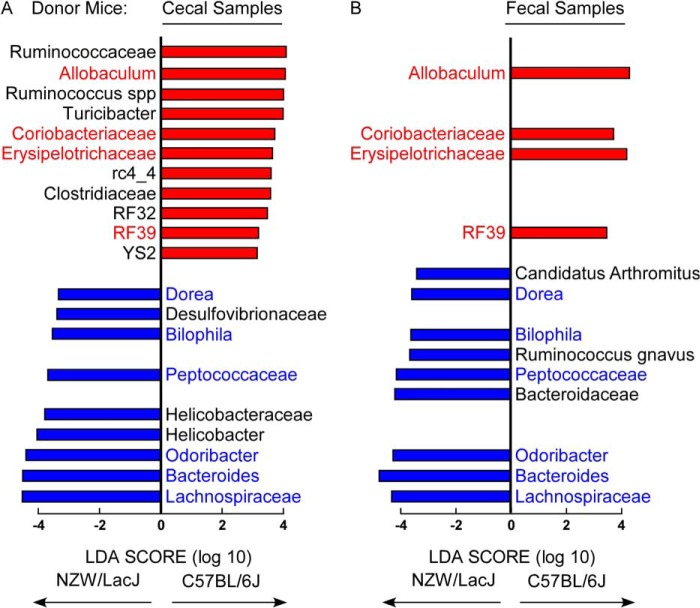
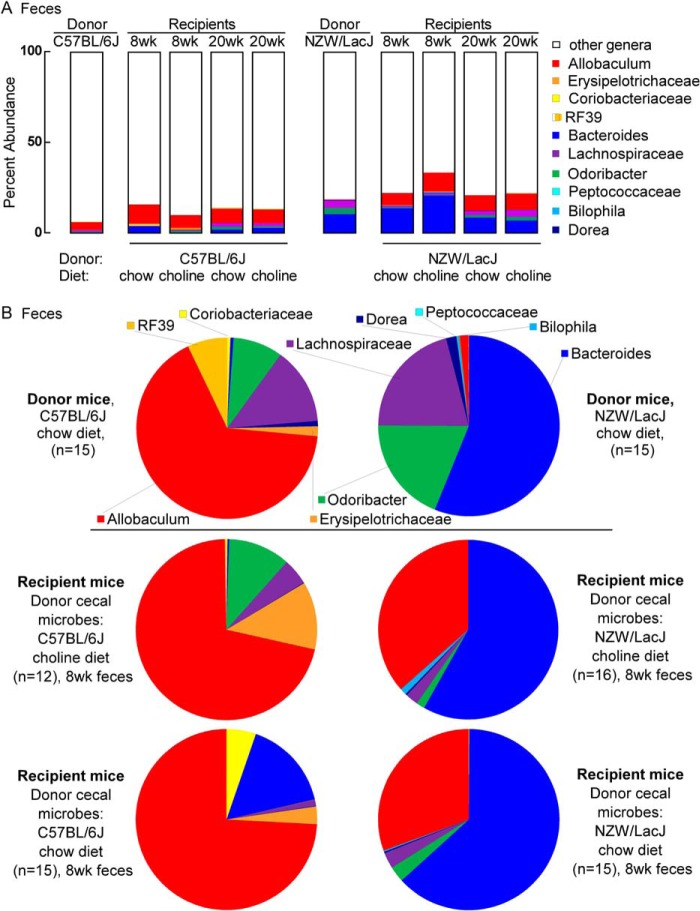
We next further examined the fecal microbial compositions of the donor mice, which could be compared with the fecal microbial compositions of recipients collected over the course of the transplantation studies. To visualize overall differences, the percent abundances of genera within each of the two donor strains (n = 15 C57BL/6J, and n = 16 NZW/LacJ) are plotted with taxa color-coded for the strains containing greater abundance (reds for C57BL/6J and blues for NZW/LacJ) (Fig. 6). Visual inspection identifies clear differences, but we also sought to perform quantitative analyses to more robustly identify microbial taxa to help discriminate between C57BL/6J versus NZW/LacJ donors when examined in both fecal and cecal microbial compositions. Fecal samples could be monitored in recipients longitudinally over the course of the transplantation studies, and cecal samples were available at the end of the study. For these analyses, we used the recently described LEfSe algorithm (26) for comparative microbial composition analyses, permitting the characterization of microbial taxa whose proportions are characteristic of differences between donor microbial communities. Initial global comparisons between cecal microbial communities from the C57BL/6J versus NZW/LacJ donors revealed 20 genus level taxa characteristic of donor inbred strain (Fig. 7A). Parallel analyses of fecal microbial communities revealed 13 donor strain characteristic taxa, 10 of which overlapped with the characteristic taxa identified in cecal microbiota analyses (Fig. 7B). Overlapping taxa characteristic of donor strain (higher proportions) in both cecum and feces are labeled in red for C57BL/6J or in blue for NZW/LacJ donor mice.
FIGURE 6.
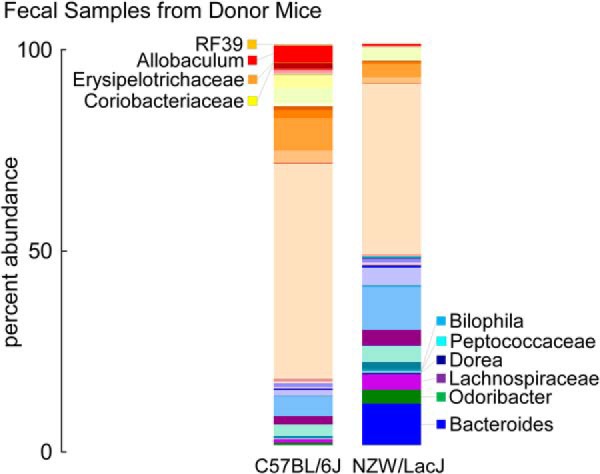
Fecal microbial compositions of donor strains. Percent abundances of genus level taxa displayed in a stacked bar graph show differences between inbred donor strains (data for chow diet shown). Taxa in higher abundance in C57BL/6J donors are colored in reds, and those higher in NZW/LacJ donors are displayed in blues. Results shown represent averages among C57BL/6J (n = 15) and NZW/LacJ (n = 16) donors.
FIGURE 7.
Defining donor characteristic taxa in cecal and fecal microbiota. A and B, microbial DNA encoding 16 S rRNA was analyzed from C57BL/6J and NZW/LacJ cecum (A) and feces (B) (n = 15 for both donor groups). Shown is a histogram displaying LDA scores calculated with the LEfSe algorithm identifying taxa most characteristic (increased abundance) of donor strain type, as described under “Experimental Procedures.” Taxa enriched in C57BL/6J are indicated with a positive LDA score (red), and taxa enriched in NZW/LacJ donors have a negative LDA score (blue). Only taxa meeting an LDA significance threshold >2 are shown. Donor characteristic taxa common to both cecal and fecal microbiota (10 overlapping taxa were identified) are indicated with color label: red for C57BL/6J characteristic donor; blue for NZW/LacJ characteristic donor.
We then examined the distribution of percent abundances of the 10 overlapping donor characteristic taxa in the recipient groups at different time points. The bar graphs in Fig. 8A show the percent abundances accounted for by the 10 characteristic taxa in the context of genera (including the genus level taxa that did not qualify for further analysis) for donors and recipients. The 10 donor characteristic taxa represent 6.2% of the microbial community detected in C57BL/6J donor mice feces, and 13.8–15.5 and 9.8–13.3% (range observed in 8–20-week samples) in the feces of recipient mice on chow and choline diets that were gavaged with C57BL/6J cecal microbes (Fig. 8A). Graphical representation of the proportions of these 10 taxa in pie charts illustrates that Allobaculum is a taxa more abundant among the 10 taxa examined within the C57BL/6J but not NZW/LacJ donors (red) (Fig. 8B), and whose proportions in recipients of C57BL/6J cecal microbes remain prominent at both early (8-week; Fig. 8B) and late (20-week; Fig. 9) time points, regardless of dietary arm. For NZW/LacJ donor mice, these same 10 taxa represent 18.4% of fecal microbial 16 S rRNA encoding DNA, and 20.7–22.3 and 22.0–32.4% (range observed in 8–20-week samples) of fecal microbes within recipients that were gavaged with NZW/LacJ cecal microbes (Fig. 8A, right side). Bacteroides (blue) is an abundant taxa characteristic of the NZW/LacJ donors, and the proportions of this taxa were more prominent in the recipients receiving the NZW/LacJ cecal microbes, regardless of dietary arm and time point examined (Figs. 8B and 9A). The overall differences between C57BL/6J and NZW/LacJ recipient groups confirm that the cecal microbial transplant protocol used resulted in substantial reorganization of the community structures in the host. Examination of the overall proportions of fecal bacterial taxa characteristic of the donor inbred strain revealed that, like the functional studies examining plasma TMA and TMAO levels, the characteristic differences over time after cecal transplantation became less pronounced (Fig. 9A). Similar analyses were carried out on the Apoe−/− weanlings prior to microbiota suppression with antibiotic administration (Fig. 9B). Substantial differences in community structures between the recipients prior to antibiotics, versus study end point, are noted in all recipient groups regardless of diet or donor cecal microbes gavage.
FIGURE 8.
Donor microbial community features transfer to recipients. The 10 overlapping donor characteristic taxa from LEfSe analysis of donor cecal and fecal samples (Fig. 7) were selected for further investigation in recipient mice. A, stacked bar graphs showing the percent abundance accounted for by the 10 selected taxa within DNA encoding the 16 S rRNA recovered from feces from the indicated donors and recipients. The key at the right lists the taxa with assigned color. Red, orange, and yellow colors indicate taxa higher in abundance in C57BL/6J donors, and blue, purple, and green colors indicate taxa higher in abundance in NZW/LacJ donors. B, pie charts of average abundance of these 10 taxa for the two donor groups (top) and the four recipient groups in feces recovered at 8 weeks of age.
FIGURE 9.
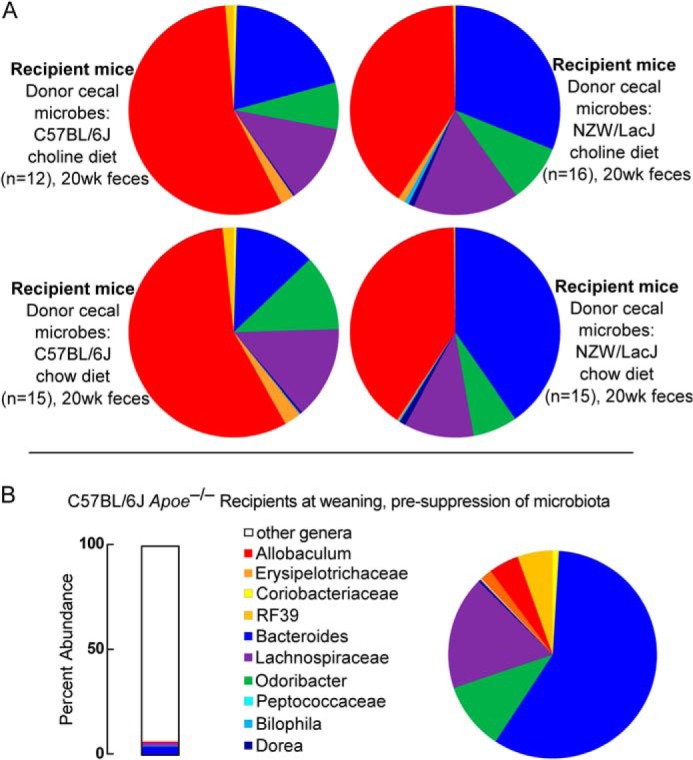
Distribution of 10 identified donor characteristic taxa in cecum of recipients at 20 weeks. A, 10 donor characteristic taxa (Fig. 7) were identified whose proportions in either cecum or feces remained indicative of the donor, as described under “Results.” Shown here are the proportions of these 10 characteristic taxa observed at 20 weeks of age in cecal samples recovered from the indicated recipient groups. B, left, bar graph illustrating the proportions of the 10 donor characteristic taxa, which make up 6.8% of the composition of the fecal microbes within C57BL/6J Apoe−/− recipient female mice before antibiotic treatment. Right, the pie chart shows the proportions among the 10 donor characteristic taxa in the C57BL/6J apoE−/− recipient female mice before antibiotic treatment.
Proportions of Select Transplanted Microbial Taxa Are Correlated within Recipient Plasma TMAO and Atherosclerosis
We next examined the relationship between proportions of fecal bacterial taxa and plasma TMAO levels in both donors and 8-week-old recipients, the time point in recipients when the greatest diet- and donor-dependent differences in plasma TMAO were observed. Proportions of Prevotella were strongly associated with TMAO levels (r = 0.44, p = 0.0003; Fig. 10A), a genus we recently reported was associated with TMAO levels in cecal microbes from carnitine-supplemented mice (6). Because of the observed association between the relative abundance of Prevotella and plasma TMAO, cecal microbial donor, and dietary groups at this early time point, we examined the abundance of Prevotella at later time points to see whether differences among the groups were maintained (Fig. 10B). By 16 weeks, the effect of choline diet on relative abundances of Prevotella were non-significant (i.e. recipients of C57Bl/6J cecal microbes on chow versus choline diets no longer showed significant differences), similar to the changes in levels of TMAO observed (Fig. 2). Also, by the end of the study, the relative abundances of Prevotella in all four groups were overlapping. We also examined whether Prevotella proportions were associated with atherosclerosis plaque quantification in the mice. Across all four groups of mice, no significant correlation was observed (r = 0.06, p = 0.6). However, a strong positive correlation was noted with atherosclerosis extent among the two recipient groups on the choline-supplemented diet (r = 0.37, p = 0.02; Fig. 10C), and a significant inverse association was observed among the two recipient mice groups receiving chow (low choline) diet (r = −0.33, p = 0.03).
FIGURE 10.
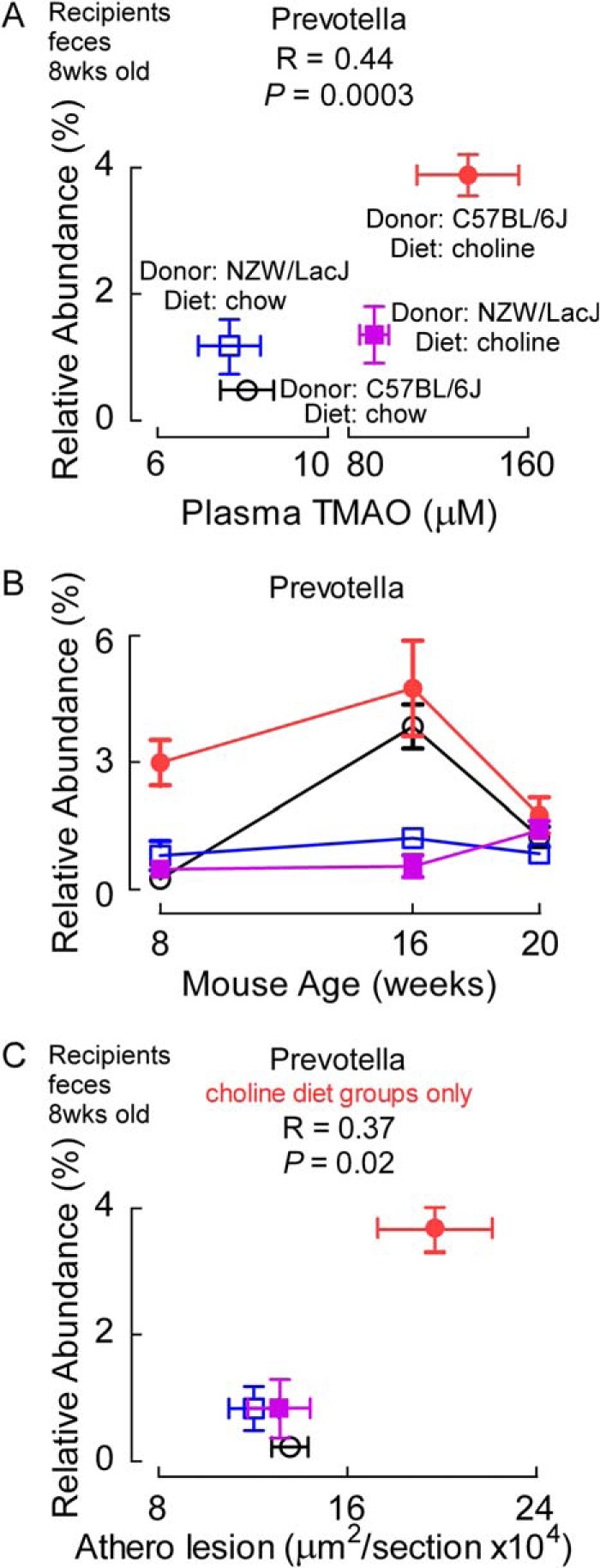
Proportions of the genus Prevotella in feces are associated with plasma TMAO plasma levels and aortic root atherosclerosis extent. A and C, the proportion of Prevotella in fecal samples recovered from the indicated 8-week-old recipient groups is plotted (y axis) versus plasma TMAO levels (A) or aortic root atherosclerotic plaque level (C) (x axis), with each data point representing mean ± S.E. from n > 12 mice. A positive correlation (Spearman) is observed (r = 0.44, p = 0.0003) between Prevotella proportions and TMAO levels across all four recipient groups, and between the proportion of Prevotella and atherosclerosis extent among recipients provided the high choline-supplemented diet (r = 0.37, p = 0.02). B, relative abundances of Prevotella throughout the course of the study followed the same trend as the TMAO levels in that the values started to converge by 16 weeks and at the end point.
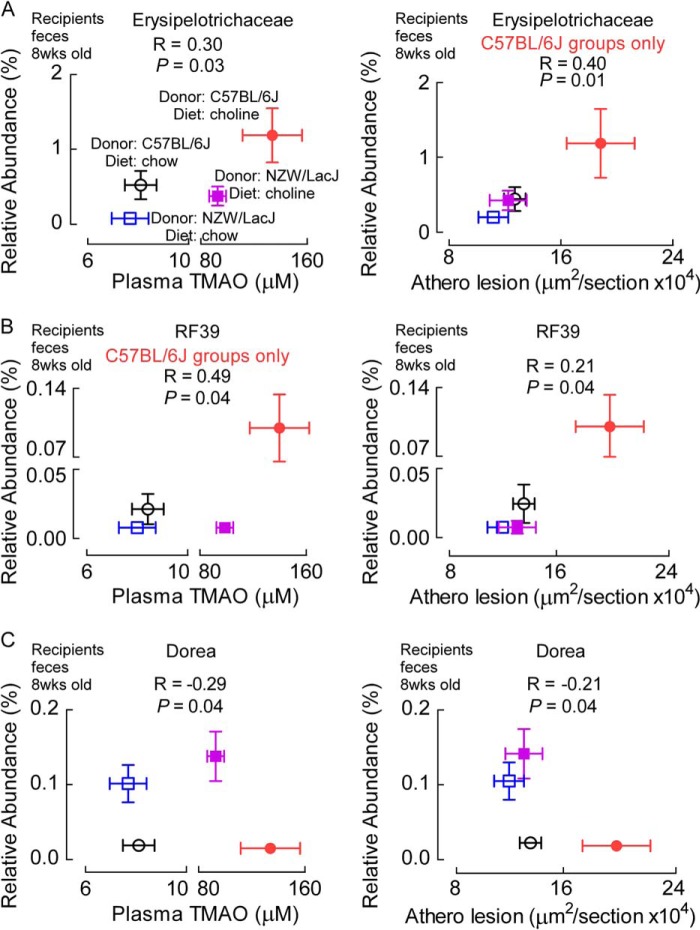
Further analyses revealed several additional microbial taxa whose proportions were characteristic of the donor microbial community and TMAO levels. In general, C57BL/6J donor strain characteristic taxa tended to be positively correlated with TMAO, and NZW/LacJ characteristic taxa were negatively correlated with TMAO values. For example, when the relationship with TMAO levels was interrogated in recipients (8 week, feces), restricting examination to the 10 donor characteristic taxa identified by LEfSe analysis of both cecal and fecal donor microbial communities (Fig. 7), the proportions of 5 of the 10 taxa (50%) showed a significant (p < 0.05) correlation with plasma TMAO levels. Similarly, proportions of the 10 donor characteristic taxa within recipient fecal (8-week) microbes showed that half of the taxa significantly (p < 0.05) correlated with atherosclerosis extent.
An example of a “C57BL/6J donor characteristic” taxa that was positively correlated with plasma TMAO from 8-week-old recipients was the Erysipelotrichaceae family (r = 0.30, p = 0.03; Fig. 11A). The proportions of Erysipelotrichaceae within recipient feces were also significantly positively correlated with aortic root atherosclerotic plaque development among recipients of atherosclerosis-prone cecal microbes (C57BL/6J) (r = 0.40, p = 0.01; Fig. 11A), and a borderline inverse association was observed among NZW/LacJ recipient groups (r = −0.26, p = 0.05). No significant association was noted with atherosclerosis among all four groups (r = 0.05, p = 0.6). Similarly, the proportions of the taxa (order) RF39 in feces from 8-week-old recipient mice, a “C57BL/6J characteristic” taxa identified in fecal and cecal microbiota analyses (Fig. 7), demonstrated positive association with atherosclerosis extent among all groups of mice (r = 0.21, p = 0.04; Fig. 11B), and a strongly positive association with plasma TMAO levels among recipients of C57BL/6J cecal microbes (r = 0.49, p = 0.04; Fig. 11B). However, RF39 did not show up as a taxa associated with TMAO levels in the analyses looking across all four recipient groups (r = 0.11, p = 0.4). Only one “NZW/LacJ characteristic” taxa identified by LEfSe analysis of both cecal and fecal donor microbial communities (Fig. 7) was associated with atherosclerosis extent and TMAO levels. Proportions of the genus Dorea within recipient feces (8 weeks) were inversely correlated with both plasma TMAO levels and atherosclerosis extent (r = −0.29, p = 0.04, and r = −0.21, p = 0.04, for plasma TMAO and atherosclerosis, respectively; Fig. 11C).
FIGURE 11.
Examples of donor characteristic taxa that associate with plasma TMAO levels and atherosclerosis in recipients. The graphs in the left column plot plasma TMAO concentrations on the x-axes, whereas graphs in the right column plot aortic atherosclerotic lesion extent on the x-axes of the indicated recipient groups. All y-axes contain the relative abundance of the indicated donor characteristic taxa as identified from the LEfSe analysis (Fig. 7). Spearman correlation coefficients and false discovery rate adjusted p values are shown. A, taxa Erysipelotrichaceae, characteristic (more abundant) of C57BL/6J donor mice, is positively correlated with TMAO across all four recipient groups and with atherosclerosis extent for recipients of atherosclerosis-prone C57BL/6J cecal microbes. B, taxa RF39, also characteristic of C57BL/6J donors, is positively correlated with both atherosclerosis extent (all recipients) and TMAO levels among C57BL/6J recipient groups. C, proportions of the taxa Dorea, characteristic of NZW/LacJ donors, demonstrates negative associations with both plasma TMAO levels and atherosclerosis extent across all recipient groups. Data points represent mean ± S.E.
No other donor characteristic microbial taxa, as defined by our criterion for observation in both cecum and feces (Fig. 7), showed significant correlations with both atherosclerosis extent and TMAO levels. However, some of the taxa that were identified as donor characteristic in either feces or cecum, but not both (Fig. 7), did show notable associations with either TMAO and/or atherosclerosis. For example, the recipient proportions (feces, 8 weeks) of the taxa (order) RF32, whose abundance is characteristic of C57BL/6J in donor cecal microbial analyses (Fig. 7A), were highly positively correlated with plasma TMAO levels among all recipient groups (r = 0.44, p = 0.0003), and both borderline positively associated with atherosclerosis extent for choline dietary groups (r = 0.32, p = 0.05) and strongly inversely associated among low choline (chow) recipient groups (r = −0.40, p = 0.009; all four groups, r = 0.07, p = 0.5) (Fig. 12).
FIGURE 12.
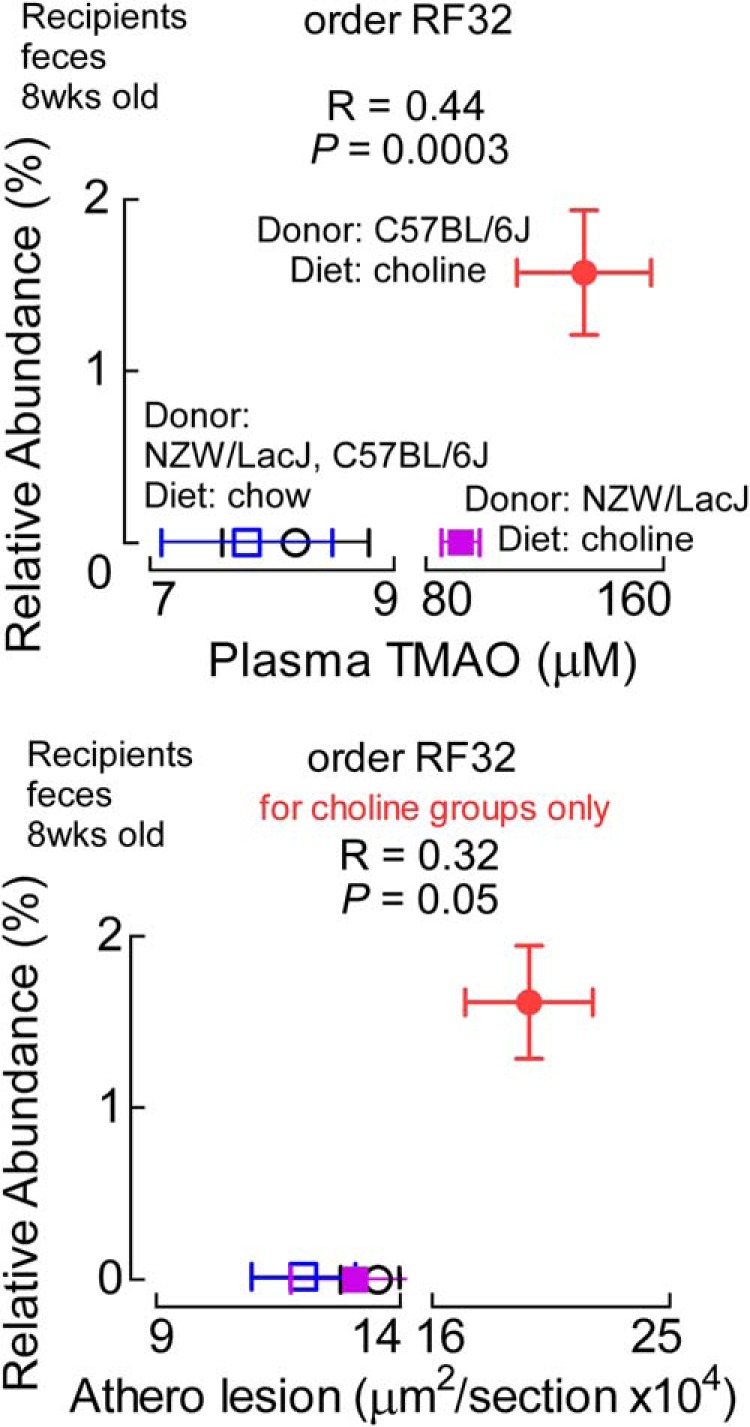
Example of donor characteristic taxa whose proportions are associated with plasma TMAO levels and atherosclerosis extent in recipients. Proportions of order RF32 (Alphaproteobacteria class), noted as a characteristic taxa of C57BL/6J donors from cecal samples, are associated positively with TMAO across all four recipient groups (top) and with atherosclerosis among the two recipient groups on the choline diet (bottom). Data points represent mean ± S.E.
DISCUSSION
Over a century ago, Robert Koch put forth a series of postulates to establish causality between a microbe and a disease (11–13). Although not universally fulfilled in infectious disease etiology, the conceptual framework they provide serves as a guide for proving a causal relationship between microbial species and disease. In this regard, despite studies demonstrating that the direct provision of a gut microbe-dependent product, TMAO, can itself foster accelerated atherosclerosis development and phenotypes (5, 6), and that dietary supplementation with the nutrient precursors to TMAO, choline and carnitine, similarly foster a pro-atherosclerotic phenotype in the presence, but not the absence, of intact gut microbiota and TMAO formation (5, 6), no studies to date have directly demonstrated transmission of atherosclerosis susceptibility by gut microbial transplantation. The present studies provide, for the first time, direct evidence that atherosclerosis susceptibility within a host may be influenced by gut (cecal) microbial transplantation. The studies thus fulfill an essential Koch's postulate, demonstrating that provision of microorganisms can cause or impact disease phenotype when introduced into a recipient organism.
Recent studies have shown that intestinal microbes serve as critical participants in development of complex metabolic phenotypes such as obesity and insulin resistance, and that traits like percentage of body fat and capacity for energy harvest are transmissible via inoculation of donor intestinal microbiota into recipient mice (28, 29). We reasoned that at least a portion of the differences in atherosclerosis susceptibility observed among distinct inbred strains of mice could similarly arise from their distinct microbial communities and ability to generate TMAO, a possibility further suggested by the striking association observed between atherosclerosis plaque development and plasma TMAO levels among different inbred strains of mice. As part of our study design, we selected relative extremes in the spectrum of atherosclerosis susceptibility, using C57BL/6J mice as an atherosclerosis-prone strain, and NZW/LacJ mice as an atherosclerosis-resistant strain to serve as microbial donors. By first suppressing endogenous microbes in recipients with a mixture of poorly absorbed oral antibiotics, we were able to show that recipients receiving cecal microbial suspensions from the atherosclerosis-prone donors demonstrated enhanced atherosclerosis relative to recipients receiving cecal microbes from atherosclerosis-resistant donors. Interestingly, the increase in atherosclerosis was only observed in the presence of a high choline diet, where differences in TMA and TMAO plasma levels, at least initially in the post-transplantation period, were observed. Thus, both atherosclerosis extent and choline diet-dependent increases in TMA and TMAO were each shown to be, in part, transmissible traits, although differences in TMA/TMAO production were relatively modest and not durable. Also of note, the recipients of the atherosclerosis-resistant NZW/LacJ cecal microbes failed to demonstrate increase in atherosclerosis on the high choline diet, despite a significant elevation in TMAO generation. We speculate that the mechanism for this may involve transmission of protective “anti-atherosclerotic” microbial taxa from the atherosclerosis-resistant donor with the transplantation. It should also be noted that the host (Apoe−/−) genetic background of the recipient may also contribute to choline diet-induced changes in plasma TMA/TMAO upon colonization with donor microbiota. Importantly, differences in atherosclerosis observed in the recipient mice following cecal microbial transplantation were not a result of changes in lipids/lipoprotein levels, or changes in other recognized risk factors for atherosclerosis (e.g. glycemic control, adiposity). Collectively, the present studies show that atherosclerosis susceptibility in the mouse model is influenced by a dynamic interaction between genetics, environment, and gut microbiota, which are uniquely and anatomically situated at the interface between these factors (Fig. 13). Indeed, gut microbiota serve as a filter of our largest environmental exposure, the food we eat, and are influenced, in turn, by both host genetics and dietary nutrients (Fig. 13).
FIGURE 13.
Summary schematic illustrating reciprocal interactions between genetics, environment, and intestinal microbiota, all of which impact on atherosclerosis susceptibility.
When designing the present studies, although not necessary conceptually to prove microbial transmission of phenotype, we would have ideally liked to have used germ-free mice as recipients to increase the odds of seeing a positive outcome. However, germ-free mouse genotypes suitable for atherosclerosis studies (e.g. Apoe−/−) were not available to us at the time. Difficulty in maintaining new (non-native) microbiota in recipient mice, whether germ-free or those in which gut microbiota suppression was initially performed with oral mixture of poorly absorbed antibiotics, has been reported in other studies (30–32). We therefore were concerned that the duration of successful transplantation of donor microbial community structure and function into the recipients might not be sufficient to permit observation of transmissibility of the desired traits: atherosclerosis and choline diet-dependent plasma TMA and TMAO levels. However, our results clearly show that under the experimental conditions used, we were able to functionally demonstrate choline diet-dependent enhancement in atherosclerosis. Further, choline diet-induced differences in plasma TMA and TMAO levels were observed, albeit only transiently. Interestingly, enhanced atherosclerosis was not observed in recipient mice that received the atherosclerosis-prone cecal microbes when maintained on a relatively low choline content diet, yet enhanced atherosclerosis was observed to be a transmissible trait in the recipient mice that both received the atherosclerosis-prone cecal microbes and were maintained on a high choline diet. Microbiota composition analyses in parallel revealed that multiple characteristic taxonomic features of the donors were transmitted to the recipients, and although the durability of these features became attenuated over time, donor characteristic taxa in both cecal and fecal communities remained visible within the recipients in both dietary arms throughout the study time course.
We think that several additional points are also worth noting. First, it is interesting that the differences in choline diet-induced enhancement in TMAO levels were not durable beyond the initial period after cecal microbial transplantation, yet choline diet-enhanced atherosclerosis was observed at the end of study duration. Other atherosclerosis studies have shown that there exist windows of time in mouse models where exposure to a trait may influence long term susceptibility for atherosclerosis development. For example, selective depletion of neutrophil levels in Apoe−/− mice for a relatively brief window of time, during the first 4 weeks of a high fat diet, but not after 3 or 11 months of a high fat diet, significantly inhibits long term atherosclerosis development during chronic dietary exposure (33). Similarly, recent studies by Blaser and colleagues (34) show that affecting microbiota in early windows of critical development can affect metabolism long term. Specifically, they have identified several taxa transiently disrupted after low-dose penicillin administration at mouse weaning age to be linked to persistent metabolic alterations such as enhanced dietary-induced fat mass accumulation and decreased intestinal-based immunity (34). In our own studies, we also observed more transient changes in microbiota early on that were not sustained to the study end point (e.g. differences in Allobaculum, Bacteroides, and Prevotella), and yet we still observed divergent functional outcomes. It is thus possible that the window of relatively reduced TMA/TMAO exposure in the recipient mice that were inoculated with NZW/LacJ cecal microbes and placed on the high choline diet was sufficient to influence overall atherosclerosis development. It is of course also possible that other choline diet-induced microbial traits were transplanted, yet not monitored, that contributed to the outcome. Indeed, the present study design does not exclude the possibility that factors alternative or in addition to TMA and TMAO participate in the enhanced choline diet-induced atherosclerosis observed (or suppression of atherosclerosis, in the case of transplant from NZW/LacJ). In addition, it is worth noting that cecal contents consist of not only microbes but also food matter and a host of unknown, potentially biochemically active microbial products. Mass spectrometry analyses of the TMA and TMAO content of the cecal slurries used for gavages showed that the total content of TMA + TMAO was negligible (“Experimental Procedures”). Whether other unmeasured factors beyond microbes were transferred with the gavages and might impact atherosclerosis susceptibility remains unknown. However, if such a factor(s) exists, it too is transmissible (or influenced by something transmissible) with cecal luminal contents used in the microbial transplantation studies, and is choline diet-dependent.
The present studies not only affirm a contributory role, at least in part, of gut microbiota to atherosclerosis susceptibility, but also suggest that microbe-targeted therapies may be of relevance to the treatment of atherosclerosis. Although few therapies targeting gut microbiota have translated into clinical use, the concept has been in existence for quite some time. Shifting gut microbial composition in humans with the aim of changing the course of disease was reported in 1958 by surgeons who treated patients suffering from antibiotic-induced diarrhea colitis by introducing microbiota from healthy donors (35). There has been a resurgence in interest in the potential of fecal transplantation for the treatment of recurrent Clostridium difficile infection because it was recently reported to be superior to administration of antibiotics (vancomycin) in direct head-to-head comparison studies (36). Remarkably, recent clinical studies have also shown that even complex metabolic traits such as insulin sensitivity may be influenced in subjects with microbial transplantation because subjects with metabolic syndrome can show improvement in insulin sensitivity by microbial (fecal) transplantation from lean healthy donors (37). The present studies raise the potential to apply gut microbiome-altering therapies for atherosclerotic heart disease. Although there are numerous challenges that need to be overcome before this can become a reality, the prospects are exciting.
Acknowledgments
We thank Denise Hatala for assistance with immunohistochemical staining. Mass spectrometry studies were performed on instrumentation housed within a facility partially supported by a Center of Innovation Award by AB SCIEX.
Note Added in Proof
Fig. 3A was incorrectly labeled “0.07% choline” in the version of this article that was published as a Paper in Press on December 30, 2014. The correct label, “1.06% choline,” is now shown above the bottom right corner image.
This work was supported, in whole or in part, by National Institutes of Health Grants R01 HL103866 (to S. L. H.), P20 HL113452 (to S. L. H.), and HL28481 and HL30568 (to A. J. L.) from the Office of Dietary Supplements. Drs. Hazen, Wang, and Levison are named as co-inventors on pending and issued patents held by the Cleveland Clinic relating to cardiovascular diagnostics and therapeutics. Dr. Hazen reports he has been paid as a consultant or speaker by the following companies: Cleveland Heart Lab, Inc., Esperion, Liposciences Inc., Merck & Co., Inc., Pfizer Inc., and Proctor & Gamble. Dr. Hazen reports he has received research funds from Abbott, Cleveland HeartLab, Liposciences, Inc., Proctor & Gamble, and Takeda. Drs. Hazen, Wang, and Levison have the right to receive royalty payments for inventions or discoveries related to cardiovascular diagnostics or therapeutics from Cleveland HeartLab, Inc., Esperion, and/or Frantz Biomarkers.
- TMA
- trimethylamine
- TMAO
- trimethylamine N-oxide
- LEfSe
- LDA effect size
- LDA
- linear discriminate analysis
- FMO
- flavin-containing monooxygenase.
REFERENCES
- 1. Brown J. M., Hazen S. L. (2014) Metaorganismal nutrient metabolism as a basis of cardiovascular disease. Curr. Opin. Lipidol. 25, 48–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bäckhed F., Ley R. E., Sonnenburg J. L., Peterson D. A., Gordon J. I. (2005) Host-bacterial mutualism in the human intestine. Science 307, 1915–1920 [DOI] [PubMed] [Google Scholar]
- 3. Cho I., Blaser M. J. (2012) The human microbiome: at the interface of health and disease. Nat. Rev. Genet. 13, 260–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kau A. L., Ahern P. P., Griffin N. W., Goodman A. L., Gordon J. I. (2011) Human nutrition, the gut microbiome and the immune system. Nature 474, 327–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang Z., Klipfell E., Bennett B. J., Koeth R., Levison B. S., Dugar B., Feldstein A. E., Britt E. B., Fu X., Chung Y. M., Wu Y., Schauer P., Smith J. D., Allayee H., Tang W. H., DiDonato J. A., Lusis A. J., Hazen S. L. (2011) Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472, 57–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Koeth R. A., Wang Z., Levison B. S., Buffa J. A., Org E., Sheehy B. T., Britt E. B., Fu X., Wu Y., Li L., Smith J. D., DiDonato J. A., Chen J., Li H., Wu G. D., Lewis J. D., Warrier M., Brown J. M., Krauss R. M., Tang W. H., Bushman F. D., Lusis A. J., Hazen S. L. (2013) Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 19, 576–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tang W. H., Wang Z., Levison B. S., Koeth R. A., Britt E. B., Fu X., Wu Y., Hazen S. L. (2013) Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 368, 1575–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bennett B. J., de Aguiar Vallim T. Q., Wang Z., Shih D. M., Meng Y., Gregory J., Allayee H., Lee R., Graham M., Crooke R., Edwards P. A., Hazen S. L., Lusis A. J. (2013) Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 17, 49–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang Z., Tang W. H., Buffa J. A., Fu X., Britt E. B., Koeth R. A., Levison B. S., Fan Y., Wu Y., Hazen S. L. (2014) Prognostic value of choline and betaine depends on intestinal microbiota-generated metabolite trimethylamine-N-oxide. Eur. Heart J. 35, 904–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tang W. H. W., Wang Z., Fan Y., Levision B., Hazen J. E., Donahue L. M., Wu Y., Hazen S. L. (2014) Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: refining the gut hypothesis. J. Am. Coll. Cardiol. 64, 1908–1914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Koch R. (1880) Investigations into the Etiology of Traumatic Infective Diseases, The New Sydenham Society, London, UK [Google Scholar]
- 12. Dolman C. E. (1973) Heinrich Hermann Robert Koch, in Dictionary of Scientific Biography (Gillespie C., ed), pp. 420–435, Charles Scribner, New York [Google Scholar]
- 13. Koch R. (1882) Die aetiologie der tuberculose. Berl Klin Wochenschr. 19, 221–230 [Google Scholar]
- 14. Wang Z., Levison B. S., Hazen J. E., Donahue L., Li X. M., Hazen S. L. (2014) Measurement of trimethylamine-N-oxide by stable isotope dilution liquid chromatography tandem mass spectrometry. Anal. Biochem. 455, 35–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bennett B. J., Orozco L., Kostem E., Erbilgin A., Dallinga M., Neuhaus I., Guan B., Wang X., Eskin E., Lusis A. J. (2012) High-resolution association mapping of atherosclerosis loci in mice. Arterioscler. Thromb. Vasc. Biol. 32, 1790–1798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rehg J. E., Bush D., Ward J. M. (2012) The utility of immunohistochemistry for the identification of hematopoietic and lymphoid cells in normal tissues and interpretation of proliferative and inflammatory lesions of mice and rats. Toxicol. Pathol. 40, 345–374 [DOI] [PubMed] [Google Scholar]
- 17. Köhler C. (2007) Allograft inflammatory factor-1/Ionized calcium-binding adapter molecule 1 is specifically expressed by most subpopulations of macrophages and spermatids in testis. Cell Tissue Res. 330, 291–302 [DOI] [PubMed] [Google Scholar]
- 18. Hamady M., Walker J. J., Harris J. K., Gold N. J., Knight R. (2008) Error-correcting barcoded primers for pyrosequencing hundreds of samples in multiplex. Nat. Methods 5, 235–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Caporaso J. G., Lauber C. L., Walters W. A., Berg-Lyons D., Huntley J., Fierer N., Owens S. M., Betley J., Fraser L., Bauer M., Gormley N., Gilbert J. A., Smith G., Knight R. (2012) Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 6, 1621–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Caporaso J. G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F. D., Costello E. K., Fierer N., Peña A. G., Goodrich J. K., Gordon J. I., Huttley G. A., Kelley S. T., Knights D., Koenig J. E., Ley R. E., Lozupone C. A., McDonald D., Muegge B. D., Pirrung M., Reeder J., Sevinsky J. R., Turnbaugh P. J., Walters W. A., Widmann J., Yatsunenko T., Zaneveld J., Knight R. (2010) QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7, 335–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bokulich N. A., Subramanian S., Faith J. J., Gevers D., Gordon J. I., Knight R., Mills D. A., Caporaso J. G. (2013) Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 10, 57–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Edgar R. C. (2010) Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26, 2460–2461 [DOI] [PubMed] [Google Scholar]
- 23. McDonald D., Price M. N., Goodrich J., Nawrocki E. P., DeSantis T. Z., Probst A., Andersen G. L., Knight R., Hugenholtz P. (2012) An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 6, 610–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lozupone C. A., Knight R. (2008) Species divergence and the measurement of microbial diversity. FEMS Microbiol. Rev. 32, 557–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pirrung M., Kennedy R., Caporaso J. G., Stombaugh J., Wendel D., Knight R. (2011) TopiaryExplorer: visualizing large phylogenetic trees with environmental metadata. Bioinformatics 27, 3067–3069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Segata N., Izard J., Waldron L., Gevers D., Miropolsky L., Garrett W. S., Huttenhower C. (2011) Metagenomic biomarker discovery and explanation. Genome Biol. 12, R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rooks M. G., Veiga P., Wardwell-Scott L. H., Tickle T., Segata N., Michaud M., Gallini C. A., Beal C., van Hylckama-Vlieg J. E., Ballal S. A., Morgan X. C., Glickman J. N., Gevers D., Huttenhower C., Garrett W. S. (2014) Gut microbiome composition and function in experimental colitis during active disease and treatment-induced remission. ISME J. 8, 1403–1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Turnbaugh P. J., Ley R. E., Mahowald M. A., Magrini V., Mardis E. R., Gordon J. I. (2006) An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1031 [DOI] [PubMed] [Google Scholar]
- 29. Bäckhed F., Ding H., Wang T., Hooper L. V., Koh G. Y., Nagy A., Semenkovich C. F., Gordon J. I. (2004) The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. U.S.A. 101, 15718–15723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ellekilde M., Selfjord E., Larsen C. S., Jakesevic M., Rune I., Tranberg B., Vogensen F. K., Nielsen D. S., Bahl M. I., Licht T. R., Hansen A. K., Hansen C. H. (2014) Transfer of gut microbiota from lean and obese mice to antibiotic-treated mice. Sci. Rep. 4, 5922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cho I., Yamanishi S., Cox L., Methé B. A., Zavadil J., Li K., Gao Z., Mahana D., Raju K., Teitler I., Li H., Alekseyenko A. V., Blaser M. J. (2012) Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 488, 621–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chung H., Pamp S. J., Hill J. A., Surana N. K., Edelman S. M., Troy E. B., Reading N. C., Villablanca E. J., Wang S., Mora J. R., Umesaki Y., Mathis D., Benoist C., Relman D. A., Kasper D. L. (2012) Gut immune maturation depends on colonization with a host-specific microbiota. Cell 149, 1578–1593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Drechsler M., Megens R. T., van Zandvoort M., Weber C., Soehnlein O. (2010) Hyperlipidemia-triggered neutrophilia promotes early atherosclerosis. Circulation 122, 1837–1845 [DOI] [PubMed] [Google Scholar]
- 34. Cox L. M., Yamanishi S., Sohn J., Alekseyenko A. V., Leung J. M., Cho I., Kim S. G., Li H., Gao Z., Mahana D., Zárate Rodriguez J. G., Rogers A. B., Robine N., Loke P., Blaser M. J. (2014) Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 158, 705–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Eiseman B., Silen W., Bascom G. S., Kauvar A. J. (1958) Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery 44, 854–859 [PubMed] [Google Scholar]
- 36. van Nood E., Vrieze A., Nieuwdorp M., Fuentes S., Zoetendal E. G., de Vos W. M., Visser C. E., Kuijper E. J., Bartelsman J. F., Tijssen J. G., Speelman P., Dijkgraaf M. G., Keller J. J. (2013) Duodenal infusion of donor feces for recurrent Clostridium difficile. N. Engl. J. Med. 368, 407–415 [DOI] [PubMed] [Google Scholar]
- 37. Vrieze A., Van Nood E., Holleman F., Salojärvi J., Kootte R. S., Bartelsman J. F., Dallinga-Thie G. M., Ackermans M. T., Serlie M. J., Oozeer R., Derrien M., Druesne A., Van Hylckama Vlieg J. E., Bloks V. W., Groen A. K., Heilig H. G., Zoetendal E. G., Stroes E. S., de Vos W. M., Hoekstra J. B., Nieuwdorp M. (2012) Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 143, 913–916 [DOI] [PubMed] [Google Scholar]