Abstract
DNA damage induces cell cycle arrest and DNA repair or apoptosis in proliferating cells. Terminally differentiated cells are permanently withdrawn from the cell cycle and partly resistant to apoptosis. To investigate the effects of genotoxic agents in postmitotic cells, we compared DNA damage-activated responses in mouse and human proliferating myoblasts and their differentiated counterparts, the myotubes. DNA double-strand breaks caused by ionizing radiation (IR) induced rapid activating autophosphorylation of ataxia-teleangiectasia-mutated (ATM), phosphorylation of histone H2AX, recruitment of repair-associated proteins MRE11 and Nbs1, and activation of Chk2 in both myoblasts and myotubes. However, IR-activated, ATM-mediated phosphorylation of p53 at serine 15 (human) or 18 (mouse) [Ser15(h)/18(m)], and apoptosis occurred in myoblasts but was impaired in myotubes. This phosphorylation could be enforced in myotubes by the anthracycline derivative doxorubicin, leading to selective activation of proapoptotic genes. Unexpectedly, the abundance of autophosphorylated ATM was indistinguishable after exposure of myotubes to IR (10 Gy) or doxorubicin (1 μM/24 h) despite efficient phosphorylation of p53 Ser15(h)/18(m), and apoptosis occurred only in response to doxorubicin. These results suggest that radioresistance in myotubes might reflect a differentiation-associated, pathway-selective blockade of DNA damage signaling downstream of ATM. This mechanism appears to preserve IR-induced activation of the ATM-H2AX-MRE11/Rad50/Nbs1 lesion processing and repair pathway yet restrain ATM-p53-mediated apoptosis, thereby contributing to life-long maintenance of differentiated muscle tissues.
The surveillance pathways that preserve genome integrity in cells exposed to genotoxic agents represent a crucial mechanism for multicellular organisms to ensure correct embryonic development and tissue homeostasis (18, 50, 54). Since mutations, chromosomal rearrangements, and changes in chromosome number are common features of malignant cells, efficient DNA repair has long been proposed to play an important role in cellular homeostasis. Failure to eliminate DNA lesions leads to the formation of genetically unstable cellular progenies with a tendency to malignant transformation (62).
While a number of studies have investigated the responses of proliferating cells to genotoxic agents, very little information exists on the effect of DNA damage in terminally differentiated tissues. Proliferating cells activate the cell cycle checkpoints in response to DNA damage to transiently arrest proliferation and allow time for repair of the lesion (21, 57), thereby preventing the spread of mutations to progeny (62). When proliferation is aimed at generating differentiated tissues in differentiation-committed progenitors, the DNA damage-dependent cell cycle arrest is coupled with a transient block of transcription of differentiation-associated genes (36, 40). This “differentiation checkpoint” is proposed to allow global genome repair before the differentiation program begins.
In the skeletal muscle lineage, myoblasts proliferate, whereas terminally differentiated myotubes are irreversibly confined in a postmitotic state (23) and hence do not need to activate cell cycle and differentiation checkpoints in response to genotoxic agents (40). Yet terminally differentiated cells are transcriptionally active and retain the need to preserve the integrity of the transcribed genome throughout the life span (25) by specific strategies, such as transcription-coupled repair (33). Nonetheless, little information is available on the ability of proliferating versus terminally differentiated cells to detect the lesion and propagate the DNA damage-activated signals (2, 24). Furthermore, while the apoptotic response of proliferating cells following DNA damage has been recognized as an alternative pathway to prevent the formation of genetically unstable progeny, it is still unclear whether terminally differentiated cells undergo programmed cell death in response to genotoxic agents. This issue appears to be of particular relevance given the relative resistance of differentiated tissues to apoptotic stimuli (52), a feature possibly related to the limited self-regenerative potential of these cells. Since differentiated tissues account for the largest component of the body in multicellular organisms, impaired apoptotic signaling in differentiated cells may reflect a conserved mechanism to maintain the body mass and long-term organ function.
DNA damage activates a variety of cellular cascades leading to cell cycle arrest, DNA repair, and/or apoptosis (55). Depending on the nature of the DNA damage, defined nuclear pathways transduce the signal towards a specific response. For instance, in response to ionizing radiation (IR), which produces double-strand breaks (DSBs), phosphorylation of histone H2AX (γ-H2AX) marks the actual site of damage (35). This is believed to help assemble and/or stabilize multiple protein complexes involved in DNA damage signaling (14). One crucial activator of DNA damage response in cells exposed to IR is the ataxia-teleangiectasia-mutated (ATM) kinase (46, 58). Upon cellular irradiation, ATM rapidly autophosphorylates at serine 1981, leading to dimer dissociation and triggering kinase activity toward H2AX and a number of transducers and effectors of DNA damage-activated pathways (6). For instance, ATM activates the Chk2 kinase by direct phosphorylation at Thr68 (1, 30-32). Activated Chk2 coordinates a number of cellular processes in response to DSBs (10), including cell cycle checkpoints as well as apoptosis by phosphorylating downstream effectors, such as Cdc25A, Cdc25C, BRCA1, Pml1, and p53 (9, 11, 19).
In a parallel pathway, ATM directly phosphorylates p53 at one specific, conserved residue, mouse serine 18 and human serine 15, hereafter referred to as Ser15(h)/18(m), as well as the p53 inhibitor MDM2, inhibiting p53-MDM2 interaction and promoting p53-dependent gene expression (3, 5, 42, 46). Phosphorylation of Ser15(h)/18(m) is also required for acetylation of the C-terminal domain of p53 by transcriptional coactivators and contributes to IR-induced expression of p53-dependent proapoptotic genes (15). The essential role of ATM and Chk2 in the activation of cellular responses to IR by p53 induction is underscored by the radioresistance displayed by certain cell types in ATM- and Chk2-deficient mice (7, 8, 49, 58, 59). In addition to ATM- and/or Chk2-dependent phosphorylation, recent studies indicate that a combination of additional posttranslational modifications (e.g., acetylation) and protein-protein interactions contribute to activate p53, and the functional homologue p73, toward the selective induction of a subset of genes which ultimately dictate the final cellular response, cell cycle arrest or apoptosis (3, 17, 51).
In this study, we used a model of skeletal muscle differentiation to investigate the responses of undifferentiated versus terminally differentiated cells to genotoxic agents. In this setting, undifferentiated myoblasts proliferate in the presence of mitogens and differentiate into multinucleated myotubes upon serum withdrawal. Myoblast-to-myotube transition entails a number of sequential stages that reflect the genome reprogramming toward differentiation (23, 41). Essential events for terminal differentiation are irreversible exit from the cell cycle, repression of cell proliferation-associated genes, and expression of muscle-specific genes (53).
Downregulation and functional inactivation of genes implicated in cell cycle checkpoint signaling, such as Chk1, ATR, BRCA1, c-Jun, and E2F1, is a specific feature of myotubes (26, 41). Other effectors of DNA damage-activated responses, such as c-Abl, are confined to the cytoplasm of myotubes (40). This evidence underscores the extensive reprogramming that occurs during terminal differentiation and suggests that undifferentiated and differentiated cells might differ significantly in their responses to genotoxic insults.
We performed a comparative analysis of the responses to IR in muscle cells both before and after terminal differentiation. This analysis revealed that the IR-activated pathway is interrupted in myotubes at the level of Ser15(h)/18(m) phosphorylation of p53, leading to the acquisition of an apoptosis-resistant phenotype upon IR exposure. Among several genotoxic agents that we tested, the anthracycline derivative Adriamycin (doxorubicin), which causes cardiotoxicity by inducing apoptosis in differentiated cardiomyocytes (4, 22, 47), could enforce Ser15(h)/18(m) phosphorylation of p53, leading to selective activation of proapoptotic genes and apoptosis in myotubes.
MATERIALS AND METHODS
Cell culture and DNA damage.
The murine C2C12 skeletal muscle cell line was cultured in growth medium (GM; Dulbecco's modified Eagle's medium supplemented with 15% fetal bovine serum, 2 mM glutamine, and antibiotics). Differentiation was induced by incubating the cells in differentiation medium (DM; Dulbecco's modified Eagle's medium supplemented with 2% horse serum) and was typically completed within 3 days. Quiescent cells were obtained by culturing C2C12 cells to confluence and replating them in Dulbecco's modified Eagle's medium supplemented with 0.5% fetal bovine serum for 36 h; at this time, 98% of the cells are in a nonproliferating state, as determined by 5-bromo-2-deoxyuridine incorporation.
The human and mouse primary satellite cells were cultured in D-Val MEM (minimal essential medium supplemented with 10% fetal bovine serum, nonessential amino acids, 0.5% chicken embryo extract, 2 mM glutamine, and antibiotics). Differentiation was induced by incubation in DM and completed after 10 (human) or 4 (mouse) days. Mouse embryonic fibroblasts (MEFs) from wild-type, p53 null, and p53 Ser18-to-Ala knock-in mice (15) were propagated in GM and converted into muscle cells by infection with adenovirus encoding the MyoD gene. After infection, confluent cells were incubated in DM to generate multinucleated, differentiated myotubes, which were exposed to genotoxic agents to evaluate the extent of apoptosis (see below).
When described, cells were treated with 10 ng of leptomycin B per ml and 5 μM MG132. IR was delivered with an X-ray generator (RT100; Philips Medico; 100 kV, 8 mA; dose rate, 0.92 Gy min−1), and cell extracts were prepared at the indicated times after radiation. When indicated, cells were exposed to 0.5 to 1 μM doxorubicin for 24 to 36 h.
Plasmids, transfections, and luciferase assay.
The p53-responsive luciferase (luc) reporter genes PGL3p21-luc, Bax-luc (from S. Soddu), and pAE21Mdm2-luc, along with pbabe-puro cDNA, were transfected into the C2C12 cell line by electroporation with the Bio-Rad Gene Pulser electroporator at 0.27 kV, 125 μFD, and 200 Ω. After gene transfer, cells were cultured in GM supplemented with 1 μg of puromycin per ml to establish C2C12 polyclones with chromatin-integrated reporter genes as described above. Luciferase activity in these cells was measured with a Lumat LB 9501 luminometer (Berthold) by following the instructions of the Promega luciferase assay system. Primary mouse satellite cells were transfected with pEGFP-F (green fluorescent protein [GFP] conjugated to a farnesylation signal; BD Clontech) along with empty vector, p53wt, or the Ser15ala mutant (5), with the Lipofectamine 2000 reagents.
Northern blotting and RT-PCR.
Northern blotting was performed by extracting total RNA with an RNA extraction kit (Oncor). RNA samples (10 μg) were run on formaldehyde gels, transferred, and hybridized with the Quik-Hyb hybridization solution from Stratagene according to the instructions. Full-length cDNAs were used as probes for p21Waf1 and Mdm2, and internal cDNA fragments were used as probes for Bax and glyceraldehhyde-3-phosphate dehydrogenase (GAPDH).
Expression of IR- and doxorubicin-induced genes was analyzed by reverse transcription-PCR (RT-PCR) amplification. Total cellular RNA was extracted with Trizol reagent (Invitrogen) according to the manufacturer's protocol from myoblasts and myotubes treated with 0.5 μM doxorubicin for 24 h; 50 ng of RNA was reverse transcribed and amplified with the SuperScript One-Step RT-PCR (Invitrogen). The sequences of the oligonucleotide pairs were as follows: Bax sense, 5′-CCCAAGCTTGGGCGAATTGGAGATGAACTGGAT-3′, and antisense, 5′-CCGCTCGAGCGGCATCTTCTTCCAGATGGTGAG-3′; p21 sense, 5′-CCCAAGCTTGGGGACACCAGTGTCCACACTCTT-3′, and antisense, 5′-CCGCTCGAGCGGCTAAAAACTTCTCTGAGAAGGC-3′; Mdm2 sense, 5′-CCCAAGCTTGGGGAGTCTCTGGACTCGGAAGAT-3′, and antisense, 5′-CCGCTCGAGCGGCGTCTGTTCGGCCTTCTCCTC-3′; p53BP (53BP1) sense, 5′-CCCAAGCTTGGGCAGCATTGAAAAAGAAGGCCA-3′, and antisense, 5′-CCGCTCGAGCGGTCACAGGGGCTCACAAACTCT-3′; ATM sense, 5′-CAGCAAAATCAAATGTATCAGC-3′, and antisense, 5′-TGACAGCATGAATTATTCTAGC-3′; and GAPDH, 5′-GGTGTCTTCACCACCATGGAGA-3′, and antisense, 5′-GATGTCATCATACTTGGCAGG-3′.
Amplified PCR products were separated on a 2% agarose gel.
Western blotting and immunofluorescence analysis.
Mouse monoclonal antibodies DCS-270 and DCS-273, both specific for Chk2, were raised as previously described (26). Other antibodies used in this study were against total p53 (Ab7 from Oncogene Research Products), phospho-p53 Ser15(h)/18(m) (Cell Signaling), tubulin (Ab4 from NeoMarkers), phospho-HA2X (Ser139, from Upstate), Nbs1 and MRE11 (from J. Petrini), phospho-Chk2 (Thr68, from S. Elledge), muscle-specific myosin heavy chain (MF20 from M. Crescenzi), and ATM phosphorylated at serine 1981 (from Rockland).
Procedures for gel electrophoresis, immunoblotting, and immunofluorescence staining of cells cultured on coverslips were described previously (19, 26, 27), except that ATM blotting was performed after electrophoresis in 6% acrylamide gels.
Immunoprecipitation and kinase assay.
Chk2 kinase activity was measured in human satellite cells transfected with Myc-tagged Chk2. Recombinant glutathione S-transferase-Cdc25A protein was used as a substrate. Myc-tagged or endogenous Chk2 was immunoprecipitated from whole-cell lysates of myoblasts and myotubes, and Chk2 activity was determined as described before (19).
Cell viability and TUNEL assay.
Cell survival was measured with the rapid cell viability assay (Oncogene Research Products). Cultured cells were grown in a 96-well microtiter plate and tested for their viability for several days after IR. Apoptosis was detected with the in situ fluorescein cell death detection kit (Roche) on cytocentrifuge slides. Both mock-treated and treated cells were collected at 36 h after doxorubicin treatment (1 μM) or 48 h after IR (20 Gy), cytocentrifuged onto a microscope slide, and stained for terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL).
RESULTS
DNA damage-dependent phosphorylation of histone H2AX and recruitment of MRE11-Nbs1 at the sites of DNA damage in both proliferating myoblasts and terminally differentiated myotubes.
We investigated the response to DNA damage in undifferentiated versus terminally differentiated cells with mouse C2C12 and primary human muscle cells as in vitro models of skeletal myogenesis. In these systems, differentiation is recapitulated by the fusion of mononucleated, undifferentiated myoblasts into multinucleated differentiated myotubes (41).
The ability of proliferating cells to recognize the DNA lesion inflicted by genotoxic agents is reflected by the rapid phosphorylation of histone H2AX (γ-H2AX), a signal to coordinate assembly of the DNA repair machinery, such as Nbs1 and MRE11 (12, 35, 56).
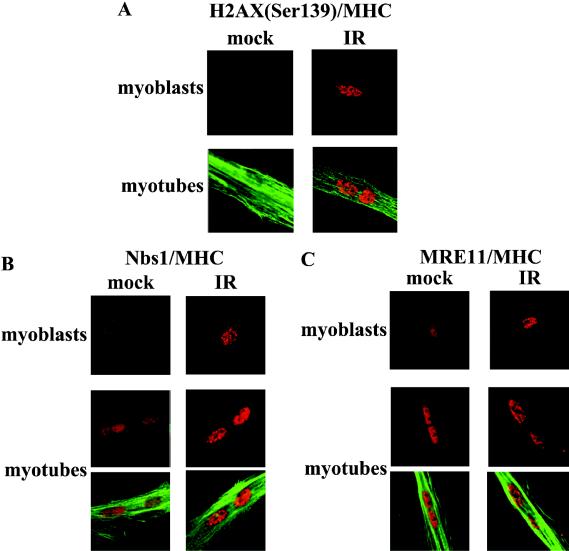
In situ immunostaining analysis of human satellite myoblasts and myotubes revealed comparable induction of nuclear foci containing phosphorylated γ-H2AX upon exposure to IR (Fig. 1A). Myosin heavy chain counterstaining was used as a marker of differentiation in myotubes. The nuclear redistribution of MRE11 and Nbs1, from a diffuse staining pattern to discrete foci, is a typical cellular response to IR-induced DNA damage and reflects their involvement in DNA repair and checkpoint signaling (29). MRE11 and Nbs1 displayed a diffuse nuclear distribution in untreated cells and readily redistributed into nuclear foci in both myoblasts and myotubes exposed to IR (Fig. 1B and C).
FIG. 1.
IR induces H2AX phosphorylation and recruitment of MRE11 and Nbs1 in both myoblasts and myotubes. In situ analysis of proteins involved in the DNA damage response is shown. Human satellite cells were grown to obtain both myoblasts and myotubes. Both were mock or gamma irradiated (10 Gy); fixed at 2 h post-IR; stained with antibodies against H2AX (phospho-Ser139) (A), Nbs1 (B), and MRE11 (C); and visualized with rhodamine-conjugated secondary antibodies. Myosin heavy chain (MHC) counterstaining was used as a marker of differentiation and revealed with a fluorescein-conjugated secondary antibody.
These results demonstrate that DNA damage-dependent recruitment of lesion processing and repair proteins at DSB sites operates in undifferentiated as well as terminally differentiated muscle cells, suggesting that upstream events in the pathway responsible for damage detection, repair, and early signaling are maintained throughout the establishment of terminal differentiation.
Activation of Chk2 in response to DNA damage in both proliferating myoblasts and differentiated myotubes.
Among the DNA damage-induced kinases, Chk1 and Chk2 are Ser/Thr kinases that coordinate cell cycle progression with DNA repair and cell survival or death (9). We have previously shown that, while Chk1 expression declines during the differentiation process, Chk2 levels are maintained in differentiated myotubes and the protein retains the ability to be activated (26).
We investigated IR-induced phosphorylation of Chk2 by ATM in muscle cells. ATM phosphorylates Chk2 on Thr68 (1, 32), and this modification is an activating signal in response to DNA damage (9, 16, 31). Caffeine inhibits ATM kinase activity and abolishes cell cycle checkpoints in several mammalian cell types (13, 43, 44, 48, 61). In both C2C12 myoblasts and myotubes, exposure to IR caused a prominent shift of Chk2, possibly reflecting protein phosphorylation, which was reversed by treatment with caffeine (Fig. 2A), suggesting that Chk2 undergoes ATM-dependent phosphorylation in response to IR regardless of the differentiation status. Specific Chk2 phosphorylation on Thr68 can be detected by Western blotting with antibodies selectively raised against phosphorylated Thr68. Since the phosphorylation-specific antibody can recognize only the human residue, a human Chk2-Myc-expressing vector was transfected into the mouse C2C12 cell line. After transfection, cells were maintained in either the presence or absence of mitogens to achieve the formation of myoblasts and myotubes, respectively.
FIG. 2.
Chk2 is activated by IR in both myoblasts and myotubes. (A) C2C12 skeletal muscle cells were cultured to obtain myoblasts (lanes 1 to 3) or myotubes (lanes 4 to 6); cells were irradiated with 10 Gy (lanes 2, 3, 5, and 6) and, in order to inhibit the checkpoint kinase ATM, pretreated with 5 mM caffeine (lanes 3 and 6). (A) Western blotting analysis was performed to detect Chk2 and its phosphorylation status (upper band). The slower-migrating upper band was abolished by caffeine treatment. (B) Proliferating C2C12 cells were transfected with 10 μg of Chk2-Myc-expressing vector and subsequently maintained in the presence of mitogens (myoblasts; lanes 2 to 4) or shifted to differentiation medium for 3 days (myotubes; lanes 5 to 7). Both were either mock-treated (lanes 2 and 5) or irradiated (lanes 3, 4, 6, and 7), together with a pretreatment with 5 mM caffeine when indicated (lanes 4 and 7). Samples were collected 2 h posttreatment and immunoprecipitated with Myc antibody, followed by immunoblotting analysis with a specific antibody for phospho-Thr68 Chk2 (upper panel) or total immunoprecipitated Chk2 (lower panel). (C) Human satellite cells were cultured to obtain either myoblasts or myotubes; both were mock or gamma irradiated (10 Gy), fixed 2 h later, and double stained with antibodies to Thr68-phosphorylated Chk2 and myosin heavy chain (MHC; differentiation marker). (D) The Chk2 kinase assay was performed with both myoblasts (lanes 1 to 5) and myotubes (lanes 7 to 11) derived from human satellite cells, irradiated, and collected at the indicated time points after IR; the Chk2-related kinase activity was measured, and the activation is indicated below the panel.
By immunoprecipitation with anti-Myc antibodies, human Chk2 was isolated from both proliferating and differentiated cells, and phosphorylation on Thr68 was detected by Western blotting with a phosphorylation-specific antibody (Fig. 2B, lanes 3 and 6). Pretreatment with caffeine reversed this phosphorylation (Fig. 2B, compare lanes 3 and 4 and lanes 6 and 7). To confirm the IR-induced phosphorylation on Th68 of endogenous Chk2 in human myoblasts, we performed an in situ analysis with human satellite cells (Fig. 2C). Both human myoblasts and myotubes were irradiated, fixed, and stained with the phosphorylation-specific Thr68 antibody; the immunofluorescence analysis confirmed that endogenous Chk2 was phosphorylated in both cycling and differentiated cells after IR. This modification was again reversed by caffeine pretreatment (data not shown).
The activation of Chk2 kinase following DNA damage-induced Thr68 phosphorylation was further demonstrated in human satellite cells by measuring the kinase activity of endogenous Chk2 toward the Cdc25A fragment in an in vitro kinase assay (Fig. 2D). The results presented in Fig. 2 show that Chk2 kinase is activated after IR by a caffeine-sensitive pathway in both proliferating and postmitotic cells, albeit with moderately different kinetics. Collectively, these data demonstrate that Chk2 is expressed, localized, and activated by DSBs in a comparable fashion during the cell cycle and after terminal differentiation of muscle cells, indicating that the pathway of Chk2 activation by DNA damage is preserved during the differentiation process.
Impaired p53 Ser15(h)/18(m) phosphorylation and stabilization in terminally differentiated myotubes exposed to IR.
To further investigate the signaling cascade evoked by IR exposure in proliferating versus differentiated cells, we analyzed posttranslational modifications and protein stability of p53, a downstream transcriptional effector of DNA damage-activated signaling.
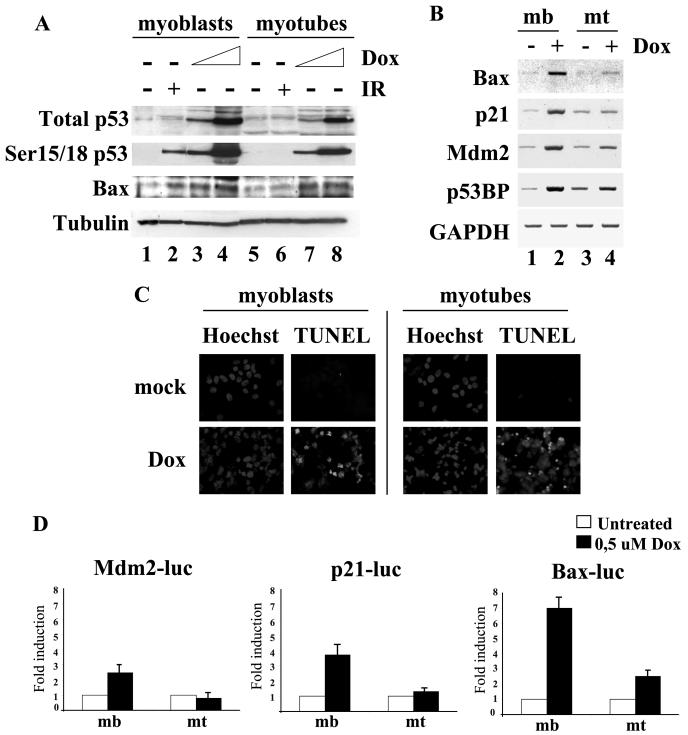
Figure 3A shows that in nonirradiated proliferating myoblasts, the p53 protein was almost undetectable, and as expected, its expression was induced upon exposure to IR. In terminally differentiated myotubes, the basal level of p53 was higher than in untreated myoblasts (Fig. 3A, compare lanes 1 and 3). Interestingly, IR exposure did not cause any further increase in p53 protein abundance. This result suggests that differentiated muscle cells become refractory to further stabilization of p53 in response to DSBs and prompted us to investigate the signaling leading to p53 activation in response to IR in myoblasts and myotubes.
FIG. 3.
p53 is differentially modified and regulated in proliferating and differentiated cells. (A) C2C12 cells were used to obtain both myoblasts (mb) and myotubes (mt). Cells were mock or gamma irradiated (10 Gy), harvested 4 h post-IR, and analyzed by immunoblotting with p53 or tubulin (loading control) antibodies. (B) Myoblasts (lanes 1 to 4) and myotubes (lanes 5 to 8) were mock or gamma irradiated, collected at 30 and 60 min after IR exposure, and treated with 5 mM caffeine (lanes 4 and 8). An immunoblotting analysis was performed with the phospho-p53 (specific for Ser15 or 18) and tubulin antibodies. (C) Time course immunoblotting analysis of C2C12 cell-derived myoblasts (lanes 1 to 6), myotubes (lanes 7 to 12), and quiescent cells (lanes 13 to 18) irradiated with 10 Gy. Treatment with 5 mM caffeine (lanes 6, 12, and 18) was initiated 10 min before exposure to IR. The upper panels show p53 phosphorylated on Ser15(h)/18(m); the middle panels show total p53; and the lower panels present the tubulin loading control.
Phosphorylation of p53 at Ser15(h)/18(m) by ATM (or other DNA damage-activated kinases, such as ATR and DNA-PK) is a well-known activating signal in response to IR. In proliferating C2C12 myoblasts, p53 phosphorylation at Ser18 could be detected after exposure to IR and was reduced by caffeine pretreatment (Fig. 3B, lanes 1 to 4). In contrast, no phosphorylation of Ser18 was observed in C2C12 myotubes exposed to IR (Fig. 3B, lanes 5 to 8). However, the experiment reported in Fig. 3B does not distinguish between the acquisition of a terminally differentiated phenotype in postmitotic myotubes and the reversible cell cycle arrest occurring in myoblasts prior to terminal differentiation.
To address this issue, we tested whether the inability to phosphorylate p53 on Ser18 is a feature of terminal differentiation or simply reflects exit from the cell cycle by comparing proliferating myoblasts, differentiated myotubes, and undifferentiated myoblasts rendered quiescent by serum starvation (39). Although differentiated myotubes and quiescent myoblasts are both arrested in the G0/G1 phase of the cell cycle (with DNA content typical of G0/G1 in approximately 92% of myotube nuclei and in approximately 91% of serum-starved myoblasts), the latter cells do not express differentiation markers and are able to reenter the cell cycle upon mitogenic stimulation (39).
A time course analysis comparing IR-induced Ser18 phosphorylation of p53 in proliferating, differentiated, and quiescent C2C12 cells revealed that this signal is impaired only in differentiated myotubes, while quiescent myoblasts are still competent to activate Ser18 phosphorylation (Fig. 3C, upper panels). These results indicate that the resistance to IR-induced p53 phosphorylation at Ser18 is coupled to a concomitant irreversible cell cycle withdrawal and the establishment of terminal differentiation.
Interestingly, Fig. 3C shows that increased Ser18 phosphorylation in response to DNA damage correlates with the accumulation of p53 in cycling myoblasts. This suggests that p53 activation by IR can be achieved in myoblasts by both protein accumulation and phosphorylation. In contrast, in quiescent myoblasts and in myotubes, the amount of p53 accumulated in response to mitogens withdrawal was not further upregulated by IR, yet quiescent myoblasts are permissive for Ser18 phosphorylation in response to DSBs (Fig. 3C, middle panels). Interestingly, phosphorylation of p53 at Ser18 in quiescent myoblasts exposed to IR was sufficient to trigger an apoptotic response, although of a magnitude reduced by 20% compared to the apoptosis observed in myoblasts (data not shown).
Accumulation of p53 in myotubes following proteasome or nuclear export inhibitors does not correlate with functional activation.
The data presented in the previous section indicate that p53 is subjected to regulation specific to the different stages of myogenic differentiation. The results also indicate that p53 accumulates in terminally differentiated cells; however, these cells lose the ability to further activate p53 in response to IR.
To investigate the potential of differentiated cells to activate p53 by IR-independent mechanisms, we compared the ability of myoblasts versus myotubes to accumulate p53 and promote its function in response to stimuli that interfere with p53 localization and degradation.
Accumulation of nuclear p53 was experimentally induced by treatment with either the general inhibitor of the nuclear export leptomycin B or the proteasome inhibitor MG132. Both proliferating and differentiated C2C12 cells were exposed to these agents with and without IR; p53 accumulation was measured 4 h after treatment and is plotted in the diagram in Fig. 4A. Upon leptomycin B and MG132 treatment, p53 protein levels increased in myoblasts and, to a lesser extent, also in myotubes. The same setting was employed to monitor p53 transcriptional activity with C2C12 cells stably transfected with the Mdm2-luciferase reporter gene (Fig. 4B). While an increased amount of p53 in myoblasts correlated with augmented transcriptional activity, in myotubes the p53 accumulated in response to leptomycin B or MG132 treatment failed to activate a p53-responsive template (Fig. 4B). Taken together, these data show that myotubes are permissive for p53 accumulation over the basal levels, but the protein increase does not correspond to augmented p53 transcriptional activity.
FIG. 4.
p53 accumulates in both myoblasts and myotubes, but in differentiated cells p53 is no longer activatable. (A) p53 protein level after leptomycin B and MG132 treatments. C2C12-derived myoblasts (open bars) and myotubes (solid bars) were mock (lanes 1 to 3) or gamma irradiated (lanes 4 to 6) before treatment either with the nuclear export inhibitor leptomycin B (LMB) (lanes 2 and 5) or the proteasome inhibitor MG132 (lanes 3 and 6) and collected 2 and 4 h after the treatment, respectively. p53 and tubulin protein levels were detected by immunoblotting analysis, measured, and normalized with a densitometer; the resulting values were plotted in the graph, setting p53 basal levels in myoblasts and myotubes to 100. These values are from one experiment representative of four different experiments carried out under the same conditions. (B) p53 transcriptional activity after leptomycin B and MG132 treatments. C2C12 cells stably transfected with the Mdm2-luc vector were treated as in panel A, and luciferase activity was measured; the graph shows the relative induction of luciferase activity after the treatment(s).
Terminally differentiated cells do not activate p53-dependent transcription in response to IR.
Next, we compared the ability of IR to activate transcription of p53 target genes in myoblasts and myotubes. To this end, we generated polyclonal C2C12 cell populations stably transfected with luciferase reporter genes under the control of different p53-responsive promoters. We analyzed the promoters of genes involved in propagating three distinct functions of p53: p21 (cell cycle arrest) (28), Mdm2 (regulator of p53 turnover) (38), and Bax (apoptosis) (45). The cells with stably integrated reporters were either cultured as myoblasts or induced to differentiate into myotubes, irradiated, and collected at the indicated times after IR. The activity of all three p53-dependent reporters invariably increased after IR only in myoblasts. In contrast, myotubes were resistant to IR induction of p53-dependent transcription despite showing somewhat increased higher basal activity of the luciferase templates, as reported previously (37, 40) (Fig. 5A, B, and C). Consistently, Northern blot analysis revealed that IR activated the expression of endogenous p21, Mdm2, and Bax mRNA in myoblasts, with a peak of induction within the first 4 h following irradiation, but failed to stimulate transcription of these genes in myotubes (Fig. 5D).
FIG. 5.
IR does not induce p53 activity in myotubes and this correlates with the impaired activation of p53 target genes in differentiated cells. (A, B, and C) Luciferase assay. C2C12 polyclones were established after transfection with (A) p21-luciferase, (B) Mdm2-luciferase, and (C) Bax-luciferase reporter vectors and antibiotic selection (see Materials and Methods). These cells were grown to obtain both myoblasts (open bars) and myotubes (solid bars), exposed to IR (10 Gy), and analyzed at the indicated time after IR. The values are expressed as induction relative to basal levels (1 RLU) of reporter activity in myoblasts and myotubes. (D) Northern analysis. C2C12-derived myoblasts and myotubes were mock or gamma irradiated (10 Gy), and the RNA was extracted at the indicated times after IR. A Northern blot analysis was performed with the p21Waf1, Mdm2, and Bax probes; the filter was normalized by glyceraldehhyde-3-phosphate dehydrogenase (GAPDH) hybridization.
Myotubes exposed to IR are resistant to cell death.
Activation of p53 target genes in proliferating myoblasts induces either cell cycle arrest or apoptosis. Myotubes are permanently confined at the G0/G1 phase of the cell cycle, and the increased basal activity of p53 in these cells may reflect a role for p53 during induction of differentiation (37). Moreover, one p53 target gene, the cell cycle inhibitor p21, is typically upregulated in myoblasts induced to differentiate, although the contribution of p53 to such an event seems to be only marginal (34). The data presented in Fig. 5 show that p53-responsive genes are not further induced by IR in myotubes, underscoring their postmitotic phenotype but also suggesting that the lack of p53 upregulation may be causally linked to the impaired ability of myotubes to undergo apoptosis in response to IR.
To test this hypothesis, we analyzed the long-term effects of IR on the survival of myoblasts and myotubes by means of established cell death assays. During the first 4 days after irradiation, myotubes displayed higher survival than myoblasts (Fig. 6A). At 5 days post-IR, virtually all the myoblasts had died, whereas myotubes could survive for at least 10 days postirradiation (data not shown). The same information was obtained by measuring apoptosis in irradiated myoblasts and myotubes by TUNEL. Apoptosis was detected in a large proportion of irradiated myoblasts (Fig. 6B, left panels), while myotubes were resistant to apoptosis upon IR exposure (Fig. 6B, right panels).
FIG. 6.
Myotubes exposed to IR display higher survival than proliferating cells and are resistant to apoptosis. (A) C2C12-derived myoblasts (open triangles) and myotubes (solid circles) were mock or gamma irradiated, and survival was assessed by measuring MTT [3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide thiazole blue] uptake for several days after IR. The diagram shows MTT uptake as a percentage relative to the uptake of mock cells. Error bars are based on the statistical analysis of four independent experiments. (B) C2C12 myoblasts and myotubes were mock or gamma irradiated with 20 Gy, cytocentrifuged, fixed, and subjected to TUNEL analysis.
Doxorubicin induces p53 phosphorylation at Ser18 and enforces the apoptotic response in differentiated myotubes.
The data presented so far suggest that resistance to apoptosis and failure to activate p53 in postmitotic muscle cells upon irradiation can be causally linked. The radioresistance displayed by terminally differentiated cells may reflect a mechanism to protect against cell depletion in tissues with limited self-regenerative potential, such as skeletal and cardiac muscles or neurons.
In order to gain further insight into the mechanisms rendering myotubes refractory to the activation of p53-dependent apoptosis, we sought to investigate myotube responses to a class of chemotherapeutic compounds, the anthracyclines, known to trigger apoptosis in terminally differentiated cells (4, 60). To this end, myotubes were challenged with the cardiotoxic agent doxorubicin. Using a dose-response curve, we first determined the minimal concentration of doxorubicin capable of inducing apoptosis in cultured skeletal and cardiac myocytes (data not shown). Exposure to concentrations of doxorubicin ranging from 0.5 to 1 μM induced Ser18 phosphorylation and stabilization of p53 in C2C12 myoblasts (Fig. 7A) and correlated with activation of p53 target genes (Fig. 7B) and apoptosis (Fig. 7C). Importantly, the same concentration of doxorubicin could enforce Ser18 phosphorylation and accumulation of p53 in C2C12 myotubes (Fig. 7A), and this correlated with selective upregulation of Bax (Fig. 7A and B) and activation of the programmed cell death pathway (Fig. 7C). By comparing the activation of distinct p53-responsive luciferase reporters by doxorubicin in myoblasts versus myotubes, we found that doxorubicin could induce only the Bax-luc reporter in myotubes, albeit to a lower extent then myoblasts (Fig. 7D). In contrast, doxorubicin activated Mdm2-luc and p21-luc in myoblasts but not in myotubes (Fig. 7D).
FIG. 7.
Doxorubicin treatment restores p53 phosphorylation on Ser18 in C2C12 myotubes and this correlates with p53-induced transcriptional activity and apoptosis. (A) C2C12-derived myoblasts (lanes 1 to 4) and myotubes (lanes 5 to 8) were either irradiated with 10 Gy (lanes 2 and 6) and collected after 2 h or treated with increasing doses of doxorubicin (0.5 and 1 μM) for 24 h (lanes 3, 4, 7, and 8). The immunoblotting analysis was performed with antibodies that recognize total p53, phospho-Ser15/18 p53, and tubulin. (B) Myoblasts (mb) and myotubes (mt) derived from C2C12 cells were either mock-treated (lanes 1 and 3) or treated with 1 μM doxorubicin (lanes 2 and 4); after total RNA isolation, samples were reverse transcribed and subjected to PCR to amplify the indicated p53 target genes. (C) C2C12 cells were grown to obtain myoblasts and myotubes, and cells were either mock-treated or treated with 1 μM doxorubicin for 24 h and then subjected to the TUNEL assay. (D) C2C12 cells stably transfected with Mdm2-luciferase, p21-luciferase, and Bax-luciferase reporter vectors were grown to obtain myoblasts and myotubes; cells were mock-treated or treated with 0.5 μM doxorubicin for 24 h, and luciferase activity was measured. Error bars represent statistical analysis of three independent experiments.
Taken together, these results indicate that anthracyclines can enforce p53-dependent activation of apoptotic genes in myotubes and suggest that terminally differentiated cells might have restricted the repertoire of genes activated by p53 to those involved in apoptotic responses.
Phosphorylation of p53 at Ser15(h)/18(m) contributes to doxorubicin-mediated apoptosis in myotubes.
The relative contribution of p53 Ser15(h)/18(m) phosphorylation to doxorubicin-induced apoptosis in myotubes was evaluated in two independent settings by exploiting the ability of doxorubicin to trigger apoptosis in myotubes derived from either primary satellite cells, wild-type, or p53 null mice (reconstituted with p53wt or the p53 Ser15ala mutant) (5) or MEFs from wild-type, p53 null, and p53 Ser18ala knock-in mice (15) and converted into muscle cells by MyoD.
Exposure to doxorubicin induced apoptosis in primary satellite myoblasts and myotubes derived from wild-type mice; in contrast, satellite cultures from p53 null mice showed partial resistance to doxorubicin-induced apoptosis (Table 1). Reintroduction of p53wt into p53 null satellite cells restored (and even increased) the apoptotic response to doxorubicin, while reconstitution with the p53 Ser15ala mutant only partially restored apoptosis in response to doxorubicin (Table 1). Notably, the levels of p53wt and the Ser15ala mutant in reconstituted cells were comparable, albeit twofold higher than the normal endogenous levels of p53 in satellite cells (data not shown).
TABLE 1.
Apoptotic response in doxorubicin-treated muscle cells is dictated by the phosphorylation status of p53a
| Satellite cells | Myoblasts (% apoptotic nuclei)
|
Myotubes (% apoptotic nuclei)
|
||
|---|---|---|---|---|
| Expt 1 | Expt 2 | Expt 1 | Expt 2 | |
| Wt | 9 | 11 | <2 | <2 |
| Wt + Dox | 39 | 45 | 25 | 23 |
| p53−/− | 7 | 10 | <2 | <2 |
| p53−/− + Dox | 17 | 20 | 11 | 14 |
| p53−/− + p53wt | 49 | 47 | 6 | 7 |
| p53−/− + p53wt + Dox | 74 | 79 | 55 | 53 |
| p53−/− + p53s15a | 26 | 32 | 5 | 4 |
| p53−/− + p53s15a + Dox | 35 | 44 | 26 | 31 |
Primary mouse satellite cells from wild-type (Wt) and p53 null mice were propagated in GM (myoblasts) or induced to differentiate by incubation in DM (myotubes). When indicated, proliferating satellite cells were transfected with cDNA coding for the human p53 gene, either wild type or the phosphorylation-resistant Ser15Ala point mutant, along with a GFP gene conjugated to a farnesylation signal that specifically localizes in the plasma membrane, to visualize productively transfected cells without interfering with the nuclear signal. Cells were then allowed to either proliferate or differentiate into myotubes and exposed or not to doxorubicin (Dox, 1 μM). After an additional 36 h, cells were fixed and stained for the TUNEL assay in order to score the extent of apoptosis, as determined by the percentage of apoptotic nuclei among GFP-positive cells.
To eliminate any experimental caveat relative to the levels of p53 in reconstituted cells, we generated myotubes from MEFs converted into myogenic cells by infection with adenovirus-expressed MyoD. Ectopic expression of MyoD converted MEFs of different genotypes with comparable efficiencies (data not shown). Myotubes derived from wild-type MEFs were permissive for apoptosis in response to doxorubicin, while myotubes derived from p53 null MEFs were refractory to doxorubicin-dependent apoptosis, and myotubes derived from p53 Ser18ala knock-in MEFs displayed an intermediate apoptotic response to doxorubicin (Table 2). Partial resistance to apoptosis was also observed in p53 null satellite myoblasts exposed to IR or to the radiomimetic bleomycin, and it was reversed by the reintroduction of p53wt but not the p53 Ser15ala mutant (data not shown). These results demonstrate that phosphorylation of p53 at Ser15(h)/18(m) provides a critical signal to trigger a full apoptotic response in myotubes exposed to doxorubicin.
TABLE 2.
Apoptotic response in doxorubicin-treated, MyoD-converted MEF cells is dictated by the phosphorylation status of p53a
| MyoD-converted myotubes | % Apoptotic nuclei
|
|
|---|---|---|
| Expt 1 | Expt 2 | |
| Wt | <2 | <2 |
| Wt + Dox | 46 | 43 |
| p53−/− | <2 | <2 |
| p53−/− + Dox | 16 | 16 |
| p53s18a | <2 | <2 |
| p53s18a + Dox | 22 | 26 |
MEFs from from wild-type (Wt), p53 null, and p53 Ser18Ala knock-in mice were converted into myogenic cells by infection with adenoviral MyoD and induced to differentiate into multinucleated myotubes by incubation in DM. After 72 h of incubation in DM, the resulting myotubes were exposed or not to doxorubicin (Dox, 1 μM). After an additional 36 h, cells were fixed and stained for the TUNEL assay in order to score the extent of apoptosis, as determined by the percentage of apoptotic nuclei in multinucleated myotubes positive for the expression of myosin heavy chain.
Induction of ATM autophosphorylation in response to DNA damage in both myoblasts and myotubes.
Phosphorylation of p53 at Ser 15(h)/18(m) in response to DNA damage can be catalyzed by at least three different kinases, depending on the type of DNA lesion. For instance, DSBs caused by IR activate ATM-dependent phosphorylation of p53 at Ser15(h)/18(m) (6). We began to elucidate the mechanism underlying myotube-associated radioresistance by analyzing the status of activation of ATM kinase in myoblasts and myotubes exposed to IR or to doxorubicin. As doxorubicin induces phosphorylation of p53 and apoptosis in myotubes (Fig. 7), it was instrumental to elucidate the molecular link between activation of ATM and p53-dependent apoptosis in myotubes.
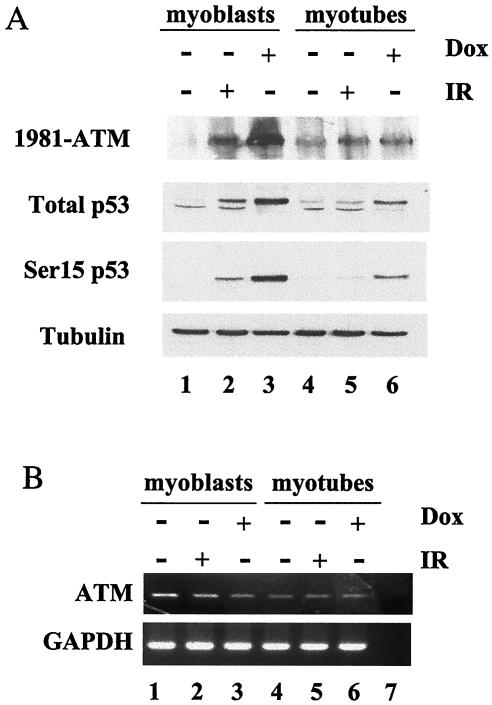
In response to DNA damage, ATM intermolecular autophosphorylation on Ser1981 triggers its kinase activity toward downstream substrates, and phosphorylated Ser1981 can be detected by specific antibodies (6). In untreated myoblasts, ATM autophosphorylation was undetectable (Fig. 8A, lane 1). Exposure to IR or doxorubicin caused ATM autophosphorylation, the magnitude of which correlated with the extent of p53 phosphorylation at Ser18; 1 μM doxorubicin induced more pronounced autophosphorylation of ATM and Ser18 phosphorylation of p53 than 0.5 μM doxorubicin (data not shown) or 10 Gy of IR (Fig. 8A, compare lanes 2 and 3). In contrast, myotubes displayed an increased basal level of autophosphorylated ATM (Fig. 8A, lane 4). Although these amounts of autophosphorylated ATM were comparable in myotubes exposed to IR (10 Gy) or treated with doxorubicin (1 μM) and only moderately reduced compared to irradiated myoblasts, these levels of ATM autophosphorylation were not sufficient to induce p53 Ser18 phosphorylation in irradiated myotubes despite being permissive for the induction of p53 phosphorylation on Ser18 in doxorubicin-treated myotubes (Fig. 8A, compare lanes 5 and 6).
FIG. 8.
DNA damage-activated autophosphorylation of ATM and p53 Ser15(h)/18(m) phosphorylation in myoblasts and myotubes. (A) C2C12-derived myoblasts (lanes 1 to 3) and myotubes (lanes 4 to 6) were either irradiated with 10 Gy (lanes 2 and 5) and collected after 2 h or treated with doxorubicin (1 μM) for 24 h (lanes 3 and 6). The immunoblotting analysis was performed with antibodies that recognize ATM autophosphorylated at Ser1981, total p53, phospho-Ser18 p53 (p53 Ser 15/18), and tubulin. (B) RNA was isolated from samples of the same experiments shown in panel A and, after reverse transcription, subjected to semiquantitative PCR in order to amplify ATM and glyceraldehhyde-3-phosphate dehydrogenase (GAPDH) (loading control) transcripts.
Immunofluorescence experiments showed that autophosphorylated ATM was localized in the nuclei of C2C12 myoblasts and myotubes, either treated or untreated, despite the observation in other experiments that total ATM could partly distribute in the cytoplasm of differentiated human myotubes (data not shown). We failed to detect total endogenous mouse ATM with any of the commercial antibodies that we used; however, we found by RT-PCR that the total RNA levels of ATM did not change in response to any of the genotoxic agents that we used in our experiments (Fig. 8B). These results indicate that, in differentiated myotubes, the impaired activation of p53 phosphorylation and apoptosis by IR (differentiation-associated radioresistance) does not reflect failure to activate ATM by autophosphorylation. The finding that doxorubicin activates p53 phosphorylation and apoptosis in myotubes despite inducing the same levels of ATM autophosphorylation observed after IR indicates that doxorubicin enforces this response by affecting a downstream or parallel pathway that converges on p53 and suggests that the differentiation-induced radioresistance lies downstream of ATM autophosphorylation.
DISCUSSION
Programmed cell death, as an ultimate response to severe genotoxic insults, is an efficient way to eliminate potentially hazardous, genetically unstable cells from cell populations in higher eukaryotes, including mammals. Consistent with their different proliferative potential for renewal and their distinct roles in development and tissue homeostasis, embryonal stem cells are the most sensitive to DNA damage-induced apoptosis; proliferating lineage-committed cells, such as myoblasts, are moderately sensitive; and terminally differentiated, nonproliferating cells (e.g., myotubes) are relatively resistant to genotoxic stresses such as IR. The molecular basis of these biologically important differences is poorly understood. Our present study provides novel mechanistic insights into the radioresistance of myotubes, a long-lived cell type with no regenerative potential, for which apoptosis represents a detrimental response that could eventually threaten the life of the whole organism.
We show that, like myoblasts, differentiated myotubes can respond to DSBs by activating a network of DNA damage sensors, signal transducers, and effectors whose impact eventually dictates the fate of the irradiated cells. Some of the key events of this ATM-regulated network (10, 46, 62), including the activating phosphorylation of ATM (6), ATM-dependent phosphorylation of histone H2AX, and the recruitment to DSB sites of MRE11 and Nbs1, two proteins involved in lesion processing, repair, and signaling (10, 14, 27, 29, 35, 46), occurred in both myoblasts and myotubes. Also, the activation of Chk2, the DNA damage-signaling kinase downstream of ATM, was similar in both cell types, albeit the kinetics of Chk2 activation was moderately impaired in myotubes. A striking difference among the ATM-mediated events was the lack of phosphorylation of Ser15(h)/18(m) of p53 in irradiated myotubes (this study), which is otherwise a prominent event that promotes cell cycle arrest or apoptosis in proliferating cells, including myoblasts (3, 15, 46, 51; this study). This ATM-mediated activating phosphorylation of p53 normally closely follows activation of ATM by various stimuli, including chromatin changes in the absence of DNA damage, when phosphorylation of lesion-associated factors such as H2AX is absent (6). Thus, our finding that ATM activation is uncoupled from p53 phosphorylation is very surprising. Together with our observation that the ATM-p53 axis operates normally in quiescent myoblasts, our data suggest the existence of a differentiation-associated mechanism that selectively silences the proapoptotic, p53-directed pathway downstream of ATM.
The barrier that prevents phosphorylation of p53 and apoptosis in myotubes could be overcome by treatment with the anthracycline derivative doxorubicin (this study), consistent with the well-known cardiotoxicity caused by this drug (4, 22, 47). Importantly, the ATM-p53 proapoptotic pathway was induced by doxorubicin even though the extent of ATM activation was the same as in IR-treated myotubes and, despite this level of ATM activation, was sufficient to phosphorylate two other ATM targets examined, histone H2AX and Chk2. Detailed mechanistic understanding of the selective blockade of p53 phosphorylation and how doxorubicin overcomes or bypasses this block are important goals for future studies. Conceptually, the selectivity of the ATM effects after IR is intriguing in the light of the different patterns of spatiotemporal redistribution of DNA damage-activated proteins (14, 27, 29, 35). Indeed, whereas H2AX (35) and Chk2 (27) are phosphorylated by ATM upon their interactions in the proximity of DSBs, phosphorylation of p53 does not appear to require its recruitment to the site of the DNA lesion (6, 27); rather, it occurs in the nucleoplasm of irradiated cells. These differential modes of phosphorylation by DSB-activated ATM suggest that myotube-associated radioresistance could be caused by a differentiation-induced block of functional interactions between ATM and its nucleoplasmic substrates (e.g., p53) despite the integrity of ATM interactions with targets that reside in the chromatin (e.g., H2AX) or require that at least transient interaction with ATM at DSB sites (e.g., Chk2) remain operational.
From a broader perspective, our study suggests the existence of hitherto unknown pathway-selective silencing that may shield some (here p53) but not other (such as H2AX) targets from being phosphorylated by ATM after irradiation of terminally differentiated cells. We propose that this selective channeling of the DNA damage-induced, ATM-mediated effects towards genome maintenance functions, such as lesion processing and repair by H2AX and MRE11/Nbs1 (29, 35, 46), may contribute to long-lasting genome integrity and proper function of differentiated tissues while avoiding tissue loss due to apoptosis. It may be rewarding to examine whether this or analogous mechanisms guard against DNA damage-induced cell death in other differentiated tissues as well. Further elucidation of the selective apoptosis-preventing mechanism reported here may help identify putative targets for intervention aimed at either counteracting muscle wasting or attenuating the cardiotoxicity induced by chemotherapy.
Acknowledgments
We thank K. H. Vousden, J. Petrini, S. Elledge, C Poizat, M. Crescenzi, and S. Soddu for providing critical reagents (antibodies, plasmids, adenoviruses, and probes) used in this work; Y. Xu kindly provided MEFs from p53wt, p53 null, and p53 Ser18ala knock-in mice; and M. Rudnicki kindly provided primary satellite cells from wild-type and p53 null mice. We are also grateful to J. Falck for stimulating discussion and M. Crescenzi, C. Poizat, and E. Borges for critically reading the manuscript.
This work was supported by the Danish Cancer Society, European Union, and Marie Curie Individual Fellowship QLGA-CT-2000-52052 (L.L.). C.S. is supported by a Telethon fellowship and the Sbarro Health Research Organization. P.L.P. was partially supported by an American Heart Association grant and a Compagnia San Paolo di Torino grant to Dulbecco Telethon Institute.
REFERENCES
- 1.Ahn, J. Y., J. K. Schwarz, H. Piwnica-Worms, and C. E. Canman. 2000. Threonine 68 phosphorylation by ataxia telangiectasia mutated is required for efficient activation of Chk2 in response to ionizing radiation. Cancer Res. 60:5934-5936. [PubMed] [Google Scholar]
- 2.Allen, D. M., H. van Praag, J. Ray, Z. Weaver, C. J. Winrow, T. A. Carter, R. Braquet, E. Harrington, T. Ried, K. D. Brown, F. H. Gage, and C. Barlow. 2001. Ataxia telangiectasia mutated is essential during adult neurogenesis. Genes Dev. 15:554-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Appella, E., and C. W. Anderson. 2001. Post-translational modifications and activation of p53 by genotoxic stresses. Eur. J. Biochem. 268:2764-2772. [DOI] [PubMed] [Google Scholar]
- 4.Arola, O. J., A. Saraste, K. Pulkki, M. Kallajoki, M. Parvinen, and L. M. Voipio-Pulkki. 2000. Acute doxorubicin cardiotoxicity involves cardiomyocyte apoptosis. Cancer Res. 60:1789-1792. [PubMed] [Google Scholar]
- 5.Ashcroft, M., M. H. Kubbutat, and K. H. Vousden. 1999. Regulation of p53 function and stability by phosphorylation. Mol. Cell. Biol. 19:1751-1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bakkenist, C. J., and M. B. Kastan. 2003. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 421:499-506. [DOI] [PubMed] [Google Scholar]
- 7.Barlow, C., K. D. Brown, C. X. Deng, D. A. Tagle, and A. Wynshaw-Boris. 1997. Atm selectively regulates distinct p53-dependent cell-cycle checkpoint and apoptotic pathways. Nat. Genet. 17:453-456. [DOI] [PubMed] [Google Scholar]
- 8.Barlow, C., S. Hirotsune, R. Paylor, M. Liyanage, M. Eckhaus, F. Collins, Y. Shiloh, J. N. Crawley, T. Ried, D. Tagle, and A. Wynshaw-Boris. 1996. Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell 86:159-171. [DOI] [PubMed] [Google Scholar]
- 9.Bartek, J., J. Falck, and J. Lukas. 2001. CHK2 kinase—a busy messenger. Nat. Rev. Mol. Cell. Biol. 2:877-886. [DOI] [PubMed] [Google Scholar]
- 10.Bartek, J., and J. Lukas. 2003. Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell 3:421-429. [DOI] [PubMed] [Google Scholar]
- 11.Bartek, J., and J. Lukas. 2001. Mammalian G1- and S-phase checkpoints in response to DNA damage. Curr. Opin. Cell Biol. 13:738-747. [DOI] [PubMed] [Google Scholar]
- 12.Bassing, C. H., K. F. Chua, J. Sekiguchi, H. Suh, S. R. Whitlow, J. C. Fleming, B. C. Monroe, D. N. Ciccone, C. Yan, K. Vlasakova, D. M. Livingston, D. O. Ferguson, R. Scully, and F. W. Alt. 2002. Increased ionizing radiation sensitivity and genomic instability in the absence of histone H2AX. Proc. Natl. Acad. Sci. USA 99:8173-8178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blasina, A., B. D. Price, G. A. Turenne, and C. H. McGowan. 1999. Caffeine inhibits the checkpoint kinase ATM. Curr. Biol. 9:1135-1138. [DOI] [PubMed] [Google Scholar]
- 14.Celeste, A., O. Fernandez-Capetillo, M. J. Kruhlak, D. R. Pilch, D. W. Staudt, A. Lee, R. F. Bonner, W. M. Bonner, and A. Nussenzweig. 2003. Histone H2AX phosphorylation is dispensable for the initial recognition of DNA breaks. Nat. Cell Biol. 7:675-679. [DOI] [PubMed]
- 15.Chao, C., M. Hergenhahn, M. D. Kaeser, Z. Wu, S. Saito, R. Iggo, M. Hollstein, E. Appella, and Y. Xu. 2003. Cell type- and promoter-specific roles of Ser18 phosphorylation in regulating p53 responses. J. Biol. Chem. 278:41028-41033. [DOI] [PubMed] [Google Scholar]
- 16.Chaturvedi, P., W. K. Eng, Y. Zhu, M. R. Mattern, R. Mishra, M. R. Hurle, X. Zhang, R. S. Annan, Q. Lu, L. F. Faucette, G. F. Scott, X. Li, S. A. Carr, R. K. Johnson, J. D. Winkler, and B. B. Zhou. 1999. Mammalian Chk2 is a downstream effector of the ATM-dependent DNA damage checkpoint pathway. Oncogene 18:4047-4054. [DOI] [PubMed] [Google Scholar]
- 17.Costanzo, A., P. Merlo, N. Pediconi, M. Fulco, V. Sartorelli, P. A. Cole, G. Fontemaggi, M. Fanciulli, L. Schiltz, G. Blandino, C. Balsano, and M. Levrero. 2002. DNA damage-dependent acetylation of p73 dictates the selective activation of apoptotic target genes. Mol. Cell 9:175-186. [DOI] [PubMed] [Google Scholar]
- 18.Dasika, G. K., S. C. Lin, S. Zhao, P. Sung, A. Tomkinson, and E. Y. Lee. 1999. DNA damage-induced cell cycle checkpoints and DNA strand break repair in development and tumorigenesis. Oncogene 18:7883-7899. [DOI] [PubMed] [Google Scholar]
- 19.Falck, J., N. Mailand, R. G. Syljuasen, J. Bartek, and J. Lukas. 2001. The ATM-Chk2-Cdc25A checkpoint pathway guards against radioresistant DNA synthesis. Nature 410:842-847. [DOI] [PubMed] [Google Scholar]
- 20.Hanawalt, P. C. 2002. Subpathways of nucleotide excision repair and their regulation. Oncogene 21:8949-8956. [DOI] [PubMed] [Google Scholar]
- 21.Hartwell, L. H., and T. A. Weinert. 1989. Checkpoints: controls that ensure the order of cell cycle events. Science 246:629-634. [DOI] [PubMed] [Google Scholar]
- 22.Kalyanaraman, B., J. Joseph, S. Kalivendi, S. Wang, E. Konorev, and S. Kotamraju. 2002. Doxorubicin-induced apoptosis: implications in cardiotoxicity. Mol. Cell. Biochem. 234-235:119-124. [PubMed] [Google Scholar]
- 23.Lassar, A., and A. Munsterberg. 1994. Wiring diagrams: regulatory circuits and the control of skeletal myogenesis. Curr. Opin. Cell Biol. 6:432-442. [DOI] [PubMed] [Google Scholar]
- 24.Lee, Y., M. J. Chong, and P. J. McKinnon. 2001. Ataxia telangiectasia mutated-dependent apoptosis after genotoxic stress in the developing nervous system is determined by cellular differentiation status. J. Neurosci. 21:6687-6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leibovitch, S. A., M. P. Leibovitch, J. Hillion, J. Kruh, and J. Harel. 1984. Alpha actin gene exist in an active structural configuration in the proliferating myoblasts as well as in differentiated myotubes of the L6 line. Biochem. Biophys. Res. Commun. 119:630-639. [DOI] [PubMed] [Google Scholar]
- 26.Lukas, C., J. Bartkova, L. Latella, J. Falck, N. Mailand, T. Schroeder, M. Sehested, J. Lukas, and J. Bartek. 2001. DNA damage-activated kinase Chk2 is independent of proliferation or differentiation yet correlates with tissue biology. Cancer Res. 61:4990-4993. [PubMed] [Google Scholar]
- 27.Lukas, C., J. Falck, J. Bartkova, J. Bartek, and J. Lukas. 2003. Distinct spatiotemporal dynamics of mammalian checkpoint regulators induced by DNA damage. Nat. Cell Biol. 5:255-260. [DOI] [PubMed] [Google Scholar]
- 28.Macleod, K. F., N. Sherry, G. Hannon, D. Beach, T. Tokino, K. Kinzler, B. Vogelstein, and T. Jacks. 1995. p53-dependent and independent expression of p21 during cell growth, differentiation, and DNA damage. Genes Dev. 9:935-944. [DOI] [PubMed] [Google Scholar]
- 29.Maser, R. S., K. J. Monsen, B. E. Nelms, and J. H. Petrini. 1997. hMre11 and hRad50 nuclear foci are induced during the normal cellular response to DNA double-strand breaks. Mol. Cell. Biol. 17:6087-6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsuoka, S., M. Huang, and S. J. Elledge. 1998. Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science 282:1893-1897. [DOI] [PubMed] [Google Scholar]
- 31.Matsuoka, S., G. Rotman, A. Ogawa, Y. Shiloh, K. Tamai, and S. J. Elledge. 2000. Ataxia telangiectasia-mutated phosphorylates Chk2 in vivo and in vitro. Proc. Natl. Acad. Sci. USA 97:10389-10394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Melchionna, R., X. B. Chen, A. Blasina, and C. H. McGowan. 2000. Threonine 68 is required for radiation-induced phosphorylation and activation of Cds1. Nat. Cell Biol. 2:762-765. [DOI] [PubMed] [Google Scholar]
- 33.Nouspikel, T., and P. C. Hanawalt. 2002. DNA repair in terminally differentiated cells. DNA Repair (Amsterdam) 1:59-75. [DOI] [PubMed] [Google Scholar]
- 34.Parker, S. B., G. Eichele, P. Zhang, A. Rawls, A. T. Sands, A. Bradley, E. N. Olson, J. W. Harper, and S. J. Elledge. 1995. p53-independent expression of p21Cip1 in muscle and other terminally differentiating cells. Science 267:1024-1027. [DOI] [PubMed] [Google Scholar]
- 35.Paull, T. T., E. P. Rogakou, V. Yamazaki, C. U. Kirchgessner, M. Gellert, and W. M. Bonner. 2000. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr. Biol. 10:886-895. [DOI] [PubMed] [Google Scholar]
- 36.Polesskaya, A., and M. A. Rudnicki. 2002. A MyoD-dependent differentiation checkpoint: ensuring genome integrity. Dev. Cell 3:757-758. [DOI] [PubMed] [Google Scholar]
- 37.Porrello, A., M. A. Cerone, S. Coen, A. Gurtner, G. Fontemaggi, L. Cimino, G. Piaggio, A. Sacchi, and S. Soddu. 2000. p53 regulates myogenesis by triggering the differentiation activity of pRb. J. Cell Biol. 151:1295-1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prives, C. 1998. Signaling to p53: breaking the MDM2-p53 circuit. Cell 95:5-8. [DOI] [PubMed] [Google Scholar]
- 39.Puri, P. L., C. Balsano, V. L. Burgio, P. Chirillo, G. Natoli, L. Ricci, E. Mattei, A. Graessmann, and M. Levrero. 1997. MyoD prevents cyclinA/cdk2 containing E2F complexes formation in terminally differentiated myocytes. Oncogene 14:1171-1184. [DOI] [PubMed] [Google Scholar]
- 40.Puri, P. L., K. Bhakta, L. D. Wood, A. Costanzo, J. Zhu, and J. Y. Wang. 2002. A myogenic differentiation checkpoint activated by genotoxic stress. Nat. Genet. 32:585-593. [DOI] [PubMed] [Google Scholar]
- 41.Puri, P. L., and V. Sartorelli. 2000. Regulation of muscle regulatory factors by DNA-binding, interacting proteins, and post-transcriptional modifications. J. Cell. Physiol. 185:155-173. [DOI] [PubMed] [Google Scholar]
- 42.Rich, T., R. L. Allen, and A. H. Wyllie. 2000. Defying death after DNA damage. Nature 407:777-783. [DOI] [PubMed] [Google Scholar]
- 43.Sarkaria, J. N., E. C. Busby, R. S. Tibbetts, P. Roos, Y. Taya, L. M. Karnitz, and R. T. Abraham. 1999. Inhibition of ATM and ATR kinase activities by the radiosensitizing agent, caffeine. Cancer Res. 59:4375-4382. [PubMed] [Google Scholar]
- 44.Schlegel, R., and A. B. Pardee. 1986. Caffeine-induced uncoupling of mitosis from the completion of DNA replication in mammalian cells. Science 232:1264-1266. [DOI] [PubMed] [Google Scholar]
- 45.Sheikh, M. S., M. Garcia, Q. Zhan, Y. Liu, and A. J. Fornace, Jr. 1996. Cell cycle-independent regulation of p21Waf1/Cip1 and retinoblastoma protein during okadaic acid-induced apoptosis is coupled with induction of Bax protein in human breast carcinoma cells. Cell Growth Differ. 7:1599-1607. [PubMed] [Google Scholar]
- 46.Shiloh, Y. 2003. ATM and related protein kinases: safeguarding genome integrity. Nat. Rev. Cancer 3:155-168. [DOI] [PubMed] [Google Scholar]
- 47.Simbre, I. V., M. J. Adams, S. S. Deshpande, S. A. Duffy, T. L. Miller, and S. E. Lipshultz. 2001. Cardiomyopathy caused by antineoplastic therapies. Curr. Treatment Options Cardiovasc. Med. 3:493-505. [DOI] [PubMed]
- 48.Steinmann, K. E., G. S. Belinsky, D. Lee, and R. Schlegel. 1991. Chemically induced premature mitosis: differential response in rodent and human cells and the relationship to cyclin B synthesis and p34cdc2/cyclin B complex formation. Proc. Natl. Acad. Sci. USA 88:6843-6847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takai, H., K. Naka, Y. Okada, M. Watanabe, N. Harada, S. Saito, C. W. Anderson, E. Appella, M. Nakanishi, H. Suzuki, K. Nagashima, H. Sawa, K. Ikeda, and N. Motoyama. 2002. Chk2-deficient mice exhibit radioresistance and defective p53-mediated transcription. EMBO J. 21:5195-5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vinson, R. K., and B. F. Hales. 2002. DNA repair during organogenesis. Mutat. Res. 509:79-91. [DOI] [PubMed] [Google Scholar]
- 51.Wahl, G. M., and A. M. Carr. 2001. The evolution of diverse biological responses to DNA damage: insights from yeast and p53. Nat. Cell Biol. 3:E277-E286. [DOI] [PubMed] [Google Scholar]
- 52.Walsh, K. 1997. Coordinate regulation of cell cycle and apoptosis during myogenesis. Prog. Cell Cycle Res. 3:53-58. [DOI] [PubMed] [Google Scholar]
- 53.Walsh, K., and H. Perlman. 1997. Cell cycle exit upon myogenic differentiation. Curr. Opin. Genet. Dev. 7:597-602. [DOI] [PubMed] [Google Scholar]
- 54.Wang, J. Y. 1998. Cellular responses to DNA damage. Curr. Opin. Cell Biol. 10:240-247. [DOI] [PubMed] [Google Scholar]
- 55.Wang, J. Y., and S. W. Ki. 2001. Choosing between growth arrest and apoptosis through the retinoblastoma tumour suppressor protein, Abl and p73. Biochem. Soc. Trans. 29:666-673. [DOI] [PubMed] [Google Scholar]
- 56.Ward, I. M., and J. Chen. 2001. Histone H2AX is phosphorylated in an ATR-dependent manner in response to replicational stress. J. Biol. Chem. 276:47759-47762. [DOI] [PubMed] [Google Scholar]
- 57.Weinert, T. 1998. DNA damage and checkpoint pathways: molecular anatomy and interactions with repair. Cell 94:555-558. [DOI] [PubMed] [Google Scholar]
- 58.Xu, Y. 1999. ATM in lymphoid development and tumorigenesis. Adv. Immunol. 72:179-189. [DOI] [PubMed] [Google Scholar]
- 59.Xu, Y., and D. Baltimore. 1996. Dual roles of ATM in the cellular response to radiation and in cell growth control. Genes Dev. 10:2401-2410. [DOI] [PubMed] [Google Scholar]
- 60.Zhang, J., J. R. Clark, Jr., E. H. Herman, and V. J. Ferrans. 1996. Doxorubicin-induced apoptosis in spontaneously hypertensive rats: differential effects in heart, kidney and intestine, and inhibition by ICRF-187. J. Mol. Cell. Cardiol. 28:1931-1943. [DOI] [PubMed] [Google Scholar]
- 61.Zhou, B. B., P. Chaturvedi, K. Spring, S. P. Scott, R. A. Johanson, R. Mishra, M. R. Mattern, J. D. Winkler, and K. K. Khanna. 2000. Caffeine abolishes the mammalian G(2)/M DNA damage checkpoint by inhibiting ataxia-telangiectasia-mutated kinase activity. J. Biol. Chem. 275:10342-10348. [DOI] [PubMed] [Google Scholar]
- 62.Zhou, B. B., and S. J. Elledge. 2000. The DNA damage response: putting checkpoints in perspective. Nature 408:433-439. [DOI] [PubMed] [Google Scholar]