Abstract
Background
Hyperglycemia, a characteristic feature of diabetes, induces glucotoxicity in pancreatic β-cells, resulting in further impairment of insulin secretion and worsening glycemic control. Thus, preservation of insulin secretory capacity is essential for the management of type 2 diabetes. In this study, we evaluated the ability of an Orthosiphon stamineus (OS) extract to prevent glucotoxicity in insulin-producing cells.
Methods
We measured insulin mRNA expression and glucose-stimulated insulin secretion (GSIS) in OS-treated INS-1 cells after exposure to a high glucose (HG; 30 mM) concentration.
Results
The hexane extract of OS elevated mRNA expression of insulin as well as pancreatic and duodenal homeobox-1 of INS-1 cells in a dose-dependent manner. The hexane OS extract also increased the levels of phosphorylated phosphatidylinositol 3-kinase (PI3K) in a concentration-dependent manner. Additionally, Akt phosphorylation was elevated by treatment with 100 and 200 µmol of the hexane OS extract. Three days of HG exposure suppressed insulin mRNA expression and GSIS; these expressions were restored by treatment with the hexane OS extract. HG elevated peroxide levels in the INS-1 cells. These levels were unaffected by OS treatment under both normal and hyperglycemic conditions.
Conclusion
Our results suggested that the hexane extract of OS elevates insulin mRNA expression and prevents glucotoxicity induced by a 3-day treatment with HG. This was associated with the activation of PI-3K and Akt.
Keywords: Glucose-stimulated insulin secretion, Insulin mRNA; Glucotoxicity; Orthosiphon stamineus
INTRODUCTION
Hyperglycemia is a key pathologic feature of type 2 diabetes that mainly results from insulin resistance and pancreatic β-cell dysfunction. However, insulin resistance alone does not induce hyperglycemia if compensatory insulin secretion is maintained. When insulin secretion is not sufficient to overcome insulin resistance, hyperglycemia develops. Moreover, elevated glucose concentrations worsen defective insulin secretion. This condition is known as "glucotoxicity" [1,2,3,4]. Thus, β-cell preservation is essential for the prevention and management of type 2 diabetes.
Insulin is secreted through an exocytotic process that releases insulin granules following the influx of calcium ions through voltage-dependent calcium channels. This results from the closure of ATP-sensitive potassium channels [5] or the activation of the phospholipase C/phosphatidyl 4,5-biphosphate/diacylglycerol/protein kinase C pathway in response to glucose or other insulin secretagogues [6,7,8]. Additionally, insulin gene expression is stimulated by glucose or signaling molecules through the activation of transcriptional factors, such as pancreatic duodenal homeobox-1 (PDX-1) and musculoaponeurotic fibrosarcoma oncogene homolog A (MafA) [9]. The expressions of PDX-1 and MafA may be associated with the phosphatidylinositol 3-kinase (PI3K)/Akt pathway in insulin-producing cells [10,11]. In general, chronic hyperglycemia suppresses both insulin mRNA expression and glucose-induced insulin secretion [4]. Thus, protecting β-cells from glucotoxicity can preserve both insulin production and secretion.
Orthosiphon stamineus (OS) has been used as a traditional medicinal herb in Southeast Asian countries. Tea made from the leaves and roots of this plant is believed to ameliorate various pathologic conditions, such as rheumatic arthritis, diabetes, hypertension, tonsillitis, epilepsy, menstrual disorders, gonorrhea, syphilis, renal calculus, and urinary lithiasis [12,13]. Recently, other beneficial effects were reported, including antidiabetic, anti-inflammatory, antiproliferative, and antiangiogenic activities [14,15,16,17,18]. Moreover, several previous reports have provided evidence for the metabolic effects of OS. Sriplang et al. [14] found that OS increases insulin secretion in perfused rat pancreas. Furthermore, Son et al. [19] reported that crude OS extract elevates plasma levels of insulin in rats, while Choi et al. [20] demonstrated the leptin stimulating effect of OS both in vitro and in vivo. Mohamed et al. [18] also showed that OS exerts antidiabetic effects in streptozotocin (STZ)-diabetic rats. However, we still do not know whether OS stimulates insulin production or protects β-cell from glucotoxicity. The purpose of the current investigation was to determine (1) whether OS elevates insulin secretion and/or production; (2) whether OS prevents glucotoxicity in vitro; and (3) which mechanisms influence these effects in insulin secreting INS-1 cells.
METHODS
INS-1 cell culture
Roswell Park Memorial Institute medium (RPMI)-1640 medium, antibiotics, and trypsin-ethylenediaminetetraacetic acid (EDTA) were purchased from Gibco (Grand Island, NY, USA). Other reagents for cell culturing were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). INS-1 cells from rat insulinoma were grown in RPMI-1640 medium containing 11.1 mM pyruvate, 10 mM 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES), 50 µM 2-mercaptoethanol, 100 U/mL penicillin, and 100 g/mL streptomycin in 5% CO2-95% air at 37℃. The RPMI-1640 medium used for all of the experiments contained the supplements noted above. All studies were performed using the INS-1 cells between passages 21 and 29. For the screening of the stimulating effect of OS in insulin mRNA expression, we treated various OS extracts for 12 hours. We found that the hexane extract of OS stimulated insulin mRNA expression. Glucotoxicity was induced by treatment with high glucose (HG; 30 mM) levels for 3 days and was confirmed by observing impaired glucose-stimulated insulin secretion (GSIS) in the INS-1 cells. The INS-1 cells cultured at HG or at the normoglycemic condition were treated with the hexane OS extract (200 µM) for 12 hours and then harvested to measure insulin mRNA expression, insulin secretory capacity, and reactive oxygen species (ROS) levels. The signaling proteins (PI3K and Akt) were analyzed after 1 hour of treatment with the hexane OS extract at 0, 50, 100, or 200 µM concentrations in the INS-1 cells cultured at a normal glucose condition.
Production of OS extract
Dried OS leaves imported from Indonesia were kindly donated by Dongbang FTL Co. (Seoul, Korea). A voucher specimen (OS201103) has been deposited at the Yeungnam University College of Pharmacy (Gyeongsan, Korea). Dried OS powder (78.6 g) was subjected to extraction with 70% ethanol by reflux for 12 hours as previously described by Choi et al. [20] The ethanol solution was then completely evaporated. The ethanol extract residue (8.8 g) was suspended in H2O (1.5 L), and the H2O layer was partitioned with n-hexane, ethylacetate, and n-butanol (each 1.5 L×3). The resulting four extracts were completely evaporated to recover the n-hexane (445 mg), H2O (2,409 mg), n-butanol (934.3 mg), and ethylacetate (1,175 mg) fractions.
Glucose stimulated insulin secretion test
The INS-1 cells were incubated in Krebs-Ringer buffer (KRB; 118 mmol/L NaCl, 4.7 mmol/L KCl, 2.5 mmol/L CaCl2, 1.18 mmol/L KH2PO4, 1.18 mmol/L MgSO4, 25 mmol/L NaHCO3, 10 mmol/L HEPES; and 0.1% bovine serum albumin (BSA, pH 7.4) that contained either normal (5.6 mM) or stimulatory (16.7 mM) concentrations of glucose for 2 hours. The incubation period was terminated by adding 1 mL of an ethanol:hydrochloric acid (95:5, v:v) solution, and the KRB was collected. Insulin levels in the buffer samples were measured with an enzyme-linked immunosorbent assay (rat insulin-specific ELISA kit; Mercodia, Uppsala, Sweden) according to the manufacturer's protocol.
Real time-polymerase chain reaction
Total RNA was obtained from the INS-1 cells using Trizol reagent (Bio Science Technology, Daejeon, Korea). cDNA was synthesized using 1 µg of total RNA with oligo-(dT) primers (Bioneer, Daejeon, Korea) and Prime RT Premix (GeNet Bio, Seoul, Korea). Real time-polymerase chain reaction was performed with a LightCycler (Roche, Mannheim, Germany) as previously described [20]. The following primers were used: 5'-ACC CAA GTC CCG TCG TGA AGT-3' (forward) and 5'-CCA GTT GGT AGA GGG AGC AGA TG-3' (reverse) for insulin, 5'-GGC TTA ACC TAA ACG CCA CA-3' (forward) and 5'-GGG ACC GTC CAA GTT TGT AA-3' (reverse) for PDX-1, and 5'-TAC TGC CCT GGC TCC TAG CA-3' (forward) and 5'-TGG ACA GTG AGG CCA GGA TAG-3' (reverse) for β-actin.
Evaluation of ROS production with flow cytometry
The intracellular peroxide levels were measured by flow cytometry using an oxidation-sensitive fluorescein-labeled dye, carboxylated dichlorodi-hydrofluorescein diacetate (carboxy-H2DCFDA; Molecular Probes, Carlsbad, CA, USA). The non-fluorescent dye is converted into a fluorescent form through an oxidative process by intracellular ROS. The INS-1 cells were labeled with 100 M carboxy-H2DCFDA for 1 hour at 37℃. After loading with the dye, the cells were washed twice with phosphate buffered saline (PBS) and further incubated for 2 hours. The INS-1 cells were then harvested, washed twice with PBS, and re-suspended in trypsin-EDTA (0.25% trypsin and 2 mM Na4-EDTA) for 5 minutes at 37℃. To disperse the cells in a single suspension, the INS-1 cells were gently passed 20 times in and out of a 1-mL pipette tip. The cells were then washed twice with ice-cold PBS and analyzed with a 488-nm argon laser EPICS XL-MCL flow cytometer with EXPO 32-ADC software (Beckman Coulter, Fullerton, CA, USA). The ROS concentrations were measured based on fluorescence intensity.
Western blotting
Proteins (30 µg) in an INS-1 cell lysate were separated in 10% polyacylamide gels and were transferred onto nitrocellulose membranes. The levels of phosphorylated PI3K, p-Akt, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were measured by incubating the blots with primary antibodies specific for these factors (1:1,000 dilution). Antibodies for p-PI3K and GAPDH were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and the anti-p-Akt antibody was obtained from Cell Signaling Technology (Danvers, MA, USA). The secondary horseradish peroxidase-conjugated anti-rabbit antibody (1:2,000 dilution) was used to detect primary antibody binding. The blots were developed with an enhanced chemiluminescence reagent (Amersham Biosciences, Little Chalfont, UK), and band intensities were quantified using Multi Gauge software (Fujifilm, Tokyo, Japan).
Data analysis
All of the results are expressed as the mean±standard error. One-way analysis of variance was used for comparisons of dose-dependent changes. Post hoc comparisons were performed using Duncan's test. Statistical comparisons of insulin mRNA expression and peroxide level between the control and OS-treated groups were evaluated by Student t-test. Values of P<0.05 were considered statistically significant.
RESULTS
Insulin mRNA expression and OS extracts
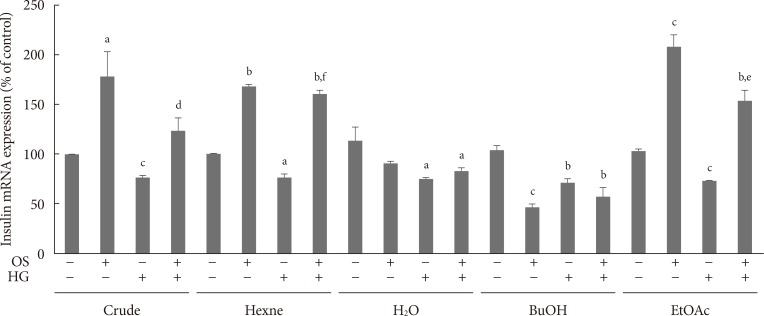
Crude OS extract elevated insulin mRNA expression in the INS-1 cells under both normal and HG conditions. Furthermore, the hexane and ethylacetate fractions elevated insulin mRNA expression in the INS-1 cells under normal and HG conditions. In contrast, the water and butanol OS fractions failed to affect insulin mRNA expression (Fig. 1). The hexane OS extract elevated insulin and PDX-1 mRNA levels in a dose-dependent manner in the INS-1 cells (Fig. 2).
Fig. 1. The effects of various fractions of Orthosiphon stamineus (OS) extract on insulin mRNA expression in INS-1 cells under normal and hyperglycemic (exposure to high glucose [HG] for 3 days) conditions. The cells were treated with each OS extract (200 µM) for 12 hours. Bars represent the mean±standard error of three separate experiments. BuOH, n-butanol; EtOAc, ethylacetate. aP<0.05, bP<0.01, and cP<0.001 versus the untreated cells under normal conditions, dP<0.05, eP<0.01, and fP<0.001 versus the untreated HG control.
Fig. 2. The effects of various concentrations of Orthosiphon stamineus (OS) extract on the mRNA expression of (A) insulin and (B) pancreatic and duodenal homeobox-1 (PDX-1) in INS-1 cells. Cells were treated with the hexane OS extract at concentrations of 0, 50, 100, and 200 µM for 12 hours. Bars represent the mean±standard error of three separate experiments.
a,b,cValues that do not share a common superscript are significantly different at P<0.05.
Glucotoxicity protecting effect of the OS extract
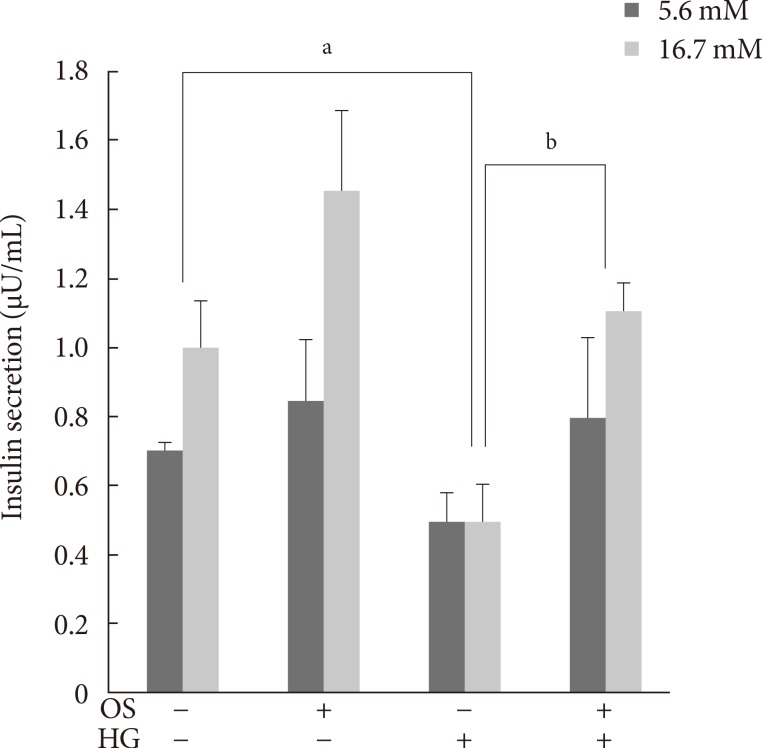
Three days of HG completely suppressed GSIS, indicating that glucotoxicity had been induced in the INS-1 cells. The hexane OS extract (200 µM) protected the INS-1 cells from glucotoxicity. Moreover, treatment with the hexane extract slightly elevated insulin secretion in both the basal and glucose-stimulated states (Fig. 3).
Fig. 3. Effect of the hexane Orthosiphon stamineus (OS) extract on glucose-stimulated insulin secretion (GSIS) in INS-1 cells. OS extract treatment (200 µM for 12 hours) restored GSIS that was completely suppressed by exposure to high glucose (HG) for 3 days. Bars represent the mean±standard error of three separate experiments. aP<0.05, bP<0.01.
OS extract and phosphorylation of PI3K and Akt
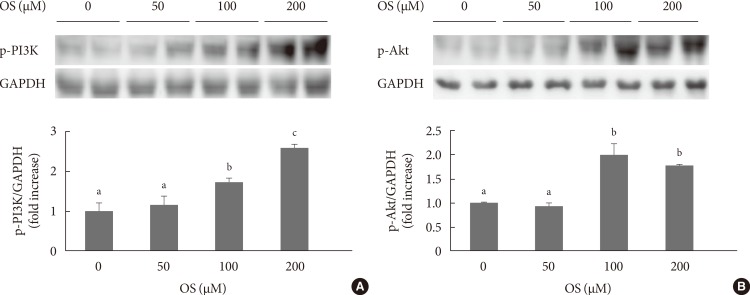
To investigate possible mechanisms underlying the induction of insulin expression by the hexane OS extract, we evaluated signaling molecules that influence insulin expression. The hexane OS extract increased p-PI3K levels in a dose-dependent manner. Akt phosphorylation was also increased by treatment with 100 and 200 µmoL of the OS extract (Fig. 4).
Fig. 4. The effects of various doses of Orthosiphon stamineus (OS) extract on (A) phosphatidylinositol 3-kinase (PI3K) and (B) Akt phosphorylation in INS-1 cells. Cells were treated with the hexane OS extract at concentrations of 0, 50, 100, and 200 µM for 12 hours. Bars represent the mean±standard error of three separate experiments. GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
a,b,cValues that do not share a common superscript are significantly different at P<0.05.
OS extract and oxidative stress
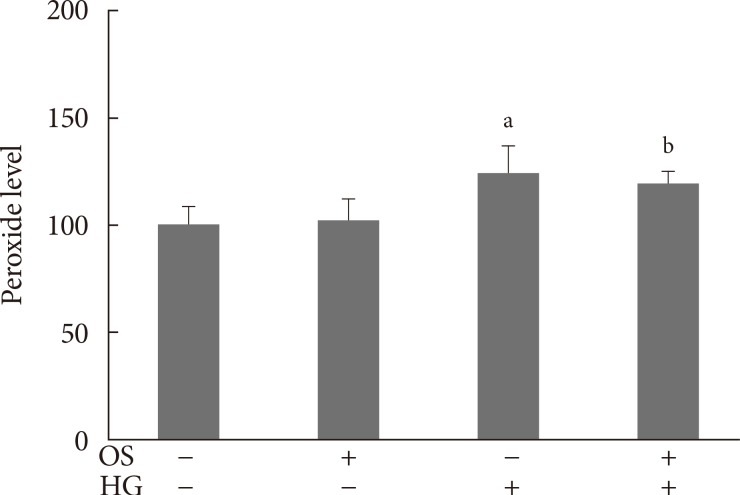
To determine whether the ability of the OS extract to protect β-cells is mediated by changes in oxidative stress, we measured the cellular peroxide levels. HG elevated the peroxide concentrations in the INS-1 cells. Treatment with the OS extract did not affect the levels in the cells under either normal or hyperglycemic conditions (Fig. 5).
Fig. 5. Effects of the hexane Orthosiphon stamineus (OS) extract on intracellular peroxide levels in INS-1 cells under normal and high glucose (HG) conditions. Cells were treated with 200 µM of the hexane extract for 12 hours. Bars represent the mean±standard error of three separate experiments. aP<0.01, bP<0.001 versus the untreated cells cultured under normal conditions.
DISCUSSION
In the present study, we demonstrated that treatment with the hexane OS extract elevated insulin mRNA expression in INS-1 cells. Additionally, the extract prevented glucotoxicity induced by HG exposure. These beneficial effects may be mediated by the activation of PI3K, Akt, and PDX-1.
Previous studies demonstrated that chronic HG impairs the insulin secretory capacity as well as insulin gene expression [4,21,22]. These findings are consistent with the results of our study, indicating that HG treatment suppressed both insulin gene expression and GSIS. Therefore, preservation of β-cell function by maintaining insulin gene expression is essential for treating type 2 diabetes. In the present investigation, we wanted to evaluate the ability of OS to protect β-cells from glucotoxicity induced by HG exposure in vitro.
Previous studies have demonstrated that OS extract stimulates insulin secretion in vivo and ex vivo [14,19]. In the present investigation, we demonstrated that the crude OS extract induces insulin mRNA expression in INS-1 cells. To determine which components of the crude OS extract are responsible for the stimulation of insulin production, we performed a fractionation. Our data indicated that the ethylacetate and hexane fractions elevated insulin mRNA expression under normal and HG conditions for 12 hours. The ethylacetate extract induced cell death when the INS-1 cells were cultured with HG levels for 3 days (data not shown). We subsequently evaluated the ability of the hexane OS extract to preserve β-cell function. The hexane OS extract stimulated insulin mRNA expression as well as insulin secretion. Moreover, the extract protected β-cells from glucotoxicity induced by HG exposure. In contrast, Mohamed et al. [18] demonstrated that the antidiabetic effect of OS is associated with the inhibition of intestinal glucose absorption and the elevation of diaphragm glucose uptake rather than the stimulation of insulin secretion. This difference may have resulted from different experimental settings. They conducted their experiment with STZ-diabetic rats that develop defective insulin secretion because their pancreatic islets are destroyed by STZ injection.
To evaluate the mechanism underlying the effect of OS on insulin mRNA expression, we next analyzed the expression and activation of several signaling molecules. There are several transcriptional factors that control insulin mRNA expression, such as PDX-1, neurogenic differentiation 1 (NeuroD1), and MafA. Among these, PDX-1 is a key factor that controls insulin gene expression [23,24]. It is also well known that Akt activates PDX-1 in insulin-producing cells [11,25,26]. Moreover, Akt deletion results in defective insulin secretion from pancreatic islet β-cells [27]. PI3K also affects PDX-1 expression in insulin-producing cells [11,28] According to Glauser and Schlegel [10], signals from the insulin receptor, insulin-like growth factor receptor, or epidermal growth factor receptor activate PI3K, which in turn activates Akt, forkhead box protein O, and PDX-1 in insulin-producing cells. We therefore evaluated the involvement of PI3K and Akt in the ability of OS to enhance insulin mRNA expression. We demonstrated that PDX-1, PI3K, and Akt are activated following treatment with the OS extracts.
Another possible mechanism underlying β-cell preservation by OS is the antioxidant activity of the extracts [29]. It is well known that oxidative stress is an important cause of glucotoxicity [1,2,3,4] and the treatment with antioxidants ameliorates glucotoxicity [2,30]. It was also reported that OS has an antioxidant effect on human mononuclear cells [31] and in an in vitro model system [32]. Furthermore, polyphenol (a major component of OS) extracted from natural products protects against cytokine-induced β-cell damage by maintaining redox homeostasis [33]. However, OS did not affect the intracellular peroxide levels of the INS1 cells in the present study under either normal or HG conditions. Although we could not explain why the hexane OS extract did not produce any antioxidant effects in our study, the results of the current investigation suggest that the hexane extract contains an insulin secretagogue.
Several groups discovered that OS contains physiologically active compounds, such as terpenoids, polyphenols, sterols, orthosiphols, saponins, flavonoids, caffeic acid, oleanolic acid, and rosmarinic acid [12,34,35]. However, it is not yet known which compound is responsible for stimulating insulin secretion. Further study is needed to identify the biologically active compounds in OS.
In summary, the results of our study demonstrated that hexane OS extract elevates insulin mRNA expression. Additionally, the extract conferred protection against glucotoxicity induced by a 3-day exposure to HG concentrations. These effects may be associated with the activation of PI-3K and Akt. Further studies are required to identify the active compounds responsible for the beneficial activities of OS.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev. 2008;29:351–366. doi: 10.1210/er.2007-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Won KC, Moon JS, Eun MJ, Yoon JS, Chun KA, Cho IH, Kim YW, Lee HW. A protective role for heme oxygenase-1 in INS-1 cells and rat islets that are exposed to high glucose conditions. J Korean Med Sci. 2006;21:418–424. doi: 10.3346/jkms.2006.21.3.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poitout V, Robertson RP. Minireview: secondary beta-cell failure in type 2 diabetes: a convergence of glucotoxicity and lipotoxicity. Endocrinology. 2002;143:339–342. doi: 10.1210/endo.143.2.8623. [DOI] [PubMed] [Google Scholar]
- 4.Kim YW, Moon JS, Seo YJ, Park SY, Kim JY, Yoon JS, Lee IK, Lee HW, Won KC. Inhibition of fatty acid translocase cluster determinant 36 (CD36), stimulated by hyperglycemia, prevents glucotoxicity in INS-1 cells. Biochem Biophys Res Commun. 2012;420:462–466. doi: 10.1016/j.bbrc.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 5.Ashcroft FM, Gribble FM. ATP-sensitive K+ channels and insulin secretion: their role in health and disease. Diabetologia. 1999;42:903–919. doi: 10.1007/s001250051247. [DOI] [PubMed] [Google Scholar]
- 6.Zawalich WS, Zawalich KC, Kelley GG. Regulation of insulin release by phospholipase C activation in mouse islets: differential effects of glucose and neurohumoral stimulation. Endocrinology. 1995;136:4903–4909. doi: 10.1210/endo.136.11.7588223. [DOI] [PubMed] [Google Scholar]
- 7.Persaud SJ, Jones PM, Howell SL. Activation of protein kinase C is essential for sustained insulin secretion in response to cholinergic stimulation. Biochim Biophys Acta. 1991;1091:120–122. doi: 10.1016/0167-4889(91)90231-l. [DOI] [PubMed] [Google Scholar]
- 8.Uchida T, Iwashita N, Ohara-Imaizumi M, Ogihara T, Nagai S, Choi JB, Tamura Y, Tada N, Kawamori R, Nakayama KI, Nagamatsu S, Watada H. Protein kinase Cdelta plays a non-redundant role in insulin secretion in pancreatic beta cells. J Biol Chem. 2007;282:2707–2716. doi: 10.1074/jbc.M610482200. [DOI] [PubMed] [Google Scholar]
- 9.Hagman DK, Latour MG, Chakrabarti SK, Fontes G, Amyot J, Tremblay C, Semache M, Lausier JA, Roskens V, Mirmira RG, Jetton TL, Poitout V. Cyclical and alternating infusions of glucose and intralipid in rats inhibit insulin gene expression and Pdx-1 binding in islets. Diabetes. 2008;57:424–431. doi: 10.2337/db07-1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glauser DA, Schlegel W. The emerging role of FOXO transcription factors in pancreatic beta cells. J Endocrinol. 2007;193:195–207. doi: 10.1677/JOE-06-0191. [DOI] [PubMed] [Google Scholar]
- 11.Furuya F, Shimura H, Asami K, Ichijo S, Takahashi K, Kaneshige M, Oikawa Y, Aida K, Endo T, Kobayashi T. Ligand-bound thyroid hormone receptor contributes to reprogramming of pancreatic acinar cells into insulin-producing cells. J Biol Chem. 2013;288:16155–16166. doi: 10.1074/jbc.M112.438192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Awale S, Tezuka Y, Banskota AH, Adnyana IK, Kadota S. Nitric oxide inhibitory isopimarane-type diterpenes from Orthosiphon stamineus of Indonesia. J Nat Prod. 2003;66:255–258. doi: 10.1021/np020455x. [DOI] [PubMed] [Google Scholar]
- 13.Awale S, Tezuka Y, Banskota AH, Adnyana IK, Kadota S. Highly-oxygenated isopimarane-type diterpenes from Orthosiphon stamineus of Indonesia and their nitric oxide inhibitory activity. Chem Pharm Bull (Tokyo) 2003;51:268–275. doi: 10.1248/cpb.51.268. [DOI] [PubMed] [Google Scholar]
- 14.Sriplang K, Adisakwattana S, Rungsipipat A, Yibchok-Anun S. Effects of Orthosiphon stamineus aqueous extract on plasma glucose concentration and lipid profile in normal and streptozotocin-induced diabetic rats. J Ethnopharmacol. 2007;109:510–514. doi: 10.1016/j.jep.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 15.Yam MF, Asmawi MZ, Basir R. An investigation of the anti-inflammatory and analgesic effects of Orthosiphon stamineus leaf extract. J Med Food. 2008;11:362–368. doi: 10.1089/jmf.2006.065. [DOI] [PubMed] [Google Scholar]
- 16.Yam MF, Lim V, Salman IM, Ameer OZ, Ang LF, Rosidah N, Abdulkarim MF, Abdullah GZ, Basir R, Sadikun A, Asmawi MZ. HPLC and anti-inflammatory studies of the flavonoid rich chloroform extract fraction of Orthosiphon stamineus leaves. Molecules. 2010;15:4452–4466. doi: 10.3390/molecules15064452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doleckova I, Rarova L, Gruz J, Vondrusova M, Strnad M, Krystof V. Antiproliferative and antiangiogenic effects of flavone eupatorin, an active constituent of chloroform extract of Orthosiphon stamineus leaves. Fitoterapia. 2012;83:1000–1007. doi: 10.1016/j.fitote.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Mohamed EA, Yam MF, Ang LF, Mohamed AJ, Asmawi MZ. Antidiabetic properties and mechanism of action of Orthosiphon stamineus Benth bioactive sub-fraction in streptozotocin-induced diabetic rats. J Acupunct Meridian Stud. 2013;6:31–40. doi: 10.1016/j.jams.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Son JY, Park SY, Kim JY, Won KC, Kim YD, Choi YJ, Zheng MS, Son JK, Kim YW. Orthosiphon stamineus reduces appetite and visceral fat in rats. J Korean Soc Appl Biol Chem. 2011;54:200–205. [Google Scholar]
- 20.Choi YJ, Park SY, Kim JY, Won KC, Kim BR, Son JK, Lee SH, Kim YW. Combined treatment of betulinic acid, a PTP1B inhibitor, with Orthosiphon stamineus extract decreases body weight in high-fat-fed mice. J Med Food. 2013;16:2–8. doi: 10.1089/jmf.2012.2384. [DOI] [PubMed] [Google Scholar]
- 21.Tajiri Y, Grill V. Aminoguanidine exerts a beta-cell function-preserving effect in high glucose-cultured beta-cells (INS-1) Int J Exp Diabetes Res. 2000;1:111–119. doi: 10.1155/EDR.2000.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roger B, Papin J, Vacher P, Raoux M, Mulot A, Dubois M, Kerr-Conte J, Voy BH, Pattou F, Charpentier G, Jonas JC, Moustaid-Moussa N, Lang J. Adenylyl cyclase 8 is central to glucagon-like peptide 1 signalling and effects of chronically elevated glucose in rat and human pancreatic beta cells. Diabetologia. 2011;54:390–402. doi: 10.1007/s00125-010-1955-x. [DOI] [PubMed] [Google Scholar]
- 23.McKinnon CM, Docherty K. Pancreatic duodenal homeobox-1, PDX-1, a major regulator of beta cell identity and function. Diabetologia. 2001;44:1203–1214. doi: 10.1007/s001250100628. [DOI] [PubMed] [Google Scholar]
- 24.Andrali SS, Sampley ML, Vanderford NL, Ozcan S. Glucose regulation of insulin gene expression in pancreatic beta-cells. Biochem J. 2008;415:1–10. doi: 10.1042/BJ20081029. [DOI] [PubMed] [Google Scholar]
- 25.Zhang SS, Hao E, Yu J, Liu W, Wang J, Levine F, Feng GS. Coordinated regulation by Shp2 tyrosine phosphatase of signaling events controlling insulin biosynthesis in pancreatic beta-cells. Proc Natl Acad Sci U S A. 2009;106:7531–7536. doi: 10.1073/pnas.0811715106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Y, Wang W, Liu Y, Guo T, Chen P, Ma K, Zhou C. alpha-lipoic acid inhibits high glucose-induced apoptosis in HIT-T15 cells. Dev Growth Differ. 2012;54:557–565. doi: 10.1111/j.1440-169X.2012.01356.x. [DOI] [PubMed] [Google Scholar]
- 27.Bernal-Mizrachi E, Fatrai S, Johnson JD, Ohsugi M, Otani K, Han Z, Polonsky KS, Permutt MA. Defective insulin secretion and increased susceptibility to experimental diabetes are induced by reduced Akt activity in pancreatic islet beta cells. J Clin Invest. 2004;114:928–936. doi: 10.1172/JCI20016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu H, MacFarlane WM, Tadayyon M, Arch JR, James RF, Docherty K. Insulin stimulates pancreatic-duodenal homoeobox factor-1 (PDX1) DNA-binding activity and insulin promoter activity in pancreatic beta cells. Biochem J. 1999;344 Pt 3:813–818. [PMC free article] [PubMed] [Google Scholar]
- 29.Yam MF, Basir R, Asmawi MZ, Ismail Z. Antioxidant and hepatoprotective effects of Orthosiphon stamineus Benth: standardized extract. Am J Chin Med. 2007;35:115–126. doi: 10.1142/S0192415X07004679. [DOI] [PubMed] [Google Scholar]
- 30.Koren-Gluzer M, Aviram M, Meilin E, Hayek T. The antioxidant HDL-associated paraoxonase-1 (PON1) attenuates diabetes development and stimulates beta-cell insulin release. Atherosclerosis. 2011;219:510–518. doi: 10.1016/j.atherosclerosis.2011.07.119. [DOI] [PubMed] [Google Scholar]
- 31.Alshawsh MA, Abdulla MA, Ismail S, Amin ZA, Qader SW, Hadi HA, Harmal NS. Free radical scavenging, antimicrobial and immunomodulatory activities of Orthosiphon stamineus. Molecules. 2012;17:5385–5395. doi: 10.3390/molecules17055385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akowuah GA, Ismail Z, Norhayati I, Sadikun A. The effects of different extraction solvents of varying polarities on polyphenols of Orthosiphon stamineus and evaluation of the free radical-scavenging activity. Food Chem. 2005;93:311–317. [Google Scholar]
- 33.Cumaoglu A, Ari N, Kartal M, Karasu C. Polyphenolic extracts from Olea europea L. protect against cytokine-induced beta-cell damage through maintenance of redox homeostasis. Rejuvenation Res. 2011;14:325–334. doi: 10.1089/rej.2010.1111. [DOI] [PubMed] [Google Scholar]
- 34.Sumaryono W, Proksch P, Wray V, Witte L, Hartmann T. Qualitative and quantitative analysis of the phenolic constituents from Orthosiphon aristatus. Planta Med. 1991;57:176–180. doi: 10.1055/s-2006-960060. [DOI] [PubMed] [Google Scholar]
- 35.Tezuka Y, Stampoulis P, Banskota AH, Awale S, Tran KQ, Saiki I, Kadota S. Constituents of the Vietnamese medicinal plant Orthosiphon stamineus. Chem Pharm Bull (Tokyo) 2000;48:1711–1719. doi: 10.1248/cpb.48.1711. [DOI] [PubMed] [Google Scholar]